Abstract
Vitronectin (Vtn) is a glycoprotein found in normal serum and pathological extracellular matrix. Given its known interactions with plasminogen activator inhibitor-1 (PAI-1) and Vtn cellular receptors, especially αvβ3 integrin and the urokinase receptor (uPAR), this study was designed to investigate its role in renal fibrogenesis in the mouse model of unilateral ureteral obstruction (UUO). Kidney Vtn mRNA levels were increased ×1.8–5.1 and Vtn protein levels ×1.9–3 on days 7, 14, and 21 after UUO compared with sham kidney levels. Groups of age-matched C57BL/6 wild-type (Vtn+/+) and Vtn−/− mice (n = 10–11/group) were killed 7, 14, or 21 days after UUO. Absence of Vtn resulted in the following significant differences, but only on day 14: fewer αSMA+ interstitial myofibroblasts (×0.53), lower procollagen III mRNA levels (×0.41), lower PAI-1 protein (×0.23), higher uPA activity (×1.1), and lower αv protein (×0.32). The number of CD68+ macrophages did not differ between the genotypes. Despite these transient differences on day 14, the absence of Vtn had no effect on fibrosis severity based on both picrosirius red-positive interstitial area and total kidney collagen measured by the hydroxyproline assay. These findings suggest that despite significant interstitial Vtn deposition in the UUO model of chronic kidney disease, its fibrogenic role is either nonessential or redundant. These data are remarkable given Vtn's strong affinity for the potent fibrogenic molecule PAI-1.
Keywords: PAI-1, myofibroblasts, fibrosis, αv integrin
vitronectin (Vtn), also known as S protein of the complement cascade, is a 70- to 83-kDa multifunctional adhesive glycoprotein primarily synthesized by hepatocytes and is an abundant circulating plasma protein. First identified in 1967 as a “serum-spreading factor” distinct from fibronectin, it was subsequently shown to be an important inhibitor of C5b-9-mediated cytolysis (10, 15, 56). Vtn may also be found as a component of the extracellular matrix that develops in response to tissue injury, especially in the early provisional matrix (19). Most published data on Vtn's role in chronic injury have focused on liver and lung disease, where its expression is closely associated with fibrosis (9, 18, 23). In chronic kidney disease, Vtn colocalizes with C5b-9, the terminal complex of the active complement cascade, and glomerular immune deposits and may be present in sclerotic glomeruli (1, 4, 28, 31, 32, 36, 39, 40, 44). Whether Vtn accumulates in the interstitium and participates in the pathogenesis of chronic kidney disease is unclear. Vtn is known to elicit several biological responses that suggest that it could either promote or attenuate fibrogenesis and downstream renal parenchymal injury, suggesting that its net effect is regulated by local environmental factors (10, 26).
Establishing the role of Vtn in the pathogenesis of chronic kidney injury is of particular interest because of its high binding affinity for the potent fibrosis-promoting molecule plasminogen activator inhibitor-1 (PAI-1) (5, 24, 37, 47). Vtn is considered as a cofactor for PAI-1 proteolytic inhibitory activities (22). When PAI-1 is bound to Vtn, its active confirmation is stabilized and its half-life is increased approximately fourfold (20). When serine proteases are present, the Vtn-PAI-1 complex efficiently inhibits plasmin generation and plasmin-mediated proteolytic responses. However, plasmin has pleiotropic and even contrasting effects that have been reported during kidney injury. Recent studies support the view that plasmin is protective in acute glomerular disease but it promotes fibrosis in chronic tubulointerstitial disease (8). Furthermore, studies from our laboratory suggest that the predominant mechanism by which PAI-1 promotes renal interstitial fibrosis involves protease-independent effects that facilitate recruitment of fibrogenic inflammatory cells and myofibroblasts (53). The mechanism is complex, and it involves PAI-1 interacting with cellular receptors, either the low-density lipoprotein receptor-related protein 1 (LRP1) or the urokinase receptor (uPAR), that work in collaboration with matrix-binding integrin receptors (2, 42). In vitro studies clearly established that Vtn can interfere with both of these cellular pathways.
Vtn can block the ability of PAI-1 to bind to LRP1 and promote chemotaxis (6, 21). Vtn interactions with the uPAR-integrin cellular pathway are more complex (41, 42, 45, 46, 52). The same region of the COOH-terminal somatomedin B domain of Vtn that binds to PAI-1 also binds to uPAR. A second site, in the Vtn RGD domain, binds directly to αv integrins, especially αvβ3, to initiate cell signaling and migration. Since uPAR itself is a nonsignaling receptor, integrins function as essential coreceptors that enable uPAR to promote cell migration. In vitro studies suggest that αvβ3 integrin and uPAR anchor fibroblasts to Vtn during fibrotic responses and that PAI-1 serves a “deadhesive” role, as it binds preferentially to Vtn. PAI-1 blocks cellular binding to Vtn by more than 80% (45). When the interaction between Vtn-uPAR-αvβ3 is blocked, fibroblasts migrate more efficiently through extracellular matrices in response to the presence of fibronectin and/or collagen. This latter movement is mediated by other integrin receptors. Consistent with this view, the number of interstitial myofibroblasts is significantly higher and fibrosis severity worse in uPAR knockout mice with chronic kidney disease induced by unilateral ureteral obstruction (UUO) (54, 55). The opposite is observed in mice genetically engineered to lack or overexpress PAI-1: more myofibroblasts and worse fibrosis in the mice with higher PAI-1 levels (27, 30). Inhibition of αvβ3 has been reported to worsen hepatic fibrosis (33). Thus, the presence of Vtn during renal fibrogenesis is expected to influence disease severity, although the net effect is difficult to predict due to the complexity of these processes and the potential for cellular, receptor, and extracellular matrix heterogeneity. However, based on these cellular responses, we predicted that Vtn should attenuate renal fibrosis, excepting one important caveat: Vtn might increase kidney PAI-1 levels with the secondary effect of enhancing fibrosis. Recent studies by Noble and colleagues (16, 17) reported therapeutic efficacy of a mutant PAI-1 “decoy” molecule in an experimental model of glomerulonephritis: glomerular matrix reduction required the expression of the Vtn-binding domain.
Vtn is predicted to increase renal PAI-1 levels during chronic kidney injury by two mechanisms: directly, through binding and trapping it within the extracellular matrix, and indirectly, by preventing access to uPAR for internalization and degradation (8, 52). The tertiary complex of uPA-uPAR and PAI-1 can be internalized and degraded via a process that involves LRP1. If PAI-1 is sequestered within Vtn-containing extracellular matrices, this important PAI-1 turnover pathway is unavailable.
