Abstract
Cyst expansion in polycystic kidney disease (PKD) results in localized hypoxia in the kidney that may activate hypoxia-inducible factor-1α (HIF-1α). HIF-1α and autophagy, a form of programmed cell repair, are induced by hypoxia. The purposes were to determine HIF-1α expression and autophagy in rat and mouse models of PKD. HIF-1α was detected by electrochemiluminescence. Autophagy was visualized by electron microscopy (EM). LC3 and beclin-1, markers of autophagy, were detected by immunoblotting. Eight-week-old male heterozygous (Cy/+) and 4-wk-old homozygous (Cy/Cy) Han:SPRD rats, 4-wk-old cpk mice, and 112-day-old Pkd2WS25/− mice with a mutation in the Pkd2 gene were studied. HIF-1α was significantly increased in massive Cy/Cy and cpk kidneys and not smaller Cy/+ and Pkd2WS25/− kidneys. On EM, features of autophagy were seen in wild-type (+/+), Cy/+, and cpk kidneys: autophagosomes, mitophagy, and autolysosomes. Specifically, autophagosomes were found on EM in the tubular cells lining the cysts in cpk mice. The increase in LC3-II, a marker of autophagosome production and beclin, a regulator of autophagy, in Cy/Cy and cpk kidneys, followed the same pattern of increase as HIF-1α. To determine the role of HIF-1α in cyst formation and/or growth, Cy/+ rats, Cy/Cy rats, and cpk mice were treated with the HIF-1α inhibitor 2-methoxyestradiol (2ME2). 2ME2 had no significant effect on kidney volume or cyst volume density. In summary, HIF-1α is highly expressed in the late stages of PKD and is associated with an increase in LC3-II and beclin-1. The first demonstration of autophagosomes in PKD kidneys is reported. Inhibition of HIF-1α did not have a therapeutic effect.
Keywords: electron microscopy
autosomal dominant polycystic kidney disease (ADPKD) is one of the most common hereditary diseases in the United States, with an estimated prevalence of 1 in 200–1,000 people (11). Over 50% of people with ADPKD develop chronic kidney disease that ultimately requires dialysis and renal transplantation (11). A better understanding of the pathophysiology of cyst formation may lead to the development of potential therapies to slow cyst formation or growth.
Cyst expansion in PKD kidneys results in localized areas of hypoxia. Evidence for the hypoxia in the kidney is the observation that PKD patients have significantly higher EPO levels than other end-stage renal disease (ESRD) patients (41). EPO gene expression is induced by hypoxia-inducible transcription factors (HIF) due to localized hypoxia in the cystic kidney. One study has demonstrated an increase in HIF-1α in humans and rat polycystic kidneys (4). Another study has shown increased expression of HIF-1α in polycystic livers (31). Hypoxic stimuli increase HIF-1α protein by inhibiting its degradation by the proteasome. Multiple studies in animal models of PKD have demonstrated activation of the mammalian target of rapamycin (mTOR) signaling pathway and the therapeutic effect of rapamycin in PKD (2, 14). Under both normoxic and hypoxic conditions, the phosphatidylinositol 3-kinase-mTOR pathway activates HIF-1α (9, 16, 22). The aim of the study was to determine HIF-1α expression in different models of PKD with varying severity of cyst formation.
HIF-1α is known to increase cell proliferation, angiogenesis, and apoptosis (9, 22). As proliferation of cyst lining epithelium and apoptosis play a causative role in cyst formation (2, 13), we also sought to determine the potential therapeutic effect of HIF-1α inhibition in PKD.
Autophagy has been described as a HIF-1α-dependent adaptive response (42). Autophagy is the process by which organelles and bits of cytoplasm are sequestered and subsequently delivered to lysosomes for hydrolytic digestion (23). Autophagy, also called “self-eating,” is a process of cell repair that usually accompanies apoptosis which is “self-killing” of the cell (19, 23). While apoptosis is a feature of most models of PKD including those for humans, rats, and mice (13), autophagy has not been described in PKD. In the current study, we sought to determine whether autophagy and its protein markers, LC3 and beclin-1, are upregulated in polycystic kidneys.
MATERIALS AND METHODS
Animal models.
The study was conducted in homozygotes (Cy/Cy), heterozygous males (Cy/+), and normal littermate control male (+/+) Han:SPRD rats. Cy/Cy rats are diagnosed by palpable kidneys at 10–14 days of age. Cy/Cy rats develop massive polycystic kidneys with a 20-fold increase in two- kidney weight-to-total body weight ratio (2K/TBW) and die from renal failure at ∼4 wk of age. Cy/+ rats develop a twofold increase in 2K/TBW and have mild renal failure at 8 wk of age. A colony of Han:SPRD rats was established in our animal care facility from a litter that was obtained from the Polycystic Kidney Program at the University of Kansas Medical Center.
cpk/+ mice in the C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). The cpk mouse is a model of ARPKD. Thus heterozygous cpk mice (cpk/+) do not have PKD, whereas homozygous cpk mice have massive polycystic kidneys with a 20-fold increase in 2K/TBW and usually die from renal failure at ∼4 wk of age. In this manuscript, the term “cpk” is used to refer to homozygous mice carrying two copies of the cpk gene (cystic cpk/cpk mice).
