Abstract
Monocyte chemoattractant protein 1 (MCP-1) is a CC cytokine that fundamentally contributes to the pathogenesis of inflammatory renal disease. MCP-1 is highly expressed in cytokine-stimulated mesangial cells in vitro and following glomerular injury in vivo. Interventions to limit MCP-1 expression are commonly effective in assorted experimental models. Fish oil, an abundant source of n-3 fatty acids, has anti-inflammatory properties, the basis of which remains incompletely defined. We examined potential mechanisms whereby fish oil reduces MCP-1 expression and thereby suppresses inflammatory responses to tissue injury. Cultured mesangial cells were treated with TNF-α in the presence of the n-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA); equimolar concentrations of the n-6 fatty acids LA and OA served as controls. MCP-1 mRNA expression was assessed by Northern blotting, and transcriptional activity of the MCP-1 promoter was assessed by transient transfection. The involvement of the ERK and NF-κB pathways was evaluated through transfection analysis and the use of the MEK inhibitor U0126. DHA and EPA decreased TNF-α-stimulated MCP-1 mRNA expression by decreasing transcription of the MCP-1 gene. DHA and EPA decreased p-ERK expression and nuclear translocation of NF-κB, both of which are necessary for TNF-α-stimulated MCP-1 expression. Both NF-κB and AP-1 sites were involved in transcriptional regulation of the MCP-1 gene by DHA and EPA. We conclude that DHA and EPA inhibit TNF-α-stimulated transcription of the MCP-1 gene through interaction of signaling pathways involving ERK and NF-κB. We speculate that such effects may contribute to the salutary effect of fish oil in renal and vascular disease.
Keywords: fish oil, inflammation, transcriptional regulation
glomerular mesangial cells (MCs) play a critical role in the initiation and progression of renal injury. MCs respond to a variety of proinflammatory mediators by proliferating, synthesizing extracellular matrix, and producing a number of cytokines that propagate the inflammatory response. Among the proinflammatory cytokines, monocyte chemoattractant protein 1 (MCP-1) has emerged as a predominant mediator of inflammation and fibrosis in chronic renal disease (60). MCP-1 is a member of the CC chemokine family and serves as a chemotactic factor for the recruitment of monocytes and subpopulations of T cells during inflammation (56, 63, 66). Human and experimental studies showed that MCP-1 expression correlates with glomerular and interstitial infiltration of macrophages and T cells during the development and progression of renal disease (7, 14, 19, 40, 70, 75). In vitro, MCs produce MCP-1 in response to a number of growth factors and cytokines, including lipopolysaccharide (LPS), IL-1, TNF-α, and TGF-β1 (9, 23, 61, 64). In a mesangial proliferative glomerulonephritis model, MCP-1 is rapidly induced after acute injury and promotes the influx of macrophages (67). Although this has not been previously established in MCs, TNF-α stimulates transcription of the MCP-1 gene through activation of a number of intracellular signaling pathways, including NF-κB, ERK, and AP-1 (43). Based on these considerations, therapies that target MC production of MCP-1 may be effective in preventing the progression of inflammatory renal disease.
Fish oil, which is rich in the n-3 polyunsaturated fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), has been used to treat mesangial proliferative and sclerosing glomerulonephritis associated with IgA nephropathy (17, 18). In addition to IgA nephropathy, n-3 fatty acids have been used to treat a variety of inflammatory and autoimmune conditions (8, 36, 51). Potential mechanisms underlying this protective effect have not been well-established (22).
One potential mechanism may relate to the antiproliferative effects of fish oil. In cultured MCs, we previously demonstrated that n-3 fatty acids block growth factor-stimulated mitogenesis through inhibition of ERK activation, reduction of cyclin E kinase activity, and induction of the cell cycle inhibitor p21 (78). In the antithymocyte serum model of mesangioproliferative glomerulonephritis, we demonstrated that n-3 fatty acids inhibit MC activation and proliferation, reduce proteinuria, and decrease histologic evidence of acute glomerular damage (22).
In addition to the antiproliferative effects of n-3 fatty acids, there is considerable experimental and clinical evidence indicating that n-3 fatty acids suppress inflammatory response to tissue injury. n-3 Fatty acids inhibit TNF-α production by peripheral blood mononuclear cells obtained from healthy volunteers (20, 25). In vivo, n-3 fatty acids inhibit the production of proinflammatory cytokines in response to injury (1, 33, 52). TNF-α and other cytokines stimulate transcription of a number of proinflammatory genes, at least in part through activation of the NF-κB signaling pathway (35, 42, 52). We previously demonstrated that fish oil reduces NF-κB activation, inflammation, and renal damage in a salt-sensitive model of hypertension, an effect that is only in part due to reduction in blood pressure (16). A number of in vitro studies implicated activation of ERK, NF-κB, and/or AP-1 in the transcriptional regulation of MCP-1 in response to a variety of proinflammatory cytokines (38, 43, 46, 47, 52, 73, 79).
Several correlative in vivo studies suggest that the anti-inflammatory effects of fish oil may at least in part relate to decreased MCP-1 expression. For example, in a 5/6 nephrectomy model, fish oil reduced the injury-induced activation of ERK and NF-κB and decreased MCP-1 expression (2). In the KKA/y/Ta murine model of type 2 diabetes, the fish oil constituent EPA suppressed ERK1/2 phosphorylation and MCP-1 expression (26). However, the precise mechanism through which fish oil suppresses MCP-1 production in MCs has not been defined.
Based on these considerations, we sought to test the hypothesis that fish oil decreases MCP-1 production through cross talk with the ERK, NF-κB, and AP-1 signaling pathways. We demonstrate that, in MCs, ERK and NF-κB activation are both required for TNF-α-mediated transcriptional regulation of the MCP-1 gene. Although AP-1 is not involved in TNF-α-stimulated MCP-1 transcription, DHA and EPA appear to target AP-1 in regulation of basal MCP-1 expression. We propose that the inhibitory effects of fish oil on TNF-α-stimulated MCP-1 transcription involve suppression of ERK and NF-κB.
MATERIALS AND METHODS
Reagents.
Waymouth's MB752/1 medium, DMEM, heat-inactivated FBS, HEPES, sodium pyruvate, nonessential amino acids, l-glutamine, premixed insulin, transferrin, selenium + bovine serum albumin (ITS+), and other cell culture reagents were purchased from Invitrogen (Carlsbad, CA). DHA, EPA, oleic acid (OA), and linoleic acid (LA) were obtained from Cayman Chemical (Ann Arbor, MI). Essentially, fatty acid-free BSA was purchased from Sigma (St. Louis, MO). Human recombinant TNF-α was purchased from Calbiochem (La Jolla, CA). U0126 was obtained from Promega (Madison, WI). Rabbit anti-p65/NF-κB antibody was obtained from Active Motif (Carlsbad, CA). FITC-conjugated swine anti-rabbit antibody was purchased from DAKO (Carpinteria, CA). Other reagents, all of highest purity grades, were purchased from standard suppliers.
