Abstract
Previously, we demonstrated that maternal diabetes reduced the excitability and increased small-conductance Ca2+-activated K+ (SK) currents of parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA). In addition, blockade of SK channels with apamin completely abolished this reduction. In the present study, we examined whether maternal diabetes affects large-conductance Ca2+-activated K+ (BK) channels and whether BK channels contribute to the attenuation of PCMN excitability observed in neonates of diabetic mothers. Neonatal mice from OVE26 diabetic mothers (NMDM) and normal FVB mothers (control) were used. The pericardial sac of neonatal mice at postnatal days 7–9 was injected with the tracer X-rhodamine-5 (and 6)-isothiocyanate 2 days prior to the experiment to retrogradely label PCMNs in the NA. Whole cell current- and voltage-clamps were used to measure spike frequency, action potential (AP) repolarization (half-width), afterhyperpolarization potential (AHP), transient outward currents, and afterhyperpolarization currents (IAHP). In whole cell voltage clamp mode, we confirmed that maternal diabetes increased transient outward currents and IAHP compared with normal cells. Using BK channel blockers charybdotoxin (CTx) and paxilline, we found that maternal diabetes increased CTx- and paxilline-sensitive transient outward currents but did not change CTx- and paxilline-sensitive IAHP. In whole cell current-clamp mode, we confirmed that maternal diabetes increased AP half-width and AHP, and reduced excitability of PCMNs. Furthermore, we found that after blockade of BK channels with CTx or paxilline, maternal diabetes induced a greater increase of AP half-width but similarly decreased fast AHP without affecting medium AHP. Finally, blockade of BK channels decreased spike frequency in response to current injection in both control and NMDM without reducing the difference of spike frequency between the two groups. Therefore, we conclude that although BK transient outward currents, which may alter AP repolarization, are increased in NMDM, BK channels do not directly contribute to maternal diabetes-induced attenuation of PCMN excitability. In contrast, based on evidence from our previous and present studies, reduction of PCMN excitability in neonates of diabetic mothers is largely dependent on altered SK current associated with maternal diabetes.
Keywords: BK channels, SK channels, repolarization, afterhyperpolarization
there is an increased risk for development of malformations of the central nervous system in human infants born from diabetic mothers (8, 35). In the developing spinal cord of embryos from streptozotocin-induced diabetic mouse mothers (15), neuroepithelial cells have decreased proliferation and increased apoptosis. Offspring of streptozotocin-induced diabetic mouse mothers have impaired sympathetic and parasympathetic development (14, 55) and attenuated baroreflex control of heart rate (51). Previously, we established that there are strong projections to the cardiac ganglia from the parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) and that baroreflex control of heart rate is predominantly regulated by the NA (5, 6). Since PCMNs in the NA are one of the key components in regulating cardiac function, it is critically important to study the maternal diabetes-induced pathophysiological changes of the PCMNs.
In many central neurons, potassium (K+) channels activated by influx of calcium (Ca2+) during action potentials (APs) generate currents that contribute to AP repolarization and afterhyperpolarization potential (AHP) (23, 37, 47). Ca2+- activated K+ channels are split into two major divisions: 1) small-conductance (SK) voltage-insensitive channels that are selectively blocked by the bee toxin apamin (4); and 2) large (or big) conductance Ca2+-activated K+ (BK) voltage-sensitive channels that are potently blocked by the scorpion venom toxin charybdotoxin (CTx) and selectively blocked by paxilline (49, 50). SK currents largely contribute to medium AHP (mAHP; tens to several hundred milliseconds), which significantly mediates firing frequency (29) and patterns (38).
BK channels are widely expressed throughout the vertebrate nervous system (19, 21) and are sensitive to membrane potentials and intracellular Ca2+ concentration. Activation of BK channels during APs regulates AP repolarization, spike-frequency adaptation, and excitability in many central neurons (1, 22, 42, 43, 47). Our aim in this paper was to study the cellular mechanism for the maternal diabetes-induced decrease in excitability of PCMNs. Previously, we reported the effects of maternal diabetes on PCMNs in the neonates from diabetic mothers (27). We demonstrated that maternal diabetes alters AP properties and reduces excitability of PCMNs in the NA. In addition, maternal diabetes increases SK transient outward currents and afterhyperpolarization currents (IAHP). Blockade of SK channels with apamin completely abolished this reduction of PCMN excitability in the neonates from diabetic mothers, indicating that SK channels significantly contribute to maternal diabetes-attenuated excitability. However, whether BK channels contribute to the firing properties of PCMNs in NA has not been well established. In a previous study, Mendelowitz (32) found that blockade of BK channels with CTx did not affect the excitability of PCMNs. Since BK channels contribute to the intrinsic firing properties and regulation of excitability in many other central neurons (2, 12, 37, 47, 52), we aimed to examine whether maternal diabetes alters BK currents and whether these changes of BK currents play a role in maternal diabetes-induced reduction of excitability.
MATERIALS AND METHODS
Animal models.
Neonatal mice from females of wild-type (FVB; n = 12) and OVE26 diabetic mice (NMDM; n = 12) (1.5–2 mo), which had been mated with wild-type FVB males (2–3 mo; n = 8) were used. Each male was mated with three females in the same cage. Female mice were separated into individual cages after they became pregnant. OVE26 mice develop type 1 diabetes due to specific overexpression of the calmodulin transgene in pancreatic beta cells (10, 11). The OVE26 mouse model has been recently used to study diabetes-induced baroreflex impairment and associated remodeling of the baroreflex arc (17, 25, 28, 54) as well as diabetes-induced complications in the heart and kidney (26, 56) . Two groups of neonatal mice were used: 1) neonates from FVB mothers (control) and 2) neonatal mice from OVE26 diabetic mothers (NMDM). Animals had free access to food and water, and no insulin therapy was given. All procedures were approved by the University of Central Florida Animal Care and Use Committee and followed the guidelines of the National Institutes of Health. Efforts were made to reduce the number of animals used.
