Abstract
Cisplatin cytotoxicity is dependent on cyclin-dependent kinase 2 (Cdk2) activity in vivo and in vitro. We found that an 18-kDa protein identified by mass spectrometry as p21WAF1/Cip1 was phosphorylated by Cdk2 starting 12 h after cisplatin exposure. The analysis showed it was phosphorylated at serine 78, a site not previously identified. The adenoviral transduction of p21 before cisplatin exposure protects from cytotoxicity by inhibiting Cdk2. Although cisplatin causes induction of endogenous p21, the protection is inefficient. We hypothesized that phosphorylation of p21 at serine 78 could affect its role as a Cdk inhibitor, and thereby lessen its ability to protect from cisplatin cytotoxicity. To investigate the effect of serine 78 phosphorylation on p21 activity, we replaced serine 78 with aspartic acid, creating the phosphomimic p21S78D. Mutant p21S78D was an inefficient inhibitor of Cdk2 and was inefficient at protecting TKPTS cells from cisplatin-induced cell death. We conclude that phosphorylation of p21 by Cdk2 limits the effectiveness of p21 to inhibit Cdk2, which is the mechanism for continued cisplatin cytotoxicity even after the induction of a protective protein.
Keywords: cell death
cisplatin is a chemotherapeutic drug used as a first-line component in the treatment of several solid tumors, including testicular, head and neck, and ovarian and cervical cancers (14). Its dosage and efficacy are limited by its side effects, especially nephrotoxicity (39). Cisplatin cytotoxicity in kidney cells is dependent on cyclin-dependent kinase 2 (Cdk2) activity in vivo and in vitro (42, 43). This dependence was shown by Cdk2 inhibitory drugs, dominant-negative Cdk2 transduction, and expression of the cyclin-dependent kinase inhibitor (CDKI), p21WAF1/Cip1, all of which protected from cisplatin-induced cell death. Cdk2 is a serine/threonine protein kinase, whose main described function is the phosphorylation of substrates necessary for cell cycle progression (37). The Cdk2 pathways involved in cisplatin-induced cell death are not yet understood, and one substrate in these pathways is described below.
Cdk2 activity is regulated by several mechanisms, including binding to cyclins, positive and negative phosphorylation, and binding of CDKIs (38). Apart from its role in cell cycle progression, various studies showed increased Cdk2 activity associated with programmed cell death (apoptosis) (21, 36, 43, 45, 54, 65, 66), and inhibition of Cdk2 activity in vitro has been shown to protect cultured cells from apoptosis (40, 42, 43, 61).
In the present study, we identified a Cdk2 substrate induced after cisplatin exposure. An 18-kDa protein accumulated and was phosphorylated by Cdk2 starting 12 h after cisplatin exposure, which coincided with the time when Cdk2 inhibition no longer protected from cell death and preceded caspase-3 activation. Mass spectrometry identified the 18-kDa band as p21WAF1/Cip1 with a novel phosphorylation site at serine 78. To investigate the effect of this phosphorylation on p21 function, we mimicked p21 phosphorylation by replacing serine 78 with aspartic acid, creating p21S78D. Although wild-type p21 transduction inhibited Cdk2 and protected from cisplatin cytotoxicity, mutant p21S78D was an inefficient inhibitor of Cdk2 and was inefficient at protecting from cisplatin-induced cell death. Our data suggest that phosphorylation of serine 78 in p21 reduced its function as a CDKI, which diminished its ability to protect against cisplatin-induced cell death. In this manner, rather than being inhibited by p21, Cdk2 acted as a feedback inhibitor of the CDKI, effectively controlling its own inhibition.
MATERIALS AND METHODS
Cell culture and treatment.
Mouse kidney proximal tubule cells (TKPTS) (11) were cultured in DMEM + Ham's F-12 medium supplemented with 50 μU/ml insulin and 7% FBS and grown at 37°C in 5% CO2. Cisplatin was added to cultures, where indicated, to a final concentration of 25 μM when cells were ∼75% confluent, and the cells were grown for an additional 24 h. Purvalanol was dissolved in DMSO and added either with or after cisplatin to a final concentration of 9 μM. Adenoviruses expressing analog-sensitive Cdk2 (as-Cdk2), cyclin A, wild-type p21, and mutant p21S78D were added where indicated to a final multiplicity of infection of 100, which resulted in infection of over 95% of the cells.
Animals and administration of cisplatin.
Experiments were performed on 10- to 12-wk-old wild-type 129Sv mice that weighed 22 to 28 g. The mice were maintained on a standard diet, and water was available freely. Cisplatin was administered by a single intraperitoneal injection of 20 mg/kg, a dosage that produces severe acute renal injury in mice (34). Animals were killed painlessly with methods of euthanasia approved by the Panel on Euthanasia of the American Veterinary Medical Association. The induction of acute kidney injury was monitored by following blood urea nitrogen (BUN) levels in serum and creatinine concentration in serum that were obtained by retroorbital bleeding using commercial kits (Biotron Diagnostics and Sigma Diagnostics, respectively).
Adenoviruses.
Cyclin A adenovirus was a gift from Dr. Gerald Denis (Boston Medical School, Boston, MA). Mouse wild-type p21 cDNA plasmid was obtained from Dr. Bert Vogelstein (Johns Hopkins, Baltimore, MD). The as-Cdk2 was created by site-directed mutagenesis to change the codon for phenylalanine 80 (TTT) to glycine (GGG) (2) in a human wild-type Cdk2 cDNA plasmid (59). The as-Cdk2 adenovirus was constructed by insertion of a BamHI fragment that contained as-Cdk2 cDNA into the BglII site of the pAdTrack-CMV plasmid as described (22). The as-Cdk2 adenovirus used in these studies was constructed as an mCherry fusion protein to assist in immunoprecipitation. mCherry is a red fluorescent protein derived from Discosoma red fluorescent protein (DsRed) (52). Briefly, as-Cdk2mCherry fusion protein was constructed by inserting the BamHI-HindIII mCherry cDNA fragment into the BamHI/HindIII window of pAd-Track-CMV-as-Cdk2 plasmid. The phosphomimic mutant of mouse p21 (p21S78D) was generated by site-directed mutagenesis (Stratagene, La Jolla, CA) to change the codon for serine 78 (AGC) to aspartic acid (GAT). The primers (Integrated DNA Technologies, Coralville, IA) used were 5′-CCC AAG GTC TAC CTG GAT CCT GGG TCC CGC AGC-3′, and 5′-GCT GCG GGA CCC AGG ATC CAG GTA GAC CTT GGG-3′. The as-Cdk2, wild-type p21, and mutant p21S78D adenoviruses were constructed in our laboratory according to protocols and materials supplied by Dr. Bert Vogelstein (Johns Hopkins, Baltimore, MD) (19). Adenoviruses were amplified in HEK-293 cells and purified by CsCl banding as described previously (22).