The study was also conducted in Pkd2WS25/− mice and normal littermate control (+/+) mice. The development of PKD and renal failure in Pkd2WS25/− mice has been described in detail (10, 38, 39). A colony of Pkd2WS25/− mice was established in our animal care facility from a litter that was obtained from Stefan Somlo at Yale University.
The study protocol was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. Mice and rats had free access to tap water and standard mouse and rat chow.
Genotyping.
The cpk gene encodes a hydrophilic, 145-amino acid protein termed cystin (15). In the cpk mouse, there is a tandem deletion of 12 and 19 bp in exon 1 of the cpk gene. cpk mutations are identified using a PCR primer set flanking the deletions. The following cpk exon1 primer set amplified a 351-bp product from the wild-type cpk gene and a 320-bp product from a mutant cpk gene: 5′CPK: 5′TCC TCC CTC CCT ATC TCT CCA3′; 3′ CPK: 5′ATC CAG CAG GCG TAG GGT CTC3′.
C57BL/6 Pkd2+/− and Pkd2WS25/+ mice were used as breeding pairs to generate Pkd2WS25/− mice for the study. Mice were genotyped by Southern blotting (1, 39). Briefly, the genotype of Pkd2WS25/− mice is determined by hybridizing the Pst1-digested tail DNA with a 32P-labeled probe (39). The size of the wild-type allele is 4.0 kb, the WS25 allele is 2.5 kb, and the null allele is 1.8 kb.
Cyst volume density.
Hematoxylin-eosin-stained sections were used to determine the cyst volume density. This was performed by a reviewer blinded to the identity of the treatment modality, using point-counting stereology (8). At least 10 areas of the medulla at 90, 180, and 270° from the hilum of each section were selected to guard against field-selection variation.
HIF-1α measurements.
HIF-1α was detected by electrochemiluminescence using an ultrasensitive singleplex kit from Meso Scale Discovery (MSD) as per the manufacturer's instructions.
Immunoblotting.
Immunoblot analysis was performed as we have previously described (33). The renal cortex was homogenized in lysis buffer (in mM: 5 Na2HPO4, 5 NaH2PO4, 150 NaCl, 1 EDTA, 0.1% Triton X-100, 50 NaF, and 0.2 Na3VO4, and 0.1% β-mercaptoethanol, pH 7.2) plus proteinase inhibitors 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 15 μM pepstatin A, 14 μM l-trans-epoxysuccinyl-leucylamide-(4-guanido)-butane (E-64), 40 μM bestatin, 22 μM leupeptin, and 0.8 μM aprotonin. The homogenates were centrifuged (14,000 rpm at 4°C for 10 min) to remove unbroken cells and debris. Supernatants were mixed with sample buffer containing 50 mM Tris-base (pH 6.8), 0.5% glycerol, 0.01% bromphenol blue, and 0.75% SDS and heated at 95°C for 5 min. Equal amounts of protein (60 μg/lane) were fractionated by Tris-glycine-SDS-15% PAGE. The electrophoretically separated proteins were then transferred to a nitrocellulose membrane (Millipore, Bedford, MA) by wet electroblotting. The membranes were blocked with 5% nonfat dry milk in TBST [50 mM Tris (pH 7.5), 150 mM NaCl, and 0.1% Tween buffer, pH 7.5] overnight at 4°C. Immunoblot analyses were performed with the following antibodies: 1) an LC3 antibody (catalog no. 2775, Cell Signaling Technology, Beverly, MA) that detects endogenous levels of total LC3 protein; LC3 initially yields a cytosolic form, LC3-I, recognized as a 16-kDa protein, and during autophagy LC3-I is converted to LC3-II recognized as a 14-kDa protein; 2) a beclin-1 antibody (catalog no. NB500–249, Novus Biologicals, Littleton, CO) that detects levels of beclin-1 protein recognized as a 52-kDa protein; beclin-1 is an earlier marker that participates in the initiation and elongation processes of autophagosome formation; and 3) HIF-1α antibody (catalog no. NB100-134, Novus Biologicals).
Immunofluorescence studies.
Kidney tissues were embedded in OCT, snap-frozen in liquid nitrogen, and stored at −80°C until sectioning. Five-micrometer cryostat sections were fixed in 70% acetone/30% methanol and prepared for immunofluorescence studies as described previously (26). The following primary antibodies were used: 1) LC3 (same antibody as described for immunoblot) and 2) HIF-1α antibody (catalog no. NB100-134, Novus Biologicals).
Transmission electron microscopy.
For transmission electron microscopy (TEM) analysis, samples were fixed in McDowell fixative, washed in cacodylate buffer (pH 7.2), and postfixed in 2% osmium tetroxide in the same buffer. The samples were dehydrated in graded acetone series and embedded in agar epoxy resin. Semithin sections were stained with toluidine blue. Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Jeol 400 transmission electron microscope at 100 kV.
2-Methoxyestradiol and bafilomycin treatments.
Rats and mice were treated with the HIF-1α inhibitor 2-methoxyestradiol (2ME2; 2 mg·kg−1·day−1 ip, product no. M6383, Sigma-Aldrich, St. Louis, MO) or vehicle (1% DMSO in PBS). The dose of 2ME2 is based on studies that demonstrate a protective effect of 2ME2 in rodent models of cerebral ischemia (8) and rheumatoid arthritis (27).
Mice were treated with bafilomycin A1 (2 mg·kg−1·day−1 ip, Wako Chemicals, Richmond, VA) or vehicle (10% ethanol in normal saline) for 4 days and then killed for determination of LC3-II.