Thus, despite considerable evidence that Vtn is a common constituent of pathological extracellular matrix and may be involved in polarizing tissue repair responses by either enhancing or attenuating fibrosis severity (depending on the expression and function of extracellular molecules with which it binds), whether Vtn plays an essential role in chronic kidney disease is unknown. The present study was designed to answer this question by investigating the severity of renal fibrosis that develops in wild-type (Vtn+/+) and knockout (Vtn−/−) mice in response to UUO.
MATERIALS AND METHODS
Animal models.
Vtn−/− mice on a C57BL/6 background were previously generated by the Ginsberg laboratory and are known to be phenotypically normal and fertile (56). Frozen heterozygous embryos were rederived at the Jackson Laboratories. Breeders were maintained in our vivarium. The genotype of each study mouse was confirmed by PCR analysis of genomic DNA isolated from tails using the following primers: Vtn5neo 5′-CTT GGG TGG AGA GGC TAT TC-3′, Vtn3neo 5′-AGG TGA GAT GAC AGG AGA TC-3′, Vtn5ko 5′-GTG CCA GTG CTT GGA ATT TGG-3′, and Vtn3ko 5′-GTA GTG GGT CAG AGT CTA TTC TTC-3′ (detecting a 442-bp wild-type and a 280-bp mutant allele). The Vtn−/− genotype was reconfirmed by immunoblotting using an affinity-purified rabbit anti-mouse Vtn antibody that was provided to us by Dr. R.-P. Czekay (Center for Cell Biology and Cancer Research, Albany Medical College, NY) (7), demonstrating the absence of a Vtn protein band in each harvested kidney. C57BL/6 Vtn+/+ wild-type mice were purchased from the Jackson Laboratories.
UUO or sham surgery was performed in groups of Vtn+/+ and Vtn−/− age-matched male mice as previously described (30). Groups of 10–11 mice of each genotype were killed 7, 14, and 21 days later. The kidneys were harvested after exsanguination under general anesthesia and the capsules were removed; next, total kidney weight was measured and the kidney was then subdivided in an identical fashion into pieces for total kidney collagen, zymography, RNA and protein isolation and histological studies. All animal studies were performed in compliance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Seattle Children's Research Institute.
Primary antibodies.
Primary antibodies were peroxidase-conjugated mouse anti-human α-smooth muscle actin (SMA; Sigma, St. Louis, MO), rat anti-mouse CD68 (AbD Serotec, Raleigh, NC), sheep anti-mouse PAI-1 (American Diagnostics, Stamford, CT), rabbit anti-αv integrin (Millipore, Billerica, MA), DyLight 549-conjugated rabbit anti-laminin (Novus Biologicals, Littleton, CO), and mouse monoclonal anti-β-actin (Sigma).
Kidney fibrosis severity.
Total kidney collagen was measured in kidney samples from each individual animal using the hydroxyproline assay as previously described (30). Results were expressed as micrograms of collagen per milligram of wet kidney weight. As a second measure of the degree of renal fibrosis, the area of the interstitium occupied by picrosirius red-stained collagen was determined in 4-μm sections of paraffin-embedded kidney using computer-assisted image analysis and the Image-Pro software program from Media Cybernetics (Bethesda, MD). Glomeruli and large vessels were excluded from the analysis.
Western blotting.
Vtn, PAI-1, αv integrin, and β-actin kidney protein levels were measured by Western blotting using protein (20 μg/lane) isolated from homogenized frozen kidney samples and our standard laboratory procedures. Secondary antibodies were Alexa 680-conjugated goat-anti-rabbit IgG, Alexa 680-conjugated donkey anti-sheep IgG (Invitrogen, Life Technologies, Carlsbad, CA), and IRDye 800-conjugated donkey anti-mouse IgG (Li-Cor Biosciences, Lincoln, NE). Specific bands were visualized and quantified using the Li-Cor Odyssey infrared imaging system (Li-Cor Biosciences). The density of each band was corrected for protein loading using the β-actin band density.
Immunohistology.
Immunostaining was performed on cryosections of snap-frozen kidney tissue for confocal microscopy or on sections of paraffin-embedded kidney tissue for immunohistochemistry using VECTASTAIN Elite (Vector Labs, Burlingame, CA) and the AEC Chromagen kit (Dako, Carpinteria, CA). The secondary antibodies were biotinylated goat anti-rabbit IgG and biotinylated rabbit anti-rat IgG (Vector Labs), donkey anti-sheep DyLight 488 and horseradish peroxidase-conjugated anti-rat IgG (Jackson ImmunoResearch Laboratory, West Grove, PA). Microscopy was performed using a Zeiss LSM5 Pascal confocal microscope or a Leica DM400 microscope. Kidney tissue sections were confirmed to be negative when stained with secondary antibodies only. Quantitative analyses were performed by measuring the positive interstitial area in 8–12 randomly selected fields per animal by computer-assisted image analysis using the Image-Pro software. The observer (SC) was blind to the animal group at the time of analysis.
Kidney mRNA levels.
The automated Maxwell 16 System (Promega, Madison, WI) was used to isolate total RNA of kidney tissue samples of individual experimental animals. RNA integrity and concentration were determined using the Agilent RNA6000 Nano Chip kit and the Agilent 2100 Bioanalyzer System (Agilent Technologies, Foster City, CA). Samples (1 μg) with a RNA integrity number >8 were reverse transcribed with MMLV reverse transcriptase using either the cDNA synthesis kit from Bioline (Taunton, MA) and a combination of random hexamers and oligo-dTs or the Bioline iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA). Semiquantitative real-time qPCR was performed according to the IQ SYBR Green Superkit (Bio-Rad Laboratories) instructions using gene-specific primers for PAI-1: forward TCAGAGCAACAAGTTCAACTACACTGAG; reverse CCCACTGTCAAGGCTCCATCACTTGCCCCA; procollagen 1 (αI): forward AGAAGTCTCAAGATGGTGGCCG; reverse GGTCACGAACCACGTTAGCATC; procollagen 3 (αI): forward CAGCTATGGCCCTCCTGATCTT; reverse GTAATGTTCTGGGAGGCCCG; Vtn: forward CCCCTGAGGCCCTTTTTCATA; reverse CAAAGCTCGTCACACTGACA; uPAR: forward GCGTCTCTCATCAACTGCCG; reverse TCCCAGCACATCTAAGCCTGTAG; and 18S: forward GGTGAAATTCTTGGACCGGC; reverse GACTTTGGTTTCCCGGAAGC. Real-time qPCR reactions containing 1.5 μl of cDNA, 0.2 μM primers, and the 2× SYBR Green Supermix were run in the Bio-Rad iQ thermal cycler with programs specific for the respective gene. Reactions were run in triplicate and genes of interest were normalized to the 18S housekeeping gene. Absence of primer dimers was confirmed for primer specificity. Single amplicons were confirmed by gel electrophoresis. Data analysis was performed using the Pfaffl algorithm with the REST analysis software version 1.9.9 (Corbett Research). Final results were expressed as fold-increase relative to wild-type sham samples that were assigned an arbitrary mean value of 1.0.
