Abstract
Bone loss in type 1 diabetes is accompanied by increased marrow fat, which could directly reduce osteoblast activity or result from altered bone marrow mesenchymal cell lineage selection (adipocyte vs. osteoblast). CCAAT/enhancer binding protein beta (C/EBPβ) is an important regulator of both adipocyte and osteoblast differentiation. C/EBPβ-null mice have delayed bone formation and defective lipid accumulation in brown adipose tissue. To examine the balance of C/EBPβ functions in the diabetic context, we induced type 1 diabetes in C/EBPβ-null (knockout, KO) mice. We found that C/EBPβ deficiency actually enhanced the diabetic bone phenotype. While KO mice had reduced peripheral fat mass compared with wild-type mice, they had 5-fold more marrow adipocytes than diabetic wild-type mice. The enhanced marrow adiposity may be attributed to compensation by C/EBPδ, peroxisome proliferator-activated receptor-γ2, and C/EBPα. Concurrently, we observed reduced bone density. Relative to genotype controls, trabecular bone volume fraction loss was escalated in diabetic KO mice (−48%) compared with changes in diabetic wild-type mice (−22%). Despite greater bone loss, osteoblast markers were not further suppressed in diabetic KO mice. Instead, osteoclast markers were increased in the KO diabetic mice. Thus, C/EBPβ deficiency increases diabetes-induced bone marrow (not peripheral) adipose depot mass, and promotes additional bone loss through stimulating bone resorption. C/EBPβ-deficiency also reduced bone stiffness and diabetes exacerbated this (two-way ANOVA P < 0.02). We conclude that C/EBPβ alone is not responsible for the bone vs. fat phenotype switch observed in T1 diabetes and that suppression of CEBPβ levels may further bone loss and decrease bone stiffness by increasing bone resorption.
Keywords: type 1 diabetes, osteoblast, adipocyte, differentiation
type 1 (t1-) diabetes affects ∼1 million people in the United States and is characterized by a loss of insulin-secreting pancreatic β-cells and hyperglycemia that must be controlled by insulin delivery in humans. In addition to other well-known complications, T1-diabetes also causes bone loss in humans and animal models (3, 26, 32). Streptozotocin (STZ) is a pharmacologic agent that can be used to induce pancreatic β-cell death and subsequent diabetes (57). Hyperglycemia is detectable as early as 5 days after diabetes-induction begins. In STZ mouse and rat models, as well as in spontaneous mouse models (5), T1-diabetes causes bone loss and impaired bone healing (4, 18, 29, 39, 51, 52). The subsequent osteoporosis is marked by decreased bone formation in both humans and animals (4, 7, 14), while reported effects on bone resorption by osteoclasts have been variable. Altered mesenchymal stem cell lineage selection toward adipocyte rather than osteoblast has been suggested to be a mechanism for bone loss in diabetes (4, 5, 36), aging (44, 62), and unloading (1). In STZ-diabetic mice, this hypothesis is supported by an increase in expression of adipocyte-specific genes in bone [peroxisome proliferator-activated receptor (PPAR) γ2 and fatty-acid binding protein (FABP) 4] and visible adipocytes in the marrow (4). Marrow adiposity is not a direct result of STZ because nonobese diabetic mice also have increased adipocyte accumulation accompanying bone loss (5).
Inhibition of marrow adiposity may provide insight as to whether adiposity could be targeted to treat diabetic osteoporosis. Previously, we demonstrated that treatment with the PPARγ2 inhibitor, bisphenol-A-diglycidyl ether (BADGE), prevents STZ-diabetic marrow adiposity, but does not protect against bone loss (6). One interpretation of this study is that mesenchymal stem cells still differentiate toward the adipocyte lineage, but BADGE forces them to remain in an early-adipocyte stage. Therefore, inhibition of adipocyte differentiation prior to PPARγ2 induction (at the level of C/EBPβ) could potentially rescue bone loss from T1-diabetes.
CCAAT/enhancer binding protein beta (C/EBPβ) is a member of the basic region-leucine zipper (bZIP) class of transcription factors. A key regulator of mesenchymal stem cell adipocyte lineage selection, it is transiently expressed during early adipocyte differentiation with C/EBPδ and then followed by expression of PPARγ2 and C/EBPα (9, 12, 48). C/EBPβ exists in three isoforms: transcriptionally active liver activator protein (LAP)-1 and LAP-2 and generally inactive liver inhibitory protein (LIP) (48). Overexpression of C/EBPβ alone induces adipocyte differentiation in NIH-3T3 fibroblasts, while LIP overexpression can prevent it (64, 66). Total deficiency of C/EBPβ protects mice from obesity and reduces body fat mass (40, 50, 54). Combined knockout of C/EBPβ and C/EBPδ leads to an even greater block to the adipocyte phenotype and adiposity (58).
C/EBPβ is also an important regulator of osteoblast lineage selection and differentiation. These effects are dependent on when (early vs. late differentiation), where (local vs. systemic), and which (LAP vs. LIP, or both) C/EBPβ is expressed. C/EBPβ is normally expressed during early and late stages of osteoblast differentiation, with decreased expression during middle stages (17, 23). Complete knockout of C/EBPβ in mice results in decreased total body mass and total bone mineral density (BMD) (54). Similarly, targeted expression of the C/EBPβ inactive form, LIP, to osteoblasts results in bone loss due to decreased bone formation (19).
To examine the balance of these C/EBPβ functions (regulating adipocyte and osteoblast differentiation) in the diabetic context, we measured C/EBPβ expression during diabetes onset and tested the role of C/EBPβ in this process by inducing type I diabetes in C/EBPβ knockout (KO) mice. Indeed, C/EBPβ expression increased in bone with the onset of diabetes. While C/EBPβ could have played a role in the diabetic bone pathology by promoting bone marrow stem cells toward the adipocyte lineage and increasing marrow adiposity, we found that C/EBPβ deficiency actually exacerbated diabetic marrow adiposity, despite causing peripheral fat loss. In addition, C/EBPβ deficiency increased bone loss; however, unlike bone loss in wild-type diabetic mice, additional bone loss in KO mice was due to enhanced bone resorption rather than further suppression of osteoblast activity.
METHODS
Animals.
Male BALB/c mice were obtained from Harlan Sprague Dawley (Indianapolis, IN). Heterozygous C/EBPβ (C/EBPβ+/−) mice were obtained from Peter F. Johnson (National Cancer Institute, Frederick, MD) (55). Heterozygous mice are bred to yield mice with a global knockout of C/EBPβ. The mice used in this study were bred into the BALB/c strain, backcrossed more than eight times and genotyped at Michigan State University by Jeffrey Leipprandt in the laboratory of Sandra Z. Haslam. All mice were maintained on a 12:12-h light-dark cycle at 23°C, were given standard lab chow and had food and water ad libitum. All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee.
