Abstract
The reduction in infant birth weight and increased frequency of preeclampsia (PE) in high-altitude residents have been attributed to greater placental hypoxia, smaller uterine artery (UA) diameter, and lower UA blood flow (QUA). This cross-sectional case-control study determined UA, common iliac (CI), and external iliac (EI) arterial blood flow in Andeans residing at 3,600–4,100 m, who were either nonpregnant (NP, n = 23), or experiencing normotensive pregnancies (NORM; n = 155), preeclampsia (PE, n = 20), or gestational hypertension (GH, n = 12). Pregnancy enlarged UA diameter to ∼0.62 cm in all groups, but indices of end-arteriolar vascular resistance were higher in PE or GH than in NORM. QUA was lower in early-onset (≤34 wk) PE or GH than in NORM, but was normal in late-onset (>34 wk) illness. Left QUA was consistently greater than right in NORM, but the pattern reversed in PE. Although QCI and QEI were higher in PE and GH than NORM, the fraction of QCI distributed to the UA was reduced 2- to 3-fold. Women with early-onset PE delivered preterm, and 43% had stillborn small for gestational age (SGA) babies. Those with GH and late-onset PE delivered at term but had higher frequencies of SGA babies (GH=50%, PE=46% vs. NORM=15%, both P < 0.01). Birth weight was strongly associated with reduced QUA (R2 = 0.80, P < 0.01), as were disease severity and adverse fetal outcomes. We concluded that high end-arteriolar resistance, not smaller UA diameter, limited QUA and restricted fetal growth in PE and GH. These are, to our knowledge, the first quantitative measurements of QUA and pelvic blood flow in early- vs. late-onset PE in high-altitude residents.
Keywords: adaptation, Andean, Bolivia, Doppler ultrasound, hypoxia, intrauterine growth restriction, small for gestational age, vascular impedance
high-altitude provides a natural laboratory for investigating the effects of lowered oxygen availability, which cannot be duplicated in other circumstances for ethical reasons. Pregnancy at high altitude is complicated by an increased incidence of low birth weight babies, who are small for their gestational age (SGA), and of preeclampsia (PE), both of which have been attributed to placental hypoxia and reduced uteroplacental blood flow (16, 20, 26, 35, 43). High-altitude hypoxia affects the uteroplacental circulation both upstream at the level of the proximal uterine artery (UA) and downstream at the terminal arterioles. Reduced intervillous oxygen tension increases placental hypoxia-inducible factor (HIF) expression (41) and impairs angiogenesis in terminal arterioles (36) in a manner that resembles changes observed in pregnancies complicated by PE and SGA at sea level (33–35). At the same time, residence at high altitude reduces the pregnancy-associated increase in cardiac output, the fraction of common iliac blood flow distributed to the UA, and UA diameter and volumetric flow (14, 43). These changes occur early in pregnancy well before the onset of altitude-associated SGA (11) and, therefore, likely play a causative role.
SGA and PE share a similar pathology involving vascular endothelial dysfunction and impaired trophoblast invasion of maternal spiral arteries, resulting in increased UA vascular resistance and lower mean flow velocity, both of which precede the onset of clinical symptoms (4, 9, 31). Proximal UA diameter and volumetric flow are also decreased in normotensive (NORM) pregnancies complicated by SGA (18), but it is not known whether similar changes occur in PE.
High-altitude ancestry influences maternal vascular responses to pregnancy and fetal growth. Multigenerational high-altitude native Andean or Tibetan women have larger UA diameter and higher volumetric flow during pregnancy than do women of European or Han (“Chinese”) descent, which likely contribute to the heavier (more normal) birth weights and lower incidence of SGA babies seen in Andeans or Tibetans compared with European or Han (“Chinese”) high-altitude residents (12, 13, 27). However, Andean or Tibetan high-altitude natives still have a 2–4 times higher incidence of PE than their low-altitude counterparts (16, 24), suggesting that genetic factors affecting susceptibility to altitude-associated SGA do not protect against PE, and supporting the idea that the etiologies of SGA and PE differ (33, 34).
In this study, we asked whether native Andean high-altitude residents with PE or gestational hypertension (GH) had lower UA blood flow than their normotensive counterparts and, if so, whether the magnitude of flow reduction was related to the severity and occurrence of PE or other pregnancy complications such as preterm delivery and SGA. Secondarily, we sought to determine whether such alterations were due to differences in the proximal vs. terminal portions of the uterine vasculature. We hypothesized that UA blood flow was reduced in PE and SGA compared with normal pregnancies, with the degree of reduction being greatest in pregnancies with the poorest fetal outcomes. We considered that these studies could shed new light on how changes in the proximal vs. terminal uterine circulation contribute to PE and SGA. Such observations, in turn, could be of relevance for identifying predictive tests or new therapies for these pregnancy disorders in any setting but especially in developing countries such as Bolivia, where a high proportion of maternal deaths are due to preeclampsia/eclampsia (16).
MATERIALS AND METHODS
Study Design
This was a cross-sectional, case-control study of women with singleton pregnancies between 20 and 38 wk of pregnancy, and nonpregnant controls who presented for obstetrical care at five hospitals in La Paz (3,600 m), and El Alto (4,100 m), Bolivia. We excluded smokers, those who drank more than two alcoholic beverages weekly before pregnancy, any with active infections, and women at known risk of developing PE or GH (e.g., preexisting hypertension, cardiovascular, or renal diseases, gestational diabetes, and multiple gestation). Women selected for inclusion were self-identified as lifelong high-altitude (≥3,600 m) residents of Aymara or Quechua descent. Ancestry was confirmed by parental and grandparental surnames, and a panel of Ancestry Informative Markers, as previously described (40).
All subjects completed a health and demographic questionnaire, underwent an obstetrical exam, and gave written informed consent to study procedures that had been approved by the Colorado Multiple Institutional Review Board and the Colégio Médico, its Bolivian counterpart. During the physical exam, we measured resting heart rate, bilateral upper extremity blood pressures (BP), height, and weight; we estimated adiposity by the sum of biceps, triceps, and subscapular skin-fold thicknesses using Lange calipers (Beta Technology, Santa Cruz, CA); collected urine samples to screen for infection and proteinuria; drew venous blood for storage of serum or plasma; and conducted the Doppler ultrasound exam. Birth weights, newborn characteristics, and occurrence of perinatal or maternal complications were obtained from medical records and postnatal follow-up interviews.