Plasminogen gel zymography.
Kidney urokinase-type plasminogen activator (uPA) activity was measured in kidney protein samples (10 μg) by plasminogen-casein gel zymography as previously described (30). The gel was stained with Coomassie brilliant blue and the size of each lytic band was measured using the ImageQuant software program (Molecular Dynamics, Sunnyvale, CA). Each gel included a lane loaded with molecular weight standards (Fermentas Life Sciences, Glen Burnie, MD) and a low-molecular-weight human uPA standard (Calbiochem-Novabiochem, La Jolla, CA). Amiloride (1 mmol/l; Sigma) was added to the Triton solution and glycine buffer in some assays to confirm the identity of the uPA band, as uPA but not tissue-type plasminogen activator (tPA) is inhibited by amiloride.
Statistics.
All data are presented and means ± SD. A nested ANOVA was used for data generated by computer-assisted image analysis. For image analysis data, the arithmetic mean of all randomly selected images from each slide (6 to 9) for each individual animal was used to calculate the reported mean for each group. Comparisons between two experimental groups used an unpaired Student's t-test for parametric data and the Mann Whitney U-test for nonparametric data. A P value <0.05 was considered statistically significant.
RESULTS
Kidney Vtn expression is increased after UUO.
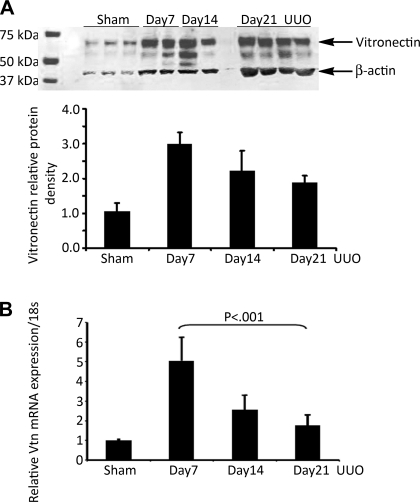
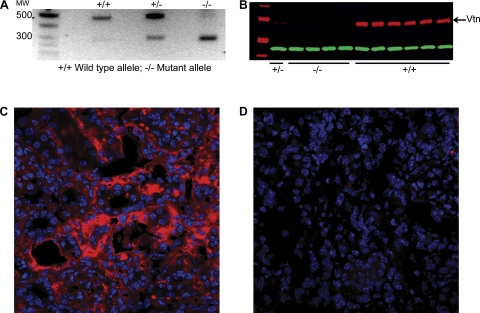
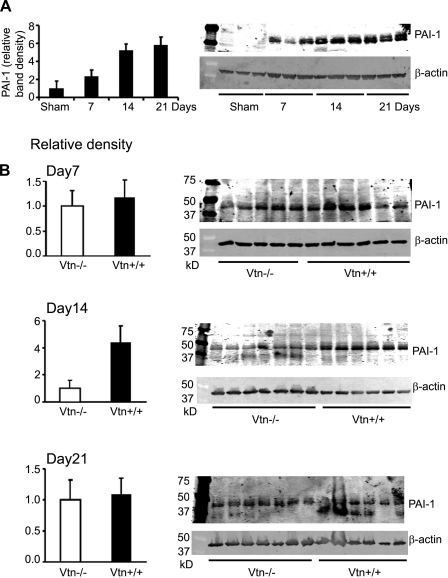
Normal kidneys exposed to sham surgery expressed low levels of a ≈70-kDa Vtn protein detectable by Western blotting (Fig. 1A). Protein levels were significantly increased ×3.0, ×2.2, and ×1.9 relative to sham levels at 7, 14, and 21 days after UUO surgery, respectively. By real-time RT-PCR, kidney Vtn mRNA levels were increased ×5.1, ×2.5, and ×1.8 relative to sham levels at these time points (Fig. 1B). The Vtn genotype of each study mouse was determined by PCR analysis of genomic DNA (Fig. 2A) and reconfirmed by kidney protein immunoblotting (Fig. 2B). By immunostaining, Vtn was localized to the renal interstitium in UUO kidneys (Fig. 2C). All Vtn−/− study mice were confirmed to lack detectable Vtn protein in the kidney after UUO surgery (Fig. 2D).
Fig. 1.
Vitronectin (Vtn) expression is increased in unilateral ureteral obstruction (UUO) nephropathy. A: Vtn Western blot illustrates increased kidney protein expression in wild-type (Vtn+/+) mice 7, 14, and 21 days after UUO compared with sham kidneys. B: graph shows Vtn band densities (means ± SD) corrected for protein loading using β-actin densities. Kidney Vtn mRNA levels measured by real-time RT-PCR (means ± 1 SD, 5 samples/time point) confirmed significantly increased levels after UUO compared with the sham kidney levels.
Fig. 2.
Vtn genotyping and protein immunolocalization after UUO. A: representative gel of genomic DNA analyzed by PCR illustrates the 442-bp wild-type (+/+) allele and the 280-bp mutant (−/−) allele. B: representative Western blot of kidney protein samples 14 days after UUO illustrates a distinct ≈70-kDa band in the Vtn+/+ mice and an absence of this band in the Vtn−/− mice. Immunofluorescence confocal photomicrographs illustrate interstitial Vtn accumulation in a Vtn+/+ kidney 14 days after UUO (C) and absence of the same protein in a Vtn−/− kidney (D). Magnification ×400.
Vtn receptors uPAR and αvβ integrin are upregulated after ureteral obstruction.