To induce diabetes, 14-wk-old male BALB/c mice, C/EBPβ KO (C/EBPβ−/−) mice, and wild-type littermate controls were injected with either 40 mg/kg STZ (Sigma, St. Louis, MO) or 0.1 M citrate buffer, pH 4.5, vehicle for 5 consecutive days. Diabetes was confirmed 12 days post-first STZ injection (DPI) using a drop of blood from the saphenous vein and an Accu-Chek Compact glucometer (Roche Diagnostics, Indianapolis, IN), with blood glucose greater than or equal to 300 mg/dl, indicating diabetes. Body mass was monitored during diabetes induction and throughout the experiment. BALB/c mice were euthanized at 5, 19, 28, and 40 DPI. C/EBPβ−/− mice and wild-type littermate controls were euthanized at 40 DPI. Whole liver, tibialis anterior muscles, and peripheral fat pads (specifically the femoral fat pad, located outside of the femur) were isolated, and their mass was assessed at the time of harvest.
RNA analyses.
Immediately after euthanasia, tibias were cleansed of muscle and connective tissue, snap frozen in liquid nitrogen, and stored at −80°C. Frozen tibias were crushed under liquid nitrogen conditions with a Bessman Tissue Pulverizer (Spectrum Laboratories, Rancho Dominguez, CA). RNA was isolated with Tri Reagent (Molecular Research Center, Cincinnati, OH), and integrity was assessed by formaldehyde-agarose gel electrophoresis. cDNA was synthesized by reverse transcription with Superscript II Reverse Transcriptase Kit and oligo dT(12–18) primers (Invitrogen, Carlsbad, CA) and amplified by real-time PCR with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA), and gene-specific primers were synthesized by Integrated DNA Technologies (Coralville, IA). Hypoxanthine guanine phosphoribosyl transferase (HPRT) mRNA levels do not fluctuate in diabetes or with deletion of C/EBPβ and were used as an internal control. HPRT was amplified using 5′-AAG CCT AAG ATG AGC GCA AG-3′ and 5′-TTA CTA GGC AGA TGG CCA CA-3′ (61). C/EBPβ was amplified using 5′-CAA GCT GAG CGA CGA GTA CA-3′ and 5′-CAG CTG CTC CAC CTT CTT CT-3′ (46). FABP4 (aP2) was amplified using 5′-GCG TGG AAT TCG ATG AAA TCA-3′ and 5′-CCC GCC ATC TAG GGT TAT GA-3′ (33). C/EBPδ was amplified using 5′-CGC AGA CAG TGG TGA GCT TG-3′ and 5′-CTT GCG CAC AGC GAT GTT GTT-3′ (46). PPARγ2 was amplified using 5′-TGA AAC TCT GGG AGA TTC TCC TG-3′ and 5′-CCA TGG TAA TTT CTT GTG AAG TGC-3′ (25). C/EBPα was amplified using 5′-GAA CAG CAA CGA GTA CCG GGT-3′ and 5′-GCC ATG GCC TTG ACC AAG GAG-3′ (46). Osteocalcin was amplified using 5′-ACG GTA TCA CTA TTT AGG ACC TGT G-3′ and 5′-ACT TTA TTT TGG AGC TGC TGT GAC-3′ (45). Tartrate-resistant acid phosphatase (TRAP5) was amplified using 5′-AAT GCC TCG ACC TGG GA-3′ and 5′-CGT AGT CCT CCT TGG CTG CT-3′ (63). Cathepsin K was amplified using 5′-GCA GAG GTG TGT ACT ATG-3′ and 5′-GCA GGC GTT GTT CTT ATT-3′ (67). Amplicons were compared with a 100 bp DNA ladder (Invitrogen, Carlsbad, CA) on a 2% agarose gel to verify RT-PCR results and to verify the absence of C/EBPβ in the knockout animals.
Bone histology and histomorphometry.
Femurs and tibias were fixed in 10% formalin and transferred to 70% ethanol after 24 h. Fixed samples were processed on an automated Thermo Electron Excelsior tissue processor for dehydration, clearing, and infiltration using a routine overnight processing schedule. Samples were then embedded in Surgipath-embedding paraffin on a Sakura Tissue Tek II-embedding center. Paraffin blocks were sectioned at 5 μm on a Reichert Jung 2030 rotary microtome. Slides were stained for TRAP activity and counterstained with hematoxylin according to manufacturer protocol (387A-1KT, Sigma, St. Louis, MO). Osteoclast surface area was measured and expressed as a percentage of total bone surface in the tibia trabecular region ranging from the proximal growth plate to 2 mm distal. Visible adipocytes, greater than 30 μm diameter, were counted in the same region of the tibia and in the region of the femur immediately proximal to the distal growth plate, extending distally 2 mm.
Microcomputed tomography analyses.
Fixed tibias were scanned using a GE Explore Locus microcomputed tomagraphy (μCT) system at a voxel resolution of 20 μm obtained from 720 views. Beam angle of increment was 0.5, and beam strength was set at 80 peak kV and 450 μA. Each run included control and diabetic, wild type (WT) and C/EBPβ−/− bones, and a calibration phantom to standardize grayscale values and maintain consistency. On the basis of autothreshold and isosurface analyses of multiple bone samples, a fixed threshold (800) was used to separate bone from bone marrow. Trabecular bone analyses were performed in a region of trabecular bone defined at 0.17 mm (∼1% of the total length) distal to the growth plate of the proximal tibia extending 2 mm toward the diaphysis, and excluding the outer cortical shell. Trabecular bone mineral content (BMC), BMD, bone volume fraction (BVF), thickness (TbTh), spacing (TbSp), and number (TbN) values were computed by a GE Healthcare MicroView software application for visualization and analysis of volumetric image data. Trabecular isosurface images were taken from a cylindrical region in the tibia immediately distal to the proximal growth plate measuring 1.0 mm in length and 1.0 mm in diameter. Fixed femurs were prepared and scanned in an identical manner to above. Cortical measurements were performed in a 2 × 2 × 2 mm cube centered midway down the length of the bone using a fixed threshold of 1400 to separate bone from marrow.
Mechanical testing.
Mouse femurs were subjected to three-point bending to determine their mechanical properties (21, 22, 24, 47, 49). Specimens were thawed at room temperature prior to testing and kept wet in saline solution. The bones were placed on the support of the apparatus with the medial side facing up and loaded using an MTS Insight at 0.05 mm/s until failure. The stiffness was determined by calculating the slope of the load vs. displacement curve. The elastic modulus was calculated using elementary beam theory (Eq. 1) (24).
| (1) |
where P is the load, L is the span length of the three-point bend support (10 mm), I is the moment of inertia, and δ is the deflection at the center of the bone. The load and displacement values were determined using the linear portion of the load vs. displacement curve. The ultimate stress was calculated using Eq. 2 (24).
| (2) |
In Eq. 2, P is the ultimate load, L is the span length, c is the half the bone diameter along the loading axis, and I is the moment of inertia. The femur was modeled as a hollow ellipsoid. The thickness of the bone was calculated using μCT in a region of interest 1.0 mm thick and centered halfway along the length of the bone. The moment of inertia for the femur was calculated using Eq. 3.
| (3) |
In Eq. 3, B and D are the outer major and minor diameters, respectively, and b and d are the inner major and minor diameters, respectively.
Statistical analyses.
All measurements are presented as the mean ± SE. Statistical significance was determined with a Student's t-test (assuming equal variance) using Microsoft Excel (Microsoft, Redmond, WA).