ATP analog synthesis.
N6-benzyl-ADP was a gift from Dr. David O. Morgan (University of California, San Francisco, CA). Radiolabeled analog N6-benzyl-[γ-32P]ATP was prepared from N6-benzyl-ADP by a phosphate transfer reaction utilizing nucleotide diphosphate kinase (NDPK; Sigma) (30). The reaction was performed in two steps. The first step involved a phosphotransfer reaction from [γ-32P]ATP to NDPK. The second step involved phosphotransfer from NDPK to N6-benzyl-ADP resulting in the production of N6-benzyl-[γ-32P]ATP. Two hundred units of NDPK and 500 μCi [γ-32P]ATP were mixed in a 100-μl reaction containing 20 mM HEPES, pH 7.4, and 150 mM NaCl (HBS) plus 5 mM MgCl2. The reaction was allowed to equilibrate at 30°C for 5 min. The 32P-labeled NDPK was purified from unused [γ-32P]ATP by two successive rounds of Microspin G50 columns (GE Healthcare). One nanomole of N6-benzyl-ADP was added to the 32P-labeled NDPK in HBS and incubated for 20 min at 30°C. The N6-benzyl-[γ-32]ATP was removed from the reaction on a Microcon YM30 column (Millipore) by centrifugation at 13,000 g for 15 min at 4°C.
Immunoprecipitation.
For the analysis of Cdk2-bound proteins, TKPTS cells were lysed in hypotonic buffer (10 mM HEPES, pH 7.5, 10 mM NaCl, 3 mM MgCl2, 1 mM EGTA) for 10 min on ice followed by 20 strokes of a Kontes glass homogenizer. Nuclei were pelleted at 550 g for 5 min. The postnuclear supernatant was incubated in lysis buffer [1% NP40, 50 mM HEPES, pH 7.5, 150 mM NaCl, 0.5 mM EDTA containing protease and phosphatase inhibitor cocktail (Sigma)] for 30 min on ice and the insoluble material was removed by centrifugation at 13,000 g for 15 min. For Cdk2 immunoprecipitation, 300 μg of lysate protein were precleared by incubation with 1 μg of normal rabbit IgG coupled to 30 μl of protein A+G agarose beads (Santa Cruz Biotechnology) for 1 h with constant agitation, followed by centrifugation at 3,000 g for 1 min. Cdk2 was immunoprecipitated from the precleared lysate with 1 μg of rabbit polyclonal anti-Cdk2 antibody (Abcam) coupled to 30 μl of protein A+G agarose beads by gentle rotation at 4°C overnight. For experiments using as-Cdk2, TKPTS cells were transduced with both as-Cdk2mCherry and cyclin A adenoviruses and lysed as described above. For as-Cdk2 immunoprecipitation, 300 μg of lysate protein were precleared as described and as-Cdk2 was immunoprecipitated using 1 μg of DsRed antibody (CLONTECH).
For the analysis of Cdk2-bound proteins in mouse kidney extracts, kidneys were homogenized on ice in lysis buffer using a Dounce homogenizer. The lysate was clarified by centrifugation at 13,000 g for 15 min. Cdk2 was immunoprecipitated from 500 μg of tissue lysate as described above.
In vitro kinase assays.
Cdk2 or as-Cdk2 immunoprecipitates were washed three times with 0.5 ml lysis buffer containing 0.1% NP-40 and once with 0.5 ml of kinase buffer (20 mM HEPES, pH 7.5, 10 mM MgCl2, 1 mM DTT). Kinase assays for Cdk2 immunoprecipitates were carried out in 40 μl of kinase buffer with the addition of 10 μM ATP or N6-benzyl- ATP and 5 μCi of either [γ-32P]ATP or N6-benzyl-[γ-32P]ATP at 30°C for 30 min. The reaction was stopped by the addition of an equal volume of 2× Laemmli buffer followed by boiling for 5 min. Samples were resolved using 12% SDS-PAGE and transferred to nitrocellulose membranes. Phosphorylated components of the reaction were visualized by autoradiography. For measuring Cdk2 kinase activity, Cdk2 was immunoprecipitated as described above and in vitro kinase assay was performed at 30°C for 30 min with histone H1 (Upstate Biotechnology, Billerica, MA) as a substrate. Proteins were resolved using 12% PAGE and the gel was exposed to X-ray film.
Western blot.
TKPTS cells were washed with ice-cold PBS, scraped, and collected in 200 μl of buffer (50 mM Tris·HCl, pH 7.4, 50 mM NaCl, 0.5% NP-40) with protease and phosphatase inhibitors (Sigma). After incubation on ice for 30 min, samples were centrifuged for 20 min at 10,000 g. Proteins were separated on 12% SDS-PAGE and Western blot was done as described previously (22). The primary antibodies used were Cdk2 (M2) polyclonal antibody (Santa Cruz Biotechnology), caspase-3 polyclonal antibody (Cell Signaling, Danvers, MA), and p21 (F5) monoclonal antibody (Santa Cruz Biotechnology). For determination of Cdk2-bound p21, the gel used for kinase assay was used for Western transfer.
Isoelectric focusing and two-dimensional gel electrophoresis.