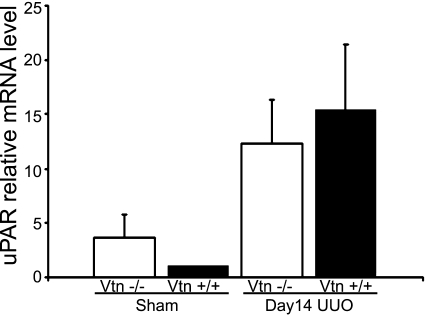
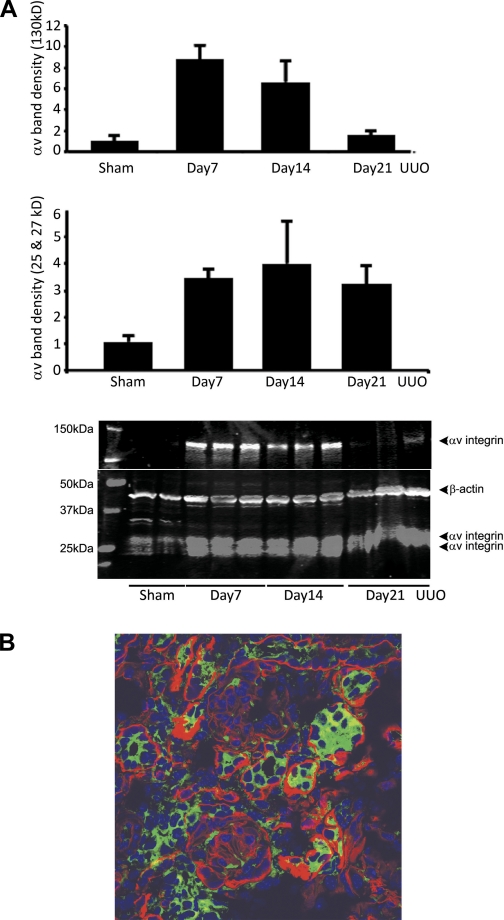
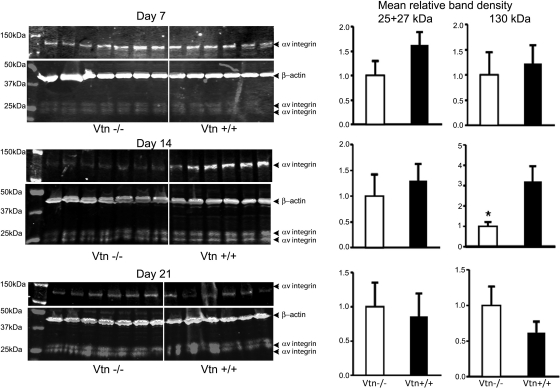
By real-time RT-PCR, uPAR mRNA levels were confirmed to be increased 14 days after UUO as we previously reported (54) (Fig. 3). Levels were similar in Vtn+/+ and Vtn−/− mice. Immunoblotting for αv integrin detected 25- and 27-kDa bands representing the light chain and a 130-kDa heavy chain. After UUO surgery, αv was upregulated, with highest levels of the 150-kDa band observed on day 7 (×8.8 increase compared with sham) and highest levels of the 25- and 27-kDa bands on day 14 (×4.0 increase compared with sham; Fig. 4A). By immunostaining, the αv integrin was localized to tubular and interstitial cells with focal glomerular staining (Fig. 4B). Renal αv integrin expression levels were not significantly different between Vtn+/+ and Vtn−/− mice 7, 14, and 21 days after UUO with the single exception that the 130-kDa band was significantly lower in the Vtn−/− mice on day 14 (Fig. 5).
Fig. 3.
Urokinase receptor (uPAR) mRNA is upregulated after UUO. Kidney uPAR mRNA levels measured by real-time RT-PCR are significantly increased 14 days after UUO; levels were similar between Vtn+/+ and Vtn−/− animals. Results are means ± SD, n = 5 samples/group. Results between the genotypes are not statistically significant.
Fig. 4.
αv Integrin is upregulated after UUO. Western blotting for αv expression in kidney protein isolates performed under reducing conditions detects 2 light chains (25 and 27 kDa) and a heavy chain (130 kDa). A: protein levels for both bands are increased after UUO. The bar graph represents mean band densities ± 1 SD after protein loading correction using the β-actin band density. B: by immunofluorescence confocal microscopy, αv integrin was expressed by tubular epithelial and interstitial cells 14 days after UUO. To distinguish between αv expression by tubular and interstitial cells, the basement membrane protein laminin is stained red. Magnification ×600.
Fig. 5.
Effect of Vtn expression on kidney αv integrin levels. The Western blotting illustrates similar levels of αv light chain protein 7, 14, and 21 days after UUO while αv heavy chain levels were significantly lower in the Vtn−/− mice on day 14 but not on days 7 or 21. Bar graphs represent mean band density ± 1 SD (n = 6–7/group). *P < 0.05.
Absence of Vtn has a modest effect on interstitial myofibroblast recruitment after UUO.
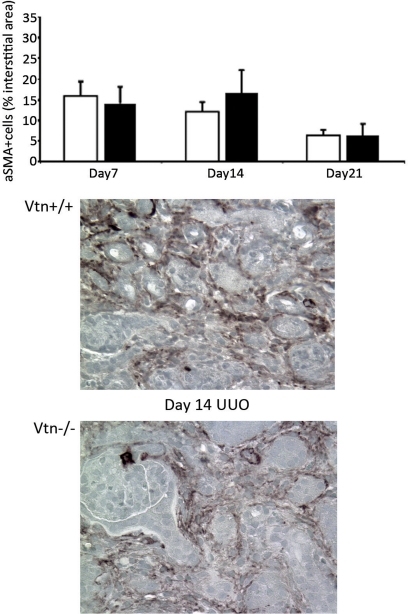
Interstitial myofibroblasts, identified as interstitial cells expressing α-SMA, are the primary source of the extracellular matrix proteins within fibrotic renal interstitial spaces. The number of α-SMA+ interstitial cells was similar between Vtn+/+ and Vtn−/− mice 7 and 21 days after UUO; levels were slightly lower (47%, P = 0.04) on day 14 (Fig. 6).
Fig. 6.
Effect of Vtn expression on interstitial α-smooth muscle actin (SMA)+ myofibroblast numbers after UUO. α-SMA+ interstitial myofibroblasts appear de novo in the interstitium after UUO. The number of α-SMA+ interstitial cells was significantly lower in the Vtn−/− mice 14 days after UUO, but numbers were similar on days 7 and 21. Bars represent means ± 1 SD (n = 6–11/group). Representative day 14 immunohistochemical photomicrographs illustrate fewer α-SMA+ interstitial cells in a Vtn−/− kidney compared with Vtn+/+ kidney. Magnification ×500.
Absence of Vtn does not alter the severity of UUO-induced renal fibrosis.
