Abstract
Rats with bilateral lesions of the lateral hypothalamus (LH) fail to exhibit sodium appetite. Lesions of the parabrachial nuclei (PBN) also block salt appetite. The PBN projection to the LH is largely ipsilateral. If these deficits are functionally dependent, damaging the PBN on one side and the LH on the other should also block Na appetite. First, bilateral ibotenic acid lesions of the LH were needed because the electrolytic damage used previously destroyed both cells and axons. The ibotenic LH lesions produced substantial weight loss and eliminated Na appetite. Controls with ipsilateral PBN and LH lesions gained weight and displayed robust sodium appetite. The rats with asymmetric PBN-LH lesions also gained weight, but after sodium depletion consistently failed to increase intake of 0.5 M NaCl. These results dissociate loss of sodium appetite from the classic weight loss after LH damage and prove that Na appetite requires communication between neurons in the LH and the PBN.
Keywords: ibotenic acid, lateral hypothalamus, asymmetric lesions, parabrachial nuclei
salt appetite arises during sodium need or its hormonal mimics. It is usually assessed by measuring NaCl intake at concentrations rejected by animals in hydromineral balance (5, 38). Although the hormonal precursors of the Na (sodium) appetite and their neural targets are yielding to investigation, the neural mechanisms that change the hedonic value of the sapid stimulus remain elusive (6, 10). Bilateral lesions centered in the parabrachial nuclei (PBN), the second central gustatory relay in rodents, eliminate the expression of salt appetite (7, 8, 41, 43–45). Chronic decerebrate rats that have an intact PBN but no connections between the hind- and forebrain also fail to express Na appetite (12). Thus, the PBN is necessary for the expression of sodium appetite, but is not sufficient unless its axonal connections to the forebrain are intact. In the forebrain, however, neither the thalamic nor the cortical gustatory areas are required for the expression of this behavior (7, 8, 41, 51, 55). Based on these observations, it follows that the gustatory neural activity required for salt appetite reaches the forebrain via the PBN projections to the limbic system (29). Nevertheless, the intermediate structures and functional relationships remain to be deciphered.
The major terminal areas of the PBN in the limbic system: 1) amygdala, 2) bed nucleus of the stria terminalis, and 3) lateral hypothalamus (LH), each influence Na appetite to one degree or another (9, 36, 42, 56). These same areas also are connected to the PBN reciprocally, to one another (3, 21), and to key components in a proposed reward system: the prefrontal cortex, ventral tegmental area, and nucleus accumbens (22, 24, 47). This anatomical complexity confounds any simple prediction about the mechanisms through which the hedonic sign of parabrachial gustatory activity is altered by body sodium deficit.
The conventional approach to such an embarrassment of riches is to damage each of the forebrain targets in turn. As mentioned above, this approach has yielded valuable results. Bilateral damage to either the central nucleus of the amygdala (CNA) or the bed nucleus of the stria terminalis reduces the magnitude of a sodium appetite induced by body sodium deficits (9, 56). Bilateral electrolytic damage to the LH, however, essentially eliminates Na appetite, at least in naïve rats (42). This latter effect parallels the absence of the appetite after bilateral PBN damage (41). The first experiments (experiments 1A and 1B) in the current series parallel the earlier LH study but use excitotoxic rather than electrolytic lesions to determine whether the sodium appetite deficit results from damaging neurons or fibers of passage.
Despite the anatomical connections between the PBN and LH, similar or even identical effects do not prove that a functional relationship exists between the two structures in the elaboration of salt appetite. In the rat, the thalamocortical limb of the gustatory system has bilateral representation with considerable crossing of PBN axons in the massa intermedia (20, 29, 34, 33). The PBN projections to the limbic system, however, are substantially ipsilateral (20, 29). For this reason, it is possible to test the functional relationship between the PBN and the LH. If the connections between the two structures are necessary for Na appetite and if these connections are functionally ipsilateral, then damaging the PBN on one side and the LH on the other should have the same behavioral effect as bilateral lesions of either one (4).
The second experiment (experiment 2) in this series tests this premise using rats with either asymmetrical damage to the PBN and LH, ipsilateral lesions of the same areas, or no damage, i.e., surgical controls. The ipsilateral group provides an almost perfect control in that the same two structures receive infusions of ibotenic acid (IBO), but on the same side of the brain. Thus, the contralateral PBN and LH and their interconnections remain intact. If the premise is correct, these rats should express a near normal Na appetite.
The animals used in this study were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the College of Medicine of The Pennsylvania State University.
EXPERIMENT 1A
Methods
The methods and protocols employed were virtually identical in experiments 1A, 1B, and 2. Therefore, we describe the methods and protocols in detail for experiment 1A and only what differs for experiments 1B and 2. Similarly, the results for each experiment are summarized separately, and the accompanying discussions emphasize the differences between the current and prior results.
Animals and maintenance.
Adult male Sprague-Dawley rats (n = 35; Charles River, Wilmington, MA) weighed between 476 and 517 g at the time of surgery. The rats were assigned to groups by matching body weights, and they were individually housed in stainless steel hanging wire-mesh cages in a colony room with automatically controlled temperature (21°C), humidity, ventilation, and light cycle (12:12-h light-dark, lights on at 0700 h). The salt appetite tests were conducted in metabolism cages. All tests occurred during the light phase.
Before testing, the rats ate standard laboratory chow (rodent diet cat. no. W8604; Harlan Teklad, Madison, WI). During the salt appetite experiments, they were given an Na-free powdered diet (Harlan-Teklad, diet cat. no. 90228; 0.02% Na, wt/wt) with or without added NaCl. Deionized distilled water (dH2O) was available ad libitum in graduated cylinders. Fluid intake (to 0.5 ml), food consumption, and body weight were measured daily.
Surgery.
The rats were water deprived overnight, and ∼15 min prior to surgery they were weighed and injected with atropine (0.1 mg/kg ip) and gentamycin (6.0 mg ip). Subsequently, they were anaesthetized with pentobarbital sodium (50 mg/kg ip) and mounted in a stereotaxic apparatus equipped with blunt ear bars (David Kopf Instruments, Tujunga, CA). Supplemental doses of barbiturate (5 mg; typically every 45 min) were given to maintain surgical levels of anesthesia throughout the procedure. Body temperature was monitored and maintained at 36 ± 1°C with a heating pad. The scalp was opened with a midline incision, and the skull leveled between β and λ by adjusting the bite bar. Two holes (≅1.0 mm diameter) were drilled into the skull positioned bilaterally above the LH centered at −3.0 mm from β and 2.0 mm lateral to the midline. The skull surface and dura were moistened frequently with physiological saline.