RESULTS
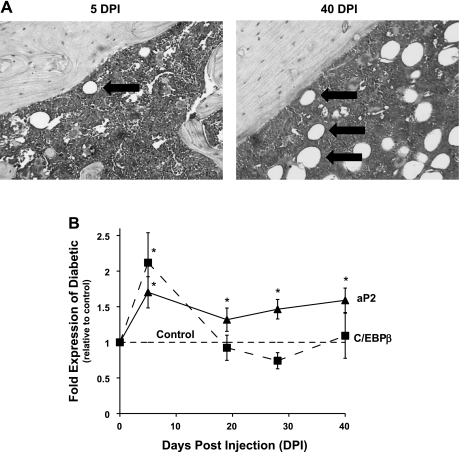
To determine the temporal pattern of C/EBPβ expression in T1-diabetic bone, we treated male mice with STZ or vehicle to induce T1-diabetes, and harvested tibiae at 5, 19, 28, and 40 DPI. At 40 DPI, adipocyte numbers are clearly increased in diabetic bone marrow (Fig. 1A) (4–6, 13, 43). However, at 5 DPI, the point at which blood glucose levels become significantly elevated in diabetic mice (190 ± 12 mg/dl vs. 152 ± 5 mg/dl in controls), adiposity markers are already beginning to increase at the RNA level (Fig. 1B) (42). Therefore, transient expression of transcription factors involved in early adipogenesis should be evident at this early time point. Consistent with this hypothesis, C/EBPβ expression was elevated at 5 DPI, concurrent with an increase in aP2, a marker of mature adipocytes, which remained elevated throughout the time course (Fig. 1B). C/EBPβ mRNA levels did not remain elevated, which corresponds with its expression pattern during adipocyte maturation (30).
Fig. 1.
CCAAT/enhancer binding protein beta (C/EBPβ) expression is increased in diabetic bone in conjunction with aP2 expression and is followed by increased marrow adiposity. BALB/c mice were injected with streptozotocin (STZ) to induce diabetes. Mice were harvested 5, 19, 28, and 40 days post injection (DPI) with STZ. A: fixed femurs from diabetic mice at 5 and 40 DPI were stained with hematoxylin. Large white circular features (denoted by arrows) are adipocytes. B: mRNA was extracted from frozen tibias and made into cDNA, which was amplified by RT-PCR with primers specific to C/EBPβ (dashed line), aP2 (solid line), and hypoxanthine guanine phosphoribosyl transferase (HPRT; a housekeeping gene control). Data points represent means ± SE of diabetic mRNA levels expressed relative to vehicle-treated control values (normalized to one, dashed horizontal line). *P < 0.05 by Student's t-test.
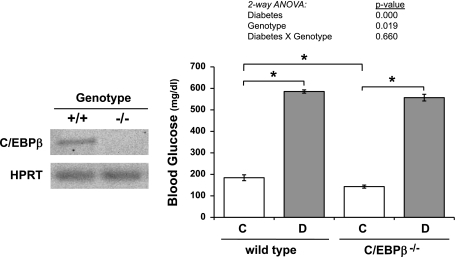
We then induced diabetes in C/EBPβ KO mice and wild-type littermate controls to understand the role of C/EBPβ in diabetic bone pathogenesis. Deletion of C/EBPβ was confirmed by RT-PCR of RNA isolated from tibiae (Fig. 2). Consistent with previous studies, the nondiabetic C/EBPβ−/− mice had lower glucose levels than nondiabetic wild-type mice (34). However, this hypoglycemia was not significant enough to affect diabetes induction in KO mice. Blood glucose levels were increased to greater than 500 mg/dl in both wild-type and C/EBPβ−/− diabetic mice (Fig. 2) and were not significantly different from each other.
Fig. 2.
Diabetes induction did not differ between C/EBPβ KO and wild type mice. Left: to confirm genotypes, mRNA was extracted from frozen tibias and was made into cDNA with a reverse transcriptase reaction. cDNA was amplified by RT-PCR with primers specific to C/EBPβ, and values are expressed relative to HPRT, a housekeeping gene control. Amplicons were separated on 1.5% agarose DNA gel and visualized by ethidium bromide staining. Absence of a C/EBPβ-specific amplicon was indicative of C/EBPβ knockout. Right: blood glucose was measured in control (C, white bars), diabetic (D, gray bars), wild type (WT) and C/EBPβ−/− mice at 40 DPI. Bars represent mean ± SE. *P < 0.05 by Student's t-test; n ≥ 5 per condition.
C/EBPβ−/− mice were smaller than wild-type mice: they have lower total body, muscle (tibialis anterior), and peripheral fat pad mass (femoral fat pad, located outside of the femur) (Table 1), as seen in past studies (34, 54). Consistent with a diabetic phenotype, both wild-type and KO diabetic mice lost weight. However, the KO diabetic mice lost more weight: −16% vs. −7% in diabetic wild-type mice (Table 1). Two-way ANOVA indicates a strong genotype and phenotype effect, while a diabetes × genotype effect approached significance (P < 0.071) (Table 1). A portion of the diabetic weight loss can be accounted for by muscle loss (−16% in wild-type and −22% in KO mice) and peripheral fat pad loss (−48%) in both wild-type and KO mice (Table 1). Liver mass increased 28% in wild-type diabetic compared with control mice, whereas it increased only 10% in KO diabetic compared with control mice (Table 1).
Table 1.
Body and tissue masses of 28-day diabetic and untreated wild-type and C/EBPβ−/− mice
| Wild Type |
C/EBPβ−/− |
Two-Way ANOVA P values |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 5) | Diabetic (n = 9) | Control (n = 9) | Diabetic (n = 12) | Diabetes | Genotype | Genotype × Diabetes | |
| Total body mass, g | 27.1 ± 0.5 | 25.1 ± 0.5* | 25.7 ± 0.3† | 21.6 ± 0.6*† | 0.000 | 0.000 | 0.071 |
| Tibialis anterior, mg | 49 ± 1 | 41 ± 3* | 41 ± 2† | 32 ± 2*† | 0.001 | 0.030 | 0.775 |
| Peripheral fat pad, mg | 259 ± 11 | 134 ± 6* | 202 ± 14† | 105 ± 31* | 0.000 | 0.000 | 0.951 |
| Liver, g | 1.27 ± 0.06 | 1.62 ± 0.06* | 1.25 ± 0.05 | 1.37 ± 0.04† | 0.000 | 0.023 | 0.056 |
P < 0.05 compared to genotype-matched control.
P < 0.05 compared to treatment-matched wild type.