MC isolation and culture.
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the study protocol was approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee. Primary MCs were obtained from 100- to 150-g male Sprague-Dawley rats by differential sieving, as described previously (12, 23, 45). Briefly, rats were euthanized by intraperitoneal injection of pentobarbital sodium (100 mg/kg), the kidneys were excised, renal capsules were removed, and cortical tissue was minced and passed through a stainless steel sieve (200-μm-pore size). The homogenate was sequentially sieved through nylon meshes of 390-, 250-, and 211-μm-pore openings. The cortical suspension was then passed over a 60-μm sieve to collect glomeruli. Purity of glomerular preparations was evaluated by light microscopy. Preparations typically contained >90% glomeruli. Glomeruli were seeded on plastic tissue culture dishes and grown in complete Waymouth's medium (Waymouth's medium supplemented with 20% heat-inactivated FBS, 15 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM l-glutamine, 1% ITS+, 100 IU/ml penicillin, and 100 μg/ml streptomycin). Fresh medium was added every 3 days. Cell outgrowths were characterized as MCs by positive immunostaining for vimentin and smooth muscle-specific actin, and negative stains for cytokeratin, factor VIII-related antigen, and leukocyte-common antigen (antibodies from DAKO). MCs were passed once a week following treatment with trypsin- EDTA (0.25%). Cells used in experiments were from passages 5–15.
Parallel studies were conducted in a pSV3-Neo immortalized rat MC line (CRL-2573; American Type Culture Collection, Manassas, VA) to confirm a response to TNF-α similar to that of primary cultures of MCs. These transformed cells have been shown to exhibit characteristics similar to those of primary rat MC cultures (9, 11). CRL-2573 cells were cultured in DMEM containing 4,500 mg/l glucose and 15% FBS.
Preparation of fatty acid-albumin complexes.
Conjugation of fatty acids to albumin was performed as previously described, with some modification (78). Briefly, stocks of fatty acids (200 μM DHA, EPA, OA, or LA in ethanol) were added dropwise to 0.29 mM essentially fatty acid-free BSA in DMEM and stirred at 4°C for 8 h under nitrogen to avoid oxidation. A negative control solution containing equimolar BSA was prepared in the same manner using ethanol alone. The conjugated solutions containing 2 mM fatty acid were aliquoted, stored under nitrogen at −20°C, and thawed immediately before use.
Northern blot analysis.
MCs were plated in 10-cm dishes (2 × 106 cells/dish) in complete medium and grown to 75% confluence. For fatty acid experiments, cells were rendered quiescent in serum-depleted (0.5% FBS) medium containing 20 μM n-3 fatty acids (DHA or EPA), 20 μM n-6 fatty acids (OA or LA), or equimolar BSA for 24 h before stimulation with TNF-α (10 ng/ml) for 6 h. For MEK inhibitor studies, cells were withdrawn in serum-depleted medium alone and then pretreated with vehicle (0.1% DMSO) or the MEK inhibitor U0126 (25 μM) for 1 h followed by TNF-α (10 ng/ml) for 6 h. Total cellular RNA was isolated using the RNeasy Total RNA Isolation Kit (Qiagen, Valencia, CA), according to the manufacturer's protocol. RNA (10–20 μg/lane) was electrophoresed through a 1% agarose, 2.2 M formaldehyde-denaturing gel and transferred to nylon membranes (Schleicher & Schuell, Keene, NH) as previously described (21, 24). Northern blot analysis was performed with a rat MCP-1 cDNA probe obtained as previously described (29) using RT-PCR. The probe was labeled with [α-32P] dCTP by the random primer method, and blots were hybridized at 65°C overnight in a 0.5 M sodium phosphate buffer, pH 7.0, containing 1 mM EDTA, 7% SDS, and 1% BSA, as described by Church and Gilbert (13). Autoradiograms were analyzed by computer-assisted video densitometry. To control for gel loading and transfer efficiency, data were normalized to 18S rRNA using negative images of ethidium bromide-stained membranes.
Plasmid constructs.
For MCP-1 promoter activity studies, the MCP-1 promoter and flanking region (bp 1220 to 3635, GenBank accession no. AF079313) was amplified from rat genomic DNA by PCR using the forward primer 5′-TGTGAGAGCTGCTTGGCTGTAAC-3′ and the reverse primer 5′-TCTGGCTTCAGTGAGAGTTGGC-3′. A truncated MCP-1 construct with deletion of the two NF-κB binding sites in the distal enhancer region (AF079313 bp 1293 to 1302 and 1319 to 1328) was produced by amplification of bp 1335 to 3635 using the forward primer 5′-TTATCCTACTCTGCCTCTG-3′ and the reverse primer above. PCR products were sequenced and subcloned into the firefly luciferase reporter pGL3-Basic (Promega). The AP-1 site in the proximal promoter region of the constructs was deleted by sited-directed mutagenesis with the QuickChange II Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) using the forward primer 5′-CCTTGGCACCAACCACCCTGCCTCTGGCTTACAATAAAAGGC-3′ (bp 3506 to 3558, less the AP-1 site at bp 3526 to 3536) and its reverse and compliment.
Transfection studies.
MCs were seeded in 24-well culture plates at 8 × 104 cells/well in complete growth medium and incubated for 24 h. Transfections were performed using FuGENE6 Transfection Reagent (Roche), according to the manufacturer's instructions. NF-κB transcription studies were carried out with slight modification as described by Trushin et al. (74) using a NF-κB/pGL2 firefly luciferase reporter. ERK activation was measured using the PathDetect Elk1 Signal Transduction trans-Reporting System (Stratagene), which consists of the firefly luciferase reporter vector pFR-Luc and the transactivator plasmid pFA2-Elk1. The Renilla luciferase reporter vector phRG-Basic (Promega) was used to control for transfection efficiency in all experiments. Transfected cells were withdrawn for 24 h in serum-depleted medium alone or containing 20 μM fatty acids and then treated with TNF-α for 6 h. For MEK inhibitor studies, quiescent cells were pretreated with U0126 1 h before TNF-α. Cells were lysed and assayed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega).
NF-κB nuclear translocation.