The experimental group (n = 26) received bilateral LH lesions (LHx) positioned stereotaxically at −3.0 mm from β, 2.0 mm lateral, and 8.8 mm deep to the skull surface. Each injection occurred over 25 min via the needle of a 1.0-μl Hamilton syringe filled with freshly prepared IBO (0.5 μl, 20 μg/μl in PBS, pH 7.4; Research Biochemicals, Natick, MA). Infusions were made manually. Following both infusions, the skull holes were packed with Gelfoam, and the scalp was closed with wound clips. Three rats failed to recover from surgery, leaving the final number at 23. Nine additional rats served as controls; of these, six underwent sham surgery (sham group) as described above, except they received a saline injection (0.5 μl) instead of IBO, and the remaining three served as nonsurgical controls. After surgery, all animals were returned to their individual cages with ad libitum access to standard lab chow and water for at least 2 wk. Analgesia (Rimadyl, 5.0 mg/kg sc) and a prophylactic antibiotic (gentamycin, 4 mg/kg im) were administered daily for a minimum of 1 wk.
Salt appetite protocol.
For each of three replications, during a 7-day baseline period all rats had ad libitum access to dH2O, 0.51 M NaCl (3% wt/vol), and sodium-free diet with 1% salt added (wt/wt). On day 8, the NaCl was removed and unadulterated Na-free diet was provided. At noon, furosemide (Furo) was injected to induce a salt appetite. In experiments 1A and B, the rats received a fixed 7.0-mg dose sc. On the morning of day 9, food was withdrawn, and fresh water and 0.51 M NaCl was offered. Intake of both fluids was measured at 15, 30, 60, 120 min and at 24 h. At 2 h, Na-free food with1% NaCl added was offered again.
In experiment 1A, bilateral LHx, the same regimen was maintained for two more cycles, i.e., a week of baseline access to NaCl and dH2O, and then an Na appetite trial. On trial 2 the rats received a Furo injection; on trial 3, they were injected with an equivalent volume of saline (sc) instead of the diuretic. For this saline trial, the Na-free diet had 0.1% NaCl added to maintain body sodium balance. Experiment 1B consisted of a single Furo trial followed a week later by a single saline trial. In experiment 2 (asymmetric PBN/LHx), four test cycles were run. On trials 1, 2, and 4, the rats received Furo injections; on trial 3, saline was injected.
Data analysis.
The data were analyzed using repeated-measures ANOVA (GLM procedure, Statistica version 8.0, Statsoft). When appropriate, post hoc assessments were conducted with the Newman-Keuls test (N-K). The criterion for statistical significance was set at P < 0.05.
Tracing and histology.
Excitotoxins such as IBO are used to produce lesions because of their reputed ability to destroy somata while preserving fibers of passage. Nevertheless, questions have been raised about the completeness of this axon sparing (2, 46). These experiments sought to determine the role of the LH interacting with the PBN while leaving projections to other nuclei intact. The integrity of parabrachial axons passing through the LHx was verified by injecting the neuronal tracer wheat germ agglutinin-horseradish peroxidase (WGA-HRP) into the PBN. Following all behavioral tests, six LHx and three sham rats received injections of WGA-HRP in the PBN 3 days prior to being killed. The surgery proceeded as described above. Each rat was pretreated with atropine, anesthetized, and placed in the stereotaxic apparatus, and the skull was exposed and leveled. Two holes were drilled in the skull centered 12 mm behind β and 2 mm lateral to the midsagittal suture.
Gustatory neurons in the PBN were located bilaterally by recording multiunit responses with a glass-insulated tungsten electrode (Z = 0.5–1.2 MΩ at 1.0 kHz) while stimulating the anterior tongue with 0.3 M NaCl and rinsing with dH2O. Initial penetrations were placed 12.0 mm posterior to β and 1.8 mm lateral to the midsagittal suture with the search electrode oriented 20 degrees off vertical with the tip rostral, thus avoiding the transverse sinus. Neural activity was amplified conventionally and monitored with an oscilloscope and speaker. Testing for gustatory responses began following a conspicuous drop in background activity, indicating that the electrode had passed through the cerebellum into the pons. Once the gustatory zone was identified, the search electrode was replaced with a double-barrel micropipette with a tungsten electrode on one side (Z = 0.3–1.0 MΩ at 1.0 kHz) and the other side glued onto the needle of a 2.0-μl Hamilton syringe filled with mineral oil. Immediately prior to injecting, the syringe was backfilled with a 2% WGA-HRP solution (Sigma). The gustatory zone of the PBN was relocated with the attached electrode, and then 0.2 μl WGA-HRP was injected with a manual microdrive over 10 min. The pipette was left in situ 10 min more before removal, and then the holes in the skull were filled with Gelfoam, and the skin incision was closed.
The rats were allowed to survive for 72 h—ample time for anterograde and retrograde axonal transport—and were then anesthetized with pentobarbital sodium (50–75 mg ip). The rats were immediately perfused transcardially with warm (37°C) physiological saline for 10 min, then a 2.5% gluteraldehyde solution in PBS for 20 min, and finally a 20-min perfusion with 5% sucrose dissolved in cold PBS. The brains were removed from the skull and stored in 5% sucrose PBS at 4°C. Subsequently, they were cut on a freezing microtome at 40 μm and then mounted in three alternating series. The sections then underwent the Gibson tetramethyl benzidine reaction, yielding a blue HRP reaction product (11, 23). One series was cleared in xylene and then coverslipped; the other two were stained with either cresyl violet or neutral red prior to covering.
The remaining rats (17 LHx and 3 sham) were anesthetized with pentobarbital sodium (50–75 mg ip) and perfused transcardially for 8 min with physiological saline, followed immediately with 10% unbuffered formalin for 20 min. The brains were then removed from the skulls and immersed in a cold 30% sucrose-10% formalin solution for 1 to 2 days. The brains were blocked, frozen, and cut coronally in 50-μm sections and then mounted in two alternating series. In experiments 1A and B, one series was stained with cresyl violet and the other with the Weil myelin staining procedure. In experiment 2, the second series was stained for the specific neuronal protein NeuN, using standard immunohistochemical techniques (17, 27). The adequacy of the lesions was judged primarily from the Cresyl violet series by comparing the areas without neurons with similar sections from the control brains (16, 20).
Results
Histology.
LESIONS.
Cresyl violet-stained sections revealed differential placement of the lesions along the anterior-posterior axis (35). The area of common damage extended from −2.6 mm to −3.7 mm posterior to β, and included the LH lateral to the fornix and mammilothalamic tracts from the level of the midventromedial nucleus to its posterior boundary (see Fig. 1A, for an example). The neuronal loss often extended dorsally into the subthalamic nucleus and the zona incerta. In individual rats, the lesions stretched > 1.2 mm anterior and 0.7 mm caudal to the common area, but these extremes were not associated with unique behavioral deficits. In one rat, the lesions missed part of the LH on both sides; its behavioral data were not included.