Fluorescent labeling of NA PCMNs and medullary slice preparation.
Neonatal mice (postnatal days 7–9) were anesthetized with 3% isoflurane (Abbott Laboratories, North Chicago, IL) and cooled to ∼4°C to decrease heart rate. After a right thoracotomy was performed, the retrograde fluorescent tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC; 2%, 4 μl; Molecular Probes, Eugene, OR) was injected into the pericardial sac at the base of the heart. After a 48-h recovery period, neonatal mice were deeply anesthetized with 4% isoflurane. The hindbrains were rapidly removed. The brain stem including PCMNs were sliced in serial sections (250 μm) using a vibrating blade microslicer (model DTK-1000; Kyoto, Japan) and maintained in an interface chamber filled with artificial cerebral spinal fluid (aCSF) containing (in mM; see Refs. 27, 29): 126 NaCl, 2.5 KCl, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 MgSO4 and 10 dextrose, equilibrated with 95% O2-5% CO2, and pH adjusted to 7.4. Slices were transferred to a recording chamber maintained at room temperature (22–25°C).
Whole cell current-clamp recording.
Brain stem slices were viewed with infrared illumination, differential interference optics (Zeiss, Göttingen, Germany), and a near-infrared sensitive camera (AxioCom MRm; Göttingen, Germany). PCMNs in the NA were identified by the presence of the fluorescent tracer in superimposed fluorescent and infrared images (Fig. 1). Whole cell current-clamp recordings were made from fluorescently labeled NA neurons. Within the recording chamber, slices were held in a stable position using a nylon net stretched over a flattened U-shaped platinum wire and were continuously superfused at room temperature with oxygenated aCSF. For the Ca2+-free solution, 2 mM CaCl2 were replaced by 3 mM MgCl2. Patch electrodes were fabricated from borosilicate glass (1.5 mm outer diameter; World Precision Instruments, Sarasota, FL) with a Flaming Brown horizontal puller (model P-97; Sutter Instruments, Novato, CA). Electrodes were heat polished to a final tip resistance of 3–5 MΩ and filled with a pipette solution containing (in mM): 120 K-gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 ATP, and 0.25 GTP, with pH adjusted to 7.3 with KOH.
Fig. 1.
A: fluorescent image of X-rhodamine-5 (and 6)-isothiocyanate-labeled parasympathetic cardiac motoneurons (PCMNs). B: differential interference contrast image of PCMNs. C: merged image of A and B. Arrows indicate a recorded PCMN. Scale bar: 40 μm.
APs were evoked by applying 10-ms depolarizing current pulses of sufficient intensity (40–100 pA) to generate a single AP (7, 29). To standardize AP recordings, neurons were depolarized to a holding potential of −65 mV by DC application through the recording electrode. APs were recorded within minutes after good recording conditions were established. Spike half-width was measured as the spike width at the half-maximal voltage. AHP amplitudes were measured at 10 and 50 ms. To investigate the firing frequency of neurons, five current injection steps (1,000 ms each) were applied from 40 to 120 pA in 20 pA increments. The mean spike frequency was calculated as the average of the inverse of all interspike intervals in response to 1 s constant current pulse. Input resistance was determined from a linear regression in the linear range (generally ±10 mV from resting potential) of the voltage-current relationship established by plotting the steady-state voltage changes in response to a series of depolarizing and hyperpolarizing current injections. To determine the membrane potential threshold, AP trains were evoked by a 1.5 s, 0.2 pA /ms ramp current from a holding potential of −65 mV in control aCSF and the threshold was measured at the beginning of the first AP.
Before seals were made on cells, offset potentials were nulled. Recordings were accepted when resting membrane potentials measured immediately after membrane rupture were less than −60 mV and the AP amplitude was ≥ 70 mV from threshold to the peak.
Whole cell voltage clamp recording.
PCMNs of the NA were again identified in brain stem slices by the presence of fluorescent tracer. To record Ca2+-activated K+ currents, cells were superfused with an oxygenated HEPES solution containing (in mM; see Refs. 27, 29): 140 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 0.0005 TTX, 1 4-aminopyridine, 0.005 glybenclamide, and 10 glucose, pH adjusted to 7.4 with NaOH. TTX, 4-aminopyridine, and glybenclamide were included routinely in the K+ recording solution unless otherwise indicated. A low concentration of 4-aminopyridine (1 mM) was chosen to reduce voltage-gated K+ currents, minimize possible nonspecific blockade on other K+ currents, and unmask Ca2+ dependence of K+ currents. Glybenclamide was used to selectively block ATP-sensitive K+ channels. Electrodes were heat polished to a final tip resistance of 2–4 MΩ and filled with pipette solution containing (in mM): 110 K-gluconate, 10 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 1 ATP, 0.2 GTP, and 0.1 leupeptin, with pH adjusted to 7.3 with KOH. For the Ca2+-free solution, 2 mM CaCl2 was replaced by 3 mM MgCl2. Pipette capacitance compensation was used in all recordings (19, 33). Membrane potential measurements were not corrected for the liquid junction potential (∼15 mV), because it did not affect the difference of currents between the two groups. The series resistances were in the range of 10 to 20 MΩ.
Outward currents were evoked by a 250-ms series of depolarizing steps from −70 to +40 mV in +10-mV increments. The peak values of transient outward currents were measured. Current-voltage relationships were then plotted. The mean peak values of outward currents were plotted against the membrane potential and fitted by the Boltzmann equation (44). To study the IAHP, outward currents of IAHP were evoked by a 100-ms pulse from a −70 mV holding potential to +10 mV followed by a 1.5-s pulse of −50 mV. The peak amplitude of IAHP was measured as the peak current after the onset of the −50-mV pulse.