MCs were plated on LabTek-II chamber slides (Nalge Nunc International, Naperville, IL) in complete growth medium. Cells were withdrawn for 24 h in the presence or absence of fatty acids, as described above, and then treated with TNF-α for 1 h. For MEK inhibitor experiments, withdrawn cells were pretreated with U0126 30 min before TNF-α. Immunofluorescence staining was performed as follows: cells were fixed in PBS with 4% paraformaldehyde for 10 min at 37°C, quenched in PBS containing 50 mM glycine for 5 min at room temperature, and permeabilized with methanol for 2 min at room temperature. They were then blocked in PBS containing 5% goat serum and 5% glycerol for 30 min, followed by incubation with rabbit anti-p65/NF-κB primary antibody (1:100 dilution in PBS containing 0.09% sodium azide, 1% BSA, and 0.1% Tween 20) for 1 h at room temperature, and FITC-conjugated swine anti-rabbit IgG (1:1,000) containing 10 μg/ml DAPI (4′,6-diamidino-2-phenylindole; Sigma) for 1 h at room temperature. Finally, the slides were coverslipped and analyzed in a Zeiss LSM 510 Confocal Laser-Scanning Microscope System using ×400 magnification.
Statistical analysis.
Data presented are representative of at least three independent experiments performed in duplicate or triplicate, as indicated in figure legends. Pair-wise comparisons were evaluated by the Mann-Whitney U-test. Differences were considered to be significant when P < 0.05.
RESULTS
n-3 Fatty acids suppress TNF-α-induced MCP-1 expression in MCs.
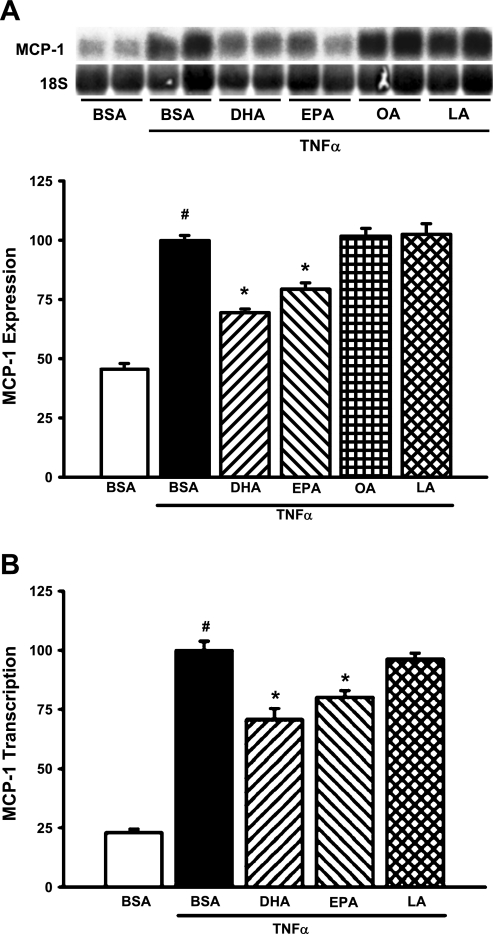
TNF-α is a well-recognized mediator of MCP-1 production in a variety of cell types, including MCs. To test the ability of n-3 fatty acids to inhibit TNF-α-mediated induction of MCP-1, MCs were treated with n-3 fatty acids (DHA and EPA, 20 μM) or n-6 fatty acids (OA and LA, 20 μM) for 24 h before TNF-α exposure. TNF-α, as expected, increased MCP-1 mRNA levels by 118% (Fig. 1A). Pretreatment with DHA or EPA significantly decreased TNF-α-stimulated MCP-1 expression (30 and 20%, respectively; P < 0.05); OA and LA were without effect (Fig. 1A). Previous studies demonstrated that the primary mechanism for increased MCP-1 production in response to cytokines is through increased transcription of the MCP-1 gene. Transient transfection of MCs with a chimeric MCP-1 promoter/luciferase reporter construct revealed that TNF-α-mediated MCP-1 transcription was significantly decreased following pretreatment with DHA or EPA (27 and 20%, respectively; P < 0.005; Fig. 1B).
Fig. 1.
n-3 Fatty acids [docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)], but not n-6 fatty acids [oleic acid (OA) and linoleic acid (LA)], decrease TNF-α-stimulated monocyte chemoattractant protein 1 (MCP-1) expression. A: n-3 fatty acids block TNF-α-stimulated MCP-1 mRNA levels. Glomerular mesangial cells (MCs) were treated with 20 μM BSA-conjugated fatty acids (n-3 = DHA and EPA; n-6 = OA and LA) or equimolar BSA alone for 24 h, followed by stimulation with TNF-α (10 ng/ml) for 6 h. Total RNA was isolated and Northern blots were hybridized with a cDNA probe specific for rat MCP-1. Data were normalized to 18S rRNA to control for gel loading and transfer efficiency. Values are expressed as means ± SE relative to TNF-α-treated cells; n = 3 experiments. #P < 0.05 vs. BSA control. *P < 0.05 vs. TNF-α-treated cells. Inset: representative Northern blot. B: n-3 fatty acids block TNF-α-stimulated MCP-1 transcription. To assess the effect of n-3 vs. n-6 fatty acids on TNF-α-stimulated MCP-1 transcription, MCs were cotransfected with a chimeric MCP-1 promoter-luciferase construct (MCP-1/pGL3, 350 ng) and a Renilla luciferase control plasmid (phRG-Basic, 5 ng). Cells were treated as described above, and luciferase activity was assessed using the Dual-Luciferase Reporter Assay System (Promega). Values are expressed as means ± SE relative to TNF-α-treated cells; n = 3 experiments. #P < 0.05 vs. BSA control. *P < 0.05 vs. TNF-α-treated cells.
n-3 Fatty acids block TNF-α-induced ERK activation.