Fig. 1.
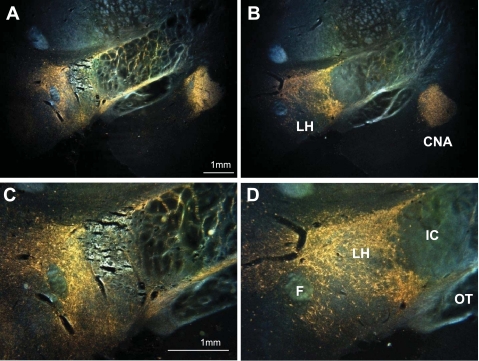
Darkfield photomicrographs of coronal sections showing wheat germ agglutinin-horseradish peroxidase (WGA-HRP) reaction product in the ventral forebrain after an infusion of the tracer into the pontine parabrachial nuclei. Dorsal is up, medial to the left. Top: reaction product (orange) is visible in the lateral hypothalamus (LH) and the central nucleus of the amygdala (CNA). A: LH lesion. B: control. Note that the CNA is filled with reaction product in both brains. Bottom: higher power photomicrographs of the LH reveal a pattern of dots and haze. The dots represent retrogradely labeled cell bodies; the haze is a mixture of retrograde and anterogradely labeled neural processes, i.e., axons and preterminal arbors. C: LH lesion. D: control. The core of the LH lesion eliminated all retrograde and anterograde labels. More medially, in the perifornical region, considerable reaction product remains. F, fornix; IC, internal capsule; OT, optic tract.
AXON SPARING.
In lesioned rats, the WGA-HRP tracing procedure demonstrated intact PBN axons passing through the LH (Fig. 1, A–D). Additionally, there was retrograde and anterograde labeling apparent in the bed nucleus of the stria terminalis, amygdala, and thalamus, as well as some retrogradely labeled neurons in the insular cortex. Thus, both ascending and descending projections between the forebrain and the PBN remained intact. There was no observable difference in the quality of reaction product in these nuclei between sham and LHx rats except in the LH itself. The rats with LHx appeared to have less reaction product there than the surgical controls, presumably due to the cell damage produced by the IBO, i.e., the lack of retrogradely labeled neurons.
Behavior.
FOOD, WATER, AND BODY WEIGHT.
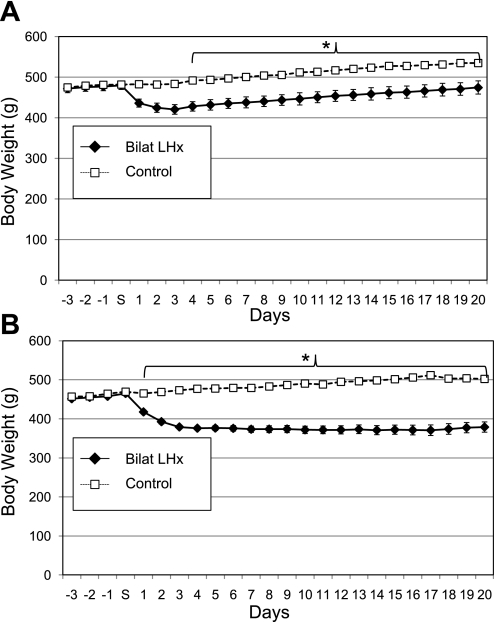
Compared with controls, the 23 rats with LHx drank less water for the first two postoperative days [F(46,667) = 2.6, P < 0.001] and ate less food for four postoperative days [F(46,667) = 8.18, P < 0.001 both with N-K post hoc tests]. Unlike the two control groups, the LHx rats lost weight following surgery (48.8 ± 10.1 g) between the four presurgical days and four postsurgical days [F(46,667) = 10.55, P < 0.001, Fig. 2A]. This loss persisted for 10 days. For the remaining 10 days prior to the first test period, they regained weight but never exceeded their presurgical means. By the end of the same 20-day period, both control groups exceeded their preoperative weights but did not differ from one another [F(1,7) = 2.65, P = 0.15]. Rats with LHx were lighter than the combined control group from postoperative day 4 until postoperative day 20 [F(23,690) = 16.3, P < 0.001, Fig. 2A].
Fig. 2.
Body weights of rats (means ± SE) with bilateral LH lesions (Bilat LHx) compared with their combined surgical and intact controls for 3 days before and 20 days following surgery (S). A: experiment 1A, LHx = 23, controls = 9. B: experiment 1B LHx = 18, controls = 7. *P ≤ 0.001.
SODIUM APPETITE.
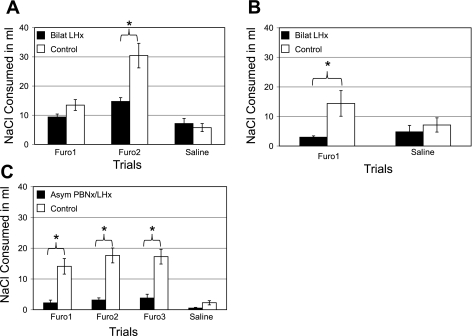
The control groups (n = 6 and n = 3) did not differ from one another on any of the Na appetite measures [F (1,7) = 0.28, P = 0.61], and thus their data were combined for the remaining statistical analysis (n = 9). There were three trials, spaced a week apart. In the first two, all of the rats received Furo; in the third, they all received an injection of saline. The ANOVA comparing groups across test days revealed significant main effects and interactions during NaCl consumption [F(2,60) = 9.99, P < 0.001, Fig. 3A]. The specific effects derive from post hoc N-K tests. Compared with their own saline trial, in the first Furo test the rats with LHx failed to express an Na appetite (P = 0.42). On the second Furo trial, their intake of 0.5 M NaCl did exceed the saline test (P < 0.04). The combined control group displayed a significant sodium appetite on both Furo trials (P < 0.035 and P < 0.001, respectively). Both groups increased their NaCl intake from trial 1 to trial 2, but the change was significant only for the controls (LHx, P > 0.135; control, P < 0.001). Finally, in trial 3 (saline injection), the LHx and control groups ingested equivalent amounts of NaCl, P > 0.60. In each of the Furo tests, however, the LHx rats licked less (0.5 M) salt than the controls, but the difference was significant only in trial 2 (Furo, trial 1: LHx/controls = 70%, P > 0.15; Furo, trial 2: LHx/controls = 49%, P < 0.001). At the earlier measurements (15, 30, 60, and 120 min) the trends were similar to those at 24 h, but few of the differences were statistically significant.