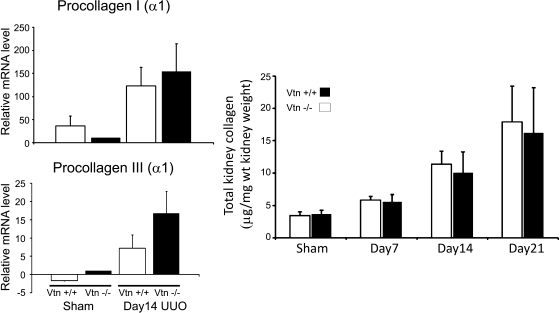
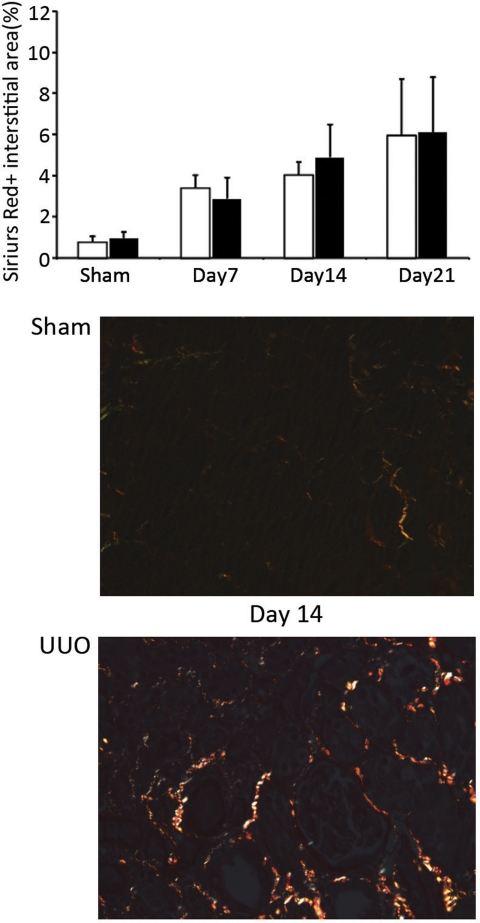
Interstitial collagens are the primary constituents of fibrotic regions, especially collagens I and III. On day 14 post-UUO, kidney procollagen α1(III), but not procollagen α1(I), mRNA levels measured by real-time RT-PCR were significantly lower (P = 0.03) in the Vtn−/− mice (Fig. 7A). Kidney collagen levels, measured using the hydroxyproline assay, progressively increased after UUO (from days 7 to 21), and levels were similar between Vtn+/+ and Vtn−/− mice at all time points (Fig. 7B). The interstitial area occupied by sirius red-positive collagen progressively increased after UUO and levels did not differ between Vtn+/+ and Vtn−/− mice (Fig. 8).
Fig. 7.
Kidney collagen levels are similar in Vtn+/+ and Vtn−/− mice after UUO. Left: kidney collagen I and III mRNA levels measured by real-time RT-PCR (n = 5/group) are significantly increased 14 days after UUO compared with sham-operated kidneys (P < 0.05). The day 14 levels were significantly lower for procollagen III but not for procollagen I. Right: total kidney collagen levels measured using the hydroxyproline assay 7, 14, and 21 days after UUO were similar in the Vtn+/+ and Vtn−/− groups. Results are expressed as means ± 1 SD and are not statistically different between the genotypes at each time point (n = 10–11 UUO mice/group).
Fig. 8.
Interstitial fibrosis is similar in Vtn+/+ and Vtn−/− mice after UUO. The area of the interstitium occupied by picrosirius red-positive interstitial collagen fibrosis quantified by computer-assisted image analysis of stained kidney sections progressively increased with time after UUO. Differences between the Vtn+/+ and Vtn−/− mice (n = 5–12/group) were not significantly significant on days 7, 14, and 21. Top: bar graph represents results expressed as a mean positive interstitial area ± 1 SD. Bottom: representative photomicrographs of tissue sections illustrate the increase in sirius red-positive interstitial collagen deposits 14 days after UUO surgery compared with sham surgery. Magnification ×200.
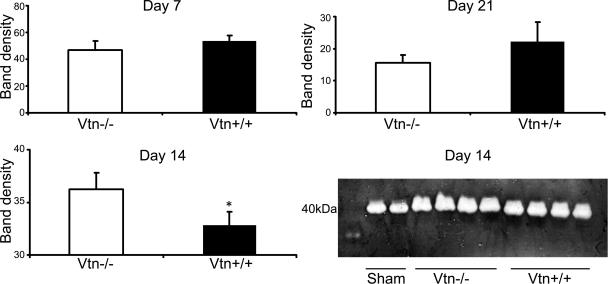
Absence of Vtn has minimal effect on kidney PAI-1 levels and plasminogen activator activity.
Minimally detectable in normal kidneys, PAI-1 protein levels are increased five- to sixfold in response to UUO-induced chronic injury (Fig. 9A). Although PAI-1 binds avidly to Vtn, kidney PAI-1 protein levels were similar in Vtn+/+ and Vtn−/− mice 7 and 21 days after UUO, but they were significantly lower (77%, P = 0.004) on day 14 (Fig. 9B). Kidney PAI-1 mRNA levels were also reduced by 46% in Vtn−/− mice on day 14 UUO (P = 0.02, data not shown). Vtn-bound PAI-1 is stabilized in its active conformation, which is predicted to enhance its protease inhibitory effects on uPA and tPA. Renal uPA activity, measured by plasminogen gel zymography, was 11% higher in the Vtn−/− mice on day 14 (P = 0.02) but no differences were observed on days 7 and 21 (Fig. 10).
Fig. 9.
Effect of Vtn expression on kidney plasminogen activator inhibitor-1 (PAI-1) levels after UUO. A: representative time course Western blot illustrates the increase in kidney PAI-1 protein levels after UUO. B: comparison Western blots illustrate that kidney PAI-1 protein levels (n = 6–7/group) were similar in Vtn+/+ and Vtn−/− mice at 7 and 21 days while significantly lower in Vtn−/− mice on day 14. *P < 0.05. Each bar graph represents the mean band density ± 1 SD, after correcting for protein loading using the β-actin band density.
Fig. 10.
Kidney urokinase-type plasminogen activator (uPA) activity. A representative casein-plasminogen zymogram (Day 14 UUO) illustrates plasminogen lytic activity of the 40-kDa uPA protein. Each bar graph represents mean activity ± 1 SD (n = 4/time point/genotype). *P < 0.05.