Drugs and chemicals.
All the channel blockers used in the present study were purchased from Sigma-Aldrich (St. Louis, Mo). Blockers were applied by directly adding them to the superfusate from stock solutions.
Data acquisition and analysis.
Data acquisition was controlled using the ClampEx program in the pClamp 9 software package (Axon Instruments, Foster City, CA). Signals were recorded using a MultiClamp 700B patch-clamp amplifier (Axon Instruments). Responses were low-pass filtered at 3 kHz and digitized at 50 kHz with a 16-bit analog-to-digital data acquisition systems (Digidata 1322A; Axon Instruments). Data were presented as means ± SE. Student's t-test and two-way ANOVA with repeated measures followed by a Tukey-Kramer post hoc test were used. Differences were considered significant at P < 0.05.
RESULTS
In the present study, 113 cells from 38 control mice and 105 cells from 31 NMDM mice were analyzed. All neurons selected for recording were strongly retrogradely labeled from the tracer XRITC injection into the pericardial sac. Fig. 1 shows XRITC-labeled PCMNs (Fig. 1A) and a recording of one of these labeled neurons using a pipette electrode (Fig. 1, B and C).
General conditions.
The body weights and blood glucose levels of the two different groups were measured on the experimental day. We randomly selected 12 animals from each experimental group to compare body weight and blood glucose level between groups. Compared with control group (5.1 ± 0.3 g), body weights in NMDM were not significantly different (4.7 ± 0.2 g; P > 0.05). The blood was sampled from the tail of nonfasting neonates, and blood glucose levels were measured using a blood glucose monitoring meter (Nova Biomedical, Waltham, MA). Blood glucose levels in the NMDM group (172.5 ± 9.2 mg/dl) were significantly higher than those in the control group (143.9 ± 7.3 mg/dl; P < 0.05). As shown in Zheng et al. (56), the blood glucose levels of normal FVB control mice were < 180 mg/dl. In addition, as shown in the previous study (27), the variations of blood glucose levels in the range of 131.9–197.3 mg/dl between groups did not affect spike frequency and outward currents of PCMNs. Therefore, we consider that the neonatal FVB and NMDM mice used in the present experiments were all within the normal blood glucose range.
Membrane properties.
Table 1 lists the passive membrane properties and the amplitudes of APs of PCMNs in control and NMDM mice. The average resting membrane potentials, input resistances, membrane capacitances, AP thresholds, and AP amplitudes did not differ between the two groups.
Table 1.
Passive membrane properties and action potentials (AP) of parasympathetic cardiac motoneurons of the nucleus ambiguus
| Group | Vm, mV | Rin, MΩ | Cm, pF | AP threshold, mV | AP amplitude, mV |
|---|---|---|---|---|---|
| Control | −70.4 ± 0.5 | 250.9 ± 8.8 | 63.8 ± 0.9 | −35.9 ± 1.5 | 82.4 ± 1.0 |
| NMDM | −71.0 ± 0.8 | 253.6 ± 11.4 | 64.8 ± 1.0 | −35.6 ± 1.6 | 83.3 ± 1.0 |
Values are means ± SE. No significant differences were found between control and neonatal mice from OVE26 diabetic mothers (NMDM). Vm, resting membrane potential; Rin, input resistance; Cm, membrane capacitance; n = 15/group.
Maternal diabetes increased BK currents.
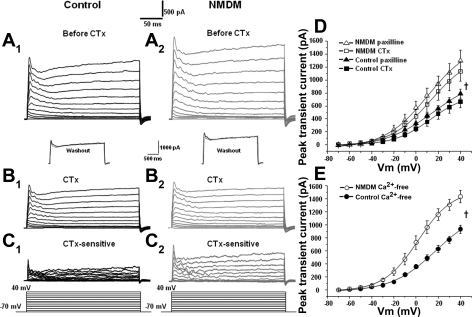
Whole cell voltage-clamp experiments were performed using K+-filled pipettes to characterize the K+ currents evoked by depolarization. Outward currents were first evoked by a series of voltage steps of +10 mV from −70 mV to +40 mV (Fig. 2, A1 and A2). Figure 2, A1 and A2 shows examples of recorded outward K+ currents in control and NMDM. To isolate BK currents, we used BK channel blockers. Following administration of 100 nM CTx, the outward currents were evoked again (Fig. 2, B1 and B2). CTx-sensitive currents (Fig. 2, C1 and C2) were obtained by subtracting outward currents evoked after CTx from those recorded before CTx (Fig. 2, A1–B1, and A2–B2). The current-voltage curves are plotted in Fig. 2D. Maternal diabetes significantly increased CTx-sensitive peak transient currents (Fig. 2D; ANOVA, P < 0.05). The peak transient current at +40 mV was 668.2 ± 94.3 pA in control and 1,129.3 ± 148.9 pA in NMDM. The current-voltage curves fitted by the Boltzmann equation were shifted to the left in NMDM compared with control (Fig. 2D). The midpoint potential (V1/2) was different in NMDM mice compared with control (control: 15.2 ± 1.0 mV vs. NMDM: 10.9 ± 1.1 mV; P < 0.05).
Fig. 2.
Membrane potential was held at −70 mV and stepped from −70 to +40 mV for 250 ms with 10-mV increments every 5 s as shown at the bottom of the traces. A1–C1, control. Outward currents evoked by voltage steps before (A1) and after (B1) application of charybdotoxin (CTx). C1, CTx-sensitive current (subtraction of A1 and B1). A2–C2: data for neonatal mice from OVE26 diabetic mothers (NMDM) showing outward currents evoked by voltage steps before (A2) and after (B2) application of CTx. C2: CTx-sensitive currents (subtraction of A2 and B2). B1 and B2, insets: black trace (before CTx) and gray trace (after CTx washout) show that outward currents were completely reversible after washout of CTx application, indicating the stability of recorded cells during the time course of experiment. D: CTx-sensitive (n = 9/group) and paxilline-sensitive (n = 8/group) transient outward currents were significantly increased in NMDM compared with control. E: Ca2+-sensitive transient outward currents also significantly increased in NMDM compared with control (n = 10/group). Vm, membrane potential. †ANOVA, P < 0.05.