Group Definitions
The study groups comprised women with NORM pregnancies (n = 155), those newly diagnosed with preeclampsia (PE, n = 20) or gestational hypertension (GH, n = 12), and nonpregnant controls (n = 23), who were either nulliparous, or more than 6 mo postpartum. NORM women were, by definition, normotensive and without any pregnancy complications, although a small number (6%) had 1+ proteinuria at the time of study. Women with PE and GH were normotensive before pregnancy, but developed elevated systolic (>140 mmHg) and diastolic (>90 mmHg) pressures after the 20th wk of pregnancy. These women also reported clinical symptoms, including sudden-onset facial or upper extremity edema, pitting lower extremity edema, headache, blurred vision, epigastric pain, nausea, and repeated vomiting that began within 24 h before presentation. It was the abruptness and severity of clinical symptoms that prompted these women to seek medical attention. PE was distinguished from GH by the presence of ≥1+ proteinuria at presentation and confirmed by ≥300 mg in a 24-h collection. Women with GH did not demonstrate proteinuria. We classified women as having either early-onset or late-onset disease depending on whether clinical manifestations of illness developed before or after 34 wk of pregnancy, respectively. All women with PE or GH were admitted to the hospital, placed on strict bed rest, and treated with alpha-methyldopa and continuous infusions of magnesium sulfate according to the local standard of medical care. Pretreatment systolic and diastolic BP ranged between 150 and 210 mmHg and between 95 and 120 mmHg, respectively, with values tending to be higher among women with PE. Indicating the severity of disease present, one subject who was not included in the data presented here was diagnosed with eclampsia at wk 36 and had a BP of 210/120 mmHg. While waiting for an operating room to become available, we observed exceedingly low values for bilateral QUA (255 ml/min) and flow reversal in the umbilical artery. At no time were treatments or interventions delayed or withheld for this or any of our studies.
Newborn Data
Newborn data are reported from all PE and GH pregnancies, but from only 125 NORM because 30 delivered under conditions in which birth weights were not recorded. None of these NORM women delivered prematurely or had peripartum complications. All women with GH and PE delivered in hospitals. Infants were considered preterm if born <37 wk gestation and term at 37–42 wk, calculated from the last menstrual period. Infants were classified as appropriate (AGA) or small (SGA) for gestational age based on whether their birth weights were above or below the 10th percentile of sea-level values for their gestational age and sex (39). While reduced relative to sea-level values, birth weights for babies born to Andean women at high altitude are greater than those of babies born to high-altitude residents of European or other populations of low-altitude ancestry (12).
Ultrasound Studies
Using a MicroMaxx (SonoSite, Bothell, WA) equipped with C60e and L38e transducers, we imaged bilateral maternal common iliac (CI) and external iliac (EI) arteries 2–4 cm from their bifurcation, and the uterine arteries (UA) at the anatomic level, where the UA appears to cross the EI (18, 19, 30, 40). Mean vessel diameters were calculated as (2 × end-diastolic + peak systolic diameter)/3. Flow velocities were measured from traces containing at least six consecutive cardiac cycles of good quality, as described and validated previously (30, 40). Peak systolic (PSV), end-diastolic velocity (EDV), resistive index (RI), pulsatility index (PI), and systolic to diastolic (S/D) velocity ratios were recorded in the UA, while PSV and minimum diastolic velocities were recorded for the CI and EI (Fig. 1). Total UA blood flow (QUA, l/min) and net antegrade CI and EI flow were reported as the sum of left- and right-sided flows calculated as the product of time-averaged mean flow velocity (TAM, cm/s) and vessel cross-sectional area (cm2) using the equation: Q = πr2 × TAM × 0.06, where r is the mean vessel radius. Because of the triphasic nature of the CI and EI waveforms, the MicroMaxx systematically underestimated total blood flow. Therefore, we normalized the flows and flow ratios for these vessels to nonpregnant values. UA vascular resistance was calculated as mean arterial pressure (MAP)/QUA. To improve reproducibility and minimize error, a single operator performed all measurements, which were later reviewed for accuracy by three of the coauthors. Placental location was recorded in 100 subjects. In more than half of the cases (n = 57), the placenta was centrally located (29 anterior, 17 posterior, 11 fundal); the remainder were laterally placed (19 left, 24 right).
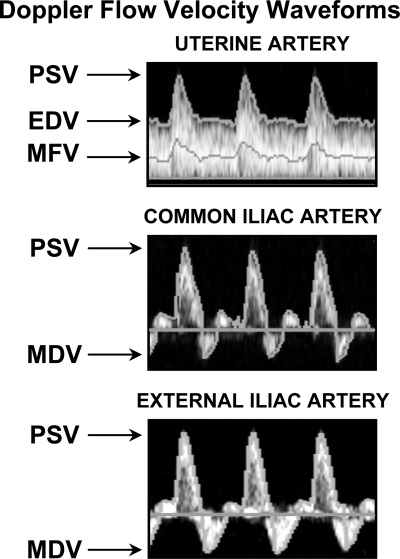
Fig. 1.
Shown are representative Doppler flow-velocity waveforms from uterine (UA), common iliac (CI), and external iliac (EI) arteries. UA blood flow (QUA) is continuous throughout the cardiac cycle and demonstrates a low-resistance pattern characterized by high peak systolic (PSV), end-diastolic (EDV), and mean flow (MFV) velocities. QCI and QEI demonstrate a high-resistance triphasic, pulsatile pattern with an antegrade midsystolic peak (PSV) followed rapidly by a retrograde minimum early-diastolic velocity (MDV), and then by resumption of forward flow that tapers off in late diastole.
Statistics
In the text, tables, and figures, data are expressed as means ± SE or percentages with 95% confidence intervals after normality was affirmed using Kolmogorov and Smirnov tests. Since groups were independent, we used Student's t-test or ANOVA with Bonferroni corrections for multiple comparisons. Birth weights were adjusted for gestational age and sex, and frequencies of preterm and SGA births were compared using Fisher's exact test. All analyses were conducted using InStat and Prism (GraphPad Software, San Diego, CA) and considered significant when P < 0.05.