Fig. 3.
Mean ± SE 24-h intake of 0.51 M NaCl across Na appetite trials. A: experiment 1A: rats with bilateral ibotenic acid LHx (n = 23) ingested less NaCl than controls (n = 9) in the second furosemide (Furo) trial (*P ≤ 0.05), but they did not differ during the saline trial. During the first Furo trial, the NaCl intake of the LHx rats was similar to that during the second Furo and saline trials (P = 0.14; 0.43). Control rats consumed more NaCl during the second Furo trial than the first (P ≤ 0.0001), but their intake during both Furo trials was more than the saline trial (P ≤ 0.03; 0.0001). B: experiment 1B: rats with larger bilateral LHx (n = 18) ingested less NaCl than the controls (n = 7) during the Furo trial (*P ≤ 0.05) but not during the saline trial. Rats with LHx consumed equivalent volumes of NaCl during both the Furo and saline trials (P = 0.51). The controls, however, ingested more NaCl after the Furo injection than after saline (P = 0.01). C: experiment 2: rats with asymmetric lesions of the parabrachial nuclei and the LH (n = 13, Asym) ingested less NaCl than their controls (n = 10) on each of 3 Furo trials but not after the saline test (*P ≤ 0.05). The Asym group actually ingested equivalent amounts of NaCl on 3 Furo and the saline trials. The controls, however, consumed more NaCl during the second and third Furo trials than the first. The saline test was done between the second and third Furo trials, but the results are depicted last in the graph for clarity.
WATER CONSUMPTION.
On each of the three trial days, the rats had simultaneous access to water and 0.51 M NaCl. After both Furo and the saline injections, the rats with LHx and their controls drank similar amounts of water [F(2,60) = 0.63, P = 0.54].
Discussion.
Bilateral IBO lesions of the LH blunt sodium appetite but do not eliminate it. The present data replicate earlier experiments with electrolytic lesions but, in those studies, sodium appetite was virtually eliminated (54). The difference could reflect the axonal destruction produced with electrolytic damage, the location of the lesions, or their volume. We demonstrated that many PBN axons survive IBO LHx, but this does not prove that fibers of passage account for the differential effect on NaCl intake. The rationale for tracing axons from the PBN in this experiment arises from the fact that bilateral damage to these pontine nuclei also eliminates Na appetite (7, 41) and that parabrachial axons pass through areas normally destroyed by LHx (54, 29). While reliable and often dramatic, tracing axonal projections is not quantifiable. Because the current deficit was merely less severe than after electrolytic lesions and it remains possible that some fibers of passage were damaged, we cannot conclude that the decrease in sodium appetite resulted from damaging intrinsic neurons of the hypothalamus.
Location seems an unlikely factor for explaining the difference in the degree of Na appetite, because collectively our lesions spanned the length of the LH; most overlapped those of Wolf (54) and their relative position did not correlate with the severity of the sodium appetite deficit. The relative size of the lesions is difficult to assess because the two methods of producing them result in differential scaring. Experiment 1B addresses this issue by using IBO to induce more extensive LH damage.
EXPERIMENT 1B
Introduction
Based on their location, the lesions in the experiment 1A rats were classified as either anterior or posterior. These differences in the LH damage had little or no effect on the degree of reduction in Na appetite. They did influence the behavioral results on several other tests, which are not reported here. This prompted a second iteration with the same paradigms in three groups of rats with lesions targeted anterior to those in experiment 1A (Ant), posterior to experiment 1A (Post), and double, i.e., both anterior and posterior IBO infusions (Dbl). Again, the Na appetite results did not differ across groups [F(2,14) = 0.44, P = 0.65], so they were collapsed into a single group (LHx).
Methods
Surgery.
Male, Sprague-Dawley rats (n = 26) weighing between of 440 and 490 g at surgery were used in this experiment. The procedures followed those used for experiment 1A except for the following details. The anterior IBO injections (0.5 μl, 20 μg/μl) were placed at −2.5 mm caudal to β and the posterior ones at −3.1 mm. The double injections were made at the same two locations, but only 0.4 μl was infused at each site. As previously, in all cases the needle was positioned 2.0 mm right and left of the midsagittal suture and 8.8 mm below the skull surface. Three rats had double infusions but saline was used instead of IBO; they served as surgical controls (sham). The remaining four rats were nonsurgical controls. Five Ant, 4 Post, and 6 Dbl rats died within 10 days following surgery. Of the 24 surviving rats, the group numbers were 6 Ant, 5 Post, 6 Dbl, 3 sham, and 4 nonsurgical controls.
Histology.
Two Dbl, 1 Ant, 1 Post, and 1 sham rat underwent the WGA-HRP tracing protocol. The remaining surgical rats were perfused with formalin, and the brains were sectioned and stained in alternating series with cresyl violet or Weil as in experiment 1A.
Sodium appetite test.
The sodium appetite protocol was identical to that described in experiment 1A, except that only a single Furo trial was administered followed 1 wk later by a saline trial.
RESULTS
Histology.
On average, these lesions were larger than those in experiment 1A. Nevertheless, all of them were confined below the medial lemniscus, and none spread laterally into the amygdala. In four rats the damage did extend into the medial hypothalamus. The remaining 14 LHx rats had lesions consistent with those described for experiment 1A. In two rats (1 Dbl and 1 Ant), the WGA-HRP tracing pattern was similar to that described for experiment 1A. The remaining two brains (1 Dbl and 1 Post) failed to show transport in the brain. Some reaction product lined the ventricles, however, suggesting that the tracer flowed up along the pipette shaft rather than diffusing into the PBN.
Food and body weight.
Six rats died following surgery. The remaining animals were closely monitored for food and water intake. Each rat received sweetened wet mash (50 g powdered Teklad chow with 20 ml of an equal mixture of dH2O and sweetened condensed milk; Carnation). If a rat did not resume drinking after 2 days, it was given 9.0 ml of the milk-H2O mixture twice a day by gavage until fluid intake resumed. Despite this intervention, nine more rats died. From the first postoperative day, the remaining rats with LHx were lighter than the combined control group [F(23,552) = 21.2, P < 0.001, Fig. 2B]. Within 4 days, the LHx rats had lost more than 80 g from their presurgical weight, and they failed to regain it over the 20-day observation period (weight difference at day 20 = 85.9 ± 14.0 g). Throughout this period the controls were gaining weight so that by day 20 they were 123.62 ± 14.8 g (24.6%) heavier than the LHx group.
Sodium appetite.