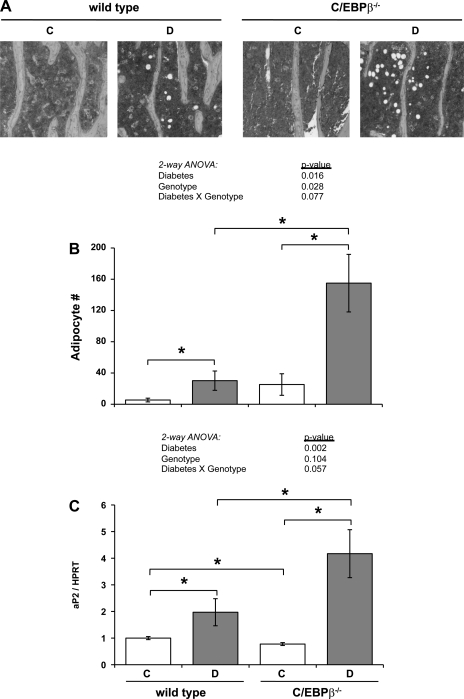
To determine whether C/EBPβ deficiency could prevent adipocyte accumulation in the diabetic marrow, we counted adipocytes in hematoxylin-stained tibia sections (Fig. 3, A and B). Marrow adipocyte number did not differ significantly between KO and wild-type mice, although KO mice tended to have more adipocytes (Fig. 3B). Diabetes increased adipocyte numbers in both genotypes, which corresponded to an increase in aP2 expression (Fig. 3C). Interestingly, KO diabetic mice had more than five-fold more adipocytes than wild-type diabetic mice. Two-way ANOVA analysis indicated a strong diabetes and genotype effect on marrow adipocyte number, while a diabetes × genotype effect approached significance (P < 0.077). Despite C/EBPβ−/− control mice having significantly lower aP2 expression than wild-type control mice, diabetes induced aP2 expression in KO mice to levels significantly higher than all other groups.
Fig. 3.
Diabetic marrow adiposity was increased by C/EBPβ deficiency. A: representative photomicrographs of tibia sections stained with hematoxylin from control (C) and diabetic (D) wild type and C/EBPβ−/− tibias. Photographs were taken 1 mm distal to the proximal growth plate. B: visible adipocytes were counted in the marrow portion of tibia sections in the area 2 mm distal to the proximal growth plate. C: mRNA was extracted from frozen tibias and was made into cDNA with a reverse transcriptase reaction. cDNA was amplified by RT-PCR with primers specific to aP2, an adipocyte marker, and values were expressed relative to HPRT, a housekeeping gene control. Bars represent mean ± SE of control (C, white bars), diabetic (D, gray bars), wild type and C/EBPβ−/− mice. *P < 0.05 by Student's t-test; n ≥ 5 per condition.
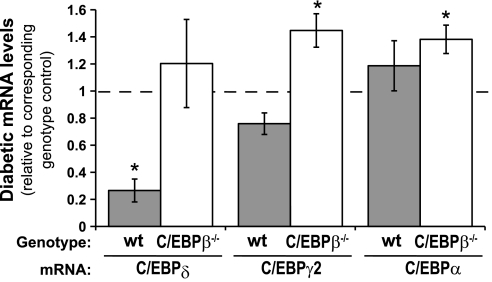
To understand how deletion of C/EBPβ, a transcription factor important for adipocyte differentiation, could enhance diabetic marrow adiposity, we hypothesized that compensation by other adipogenic transcription factors may have occurred. Tibia expression of C/EBPδ, which is normally expressed with C/EBPβ early in adipocyte differentiation, was significantly decreased (nearly five-fold) in diabetic wild-type compared with control bone (Fig. 4). This decrease did not occur from diabetes in the KO mice. PPARγ2 and C/EBPα, both transcription factors present later in adipocyte differentiation, were unchanged in diabetic wild-type mice compared with wild-type controls but were significantly elevated in KO diabetic mice compared with KO controls. These data support the notion that heightened adipogenic transcription factors may compensate for the lack of C/EBPβ and be responsible for increased marrow fat.
Fig. 4.
Adipogenic transcription factors are elevated in diabetic C/EBPβ−/− compared with wild-type diabetic bone. mRNA was extracted from frozen tibias and was made into cDNA with a reverse transcriptase reaction. cDNA was amplified by RT-PCR with primers specific to C/EBPδ, peroxisome proliferator-activated receptor-γ2 (PPARγ2), and C/EBPα. Diabetic levels were expressed relative to controls for the corresponding genotype. Bars represent mean ± SE of diabetic wild-type (gray bars) and diabetic C/EBPβ−/− (white bars) bone. *P < 0.05 compared with genotype-matched control by Student's t-test; n ≥ 5 per condition.
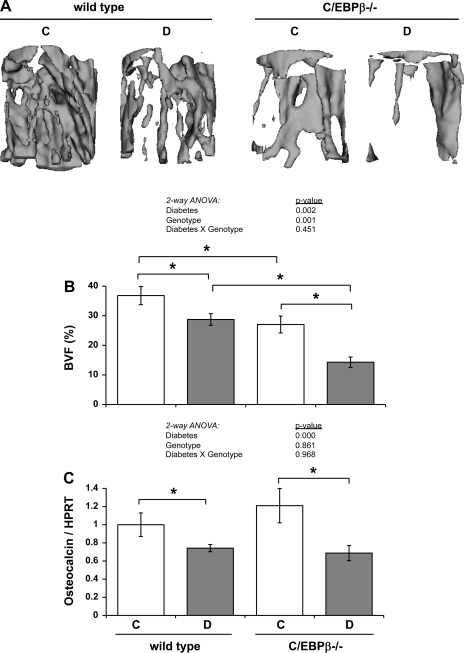
Next, we examined trabecular bone density parameters in the tibiae of these mice to determine whether C/EBPβ deficiency had an impact on the diabetic bone phenotype. Control C/EBPβ KO mice had significantly lower BMC, BMD, BVF, and TbN than control wild-type mice, similar to recent findings (54, 68). Diabetes caused trabecular bone loss in both wild-type and KO mice, as demonstrated by lower trabecular BMC, BMD, BVF, and TbTh (Fig. 5A, Table 2). Thus, diabetic bone loss was not prevented, by the absence of C/EBPβ. Interestingly, T1-diabetes caused BMD to be decreased in diabetic KO by 66 mg/cc (31%) vs. 39 mg/cc (15%) in wild-type mice. BVF loss was 48% in diabetic KO mice, while only 22% in diabetic wild-type mice (Table 2, Fig. 5, A and B). Trabecular thickness decreased 38% in diabetic KO mice vs. 20% in diabetic wild-type mice. Although, trabecular number was unchanged in the diabetic wild-type mice compared with control wild-type mice, it was significantly lower (27% decrease) in the diabetic KO mice compared with control KO mice (Table 2). Taken together, these data indicate that bone loss was more severe in KO mice than in wild-type mice. Two-way ANOVA analyses indicated that the changes are due to a diabetes and a genotype effect, but not a genotype × diabetes effect, for all parameters.
Fig. 5.
Absence of C/EBPβ exacerbated T1-diabetic bone loss without altering osteocalcin expression. A: representative three-dimensional isosurface μCT images were taken in a cylindrical region of interest (1 mm diameter × 1 mm length) immediately distal to the proximal growth plate of the tibia. B: bone volume fraction (BVF) was measured by μCT in fixed tibias immediately distal to the proximal growth plate. C: mRNA was extracted from frozen tibias and was made into cDNA with a reverse-transcriptase reaction. cDNA was amplified by RT-PCR with primers specific to osteocalcin (an osteoblast marker), and values are expressed relative to HPRT, a housekeeping gene control. Bars represent means ± SE of control (C, white bars), diabetic (D, gray bars), wild type and C/EBPβ−/− mice. *P < 0.05 by Student's t-test; n ≥ 5 per condition.
Table 2.
