Abstract
The endoplasmic reticulum (ER) folds and modifies proteins; however, during conditions of cellular stress, unfolded proteins accumulate in the ER and activate the unfolded protein response (UPR). The UPR, also referred to as the ER stress response, activates three distinct signaling cascades that are designed to globally reduce transcription and translation. The three major arms of the mammalian UPR include 1) protein kinase RNA (PKR)-like ER kinase (PERK), 2) inositol-requiring protein-1 (IRE1α), and 3) activating transcription factor-6 (ATF6) pathways. The PERK pathway rapidly attenuates protein translation, whereas the ATF6 and IRE1α cascades transcriptionally upregulate ER chaperone genes that promote proper folding and ER-associated degradation (ERAD) of proteins. This integrated response in turn allows the folding machinery of the ER to catch up with the backlog of unfolded proteins. The ER stress response plays a role in a number of pathophysiological processes, including pancreatic β-cell failure and apoptosis. The goals of the current review are to familiarize investigators with cellular and tissue activation of this response in the rodent and human diabetic kidney. Additionally, we will review therapeutic modulators of the ER stress response and discuss their efficacy in models of diabetic kidney disease. The ER stress response has both protective and deleterious features. A better understanding of the molecular pathways regulated during this process in a cell- and disease-specific manner could reveal novel therapeutic strategies in chronic renal diseases, including diabetic kidney disease.
Keywords: unfolded protein response
Overview of the Endoplasmic Reticulum Stress Response
the normal functional role of the endoplasmic reticulum (ER) is to fold, modify, and degrade secretory and transmembrane proteins. Pathophysiological stress conditions, including nutrient deprivation, nutrient excess, altered protein glycosylation, reducing agents, and changes in ER calcium content and oxidative stress, are associated with interference of normal protein folding. Accumulation of misfolded and unfolded proteins induces their aggregation and subsequent cellular toxicity (96). Accordingly, cells have evolved intricate signaling networks to respond to the accumulation of unfolded proteins and to regulate ER membrane structure and secretory protein processing capacity (15, 88). In 1988, investigators first described the unfolded protein response (UPR) that was activated when misfolded proteins accumulated in the ER. These investigators observed that the UPR was associated with increased synthesis of ER chaperone proteins, including glucose-regulated protein 78 (GRP78/BiP) (48). Although this pathway was first described in mammalian cells, elucidation of the mechanisms of the mammalian UPR was pioneered using the power of yeast genetics (71, 89). The UPR is a normal homeostatic process; however, when the cell is unable to deal with the increased unfolded protein load, then it is considered as the ER stress response (34, 88, 117).
Mammalian UPR
In mammalian cells, there are three major arms of the UPR: 1) protein kinase RNA (PKR)-like ER kinase (PERK), 2) inositol-requiring protein-1 (IRE1α), and 3) activating transcription factor-6 (ATF6) pathways. The PERK pathway rapidly attenuates protein translation, whereas the ATF6 and the IRE1α cascades transcriptionally upregulate ER chaperone genes that promote proper folding and ER-associated degradation (ERAD) of proteins, allowing the folding machinery of the ER to catch up with the backlog of unfolded proteins. These pathways are designed to relieve the accumulation of misfolded ER proteins; however, when these pathways are overwhelmed by sustained ER stress, the UPR initiates proapoptotic pathways (45, 62, 69, 98, 108).
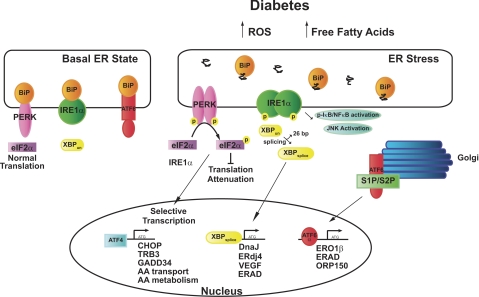
GRP78/BiP (BiP), an ER chaperone protein, is a central regulator of ER homeostasis and is involved in activation of the ER stress response. In resting, unstressed cells, BiP binds to the ER luminal domains of the ER stress sensors IRE1α, PERK, and ATF-6 (6, 94) and maintains them in an inactivated state. During ER stress, BiP preferentially binds to unfolded and misfolded proteins and dissociates from the transmembrane sensors, facilitating their activation (Fig. 1). After BiP dissociation, it is not clear whether full activation of the ER stress response requires subsequent binding of unfolded proteins to the luminal domains of IRE1α, PERK, and ATF-6 (reviewed in Refs. 84 and 88).
