Abstract
Renal epithelial cell primary cilia act as mechanosensors in response to changes in luminal fluid flow. To determine the role of cilia bending in the mechanosensory function of cilia, we performed proteomic analysis of collecting duct cell lines with or without cilia that were kept stationary or rotated to stimulate cilia bending. Expression of the Raf-1 kinase inhibitor protein (RKIP), an inhibitor of the MAPK pathway, was significantly elevated in rotated cilia (+) cells. This was compared with RKIP levels in cilia (−) cells that were stationary or rotated as well as in cilia (+) cells that were stationary. This result was confirmed in cilia knockout adult mice that had lower renal RKIP levels compared with adult mice with cilia. Downstream of RKIP, expression of phosphorylated ERK was decreased only in cells that had cilia and were subjected to constant cilia bending. Furthermore, elevated RKIP levels were associated with reduced cell proliferation. Blockade of PKC abrogated ciliary bending-induced increases in RKIP. In summary, we found that ciliary movement may help control the expression of the Raf-1 kinase inhibitor protein and thus maintain cell differentiation. In terms of polycystic kidney disease, loss of cilia and therefore sensitivity to flow may lead to reduced RKIP levels, activation of the MAPK pathway, and contribute to the formation of cysts.
Keywords: polycystic kidney disease, renal epithelial cells, renal cysts
primary cilia are nonmotile projections from polarized, highly differentiated epithelial cells (31). In the kidney, primary cilia are present on nearly all epithelial cells and extend from the apical surface into the tubular lumen, where they respond to luminal fluid flow. Numerous studies have demonstrated that cilia bending with changes in apical flow leads to various intracellular signaling events including increases in intracellular calcium concentrations, thereby acting as mechanosensors (20, 23, 30, 32, 36, 38, 40). Recent studies suggest that primary cilia also serve an important role in the maintenance of cell differentiation (3).
Polycystic kidney disease (PKD) is a ciliopathic disorder associated with increased rates of cell proliferation and apoptosis (17), aberrant cellular polarity (29), and abnormalities in protein trafficking (7). The initiation and progression of PKD has been partially attributed to activation of the MAPK pathway (25, 47, 48). This pathway regulates cell proliferation, apoptosis, and protein synthesis, all of which are altered in PKD (22). In models of PKD, it has been proposed that the altered structure/function of cilia may cause activation of various pathways including the MAPK pathway, leading to cell dedifferentiation and proliferation as well as the other phenotypic characteristics of cystic cells.
Since cilia are unable to synthesize proteins, proteins must be able to travel into and out of the cilium for the formation and proper function of cilia (6). Protein transport occurs in the cilium by intraflagellar transport (IFT). Disruption of this system by mutating genes such as ift88 (formally called Tg737), which encodes for the IFT protein polaris, results in cilia defects including loss of or severely stunted cilia (53, 54). The Oak Ridge Polycystic Kidney (orpk) mouse is a hypomorph of the ift88 gene and has severe renal cystic disease as well as other developmental abnormalities. A SV40-temperature-sensitive immortalized orpk collecting duct cell line lacking cilia was developed from this mouse model, along with a cell line in which the wild-type ift88 gene was reinserted to create a cell line that had cilia (54).
In the present study, we used orpk-derived collecting duct cilia (+) and cilia (−) cell lines to identify differences in proteomic profiles between these two cell lines when kept stationary or subjected to constant rotation to induce cilia bending. To our knowledge, proteomic analyses in response to flow-induced bending of cilia have not been previously performed. Proteins were identified with altered levels of expression between cilia (+) and cilia (−) cells and between stationary and rotated cells. Of note, we found that the levels of expression of the Raf-1 kinase inhibitor protein (RKIP) appeared to be affected by ciliary motion. This was of interest as RKIP is an inhibitor of MAPK signaling and has been shown to play a major role in the pathogenesis of certain forms of cancer. This prompted us to perform additional in vitro and in vivo studies to more closely examine the association between RKIP and cilia.
MATERIALS AND METHODS
Cell culture.