Trabecular bone μCT measurements from the tibia of 28-day diabetic and untreated wild-type and C/EBPβ−/− mice
| Wild Type |
C/EBPβ−/− |
Two-Way ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 5) | Diabetic (n = 9) | Control (n = 9) | Diabetic (n = 12) | Diabetes | Genotype | Genotype × Diabetes | |
| BMC, mg | 0.75 ± 0.04 | 0.64 ± 0.02* | 0.62 ± 0.03† | 0.43 ± 0.03*† | 0.000 | 0.000 | 0.651 |
| BMD, mg/cc | 251 ± 12 | 212 ± 8* | 214 ± 11† | 148 ± 10*† | 0.000 | 0.001 | 0.589 |
| BVF, % | 37 ± 3 | 29 ± 2* | 27 ± 3† | 14 ± 2*† | 0.002 | 0.001 | 0.454 |
| TbTh, μm | 65 ± 5 | 52 ± 2* | 58 ± 7 | 36 ± 2*† | 0.029 | 0.053 | 0.738 |
| TbSp, μm | 174 ± 16 | 204 ± 31 | 298 ± 33† | 369 ± 25†‡ | 0.008 | 0.000 | 0.467 |
| TbN, 1/mm | 6.4 ± 0.2 | 6.0 ± 0.3 | 5.2 ± 0.3† | 3.8 ± 0.3*† | 0.003 | 0.000 | 0.208 |
BMC, bone mineral content; BMD, bone mineral density; BVF, bone volume fraction; Tb, trabecular; Th, thickness; Sp, spacing; N, number.
P < 0.05 compared to genotype-matched control.
P < 0.05 compared to treatment-matched wild type.
P < 0.1 compared to genotype-matched control.
To determine the mechanism of heightened bone loss in C/EBPβ-deficient mice, we examined expression of osteocalcin and TRAP5, markers of bone formation and resorption, respectively. Tibia expression of osteocalcin was decreased 26% in diabetic wild-type mice compared with control wild-type mice, as we have demonstrated previously (4, 42, 43), indicating decreased osteoblast activity with diabetes (Fig. 5C). Although control KO mice had less bone than control wild-type mice, osteocalcin expression was not significantly different. Diabetic KO mice also had a decrease in osteocalcin expression (43%) compared with control KO mice; however, diabetic KO mice levels were not significantly lower than those of diabetic wild-type mice. Thus, two-way ANOVA detected only a diabetes effect on osteocalcin levels.
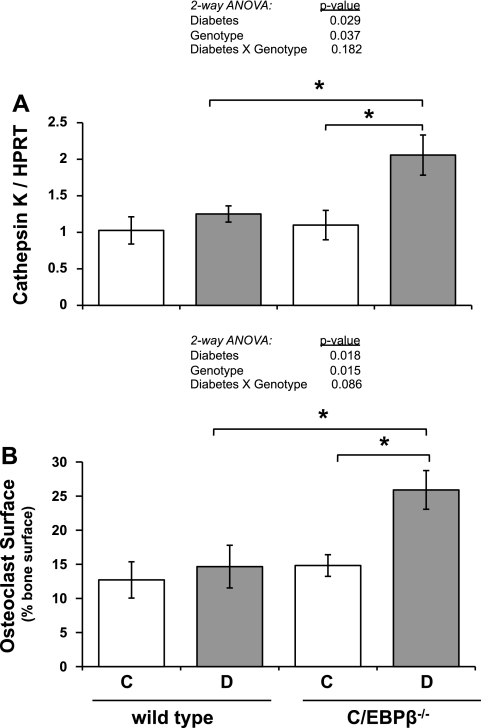
Since knockout of C/EBPβ (genotype effect) did not alter diabetic changes in osteocalcin expression, we examined osteoclast parameters to determine whether resorption was increased (to account for the drop in %BVF in diabetic KO mice). Tibia RNA levels cathepsin K, a marker of osteoclast activity, were unchanged in diabetic compared with control wild-type mice (Fig. 6A), similar to previous findings (4, 42). Similarly, cathepsin K mRNA levels were unchanged in C/EBPβ−/− bone. In contrast, cathepsin K mRNA was elevated twofold in diabetic KO mice compared with control KO mice; this led to the identification of a diabetes and genotype effect by two-way ANOVA (P < 0.029 and P < 0.037, respectively). To account for the enhanced bone loss and clarify the status of bone resorption in the diabetic KO mice, acid phosphatase-positive osteoclasts were measured (Fig. 6B). Percent osteoclast surface was 73% higher in diabetic KO mice than control KO mice. This is consistent with elevated cathepsin K mRNA levels and indicates a portion of the bone loss in diabetic C/EBPβ−/− mice is likely due to increased resorption. Two-way ANOVA analysis indicated a strong diabetes and genotype effect, while a diabetes × genotype effect approached significance (P < 0.086).
Fig. 6.
C/EBPβ knockout causes increased bone resorption in type 1 diabetes. A: mRNA was extracted from frozen tibias and was made into cDNA with a reverse transcriptase reaction. cDNA was amplified by RT-PCR with primers specific to cathepsin K (osteoclast marker) and expressed relative to HPRT, a housekeeping gene control. B: decalcified tibia sections were stained for TRAP activity to identify osteoclasts. Trabecular bone surface in contact with osteoclasts was measured and expressed relative to the total bone surface examined. Bars represent means ± SE of control (C, white bars), diabetic (D, gray bars), wild type and C/EBPβ−/− mice. *P < 0.05 by Student's t-test; n ≥ 5 per condition.
We next examined whether diabetes-induced bone loss would be present in the cortical bone of C/EBPβ−/− mice (Table 3). Cortical bone parameters can be variable, and they can either decrease (5, 36, 43) or not be altered (4, 5, 43) in response to type 1 diabetes in wild-type mice. Here, cortical parameters were not different in diabetic wild-type mice compared with control wild-type mice. Additionally, nondiabetic C/EBPβ−/− mice had cortical bone density parameters similar to those of nondiabetic wild-type mice. Consistent with trabecular bone changes, the combination of diabetes with the deletion of C/EBPβ reduced cortical thickness and cortical area (Table 3). Although inner perimeter and marrow area were unchanged in diabetic C/EBPβ−/− mice compared with nondiabetic C/EBPβ−/− and wild-type mice, outer perimeter was decreased, indicating uncoupled bone remodeling in the periosteum. The moment of inertia (MOI) was consistent with cortical μCT findings in that it was similar between control and diabetic wild-type mice, and control C/EBPβ−/− mice, but decreased in diabetic C/EBPβ−/− mice, suggesting reduced strength (Table 3).
Table 3.