RESULTS
Maternal Physical Characteristics
The women were overwhelmingly of Amerindian ancestry by self-report and analysis of gene markers; modest differences between groups were due to one or two subjects with a higher proportion of European genes (Table 1). Women with GH or PE were older, heavier, and of higher gravidity than NORM women, but similar in terms of parity, height, and adiposity (data not shown). At the time of ultrasound study, resting heart rate was similar in all groups, but blood pressures were higher in women with GH or PE, despite treatment with alpha-methyldopa and magnesium sulfate.
Table 1.
Maternal and newborn characteristics in nonpregnant controls, normal pregnancies with term or preterm delivery, gestational hypertension, and preeclampsia before and after 34 wk of pregnancy
| Nonpregnant |
Normal Pregnancy |
GH |
PE ≤34wk |
PE > 34wk |
||
|---|---|---|---|---|---|---|
| Pregnancy Stage at Delivery | N/A (n = 23) | Term (n = 119) | Preterm (n = 6) | Term (n = 12) | Preterm (n = 7) | Term (n = 13) |
| Maternal Characteristics | ||||||
| Ancestry, % | ||||||
| Amerindian | 83.6 ± 1.2 | 84.6 ± 0.4 | 79.5 ± 6.8 | 78.8 ± 2.6† | 75.4 ± 5.4*† | 83.2 ± 1.6 |
| European | 8.6 ± 1.3 | 7.1 ± 0.4 | 12 ± 5.3 | 14.3 ± 2.4† | 17.6 ± 5.7*† | 8.4 ± 1.6 |
| West African | 7.8 ± 0.7 | 8.3 ± 0.3 | 8.5 ± 1.7 | 6.9 ± 1.0 | 7.0 ± 0.7 | 8.4 ± 1.6 |
| Age, yr | 27 ± 1.3 | 26 ± 0.5 | 23 ± 2.8 | 28 ± 2.7 | 27 ± 4.6 | 31 ± 1.6†§ |
| Gravidity, no. of pregnancies | 1.4 ± 0.3 | 2 ± 0.14 | 2 ± 0.4 | 3 ± 0.98 | 3 ± 0.72 | 3 ± 0.6* |
| Parity, no. of live births | 1.1 ± 0.2 | 1 ± 0.13 | 1 ± 0.4 | 2 ± 0.94 | 2 ± 0.62 | 2 ± 0.6 |
| Height, cm | 152.6 ± 1.3 | 150 ± 0.4 | 150 ± 0.8 | 153 ± 1.8 | 151 ± 2.8 | 151 ± 2.1 |
| Weight, kg | 55.2 ± 1.4 | 62 ± 0.8* | 61 ± 3.3 | 72 ± 3.4*† | 68 ± 4.1*§ | 81 ± 3.4*‡ |
| Heart rate, beats/min | 70 ± 2 | 77 ± 1 | 79 ± 4 | 78 ± 2 | 78 ± 6 | 80 ± 3 |
| Blood pressure, mmHga | ||||||
| Systolic | 89 ± 1 | 91 ± 1 | 96 ± 2 | 113 ± 3*† | 137 ± 5*†‡ | 127 ± 3*‡§ |
| Diastolic | 63 ± 1 | 60 ± 1 | 65 ± 2 | 79 ± 3*† | 93 ± 3*†‡ | 86 ± 2*‡ |
| Mean | 72 ± 1 | 71 ± 1 | 75 ± 3 | 90 ± 3*† | 108 ± 4*†‡§ | 100 ± 3*‡ |
| Newborn Characteristics | ||||||
| Gestational age, wk | 39.1 ± 0.1 | 33.8 ± 0.5§ | 38.1 ± 0.4† | 30.2 ± 1.2†§ | 38.8 ± 0.4 | |
| AGA | 39.1 ± 0.1 | 33.8 ± 0.5§ | 38.0 ± 0.6 | 32.7 ± 0.6§ | 38.8 ± 0.4 | |
| SGA | 39.1 ± 0.2 | 38.3 ± 0.6 | 27.0 ± 0.7§ | 38.3 ± 0.7 | ||
| Birth weight, g | 3182 ± 40 | 2136 ± 236§ | 2748 ± 46† | 1285 ± 238*‡§ | 2742 ± 125* | |
| AGA | 3286 ± 38 | 2136 ± 236§ | 3130 ± 83 | 1750 ± 168‡§ | 3126 ± 107 | |
| SGA | 2613 ± 37 | 2366 ± 44† | 667 ± 83*‡§ | 2378 ± 106* | ||
| SGA babies, % | 18 (15%) | 0 (0%) | 6 (50%)† | 3 (43%)† | 6 (46%)* | |
| Fetal deaths | 1 | 1 | 0 | 3 | 0 | |
Values are expressed as means ± SE. aBlood pressure was taken at the time of ultrasound study. (All women with PE or GH had BP >140/90 at the time of admission.) Blood pressures reported for gestational hypertension (GH) and preeclampsia (PE) groups were measured after treatment with alpha-methyldopa and magnesium sulfate. AGA, appropriate for gestational age; SGA, small for gestational age.
P < 0.05, nonpregnant vs. pregnant;
P < 0.05, normal pregnancy vs. GH or PE;
P < 0.05, GH vs. PE; and
P < 0.05, term vs. preterm delivery.
Maternal Blood Flow Characteristics
Uterine artery.
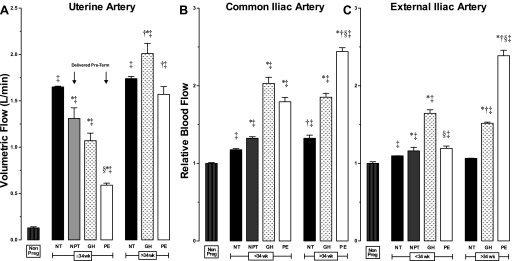
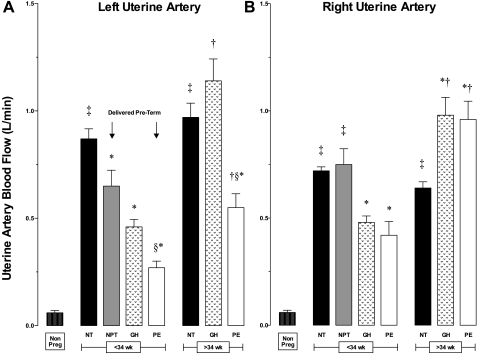
Pregnancy raised UA diameters equally in all groups, and values were similar at mid (20–24 wk) and late (≥36 wk) gestation (Table 2). Compared with the nonpregnant state, pregnancy lowered UA vascular resistance and indices of end-arteriolar vascular impedance (RI, PI, S/D ratios), but these values were much higher in PE than NORM (Table 2). Women with PE or GH had lower UA blood flow (QUA) than NORM (Fig. 2A) and a higher frequency of bilateral early diastolic notches (PE = 85%, GH = 65% vs. NORM = 8%, both P < 0.01), consistent with higher downstream vascular impedance. NORM women who delivered preterm also had lower QUA at ≤ 34 wk (Fig. 2A) that was due entirely to reduced left QUA (Fig. 3). In late-onset PE or GH, QUA was similar to NORM (Fig. 2A), but there were marked differences in unilateral blood flow (Fig. 3). In late-onset PE, left QUA was markedly reduced, and unilateral early diastolic notches were present in more than half of the women, consistent with higher left-sided vascular impedance (Table 2). In contrast, right QUA was higher in late-onset GH and PE, so that total QUA was similar to that seen in NORM. Placental location and its relationship to unilateral QUA did not differ between groups.