Fig. 1.
Endoplasmic reticulum (ER) stress response in diabetes and basal conditions. In unstressed conditions, BiP/glucose-regulated protein 78 (BiP/GRP78) binds to protein kinase RNA (PKR)-like ER kinase (PERK), inositol-requiring protein-1 (IRE1α), and activating transcription factor-6 (ATF6). Reactive oxygen species (ROS), free fatty acids, and likely other mediators associated with the diabetic milieu increase the load of unfolded proteins and oligomerization of the mammalian ER sensors PERK, IRE1α, and ATF6. PERK phosphorylation induces phosphorylation of eIF2α and attenuation of translation. However, ATF4 is selectively translated and it functions to transcribe ER stress-associated molecules, including genes involved in amino acid (AA) transport and metabolism. Activation/phosphorylation of IRE1α results in splicing of X box binding protein-1 (XBP-1) and transcription of another subset of ER chaperones and ER-associated degradation (ERAD) proteins. ATF6 is cleaved by site 1 protease and site 2 protease (S1P/S2P) in the Golgi. ATF6α then translocates to the nucleus to activate transcription of another subset of ER stress-related genes. See the text for additional definitions.
PERK is a transmembrane protein with an ER luminal stress-sensing domain that binds BiP and a cytosolic kinase domain (6). When ER stress is sensed, PERK multimerizes and phosphorylates eukaryotic translation initiation factor-α (eIF2α) (31). Phosphorylation of eIF2α inhibits general protein translation. However, a subset of genes that includes ATF4 (31) and nephrin (17) is preferentially translated by phosphorylated eIF2α. ATF4 in turn drives the transcription of specific UPR target genes, which include C/EBP homologous protein (CHOP, C/EBPζ, DDIT, GADD153) (24, 29, 57), GADD34 (58), TRB3 (73), osteocalcin, bone sialoprotein (90), receptor activator of NF-κB ligand (RANKL), E-selectin, VEGF (reviewed in Ref. 2), and genes important in amino acid metabolism (32). Recent work demonstrates that ATF4 augments expression of regulated in development and DNA damage responses 1 (Redd1) and Redd1 in turn inhibits mammalian target of rapamycin (mTOR) (40). Phosphorylated eIF2α (p- eIF2α) may also activate NF-κB; however, the precise mechanisms have not been clarified (20, 39). Other stress-associated kinase signaling pathways converge downstream of p-eIF2α, and thus p-eIF2α functions to induce an “integrated stress response” (32, 88).
CHOP, a transcription factor that can heterodimerize with other members of the C/EBP/ATF family, has traditionally been considered an inducer of apoptosis (77, 121). However, it does not promote apoptosis in all cell types (66), and CHOP's biological effects may be more dependent on CHOP's heterodimeric partner (26). CHOP can activate ERO1α, an ER oxidase, GADD34 (60), TRB3 (73, 92), and the proapoptotic factors Bim (85) and DR5 (109). CHOP also decreases expression of the prosurvival factor Bcl-2 (63). Additionally, Ron and colleagues (104) identified downstream of CHOP (DOC) genes (104). DOC1 is a stress-inducible form of carbonic anhydrase VI, DOC4 is a homolog of Drosophila Tenm/Odz, and DOC6 is a homolog of the actin-binding proteins villin and gelsolin.
The IRE1α/X box binding protein-1 (XBP-1) pathway is the most evolutionarily conserved of the ER stress pathways (64). IRE1α is a membrane-bound serine/threonine kinase with endonuclease activity (16, 65). When BiP dissociates from IRE1α (74), IRE1α is activated to splice a 26-bp intron from XBP-1. This unconventional splicing event induces a translational frame shift that generates a 371-amino acid highly active transcription factor, compared with the poorly active 267-amino acid unspliced form (13, 112). XBP-1 induces the transcription of a number of UPR-associated genes such as PERK and ATF4. XBP-1 also drives transcriptional networks involved in ER maintenance, expansion, and ERAD, in addition to genes involved in DNA repair and redox homeostasis (1, 50). Recent work suggests that unspliced XBP-1 may negatively regulate the UPR by binding and excluding spliced XBP from the nucleus (113). IRE1α also activates apoptosis signal-regulating kinase (ASK1), MAPK (JNK), and NF-κB (reviewed in Ref. 34), which are involved in apoptotic, autophagy, and inflammatory pathways (5, 44, 72, 101, 111).