Although CTx is one of the most commonly used potent inhibitors of BK channels, it might also block intermediate conductance Ca2+-activated K+ channels (20). Therefore, we also used paxilline, which is a specific BK channel blocker (40). Consistent with the CTx data, maternal diabetes significantly increased the paxilline-sensitive transient currents (ANOVA, P < 0.05, Fig. 2D). The peak transient current value at +40 mV was 789.4 ± 69.4 pA in control and 1,298.4 ± 162.3 pA in NMDM (P < 0.05, Fig. 2D). The paxilline-sensitive current-voltage curves fitted by the Boltzmann equation in NMDM were shifted to the left compared with controls. The V1/2 differed significantly between NMDM and control mice (control: 16.1 ± 1.7 mV, NMDM: 10.9 ± 2.2 mV, P < 0.05).
Maternal diabetes increased Ca2+-sensitive K+ currents.
Since BK channels are Ca2+-dependent, we also examined whether prevention of Ca2+ influx may yield a similar effect on outward currents to that seen in blockade of BK channels. The Ca2+-dependent outward currents were first evoked by a series of voltage steps of +10 mV from −70 mV to +40 mV in a 2-mM Ca2+ solution. Ca2+ was then replaced by 3 mM Mg2+ (Ca2+-free solution). The outward currents were then again evoked in Ca2+-free solution. Ca2+-sensitive currents were obtained by subtracting outward currents evoked after Ca2+-free solution from those obtained before Ca2+-free solution. Similar to CTx-sensitive and paxilline-sensitive currents, maternal diabetes significantly increased Ca2+-sensitive transient currents (ANOVA, P < 0.05, Fig. 2E). The mean peak transient current at +40 mV was 933.0 ± 63.0 pA in control and 1,433.4 ± 97.2 pA in NMDM (Fig. 2E). The curve fitted by the Boltzmann equation in NMDM showed a distinctly shifted mean V1/2 to the left from 18.3 ± 2.7 mV (control) to 1.9 ± 0.6 mV (NMDM) (P < 0.05).
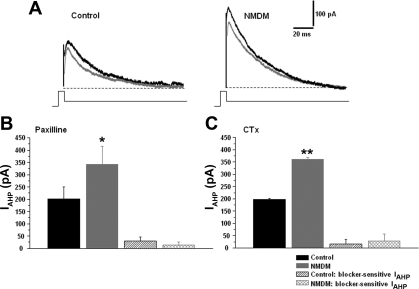
Maternal diabetes did not affect BK-mediated IAHP.
To examine whether IAHP currents were mediated by BK channels, and whether maternal diabetes altered BK channel-mediated IAHP, IAHP was evoked by a 100-ms pulse of +10 mV from a holding potential of −70 mV and was immediately followed by a 1.5-s, −50-mV pulse. The peak amplitude of IAHP was measured and compared with control and NMDM. The traces in Fig. 3A are representative recordings of IAHP in control and NMDM before (black) and after (gray) paxilline was administered. Maternal diabetes significantly increased the peak amplitude of IAHP in NMDM (341.1 ± 74.7 pA) compared with control (201.9 ± 47.4 pA, P < 0.05) (Fig. 3B). Paxilline bath application significantly decreased the peak amplitude of IAHP in both control (151.1 ± 45.1 pA) and NMDM (310.2 ± 70.4 pA, P < 0.05) groups compared with that before paxilline (Fig. 3B). However, the reduction of the peak amplitude of IAHP in control and NMDM, obtained by subtracting IAHP before and after application of paxilline, was not significantly different (control: 30.0 ± 15.7 pA, NMDM: 13.3 ± 11.7 pA) (Fig. 3B). To confirm our data by using paxilline, we also administered CTx. Consistent with paxilline, the peak amplitude of IAHP was significantly increased in NMDM (360.8 ± 8.5 pA) compared with control (198.6 ± 3.4 pA, P < 0.05) before CTx administration. CTx bath application significantly decreased the peak amplitude of IAHP in both control (174.4 ± 6.0 pA) and NMDM (324.6 ± 14.5 pA; P < 0.05) groups compared with that before CTx. Similar to paxilline, the reduction of the peak amplitude of IAHP in control and NMDM was not significantly different (control: 16.8 ± 18.4 pA, NMDM: 28.2 ± 28.4 pA) (Fig. 3C).
Fig. 3.
A: representative afterhyperpolarizaton current (IAHP) waveforms of control and NMDM before (black trace) and after (gray trace) paxilline application. B: paxilline application. The peak amplitude of IAHP significantly increased in NMDM (n = 8) compared with that in control (n = 9), but paxilline-sensitive IAHP did not differ between control and NMDM. C: CTx application. Similar to paxilline application, the peak amplitude of IAHP significantly increased in NMDM (n = 8) compared with that in control (n = 13); but again, CTx-sensitive IAHP did not differ between control and NMDM groups. *P < 0.05; **P < 0.01.
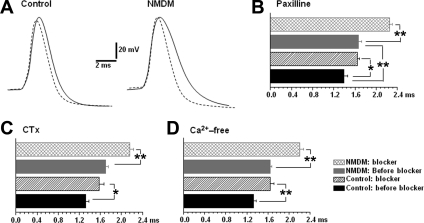
Maternal diabetes increased the contribution of BK channels to AP repolarization.