Twenty-four rats were tested (6 Dbl, 6 Ant, 5 Post, and 7 control). Across all measurement times, NaCl intake did not differ for the 3 LHx groups [F(2,14) = 0.44, P = 0.65] or between the two sets of controls [F(1,5) = 1.78, P = 0.24]. Thus, the rats with LH damage (LHx, n = 17) were combined into a single group and compared with the combined controls (n = 7). In the Furo and saline trials, the controls consumed 14.4 ± 4.3 ml and 7.14 ± 2.4 ml of NaCl over 24 h, respectively. In the same period, the LHx group ingested 3.0 ± 0.45 ml and 4.82 ± 2.17 ml, respectively [Fig. 3B; F(1,22) = 5.62, P < 0.03]. During the Furo test, the controls drank more NaCl than in the other three tests (i.e., LHx, Furo and saline; controls, saline; P < 0.05). Intake of these latter three tests did not differ from each other (P > 0.05).
Water consumption during salt appetite trials.
Rats with LHx drank less water in the Furo (21.8 ± 2.97 ml) and saline trials (14.1 ± 3.63 ml) than the controls, 66.0 ± 4.21 ml and 37.1 ± 3.58 ml, respectively [F(1,22) = 6.75, P = 0.02]. The controls consumed more water during the Furo than the saline trial (P < 0.001), but the LHx rats did not (P = 0.19).
Discussion
Compared with experiment 1A, these rats with bilateral LHx lost more weight initially than their controls (86.0 ± 14.0 g vs. 48.8 ± 10.1 g). In addition, the first group began to regain weight quickly. The second set never regained weight despite measures to encourage it, i.e., normally preferred food or even gavage. The LHx rats of experiment 1A also showed, at best, a modest reduction in sodium appetite. The more extensive LH damage in experiment 1B virtually eliminated the appetite. As expected with IBO lesions, in both sets of experimental animals the histology demonstrated that axons originating in the PBN pass intact through or near the LH. Thus, these deficits associated with LH damage appear to reflect the loss of intrinsic neurons (50).
Nevertheless, the correlation between body weight loss and lack of Na appetite leaves the nature of the deficits open. Both symptoms could reflect a broad inability to link physiological deficits with compensatory ingestive behavior such as eating or drinking (25, 48). The fact that lesion volume seems to be an important variable in the magnitude of all three deficits supports such an inference.
A common mechanism would be more difficult to support if the feeding and Na appetite effects could be experimentally dissociated. As stated previously, bilateral lesions of the PBN result in Na appetite deficits that closely match those seen after LH damage but without substantial or chronic weight loss (13, 41). Because of this similarity and the predominantly ipsilateral projections from the PBN to the ventral forebrain, we hypothesized that damaging the PBN on one side of the brain and the LH on the other would eliminate Na appetite without concomitant aphagia or weight loss. The following experiment tests this hypothesis using the same Na appetite regimen and these asymmetrical lesions.
EXPERIMENT 2
Methods
Twenty-three Sprague-Dawley rats ranging from 357 to 552 g on the day of the surgery were housed and maintained as described previously.
Surgery.
Following acclimatization, 19 rats received LH and PBN IBO lesions. One set of rats received PBN damage in one side and LH damage in the other side [asymmetrical (Asym group), n = 13]. The second group had LH and PBN lesions on the same side [ipsilateral (Ipsi group); n = 6]. In both groups lesion placement was counterbalanced. The procedures were identical to those described in experiment 1A for the LHx and PBN tracing. The sole difference was that, for the PBN, an infusion of 0.2 μl of IBO was substituted for the WGA-HRP tracer used previously. There were four unoperated controls. At all measured intervals, NaCl intake of the Ipsi group and the controls did not differ [F(1,8) = 0.07; P = 0.80], so their data were collapsed into a single control group.
Sodium appetite.
Four salt appetite trials were conducted. Trials 1, 2, and 4 were with Furo; trial 3 used a saline injection. In experiment 2, the Furo dose was 10 mg/kg sc (range 5.3–5.5 mg). Prior data indicate that the Na appetites produced by 5.0 to 7.0 mg doses do not differ significantly (19). During this experiment, urine was collected and the volume measured three times: 24 h prior to the Furo and saline injections, 3 h immediately after the injections, and again from 3 to 21 h postinjection.
Results
Surgery.
There was no postoperative mortality.
Histology.
With a single exception, the LHx of both the Asym and Ipsi rats were centered at 2.9 ± 0.36 mm posterior to β (Fig. 4B). The exception (rat no. 6–28) had a more rostral LH lesion (−1.8 mm) along with adequate PBN damage. The PBN lesions were centered 9.5 ± 0.24 mm caudal to β. Eight rats had lesions essentially confined to the medial PBN. The remaining 11 rats had complete medial PBN damage that extended into the lateral half of the nuclei to varying degrees (Fig. 4C).
Fig. 4.
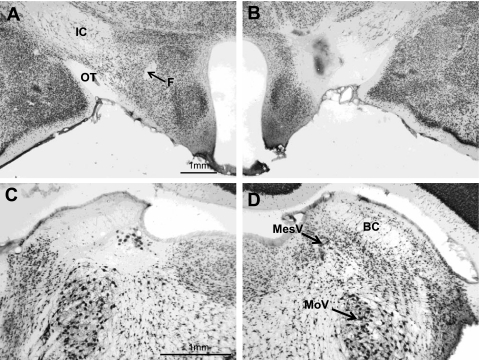
Photomicrographs of coronal sections through the LH (top) and the parabrachial nuclei (PBN; bottom) in a rat (no. 06–17) with asymmetric lesions (NeuN stain). A: intact left LH. B: lesioned right LH. C: lesioned left PBN. D: intact right PBN. The acellular area in C extends into the supratrigeminal area above the motor trigeminal nucleus (MoV) and the locus coeruleus medial to the mesencephalic trigeminal nucleus (MesV). BC, brachium conjunctivum. Magnification in C and D is double that of A and B.
The extent of the damage was almost equally distributed between the Asym and Ipsi groups. The underlying trigeminal motor nucleus was not involved in any of the lesions, but the supratrigeminal area or the locus coeruleus was encroached on in some rats. The LH and PBN lesions were adequate in all rats in both the Asym and Ipsi groups, so all of their data were included in the analysis.
Food, water, and body weight.