ATF6 is another ER stress sensor that is bound as an inactive precursor in the ER membrane. During ER stress, ATF6 is transported to the Golgi and cleaved by site 1 protease (S1P) and site 2 protease (S2P). Cleaving of ATF6 releases its cytoplasmic bZIP domain, which can translocate to the nucleus and activate the transcription of target genes (33, 52). Interestingly, the Golgi-localized proteases S1P and S2P also catalyze the proteolytic activation of a group of transcription factors, the sterol-regulatory binding proteins (SREBPs), linking the ER stress response with lipid and cholesterol synthesis (82, 110). However, it remains unclear whether the UPR and SREBP pathways function in an antagonistic or synergistic manner (88). ATF6 is also related to OASIS, CREBH, and CREB4, which have tissue-specific effects. For example, in the liver CREBH enhances expression of acute phase reactants (118).
ER Stress in Diabetes
The ER stress response plays a role in a number of pathophysiological processes, including pancreatic β cell apoptosis and failure (97). In a seminal paper, Ozcan and colleagues (80) demonstrated that in the liver, obesity (in high-fat diet-induced and ob/ob mice) activates the ER stress response. In turn, ER stress suppresses insulin receptor signaling through activation of JNK and serine phosphorylation of insulin receptor substrate-1 (IRS-1) (80, 81), linking obesity to insulin resistance. Mice with constitutive mutations in ER stress proteins develop diabetes (30, 91) and ER stress induces β cell failure (41, 43, 49, 102). Additionally, mice with a heterozygous constitutive knockin mutation of a mutant BiP have evidence of ER stress in the kidney, associated with age-related renal tubular atrophy, interstitial fibrosis, and glomerulosclerosis (46). Akita mice develop early and sustained hyperglycemia, renal hypertrophy, albuminuria, and mesangial matrix expansion and are a favored model of type 1 diabetic kidney disease (reviewed in Ref. 11). Genotypically, heterozygous Akita mice (Ins2+/C96y) have a missense mutation in the insulin gene, which interferes with protein folding and causes accumulation of misfolded insulin, activation of the ER stress response, and subsequent pancreatic β cell failure (76, 103, 114). Diabetes and obesity induce ER stress in many organs such as the pancreas, liver (59, 80), and heart, (9, 21, 51) and the nervous system, (61, 79), adipose tissue (8, 93, 100), and kidney. Precipitants of ER stress that induce accumulation of unfolded ER proteins include altered nutrient availability, free fatty acids, cytokines (TNF-α), perturbations in calcium transits, oxidative stress, and hypoxia.
The UPR in Diabetic Kidney Disease
A cell's dependence on the UPR is often a consequence of its function. Secretory cells such as plasma cells, hepatocytes, β cells, adipocytes, macrophages, and oligodendrocytes are particularly sensitive to perturbations in ER function. Additionally, aberrant metabolic conditions such as hyperlipidemia, hyperglycemia, excess cytokines, and reactive oxygen species (ROS) can differentially affect ER trafficking depending on the cell type (88). Therefore, it is important to view the ER stress response in a cell- and tissue context- specific manner.
In podocytes, tunicamycin (TM), a glycosylation inhibitor, A23187 (a calcium ionophore), S-nitroso-N-acetyl-dl-penicillamine (SNAP), complement activation, ROS, palmitate, and thapsigargin induce the ER stress response (18, 37, 66, 95). However, in these cells, neither hypoxia nor high-glucose conditions induce the ER stress response (37, 66). CHOP is a classic marker of ER stress, and Pavenstadt's group (4) was the first to show in podocytes that ROS induce CHOP expression. In their study, podocytes that retrovirally overexpressed CHOP had reduced expression of β1- and α3-integrin; unexpectedly, CHOP overexpression was associated with enhanced cell adhesion to collagen IV-coated plates. Interestingly, in podocytes, indomethacin, but not other cyclooxygenase inhibitors, induces ER stress, and this suppresses TNF-α-induced activation of NF-κB (75).