The effect of BK channels on the half-width of AP was first determined by application of paxilline. To standardize AP recordings, the membrane potential was held at −65 mV (below the threshold) and the AP was evoked by a 10-ms depolarizing current injection with an intensity that only generated a single spike. Figure 4A shows the original recordings of the spikes before (dotted trace) and after (solid trace) paxilline (10 μm). As shown in Fig. 4B, application of paxilline significantly broadened the spike half-width in control (before: 1.39 ± 0.07, after: 1.65 ± 0.04 ms, P < 0.05) and in NMDM (before: 1.65 ± 0.06, after: 2.25 ± 0.05 ms, P < 0.05). Maternal diabetes increased spike half-width compared with control (Fig. 4A, left and right dotted trace; Fig. 4B). Noticeably, application of paxilline significantly increased spike half-width in NMDM (37.1 ± 6.9%) more than that in control (19.1 ± 4.0%, P < 0.05) (Fig. 4A, left and right solid lines; Fig. 4B). To confirm our data by using paxilline, we also administered CTx. Consistent with paxilline, application of CTx significantly increased the spike half-width in both control (before: 1.32 ± 0.06 ms, after: 1.58 ± 0.09 ms, P < 0.05) and NMDM groups (before: 1.70 ± 0.06 ms, after: 2.16 ± 0.07 ms, P < 0.05) (Fig. 4C). Noticeably, application of CTx significantly increased spike half-width in NMDM (27.7 ± 4.2%) more than that in control (19.6 ± 3.2%, P < 0.05).
Fig. 4.
A: representative action potential (AP) waveforms of control and NMDM PCMNs before (dotted trace) and after (solid trace) paxilline application. B: comparison of the spike half-width of control and NMDM (n = 9/group) before and after paxilline. In both control and NMDM, paxilline significantly increased spike half-width. Paxilline induced a significantly greater broadening of the spike half-width in NMDM than in control. C: comparison of the spike half-width of control and NMDM (n = 9/group) before and after CTx. In both control and NMDM CTx significantly increased spike half-width. CTx induced a significantly greater broadening of the spike half-width in NMDM. D: comparison of the spike half-width of control and NMDM (n = 8/group) before and after Ca2+-free solution. In both control and NMDM, Ca2+-free significantly increased spike half-width. In addition, Ca2+-free induced a significantly greater broadening of the spike half-width in NMDM than in control. *P < 0.05; **P < 0.01.
Effect of Ca2+-activated K+ channels on the half-width of AP was also determined using the Ca2+-free solution to prevent Ca2+ influx. As shown in Fig. 4D, application of Ca2+-free solution significantly increased the spike half-width in both control (before: 1.33 ± 0.05 ms, after: 1.64 ± 0.07 ms; P < 0.05) and NMDM groups (before: 1.64 ± 0.03 ms, after: 2.21 ± 0.06 ms, P < 0.01). Noticeably, application of Ca2+-free solution significantly increased spike half-width in NMDM (36.5 ± 5.4%) more than that in control (21.9 ± 3.0%, P < 0.05).
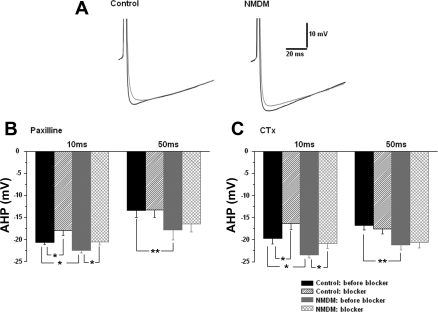
Maternal diabetes did not affect the contribution of BK channels to AHP.
To determine whether maternal diabetes affects the contribution of BK channels to spike AHP, an AP was evoked by a 10-ms depolarizing current of an intensity that was sufficient to generate a single spike. Figure 5A shows the original recordings of the AHP waveforms before (black trace) and after (gray trace) paxilline. As shown in Fig. 5, A and B, the peak amplitude of spike AHP evoked by a 10-ms depolarizing current in NMDM before paxilline application was increased compared with control at 10 ms (control: −20.7 ± 0.5 mV, NMDM: −22.5 ± 0.5 mV, P < 0.05) and at 50 ms (control: 13.4 ± 1.5 mV, NMDM: −17.9 ± 2.2 mV, P < 0.05) (Fig. 5B). Application of paxilline significantly reduced the AHP at 10 ms, but not at 50 ms in control and NMDM (Fig. 5B). Similar to the situation in paxilline application, maternal diabetes significantly increased the peak amplitude of spike AHP in NMDM before CTx application (black traces) at 10 ms (control: −19.8 ± 1.2 mV, NMDM: −23.4 ± 0.7 mV, P < 0.05) and at 50 ms (control: −16.8 ± 1.0 mV, NMDM: −21.2 ± 1.1 mV, P < 0.05; Fig. 5C). Application of CTx significantly reduced the AHP at 10 ms, but not at 50 ms in control and NMDM (Fig. 5C). Since blockade of BK channels similarly reduced fast AHP, maternal diabetes did not change BK-mediated fast AHP.
Fig. 5.
Afterhyperpolarization potential (AHP) was evoked by a current injection with an intensity that was sufficient to generate a single AP. The amplitude of AHP was measured at 10 and 50 ms. A: representative AHP waveforms of control and NMDM before (black trace) and after (gray trace) paxilline application. B: paxilline application. The amplitude of AHP significantly increased in NMDM (n = 9) compared with that in control (n = 10) at 10 and 50 ms. Application of paxilline significantly reduced the amplitude of AHP at 10 ms (fast AHP) but not at 50 ms (medium AHP) in both control and NMDM groups. C: CTx application. The amplitude of AHP significantly increased in NMDM (n = 9) compared with that in control (n = 10) at 10 and 50 ms. Application of CTx significantly reduced the amplitude of AHP at 10 ms but not at 50 ms in both control and NMDM groups. Since blockade of large conductance Ca2+-activated K+ (BK) channels similarly reduced fast AHP, maternal diabetes did not change BK-mediated fast AHP. *P < 0.05; **P < 0.01.