Statistical analysis.
Nonnormally distributed data were analyzed by an nonparametric unpaired Mann-Whitney test. Multiple group comparisons were performed using ANOVA with a Newman-Keuls posttest. A P value <0.05 is considered statistically significant. Values are expressed as means ± SE.
RESULTS
Upregulation of HIF-1α in Cy/Cy and cpk kidneys.
We determined whether HIF-1α is increased in whole kidneys of rats and mice with PKD using an ultrasensitive singleplex kit from MSD. The HIF-1α contents of different rodent species were compared (Table 1). Despite the fact that the 2K/TBW was increased in Cy/+ rats and Pkd2WS25/− mice, levels of HIF-1α in Cy/+ and Pkd2WS25/− were not statistically different from the levels in +/+ rats. Interestingly, we found large increases in HIF-1α in massive kidneys from Cy/Cy rats and cpk mice compared with their respective +/+ rats and mice (Table 1). These results in three different models of PKD establish that HIF-1α is increased in late stages of PKD when the kidneys are massive.
Table 1.
2K/TBW ratio and HIF-1α in kidneys of Cy/Cy rats and cpk mice
| P Value | ||||
|---|---|---|---|---|
| Han:SPRD rats (n = 6/group) | 8-Wk-old +/+ | 8-Wk-old Cy/+ | 4-Wk-old Cy/Cy | |
| 2K/TBW, % | 0.9 ± 0.1 | 1.8 ± 0.1* | 17.8 ± 2.2* | *P < 0.001 vs. +/+ |
| HIF-1α | 54.8 ± 4.2 | 52.3 ± 3.3 | 88.4 ± 3* | *P < 0.001 vs. +/+ |
| Cpk mice (n =7/group) | 4-Wk-old +/+ | 4-Wk-old cpk | ||
| 2K/TBW, % | 1.3 ± 0.1 | 28.7 ± 1.9* | *P < 0.001 vs. +/+ | |
| HIF-1α | 62.5 ± 4.5 | 133 ± 11* | *P < 0.001 vs. +/+ | |
| Pkd2WS25/− mice (n =4/group) | 112-Day-old +/+ | 112-Day-old Pkd2WS25/− | ||
| 2K/TBW, % | 1.5 ± 0.1 | 2.0 ± 0.2* | *P < 0.01 vs. +/+ | |
| HIF-1α | 59.7 ± 3.0 | 62.7 ± 5.8 | NS | |
Values are means ± SE.
2K/TBW, 2-kidney weight-to-total body weight ratio; HIF-1α, hypoxia-inducible factor-1α; NS, not significant. Kidney size, as indicated by 2K/TBW ratio, was massively increased in Cy/Cy rats and cpk mice compared with normal controls (+/+). HIF-1α was increased in Cy/Cy rat and cpk mouse kidneys.
Localization of HIF-1α in cyst-lining epithelial cells.
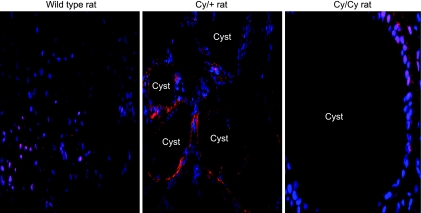
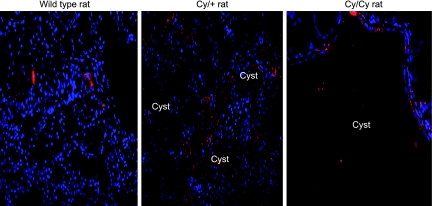
To determine the localization of HIF-1α in PKD kidneys, immunofluorescence was performed. Cystic kidneys, from +/+, Cy/+, and Cy/Cy rats were stained and analyzed by confocal microscopy. Cells lining the cysts in Cy/+ and Cy/Cy kidneys showed intense HIF-1α staining. A representative picture of HIF-1α staining from at least three separate experiments is shown in Fig. 1.
Fig. 1.
Hypoxia-inducible factor-1α (HIF-1α) immunofluorescence. To demonstrate that HIF-1α is present in the cells lining the cysts, immunofluorescence was performed. There is HIF-1α staining (red) in tubular epithelial cells of normal controls. There is HIF-1α staining (red) in cells lining the cysts in Cy/+ and Cy/Cy rats. HIF-1α is known to be a nuclear protein. Nuclei are represented by blue (DAPI) staining. Superimposed red and blue staining indicates localization of HIF-1α staining to the nucleus.
Autophagy.
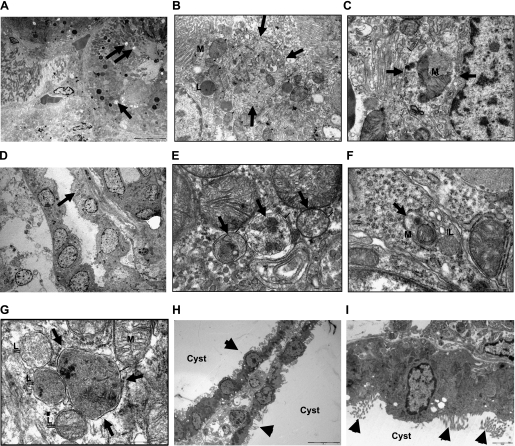
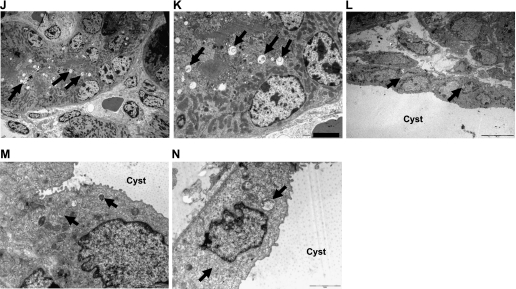
To visualize autophagy in PKD kidneys, electron microscopy was performed. Electron micrographs of autophagosomes, mitochondria in a autophagosome (mitophagy), autophagosomes fusing with lysosomes, and autophagosomes in the epithelial cells lining cysts in +/+, Cy/+, and Cy/Cy rats and cpk mice are demonstrated in Fig. 2, A–N.