A number of groups, including ours, have documented activation of the ER stress response in the kidney (66). Lui and colleagues (54) were the first to evaluate the ER stress response in a mammalian model of diabetes. In streptozotocin (STZ)-treated rats (65 mg/kg STZ ip once), they demonstrated increased expression of BiP in glomerular and tubular cells and enhanced kidney cell apoptosis, CHOP, JNK, and caspase-12 expression (54). Additionally, BiP expression is dysregulated by high glucose (HG) in human mesangial cells (HMC) (87). Recently, the ER stress response was studied in 9- and 22-mo-old STZ-treated mice (50 μg/g STZ for 5–8 injections). In this study, expression of BiP, CHOP, p-PERK, and p-eIF2α was increased in the diabetic mice, predominately at 22 mo compared with the 9-mo-old diabetic mice and controls. Diabetic CHOP knockout mice seemed to be protected as they had less proteinuria compared with the wild-type controls (106).
TM potently and quickly activates the ER stress response. A single dose of TM (1 μg/g body wt) induces apoptosis of the juxtamedullary renal tubular cells and acute tubular necrosis (ATN) (121). However, mice with a deletion in the C terminal of GADD34 (an eIF2α phosphatase) and CHOP-deficient mice are protected from TM-induced ATN (60, 121). Recent work has shown that disruption of TNF receptor 1 signaling reduces phosphorylation of eIF2α and increases acute TM-induced kidney injury (35). In humans and rats with nephrotic syndrome, ER stress markers are increased in tubular epithelial cells (37, 107). Moreover, microarray studies of biopsies obtained from patients with diabetes demonstrate higher expression of BiP (HSPA5), oxygen-regulated protein 150 (ORP150/HYOU1), S1P (MBTPS1), calnexin, and XBP-1 in patients with established diabetes compared with mild diabetes. These results suggest that there is activation of the ER stress response in the tubulointerstitium of patients with established diabetic kidney disease (53).
Modulators of ER Stress
Over the last decade, there has been considerable interest in discovering compounds that modulate the ER stress response (Table 1). Chemical chaperones that improve ER folding capacity such as 4-phenylbutyric acid (PBA), taurine-conjugated ursodeoxycholic acid (TUDCA), and the ER chaperone ORP150, have been used successfully to reduce ER stress and restore glucose tolerance and improve insulin action and sensitivity (70, 78, 81). Similarly, overexpression of ORP150 improves survival of the thick ascending limb cells in a murine model of ischemia-reperfusion (3). Salubrinal prevents the dephosphorylation of eIF2α and protects rat pheochromocytoma cells and proximal tubular cells from ER stress-mediated apoptosis (10, 42). Incretins, such as exenedin-4 (an agonist of glucagon-like peptide 1 receptor), induce ATF4 and Gadd34 (60) expression, thereby enhancing insulin synthesis and secretion (115).
Table 1.
Modulators of ER stress
| ER Stress Modulator | Mechanism | Cell Type | Disease | Outcome | Reference |
|---|---|---|---|---|---|
| 4 Phenyl-butyric acid (4-PBA) | ER chaperone | STZ-induced diabetes | Attenuates proteinuria, oxidative stress markers p-JNK, MCP-1, and TGF-β1 | 56 | |
| 86 | |||||
| 4 Phenyl-butyric acid (4-PBA) | ER chaperone | 293FT cells | Improves trafficking of mutated nephrin | 55 | |
| Oxygen-regulated protein-150 (ORP150) | ER chaperone | MDCK cells | ORP150 overexpression in rodent ischemia-reperfusion model | Improved viability of TAL and renal function | 3 |
| Salubrinal | Increased phosphorylation eIF2α | Proximal tubular | Decreased palmitic acid-induced apoptosis | 42 | |
| Exenedin-4 | Increased ATF4, sXBP-1, GADD34 | Insulinoma cells | Reduced ER stress-associated pancreatic β cell death | 115 | |
| Exenedin-4 | Decreased CHOP, sXBP-1 | Pancreatic β cells | db/db Mice β cells | Improved glycemic control | 115 |
| Imatinib | Reduced ER stress markers, activation of NF-κB | Liver cells, pancreatic β cells | db/db Mice, STZ-induced and nonobese diabetic mice | Induced remission of diabetes, suppressed islet cell death | 27 |
| 25 | |||||
| Subtilase cytotoxin (SubAB) | Cleaved BiP/GRP78 | Macrophages | LPS-induced sepsis, arthritis | Lower MCP-1, TNF-α, improved sepsis, arthritis | 28 |
| BiP/GRP78 | Induction of TH2 cytokines including IL-4 | T Lymphocytes | Collagen-induced arthritis | Suppressed arthritis | 12 |
| Preconditioning with low doses of TM and adriamycin | Preconditioning | Glomerular epithelial cells | Heymann's nephritis | Reduced proteinuria | 18 |
| Subnephritogenic TM, TG | Preconditioning increased BIP/GRP78 and ORP150 | Mesangioproliferative GN | Reduced proteinuria and histological manifestations | 36 | |
| Low-dose LPS | Suppressed CHOP expression | Proximal tubular cells | TM-induced ATN | Improved proteinuria and creatinine | 105 |
| Unsaturated fatty acids | Attenuated palmitic acid-induced upregulation of CHOP | Podocytes | Reduced palmitic acid-induced apoptosis | 95 |
ER, endoplasmic reticulum; TGF, transforming growth factor; TAL, thick ascending limb; GN, glomerulonephritis; TM, tunicamycin; TG, thapsigargin; ATN, acute tubular necrosis; STZ, streptozotocin; MDCK, Madin-Darby canine kidney.