In the 5-day baseline before each of the 2-day Na appetite tests, body weight did not differ by group, even though the rats did gain weight across the entire observation period [groups: F(1,21) = 0.10, P = 0.75; days: F(19,399) = 56.7, P < 0.001]. Similarly, between groups, average food intake differed by only a gram [Asym = 21.9 ± 1.71 g/day, controls = 22.9 ± 1.90 g/day; F(1,21) = 3.26, P = 0.09]. Nevertheless, eating did increase across the experiment [F(19,399) = 41.70, P < 0.001]. Despite the parallels in weight and food intake, baseline drinking did differ [Asym = 18.4 ± 1.36 ml/day, control = 24.7 ± 2.00 ml/day; F(1,21) = 17.0, P < 0.001].
In the 24 h prior to the Furo injections, the Asym rats continued to drink less than their controls [23.6 ± 3.61 ml vs. 33.8 ± 3.16 ml; F(1,21) = 17.3, P < 0.001]. The following day, in the Na appetite test, both groups drank similar amounts during the first and third tests, but the Asym rats ingested less water than controls during the second test [16.2 ± 1.9 ml vs. 33.9 ± 2.4 ml; F(1,21) = 6.7, P < 0.02; N-K post hoc: test 1, P = 0.82; test 2, P = 0.012; test 3, P = 0.81].
Urine output.
Three hours following the Furo injection, urine volumes of both groups of rats were essentially identical [17.1 ± 0.75 vs. 17.5 ± 1.02; F(1,21) = 0.20, P = 0.66]. The post-Furo urine volumes (3–21 h), however, did differ [F(1,21) = 8.42, P = 0.01]. The urine output of rats with asymmetrical lesions (12.8 ± 1.5 ml) was lower than the control group (19.0 ± 2.6 ml). Urine volumes of the rats with asymmetrical lesions showed a similar difference from controls during the 24-h collection period prior to the Furo injection [11.9 ± 0.8 ml vs.16.9 ± 1.5 ml, respectively, F(1,21) = 14.13, P < 0.001].
Sodium appetite.
During baseline, the mean NaCl consumption of the Asym rats (n = 13, 0.7 ± 0.3 ml) and controls (n = 10, 1.0 ± 0.4 ml) did not differ [F(1,21) = 1.18, P = 0.3]. In the three Furo trials, after 24 h the control rats consumed 14.1 ± 2.5 ml, 17.7 ± 2.4 ml, and 17.3 ± 2.4 ml of 0.5 M NaCl, respectively. In the same period, the Asym group ingested 2.23 ± 0.89 ml, 3.15 ± 0.69 ml, and 3.77 ± 1.26 ml NaCl. During the saline trial, the control rats ingested 2.3 ± 0.7 ml of NaCl in 24 h and the Asym rats, 0.5 ± 0.2 ml [Fig. 3C; F(3,63) = 16.61, P < 0.001, N-K, P < 0.02]. At each of the four earlier measurements (15, 30, 60, and 120 min), on all three Furo trials the NaCl intake of the control rats exceeded the Asym group by a factor of > 5.0. All save one of these comparisons was significant (N-K: P < 0.05 to < 0.001). For the single exception, Furo 1 at 30 min, the control to Asym intake ratio was 5.8:1 (2.9:0.5 ml), but due to variance among the controls, the difference was marginal (P < 0.07).
After a saline injection (trial 3), the rats with Asym lesions ingested similar amounts of NaCl compared with their own three Furo tests (N-K: P = 0.25, 0.28, 0.18, respectively). Their Furo NaCl intakes also were similar to the control values during the saline trial (P > 0.75). The control intake during the saline trial, however, was substantially less than on their own Furo trials (P < 0.001, for each comparison).
Discussion
Asymmetrical lesions of the PBN on one side and the LHx on the other eliminate the expression of sodium appetite elicited by Furo. Following a Furo injection, sodium consumption of rats with asymmetrical lesions was siginifcantly lower than controls at 15, 30, 60, 120 min, and 24 h. Essentially, identical lesions of the same structures, but ipsilateral to one another, had little or no effect on the behavior. Bilateral damage to either the LH or PBN also blocks Na appetite (7, 8, and 42). Thus, to raise a sodium appetite, a functional connection must exist between these two areas.
Prior anatomical and electrophysiological evidence supports this inference. Axons arising from the PBN reach the LH and neurons in the LH project back to the PBN (29, 39). Some of these connections are related to gustatory function because PBN taste neurons can be both ortho- and antidromically driven from the LH (3, 21, 28, 29). Neither the current data nor the prior investigations determined which connections are critical, but the projections from the PBN are strongly ipsilateral and some do carry gustatory afferent activity.
The functions of the reciprocal LH connections remain obscure and are complicated by similar, extensive projections from other forebrain areas, such as the amygdala and insular cortex (3, 21; in hamster). Electrical stimulation of the central nucleus of the amygdala considerably sharpens the response profiles of PBN taste cells, but similar data are not yet available for the LH-PBN interaction (21).
Response profile sharpening via centrifugal connections is common in sensory physiology, so its occurrence within the gustatory system is not surprising. Sharpening presumably produces better sensory discrimination, perhaps permitting an animal to better focus attention. When the reciprocal influence arises from the limbic system, the elements of attention involved could be the motivational and hedonic attributes of gustatory stimuli (32). In fact, a forebrain index of reward, dopamine release in the nucleus accumbens produced when rats lick sucrose, is blunted by PBN lesions but not by damage to the thalamic taste area (16).
Another, more prosaic explanation for the effects of asymmetric PBN-LHx on Na appetite arises from the well-established integrative control of the hypothalamus on regulatory systems combined with the restricted goal of this particular need state. The deficit needing restitution is an element (sodium) that is detected in the external environment only by the gustatory system. In this scenario, the LH orchestrates the appropriate behavior (as well as endocrine and autonomic responses) based on the animal's physiological need. The PBN taste neurons supply the sensory information needed to guide the behavior. Because each system is required for the behavioral adjustment to proceed (and both are effectively ipsilateral), damaging them on opposite sides of the brain cashiers the response.
The logic supporting this explanation appears straightforward, but it is both limited and fragile. The autonomic and endocrine aspects of hypothalamic regulatory functions, including Na appetite, are neither ipsilateral nor confined to the brain. Indeed, the behavior involved in seeking out and ingesting sodium is not ipsilateral. This restricts the critical hypothalamic functions to processing neural information that is ipsilateral, perhaps just gustatory, afferent activity.
Not even all taste activity fits that bill. Bilateral PBN lesions that prevent the expression of Na appetite do not necessarily reduce gustatory detection thresholds for sucrose or NaCl (43). Thus, rats with PBN lesions can use gustatory information in other tasks. In addition, if rats have had experience with Na appetite prior to surgery, then bilateral PBN damage fails to prevent its subsequent expression (41). These observations increase the specificity of the sensory information that needs to be processed via these reciprocal PBN-LH connections: not all gustatory information, nor all gustatory projections, nor memories related to the Na appetite experience.