Table 2.
Uterine, common iliac, and external iliac artery Doppler ultrasound indices in nonpregnant controls, normal pregnancy, gestational hypertension, and preeclampsia presenting before and after 34 wk of pregnancy
| Nonpregnant | Pregnant Groups | ≤34 Week | >34 Week | |
|---|---|---|---|---|
| Uterine Artery | ||||
| Diameter, cm | 0.38 ± 0.01 (23) | Normal pregnancy | 0.62 ± 0.01 (115)‡ | 0.61 ± 0.01 (40)‡ |
| GH | 0.57 ± 0.03 (4)‡ | 0.66 ± 0.02 (8)‡ | ||
| Preeclampsia | 0.61 ± 0.02 (7)‡ | 0.62 ± 0.03 (14)‡ | ||
| PSV | 68.5 ± 5.5 (23) | Normal pregnancy | 115.3 ± 3.1 (115)‡ | 131.9 ± 6.2 (40)*†‡ |
| GH | 128.3 ± 26.1 (4)‡ | 166.5 ± 19.7 (8)†‡ | ||
| Preeclampsia | 93.2 ± 8 (7)*‡ | 132.4 ± 12.4 (14)*†‡ | ||
| End-diastolic velocity | 8.04 ± 0.26 (23) | Normal pregnancy | 58.4 ± 1.8 (115)‡ | 68.4 ± 3.3 (40) †‡ |
| GH | 53.3 ± 5.1 (4)‡ | 86.9 ± 5.8 (8)*†‡ | ||
| Preeclampsia | 23.9 ± 2.9 (7)*‡§ | 64.1 ± 6.9 (14)†‡§ | ||
| TAM, cm/s | 8.8 ± 0.9 (23) | Normal pregnancy | 38.4 ± 1.1 (115)‡ | 43.9 ± 2.1 (40)†‡ |
| GH | 33.5 ± 3.1 (4)‡ | 48.6 ± 3.1 (8)†‡ | ||
| Preeclampsia | 18.6 ± 3.3 (7)*§ | 36.8 ± 4.2 (14)†‡ | ||
| Resistance index | 0.89 ± 0.02 (23) | Normal pregnancy | 0.52 ± 0.01 (115)‡ | 0.49 ± 0.01 (40)‡ |
| GH | 0.53 ± 0.01 (4)‡ | 0.48 ± 0.03 (8)‡ | ||
| Preeclampsia | 0.72 ± 0.04 (7)*†‡ | 0.54 ± 0.03 (14)‡ | ||
| Pulsatility index | 3.92 ± 0.33 (23) | Normal pregnancy | 0.79 ± 0.02 (115)‡ | 0.73 ± 0.03 (40)‡ |
| GH | 0.92 ± 0.03 (4)‡ | 0.85 ± 0.10 (8)‡ | ||
| Preeclampsia | 1.89 ± 0.22 (7)*†‡§ | 1.05 ± 0.10 (14)*‡ | ||
| S/D ratio | 25.18 ± 3.45 (23) | Normal pregnancy | 2.20 ± 0.05 (115)‡ | 2.01 ± 0.05 (40)†‡ |
| GH | 2.19 ± 0.05 (4)‡ | 2.10 ± 0.06 (8)‡ | ||
| Preeclampsia | 4.38 ± 0.61 (7)*†‡§ | 2.42 ± 0.17 (14)*‡ | ||
| Vascular resistance, mmHg·ml−1·min | 1.93 ± 0.22 (46) | Normal pregnancy | 0.14 ± 0.01 (115)‡ | 0.13 ± 0.01 (40)‡ |
| GH | 0.20 ± 0.02 (4)‡ | 0.18 ± 0.07 (8)‡ | ||
| Preeclampsia | 0.52 ± 0.01 (7)*‡§ | 0.33 ± 0.08 (14)*‡ | ||
| Common Iliac Artery | ||||
| Diameter, cm | 0.70 ± 0.01 (23) | Normal pregnancy | 0.74 ± 0.01 (115) | 0.75 ± 0.02 (40) |
| GH | 0.81 ± 0.03 (4)*‡ | 0.76 ± 0.03 (8) | ||
| Preeclampsia | 0.82 ± 0.03 (7)*‡ | 0.78 ± 0.02 (14)‡ | ||
| PSV | 107.2 ± 4.4 (23) | Normal pregnancy | 102.2 ± 1.6 (115) | 95.7 ± 2.6 (40)‡ |
| GH | 102.6 ± 13.1 (4) | 102.9 ± 4.5 (8) | ||
| Preeclampsia | 87.5 ± 5.6 (7)*‡ | 109.4 ± 6.1 (14) | ||
| MDV | −23.8 ± 3.3 (23) | Normal pregnancy | −37.6 ± 0.7 (115)‡ | −36.1 ± 1.3 (40)‡ |
| GH | −28.2 ± 2.1 (4)* | −28.8 ± 1.8 (8)* | ||
| Preeclampsia | −23.6 ± 2.5 (7)* | −23.7 ± 2.1 (14)* | ||
| TAM, cm/s | 7.4 ± 0.6 (23) | Normal pregnancy | 7.3 ± 0.3 (115) | 7.6 ± 0.6 (40) |
| GH | 11.1 ± 1.4 (4)*‡ | 11.7 ± 1.6 (8)*‡ | ||
| Preeclampsia | 9.5 ± 1.3 (7) | 14.6 ± 1.2 (14)*†‡ | ||
| External Iliac Artery | ||||
| Diameter, cm | 0.62 ± 0.01 (23) | Normal pregnancy | 0.71 ± 0.01 (115)‡ | 0.71 ± 0.02 (40)‡ |
| GH | 0.80 ± 0.04 (4)‡ | 0.72 ± 0.03 (8)‡ | ||
| Preeclampsia | 0.76 ± 0.03 (7)‡ | 0.79 ± 0.02 (14)‡ | ||
| PSV | 112.6 ± 4.8 (23) | Normal pregnancy | 110.3 ± 1.6 (115) | 105.7 ± 3.1 (40) |
| GH | 97.1 ± 9.4 (4) | 103.9 ± 6.3 (8) | ||
| Preeclampsia | 89.6 ± 8.1 (7)*‡ | 105.6 ± 5.0 (14)† | ||
| MDV | −27.6 ± 1.9 (23) | Normal pregnancy | −39.9 ± 0.6 (115)‡ | −38.3 ± 1.1 (40)‡ |
| GH | −31.9 ± 2.4 (4)* | −26.2 ± 2.3 (8)* | ||
| Preeclampsia | −23.2 ± 2.4 (7)* | −27.6 ± 1.6 (14)* | ||
| TAM, cm/s | 8.38 ± 0.77 (23) | Normal pregnancy | 6.9 ± 0.3 (115) | 6.9 ± 0.4 (40) |
| GH | 8.6 ± 0.9 (4) | 9.5 ± 0.9 (8)* | ||
| Preeclampsia | 7.9 ± 1.2 (7) | 11.5 ± 0.9 (14)*†‡ | ||