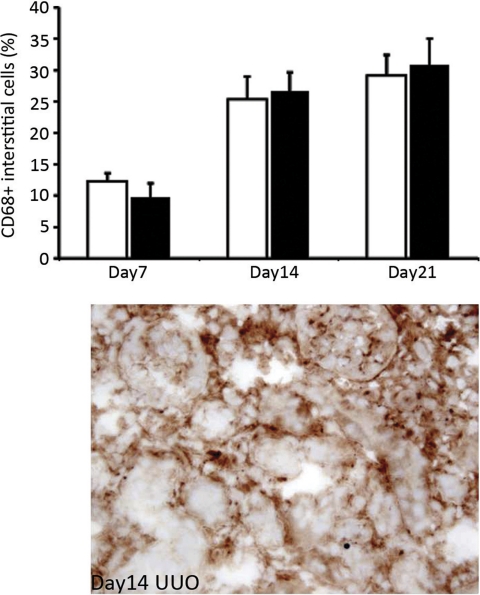
Absence of Vtn does not modify interstitial macrophage recruitment after UUO.
Since kidney PAI-1 levels showed a significant positive correlation with kidney macrophage numbers in several studies and PAI-1 can serve as a chemoattractant molecule for monocytes/macrophages, the number of CD68+ cells was quantified and shown to be similar in Vtn+/+ and Vtn−/− mice 7, 14, and 21 days after UUO (Fig. 11).
Fig. 11.
Vtn expression does not influence kidney macrophage recruitment in response to UUO. The graph illustrates the mean number of CD68+ interstitial macrophages (n = 8–10/group) 7, 14, and 21 days after UUO. Numbers are similar in Vtn+/+ and Vtn−/− mice. Representative immunohistochemical photomicrograph illustrates numerous CD68+ interstitial macrophages in a Vtn+/+ mouse 14 days after UUO. Magnification ×500.
DISCUSSION
The results of this study demonstrate that, despite significant accumulation in the renal interstitium in an experimental model of chronic kidney disease, Vtn does not play an essential role in the fibrogenic response. Although a modest reduction in the number of interstitial myofibroblasts and PAI-1 protein levels was observed at a single time point (day 14), these differences were not associated with significant differences in interstitial fibrosis severity.
Vtn accumulates within the interstitium of chronically damaged kidneys as shown in the present study of UUO-induced injury. Based on elevated Vtn mRNA levels, de novo synthesis occurs within the kidney, although it is possible that Vtn leakage from interstitial capillaries may also contribute, as early increases in impermeability are a feature of this model (49). Despite the consistent pattern of changes, fewer myofibroblasts, lower procollagen III mRNA levels, lower αv integrin protein, lower PAI-1 protein levels, and higher uPA activity, observed in the Vtn−/− mice on day 14, the overall degree of renal fibrosis was similar between Vtn+/+ and Vtn−/− mice at all time points and these differences were not detected at early and later time points (days 7 and 21). These findings, together with the normal development and phenotype of the Vtn−/− mice (56), suggest that other extracellular matrix proteins share overlapping functions and/or compensate for the absence of Vtn. These data are particularly surprising given the known important interactions between Vtn and PAI-1. It is possible that Vtn is more important in the circulation, where most PAI-1 is thought to be Vtn-bound (5, 24, 37, 47). Additionally, although the αv Vtn receptors have been considered essential coreceptors for uPAR-dependent fibroblast migration (41, 42, 52), Vtn deficiency only had a transient effect on interstitial (myo)fibroblast numbers after UUO.
Given that Vtn is known to be expressed during several fibrotic responses, in the liver, lung, skin, and kidney, and has pleiotropic functions, the possibility remains that the biological effects of Vtn are highly context-dependent and may still serve to polarize the tissue repair response under specific circumstances; because so many other dominant pathways are operative in chronic kidney disease, it may be that alternative pathways readily compensate for the absence of Vtn. The same response may not ensue in other pathological conditions. For example, dermal wound healing was found to be slightly delayed in Vtn−/− mice (20). It is also possible that in glomerular diseases characterized by upregulated uPAR or αv integrin expression, Vtn may serve a primary pathogenetic function.
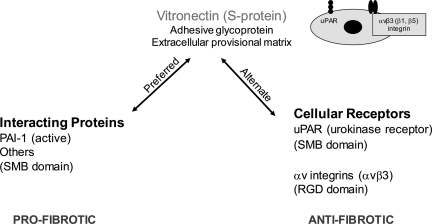
An alternative possibility is that Vtn mediates significant but opposing responses during renal fibrogenesis, some promoting and others attenuating, such that the net effect on overall fibrosis severity is negligible. These potential effects can be considered in two broad categories: Vtn interactions with extracellular molecules and Vtn receptor-dependent cellular activities (Fig. 12). PAI-1 is considered the prototype of the first category. Although the present study detected significantly lower kidney PAI-1 protein levels in the Vtn−/− mice on day 14, it is not clear why these differences were not present on days 7 and 21, given abundant Vtn within the interstitium of the wild-type mice. Significantly lower PAI-1 mRNA levels on day 14 raise the possibility that decreased PAI-1 synthesis, perhaps due to fewer interstitial myofibroblasts, contributed to the lower levels observed at this single time point. It is also possible that higher PAI-1 protein levels on day 14 in the Vtn+/+ mice represent the combined effects of enhanced PAI-1 interstitial trapping and delayed uPAR-uPA-LRP1-mediated PAI-1 degradation in the Vtn+/+ mice. In addition to PAI-1, Vtn has been reported to bind several other molecules that may contribute to the fibrogenic response, and together these extracellular interactions may result in a negligible overall effect on fibrosis severity. The potential list of Vtn-interacting molecules includes transforming growth factor-β, hepatocyte growth factor, connective tissue growth factor, epidermal growth factor, vascular endothelial growth factor, fibroblast growth factor, insulin-like growth factor II, anti-thrombin complexes, and proteoglycans (10, 11, 35, 38, 43).
Fig. 12.
Schematic overview of the competing fibrogenic effects of Vtn. Vtn binds with high affinity to PAI-1 via its NH2-terminal somatomedin B domain (SMB), an interaction that is preferred over Vtn receptor engagement, and results in PAI-1 stability in its active conformation. PAI-1 is a potent renal fibrosis-promoting molecule, mediated at least in part by its ability to enhance interstitial myofibroblast recruitment. Although less extensively investigated, Vtn has been reported to bind an array of other molecules implicated in chronic kidney disease, including transforming growth factor-β, hepatocyte growth factor, connective tissue growth factor, epidermal growth factor, vascular endothelial growth factor, fibroblast growth factor, insulin-like growth factor II, anti-thrombin complexes, and proteoglycans. Alternatively, Vtn can interact directly with cell membrane receptors, most notably the classical uPAR via the SMB domain and αv integrins (mainly αvβ3) via its RGD domain. Both uPAR and αvβ3 have anti-fibrotic effects based on in vivo studies comparing outcomes between wild-type and receptor-deficient or -inhibited mice.