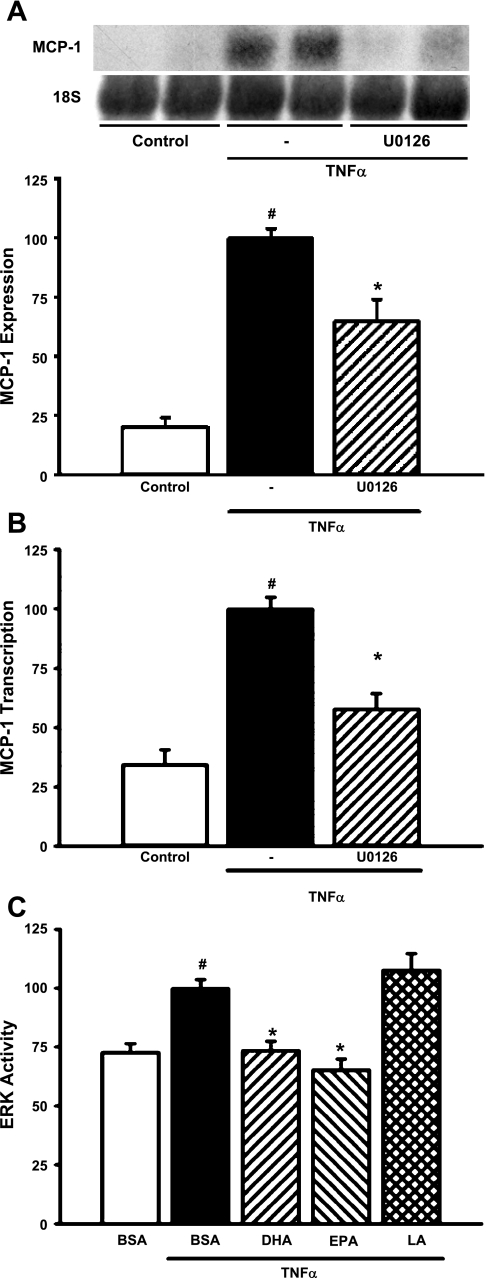
We previously showed that n-3 fatty acids inhibit basal ERK activity in MCs (78). We therefore sought to test the hypothesis that the inhibitory effects of n-3 fatty acids on TNF-α-stimulated MCP-1 induction occur at least in part through suppression of ERK activation. Inhibition of the ERK pathway with U0126 (25 μM) decreased TNF-α-stimulated MCP-1 mRNA expression by 35% (P = 0.0006; Fig. 2A). TNF-α-mediated transcription of the MCP-1 gene was blocked by U0126 (42% reduction in promoter activity; P = 0.0022; Fig. 2B), indicating that the ERK pathway is involved in transcriptional regulation of the MCP-1 gene by TNF-α. The effects of TNF-α on ERK activity were directly assessed by transfection using an Elk-1 Trans-reporting system. As expected, TNF-α activated the ERK pathway (37%; P = 0.0002; Fig. 2C). TNF-α-mediated activation of the ERK pathway was significantly inhibited by pretreatment of MCs with DHA or EPA (26 and 35%, respectively; P < 0.0005); LA did not significantly alter TNF-α-mediated ERK activation (Fig. 2C).
Fig. 2.
n-3 Fatty acids inhibit TNF-α-stimulated MCP-1 expression at least in part through suppression of ERK activity. A: U0126 blocks TNF-α-stimulated MCP-1 mRNA expression. MCs were pretreated with U0126 (25 μM) for 1 h before exposure to TNF-α (10 ng/ml) for 6 h. Northern blots of total RNA were hybridized with a rat MCP-1 probe, and data were normalized to 18S rRNA. Values are expressed as means ± SE relative to TNF-α-treated cells; n = 4 experiments. #P < 0.05 vs. control. *P < 0.05 vs. TNF-α-treated cells. Inset: representative Northern blot. B: U0126 blocks TNF-α-stimulated transcription of the MCP-1 gene. MCs were cotransfected with MCP-1/pGL3 and a Renilla luciferase control plasmid (phRG-Basic) for 8 h and then pretreated with U0126 (25 μM) for 1 h and exposed to TNF-α (10 ng/ml) for 6 h. Luciferase activity was assessed using the Dual Luciferase Reporter Assay System (Promega). Values are expressed as means ± SE relative to TNF-α-treated cells; n = 3 experiments. #P < 0.05 vs. control. *P < 0.05 vs. TNF-α-treated cells. C: n-3 fatty acids block TNF-α induction of ERK activity. ERK activity in MCs was assessed by a transfection-based in vivo kinase assay (Stratagene) as described in materials and methods. Transfected MCs were treated for 24 h with 20 μM DHA, EPA, or LA and exposed to TNF-α (10 ng/ml) for 6 h. Luciferase activity was assessed using the Dual Luciferase Reporter Assay System (Promega). Values are expressed as means ± SE relative to TNF-α-treated cells; n = 4 experiments. #P < 0.05 vs. BSA control. *P < 0.05 vs. TNF-α-treated cells.
Inhibitory effects of n-3 fatty acids on TNF-α-induced MCP-1 expression involve cross talk between the ERK and NF-κB pathways.
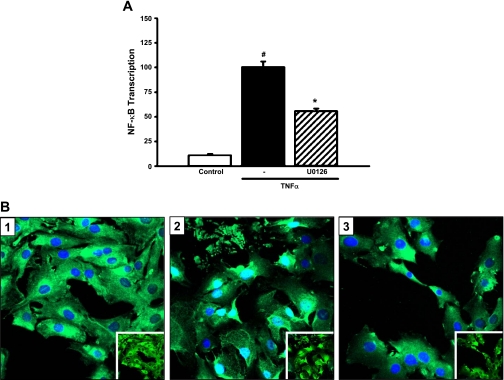
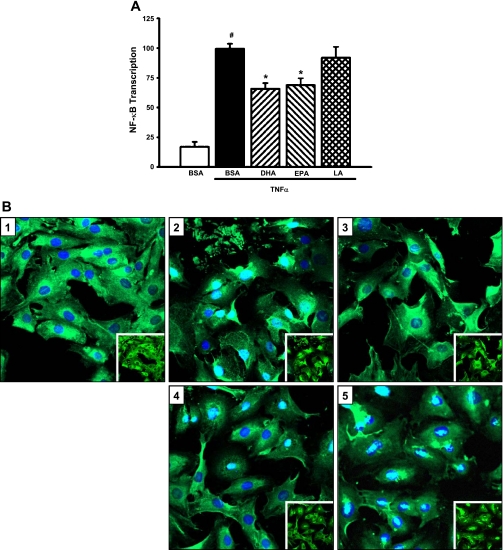
In other cell systems, studies showed that NF-κB is a critical mediator of cytokine-stimulated MCP-1 expression. To determine whether the inhibitory effect of DHA and EPA on MCP-1 expression involves cross talk between the ERK and NF-κB signaling pathways, MCs were transfected with a chimeric NF-κB promoter/luciferase reporter construct and treated with U0126 followed by TNF-α. As expected, TNF-α markedly induced NF-κB activity (by 809%; P = 0.0001; Fig. 3A). Treatment with U0126 significantly decreased TNF-α-stimulated NF-κB transcription (by 44%; P < 0.0001; Fig. 3A). U0126 blocked TNF-α-induced nuclear translocation of the p65 subunit of NF-κB (Fig. 3B). DHA and EPA significantly suppressed TNF-α-mediated activation of NF-κB (34 and 30%, respectively; P < 0.0001; Fig. 4A). TNF-α-stimulated nuclear translocation of p65 was inhibited by DHA and EPA, but not LA (Fig. 4B). These studies demonstrate that TNF-α-stimulated MCP-1 expression is mediated through cross talk between the ERK and NF-κB pathways and that n-3 fatty acids target these pathways in blocking TNF-α-mediated induction of MCP-1.
Fig. 3.