Values are expressed as means ± SE; n = number in parentheses. Peak systolic (PSV), minimum diastolic (MDV), end-diastolic (EDV), and time-averaged mean (TAM) velocities. Resistance index (S-D)/S. Pulsatility index, (S-D)/mean. Systolic to diastolic (S/D) velocity ratio. UA, uterine artery; CI, common iliac artery; EI, external iliac (artery); GH, gestational hypertension. Calculated vascular resistance = MAP/total uterine artery volumetric flow.
P < 0.05, normal pregnancy vs. GH or PE;
P < 0.05, ≤ 34 wk vs. >34 wk;
P < 0.05, nonpregnant vs. pregnant;
P < 0.05, GH vs. PE.
Fig. 2.
Blood flow in the UA, CI, and EI arteries in nonpregnant controls (Non Preg), normotensive pregnant women who delivered at term (NT) or preterm (NPT), and women with gestational hypertension (GH) or preeclampsia (PE) who were ≤34 wk or >34 wk of pregnancy at the time of ultrasound study. A: compared with NT, uterine artery blood flow (QUA) was lower in NPT and markedly reduced in early-onset but not in late-onset GH and PE. B and C: women who developed PE and GH had greater pregnancy-associated increases in QCI and QEI than NT. P < 0.01 in *NT vs. NPT, GH, or PE; †≤34 wk vs. >34 wk; ‡Nonpregnant vs. pregnant; and §GH vs. PE.
Fig. 3.
Left and right QUA were equal in nonpregnant women but dissimilar during pregnancy. Left QUA (A) was consistently higher than the right (B) in normotensive women who delivered at NT and in late-onset GH. The reverse occurred in normotensive women who delivered NPT and in women with PE, with left QUA being much lower than the right QUA. P < 0.01 in *NT vs. NPT, GH, or PE; †≤34 wk vs. >34 wk; ‡Nonpregnant vs. pregnant; and §GH vs. PE.
Common iliac artery.
Pregnancy raised CI blood flow (QCI) due to greater TAM (Fig. 2B, Table 2). QCI was 1.5 to 2 times higher in PE or GH than NORM due to larger diameter, lower retrograde early-diastolic blood flow, and higher TAM (Table 2). QCI was similar before and after 34 wk in NORM and GH, but much higher in late- vs. early-onset in PE due to higher TAM (Fig. 2B, Table 2).
External iliac artery.
Pregnancy raised EI diameter in all groups, but TAM increased only in late-onset PE or GH (Table 2). All women with GH and those with late-onset PE had higher EI blood flow (QEI) than NORM (Fig. 2C), as the result of lower retrograde early-diastolic blood flow and higher TAM (Table 2). QEI was much higher in late- vs. early-onset PE but was similar in NORM and GH before and after 34 wk.
Pelvic blood flow distribution.
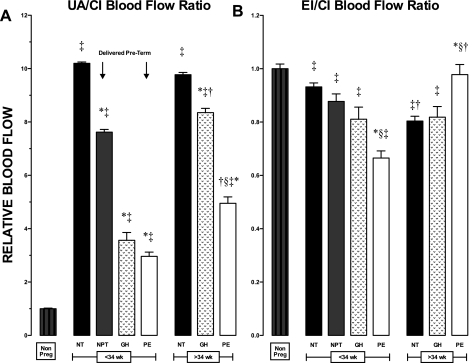
UA/CI ratios were much higher and EI/CI ratios lower in all pregnant women than in nonpregnant controls, reflecting the redistribution of CI flow to favor the uteroplacental circulation (Fig. 4). UA/CI ratios were similar before and after 34 wk in normal pregnancy but reduced in normotensive women who delivered preterm and in all women with GH or PE (Fig. 4A). UA/CI ratios were higher in late- than early-onset GH and PE due to proportionally higher QUA (Fig. 2). Compared with nonpregnant values, EI/CI ratios were lower in GH, early-onset PE, and NORM women >34 wk, likely reflecting higher peripheral vascular resistance.
Fig. 4.
CI artery blood flow is redistributed to favor the UA during pregnancy. A: compared with normotensive women who delivered at NT, UA relative to CI blood flow (UA/CI ratio) was reduced in normotensive women who delivered NPT and in women with GH or PE. B: EI relative to CI blood flow (EI/CI ratio) decreased during pregnancy and was lowest in GH, early-onset PE, and NT >34 wk. P < 0.05 in *NT vs. NPT, GH, or PE; †≤34 wk vs. >34 wk; ‡Nonpregnant vs. pregnant; and §GH vs. PE.