Kinase reactions of immunoprecipitated Cdk2 from 1 mg of postnuclear extract from control and cisplatin-treated cells were performed as described above using N6-benzyl-[γ-32P]ATP. An equal volume of 2% SDS, 0.4 M DTT was added and the mixture was heated to 90°C for 5 min. Agarose beads were removed by using a Wizard mini column (Promega) and eluted proteins were precipitated with five volumes of ice-cold acetone for 1 h at −20°C. Precipitated proteins were collected by centrifugation for 15 min at 13,000 rpm at 4°C. The pellet was air dried and dissolved in rehydration buffer {8 M urea, 1 M thiourea, 2% 3[(3-Cholamidopropyl) dimethylammonio]-propanesulfonic acid, 50 mM DTT, 0.01% bromophenol blue, and 0.2% of BioLyte 3–10 buffer (Bio-Rad)} for isoelectric focusing. Eleven-centimeters-long, 3–10 NL IPG ready strips (Bio-Rad) were incubated with 200 μl of the rehydrated samples overnight. Isoelectric focusing was performed in a Protean IEF Cell (Bio-Rad) following the manufacturer's directions. The strips were incubated in equilibration buffer (6 M urea, 2% SDS, 0.375 M Tris·HCl, pH 8.8, 20% glycerol) plus 2% DTT for 15 min followed by a 15-min incubation with equilibration buffer plus 2.5% Iodoacetamide. Proteins were then resolved on 8–10% Criterion SDS-precast gels (Bio-Rad). The gels were silver stained (32), dried, and subjected to autoradiography.
Recovery of Cdk2 substrates and LC-MS/MS analysis.
Cdk2-bound proteins were separated on one-dimensional SDS-PAGE and detected in gels by staining with Coomassie Brilliant Blue (56). Proteins were excised from the gel and in gel digested with trypsin. Briefly, protein-containing gel slices were destained in 50% methanol (Fisher), 100 mM ammonium bicarbonate (Sigma), followed by reduction in 10 mM Tris[2-carboxyethyl]phosphine (Pierce) and alkylation in 50 mM iodoacetamide (Sigma). Gel slices were dehydrated in acetonitrile, followed by addition of 100 ng porcine trypsin (Promega) in 100 mM NH4HCO3 (Sigma) and incubated at 37°C for 12–16 h. Peptide products were acidified in 0.1% formic acid (Fluka) and separated by reverse-phase HPLC on a 10-cm C18 column using a NanoLC 2D system (Eksigent) and analyzed by MS/MS using an LTQ XL mass spectrometer (Thermo). Proteins and modifications were identified from MS/MS spectra by database searching using the Mascot search engine (Matrix Science).
Light microscopy.
TKPTS cells were photographed using an inverted microscope (Nikon Eclipse TE200, Melville, NY) with Hoffman optics before harvesting (42).
Fluorescence-activated cell sorter analysis.
Cells were prepared as previously described (64) and the samples were analyzed using FACSCalibur (Becton Dickinson). Both floating and attached cells were combined for analyses. The cells were grouped into sub-G1/G0, G1/G0, S, and G2/M phases using a cell cycle analysis program (WinMDI 2.8). For each culture condition, >1 × 105 cells were analyzed and each experiment was repeated at least three times. Cells in sub-G1/G0 were considered apoptotic (8).
Statistical analysis.
Statistical significance between treated and nontreated cultures was done using Student's t-test with two-tailed distribution.
RESULTS
Substrates phosphorylated by Cdk2.
Previous data from our laboratory showed that inhibition of Cdk2 protected cells from cisplatin-induced cytotoxicity in vivo and in vitro (43). The main goal of the current studies was to understand the dependence of cisplatin cytotoxicity on Cdk2 and to identify Cdk2 substrates that could be related to cisplatin-induced cell death. Initially, we took advantage of observations that several substrates were shown to form stable complexes with cyclin-Cdks (10, 12, 13, 53, 60) that could be subsequently phosphorylated in vitro.
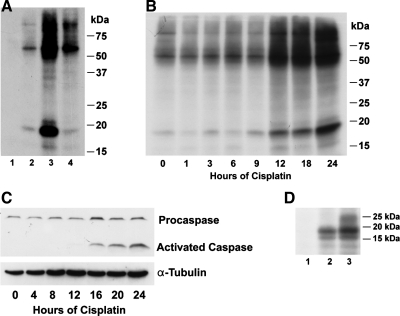
Proteins were immunoprecipitated from mouse kidney proximal tubule cells (TKPTS) before (Fig. 1A, lanes 1, 2) and after cisplatin exposure (Fig. 1A, lanes 3, 4) using either nonspecific IgG as a negative control (lane 1) or anti-Cdk2 (lanes 2–4). Proteins were also immunoprecipitated from cells treated with cisplatin and 3 h later with purvalanol, an inhibitor of Cdk2 (Fig. 1A, lane 4). After immunoprecipitation, bound proteins were phosphorylated by Cdk2 using [γ-32P]ATP. Proteins were inefficiently phosphorylated by immunoprecipitated kinases when the immunoprecipitation was performed with nonspecific IgG (Fig. 1A, lane 1). Before cisplatin exposure, several Cdk2-bound proteins were phosphorylated (Fig. 1A, lane 2) but the level of phosphorylation was greatly enhanced by cisplatin, especially the phosphorylation of an 18-kDa protein (Fig. 1A, lane 3). In the presence of the Cdk2 inhibitory drug purvalanol, the phosphorylation of these substrates was markedly decreased, even if purvalanol was added 3 h after cisplatin exposure (Fig. 1A, lane 4).
Fig. 1.
A: substrates phosphorylated by cyclin-dependent kinase 2 (Cdk2). TKPTS cells were either untreated (lanes 1, 2), exposed to 25 μM cisplatin (lanes 3, 4), or exposed to cisplatin and 3 h later treated with the Cdk2 inhibitory drug purvalanol (lane 4). Cdk2-bound proteins were immunoprecipitated with Cdk2 antibody (lanes 2–4). Immunoprecipitated proteins using nonspecific IgG were used as a negative control (lane 1). After immunoprecipitation, bound proteins were phosphorylated by Cdk2. B: time course of Cdk2 substrate phosphorylation. TKPTS cells were exposed to 25 μM cisplatin and anti-Cdk2-immunoprecipitated proteins were phosphorylated as described above. C: time course of caspase-3 activation after cisplatin exposure. Both full-length (procaspase-3) and cleaved (active) caspases-3 are indicated. α-Tubulin was used as a loading control. D: 18-kDa protein phosphorylation in mouse kidney after cisplatin injection. Immunoprecipitated Cdk2-bound proteins isolated from mouse kidney were phosphorylated using as-Cdk2+cyclin. Proteins were extracted from kidney of untreated mouse (lane 1) and kidney from mouse at 2 days (lane 2) and 3 days (lane 3) after cisplatin (25 mg/kg).