The alternative pathway we considered as potentially important pertains to the ability of Vtn to bind to the cellular receptors uPAR and αv integrins and promote cell migration. uPAR is known to be expressed by interstitial fibroblasts and is upregulated in response to chronic kidney injury (51, 54). However, in addition to Vtn, kininogen and uPA are known uPAR ligands (52). Given that both uPA deficiency and Vtn deficiency fail to alter renal fibrosis severity in response to UUO (48), it is possible that the two-chain high-molecular-weight kininogen (HKa) is more important in mediating the fibrosis-attenuating effects of uPAR (3, 14, 34).
Vtn is also known to bind to αv integrin receptors, especially αvβ3 (10). Although alterations in αv integrin expression have been reported in glomerular disease (12, 13, 50), less is known about its expression in chronic tubulointerstitial disease. Increased fibroblast αv expression has been associated with fibrosis (29). The present study reports significantly upregulated αv integrin expression by interstitial and tubular cells in the UUO model of chronic kidney disease. αv Integrin is ubiquitous, however, and can form heterodimeric receptors that bind extracellular matrix proteins other than Vtn; the predominant Vtn integrin αvβ3 itself binds numerous other ligands, including many that may be involved in fibrogenesis (25). The present study only shows that Vtn integrin receptors are available to interact with interstitial Vtn; whether different signaling pathways were activated in the presence and absence of Vtn has not been determined.
In summary, Vtn, PAI-1, and the Vtn receptors uPAR and αv integrin are upregulated in chronic kidney disease induced by UUO. Despite the myriad biological properties ascribed to Vtn, its function during renal fibrogenesis is either nonessential or redundant, possibly due to the expression of other extracellular matrix proteins that can serve similar roles when Vtn is not available. The potential pathways involved are multiple and complex. It remains possible that a different outcome might occur in other pathological conditions when Vtn expression is absent.
GRANTS
The authors gratefully acknowledge research grant support from the National Institutes of Health Grants DK54500 (to A. A. Eddy) and DK44757 (to A. A. Eddy) and Seattle Children's Research Institute. We also thank Dr. R.-P. Czekay, Center for Cell Biology and Cancer Research, Albany Medical College, NY for providing the anti-vitronectin antiserum.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The excellent technical assistance of Ayelet Goldshmid-Shagal is acknowledged.
REFERENCES
- 1. Bariety J, Hinglais N, Bhakdi S, Mandet C, Rouchon M, Kazatchkine MD. Immunohistochemical study of complement S protein (Vitronectin) in normal and diseased human kidneys: relationship to neoantigens of the C5b-9 terminal complex. Clin Exp Immunol 75: 76–81, 1989 [PMC free article] [PubMed] [Google Scholar]
- 2. Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett 584: 1923–1930, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Colman RW, Pixley RA, Najamunnisa S, Yan W, Wang J, Mazar A, McCrae KR. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J Clin Invest 100: 1481–1487, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dahlback K, Lofberg H, Dahlback B. Immunohistochemical demonstration of vitronectin in association with elastin and amyloid deposits in human kidney. Histochemistry 87: 511–515, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Declerck PJ, De Mol M, Alessi MC, Baudner S, Paques EP, Preissner KT, Muller-Berghaus G, Collen D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin). J Biol Chem 263: 15454–15461, 1988 [PubMed] [Google Scholar]
- 6. Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem 279: 22595–22604, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol 134: 1563–1571, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eddy AA, Fogo AB. PAI-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol 17: 2999–3012, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Eklund AG, Sigurdardottir O, Ohrn M. Vitronectin and its relationship to other extracellular matrix components in bronchoalveolar lavage fluid in sarcoidosis. Am Rev Respir Dis 145: 646–650, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol 5: 864–868, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Francischetti IM, Kotsyfakis M, Andersen JF, Lukszo J. Cyr61/CCN1 displays high-affinity binding to the somatomedin B(1–44) domain of vitronectin. PloS One 5: e9356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hafdi Z, Lesavre P, Nejjari M, Halbwachs-Mecarelli L, Droz D, Noel LH. Distribution of alphavbeta3, alphavbeta5 integrins and the integrin associated protein–IAP (CD47) in human glomerular diseases. Cell Adhes Commun 7: 441–451, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Hafdi Z, Lesavre P, Tharaux PL, Bessou G, Baruch D, Halbwachs-Mecarelli L. Role of alpha v integrins in mesangial cell adhesion to vitronectin and von Willebrand factor. Kidney Int 51: 1900–1907, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Hermann A, Braun A, Figueroa CD, Muller-Esterl W, Fritz H, Rehbock J. Expression and cellular localization of kininogens in the human kidney. Kidney Int 50: 79–84, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Holmes R. Preparation from human serum of an alpha-one protein which induces the immediate growth of unadapted cells in vitro. J Cell Biol 32: 297–308, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Border WA, Lawrence DA, Noble NA. Mechanisms underlying the antifibrotic properties of noninhibitory PAI-1 (PAI-1R) in experimental nephritis. Am J Physiol Renal Physiol 297: F1045–F1054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest 112: 379–388, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inuzuka S, Ueno T, Torimura T, Tamaki S, Sugawara H, Sakata R, Kusaba N, Sata M, Tanikawa K. The significance of colocalization of plasminogen activator inhibitor-1 and vitronectin in hepatic fibrosis. Scand J Gastroenterol 32: 1052–1060, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Isik FF, Gibran NS, Jang YC, Sandell L, Schwartz SM. Vitronectin decreases microvascular endothelial cell apoptosis. J Cell Physiol 175: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Jang YC, Tsou R, Gibran NS, Isik FF. Vitronectin deficiency is associated with increased wound fibrinolysis and decreased microvascular angiogenesis in mice. Surgery 127: 696–704, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kamikubo Y, Neels JG, Degryse B. Vitronectin inhibits plasminogen activator inhibitor-1-induced signaling and chemotaxis by blocking plasminogen activator inhibitor-1 binding to the low-density lipoprotein receptor-related protein. Int J Biochem Cell Biol 41: 578–585, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Konstantinides S, Schafer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation 103: 576–583, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Koukoulis GK, Shen J, Virtanen I, Gould VE. Vitronectin in the cirrhotic liver: an immunomarker of mature fibrosis. Hum Pathol 32: 1356–1362, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Lindahl TL, Sigurdardottir O, Wiman B. Stability of plasminogen activator inhibitor 1 (PAI-1). Thromb Haemost 62: 748–751, 1989 [PubMed] [Google Scholar]