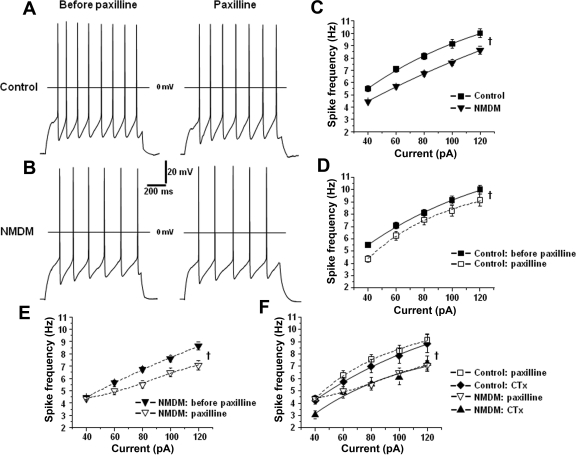
Blockade of BK channels reduced the firing frequency of PCMNs.
To evaluate the effect of maternal diabetes on the excitability of PCMNs, spike trains were evoked by 1-s depolarizing currents (40–120 pA in 20-pA increments) from a holding potential of −65 mV in control (Fig. 6A, left) and NMDM groups (Fig. 6B, left). As shown in Fig. 6C, spike frequency increased as a function of current intensity in both control and NMDM, but maternal diabetes significantly reduced the spike frequency compared with the control group (ANOVA; P < 0.05). To clarify whether BK channels regulate the spike firing frequency, paxilline (Fig. 6A and B, right) was applied to block the BK channels. Blockade of BK channels by paxilline significantly reduced the spike firing frequency in response to current injection in both control and NMDM groups (Fig. 6, D and E; ANOVA: P < 0.05). The effect of CTx on spike trains was also tested in both control and NMDM. Blockade of BK channels by CTx in control and NMDM significantly reduced the spike firing frequency in response to current injection (ANOVA: P < 0.05). Noticeably, the effect of CTx and paxilline in control or in NMDM was similar (Fig. 6F). After CTx and paxilline application, spike firing frequency was significantly lower in NMDM than that in control (Fig. 6F, P < 0.05).
Fig. 6.
A and B: representative traces of AP trains evoked by 1-s depolarizing current (80 pA) injection before and after paxilline application in control (A) and NMDM (B). C: maternal diabetes reduced spike firing frequency. D: application of paxilline significantly reduced spike firing frequency in control. E: application of paxilline significantly reduced spike firing frequency in NMDM. F: blockade of BK channels with CTx (n = 9/group) and paxilline (n = 9/group) equally decreased the firing rate in control and NMDM. †ANOVA, P < 0.05.
DISCUSSION
Previously, we demonstrated that in the NA of neonatal mice, maternal diabetes reduced the excitability and increased their SK currents of PCMNs (27). In addition, blockade of SK channels with apamin substantially increased PCMN firing rate and completely abolished the difference of firing-rate between control and NMDM, indicating that SK channels significantly contribute to the reduction of excitability of PCMNs. Since BK channels modulate AP properties and excitability in other central neurons, we initially hypothesized that maternal diabetes may alter BK channel properties, which might also contribute to maternal diabetes-induced reduction of excitability of PCMNs. In the present study, we found that BK channels do indeed regulate AP repolarization, AHP, and excitability. In addition, maternal diabetes increases the contribution of BK channels to AP repolarization, but not to AHP. Since blockade of BK channels with CTx and paxilline equally reduced excitability in control and NMDM, we conclude that although BK transient outward currents, which may alter AP repolarization are increased in NMDM, the increase of BK channel activity associated with maternal diabetes does not directly contribute to the attenuated excitability of PCMNs.
Fast, medium, and slow AHP of PCMNs.
In many central neurons, AHP can be divided into the fast, medium, and slow components (fAHP, mAHP, and sAHP). The fAHP that follows a single AP has a duration lasting from a few to 10 ms (38, 48). The mAHP and sAHP are much more persistent than fAHP (mAHP: from tens to several hundred ms; sAHP: from several hundred ms to seconds) (12, 39). The mAHP largely determines spike frequency, and spike train adaption is influenced by the sAHP (12). Unlike findings in other reported neurons (34, 36), PCMNs did not have clearly distinguished boundaries between fAHP, mAHP, and sAHP traces as seen in some other central neurons (24, 45). In our study, we measured AHP at 10 and 50 ms. Approximately 10 ms is probably in the fast or early medium duration range of AHP, and 50 ms is in the range of medium duration AHP. In PCMNs, the AP width at the baseline was ∼3 ms during aCSF and 5 ms during BK channel blocker application. The AHP in both conditions reached the peak amplitudes at about 10 ms, and then AHP started to go back to the resting membrane potential in ∼100 ms. Although we could not draw a clear boundary between the fAHP and mAHP, the measurement around the AHP peak is very likely to be in the range of fAHP as shown in many other central neurons (38, 48), whereas the measurement at 50 ms after single AP is in the mAHP range in many neurons (12, 39). We do not believe that PCMNs have a significant sAHP, since AHP almost returned to the resting membrane potentials at ∼100 ms after spikes in control and NMDM.
Maternal diabetes increased the contribution of BK channels to spike repolarization but not to AHP.
Previously, it has been reported in other central neurons that BK channels mainly promote spike repolarization and fAHP without a significant contribution to mAHP (for a review, see Ref. 13). Consistent with these central neurons, we have demonstrated that BK channels contribute significantly to spike repolarization and fAHP because blockade of BK channels induces a significant increase in spike half-width of PCMNs and decreased amplitude of fAHP in both control and NMDM. Furthermore, we found maternal diabetes increased BK transient outward currents, which may facilitate repolarization (16, 31, 53).