TNF-α stimulation of MCP-1 expression involves cross talk between the ERK and NF-κB pathways. A: U0126 downregulates TNF-α-stimulated NF-κB transcription. MCs were cotransfected with a chimeric NF-κB promoter-luciferase construct (NF-κB/pGL2) and a Renilla luciferase control plasmid (phRG-Basic) for 8 h and treated with U0126 (25 μM) for 1 h followed by TNF-α (10 ng/ml) for 6 h. Luciferase activity was assessed using the Dual Luciferase Reporter Assay System (Promega). Values are expressed as means ± SE relative to TNF-α-treated cells; n = 3 experiments. #P < 0.05 vs. control. *P < 0.05 vs. TNF-α-treated cells. B: U0126 decreases TNF-α-stimulated nuclear translocation of NF-κB. MCs were pretreated with U0126 for 30 min and then stimulated with TNF-α for 1 h. Cells were fixed and immunostained for the p65 subunit of NF-κB, and nuclei were stained with DAPI. Slides were analyzed by confocal microscopy. 1: Vehicle control. 2: Vehicle + TNF-α. 3: U0126 + TNF-α. Insets: same images without DAPI stain overlay.
Fig. 4.
n-3 Fatty acids block TNF-α-induced NF-κB activity. A: n-3 fatty acids downregulate TNF-α-stimulated NF-κB transcription. MCs were cotransfected with NF-κB/pGL2 and a Renilla luciferase control plasmid (phRG-Basic) for 8 h, pretreated with fatty acids (20 μM) for 24 h, and stimulated with TNF-α (10 ng/ml) for 6 h. Luciferase activity was assessed using the Dual Luciferase Reporter Assay System (Promega). Values are expressed as means ± SE relative to TNF-α-treated cells; n = 4 experiments. #P < 0.05 vs. BSA control. *P < 0.05 vs. TNF-α-treated cells. B: n-3 fatty acids decrease TNF-α-stimulated nuclear translocation of NF-κB. MCs were pretreated with fatty acids (20 μM) for 24 h and stimulated with TNF-α for 1 h. Cells were fixed and immunostained for the p65 subunit of NF-κB, and nuclei were stained with DAPI. Slides were analyzed by confocal microscopy. 1: BSA alone. 2: BSA + TNF-α. 3: DHA + TNF-α. 4: EPA + TNF-α. 5: LA + TNF-α. Insets: same images without DAPI stain overlay.
Inhibitory effects of n-3 fatty acids on TNF-α-stimulated transcription of MCP-1 involve NF-κB and AP-1 binding sites.
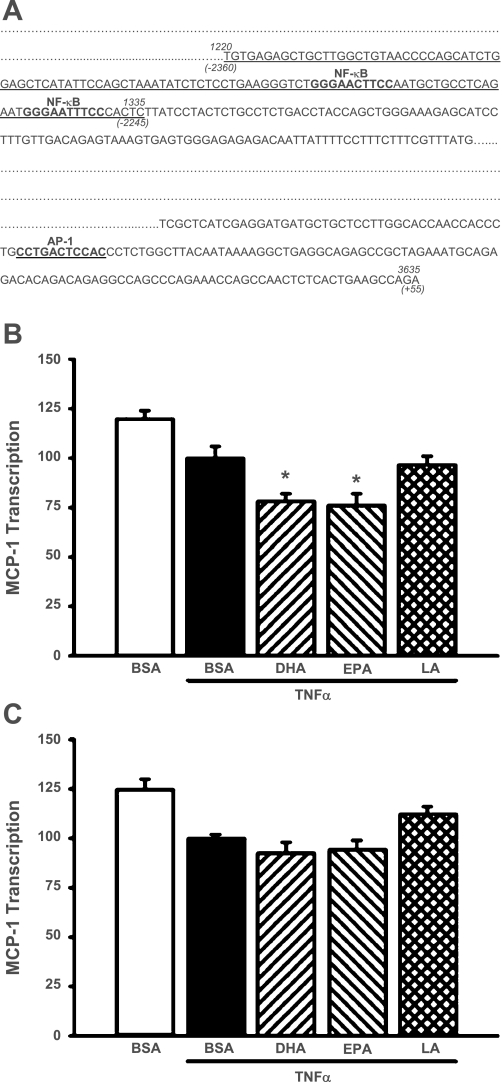
MCs were transfected with an NF-κB deletion mutant of the MCP-1 promoter/luciferase reporter construct (Fig. 5A) and were treated with DHA, EPA, or LA followed by TNF-α. The construct was unresponsive to TNF-α as expected, but DHA and EPA significantly decreased basal reporter activity of the MCP-1 construct (22 and 24%, respectively; P < 0.05); LA was without significant effect (Fig. 5B). These studies suggest that the inhibitory effects of DHA and EPA may involve additional regulatory sequences within the MCP-1 promoter. The proximal promoter region contains an AP-1 site that has been shown to be important in transcriptional regulation of the MCP-1 gene in some cell types. Deletion of this AP-1 site decreased basal transcriptional activity of the MCP-1 construct by 52% (P = 0.0019). This construct responded to TNF-α treatment with a 180% increase in transcriptional activity (P < 0.05). Transcriptional activity of an MCP-1 construct containing deletions of both the κB1 and κB2 sites and the AP-1 site showed no stimulatory response to TNF-α and no inhibitory response to DHA and EPA (Fig. 5C).
Fig. 5.
A: map of the promoter region of the rat MCP-1 gene, including the consensus NF-κB and AP-1 binding sites. The 2 NF-κB sites located in the distal enhancer and the AP-1 site located in the proximal promoter are shown in bold. The sequences deleted to prepare the NF-κB and NF-κB/AP-1 deletion mutants are underlined. B: deletion of the NF-κB site within the MCP-1 promoter region abolishes TNF-α-stimulated MCP-1 transcription. MCs were cotransfected with an NF-κB deletion mutant of MCP-1/pGL3 and a Renilla luciferase control plasmid (phRG-Basic) for 8 h, pretreated for 24 h with 20 μM fatty acids, and then exposed to TNF-α (10 ng/ml) for 6 h. *P < 0.05 vs. TNF-α-treated cells. C: deletion of both the NF-κB and AP-1 sites prevents n-3 fatty acid-mediated suppression of MCP-1 transcription. MCs were cotransfected with an AP-1/NF-κB deletion mutant of MCP-1/pGL3 and a Renilla luciferase control plasmid (phRG-Basic) for 8 h, pretreated with DHA, EPA, or LA (20 μM) for 24 h, and exposed to TNF-α (10 ng/ml) for 6 h. Luciferase activity was assessed using the Dual Luciferase Reporter Assay System (Promega). Values are expressed as means ± SE relative to TNF-α-treated cells; n = 3 experiments.
DISCUSSION
Infiltration of monocytes/macrophages into the glomerulus plays a major role in glomerular injury in many forms of glomerulonephritis. Of the inflammatory mediators, MCP-1 has emerged as a critical mediator of glomerular and interstitial inflammation during progressive renal disease by virtue of its potent chemotactic effects and by its induction in response to a variety of growth factors and cytokines, all relevant to progressive renal injury (9, 23, 61, 64). Blockade of MCP-1 attenuates acute and chronic injury in a number of renal disease models. However, potential mechanisms whereby n-3 fatty acids decrease cytokine-induced MCP-1 expression have not been previously established in MCs.