Femur cortical bone μCT measurements from 28-day diabetic and untreated wild-type and C/EBPβ−/− mice
| Wild Type |
C/EBPβ−/− |
Two-Way ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Control (n = 6) | Diabetic (n = 6) | Control (n = 6) | Diabetic (n = 5) | Diabetes | Genotype | Genotype × Diabetes | |
| Cortical thickness, μm | 302 ± 5 | 304 ± 3 | 286 ± 10 | 254 ± 8*† | 0.774 | 0.035 | 0.646 |
| Inner perimeter, mm | 2.86 ± 0.05 | 2.86 ± 0.05 | 2.90 ± 0.08 | 2.73 ± 0.09 | 0.308 | 0.833 | 0.558 |
| Outer perimeter, mm | 4.92 ± 0.11 | 5.11 ± 0.08 | 4.78 ± 0.10 | 4.37 ± 0.06*† | 0.363 | 0.054 | 0.821 |
| Marrow area, mm2 | 0.54 ± 0.02 | 0.52 ± 0.02 | 0.58 ± 0.03 | 0.52 ± 0.04 | 0.311 | 0.719 | 0.598 |
| Cortical area, mm2 | 1.07 ± 0.03 | 1.05 ± 0.02 | 1.02 ± 0.03 | 0.84 ± 0.02*† | 0.777 | 0.012 | 0.753 |
| Total area, mm2 | 1.61 ± 0.04 | 1.57 ± 0.04 | 1.60 ± 0.03 | 1.37 ± 0.05*† | 0.450 | 0.021 | 0.963 |
| MOI, mm4 | 0.146 ± 0.014 | 0.131 ± 0.005 | 0.127 ± 0.007 | 0.093 ± 0.007*† | 0.611 | 0.899 | 0.828 |
MOI, moment of inertia.
P < 0.05 compared to genotype-matched control.
P < 0.05 compared to treatment-matched wild type.
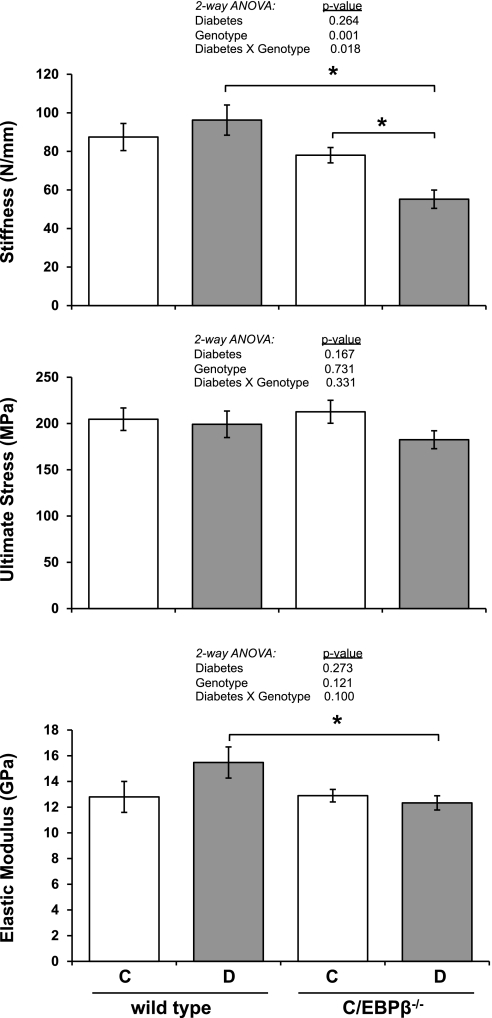
To determine whether these cortical bone alterations had any impact on the actual mechanical properties of the whole bone, we performed 3-point bending tests on femurs. Consistent with cortical measurements, the stiffness, ultimate stress, and elastic modulus were unchanged in wild-type diabetic mice compared with wild-type controls (Fig. 7). Similarly, all three parameters were also unchanged in nondiabetic C/EBPβ−/− mice compared with nondiabetic wild-type mice. However, consistent with reduced cortical thickness, outer perimeter and MOI, the stiffness and elastic modulus were all lower in diabetic C/EBPβ−/− mice compared with diabetic wild-type mice. When analyzed by two-way ANOVA, genotype and diabetes × genotype effects impacted bone stiffness (P < 0.001 and P < 0.018, respectively).
Fig. 7.
Absence of C/EBPβ reduces stiffness and elastic modulus of diabetic mouse femurs. Femurs from mice were subjected to three-point bending to test their mechanical properties: stiffness (top), ultimate stress (middle), and elastic modulus (bottom). Bars represent means ± SE of control (C, white bars), diabetic (D, gray bars), wild type and C/EBPβ−/− mice. *P < 0.05 by Student's t-test; n ≥ 5 per condition.
DISCUSSION
T1-diabetes results in decreased bone formation and increased marrow adiposity, which could be caused by mesenchymal stem cell lineage selection preference for the adipocyte rather than the osteoblast lineage (4, 38). The aim of this study was to determine whether C/EBPβ was required for diabetic marrow adiposity and whether deletion of C/EBPβ could ameliorate diabetic bone loss. Rather than preventing altered mesenchymal stem cell lineage selection, C/EBPβ−/− mice had enhanced bone marrow adiposity and bone loss in response to T1-diabetes.
Despite the enhanced marrow adiposity, C/EBPβ diabetic KO mice lost the same percentage of peripheral fat as diabetic wild type mice (Table 1). Bone marrow adipocytes and peripheral adipose depots often have reciprocal phenotypes. T1-diabetes generally induces a loss of peripheral and visceral fat and an increase in bone marrow fat (4). This reciprocal relationship between peripheral/visceral fat and marrow adiposity is also present in models of alcohol consumption (35), anorexia (8), and aging (28). Here, however, the enhanced adiposity of the bone marrow in the diabetic KO mice was not accompanied by enhanced peripheral fat loss, suggesting independent regulation of the two depots in the C/EBPβ KO mice. This idea is also supported by the fact that control C/EBPβ KO mice have less peripheral fat with no significant change in bone marrow adiposity compared with their wild-type counterparts. Perhaps under normal conditions, C/EBPβ plays an important role in adipose deposition in the periphery, but not in the marrow. In contrast, our study indicates that C/EBPβ is important in regulating diabetic bone marrow fat accumulation, while having no effect on diabetic peripheral fat loss.
Although many studies have demonstrated the importance of C/EBPβ for adipocyte phenotype in vitro (9, 12, 64, 66) and in vivo (40, 50, 54), effects of C/EBPβ on bone marrow adiposity have not been extensively examined. Our findings demonstrate no significant difference between marrow adipocyte numbers in wild-type and C/EBPβ−/− mice (Fig. 3), consistent with a recent study (68). However, expression of the adipocyte marker aP2 is significantly decreased in KO compared with wild-type control mice (Fig. 3C). In spite of C/EBPβ's role in adipocyte lineage selection and maturation, the loss of C/EBPβ enhanced T1-diabetic bone marrow adiposity (Fig. 3) and aP2 expression. Our studies indicate that altered expression of other adipogenic transcription factors may contribute to this outcome. Specifically, we observed the maintenance of C/EBPδ expression in diabetic C/EBPβ KO mice (Fig. 4), which may be sufficient for the modest increase in C/EBPα and PPARγ2 expression that we observed (Fig. 4).
C/EBPδ expression has previously been reported to be IFN-γ/LPS inducible in C/EBPβ KO, but not wild-type, macrophages (15). We previously reported inflammatory effects of T1-diabetes in bone (42). While most inflammatory effects were observed at diabetic onset, elevated IL-1α expression was observed at later times. IL-1 is known to stimulate C/EBPδ expression (2, 16, 20, 37, 59), as well as enhance its activity (56). As both C/EBPα (31) and PPARγ2 (11) transcription can be directly regulated through C/EBP transcription factors, a chain of events where IL-1 stimulates C/EBPδ expression and activity, which, in turn, stimulates C/EBPα and PPARγ2 expression to promote adipogenesis is plausible in the diabetic C/EBPβ KO animals.