A time course for substrate phosphorylation showed that the increase in phosphorylation started 12 h after cisplatin exposure (Fig. 1B), preceding the activation of caspase 3, which started 16 h after cisplatin exposure (Fig. 1C).
To determine whether the phosphorylation of the 18-kDa protein also occurred in vivo after cisplatin administration, protein extracts from untreated (Fig. 1D, lane 1), 48-h cisplatin (lane 2), and 72-h cisplatin (lane 3)-treated mice were immunoprecipitated and phosphorylated as above using [γ-32P]ATP. At 0, 48, and 72 h after cisplatin administration, BUN values averaged 28.3 ± 1.7, 88.6 ± 4.1, and 149.8 ± 3.5 mg/dl, respectively, and creatinine levels averaged 0.31 ± 0.02, 1.33 ± 0.11, and 2.17 ± 0.19 mg/dl, respectively, indicating the presence of acute kidney injury. The results confirmed that phosphorylation of the 18-kDa protein occurred in vitro and in vivo after cisplatin exposure.
Identification of Cdk2-specific substrates by as-Cdk2.
Several proteins in a Cdk2 immunoprecipitate were phosphorylated by an endogenous kinase after cisplatin exposure (Fig. 1, A and B). To confirm that these proteins were authentic Cdk2 substrates, we adapted a method developed by Shah and Shokat (51). We generated a mutant Cdk2 by replacing a conserved phenylalanine residue within the ATP-binding pocket with a glycine to create as-Cdk2 as described previously (2, 22). This mutation should enlarge the ATP-binding pocket to enable the as-Cdk2, but not wild-type kinases, to use bulky ATP analogs to label Cdk2 substrates specifically.
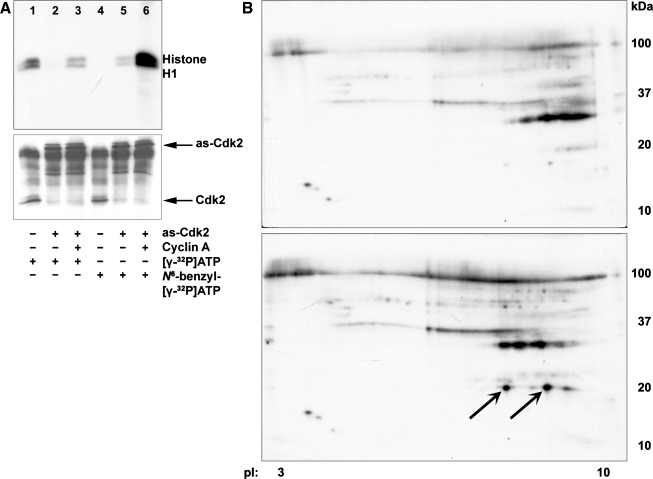
The activity of wild-type Cdk2 and as-Cdk2 was studied with histone H1 using [γ-32P]ATP (Fig. 2A, top, lanes 1–3) and N6-benzyl-[γ-32P]ATP (Fig. 2A, top, lanes 4–6). Wild-type Cdk2 (Fig. 2A, lanes 1, 4) or as-Cdk2 (Fig. 2A, lanes 2, 3, 5, 6) was immunoprecipitated from cells transduced either with as-Cdk2mCherry (Fig. 2A, lanes 2, 5) or with as-Cdk2mCherry plus cyclin A (Fig. 2A, lanes 3, 6). An immunoblot using Cdk2 antibody showed that similar amounts of Cdk2 were immunoprecipitated either with anti-Cdk2 (Fig. 2A, bottom, lanes 1, 4) or anti-DsRed (Fig. 2A, bottom, lanes 2, 3, 5, 6). Wild-type Cdk2 was able to use ATP (Fig. 2A, lane 1) but not analog-ATP (lane 4) as phosphate donor; as-Cdk2 without added cyclin could not use ATP (Fig. 2A, lane 2) and had low activity with analog-ATP (Fig. 2A, lane 5). The as-Cdk2 plus cyclin could use ATP (Fig. 2A, lane 3) and could best incorporate 32P into the substrate with analog N6-benzyl-[γ-32P]ATP (Fig. 2A, lane 6). Proteins were phosphorylated using as-Cdk2 plus cyclin and analog ATP to label proteins before (Fig. 2B, top) and after cisplatin exposure (Fig. 2B, bottom). Proteins were analyzed by two-dimensional (2D) electrophoresis, visualized by silver staining, and phosphorylated proteins were identified by autoradiography. The as-Cdk2 specifically labeled the 18-kDa protein after cisplatin exposure (indicated by arrows). Parallel 2D gels were transferred to nitrocellulose membranes and immunoblotted using p21 (F5) antibody (Santa Cruz Biotechnology). Proteins identified as p21 are labeled with arrows (Supplementary Fig. S1; the online version of this article contains supplemental data).
Fig. 2.
A: activities of Cdk2s using ATP analogs. The activity of wild-type Cdk2 and analog-sensitive Cdk2 (as-Cdk2) was determined by histone H1 kinase assay using [γ-32P]ATP (lanes 1–3) or N6-benzyl-[γ-32P]ATP (lanes 4–6). Cells were either untreated (lanes 1, 4), transduced with as-Cdk2mCherry (lanes 2, 5), or transduced with as-Cdk2mCherry plus cyclin A adenoviruses (lanes 3, 6). Wild-type Cdk2 was immunoprecipitated by Cdk2 antibody (lanes 1, 4). The as-Cdk2 was immunoprecipitated using Discosoma red fluorescent protein (DsRed; anti-mCherry) antibody (lanes 2, 3, 5, 6). An immunoblot using Cdk2 antibody showed that similar amounts of Cdk2 were immunoprecipitated either with anti-Cdk2 (bottom, lanes 1, 4) or anti-DsRed (bottom, lanes 2, 3, 5, 6). B: 2-dimensional (2D) gel electrophoresis. Cdk2-bound TKPTS cellular proteins were immunoprecipitated from untreated cells (top) or cells exposed to cisplatin for 24 h (bottom). Proteins were separated using 2D electrophoresis. Proteins were identified as p21 labeled with arrows.
Identification of the 18-kDa band as mouse p21 with phosphorylation at serine 78.