We demonstrate that TNF-α induction of MCP-1 requires activation of ERK1/2 in cultured rat MCs. This finding is in accord with observations in other cell systems. In vascular smooth muscle cells, TNF-α induces ERK phosphorylation (39), particularly in cells obtained from older rats. In renal tubular epithelial cells, the MEK inhibitor U0126 prevents induction of MCP-1 in response to a variety of stimuli, including TNF-α, albumin, and osmotic stress (27, 34, 71). In MCs, a dominant-negative ERK construct inhibits PDGF-stimulated MCP-1 production (31). We previously demonstrated that n-3 fatty acids decrease EGF-stimulated ERK activation in cultured MCs (78). In the current study, DHA and EPA potently inhibited TNF-α-stimulated ERK activity, indicating that the suppressive effects of n-3 fatty acids on MCP-1 are at least in part through inhibition of ERK.
In many cell systems, proinflammatory cytokines including TNF-α and IL-1 promote MCP-1 expression through activation of the NF-κB signaling pathway. NF-κB is a transcription factor complex widely distributed in most cell types, including MCs (37, 44). NF-κB consists of homo- or heterodimers formed from five possible units of the Rel family, with the p65/p50 heterodimer being the most common form. The complex exists in an inactive form within the cytoplasm associated with the inhibitory protein IκB (5). Activation of NF-κB results from release of the IκB subunit from the heterotrimeric complex and is followed by translocation of the dimer into the nucleus, initiating transcription of target genes.
We demonstrate that DHA and EPA inhibit activation of MCP-1 at least in part through inactivation of NF-κB. In other cell types, n-3 fatty acids inhibit NF-κB activation (32, 41, 69, 81). Furthermore, the MEK inhibitor U0126 prevented NF-κB activation in MCs, suggesting that TNF-α-mediated induction of MCP-1 requires sequential activation of ERK and NF-κB. A similar ERK→NF-κB pathway has been established in aortic smooth muscle cells (65) and in melanoma cells (15).
Recent studies led to the identification of central regulatory regions within the MCP-1 promoter and flanking regions that are necessary for cytokine and growth factor-stimulated MCP-1 expression (6, 58, 59). A distal enhancer region contains two consensus NF-κB binding sites (κB1 and κB2). We verify that the κB1 and κB2 sites are essential for transcriptional induction of the MCP-1 gene by TNF-α in MCs, since a construct in which these elements are deleted showed no transcriptional response to TNF-α. Although this construct did not respond to TNF-α, DHA and EPA suppressed basal transcriptional activity. These studies suggest that DHA and EPA target sequences in addition to the κB1 and κB2 sites in regulating MCP-1 expression.
Based on studies in other cell systems, we hypothesized that an AP-1 binding site located within the proximal promoter region may be involved in n-3 fatty acid-mediated inhibition of TNF-α-stimulated MCP-1 transcription (4, 43, 59, 72, 76, 80). We found that a mutant MCP-1 construct with deletion of an AP-1 site located within the promoter region in addition to the distal κB1 and κB2 elements shows no transcriptional activation in response to TNF-α, as expected, and no transcriptional suppression in response to DHA and EPA. Based on these considerations, we propose that n-3 fatty acids target both the AP-1 site, which regulates basal transcription of the MCP-1 gene, and the κB1 and κB2 sites, which regulate TNF-α-stimulated transcription of the MCP-1 gene.
The role of the NF-κB and AP-1 sites in transcriptional regulation of the MCP-1 gene appears to be stimulus-specific. For example, in glomerular endothelial cells, TNF-α-induced transcription of the MCP-1 gene requires cooperative interaction of NF-κB and AP-1 (55). However, the NF-κB sites do not appear to be involved in transcriptional regulation of the MCP-1 gene in response to PDGF in murine fibroblasts (58) or to TGF-β in rat MCs (9).
The precise mechanism through which n-3 fatty acids suppress ERK and NF-κB activation has not yet been established. n-3 Fatty acids are substrates for resolvins and protectins, which are potent anti-inflammatory compounds (3, 30, 68). Recent studies identified GPR120 as a receptor for n-3 fatty acids (54, 77). Activation of GPR120 antagonizes the proinflammatory effects of TNF-α and LPS in a macrophage cell line, an effect that is associated with inhibition of NF-κB signaling. Engagement of GPR120 on macrophages may promote transition from a proinflammatory M1 phenotype–a phenotype characterized by increased MCP-1 production–to an anti-inflammatory/repair M2 phenotype (54). GPR120 is highly expressed on macrophages and adipocytes (62); it is not yet clear whether this receptor is highly expressed on MCs or other parenchymal cells. Further studies are needed to address this issue.
Recent studies called attention to the inductive effect of fish oil on heme oxygenase (HO)-1 (57), and that an oxidative product of n-3 fatty acids, 4-hydroxy hexenol, induces HO-1 (28). In this regard, our prior studies demonstrated that HO-1 is an inhibitor of MCP-1 expression (48–50). For example, HO-1 deficiency is associated with increased inflammatory responses and increased activation of NF-κB and MCP-1 (49). Conversely, overexpression of HO-1 suppresses albumin-induced NF-κB activation and MCP-1 expression through a mechanism that involves inhibition of an ERK-dependent NF-κB-dependent pathway (48). Products of HO-1 such as bile pigments and carbon monoxide are anti-inflammatory in nature (50). We offer the speculation that it is possible that the inhibitory effect of n-3 fatty acids on MCP-1 expression may involve induction of HO-1 and the products of such HO activity.
In summary, we propose that n-3 fatty acids suppress TNF-α-stimulated MCP-1 transcription by preventing NF-κB activation in an ERK-dependent fashion. We identified two consensus NF-κB binding sites in the distal enhancer region that are involved in transcriptional activation of the MCP-1 gene. n-3 Fatty acids block transcriptional activation of the MCP-1 gene by preventing nuclear translocation of NF-κB subunits. n-3 Fatty acids may also suppress MCP-1 transcription through interaction with an AP-1 site located within the proximal promoter region of the MCP-1 gene. Inhibition of MCP-1 production may represent an important mechanism for the well-established anti-inflammatory properties of n-3 fatty acids.
GRANTS
These studies were supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute Grant HL85307 and NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK55603, DK70124, and DK47060.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Cherish Grabau for excellent secretarial assistance.