Fetal Outcomes
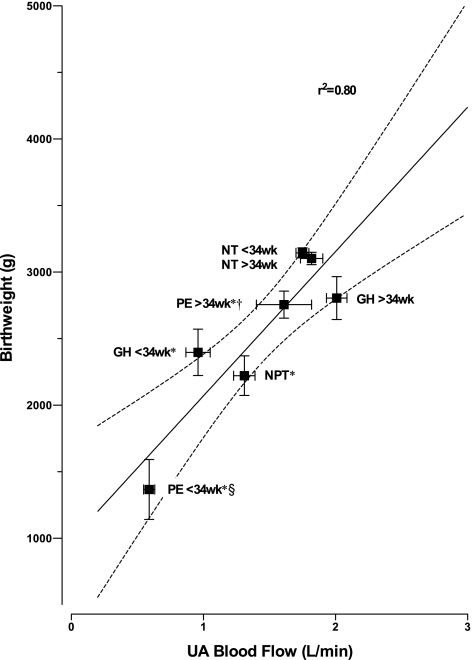
Women with early-onset PE delivered preterm, and 43% had stillborn SGA babies (Table 1). Although premature AGA babies were similar in gestational age and weight, those born to women with early-onset PE were younger and more severely growth-restricted than those born to NORM women (Table 1). Women with GH and late-onset PE delivered at term, but had a three-fold greater frequency of SGA (GH = 50%, PE = 46%, NORM = 15%, P < 0.01). Overall, the frequency of low-birth weight infants (<2500 g) was 6.3-fold higher in women with PE and GH than in NORM (44% vs. 7%, P < 0.0001) and was inversely related to UA blood flow (Fig. 5). Across all groups, QUA was strongly and positively associated with infant birth weight (R2 = 0.80, P < 0.01, Fig. 5). Furthermore, 76% of women who had reduced UA blood flow at ≤34 wk of pregnancy delivered prematurely, and 59% had SGA babies (Table 1), underscoring the importance of reduced QUA.
Fig. 5.
Uterine artery (UA) artery blood flow (QUA) at the time of ultrasound study are strongly related to average birth weight when comparing women who remained normotensive and delivered at term (NT) or preterm (NPT), and those who developed GH or PE. Reduced QUA before 34 wk was associated with lower birth weight and an increased risk of premature delivery. The solid line with dotted 95% CI shows the overall correlation, and the R2 value of 0.80 indicates that 80% of the variation in birth weight can be explained by QUA across all groups. P < 0.05 in *NT vs. NPT, GH, or PE; †≤34 wk vs. >34 wk; and §GH vs. PE.
DISCUSSION
This is the first study to compare UA and pelvic flow in Andean high-altitude residents experiencing normal pregnancies, PE, or GH. Our principal findings were that 1) high-end arteriolar vascular resistance, not smaller UA diameter, reduced QUA in PE and GH compared with NORM and 2) the magnitude of QUA reduction correlated with the degree of disease severity and adverse fetal outcomes. Compensatory increases in CI blood flow and dynamic balancing of left and right QUA permitted women with late-onset PE or GH to achieve normal total QUA and maintain pregnancy to term, with an albeit higher incidence of SGA.
Our study findings are subject to certain limitations. The cross-sectional design allowed us to sample women in larger numbers than in other reports but limited our ability to define longitudinal changes. However, the blood flow patterns in the NORM subjects were similar to those seen previously in longitudinal studies of normal pregnant Andean high-altitude residents (13, 30, 40). While both early- and late-onset hypertensive disease was studied, we recognize that the women may have been at fundamentally different time points in the course of their illness since early-onset disease is likely to be diagnosed later in the course of the disease and to be more severe. Therefore, we were careful to compare each group to NORM women whose pregnancies were at the same gestational stage. The accuracy with which volumetric flow can be measured using Doppler ultrasound is limited, but the rise in QUA is due principally to the near doubling of UA diameter, which can be easily identified, and the flow estimates observed agree with more directly measured values (see Ref. 30 for a review). Since a single operator made all measurements, the range of values within groups was narrow, and high reproducibility estimates have been observed previously (30), we consider that the error in our QUA estimates is likely to be <10%. Additional assurance is provided by the fact that our QUA values are within the expected range, given that cardiac output in pregnant high-altitude Andeans is 5–7 liters (14, 38), the uteroplacental circulation receives 20–25% of cardiac output (23), and ∼80% of total uteroplacental blood flow is derived from the UA. Because all PE and GH women received alpha-methyldopa and magnesium sulfate, both of which lower BP and UA vascular resistance (29), we concluded that the QUA reductions seen in PE or GH were real and may have been more severe than indicated by our data.
Our measurements of QUA and related parameters in NORM pregnancies are consistent with the known Andean protection from fetal growth reduction (12). The near doubling in UA diameter and rise in UA flow resembles that seen in healthy pregnant women at low altitude (19, 30), suggesting that conservation of physiological mechanisms responsible for enlarging proximal UA enlargement are involved in Andean protection from altitude-associated SGA. This makes physiological sense since the enlarged UA diameter, along with systemic changes in blood volume and cardiac output (3, 7, 23), are responsible for raising QUA (19, 30). Unlike pregnancy at low altitude, in which UA diameter continues to increase after 20 wk (18, 19), a consistent finding in this and other high-altitude studies is that UA enlargement is maximal by week 20. Since the pregnancy-associated rise in maternal cardiac output is reduced at high vs. low altitude (14), maximal enlargement of UA diameter earlier in gestation may be a physiological mechanism for bolstering uteroplacental blood flow.
A new finding was that while QUA was lower in GH or PE compared with NORM women, UA diameter was the same. To the best of our knowledge, this is the first measurement of UA diameter in pregnancies characterized by PE or GH at any altitude. These observations indicate that this component of maternal vascular response to pregnancy at high altitude remains intact in the Andean women, unlike what is seen in European high-altitude residents in whom chronic hypoxia reduces the pregnancy-associated rise in UA diameter (11, 13). It also shows that lower QUA in PE or GH vs. NORM women was due entirely to lower mean flow velocity and, in turn, higher downstream vascular impedance as demonstrated by higher RI, PI, and S/D ratios. Higher vascular resistance was further corroborated by a high prevalence of early diastolic notching, similar to that reported in PE and GH at low altitudes (4, 6) and known to correlate with reduced UA and intervillous blood flow (21). Therefore, we concluded that the low QUA in Andean women with PE and GH was due to impaired end-arteriolar growth or dilation (5, 17, 32) and that such effects were independent of influences of pregnancy or hypoxia acting upstream on the main UA.