Fig. 2.
Electron microscopy of autophagy. A–C: +/+ rats. Magnification ×2,500 (A), ×12,000 (B), and ×20,000 (C). D–G: Cy/+ rats. Magnification ×2,500 (D), ×40,000 (E), ×40,000 (F), and ×60,000 (G). H and I: Cy/Cy rats. Magnificaion ×2,500 (H) and ×8,000 (I). J and K: +/+ mice. Magnification ×2,500 (J) and ×6,000 (K). L–N: cpk mice. Magnification ×2,500 (L), ×12,000 (M), and ×12,000 (N). Autophagosomes (arrows) are demonstrated in kidneys of +/+ rats (A–C), Cy/+ rats (D–G), +/+ mice (J and K), and cpk mice (L–N). Lysosomes (L) fusing to autophagosomes are shown in F and G. Mitochondria (M) within a autophagosome (mitophagy) are shown in C and F. Autophagosomes were not seen in epithelial cells lining cysts in Cy/Cy rats (arrowheads, H and I). Autophagosomes in epithelial cells lining cysts are shown in J–L.
Increase in autophagy markers LC3 and beclin-1 in PKD.
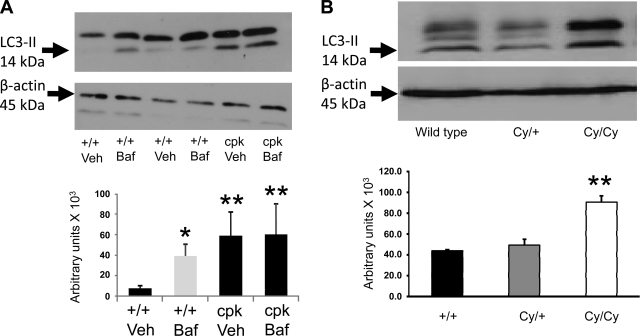
To determine “autophagic flux,” rats and mice were treated with bafilomycin A1 or vehicle, and LC3 was measured. LC3 is a mammalian homolog of yeast Apg8p that initially yields a cytosolic form LC3-I, which is converted to LC3-II during the formation of autophagosomes (23). LC3-II is the only known protein that is recruited to both inner and external surfaces of the expanding autophagosomal membranes and specifically associates with autophagosomes and not with any other vesicular structures.
Rats and mice were treated with bafilomycin A1 (2 mg·kg−1·day−1 ip) or vehicle for 4 days. Bafilomycin at a dose of 2 or 1 mg·kg−1·day−1 for 4 days resulted in the death of +/+ and Cy/Cy rats, and +/+ and cpk mice tolerated bafilomycin A1 (2 mg·kg−1·day−1 for 4 days) well. The intensity of the LC3-II bands was increased in cpk mice compared with +/+ mice (Fig. 3A). In +/+ mice treated with bafilomycin A1, there was an increase in LC3-II compared with vehicle-treated +/+ mice (Fig. 3A). In cpk mice treated with bafilomycin A1, LC3-II was not increased compared with vehicle-treated cpk mice (Fig. 3A). LC3-II was increased in Cy/Cy rats compared with +/+ and Cy/+ rats (Fig. 3B). β-Actin used as a loading control was not different between the groups (*P < 0.05 vs. controls; n = 4/group).
Fig. 3.
Immunoblotting for LC3-II. LC3-II specifically associates with autophagosomes and not with any other vesicular structures. The intensity of the LC3-II bands was increased in cpk mice compared with wild-type (+/+) mice (A). In +/+ mice treated with bafilomycin A1, there was an increase in LC3-II (A). In cpk mice treated with bafilomycin A1, LC3-II was not increased (A). LC3-II was increased in Cy/Cy rats compared with +/+ and Cy/+ rats (B) compared with respective normal littermate controls. β-Actin, used as a loading control, was not different among the groups. *P < 0.05 vs. controls; n = 4/group.
While increased LC3-II was seen on immunoblots in Cy/Cy, autophagosomes were not visualized on electron microscopy (Fig. 2, H and I) in Cy/Cy. The reason for the discrepancy between LC-3 on immunoblots and autophagosomes on electron microscopy is not clear. However, it is known that electron microscopy is not a good technique for quantifying autophagosomes (23).