M. M. Diaz-Encarnacion is currently affiliated with the Department of Nephrology, Fundación Puigvert, Barcelona, Spain.
REFERENCES
- 1. Adam O. Dietary fatty acids and immune reactions in synovial tissue. Eur J Med Res 8: 381–387, 2003 [PubMed] [Google Scholar]
- 2. An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol 297: F895–F903, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA 102: 7671–7676, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babcock TA, Kurland A, Helton WS, Rahman A, Anwar KN, Espat NJ. Inhibition of activator protein-1 transcription factor activation by omega-3 fatty acid modulation of mitogen-activated protein kinase signaling kinases. JPEN J Parenter Enteral Nutr 27: 176–180; discussion 181, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12: 141–179, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Boekhoudt GH, Guo Z, Beresford GW, Boss JM. Communication between NF-kappa B and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J Immunol 170: 4139–4147, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Boucher A, Droz D, Adafer E, Noel LH. Characterization of mononuclear cell subsets in renal cellular interstitial infiltrates. Kidney Int 29: 1043–1049, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Calder PC. n-3 Polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab 41: 203–234, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Cheng J, Diaz Encarnacion MM, Warner GM, Gray CE, Nath KA, Grande JP. TGF-β1 stimulates MCP-1 expression in mesangial cells via a PDE4-dependent process. Am J Physiol Cell Physiol 289: C959–C970, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Cheng J, Thompson MA, Walker HJ, Gray CE, Diaz Encarnacion MM, Warner GM, Grande JP. Differential regulation of mesangial cell mitogenesis by cAMP phosphodiesterase isozymes 3 and 4. Am J Physiol Renal Physiol 287: F940–F953, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Cheng J, Yusufi AN, Thompson MA, Chini EN, Grande JP. Nicotinic acid adenine dinucleotide phosphate: a new Ca2+ releasing agent in kidney. J Am Soc Nephrol 12: 54–60, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Danoff TM. Chemokines in interstitial injury. Kidney Int 53: 1807–1808, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway upregulates NF-kappa B activity in melanoma cells. J Biol Chem 277: 7920–7928, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diaz Encarnacion MM, Warner GM, Gray CE, Cheng J, Keryakos HK, Nath KA, Grande JP. Signaling pathways modulated by fish oil in salt-sensitive hypertension. Am J Physiol Renal Physiol 294: F1323–F1335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donadio JV, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. N Engl J Med 331: 1194–1199, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Donadio JV, Jr, Larson TS, Bergstralh EJ, Grande JP. A randomized trial of high-dose compared with low-dose omega-3 fatty acids in severe IgA nephropathy. J Am Soc Nephrol 12: 791–799, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int 47: 1546–1557, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JWM, Cannon JG, Rogers TS, Klempner MS, Weber PC, Schaefer EJ, Wolff SM, Dinarello CA. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 320: 265–271, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Grande J, Melder D, Zinsmeister A, Killen P. TGF-b1 induces collagen IV gene expression in NIH-3T3 cells. Lab Invest 69: 387–395, 1993 [PubMed] [Google Scholar]
- 22. Grande J, Walker H, Holub B, Warner G, Keller D, Haugen J, Donadio J, Dousa T. Suppressive effects of fish oil on mesangial cell proliferation in vitro and in vivo. Kidney Int 57: 1027–1040, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Grande JP, Jones ML, Swenson CL, Killen PD, Warren JS. Lipopolysaccharide induces monocyte chemoattractant protein production by rat mesangial cells. J Lab Clin Med 124: 112–117, 1994 [PubMed] [Google Scholar]
- 24. Grande JP, Melder DC, Zinsmeister AR. Modulation of collagen gene expression by cytokines: Stimulatory effect of transforming growth factor-b1, with divergent effects of epidermal growth factor and tumor necrosis factor-a on collagen type I and collagen type IV. J Lab Clin Med 130: 476–486, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Grimble RF, Howell WM, O'Reilly G, Turner SJ, Markovic O, Hirrell S, East JM, Calder PC. The ability of fish oil to suppress tumor necrosis factor alpha production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor alpha production. Am J Clin Nutr 76: 454–459, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, Kaneko S, Itoh T, Gohda T, Horikoshi S, Tomino Y. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant 21: 605–615, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Ho AW, Wong CK, Lam CW. Tumor necrosis factor-alpha upregulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology 213: 533–544, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Ishikado A, Nishio Y, Morino K, Ugi S, Kondo H, Makino T, Kashiwagi A, Maegawa H. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem Biophys Res Commun 402: 99–104, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Jones ML, Warren JS. Monocyte chemoattractant protein 1 in a rat model of pulmonary granulomatosis. Lab Invest 66: 498–503, 1992 [PubMed] [Google Scholar]
- 30. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem 49: 133–143, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Kawano H, Kim S, Ohta K, Nakao T, Miyazaki H, Nakatani T, Iwao H. Differential contribution of three mitogen-activated protein kinases to PDGF-BB-induced mesangial cell proliferation and gene expression. J Am Soc Nephrol 14: 584–592, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Kielar ML, Jeyarajah DR, Penfield JG, Lu CY. Docosahexaenoic acid decreases IRF-1 mRNA and thus inhibits activation of both the IRF-E and NFkappa d response elements of the iNOS promoter. Transplantation 69: 2131–2137, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Kielar ML, Jeyarajah DR, Zhou XJ, Lu CY. Docosahexaenoic acid ameliorates murine ischemic acute renal failure and prevents increases in mRNA abundance for both TNF-alpha and inducible nitric oxide synthase. J Am Soc Nephrol 14: 389–396, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Kojima R, Taniguchi H, Tsuzuki A, Nakamura K, Sakakura Y, Ito M. Hypertonicity-induced expression of monocyte chemoattractant protein-1 through a novel cis-acting element and MAPK signaling pathways. J Immunol 184: 5253–5262, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Komatsu W, Ishihara K, Murata M, Saito H, Shinohara K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic Biol Med 34: 1006–1016, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, Sherman M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum 33: 810–820, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J. Growth inhibition by TGF-b linked to suppression of retinoblastoma protein phosphorylation. Cell 62: 175–185, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Lee SK, Kim BS, Yang WS, Kim SB, Park SK, Park JS. High glucose induces MCP-1 expression partly via tyrosine kinase-AP-1 pathway in peritoneal mesothelial cells. Kidney Int 60: 55–64, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Li M, Mossman BT, Kolpa E, Timblin CR, Shukla A, Taatjes DJ, Fukagawa NK. Age-related differences in MAP kinase activity in VSMC in response to glucose or TNF-alpha. J Cell Physiol 197: 418–425, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med 185: 1371–1380, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res 82: 216–221, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Lopez-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C. Nuclear