It is important to address the fact that we do not observe elevated PPARγ2 expression in diabetic wild-type bone compared with control wild-type bone (Fig. 4), as we have seen in our past studies (4). Suppression of PPARγ2 expression occurs with adipocyte maturation; therefore, the lack of an increase in diabetic mice in this study may be an indicator of a more mature population of adipocytes in the bone marrow. This is consistent with our observation of increased marrow adiposity and aP2 mRNA levels. It is possible that the progression of marrow adiposity may be dependent upon the severity of diabetes induction, which can vary between experiments using streptozotocin (41) and that more severe diabetes would produce extended increases in PPARγ2 expression concurrent with aP2 and marrow adiposity.
Upon examination of the bone phenotype of C/EBPβ KO mice, we found that KO mice had significantly lower bone density. However, the absence of C/EBPβ did not significantly alter osteoblast parameters in control mice. This is consistent with a recent study indicating bone loss, but unchanged bone formation parameters (osteoblast surface and osteoblast number) in femurs of 12-wk-old C/EBPβ−/− mice compared with wild-type littermates (68). However, another study found reduced mineral apposition rate and bone formation rate/bone surface (BFR/BS) in 8-wk-old C/EBPβ−/− mice, suggesting C/EBPβ-deficiency suppresses osteoblast function (53). As our study was started in mice that were 14 wk old, this apparent discrepancy may be due to the age of the mice, with C/EBPβ being an essential regulator of osteoblasts during development (53, 60) but not during adult remodeling (68).
Recent studies have demonstrated that loss of C/EBPβ enhances osteoclast differentiation. This likely occurs through the absence of LAP-induced suppression of MafB (53), which negatively regulates RANKL-induced osteoclastogenesis (27). Thus, C/EBPβ−/− osteoclasts in vitro demonstrate increased expression of cathepsin K, and increased TRAP staining in vivo (specifically, larger osteoclasts were observed, with no change in osteoclast number in 8-wk-old KO mice) (53). Here, we did not observe any change in cathepsin K (Fig. 6A), percentage osteoclast surface (Fig. 6B), or in osteoclast number (not shown) in control KO bone compared with control wild-type bone. Another study using 12-wk-old, wild-type and KO mice had a similar result (no change in osteoclast parameters), but it did not examine serum or RNA markers of resorption (68). Upon induction of diabetes, we observed no change in osteoclast parameters in wild-type mice (consistent with previous studies), but we found increases in diabetic KO mice compared with control KO mice (Fig. 7). This suggests that the additional bone loss in the diabetic C/EBPβ−/− mice occurred through increased osteoclast activity, rather than suppressed bone formation. It is possible that the enhanced osteoclast activity is a direct result of the absence of C/EBPβ in osteoclasts or that it is secondary to the increased marrow fat because adipocytes can secrete factors like TNF-α that induce osteoclastogenesis (10, 65).
Perspectives and Significance
Our findings demonstrated that the absence of C/EBPβ enhanced the diabetic bone phenotype. Specifically, absence of C/EBPβ increased diabetes-induced expression of PPARγ2 and C/EBPα and subsequent marrow adiposity. Increased marrow adiposity was concurrent with reduced bone density, but osteoblast activity markers were not further suppressed. In fact, osteoclast activity was increased in diabetic C/EBPβ KO mice, which is contrary to the classic mechanism of bone loss from T1-diabetes. Our findings add to the work of others who recently have suggested a role for C/EBPβ in the inhibition of osteoclast differentiation. Finally, we conclude that C/EBPβ alone is not responsible for the bone vs. fat phenotype switch observed in T1-diabetes and that suppression of its levels may further bone loss by increasing bone resorption.
GRANTS
This study was funded by grants from National Institutes of Health (DK061184) and the American Diabetes Association (7-07-RA-105) to L. R. McCabe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Pete Johnson (National Cancer Institute) for providing breeder C/EBPβ−/− mice, Sandra Haslam, and Jeffery Leipprandt (Michigan State University) for breeding, genotyping, and backcrossing the C/EBPβ−/− mice into the BALB/c strain, and Regina Irwin and Lindsay Martin for technical assistance and critical review of the manuscript.
REFERENCES
- 1. Ahdjoudj S, Lasmoles F, Holy X, Zerath E, Marie PJ. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res 17: 668–677, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J 9: 1897–1906, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Auwerx J, Dequeker J, Bouillon R, Geusens P, Nijs J. Mineral metabolism and bone mass at peripheral and axial skeleton in diabetes mellitus. Diabetes 37: 8–12, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology 146: 3622–3631, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 148: 198–205, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 209: 967–976, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, Ravussin E. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab 80: 1194–1202, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94: 2129–2136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3–L1 cells. Genes Dev 5: 1538–1552, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 582: 117–131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clarke SL, Robinson CE, Gimble JM. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem Biophys Res Commun 240: 99–103, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273: 30057–30060, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Fowlkes JL, Bunn RC, Liu L, Wahl EC, Coleman HN, Cockrell GE, Perrien DS, Lumpkin CK, Jr, Thrailkill KM. Runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology 149: 1697–1704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes 33: 825–831, 1984 [DOI] [PubMed] [Google Scholar]
- 15. Gorgoni B, Maritano D, Marthyn P, Righi M, Poli V. C/EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mRNAs in macrophages. J Immunol 168: 4055–4062, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Greenwel P, Tanaka S, Penkov D, Zhang W, Olive M, Moll J, Vinson C, DiLiberto M, Ramirez F. Tumor necrosis factor alpha inhibits type I collagen synthesis through repressive CCAAT/enhancer-binding proteins. Mol Cell Biol 20: 912–918, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutierrez S, Javed A, Tennant DK, van Rees M, Montecino M, Stein GS, Stein JL, Lian JB. CCAAT/enhancer-binding proteins (C/EBP) beta and delta activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem 277: 1316–1323, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hamada Y, Kitazawa S, Kitazawa R, Fujii H, Kasuga M, Fukagawa M. Histomorphometric analysis of diabetic osteopenia in streptozotocin-induced diabetic mice: a possible role of oxidative stress. Bone 40: 1408–1414, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Harrison JR, Huang YF, Wilson KA, Kelly PL, Adams DJ, Gronowicz GA, Clark SH. Col1a1 promoter-targeted expression of p20 CCAAT enhancer-binding protein beta (C/EBPbeta), a truncated C/EBPbeta isoform, causes osteopenia in transgenic mice. J Biol Chem 280: 8117–8124, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Harrison JR, Kelly PL, Pilbeam CC. Involvement of CCAAT enhancer binding protein transcription factors in the regulation of prostaglandin G/H synthase 2 expression by interleukin-1 in osteoblastic MC3T3–E1 cells. J Bone Miner Res 15: 1138–1146, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Isaksson H, Tolvanen V, Finnila MA, Iivarinen J, Turunen A, Silvast TS, Tuukkanen J, Seppanen K, Arokoski JP, Brama PA, Jurvelin JS, Helminen HJ. Long-term voluntary exercise of male mice induces more beneficial effects on cancellous and cortical bone than on the collagenous matrix. Exp Gerontol 44: 708–717, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Isaksson H, Tolvanen V, Finnila MA, Iivarinen J, Tuukkanen J, Seppanen K, Arokoski JP, Brama PA, Jurvelin JS, Helminen