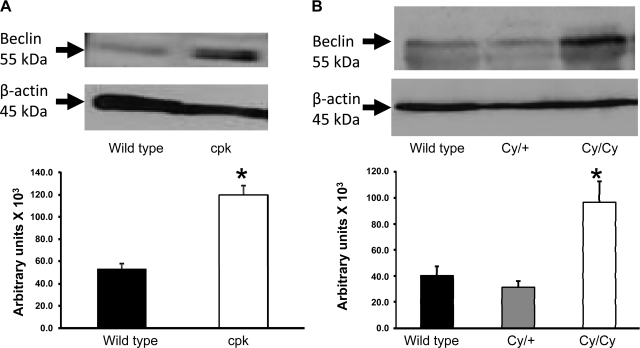
Beclin-1 is the mammalian ortholog of the yeast Apg6/Vps30 gene that participates in the initiation and elongation processes of autophagosome and has a key role in autophagy (23) (19) (37). In agreement with the LC3-II findings, increased beclin-1 expression was observed in cpk mice (Fig. 4A) and Cy/Cy rats (Fig. 4B) compared with respective normal littermate controls (+/+). These results demonstrate that beclin-1 is upregulated in PKD and may be a critical regulator of autophagy in PKD.
Fig. 4.
Immunoblotting for beclin-1. Beclin-1 participates in the initiation and elongation processes of autophagosome and has a key role in autophagy. The intensity of the beclin-1 bands was increased in cpk mice (A) and Cy/Cy rats (B) compared with respective normal littermate controls. β-Actin, used as a loading control, was not different between the groups. *P < 0.05 vs. controls; n = 4/group.
Localization of LC3 in cyst-lining epithelial cells.
To characterize the distribution of endogenous LC3 in cystic kidneys, we performed immunofluorescent analysis. In agreement with the HIF-1α findings, cells lining the cysts showed intense staining in Cy/+ and Cy/Cy (Fig. 5). Staining was mainly in the cytoplasm. A representative picture of LC3 staining from at least three separate experiments is shown in Fig. 5.
Fig. 5.
Immunofluorescence for LC3. To demonstrate that LC3 is present in the cells lining the cysts, immunofluorescence was performed. There is LC3 staining (red) in cells lining the cysts in Cy/+ and Cy/Cy rats. Nuclei are represented by blue (DAPI) staining. LC3 staining is cytosolic.
HIF-1α inhibition.
HIF-1α is significantly increased in kidneys from Cy/Cy rats and cpk mice (Table 1) and in the cells lining the cysts in Cy/Cy rats (Fig. 1). To determine the potential functional role of HIF-1α, inhibition studies were performed.
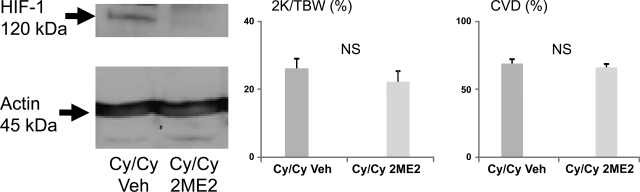
Cy/Cy rats were identified by the palpation of polycystic kidneys at day 14 of age. Rats were treated with the 2ME2 (2 mg·kg−1·day−1 ip) or vehicle from 14 to 28 days of age. Cy/Cy rats usually die from renal failure due to massively enlarged kidneys at ∼day 28 of age. Both vehicle- and 2ME2-treated Cy/Cy rats became very sick at days 27–28 of age, and the kidneys were removed just before death. Immunoblotting of Cy/Cy kidneys demonstrated a decrease in HIF-1α protein in 2ME2-treated rat kidneys compared with vehicle-treated rats (Fig. 6). However, the 2K/TBW ratio and cyst volume density were not different in 2ME2- vs. vehicle-treated Cy/Cy rats (Fig. 6).
Fig. 6.
2-Methoxyestradiol (2ME2) treatment in Cy/Cy rats. Cy/Cy rats were treated with the HIF-1α inhibitor 2ME2 or vehicle from 14 to 28 days of age. Kidneys were removed at death for determination of HIF-1α protein, kidney size as determined by 2K/TBW ratio, and cyst volume density (CVD). 2ME2 treatment (2 mg·kg−1·day−1 ip) resulted in inhibition of HIF-1α as determined by immunoblotting. There was no significant difference in 2-kidney-to-total body weight (2K/TBW) ratio and CVD between vehicle- and 2ME2-treated Cy/Cy rats. NS, not significant; n = 5/group.
+/+ and Cy/+ rats were also treated with the HIF-1α inhibitor 2ME2. Cy/Cy rats were identified by the palpation of polycystic kidneys at day 14 of age and removed from the litters. The rest of the litter consisting of +/+ and Cy/+ rats were treated with 2ME2 (2 mg·kg−1·day−1 ip) or vehicle for 5 wk from 4 to 8 wk of age. The blood urea nitrogen, 2K/TBW ratio, and cyst volume density were not different in 2ME2- vs. vehicle-treated Cy/+ rats (Table 2).
Table 2.
Effect of 2ME2 treatment on 2K/TBW, cyst volume density, and BUN in Cy/+ rats
| +/+ Vehicle | +/+ 2ME2 | Cy/+ Vehicle | Cy/+ 2ME2 | |
|---|---|---|---|---|
| 2K/TBW, % | 0.8 | 0.8 | 1.8* | 2* |
| CVD | 1 | 2 | 30.3* | 27.4* |
| BUN | 21 | 18 | 30* | 28* |
2M2, 2-methoxyestradiol; CVD, cyst volume density; BUN, blood urea nitrogen.
2ME2 treatment had no effect on 2K/TBW, CVD, and BUN in Cy/+ rats (n = 4-5/group).
P < 0.05 vs. +/+ vehicle.