factor-kB inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol 161: 1497–1505, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin T, Cardarelli PM, Parry GC, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kB and AP-1. Eur J Immunol 27: 1091–1097, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Massy ZA, Guijarro C, O'Donnell MP, Kim Y, Kashtan CE, Egido J, Kasiske BL, Keane WF. The central role of nuclear factor-kappa B in mesangial cell activation. Kidney Int Suppl 71: S76–S79, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Matousovic K, Grande JP, Chini CCS, Chini EN, Dousa TP. Inhibitors of cyclic nucleotide phosphodiesterase isozymes type-III and type-IV suppress mitogenesis of rat mesangial cells. J Clin Invest 96: 401–410, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matsuo H, Tamura M, Kabashima N, Serino R, Tokunaga M, Shibata T, Matsumoto M, Aijima M, Oikawa S, Anai H, Nakashima Y. Prednisolone inhibits hyperosmolarity-induced expression of MCP-1 via NF-kappaB in peritoneal mesothelial cells. Kidney Int 69: 736–746, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, Ghilardi M, Remuzzi G. Protein overload-induced NF-kappaB activation in proximal tubular cells requires H2O2 through a PKC-dependent pathway. J Am Soc Nephrol 13: 1179–1189, 2002 [PubMed] [Google Scholar]
- 48. Murali NS, Ackerman AW, Croatt AJ, Cheng J, Grande JP, Sutor SL, Bram RJ, Bren GD, Badley AD, Alam J, Nath KA. Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: implications for chronic kidney disease. Am J Physiol Renal Physiol 292: F837–F844, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Nath K, Vercellotti G, Grande J, Miyoshi H, Paya C, Manivel J, Haggard J, Croatt A, Payne W, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int 59: 106–117, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Nielsen GL, Faarvang KL, Thomsen BS, Teglbjaerg KL, Jensen LT, Hansen TM, Lervang HH, Schmidt EB, Dyerberg J, Ernst E. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest 22: 687–691, 1992 [DOI] [PubMed] [Google Scholar]
- 52. Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-κB inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am J Physiol Lung Cell Mol Physiol 284: L84–L89, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Oh da Y, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park SK, Yang WS, Han NJ, Lee SK, Ahn H, Lee IK, Park JY, Lee KU, Lee JD. Dexamethasone regulates AP-1 to repress TNF-alpha induced MCP-1 production in human glomerular endothelial cells. Nephrol Dial Transplant 19: 312–319, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Pawluczyk IZA, Harris KPG. Macrophages promote prosclerotic responses in cultured rat mesangial cells: a mechanism for the initiation of glomerulosclerosis. J Am Soc Nephrol 8: 1525–1536, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Peake JM, Gobe GC, Fassett RG, Coombes JS. The effects of dietary fish oil on inflammation, fibrosis and oxidative stress associated with obstructive renal injury in rats. Mol Nutr Food Res 55: 405–410, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Ping D, Boekhoudt G, Boss JM. trans-Retinoic acid blocks platelet-derived growth factor-BB-induced expression of the murine monocyte chemoattractant-1 gene by blocking the assembly of a promoter proximal Sp1 binding site. J Biol Chem 274: 31909–31916, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Ping D, Boekhoudt GH, Rogers EM, Boss JM. Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol 162: 727–734, 1999 [PubMed] [Google Scholar]
- 60. Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Rovin BH, Dickerson JA, Tan LC, Fassler J. Modulation of IL-1-induced chemokine expression in human mesangial cells through alterations in redox status. Cytokine 9: 178–186, 1997 [DOI] [PubMed] [Google Scholar]
- 62. Saltiel AR. Fishing out a sensor for anti-inflammatory oils. Cell 142: 672–674, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Satriano J, Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor alpha, immunoglobuoin G, and adenosine 3′:5′-cyclic monophosphate. J Clin Invest 94: 1629–1636, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Satriano JA, Shuldiner M, Hora K, Xing Y, Shan Z, Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADH)-dependent oxidase. J Clin Invest 92: 1564–1571, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schecter AD, Berman AB, Yi L, Ma H, Daly CM, Soejima K, Rollins BJ, Charo IF, Taubman MB. MCP-1-dependent signaling in CCR2(−/−) aortic smooth muscle cells. J Leukoc Biol 75: 1079–1085, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Schlondorff D. The role of chemokines in the initiation and progression of renal disease. Kidney Int 47: S44–S47, 1995 [PubMed] [Google Scholar]
- 67. Schneider A, Panzer U, Zahner G, Wenzel U, Wolf G, Thaiss F, Helmchen U, Stahl RA. Monocyte chemoattractant protein-1 mediates collagen deposition in experimental glomerulonephritis by transforming growth factor-b. Kidney Int 56: 135–144, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and proresolution lipid mediators. Nat Rev 8: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sethi S, Eastman AY, Eaton JW. Inhibition of phagocyte-endothelium interactions by oxidized fatty acids: a natural anti-inflammatory mechanism? J Lab Clin Med 128: 27–38, 1996 [DOI] [PubMed] [Google Scholar]
- 70. Stahl RA, Thaiss F, Disser M, Helmchen U, Hora K, Schlondorff D. Increased expression of monocyte chemoattractant protein-1 in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int 44: 1036–1047, 1993 [DOI] [PubMed] [Google Scholar]
- 71. Takaya K, Koya D, Isono M, Sugimoto T, Sugaya T, Kashiwagi A, Haneda M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am J Physiol Renal Physiol 284: F1037–F1045, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Takeshita A, Chen Y, Watanabe A, Kitano S, Hanazawa S. TGF-beta induces expression of monocyte chemoattractant JE/monocyte chemoattractant protein 1 via transcriptional factor AP-1 induced by protein kinase in osteoblastic cells. J Immunol 155: 419–426, 1995 [PubMed] [Google Scholar]
- 73. Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes. Brain Res 1287: 47–57, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trushin SA, Pennington KN, Algeciras-Schimnich A, Paya CV. Protein kinase C and calcineurin synergize to activate IkappaB kinase and NF-kappaB in T lymphocytes. J Biol Chem 274: 22923–22931, 1999 [DOI] [PubMed] [Google Scholar]
- 75. Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Upregulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58: 1492–1499, 2000 [DOI] [PubMed] [Google Scholar]
- 76. Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC. Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-kappaB dependent. Kidney Int 57: 2011–2022, 2000 [DOI] [PubMed] [Google Scholar]
- 77. Willyard C. How fish oil fights inflammation. http://news.sciencemag.org/sciencenow/2010/09/how-fish-oil-fights-inflammation.html
- 78. Yusufi A, Cheng J, Thompson M, Walker H, Gray C, Warner G, Grande J. Differential effects of low-dose docosahexaenoic acid and eicosapentaenoic acid on the regulation of mitogenic signaling pathways in mesangial cells. J Lab Clin Med 141: 318–329, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int 72: 193–201, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Zhao Y, Chen LH. Eicosapentaenoic acid prevents lipopolysaccharide-stimulated DNA binding of activator protein-1 and c-Jun N-terminal kinase activity. J Nutr Biochem 16: 78–84, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr 23: 71–78, 2004 [DOI] [PubMed] [Google Scholar]