A third explanation for the failure of Na appetite in the rats with asymmetric PBN-LH damage is parallel to, but more indirect, than the former two. In this case the brain damage could prevent the diuretic response to the Furo itself, block the normal endocrine responses to the electrolyte loss, or suppress the central responses to the endocrine fluxes. In experiment 2, the urine output 3 h after a Furo injection was almost identical in both groups, indicating that the rats with asymmetric lesions responded appropriately to the diuretic. The only data relevant to possible changes in endocrine responses is indirect at best. Although the rats with lesions drank somewhat less water than their controls, the relationship was consistent across all four test sequences and specifically before and after each Furo injection. Thus, it seems unlikely that the lesions dramatically altered endocrine responses, at least those resulting from the diuretic. With the present data, the final possibility, that the central damage interfered with central sensing of endocrine fluxes, cannot be excluded (10). Nevertheless, the fact that the destruction in the PBN and the LH was unilateral militates against this explanation. If unilateral damage to either structure was sufficient, then the rats with ipsilateral PBN-LH damage would also fail to respond. In fact, they exhibit a robust sodium appetite.
Despite this winnowing of possible hypothalamic functions, the data do not determine how the PBN-LH interaction brings about expression of the appetite. Although both the PBN and LH limbs have monosynaptic connections to the other, the effects observed with these asymmetric lesions do not depend on a closed loop. They require only that the critical neural activity be functionally confined to one side of the brain.
This latter restriction probably explains why asymmetric lesions block Na appetite but have little, if any, effect on water intake or weight regulation. Classically, bilateral LH lesions produce anorexia, weight loss, and adipsia (48). Following the equally dramatic overeating and weight gain attending medial hypothalamic damage (1), this lateral hypothalamic syndrome was the impetus for research on the neural mechanisms of energy and fluid balance regulation that continues apace (26, 52). The recognition that LH destruction also interfered with Na appetite completed the motivational triad for ingestive behavior (53).
Our bilateral IBO lesions in the LH replicated these findings. Eight rats died in the second cohort despite access to highly preferred foods and even gavage. All of the LH rats that survived weighed less than their controls. Prior work with excitotoxic lesions indicated that most, if not all, of the energy and fluid balance disruptions resulted from destroying hypothalamic neurons rather than passing axons (49). The current experiments added Na appetite to the list of intrinsic LH functions. We included tracer injections to demonstrate that, after ibotenate lesions of the LH, parabrachial axons nevertheless reached their remaining forebrain targets. In the subsequent asymmetric PBN-LH experiment, none of the rats died and their body weight did not differ from controls. The Asym rats did consistently ingest less water than their controls, but compared with the classic lateral hypothalamic syndrome, the deficit was mild. Thus, these asymmetric lesions dissociated the feeding, drinking, and body weight effects of LH damage from the control of Na appetite.
Intrinsic hypothalamic regulatory systems can be differentiated based on their neurotransmitters, peptides, and receptors (15, 52). The present series of experiments specifically tested the separation of energy and water balance from Na appetite based on extra-hypothalamic mechanisms. Sodium appetite requires gustatory afferent activity. Feeding and drinking use taste, but the sensory information needed to guide the consummatory behaviors is more heterogeneous and thus not affected by PBN damage (30).
Perspective and Significance
This observation leads to a final point. The hypothalamus has reigned over regulatory systems and basic biological motivations for more than 50 years. For a period that hegemony was challenged by evidence implicating the caudal brain stem and peripheral feedback in the behavior and autonomic outcomes of these hypothalamic functions (14, 31, and 37). Subsequent discoveries of hormonal and peptidergic regulatory circuits within the hypothalamus have not so much supplanted this broader view as overwhelmed it with molecular detail and precision (18, 40). In fact, the original challenges were demonstrations that hypothalamic functions required access to the brain stem sensorimotor apparatus for their expression. The emphasis was largely on motor functions because the focus was motivated behavior. The current experiments fill out this view by emphasizing that these same hypothalamic functions require access to peripheral sensory activity to initiate, orient, and guide motivated behavior.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Han Li for assistance with the lesions as well as K. Matyas and N. Horvarth for histology. This research was supported by National Institute on Deafness and Other Communication Disorders Grants DC-05435 and DC-008937.
Present affiliations: S. Dayawansa, Department of Neurosurgery, University of Rochester, Rochester, New York; S. Peckins, Department of Pathology and Laboratory Medicine, Pennsylvania State University, Hershey, PA; and S. Ruch, Division of General Studies, Lancaster General College of Nursing and Health Sciences, Lancaster, PA.
REFERENCES
- 1. Coscina DV, Lacombe S, Chambers JW, Dixon L, Nobrega JN. Intake of greasy diets in hypothalamic obesity: a re-assessment. Appetite 13: 15–24, 1988 [DOI] [PubMed] [Google Scholar]
- 2. Coffey PJ, Perry VH, Allen Y, Sinden J, Rawlins JNP. Ibotenic acid induced demyelination in the central nervous system: a consequence of a local inflammatory response. Neurosci Lett 84: 178–184, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Cho YK, Li C. Gustatory neural circuitry in the hamster brain. J Neurophysiol 100: 1007–1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards GL, Johnson AK. Enhanced drinking after excitotoxic lesions of the parabrachial nucleus in the rat. Am J Physiol Regul Integr Comp Physiol 261: R1039–R1044, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Epstein AN, Stellar E. The control of salt preference in adrenalectomized rat. J Comp Physiol Psychol 48: 167–172, 1955 [DOI] [PubMed] [Google Scholar]
- 6. Fluharty SJ, Epstein AN. Sodium appetite elicited by intracerebroventricular infusion of angiotensin II in the rat: II. Synergistic interaction with systemic mineral corticoids. Behav Neurosci 97: 746–758, 1983 [DOI] [PubMed] [Google Scholar]