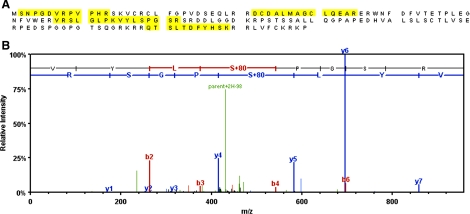
The major 18-kDa band protein induced after cisplatin exposure was excised from a Coomassie-stained gel and analyzed by mass spectrometry using an LTQ XL mass spectrometer. The analysis identified the 18-kDa band as mouse p21 with 100% certainty with a calculated molecular weight (MW) of 17,785.1 Da. The analysis identified five unique peptides and seven total spectra with 33% sequence coverage of the protein (Fig. 3A). Database searching of the MS/MS spectra of the peptides using the Mascot search engine (Matrix Science) indicated a phosphoserine at position 78 (Fig. 3B), which was not previously identified (5).
Fig. 3.
A: identification of 18-kDa protein as Cdk inhibitor, p21 (Waf1/Cip1). The 18-kDa protein identified by 2D PAGE and analyzed by mass spectrometry. Proteins and their modifications were identified from MS/MS spectra using the Mascot search engine (Matrix Science). Amino acid sequence of p21 showing 33% sequence coverage with 100% certainty. Peptides identified by MS/MS are highlighted. B: MS/MS spectrum of phosphopeptide 75-VYLSPGSR-82. Presence of a strong doubly charged neutral loss peak at 430.7 (parent+2H-98) indicates a phosphopeptide. Mass difference of 167 Da between b3-b4 and y4-y5 ions indicates a phosphoserine at position 78.
Phosphorylation of p21 at serine 78 significantly decreases its ability to protect from cisplatin toxicity.
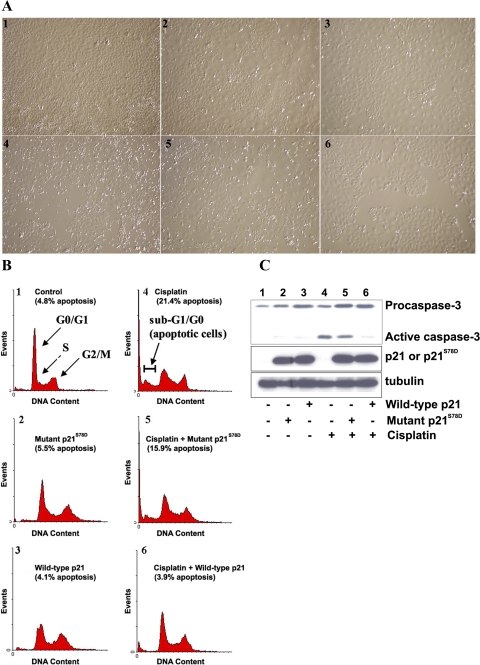
The phosphorylation of p21 at Ser78 occurs within the 310 helix, which by analogy with p27Kip1, a Cdk inhibitor in the same family as p21WAF1/Cip1, stearically blocks the ATP-binding site of the Cdk and prevents Cdk catalytic activity (41, 47). As described with p27, phosphorylation within the 310 helix could reduce the activity of p21 as a Cdk inhibitor (6, 16), which could diminish its role to protect from cisplatin cytotoxicity. To investigate the effects of this phosphorylation on p21, we generated a phosphomimic p21 at serine 78 by substituting aspartic acid (p21S78D). Mutant p21S78D lost its ability to protect cells from cisplatin-induced cell death (Fig. 4) as determined by light microscopy (Fig. 4A), FACS analysis of propidium iodide-stained cells (Fig. 4B), and Western blot analysis for caspase-3 activation (Fig. 4C).
Fig. 4.
A: apoptosis assay by light microscopy. TKPTS cells were untreated (panel 1) and exposed to 25 μM cisplatin for 24 h (panels 4–6). Cultures were transduced with adenovirus-expressing mutant p21S78D (panels 2, 5) and wild-type p21 (panels 3, 6) for 48 h. B: apoptosis assay by fluorescence-activated cell sorter (FACS) analysis. Cells were treated the same as described in A. Cells were stained with propidium iodide (PI) and analyzed using FACSCalibur. The cells were grouped into sub-G1/G0, G1/G0, S, and G2/M phases using a cell cycle analysis program (WinMDI 2.8). Cells in sub-G1/G0 were considered apoptotic. C: mutant p21S78D lost its ability to prevent caspase cleavage induced by cisplatin. Source of protein extracts as in A. Both full-length (procaspase) and cleaved (active) caspase-3 are indicated. Expression of wild-type and mutant p21 was detected by immunoblot using p21 (F5) antibody. α-Tubulin was used as a loading control.
Adenovirus transduction of TKPTS cells with wild-type p21 or mutant p21S78D had little effect on cell death (4.1 ± 0.6 and 5.5 ± 2.5% apoptosis, respectively, vs. 4.8 ± 1.4% apoptosis with untreated cells; Fig. 4, A and B, panels 1–3) or caspase-3 activation (Fig. 4C, lanes 1–3). Cisplatin exposure resulted in 21.4 ± 2.5% of cells being apoptotic (Fig. 4, A and B, panel 4) and showed a high level of caspase-3 cleavage (Fig. 4C, lane 4). Transduction of cells with wild-type p21 adenovirus protected TKPTS cells from cisplatin toxicity, which lowered apoptosis to background levels (3.9 ± 1.0%; Fig. 4, A and B, panel 6) and also prevented caspase-3 activation (Fig. 4C, lane 6). Cells transduced with mutant p21S78D adenovirus and exposed to cisplatin were not protected from cell death, which had 15.9 ± 0.8% apoptosis (Fig. 4, A and B, panel 5) and a high level of caspase-3 activation (Fig. 4C, lane 5). As noted previously, expression of p21 in these cells did not cause cell cycle arrest, but rather lengthened the doubling time of the cells (42). The expression level of either p21 or p21S78D from transducing adenovirus was approximately equal (Fig. 4C, middle). These results show that phosphorylation at serine 78 altered p21 function and severely lessened its protection against cisplatin-induced apoptosis.
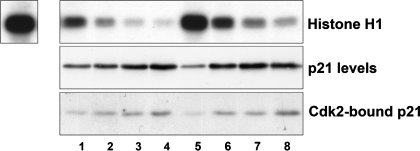
Phosphomimic p21S78D is a poor inhibitor of Cdk2.