Another modulator of the ER stress response is the subtilase cytotoxin (SubAB) produced by Shiga toxigenic Escherichia coli. SubAB cleaves BiP/GRP78, which activates the ER stress response (83). Treatment of macrophages with SubAB inhibits LPS-induced MCP-1 and TNF-α expression and protects mice from LPS-induced sepsis and the development of experimental arthritis (28). Administration of BiP may also reduce collagen-induced arthritis and is being considered a potential therapeutic agent in patients with rheumatoid arthritis (12). Reducing agents also protect cells from ER stress (32). Additionally in db/db mice and high fat-fed rats, imatinib, a tyrosine kinase inhibitor, improves insulin resistance by modulating the ER stress response (25, 27). 4-Phenyl butyric acid (PBA) is an aromatic short chain fatty acid that has chaperone-like activities and is approved for clinical use as an ammonia scavenger in children with urea cycle disorders. Recent work in STZ-treated rats demonstrates that PBA attenuates manifestations of diabetic nephropathy including markers of renal oxidative stress such as NADPH oxidase activity (56).
Various missense mutations of the nephrin gene (NPHS1) can cause defects in intracellular transport, retention of the mutant proteins in the ER, and subsequent nephrotic syndrome. Studies in 293FT cells suggest that PBA may rescue the trafficking of mutated nephrin (55). In STZ-treated rats, ER stress marker expression was reduced in PBA-treated rats, and this was associated with improved renal hypertrophy, hyperglycemia, urinary protein excretion, mesangial matrix expansion, and reduced expression of p-JNK, MCP-1, type I collagen, and TGF-β1. However, these changes were not associated with improvements in serum creatinine (86). Also, preconditioning with subnephritogenic doses of TM, thapsigargin, adriamycin, and low-dose LPS can attenuate ER stress-induced signaling in mesangioproliferative, Heymann's nephritis, and ATN (18, 36, 105). Finally, saturated free fatty acids induce ER stress, and investigators have suggested that increasing the unsaturated free fatty acids in the diet could attenuate ER stress and the manifestations of diabetic kidney disease (95).
Wolcott-Rallison disease is caused by autosomal recessive mutations in PERK (19). In this inherited defect of the UPR, children develop skeletal abnormalities, infantile diabetes, and interestingly there are reports of renal insufficiency (7, 38), including proteinuria. The proteinuria could indicate podocyte or tubular epithelial cell dysfunction, although prerenal azotemia also plays a role in renal disease.
Conclusions
In conclusion, animal models and human mutations affecting the UPR highlight the importance of ER stress in regulating efficient secretory cell function (30, 119). Diabetic nephropathy is associated with accelerated matrix deposition and release of inflammatory molecules secreted by podocytes and mesangial cells, suggesting that these cells are likely to be affected by activation and perturbations in function of the UPR.
ER stress affects multiple organs and can inhibit insulin and leptin receptor signaling (80, 120) and induce inflammation (116, 117). However, certain pathways including induction of TRB3 by CHOP and ATF4 (117) may reduce inflammation by blocking MAPK signaling (47) and expression of inflammatory mediators such as MCP-1 (66, 75). Furthermore, there is evidence of cross talk between energy-sensing pathways such as AMPK and ER stress (14, 22, 23, 61, 99). Manipulation of these signaling networks could potentially improve organ function.