HJ. Physical exercise improves properties of bone and its collagen network in growing and maturing mice. Calcif Tissue Int 85: 247–256, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Iyer VV, Kadakia TB, McCabe LR, Schwartz RC. CCAAT/enhancer-binding protein-beta has a role in osteoblast proliferation and differentiation. Exp Cell Res 295: 128–137, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Jamsa T, Jalovaara P, Peng Z, Vaananen HK, Tuukkanen J. Comparison of three-point bending test and peripheral quantitative computed tomography analysis in the evaluation of the strength of mouse femur and tibia. Bone 23: 155–161, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Kast-Woelbern HR, Dana SL, Cesario RM, Sun L, de Grandpre LY, Brooks ME, Osburn DL, Reifel-Miller A, Klausing K, Leibowitz MD. Rosiglitazone induction of Insig-1 in white adipose tissue reveals a novel interplay of peroxisome proliferator-activated receptor gamma and sterol regulatory element-binding protein in the regulation of adipogenesis. J Biol Chem 279: 23908–23915, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest 23: 295–303, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 109: 3253–3259, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 37: 757–767, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes 44: 775–782, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun 266: 677–683, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Legraverend C, Antonson P, Flodby P, Xanthopoulos KG. High level activity of the mouse CCAAT/enhancer binding protein (C/EBP alpha) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res 21: 1735–1742, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med 294: 241–245, 1976 [DOI] [PubMed] [Google Scholar]
- 33. Li J, Takaishi K, Cook W, McCorkle SK, Unger RH. Insig-1 “brakes” lipogenesis in adipocytes and inhibits differentiation of preadipocytes. Proc Natl Acad Sci USA 100: 9476–9481, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu S, Croniger C, Arizmendi C, Harada-Shiba M, Ren J, Poli V, Hanson RW, Friedman JE. Hypoglycemia and impaired hepatic glucose production in mice with a deletion of the C/EBPbeta gene. J Clin Invest 103: 207–213, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, Iwaniec UT. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int 20: 1529–1538, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Martin LM, McCabe LR. Type I diabetic bone phenotype is location but not gender dependent. Histochem Cell Biol 128: 125–133, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Massaad C, Paradon M, Jacques C, Salvat C, Bereziat G, Berenbaum F, Olivier JL. Induction of secreted type IIA phospholipase A2 gene transcription by interleukin-1β. Role of C/EBP factors. J Biol Chem 275: 22686–22694, 2000 [DOI] [PubMed] [Google Scholar]
- 38. McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem 102: 1343–1357, 2007 [DOI] [PubMed] [Google Scholar]
- 39. McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants 15: 345–354, 2000 [PubMed] [Google Scholar]
- 40. Millward CA, Heaney JD, Sinasac DS, Chu EC, Bederman IR, Gilge DA, Previs SF, Croniger CM. Mice with a deletion in the gene for CCAAT/enhancer-binding protein beta are protected against diet-induced obesity. Diabetes 56: 161–167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Motyl K, McCabe LR. Streptozotocin, Type I diabetes severity and bone. Biol Proced Online 11: 296–315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Motyl KJ, Botolin S, Irwin R, Appledorn DM, Kadakia T, Amalfitano A, Schwartz RC, McCabe LR. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol 218: 575–583, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Motyl KJ, McCabe LR. Leptin treatment prevents type I diabetic marrow adiposity but not bone loss in mice. J Cell Physiol 218: 376–384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res 13: 371–382, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Ontiveros C, McCabe LR. Simulated microgravity suppresses osteoblast phenotype, Runx2 levels and AP-1 transactivation. J Cell Biochem 88: 427–437, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Phan J, Peterfy M, Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem 279: 29558–29564, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Phillips JA, Almeida EA, Hill EL, Aguirre JI, Rivera MF, Nachbandi I, Wronski TJ, van der Meulen MC, Globus RK. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol 27: 609–618, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365: 561–575, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schriefer JL, Robling AG, Warden SJ, Fournier AJ, Mason JJ, Turner CH. A comparison of mechanical properties derived from multiple skeletal sites in mice. J Biomech 38: 467–475, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Schroeder-Gloeckler JM, Rahman SM, Janssen RC, Qiao L, Shao J, Roper M, Fischer SJ, Lowe E, Orlicky DJ, McManaman JL, Palmer C, Gitomer WL, Huang W, O'Doherty RM, Becker TC, Klemm DJ, Jensen DR, Pulawa LK, Eckel RH, Friedman JE. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J Biol Chem 282: 15717–15729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shires R, Teitelbaum SL, Bergfeld MA, Fallon MD, Slatopolsky E, Avioli LV. The effect of streptozotocin-induced chronic diabetes mellitus on bone and mineral homeostasis in the rat. J Lab Clin Med 97: 231–240, 1981 [PubMed] [Google Scholar]
- 52. Shyng YC, Devlin H, Sloan P. The effect of streptozotocin-induced experimental diabetes mellitus on calvarial defect healing and bone turnover in the rat. Int J Oral Maxillofac Surg 30: 70–74, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Smink JJ, Begay V, Schoenmaker T, Sterneck E, de Vries TJ, Leutz A. Transcription factor C/EBPβ isoform ratio regulates osteoclastogenesis through MafB. EMBO J 28: 1769–1781, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Staiger J, Lueben MJ, Berrigan D, Malik R, Perkins SN, Hursting SD, Johnson PF. C/EBPβ regulates body composition, energy balance-related hormones and tumor growth. Carcinogenesis 30: 832–840, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPβ in female reproduction. Genes Dev 11: 2153–2162, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Svotelis A, Doyon G, Bernatchez G, Desilets A, Rivard N, Asselin C. IL-1 β-dependent regulation of C/EBP delta transcriptional activity. Biochem Biophys Res Commun 328: 461–470, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50: 537–546, 2001 [PubMed] [Google Scholar]
- 58. Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J 16: 7432–7443, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tengku-Muhammad TS, Hughes TR, Ranki H, Cryer A, Ramji DP. Differential regulation of macrophage CCAAT-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine 12: 1430–1436, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Tominaga H, Maeda S, Hayashi M, Takeda S, Akira S, Komiya S, Nakamura T, Akiyama H, Imamura T. CCAAT/enhancer-binding protein beta promotes osteoblast differentiation by enhancing Runx2 activity with ATF4. Mol Biol Cell 19: 5373–5386, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci 82: 638–646, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55: 693–698, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, Jepsen KJ. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology 145: 3507–3522, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev 9: 2350–2363, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, Tan RX. NF-κB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev 20: 7–17, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 9: 168–181, 1995 [DOI] [PubMed] [Google Scholar]
- 67. Yoshimatsu M, Shibata Y, Kitaura H, Chang X, Moriishi T, Hashimoto F, Yoshida N, Yamaguchi A. Experimental model of tooth movement by orthodontic force in mice and its application to tumor necrosis factor receptor-deficient mice. J Bone Miner Metab 24: 20–27, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Zanotti S, Stadmeyer L, Smerdel-Ramoya A, Durant D, Canalis E. Misexpression of CCAAT/enhancer binding protein beta causes osteopenia. J Endocrinol 201: 263–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]