Initially, we had postulated that maternal diabetes might have significantly decreased BK transient outward currents, which could explain why maternal diabetes induced an increase of spike half-width. Opposite to our assumption, our data showed that maternal diabetes actually increased the BK transient outward currents. If transient outward currents did indeed contribute to repolarization, then the increased BK currents would have been expected to decrease the AP width more in NMDM than in control. In fact, our data in Fig. 4 show that after blockade of BK channels, the percentage increase of spike half-width in NMDM was larger than that in control. Therefore, the increased BK outward currents in NMDM may have a larger effect on sharpening the AP. However, the increased BK channel outward currents could not explain the overall AP broadening in NMDM.
Since activation of BK channels in some cell types is involved in the fast AHP (23, 37, 47), we then examined the effect of maternal diabetes on BK channel-mediated IAHP and the effect of blockade of BK channels on AHP in control and NMDM. We found that blockade of BK channels with CTx and paxilline reduced IAHP by a significant amount. However, maternal diabetes did not change CTx- and paxilline-sensitive IAHP. Blockade of BK channels revealed that BK channels equally contribute to AHP at 10 ms (fAHP), but did not affect AHP at 50 ms (mAHP) in both control and NMDM. Thus, we suggest that maternal diabetes did not alter BK channel-mediated IAHP.
Ca2+-activated K+ channels mediate a negative-feedback control system that regulates spike firing properties and excitability, using Ca2+ influx as a control signal (46). Usually, one of the purposes of preventing Ca2+ influx is to examine the Ca2+ dependency of Ca2+-activated K+ channels as a subsidiary experiment (9, 22). In the present study, we also used Ca2+-free solution to prevent Ca2+ influx to test whether elimination of Ca2+ influx would affect outward currents and spike half-width. Similar to CTx and paxilline applications, measurement in a Ca2+-free solution increased Ca2+-sensitive transient outward currents and spike half-widths.
Perspectives and Significances
In early studies (e.g., Refs. 2 and 22), activation of the BK channels reduces the spike frequency in response to current injection and limits epileptiform bursts of rat hippocampal CA1 pyramidal neurons. In contrast, it was recently reported that mice lacking BK channels (BK−/−) showed a dramatic reduction in spontaneous activity of the BK−/− cerebellar Purkinje neurons (41). Furthermore, blockade of hippocampus dentate granule neurons' BK channels with paxilline in BK channel β4-subunit knockout mouse reduces firing rate (3). More recently, the BK channels have been reported to facilitate high-frequency discharge and increase the spike frequency in response to strong depolarizing current injection in rat hippocampal CA1 pyramidal neurons (18). Consistent with these recent studies, our present study demonstrated for the first time that blockade of BK channels with CTx and paxilline significantly and equally decreased spike firing frequency of PCMNs in both control and NMDM. This indicates that activation of the BK channels of PCMNs promotes a higher firing rate. In contrast, our previous study found that blockade of SK channels with apamin significantly increased spike firing rate of PCMNs (29) and completely abolished the difference of the firing rate between control and NMDM (27). Collectively, these data suggest that increased BK currents do not significantly contribute to the decrease in excitability of PCMNs associated with maternal diabetes. In contrast, SK channels have a dominant role in causing the reduced excitability of PCMNs in the offspring of diabetic mothers.
Streptozotocin-induced diabetic mouse (30) and the offspring of streptozotocin-induced diabetic mouse mothers (51) have impaired baroreflex control of heart rate. Since PCMNs in the NA play a dominant role in baroreflex control of the heart rate, attenuated PCMN excitability may contribute to baroreflex impairment associated with maternal diabetes. Interestingly, maternal diabetes increases both BK and SK channels' currents, which oppositely affect the PCNMs' excitability; whereas augmented SK currents decrease excitability, the augmented BK currents contribute to the sharpness of spikes and hence attenuate the reduction of PCMN excitability due to augmented SK currents. Therefore, it is likely that interventions targeted at reducing SK channel and/or facilitating BK channel activity may have therapeutic benefits for disease-impaired function of PCMNs.