Many questions remain, regarding which renal cells are most affected by ER stress and whether all cells are affected similarly. Also, does activation of one pathway constitute the ER stress response, or must all three arms of the UPR be activated to be considered a true ER stress response (59)? It has also not been clarified whether specific inducers of the UPR, such as ROS or free fatty acids, activate all three arms of the UPR, nor has the time dependence or integration of the pathways been clarified. Also, it is possible there are novel ER stress inducers associated with the renal diabetic milieu. However, it is clear that the ER stress response has both protective and deleterious features. A better understanding of the molecules regulated during this process in a cell- and disease-specific manner could reveal novel therapeutic strategies in chronic diseases, including diabetic kidney disease.
GRANTS
These studies were performed with the support of a Department of Veterans Affairs Career Development Transition Award and Merit Award to R. Cunard, a VA Merit Award and National Institute of Diabetes and Digestive and Kidney Diseases Grant U01DK076133 to K. Sharma, and a University of California, San Diego Senate Grant awarded to R. Cunard.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol 40: 14–21, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bando Y, Tsukamoto Y, Katayama T, Ozawa K, Kitao Y, Hori O, Stern DM, Yamauchi A, Ogawa S. ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J 18: 1401–1403, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bek MF, Bayer M, Muller B, Greiber S, Lang D, Schwab A, August C, Springer E, Rohrbach R, Huber TB, Benzing T, Pavenstadt H. Expression and function of C/EBP homology protein (GADD153) in podocytes. Am J Pathol 168: 20–32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4: e423, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bin-Abbas B, Al-Mulhim A, Al-Ashwal A. Wolcott-Rallison syndrome in two siblings with isolated central hypothyroidism. Am J Med Genet 111: 187–190, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57: 2438–2444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borradaile NM, Buhman KK, Listenberger LL, Magee CJ, Morimoto ET, Ory DS, Schaffer JE. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol Biol Cell 17: 770–778, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Breyer MD, Bottinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Brownlie RJ, Myers LK, Wooley PH, Corrigall VM, Bodman-Smith MD, Panayi GS, Thompson SJ. Treatment of murine collagen-induced arthritis by the stress protein BiP via interleukin-4-producing regulatory T cells: a novel function for an ancient protein. Arthritis Rheum 54: 854–863, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Choudhury M, Qadri I, Rahman SM, Schroeder-Gloeckler J, Janssen RC, Friedman JE. C/EBPbeta is AMP kinase sensitive and up-regulates PEPCK in response to ER stress in hepatoma cells. Mol Cell Endocrinol 331: 102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell 8: 1805–1814, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73: 1197–1206, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Cybulsky AV, Takano T, Papillon J, Bijian K. Role of the endoplasmic reticulum unfolded protein response in glomerular epithelial cell injury. J Biol Chem 280: 24396–24403, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Cybulsky AV, Takano T, Papillon J, Khadir A, Liu J, Peng H. Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J Biol Chem 277: 41342–41351, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25: 406–409, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 24: 10161–10168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dong F, Ren J. Adiponectin improves cardiomyocyte contractile function in db/db diabetic obese mice. Obesity (Silver Spring) 17: 262–268, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation 121: 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, Wu M, Choi HC, Lyons TJ, Zou MH. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 59: 1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339: 135–141, 1999 [PMC free article] [PubMed] [Google Scholar]
- 25. Hagerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. FASEB J 21: 618–628, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Halterman MW, Gill M, DeJesus C, Ogihara M, Schor NF, Federoff HJ. The endoplasmic reticulum stress response factor CHOP-10 protects against hypoxia-induced neuronal death. J Biol Chem 285: 21329–21340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han MS, Chung KW, Cheon HG, Rhee SD, Yoon CH, Lee MK, Kim KW, Lee MS. Imatinib mesylate reduces endoplasmic reticulum stress and induces remission of diabetes in db/db mice. Diabetes 58: 329–336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harama D, Koyama K, Mukai M, Shimokawa N, Miyata M, Nakamura Y, Ohnuma Y, Ogawa H, Matsuoka S, Paton AW, Paton JC, Kitamura M, Nakao A. A subcytotoxic dose of subtilase cytotoxin prevents lipopolysaccharide-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J Immunol 183: 1368–1374, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10: 3787–3799, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol Cell 35: 551–561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang L, Zhang R, Wu J, Chen J, Grosjean F, Satlin LH, Klein JD, Sands JM, Striker GE, Tan J, Zheng F. Increased susceptibility to acute kidney injury due to endoplasmic reticulum stress in mice lacking tumor necrosis factor-alpha and its receptor 1. Kidney Int. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 36. Inagi R, Kumagai T, Nishi H, Kawakami T, Miyata T, Fujita T, Nangaku M. Preconditioning with endoplasmic reticulum stress ameliorates mesangioproliferative glomerulonephritis. J Am Soc Nephrol 19: 915–922, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Inagi R, Nangaku M, Onogi H, Ueyama H, Kitao Y, Nakazato K, Ogawa S, Kurokawa K, Couser WG, Miyata T. Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int 68: 2639–2650, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Iyer S, Korada M, Rainbow L, Kirk J, Brown RM, Shaw N, Barrett TG. Wolcott-Rallison syndrome: a clinical and genetic study of three children, novel mutation in EIF2AK3 and a review of the literature. Acta Paediatr 93: 1195–1201, 2004 [PubMed] [Google Scholar]
- 39. Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 23: 5651–5663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jin HO, Seo SK, Woo SH, Kim ES, Lee HC, Yoo DH, An S, Choe TB, Lee SJ, Hong SI, Rhee CH, Kim JI, Park IC. Activating transcription factor 4 and CCAAT/enhancer-binding protein-beta negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radic Biol Med 46: 1158–1167, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147: 3398–3407, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Katsoulieris E, Mabley JG, Samai M, Sharpe MA, Green IC, Chatterjee PK. Lipotoxicity in renal proximal tubular cells: relationship between endoplasmic reticulum stress and oxidative stress pathways. Free Radic Biol Med 48: 1654–1662, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145: 5087–5096, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, Cosford ND, Reed JC. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. J Biol Chem 284: 1593–1603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis 11: 5–13, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Kimura K, Jin H, Ogawa M, Aoe T. Dysfunction of the ER chaperone BiP accelerates the renal tubular injury. Biochem Biophys Res Commun 366: 1048–1053, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa V, Dempsey C, Caunt JC, Oxley KM, Wyllie DH, Polgar T, Harte M, O'Neill LA, Qwarnstrom EE, Dower SK. Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J Biol Chem 279: 42703–42708, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332: 462–464, 1988 [DOI] [PubMed] [Google Scholar]
- 49. Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50: 752–763, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 59: 2033–2042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li M, Baumeister P, Roy B, Phan T, Foti D, Luo S, Lee AS. ATF6 as a transcription activator of the endoplasmic reticulum stress element: thapsigargin stress-induced changes and synergistic interactions with NF-Y and YY1. Mol Cell Biol 20: 5096–5106, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlondorff D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19: 2225–2236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 370: 651–656, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Liu XL, Done SC, Yan K, Kilpelainen P, Pikkarainen T, Tryggvason K. Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J Am Soc Nephrol 15: 1731–1738, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Luo ZF, Feng B, Mu J, Qi W, Zeng W, Guo YH, Pang Q, Ye ZL, Liu L, Yuan FH. Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol Appl Pharmacol 246: 49–57, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318: 1351–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem 278: 34864–34873, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martinez de Morentin PB, Varela L, Ferno J, Nogueiras R, Dieguez C, Lopez M. Hypothalamic lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 350–361, 2010 [DOI] [PubMed] [Google Scholar]
- 62. Matsumoto M, Minami M, Takeda K, Sakao Y, Akira S. Ectopic expression of CHOP (GADD153) induces apoptosis in M1 myeloblastic leukemia cells. FEBS Lett 395: 143–147, 1996 [DOI] [PubMed] [Google Scholar]
- 63. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem 146: 743–750, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74: 743–756, 1993 [DOI] [PubMed] [Google Scholar]
- 66. Morse E, Schroth J, You YH, Pizzo DP, Okada S, Ramachandrarao S, Vallon V, Sharma K, Cunard R. TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP-1. Am J Physiol Renal Physiol 299: F965–F972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280: 847–851, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Normington K, Kohno K, Kozutsumi Y, Gething MJ, Sambrook JS. Cerevisiae encodes an essential protein homologous in sequence and function to mammalian BiP. Cell 57: 1223–1236, 1989 [DOI] [PubMed] [Google Scholar]