- 25. Lygoe KA, Norman JT, Marshall JF, Lewis MP. AlphaV integrins play an important role in myofibroblast differentiation. Wound Repair Regen 12: 461–470, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol 177: 927–939, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Murphy BF, Davies DJ, Morrow W, d′Apice AJ. Localization of terminal complement components S-protein and SP-40,40 in renal biopsies. Pathology 21: 275–278, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Norman JT, Fine LG. Progressive renal disease: fibroblasts, extracellular matrix, and integrins. Exp Nephrol 7: 167–177, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Oda T, Jung YO, Kim H, Cai X, Lopez-Guisa J, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 30: 587–596, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Ogawa T, Yorioka N, Yamakido M. Immunohistochemical studies of vitronectin, C5b-9, and vitronectin receptor in membranous nephropathy. Nephron 68: 87–96, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Okada M, Yoshioka K, Takemura T, Akano N, Aya N, Murakami K, Maki S. Immunohistochemical localization of C3d fragment of complement and S-protein (vitronectin) in normal and diseased human kidneys: association with the C5b-9 complex and vitronectin receptor. Virchows Arch 422: 367–373, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Patsenker E, Popov Y, Stickel F, Schneider V, Ledermann M, Sagesser H, Niedobitek G, Goodman SL, Schuppan D. Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology 50: 1501–1511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phipps JA, Feener EP. The kallikrein-kinin system in diabetic retinopathy: lessons for the kidney. Kidney Int 73: 1114–1119, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Rahman S, Patel Y, Murray J, Patel KV, Sumathipala R, Sobel M, Wijelath ES. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signaling pathway in endothelial cells. BMC Cell Biol 6: 8, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reilly JT, Nash JR. Vitronectin (serum spreading factor): its localisation in normal and fibrotic tissue. J Clin Pathol 41: 1269–1272, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schar CR, Jensen JK, Christensen A, Blouse GE, Andreasen PA, Peterson CB. Characterization of a site on PAI-1 that binds to vitronectin outside of the somatomedin B domain. J Biol Chem 283: 28487–28496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schoppet M, Chavakis T, Al-Fakhri N, Kanse SM, Preissner KT. Molecular interactions and functional interference between vitronectin and transforming growth factor-beta. Lab Invest 82: 37–46, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Shiiki H, Nishino T, Uyama H, Kimura T, Nishimoto K, Hashimoto T, Fujii Y, Dohi K. Alterations in extracellular matrix components and integrins in patients with preeclamptic nephropathy. Virchows Arch 427: 567–573, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Shikata K, Makino H, Morioka S, Kashitani T, Hirata K, Ota Z, Wada J, Kanwar YS. Distribution of extracellular matrix receptors in various forms of glomerulonephritis. Am J Kidney Dis 25: 680–688, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Smith HW, Marshall CJ. Regulation of cell signaling by uPAR. Nat Rev Mol Cell Biol 11: 23–36, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Stefansson S, Lawrence DA. Old dogs and new tricks: proteases, inhibitors, and cell migration. Sci STKE 2003: pe24, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Upton Z, Webb H, Hale K, Yandell CA, McMurtry JP, Francis GL, Ballard FJ. Identification of vitronectin as a novel insulin-like growth factor-II binding protein. Endocrinology 140: 2928–2931, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Van Dixhoorn MG, Salazar-Exaire D, Sato T, Daha MR, Quigg RJ, Bruijn JA, Couser WG, De Heer E. Anti-vitronectin antibodies enhance anti-Thy-1-induced proteinuria in PVG/c, but not in Wistar rats. J Am Soc Nephrol 9: 994–1007, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest 100: 58–67, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem 269: 32380–32388, 1994 [PubMed] [Google Scholar]
- 47. Wun TC, Palmier MO, Siegel NR, Smith CE. Affinity purification of active plasminogen activator inhibitor-1 (PAI-1) using immobilized anhydrourokinase. Demonstration of the binding, stabilization, and activation of PAI-1 by vitronectin. J Biol Chem 264: 7862–7868, 1989 [PubMed] [Google Scholar]
- 48. Yamaguchi I, Lopez-Guisa JM, Cai X, Collins SJ, Okamura DM, Eddy AA. Endogenous urokinase lacks antifibrotic activity during progressive renal injury. Am J Physiol Renal Physiol 293: F12–F19, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Yamaguchi I, Yamada M, Tchao BN, Burger M, Nicosia RF, Eddy AA. Structural and functional changes to renal peritubular capillaries after unilateral ureteral obstruction in mice. J Am Soc Nephrol 20: TH-PO115, 2009 [Google Scholar]
- 50. Yoon S, Gingras D, Bendayan M. Alterations of vitronectin and its receptor alpha(v) integrin in the rat renal glomerular wall during diabetes. Am J Kidney Dis 38: 1298–1306, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Zhang G, Cai X, Lopez-Guisa JM, Collins SJ, Eddy AA. Mitogenic signaling of urokinase receptor-deficient kidney fibroblasts: actions of an alternative urokinase receptor and LDL receptor-related protein. J Am Soc Nephrol 15: 2090–2102, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Zhang G, Eddy AA. Urokinase and its receptors in chronic kidney disease. Front Biosci 13: 5462–5478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang G, Kernan KA, Collins SJ, Cai X, Lopez-Guisa JM, Degen JL, Shvil Y, Eddy AA. Plasmin(ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: role of plasmin-activated signals. J Am Soc Nephrol 18: 846–859, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Zhang G, Kim H, Cai X, Lopez-Guisa J, Alpers C, Liu Y, Carmeliet P, Eddy A. Urokinase receptor deficiency accelerates fibrosis in obstructive nephropathy. J Am Soc Nephrol 14: 1254–1271, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Zhang G, Kim H, Cai X, Lopez-Guisa J, Carmeliet P, Eddy A. Urokinase receptor modulates cellular and angiogenic responses in obstructive uropathy. J Am Soc Nephrol 14: 1234–1253, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci USA 92: 12426–12430, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.