- 7. Flynn FW, Grill HJ, Schulkin J, Norgren R. Central gustatory lesions: II. Effects on sodium appetite, taste aversion learning, and feeding behaviors. Behav Neurosci 105: 944–954, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Flynn FW, Grill HJ, Schwartz GJ, Norgren R. Central gustatory lesions: I. Preference and taste reactivity tests. Behav Neurosci 105: 933–943, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Galverna OL, Deluca J, Schulkin S, Yao Z, Epstein A. Deficits in NaCl ingestion after damage to the central nucleus of amygdala in the rat. Brain Res Bull 28: 89–98, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol 93: 177–209, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Gibson AR, Hansma DI, Houk JC, Robinson FR. A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res 298: 235–241, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Grill HJ, Schulkin J, Flynn FW. Sodium homeostasis in chronic decerebrate rats. Behav Neurosci 100: 536–543, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Grigson PS, Shimura T, Norgren R. Brainstem lesions and gustatory function: II. The role of the nucleus of solitary tract and the parabrachial nucleus in the retention of a conditioned taste aversion in rats. Behav Neurosci 111: 180–187, 1997 [PubMed] [Google Scholar]
- 14. Grill HJ, Norgren R. Chronic decerebrate rats demonstrate satiation, but not bait shyness. Science 201: 267–269, 1978 [DOI] [PubMed] [Google Scholar]
- 15. Harthoorn LF, Sañé A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol 25: 1209–1223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Jongen-Relo AL. Specific neuronal protein: a new tool for histological evaluation of excitotoxic lesions. Physiol Behav 76: 449–456, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Low MJ. Role of proopiomelanocortin neurons and peptides in the regulation of energy homeostasis. J Endocrinol Invest 27: 95–100, 2004 [PubMed] [Google Scholar]
- 19. Lundy RF, Jr, Blair M, Horvath N, Norgren R. Furosemide, sodium appetite, and ingestive behavior. Physiol Behav 78: 449–458, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Lundy RF, Jr, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91: 1143–1157, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Lundy RF, Norgren R. Gustatory system . In: The Rat Nervous System (3rd ed.), edited by Paxinos G. New York: Elsevier, 2004, 891–921 [Google Scholar]
- 22. McDonald AJ. Topographical organization of amydaloid projections to the caudate-putamen, nucleus accumbens, and related striate-like areas of the rat brain. Neuroscience 44: 15–33, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117, 1978 [DOI] [PubMed] [Google Scholar]
- 24. Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol 75: 143–160, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Morrison SD, Mayer J. Adipsia and aphagia in rats after subthalamic lesions. Am J Physiol 191: 248–254, 1957 [DOI] [PubMed] [Google Scholar]
- 26. Morton G, Cummings D, Baskin D, Barsh G, Schwartz W. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Mullen RJ, Buck CR, Smith AM. NeuN a neuronal specific nuclear protein in vertebrates. Development 116: 201–211, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Norgren R. Gustatory afferents to ventral forebrain. Brain Res 81: 285–295, 1974 [DOI] [PubMed] [Google Scholar]
- 29. Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Norgren R. Sensory detection of water . In: Thirst: Physiological and Psychological Aspects. edited by Ramsay D, Booth D. New York: Springer-Verlag, 1991, p. 221– [Google Scholar]
- 31. Norgren R, Grill HJ. Brain stem control of ingestive behavior. In: Physiological Mechanisms of Motivation, edited by Pfaff DW. New York: Springer-Verlag, 1982, p. 99–113 [Google Scholar]
- 32. Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 89: 531–535, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ogawa H, Hesagawa K, Murayama N. Difference in taste quality coding between two cortical taste areas, granular and dysgranular insular areas in rats. Exp Brain Res 91: 415–424, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Ogawa H, Nomura H. Receptive field properties of thalamo-cortical taste relay neurons in the parvicellular part of the posteromedial ventral nucleus in the rat. Exp Brain Res 73: 364–370, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Paxinos G, Watson C. Atlas of the Rat Brain (2nd ed.). Orlando, FL: Academic, 1992 [Google Scholar]
- 36. Pereira da Silva RK, Saad WA, Renzi A, Menani JV, Camargo LA. Effects of lateral hypothalamus on the water and the salt intake, and sodium and urine excretion induced by activation of the median preoptic nucleus in conscious rats. J Auton Nerv Syst 53: 195–204, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev 84: 89–126, 1977 [PubMed] [Google Scholar]
- 38. Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci 101: 724–731, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res 197: 291–317, 1980 [DOI] [PubMed] [Google Scholar]
- 40. Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109: 997–1008, 1995 [PubMed] [Google Scholar]
- 42. Schulkin J, Fluharty S. Further studies on salt appetite following lateral hypothalamic lesions: effects of preoperative alimentary experience. Behav Neurosci 99: 929–935, 1985 [DOI] [PubMed] [Google Scholar]
- 43. Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci 106: 147–161, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Spector AC, Grill HJ, Norgren R. Concentration-dependent licking of sucrose and sodium chloride in rats with parabrachial gustatory lesions. Physiol Behav 53: 277–283, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Spector AC, Scalera G, Grill HJ, Norgren R. Gustatory detection thresholds after parabrachial nuclei lesions in rats. Behav Neurosci 109: 939–954. 1995 [PubMed] [Google Scholar]
- 46. Stellar JR, Hall FS, Waraczysnki M. The effects of excitotoxic lesions of the lateral hypothalamus on self stimulation reward. Brain Res 541: 29–40, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann NY Acad Sci 985: 174–184, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev 69: 74–90, 1962 [DOI] [PubMed] [Google Scholar]
- 49. Whishaw IQ, Pelis SM, Pelis VC. A behavioral study of the contributions of cells and fibers of passage in the red nucleus of the rat to postural righting, skilled movements, and learning. Behav Brain Res 52: 29–44, 1992 [DOI] [PubMed] [Google Scholar]
- 50. Winn P, Tarbuck A, Dunnett SB. Ibotenic acid lesions of the lateral hypothalamus: comparison with the electrolytic lesion syndrome. Neuroscience 12: 225–240, 1984 [DOI] [PubMed] [Google Scholar]
- 51. Wirsig CR, Grill HJ. Contribution of the rat neocortex to ingestive control: I. Latent learning for the taste of sodium chloride. J Comp Physiol Psychol 96: 615–627, 1982 [DOI] [PubMed] [Google Scholar]
- 52. Woods JS, Leibowitz SF. Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharmacol Biochem Behav 23: 431–438, 1985 [DOI] [PubMed] [Google Scholar]
- 53. Wolf G. Effect of dorsolateral hypothalamic lesions on sodium appetite elicited by deoxycorticosterone and by acute hyponateremia. J Comp Physiol Psychol 58: 396–402, 1964 [DOI] [PubMed] [Google Scholar]
- 54. Wolf G. Hypothalamic regulation of sodium intake: relations to preoptic and tegmental function. Am J Physiol 213: 1433–1438, 1967 [DOI] [PubMed] [Google Scholar]
- 55. Wolf G, Dicara LV, Braun JJ. Sodium appetite in rats after neocortical ablation. Physiol Behav 5: 1265–1269, 1970 [DOI] [PubMed] [Google Scholar]
- 56. Zardetto-Smith AM, Beltz TG, Johnson AK. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally induced salt appetite. Brain Res 645: 123–134, 1994 [DOI] [PubMed] [Google Scholar]