The mutant p21 S78D was a much less potent inhibitor of Cdk2 than wild-type mouse p21 (Fig. 5). A titration using increasing amounts of p21 expression adenovirus for transduction showed that Cdk2 activity in wild-type p21-transduced cells (lanes 1–4, top) was inhibited to a greater extent than the Cdk2 activity in p21S78D-transduced cells (lanes 5–8, top). The wild-type and the transduced p21S78D proteins were expressed at similar levels (middle), and bound Cdk2 to the same extent (bottom).
Fig. 5.
Cdk2 activity in the presence of p21. Top: Cdk2 activity was measured using histone H1 kinase assay. Cells were either transduced with increasing amounts of wild-type p21 (lanes 1–4) or mutant p21S78D (lanes 5–8) adenoviruses. Expression of p21 protein by the adenoviruses (middle) and p21 bound in the Cdk2 immunoprecipitate used for kinase activity (bottom). Top, left: control Cdk2 activity in untreated cells.
DISCUSSION
Exposure to cisplatin is cytotoxic to most cultured cells in vitro and to tissues in vivo, especially cells in kidney proximal tubules. We demonstrated that inhibiting endogenous Cdk2 activity by inhibitory drugs, p21WAF1/Cip1 expression, or dominant-negative Cdk2 expression protected cells from cisplatin cytotoxicity in vivo and in vitro (43, 44, 65). The mechanism(s) of cisplatin cytotoxicity and its dependence on Cdk2 activity are not yet clear. We showed that p21 was induced to very high levels after acute kidney injury, caused either by cisplatin exposure, ischemia-reperfusion, or ureteral obstruction (33). Its induction by cisplatin or ischemia-reperfusion was found to protect from more severe acute kidney injury (34, 35). However, it was surprising that, although p21 was induced after cisplatin exposure so that its expression was one of the most abundant mRNAs in the kidney (48), this expression did not protect more completely from cisplatin nephrotoxicity.
We investigated Cdk2 substrates, especially those present after cisplatin exposure, to address these questions. Cdk-cyclins can form stable complexes with their substrates through cyclin-Cdk-binding motifs (10, 12, 13, 53, 60) and we determined whether substrates coimmunoprecipitating with Cdk2 were dependent on cisplatin exposure. We found that the phosphorylation of an 18-kDa protein by Cdk2 increased starting 12 h after the cells were exposed to cisplatin (Fig. 1, A and B) and several h before caspase-3 was activated (Fig. 1C). Similarly, this protein was also present and phosphorylated in kidney cells after cisplatin administration in vivo (Fig. 1D). We concluded that the increase of this protein and/or its phosphorylation by Cdk2 was a component in the cisplatin cytotoxicity pathway.
To confirm that this protein was a bona fide Cdk2 substrate, we used as-Cdk2 to specifically radiolabel Cdk2-bound proteins (Fig. 2). The as-Cdk2, in the presence of excess cyclin, was able to use both ATP and bulky ATP analogs (N6-benzyl-ATP) while wild-type Cdk2 only used ATP (Fig. 2A). Substrates specifically labeled by as-Cdk2 were separated by 2D gel electrophoresis and we confirmed that the 18-kDa protein was phosphorylated by as-Cdk2 only after cisplatin exposure (Fig. 2B, bottom).
Mass spectrometry identified the 18-kDa protein as mouse p21 (Fig. 3), a protein belonging to the Cip/Kip family of Cdk inhibitors, which includes p21WAF1/Cip1, p27Kip1, and p57Kip2 (reviewed in Ref. 44). It was initially shown to be a cell cycle regulator by inhibiting cyclin-dependent kinases (17) and binding PCNA (63). It was since demonstrated that p21WAF1/Cip1 can also be a component of active Cdk complexes and participate in their assembly (1, 15). In the presence of excess cyclin, Cdk2 phosphorylation of p21 at S130 was found to destabilize the p21 (67), but excess p21 could result in its stability through cyclin/Cdk binding. Since cisplatin exposure causes p21 excess, it was unlikely that p21 destabilization results in compromised protection from cytotoxicity. However, neutralization of p21 activity as a Cdk inhibitor could be the mechanism for the continued cytotoxicity of cisplatin in the presence of excess p21.
The MS/MS spectrum of the peptides indicated a novel phosphorylation of the protein at serine 78, which was not previously reported (5). The amino acid sequence of the region near Ser78 (S*PGSR) agrees closely with the consensus phosphorylation site for Cdk: S*/T*-P-X-K/R (55). This residue lies within the 310 helix, a region that binds Cdk, inhibiting the catalytic activity of the kinase by stearically blocking the ATP-binding site of the Cdk. The 310 helix region is conserved among proteins of the Cip/Kip family, occurring at residues 85–90 in p27, 73–78 in p21 (41, 47), and 77–82 in p57 (18). (Note: amino acid residue numbers are indicated according to the mouse proteins.) Phosphorylation of p27 within its 310 helix ejects the 310 helix from the catalytic cleft of Cdk2, allowing Cdk2 activity, but it did not disrupt p27 binding to cyclin A-Cdk2 (16). Previous studies on p27 showed that phosphorylation within its 310 helix modulated its inhibitory activity in vivo (28). Tyrosine phosphorylation within the 310 helix of p27 converted it to a Cdk4-bound noninhibitor (25), and tyrosine phosphorylation within this same region impaired the Cdk2 inhibitory action of p27 (6, 16).
To investigate the effect of serine 78 phosphorylation on p21 activity, we replaced serine 78 with aspartic acid, creating the phosphomimic p21S78D. Mutant p21S78D was inefficient at protecting TKPTS cells from cisplatin-induced cell death. Since we reported that cisplatin cytotoxicity is dependent on Cdk2 activity, it is reasonable to assume that the mechanism of this inefficient protection by phosphomimic p21 is by virtue of its inefficient inhibition of Cdk2 activity (Fig. 5). Curiously, the p57 protein, which is an efficient inhibitor of cyclin/Cdk activity (18), contains a phosphomimic amino acid, either glutamic acid or aspartic acid, within its 310 helix at a similar position to the S78D mutation in p21. This negatively charged amino acid does not seem to prevent cyclin/Cdk inhibition by the p57 protein. However, as we show (Fig. 5), the p21S78D mutation does not prevent cyclin/Cdk inhibition, but it reduces the function of p21 as an inhibitor compared with wild-type p21, and it results in its ineffective protection from cisplatin cytotoxicity (Fig. 4). The p21S78D modification, while decreasing the effect of p21 on Cdk2 activity, had little effect on p21-Cdk2 binding (Fig. 5, bottom), similar to that reported by Grimmler et al. (16) in which phosphorylation within the 310 helix of p27 affected Cdk inhibition and not p27 binding to cyclin A/Cdk2.