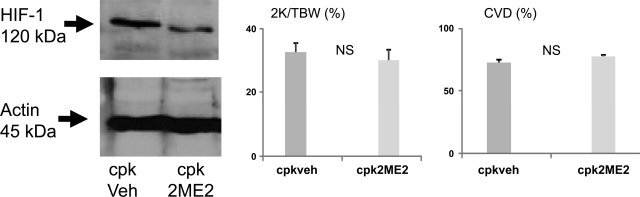
Cpk mice were identified by the palpation of polycystic kidneys at day 14 of age. Mice were treated the HIF-1α inhibitor 2ME2 (2 mg·kg−1·day−1 ip) or vehicle from 14 to 21 days of age. Cpk mice usually die from renal failure due to massively enlarged kidneys at ∼days 21–28 of age. Both vehicle- and 2ME2-treated cpk mice became very sick at day 21 of age, and the kidneys were removed just before death. Immunoblotting of cpk kidneys demonstrated a decrease in HIF-1α protein in 2ME2-treated mouse kidneys compared with vehicle-treated mice (Fig. 7). However, the 2K/TBW ratio and cyst volume density were not different in 2ME2- vs. vehicle-treated cpk mice (Fig. 7).
Fig. 7.
2ME2 treatment in cpk mice. Cpk mice were treated with the HIF-1α inhibitor 2ME2 or vehicle from 14 to 21 days of age. Kidneys were removed at death for determination of HIF-1α protein, kidney size as determined by 2K/TBW ratio, and CVD. 2ME2 treatment (2 mg·kg−1·day−1 ip) resulted in inhibition of HIF-1α as determined by immunoblotting. There was no significant difference in 2K/TBW ratio and CVD between vehicle- and 2ME2-treated cpk mice; n = 3/group.
DISCUSSION
The first aim of the study was to examine HIF-1α expression in kidneys with varying degrees of cyst formation. In kidneys from Cy/+ rats and PkdWS25/− mice that have a small but significant increase in kidney volume-to-body weight ratio, there was no increase in HIF-1α protein expression in whole kidney homogenates. However, on immunofluorescence, the cyst epithelial cells in Cy/+ rat kidneys did show HIF-1α staining. In kidneys from Cy/Cy rats and cpk mice that have a massive ∼20-fold increase in kidney volume-to-body weight ratio, there was a large increase in HIF-1α protein. In addition, HIF-1α staining on immunofluorescence was present in epithelial cells lining large cysts in Cy/Cy kidneys. On the one hand, the presence of a large increase in HIF-1α in very large cystic kidneys suggests that the increase in HIF-1α is a result of more localized hypoxia with larger cysts rather than the cause of the cyst expansion. The presence of regional areas of hypoxia around large cysts has been demonstrated (4). On the other hand, HIF-1α expression was seen in epithelial lining cells of smaller cysts in Cy/+ animals, suggesting that HIF-1α could be the cause of the cyst expansion and may increase pericystic angiogenesis.
To determine whether HIF-1α may be playing a causative role in cyst expansion, inhibition studies were undertaken. HIF-1α induces genes that play a role in many cellular functions including cell proliferation, angiogenesis, and apoptosis (9, 22). Increased cell proliferation of cyst-lining epithelium plays a causative role in cyst growth (2). Increased angiogenesis is a feature of polycystic kidneys (35). The role of apoptosis in PKD is controversial (13). It has been shown that caspase and apoptosis inhibition is protective in PKD (34). However, it has also been shown that the protective effect of rapamycin in PKD is associated with increased apoptosis in cysts (29, 30). Thus HIF-1α inhibition may be protective by inhibitory effects on cell proliferation, angiogenesis and apoptosis. 2ME2 is an endogenous metabolite of estrogen and has antiproliferative, antiangiogenic, and antitumor effects (20, 24). 2ME2 has disease-modifying effects, attributable to the inhibition of neovascular events, in a mouse model of rheumatoid arthritis (27). 2ME2 also has antiarthritis properties by inhibiting inflammation independently of its antiangiogenic properties (17). 2ME2 attenuates the hemorrhagic conversion of cerebral infarcts in rats (8). 2ME2 or its analogs inhibit tumor growth in mice (20, 25, 28). 2ME2 has been well tolerated by patients in phase I and II clinical studies (24). In the pilot study, Cy/Cy rats and cpk mice that have a significant increase in HIF-1α in their polycystic kidneys were treated with 2ME2. Inhibition of HIF-1α in the polycystic kidney by the 2 mg·kg−1·day−1 dose of 2ME2 was demonstrated. However, 2ME2 did not have a significant effect on kidney volume or cyst volume in Cy/Cy rats and cpk mice. It should be noted that treatment was started at a late stage of the disease when cyst formation was advanced and that the pilot studies were undertaken in rats and mice with severe disease. Thus a 5-wk treatment study in Cy/+ rats with milder PKD was performed. 2ME2 had no effect on blood urea nitrogen, 2K/TBW ratio, or cyst volume in Cy/+ rats compared with +/+ rats. The rats and mice tolerated 2ME2 without visible side effects.