Another novel finding was the marked asymmetry in bilateral QUA. In normotensive pregnancies, such asymmetry may be due to supine posture causing aorto-caval compression (15) or anatomic features of the aortic and iliac bifurcations, since UA diameters and impedance ratios were similar on the right and left sides. However, in PE or early-onset GH, vascular impedance was consistently higher in the UA with lower blood flow. This suggests that although PE affects both sides of the uteroplacental circulation, dysregulation of the downstream (i.e., arcuate, radial, or spiral arteries) vascular function is not uniformly distributed. This is consistent with observations that trophoblast invasion and remodeling are reduced but not uniformly distributed at high altitude (36). Hence, asymmetric blood flow may be related to regional heterogeneity in the transformation of spiral arteries into low-resistance vessels (17). Other investigators have shown that when the placenta is laterally placed, volumetric flow is higher and vascular impedance lower in the ipsilateral UA and that there is an increased incidence of PE, fetal distress, Cesarean sections, and IUGR (8, 19, 22). Because we did not observe differences in the location of the placenta among groups or any relationship between placental location and QUA, we concluded that altered trophoblast invasion and remodeling rather than placenta location was responsible for the asymmetry observed.
Pelvic blood flow was markedly altered in PE and GH, as well as in preterm deliveries. Although QCI was higher than in NORM, the fraction distributed to the UA was reduced not only in PE or GH but also in normotensive women who delivered prematurely. Blood flow was higher in the ipsilateral CI but lower in the EI on the side with the higher QUA. Likewise, peripheral resistance in the leg was consistently higher in the ipsilateral EI but lower in PE than in NORM pregnancies. This suggests that when the UA are maximally enlarged, increasing CI blood flow and decreasing blood flow to the leg may be compensatory mechanisms for raising uteroplacental volumetric flow. Previous studies have shown that the fraction of CI blood flow distributed to the UA is lower in normotensive pregnancies at high vs. low altitude (43, 44), in newcomers compared with high-altitude natives (28, 40, 44), and in late-onset PE compared with normotensive pregnancies (42). Together, these data suggest that pelvic blood flow is dynamically redistributed to optimize QUA over a wide range of conditions, including changing metabolic demands of the growing fetus, maternal posture, and activity (10, 15). These mechanisms restored QUA to normal levels in late- but not early-onset PE and GH, perhaps because of the high downstream vascular impedance or reduced cardiac output (3, 7, 37).
Consistent with previous reports, early- and late-onset PE and GH appeared to be distinct disorders. Compared with NORM, early- but not late-onset disease was typified by normal maternal weight, markedly reduced QUA, and high UA vascular impedance. Elevated QCI maintained normal QUA and even appeared to raise QEI in late-onset PE. These observations agree with previous studies in which lower cardiac output and higher peripheral vascular resistance were seen in women with early-onset PE, whereas late-onset PE or GH was characterized by higher cardiac output and low to normal vascular resistance (3, 7, 37). Thus, early disease appears to represent a failure of both central and uteroplacental vascular responses to pregnancy that serve collectively to reduce uteroplacental blood flow, whereas late-onset disease appears to be more an effort to defend uteroplacental blood flow in a setting where the maternal circulation is encumbered by excess body weight or limitations in oxygen availability, such as are present at high altitude.
Our data confirm a strong association between reduced QUA and decreased birth weight at high altitude (11, 13, 44), and they exhibit, for the first time, the strength of this association when the full range of variation in QUA flow and birth weight are taken into account by the inclusion of women with pregnancy complications. Our data are also consistent with the concept that the increased frequency and severity of SGA in PE and GH are related to high UA vascular resistance and low intervillous blood flow (4, 6, 9, 17–19, 21, 31). Thus, maintenance of high UA blood flow during pregnancy at high altitude appears to play a key role in defending fetal growth, as well as in the pathogenesis of PE and GH, but whether the benefits accrue from increased delivery of oxygen or other nutrients, defense of placental metabolism, or other factors is unknown (25, 44, 45).
Perspectives and Significance
The maternal vascular response to pregnancy in multigenerational high-altitude populations is thought to reflect key heritable adaptations that improve reproductive success by maintaining a normal increase in uteroplacental blood flow. Despite these adaptations, multigenerational high-altitude populations are equally vulnerable to effects of hypoxia acting to increase the incidence of PE and GH, underscoring the likelihood that the etiologies of SGA and PE/GH differ. Together, with previous studies, these observations suggest that Andean ancestry acts to preserve normal UA enlargement but does not prevent the impaired trophoblast invasion and remodeling of maternal end-arterioles characteristic of PE or GH. Early- vs. late-onset PE or GH are distinguished not only by the severity of impaired vascular remodeling but also by the ability to increase maternal cardiac output and redistribute QCI to the UA in an attempt to overcome downstream flow limitations. Further studies are needed to better understand what factors influence individual susceptibility to early vs. late-onset disease and, in particular, the contributions of alterations in HIF- or other oxygen-sensitive genes (1, 2), gene expression, and/or circulating levels of vasoactive or angiogenic factors. It will also be important to determine whether the changes in UA and pelvic blood flow seen in PE or GH at high altitude also occur at low altitude and whether measuring QUA in high-risk pregnancies could improve early detection or prediction of fetal outcomes.
GRANTS
This study was funded by National Heart Lung and Blood Institute Grants HL079647 and HL079647-S1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This study would not have been possible without the generous support of SonoSite, which supplied the ultrasound equipment. We thank Armando Rodriguez, Martha Aguilar, and Marcelino Guarachi at Instituto Boliviano de Biología de Altura for their technical assistance, and acknowledge the vital collaboration of the hospital administrators, OB/Gyn physicians, and nurses at Hospital de la Mujer, Hospital Materno-Infantil, Hospital Andes, Hospital Boliviano Holandes, and Hospital Corea in La Paz and El Alto, Bolivia. We also thank Jennifer Hageman, Paige Sheen, and Barbara Lommen for their logistic and administrative support.