Previous studies investigated the phosphorylation of p21 and a number of phosphorylation sites have been identified (5). However, only a few of these phosphorylation sites were identified in vivo and it is not clear which kinase(s) were involved. Phosphorylation of p21 on T145 or S146 prevented p21 from interacting with PCNA and caused p21 translocation from the nucleus to the cytosol. Although studies showed that both T145 and S146 were phosphorylated by Akt in vitro, it was not shown if these sites were targeted by Akt in vivo. The effects of T145 phosphorylation on the activity of p21 as a Cdk inhibitor are also unclear. Rossig et al. (46) showed the phosphomimic mutant T145D impaired the interaction of p21 with Cdk2 and subsequently reduced its kinase inhibition properties, while a study by Li et al. (31) showed that phosphorylation of p21 at T145 had no effect on the interaction of p21 with Cdk2 or its inhibition of the kinase. It was shown that PKC can phosphorylate p21 at S146 and at S160 in vitro (49, 50), which decreased p21 interaction with PCNA. Phosphorylation of p21 at S153 was suggested to increase cytoplasmic localization of p21. Cdk2-cyclin complexes were found to phosphorylate p21 at T57 and S130 (21, 27). Phosphorylation of p21 at T57 promoted cyclin B-Cdc2 kinase activity, but it was not reported to affect Cdk2 activity (9). Phosphorylation at S130 was reported to either stabilize p21 (29) or promote its degradation (3, 67). This modification was also proposed to cause p21 inactivation that was not attributed to degradation, but rather caused by its loss of cyclin/Cdk2 association (26).
The p21 phosphorylation sites described above are conserved in both mice and humans. Although the 310 helix is conserved in Cip/Kip proteins (41, 47), the serine at position 78 in p21 is only found in rodents. The position of phosphorylation sites in proteins is determined by a combination of protein primary structure and protein kinase specificity, and many sites participate in cell signaling pathways (7). After analyzing phosphorylation sites of Cdk1 substrates in budding yeast Saccharomyces cerevisiae, Holt et al. (23) concluded that the localization of these sites shifts position in rapidly evolving disordered regions of proteins. Previous analyses showed that the majority of Cdk phosphorylation sites occur in disordered regions or in loops that can evolve rapidly (4, 58, 62). However, the conservation of the 310 helix among Cip/Kip family member proteins, and among different vertebrate species, makes this an unlikely explanation for sequence variability in this region of the protein. Tan et al. (57) found that tyrosine residues, potential sites of tyrosine kinase phosphorylation, are selectively lost through evolution. Hunter (24) proposed that throughout evolution, there is selective pressure to maintain beneficial regulatory phosphorylation sites and remove deleterious ones. We found that in rodents, induction of the p21 cell cycle inhibitor coinciding with activation of Cdk2-dependent cell death pathways could result in Cdk2 phosphorylation of p21, and inactivation of p21 as an effective Cdk2 and cell cycle inhibitor. The evolutionary significance of the finding of this phosphorylated serine within the 310 helix of rodents and not in higher mammals is that control of Cdk2, the cell cycle, and cell fate by p21 in higher mammals will be preserved by its continued activity as a Cdk inhibitor. Our results suggest that phosphorylation of p21 at Ser 78 changed the function of the p21 so that it no longer was an efficient Cdk2 inhibitor (Fig. 4). In this manner, Cdk2 acted as a feedback inhibitor of the CDKI, effectively controlling its own inhibition. We propose that loss of this phosphorylation site at Ser 78 through evolution removes a potentially deleterious residue without affecting the beneficial function of p21 as a CDKI.
GRANTS
This work was supported in part by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-54471) and a VA Merit Review and with resources and the use of facilities at the John L. McClellan Memorial Veterans' Hospital (Little Rock, AR).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Gerald Denis (Boston Medical School, Boston, MA) for providing cyclin A adenovirus, and Dr. Bert Vogelstein (John Hopkins, Baltimore, MD) for providing the mouse wild-type p21 cDNA plasmid. N6-benzyl-ADP was a gift from Dr. David O. Morgan (University of California, San Francisco, CA). We also appreciate the gift of human wild-type Cdk2 cDNA plasmid obtained from Dr. S. van den Heuvel (Massachusetts General Hospital). We also thank Dr. Kottayil Varughese for fruitful discussions of the structures of the 310 regions of the Cdk inhibitors.