- 72. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26: 9220–9231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24: 1243–1255, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oikawa D, Kimata Y, Kohno K, Iwawaki T. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res 315: 2496–2504, 2009 [DOI] [PubMed] [Google Scholar]
- 75. Okamura M, Takano Y, Hiramatsu N, Hayakawa K, Yao J, Paton AW, Paton JC, Kitamura M. Suppression of cytokine responses by indomethacin in podocytes: a mechanism through induction of unfolded protein response. Am J Physiol Renal Physiol 295: F1495–F1503, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, Hori O, Yamasaki Y, Ogawa S. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54: 657–663, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 9: 35–51, 2009 [DOI] [PubMed] [Google Scholar]
- 80. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 81. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev 79: 683–701, 1999 [DOI] [PubMed] [Google Scholar]
- 83. Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443: 548–552, 2006 [DOI] [PubMed] [Google Scholar]
- 84. Pincus D, Chevalier MW, Aragon T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol 8: e1000415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Qi W, Mu J, Luo ZF, Zeng W, Guo YH, Pang Q, Ye ZL, Liu L, Yuan FH, Feng B. Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response. Metabolism. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 87. Ramachandra Rao SP, Wassell R, Shaw MA, Sharma K. Profiling of human mesangial cell subproteomes reveals a role for calmodulin in glucose uptake. Am J Physiol Renal Physiol 292: F1182–F1189, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Rose MD, Misra LM, Vogel JP. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 57: 1211–1221, 1989 [DOI] [PubMed] [Google Scholar]
- 90. Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2{alpha}-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem 286: 4809–4818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7: 1165–1176, 2001 [DOI] [PubMed] [Google Scholar]
- 92. Selim E, Frkanec JT, Cunard R. Fibrates upregulate TRB3 in lymphocytes independent of PPAR alpha by augmenting CCAAT/enhancer-binding protein beta (C/EBP beta) expression. Mol Immunol 44: 1218–1229, 2007 [DOI] [PubMed] [Google Scholar]
- 93. Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab 93: 4532–4541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3: 99–111, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H, Mundel P, Jehle AW. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 299: F821–F829, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sommer T, Jarosch E. BiP binding keeps ATF6 at bay. Dev Cell 3: 1–2, 2002 [DOI] [PubMed] [Google Scholar]
- 97. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: 3378–3389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7: 880–885, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 25: 9554–9575, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tsutsumi A, Motoshima H, Kondo T, Kawasaki S, Matsumura T, Hanatani S, Igata M, Ishii N, Kinoshita H, Kawashima J, Taketa K, Furukawa N, Tsuruzoe K, Nishikawa T, Araki E. Caloric restriction decreases ER stress in liver and adipose tissue in ob/ob mice. Biochem Biophys Res Commun 404: 339–344, 2011 [DOI] [PubMed] [Google Scholar]
- 101. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 102. Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. J Cell Sci 118: 3905–3915, 2005 [DOI] [PubMed] [Google Scholar]
- 103. Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103: 27–37, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of chop. EMBO J 17: 3619–3630, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 11: 1473–1480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, Vlassara H, Zheng F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 176: 2163–2176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu X, He Y, Jing Y, Li K, Zhang J. Albumin overload induces apoptosis in renal tubular epithelial cells through a CHOP-dependent pathway. Omics 14: 61–73, 2010 [DOI] [PubMed] [Google Scholar]
- 108. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279: 45495–45502, 2004 [DOI] [PubMed] [Google Scholar]
- 110. Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6: 1355–1364, 2000 [DOI] [PubMed] [Google Scholar]
- 111. Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281: 30299–30304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001 [DOI] [PubMed] [Google Scholar]
- 113. Yoshida H, Oku M, Suzuki M, Mori K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol 172: 565–575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 46: 887–894, 1997 [DOI] [PubMed] [Google Scholar]
- 115. Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4: 391–406, 2006 [DOI] [PubMed] [Google Scholar]
- 116. Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med 3: 33–40 [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124: 587–599, 2006 [DOI] [PubMed] [Google Scholar]
- 119. Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22: 3864–3874, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]