GRANTS
This study was supported by National Institutes of Health grants HL-79636 and AG-21020 (to Z. J. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature 296: 746–749, 1982 [DOI] [PubMed] [Google Scholar]
- 2. Alger BE, Williamson AA. Transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol 399: 191–205, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759 2005 [DOI] [PubMed] [Google Scholar]
- 4. Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci 12: 59–65, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Cheng Z, Powley TL. Nucleus ambiguus projections to cardiac ganglia of rat atria: an anterograde tracing study. J Comp Neurol 424: 588–606, 2000 [PubMed] [Google Scholar]
- 6. Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over vagal efferent postganglionic neurons in rat intrinsic cardiac ganglia by neurons in the nucleus ambiguus and the dorsal motor nucleus of the vagus: anatomical evidence. Am J Physiol Regul Integr Comp Physiol 286: R625–R633, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol 495: 353–366, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dheen ST, Tay SS, Boran J, Ting LW, Kumar SD, Fu J, Ling EA. Recent studies on neural tube defects in embryos of diabetic pregnancy: an overview. Curr Med Chem 16: 2345–2354, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol 548: 53–69, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early onset diabetes in transgenic mice. Cell 58: 1067–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Epstein PN, Ribar TJ, Decker GL, Yaney G, Means AR. Elevated β-cell calmodulin produces a unique insulin secretory defect in transgenic mice. Endocrinology 130: 1387–1393, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Faber ESL, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9: 181–194, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Fiorentini A, Perciaccante A, Paris A, Serra P, Tubani L. Circadian rhythm of autonomic activity in non diabetic offspring of type 2 diabetic patients. Cardiovasc Diabetol 4: 15–22, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao Q, Gao YM. Hyperglycemic condition disturbs the proliferation and cell death of neural progenitors in mouse embryonic spinal cord. Int J Dev Neurosci 25: 349–357, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvβ1.1-deficient mice with impaired learning. Learn Mem 5: 257–273, 1998 [PMC free article] [PubMed] [Google Scholar]
- 17. Gu H, Epstein PN, Li L, Wurster RD, Cheng ZJ. Functional changes in baroreceptor afferent, central and efferent components of the baroreflex circuitry in type 1 diabetic mice (OVE26). Neuroscience 152: 741–752, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580: 859–882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001 [Google Scholar]
- 20. Kaczorowski GJ, Garcia ML. Pharmacology of voltage-gated and calcium-activated potassium channels. Curr Opin Chem Biol 3: 448–458, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci 16: 955–963, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389: 187–204, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lancaster B, Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol 387: 519–548, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li L, Huang C, Ai J, Yan B, Gu H, Ma Z, Li AY, Xinyan S, Harden SW, Hatcher JT, Wurster RD, Cheng ZJ. Structural remodeling of vagal afferent innervation of aortic arch and nucleus ambiguus (NA) projections to cardiac ganglia in a transgenic mouse model of type 1 diabetes (OVE26). J Comp Neurol 518: 2771–2793, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes 51: 174–181, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Lin M, Chen QH, Wurster RD, Hatcher JT, Liu YQ, Li LH, Harden SW, Cheng ZJ. Maternal diabetes increases small conductance Ca2+-activated K+ (SK) currents which alter action potential properties and excitability of cardiac motoneurons in the nucleus ambiguus. J Neurophysiol 104: 2125–2138 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin M, Harden SW, Li L, Wurster RD, Cheng ZJ. Impairment of baroreflex control of heart rate in conscious transgenic mice of type 1 diabetes (OVE26). Auton Neurosci 152: 67–74 2010 [DOI] [PubMed] [Google Scholar]
- 29. Lin M, Hatcher JT, Chen QH, Wurster RD, Cheng ZJ. Small conductance Ca2+-activated K+ channels regulate firing properties and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus. Am J Physiol Cell Physiol 299: C1285–C1298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin M, Ai J, Harden SW, Huang C, Li L, Epstein PN, Wurster RD, Cheng ZJ. Impairment of baroreflex control of heart rate and structural changes of cardiac ganglia in conscious streptozotocin (STZ)-induced diabetic mice. Auton Neurosci 155: 39–48, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci 16: 4089–4101, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in the nucleus ambiguus. Am J Physiol Heart Circ Physiol 271: H2609–H2614, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Nicholls JG, Martin AR, Wallace BG. From Neuron to Brain (3d ed.). Sunderland, MA: Sinauer Associates, 1992. chapt. 4–5 [Google Scholar]
- 34. Nishimura Y, Asahi M, Saitoh K, Kitagawa H, Kumazawa Y, Itoh K, Lin M, Akamine T, Shibuya H, Asahara T, Yamamoto T. Ionic mechanisms underlying burst firing of layer III sensorimotor cortical neurons of the cat: an in vitro slice study. J Neurophysiol 86: 771–781, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Ramos-Arroyo MA, Rodriguez-Pinilla E, Cordero JF. Maternal diabetes: the risk for specific birth defects. Eur J Epidemiol 8: 503–508, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Saar D, Grossman Y, Barkai E. Long lasting cholinergic modulation underlies rule learning in rats. J Neuorsci 21: 1385–1392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol 68: 1834–1841, 1992 [DOI] [PubMed] [Google Scholar]
- 38. Sah P. Ca2+-activated K+ currents in neurons: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Sanchez M, McManus OB. Paxilline inhibition of the α-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA 101: 9474–9478, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol 59: 424–449, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol 77: 77–93, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385: 733–759, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 171–190, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strøbaek D, Christophersen P, Holm NR, Moldt P, Ahring PK, Johansen TE, Olesen SP. Modulation of the Ca2+-dependent K+ channel, hslo, by the substituted diphenylurea NS 1608, paxilline and internal Ca2+. Neuropharmacology 35: 903–914, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Sugg EE, Garcia ML, Reuben JP, Patchett AA, Kaczorowski GJ. Synthesis and structural characterization of charybdotoxin, a potent peptidyl inhibitor of the high conductance Ca2+-activated K+ channel. J Biol Chem 265: 18745–18748, 1990 [PubMed] [Google Scholar]
- 51. Wichi RB, Souza SB, Casarini DE, Morris M, Barreto-Chaves ML, Irigoyen MC. Increased blood pressure in the offspring of diabetic mothers. Am J Physiol Regul Integr Comp Physiol 288: R1129–R1133, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Williamson A, Alger BE. Characterization of an early afterhyperpolarization after a brief train of action potentials in rat hippocampal neurons in vitro. J Neurophysiol 63: 72–81 1990 [DOI] [PubMed] [Google Scholar]
- 53. Wilson JM, Coderre E, Renaud LP, Spanswick D. Active and passive membrane properties of rat sympathetic preganglionic neurones innervating the adrenal medulla. J Physiol 545: 945–960, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yan B, Li L, Harden SW, Epstein PN, Wurster RD, Cheng Z. Diabetes induces neural degeneration in nucleus ambiguus (NA) and attenuates heart rate control in OVE26 mice. Exp Neurol 220: 34–43, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Young JB, Morrison SF. Effects of fetal and neonatal environment on sympathetic nervous system development. Diabetes Care 21, Suppl 2: B156–B160, 1998 [PubMed] [Google Scholar]
- 56. Zheng S, Noonan WT, Metreveli NS, Coventry S, Kralik PM, Carlson EC, Epstein PN. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes 53: 3248–3257, 2004 [DOI] [PubMed] [Google Scholar]