REFERENCES
- 1.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J 18: 1223–1234, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407: 395–401, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278: 25752–25757, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Malden, MA: Blackwell, 2005. [Google Scholar]
- 5.Child ES, Mann DJ. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle 5: 1313–1319, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, Sun P, Tan CK, Hengst L, Slingerland J. p27 Phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 26: 281–294, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins MO. Evolving cell signals. Science 325: 1635–1636, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Darzynkiewicz Z, Li X, Gong J. Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol 41: 15–38, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Dash BC, El-Deiry WS. Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol Cell Biol 25: 3364–3387, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, Weinberg RA. Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73: 499–511, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Ewen ME, Faha B, Harlow E, Livingston DM. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science 255: 85–87, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Faha B, Ewen ME, Tsai LH, Livingston DM, Harlow E. Interaction between human cyclin A and adenovirus E1A-associated p107 protein. Science 255: 87–90, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Giaccone G. Clinical perspectives on platinum resistance. Drugs 59: 9–17, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Gorospe M, Cirielli C, Wang X, Seth P, Capogrossi MC, Holbrook NJ. p21Waf1/Cip1 protects against p53-mediated apoptosis of human melanoma cells. Oncogene 14: 929–935, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jäkel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 26: 269–280, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75: 805–816, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Kohri K, Kaneko Y, Morisaki H, Kato T, Ikeda K, Nakanishi M. Critical role for the 310 helix region of p57Kip2 in cyclin-dependent kinase 2 inhibition and growth suppression. J Biol Chem 273: 16544–16550, 1998 [DOI] [PubMed] [Google Scholar]
- 19.He TC, Zhou S, Da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengst L, Gopfert U, Lashuel HA, Reed SI. Complete inhibition of Cdk/cyclin by one molecule of p21Cip1. Genes Dev 12: 3882–3888, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoang AT, Cohen KJ, Barrett JF, Bergstrom DA, Dang CV. Participation of cyclin A in Myc-induced apoptosis. Proc Natl Acad Sci USA 91: 6875–6879, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodeify R, Megyesi J, Tarcsafalvi A, Safirstein RL, Price PM. Protection of cisplatin cytotoxicity by an inactive cyclin-dependent kinase. Am J Physiol Renal Physiol 299: F112–F120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt LJ, Tuch BB, Villén J, Johnson AD, Gygi SP, Morgan DO. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325: 1682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter T. Tyrosine phosphorylation: thirty years and counting. Curr Opin Cell Biol 21: 140–146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol 28: 498–510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Järviluoma A, Child ES, Sarek G, Sirimongkolkasem P, Peters G, Ojala PM, Mann DJ. Phosphorylation of the cyclin-dependent kinase inhibitor p21Cip1 on serine 130 is essential for viral cyclin-mediated bypass of a p21Cip1-imposed G1 arrest. Mol Cell Biol 26: 2430–2440, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaumot M, Estanol JM, Casanovas O, Grana X, Agell N, Bachs O. The cell cycle inhibitor p21CIP is phosphorylated by cyclin A-CDK2 complexes. Biochem Biophys Res Commun 241: 434–438, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Kaldis P. Another piece of the p27Kip1 puzzle. Cell 26: 241–244, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21Cip1 by phosphorylation. J Biol Chem 277: 29792–29802, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kumar NV, Eblen ST, Weber MJ. Identifying specific kinase substrates through engineered kinases and ATP analogs. Methods 32: 389–397, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277: 11352–11361, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Lin JF, Chen QX, Tian HY, Gao X, Yu ML, Xu GJ, Zhao FK. Stain efficiency and MALDI-TOF MS compatibility of seven visible staining procedures. Anal Bioanal Chem 390: 1765–1773, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Megyesi J, Udvarhelyi N, Safirstein RL, Price PM. The p53-independent activation of transcription of p21WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1211–F1216, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest 101: 777–782, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int 60: 2164–2172, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Meikrantz W, Gisselbrecht S, Tam SW, Schlegel R. Activation of cyclin A-dependent protein kinase during apoptosis. Proc Natl Acad Sci USA 91: 3754–3758, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13: 261–291, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Morgan DO. Principles of CDK regulation. Nature 9: 131–134, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Park DS, Levine B, Ferrari G, Greene LA. Cyclin dependent kinase inhibitors and dominant negative cyclin dependent kinase 4 and 6 promote survival of NGF-deprived sympathetic neurons. J Neurosci 17: 8975–8983, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 287: 821–828, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol 286: F378–F384, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, Megyesi J. Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol 17: 2434–2442, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenblatt J, Gu Y, Morgan DO. Human cyclin-dependent kinase 2 is activated during the S and G2 phases of the cell cycle and associates with cyclin A. Proc Natl Acad Sci USA 89: 2824–2828, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol 21: 5644–5657, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russo AA, Jeffrey PD, Patten AK, Massagué J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382: 325–331, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Safirstein R, Portilla D, Luxon B, Gorenstein D, Megyesi J, Price P. Functional genomics in cisplatin nephrotoxicity. J Am Soc Nephrol 13: 327A, 2002 [Google Scholar]
- 49.Scott MT, Ingram A, Ball KL. PDK1-dependent activation of atypical PKC leads to degradation of the p21 tumor modifier protein. EMBO J 21: 6771–6780, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott MT, Morrice N, Ball KL. Reversible phosphorylation at the C-terminal regulatory domain of p21Waf1/Cip1 modulates proliferating cell nuclear antigen binding. J Biol Chem 275: 11529–11537, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Shah K, Shokat KM. A chemical genetic approach for the identification of direct substrates of protein kinases. Methods Mol Biol 233: 253–271, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev 11: 1464–1478, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Shi L, Chen G, He D, Bosc DG, Litchfield DW, Greenberg AH. Granzyme B induces apoptosis and cyclin A-associated cyclin-dependent kinase activity in all stages of the cell cycle. J Immunol 157: 2381–2385, 1996 [PubMed] [Google Scholar]
- 55.Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol 4: 973–982, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Tal M, Silberstein A, Nusser EJ. Why does coomassie brilliant blue r interact differently with different proteins? A partial answer. J Biol Chem 260: 9976–9980, 1985 [PubMed] [Google Scholar]
- 57.Tan CS, Pasculescu A, Lim WA, Pawson T, Bader GD, Linding R. Positive selection of tyrosine loss in metazoan evolution. Science 325: 1686–1688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuch BB, Li H, Johnson AD. Evolution of eukaryotic transcription circuits. Science 319: 1797–1799, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262: 2050–2054, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J 16: 5334–5344, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, Walsh K. Resistance to apoptosis conferred by cdk inhibitors during myocyte differentiation. Science 273: 359–361, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wray GA, Hahn MW, Abouheif E, Balhoff JP, Pizer M, Rockman MV, Romano LA. The evolution of transcriptional regulation in eukaryotes. Mol Biol Evol 20: 1377–1419, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71: 505–514, 1992 [DOI] [PubMed] [Google Scholar]
- 64.Yu F, Megyesi J, Safirstein RL, Price PM. Involvement of the CDK2–E2F1 pathway in cisplatin cytotoxicity in vitro and in vivo. Am J Physiol Renal Physiol 293: F52–F59, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Yu F, Megyesi J, Safirstein RL, Price PM. Identification of the functional domain of p21WAF1/CIP1 that protects cells from cisplatin cytotoxicity. Am J Physiol Renal Physiol 289: F514–F520, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Zhou BB, Li H, Yuan J, Kirschner MW. Caspase-dependent activation of cyclin-dependent kinases during Fas-induced apoptosis in Jurkat cells. Proc Natl Acad Sci USA 95: 6785–6790, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu H, Nie L, Maki CG. Cdk2-dependent inhibition of p21 stability via a C-terminal cyclin-binding motif. J Biol Chem 280: 29282–29288, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.