Autophagy describes the process by which cytoplasmic materials including organelles reach the lysosomes for degradation (23). Thus autophagy exerts cytoprotective effects. As hypoxia, apoptosis, and mTOR signaling are modulators of autophagy (13) (18) and are also features of PKD kidneys, we determined the effect of PKD on autophagy. Autophagy occurs constitutively at low levels and plays an essential role under physiological conditions, e.g., maintenance of the amino acid pool during starvation, antiaging, regulation of innate and adaptive immunity (23). This likely explains the presence of autophagy seen on EM in normal rat and mouse kidneys (Fig. 2, A–C, J, and K). Although autophagosomes cannot be quantified by TEM, this technique provides an insight into the extent of ongoing autophagy in cells (23). As quantitation of autophagy on EM is unreliable (23), we measured LC3-II. LC3-II is the cleaved isoform of LC3 that localizes to autophagosomes and phagolysosomes during autophagy (37). LC3-II was increased in massive Cy/Cy and cpk kidneys compared with littermate controls.
The amount of LC3-II at a specific time may represent either increased autophagy or suppression of downstream steps, e.g., autophagosome-lysosome fusion. “Autophagic flux” is a term used to describe the dynamic process of autophagosome synthesis, delivery of autophagosomes to the lysosome, and degradation of autophagosomes in the lysosome (23). To measure autophagic flux, lysosomal inhibitors like bafilomycin A1 are used. The difference in LC3-II between samples in the presence and absence of bafilomycin A1 represents the amount of LC3-II that is delivered to the lysosome for degradation (autophagic flux) (23). To measure autophagic flux, wild-type and cpk mice were treated with bafilomycin A1.
In wild-type mouse kidneys, LC3-II was increased in the presence of bafilomycin A1, suggesting increased autophagic flux. LC3-II was increased in cpk compared with wild-type kidneys. The increase in LC3-II in PKD vs. wild-type kidneys suggests either increased autophagosome synthesis in PKD or decreased degradation in the lysosome in PKD. To investigate whether there was increased autophagosome synthesis or decreased degradation in the lysosome, cpk mice were treated with bafilomycin A1. If the increase in LC3-II in PKD is due to increased production, then it would be expected that bafilomycin A1 would further increase LC3-II. Alternatively, if the observed increase in LC3-II is due to a lysosomal defect, then bafilomycin would not affect LC3-II. Bafilomycin A1 had no effect on LC3-II in cpk kidneys. The lack of effect of bafilomycin A1 on LC3-II in cpk kidneys suggests a defect in autophagy in PKD resulting from a block of autophagosome-lysosme fusion and degradation.
What is the significance of autophagy in PKD? A major function of autophagy is to keep cells alive under “stressful” conditions. Increased apoptotic cell death in the tubular cells lining the cysts plays a role in disease progression in PKD (13). Autophagy in the tubular epithelial cells lining the cysts may be a response to the cell death in these cells. Alternatively, autophagy suppression is associated with certain diseases, e.g., cancer, and is a feature of aging. Bafilomycin resulted in an increase in LC3-II in wild-type but not in PKD kidneys. Thus autophagy suppression may play a role in disease progression in PKD. Further study of autophagy in PKD is merited especially in view of the fact that apoptosis plays an important role in disease progression in PKD and that there is important cross talk between apoptosis and autophagy (6, 21). Also, mTOR activation inhibits autophagy and mTOR activation is a feature of PKD (32, 40).
Beclin-1 (also known as atg6/Beclin 1) interacts with various anti-apoptotic proteins, and has recently been found to be a beclin-2-homology-3-only protein. Beclin-1 regulates both formation and maturation of autophagosomes (23). Overexpression of beclin-1 in human MCF7 breast carcinoma cells promotes autophagy and inhibits in vitro tumorigenesis in nude mice (7). Heterozygous beclin-1 +/− mice have reduced autophagy levels and increased incidence of spontaneous tumors (7). These studies clearly demonstrate a causative role for beclin-1 in autophagy. Beclin-1 was increased in Cy/Cy and cpk kidneys, suggesting that it may play a causative role in the autophagy in PKD.
Next, we considered the connection between HIF-1α and autophagy. Both HIF-1α and LC3-II were increased in the whole kidney homogenates and in the cells lining the cyst in the same Cy/Cy and cpk kidneys. HIF-1α has been shown to upregulate both apoptosis and autophagy (3, 5). In this regard, we have demonstrated increased apoptosis in both Cy/Cy kidneys (12, 33) and cpk kidneys (36). Silencing of HIF-1α in chondrocytes results in decreased beclin- 1 and decreased autophagy, suggesting that increased HIF-1α can cause autophagy (3, 5). Thus it is possible that the increased HIF-1α in the present study may play a causative role in the autophagy seen in PKD.