REFERENCES
- 1. Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, Lopez Herraez D, Brutsaert T, Parra EJ, Moore LG, Shriver MD. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 6: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, Shriver MD. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics 4: 79–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosio P, McKenna P, Conroy R, O'Herlihy C. Maternal cental hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol 94: 978–984, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bower S, Bewley S, Campbell S. Improved prediction of preecalmpsia by two-stage screeening of uterine arteries using the early diastolic notch and color Doppler imaging. Obstet Gynecol 82: 78–83, 1993 [PubMed] [Google Scholar]
- 5. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1: 177–191, 1972 [PubMed] [Google Scholar]
- 6. Dugoff L, Lynch A, Cioffi-Ragan D, Hobbins JC, Schultz L, Malone F, D'Alton M. First trimester uterine artery Doppler abnomalities predict subsequent intrauterine growth restriction. Am J Obstet Gynecol 193: 1208–1212, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Easterling T, Benedetti T, Schmucker B, Millard S. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol 76: 1061–1069, 1990 [PubMed] [Google Scholar]
- 8. Gonser M, Tillack N, Pfeiffe K, Mielke G. Placental location and incidence of preeclampsia. Ultraschall Med 17: 236–238, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Harrington K, Fayyad A, Thakur V, Aquilina J. The value of uterine artery Doppler in the prediction of uteroplacental complications in multiparous women. Ultrasound Obstet Gynecol 23: 50–55, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Jeffreys RM, Stepanchak W, Lopez B, Hardis J, Clapp JF., 3rd Uterine blood flow during supine rest and exercise after 28 weeks of gestation. BJOG 113: 1239–1247, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol 295: R906–R915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 92: F372–F377, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 296: R1564–R1575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kametas N, McAuliffe F, Krampl E, Chambers J, Nicolaides K. Maternal cardiac function during pregnancy at high altitude. BJOG 111: 1051–1058, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kauppila A, Koskinen M, Pouolakka J, Tuimala R, Kuikka J. Decreased intervillous and unchanged myometrial blood flow in supine recumbency. Obstet Gynecol 55: 203–205, 1980 [PubMed] [Google Scholar]
- 16. Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young D, Villena M, Moore LG. Intrauterine growth restriction, preeclampsia and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93: 1049–1059, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Konje J, Howarth E, Kaufmann P, Taylor D. Longitudinal quantification of uterine artery blood flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG 110: 301–305, 2003 [PubMed] [Google Scholar]
- 19. Konje J, Kaufmann P, Bell S, Taylor D. A longitudinal study of quantitative uterine blood flow with the use of color power angiography in appropriate for gestational age pregnancies. Am J Obstet Gynecol 185: 608–613, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Krampl E, Espinoza-Dorado J, Lees CC, Moscoso G, Bland JM. Maternal uterine artery Doppler studies at high altitude and sea level. Ultrasound Obstet Gynecol 18: 578–582, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lunell NO, Lewander R, Mamoun I, Nylund L, Sarby S, Thornstrom S. Uteroplacental blood flow in pregnancy induced hypertension. Scan J Clin Lab Invest Suppl 169: 28–35, 1984 [DOI] [PubMed] [Google Scholar]
- 22. Magann E, Doherty D, Turner K, Lanneau GJ, Morrison J, Newnham J. Second trimester placental location as a predictor of an adverse pregnancy outcome. J Perinatol 27: 9–14, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Metcalfe J, Ueland K. Maternal cardiovascular adjustments to pregnancy. Prog Cardiovasc Dis 16: 363–374, 1974 [DOI] [PubMed] [Google Scholar]
- 24. Miller S, Tudor C, Nyima Thorsten VR, Sonam Droyoung Craig S, Le P, Wright LL, Varner MW. Maternal and neonatal outcomes of hospital vaginal deliveries in Tibet. Int J Gynaecol Obstet 98: 217–221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore L. Uterine blood flow as a determinant of feto-placental development. In: Placenta and Fetal Programming, edited by Burton G, Barker D. Cambridge, UK: Univ Cambridge Press, 2010 [Google Scholar]
- 26. Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to high-altitude pregnancy: an experiment of nature. Placenta 25 Suppl: S60–S71, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 13: 635–644, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Oxygen transport in Tibetan women during pregnancy at 3658 m. Am J Phys Anthropol 114: 42–53, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Nelson S, Suresh M. Magnesium sulfate-induced relaxation of uterine arteries from pregnant and nonpregnant patients. Am J Obstet Gynecol 164: 1344–1350, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80: 1000–1006, 1992 [PubMed] [Google Scholar]
- 31. Papageorghiou A, Yu C, Nicolaides K. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol 18: 383–396, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Pijnenborg R, Bland J, Robertson W, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–413, 1983 [DOI] [PubMed] [Google Scholar]
- 33. Rajakumar A, Brandon H, Daftary A, Ness RB, Conrad K. Evidence for the functional activity of hypoxa-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25: 763–769, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Rajakumar A, Jeyabalan A, Markovic N, Ness RB, Gilmour C, Conrad K. Placental HIF-1α, HIF-2α, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 293: R766–R774, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90: 4299–4308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tissot van Patot M, Grilli A, Chapman P, Broad E, Tyson W, Heller DS, Zwerdlinger L, Zamudio S. Remodeling of uteroplacental arteries is decreased in high altitude placentae. Placenta 24: 326–335, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 52: 873–880, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Vargas M, Vargas E, Julian CG, Armaza JF, Rodriguez A, Tellez W, Niermeyer S, Wilson M, Parra E, Shriver M, Moore LG. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude. Am J Physiol Regul Integr Comp Physiol 293: R1303–R1312, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol 59: 624–632, 1982 [PubMed] [Google Scholar]
- 40. Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bigham A, Armaza JF, Niermeyer S, Shriver M, Vargas E, Moore LG. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol 293: R1313–R1324, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S, Perkins AV. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta 28: 846–853, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol 79: 15–22, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Zamudio S, Palmer SK, Stamm E, Coffin C, Moore LG. Uterine blood flow at high altitude. In: Hypoxia and the Brain, edited by Sutton JR, Houston CS. Burlington, VT, USA: Queen City Press, 1995, p. 112–124 [Google Scholar]
- 44. Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Armeller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol 582: 12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zamudio S, Torricos T, Fik E, Oyala M, Echalar L, Pullockaran J, Tutino E, Martin B, Belliappa S, Balanza E, Illsley NP. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One 5: e8551, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]