In summary, we report the first demonstration of autophagosomes in PKD kidneys. HIF-1α is highly expressed in the late stages of PKD in cpk mice and Cy/Cy rats. The increase in HIF-1α is associated with an increase in LC3-II and beclin-1. HIF-1α inhibition with 2ME2 did not significantly reduce kidney size in Cy/+ rats, Cy/Cy rats, and cpk mice.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK074835 and DK07483503S1 (to C. L. Edelstein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1.Amura CR, Brodsky KS, Groff R, Gattone VH, Voelkel NF, Doctor RB. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/−) mice. Am J Physiol Cell Physiol 293: C419–C428, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Belibi F, Edelstein CL. Novel targets for the treatment of autosomal dominant polycystic kidney disease. Expert Opin Investig Drugs 19: 315–328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29: 2570–2581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhardt WM, Wiesener MS, Weidemann A, Schmitt R, Weichert W, Lechler P, Campean V, Ong AC, Willam C, Gretz N, Eckardt KU. Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am J Pathol 170: 830–842, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohensky J, Shapiro IM, Leshinsky S, Terkhorn SP, Adams CS, Srinivas V. HIF-1 regulation of chondrocyte apoptosis: induction of the autophagic pathway. Autophagy 3: 207–214, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Boya P, Gonzalex-polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25: 1025–1040, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 17: 839–849, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Cowley BD, Jr, Rupp JC, Muessel MJ, Gattone VH. Gender and the effect of gonadal hormones on the progression of inherited polycystic kidney disease in rats. Am J Kidney Dis 29: 265–272, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Dery MC, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol 37: 535–540, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Doctor RB, Serkova N, Hasebroock K, Zafar I, Edelstein CL. Distinct patterns of kidney and liver cyst growth in Pkd2WS25/- mice. Nephrol Dial Transplant 25: 3496–3504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ecder T, Fick-Brosnahan G, Schrier RW. Polycystic kidney disease. In: Diseases of the Kidney and Urinary Tract, edited by Schrier RW. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007, p. 502–539 [Google Scholar]
- 12.Ecder T, Melnikov VY, Stanley M, Korular D, Lucia MS, Schrier RW, Edelstein CL. Caspases, Bcl-2 proteins and apoptosis in autosomal-dominant polycystic kidney disease. Kidney Int 61: 1220–1230, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Edelstein CL. What is the role of tubular epithelial cell apoptosis in polycystic kidney disease (PKD)? Cell Cycle 4: e141–e145, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Edelstein CL. Mammalian target of rapamycin and caspase inhibitors in polycystic kidney disease. Clin J Am Soc Nephrol 3: 1219–1226, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22: 7004–7014, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issekutz AC, Sapru K. Modulation of adjuvant arthritis in the rat by 2-methoxyestradiol: an effect independent of an anti-angiogenic action. Int Immunopharmacol 8: 708–716, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett 584: 1287–1295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9: 1004–1010, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3: 363–375, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Maiuri MC, Zalckvar E, Kimchi A, Kroemer A. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741–752, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Maxwell P. HIF-1: an oxygen response system with special relevance to the kidney. J Am Soc Nephrol 14: 2712–2722, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313–326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooberry SL. Mechanism of action of 2-methoxyestradiol: new developments. Drug Resist Updat 6: 355–361, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Moser C, Lang SA, Mori A, Hellerbrand C, Schlitt HJ, Geissler EK, Fogler WE, Stoeltzing O. ENMD-1198, a novel tubulin-binding agent reduces HIF-1alpha and STAT3 activity in human hepatocellular carcinoma(HCC) cells, and inhibits growth and vascularization in vivo. BMC Cancer 8: 206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh DJ, Dursun B, He Z, Lu L, Hoke TS, Ljubanovic D, Faubel S, Edelstein CL. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 294: F264–F271, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Plum SM, Park EJ, Strawn SJ, Moore EG, Sidor CF, Fogler WE. Disease modifying and antiangiogenic activity of 2-methoxyestradiol in a murine model of rheumatoid arthritis. BMC Musculoskelet Disord 10: 46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricker JL, Chen Z, Yang XP, Pribluda VS, Swartz GM, Van Waes C. 2-Methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res 10: 8665–8673, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spirli C, Okolicsanyi S, Fiorotto R, Fabris L, Cadamuro M, Lecchi S, Tian X, Somlo S. ERK1/2-dependent vascular endothelial growth factor signaling sustains cyst growth in polycystic-2 defective mice. Gastroenterology 138: 360–371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease (PKD). J Am Soc Nephrol 16: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Tao Y, Kim J, Stanley M, He Z, Faubel SG, Schrier RW, Edelstein CL. Pathways of caspase-mediated apoptosis in autosomal dominant polycystic kidney disease (ADPKD). Kidney Int 67: 909–919, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Tao Y, Kim J, Faubel S, Wu JC, Falk SA, Schrier RW, Edelstein CL. Caspase inhibition reduces tubular apoptosis and proliferation and slows disease progression in polycystic kidney disease (PKD). Proc Natl Acad Sci USA 102: 6954–6959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao Y, Zafar I, Falk S, Faubel S, He Z, Schrier RW, Edelstein CL. VEGF receptor (VEGFR) inhibition slows the progression of polycystic kidney disease (PKD). Kidney Int 72: 1358–1366, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Tao Y, Zafar I, Kim J, Schrier RW, Edelstein CL. Deletion of the caspase-3 gene markedly prolongs survival in the cpk mouse model of polycystic kidney disease (PKD). J Am Soc Nephrol 19: 749–755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tasdemir E, Galluzi LM, Chiara Maiuri M, Criollo A, Vitale I, Hangen E, Modjtahedi N, Kroemer G. Methods for assessing autophagy and autophagic cell death . In: Autophagosome and Phagosome, edited by Deretic V. Totowa, NJ: Humana, 2008, p. 29–76 [DOI] [PubMed] [Google Scholar]
- 38.Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75–78, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Zafar I, Belibi FA, He Z, Edelstein CL. Long-term rapamycin therapy in the Han:SPRD rat model of polycystic kidney disease (PKD). Nephrol Dial Transplant 24: 2349–2353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeier M, Jones E, Ritz E. Autosomal dominant polycystic kidney disease—the patient on renal replacement therapy. Nephrol Dial Transplant 11, Suppl 6: 18–20, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.