Collecting duct cells derived from the orpk mouse model expressing a hypomorphic allele of the Tg737 gene [pCDNA cells, cilia (−)] and genetically rescued cells with the wild-type orpkTg737 gene [BAP2 cells, cilia (+)] were generously donated by Dr. Bradley Yoder (University of Alabama at Birmingham; UAB) (54). Cells were handled identically and grown to confluence in DMEM/F12 media with 0.2 μg/ml dexamethasone, 10 nM triiodothrionine, 1× insulin-transferrin-sodium selenite, 12 U/ml IFN-γ, 268 μg/ml G418, 1% penicillin-streptomycin, and 5% FBS at 33°C, 5% CO2. When confluent, cells were placed at 37°C, 5% CO2 in complete media without IFN-γ or G418 for 5 days until differentiated. All media additives were from Sigma (St. Louis, MO) except for FBS (Thermo Scientific, Waltham, MA). Media was from Mediatech (Manassas, VA). For generation of cilia movement, differentiated cells were subjected to shear stress on an orbital rotator at 1 Hz (33).
Ift88 mouse.
Development of the ift88 (originally referred to as the Tg737 mouse) floxed allele mouse has been reported in a previous study (16). This mouse line was transferred from UAB to the Medical University of South Carolina (MUSC) where this work was performed. The ift88 conditional mutant allele was generated such that exons 4–6 would be deleted upon Cre recombinase-mediated excision, resulting in a null allele (see Refs. 10 and 16 for a complete description of this mouse). All mice were maintained in accordance with Institutional Animal Care and Use Committee regulations at the MUSC. Genotyping of mice was performed as previously described (16).
For induction of Cre activity, tamoxifen administration was performed once daily for 5 consecutive days when mice were between 8 and 12 wk of age. Tamoxifen (Sigma) dissolved in corn oil (Sigma) was administered (0.5 ml of 10 mg/ml tamoxifen ip). Mice were euthanized 3 wk after tamoxifen injection by isoflurane overdose followed by aortic transection. Kidney tissue was removed and snap-frozen in liquid nitrogen or preserved in 10% buffered formalin.
Two-dimensional gel electrophoresis.
Cilia (+) and cilia (−) cells were maintained as described and were incubated in a stationary state or rotated at 1 Hz for 12 h. Cell lysates were collected in a two-dimensional gel electrophoresis (2DE)-compatible buffer [7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS)] and lysed by tip sonication for 5 min on ice. Lysates were vortexed for 20 min at room temperature and centrifuged at 13,000 g for 10 min at 4°C. The supernatant was removed, and protein concentration was determined by Bradford assay. Rehydration buffer [7 M urea, 2 M thiourea, 4% CHAPS, 50 mM DTT, 0.2% biolytes pH 3–10 (Bio-Rad, Hercules, CA)] was added to 125 μg protein to a volume of 200 μl. Proteins were focused using immobilized pH gradient strips (IPG 4–7, Bio-Rad) in a Protean IEF cell (Bio-Rad) for 35,000 V-h with a maximum voltage of 8,000 V and a maximum current of 50 μA/strip. A second dimension separation by SDS-PAGE utilized 11 cm, precast, 4–12% Bis acrylaminde gels (Bio-Rad). Proteins were visualized by Sypro Ruby staining (Bio-Rad) and imaging (FX Pro Plus, Bio-Rad) at 50-μm resolution. Spots were automatically detected and manually edited using Same Spots Progenesis software (Nonlinear Dynamics, Durham, NC). Log-transformed spot volumes were normalized to total spot volume, and comparisons were made across the four groups. Significant protein spots were robotically excised, and proteins were in-gel digested using trypsin gold (Promega, Madison, WI). Peptides were purified by Zip Tip (Millipore, Billerica, MA) and manually spotted to matrix-assisted laser desorption/ionization (MALDI) target plates.
Mass spectrometry-protein identifications.
Proteins were identified using tandem mass spectrometry. Tryptic peptides were analyzed using a 4800 MALDI-TOF-TOF (Applied Biosystems, Carlsbad, CA) mass spectrometer. The top 12 ions were selected for tandem mass spectrometry, and data were searched in MASCOT (Matrix Science, Boston, MA) against the NCBI mammalian database. Modifications allowed include oxidation of methionine, carbamidomethylation of cysteine, one missed tryptic cleavage, and peptide tolerance of 300 ppm. Peptide mass fingerprinting was used to confirm identifications for select proteins as described previously (24). Data were analyzed through the use of Ingenuity Pathways Analysis (IPA, version 8.0; Ingenuity Systems, www.ingenuity.com). Canonical pathways analysis identified the pathways from the Ingenuity Pathways Analysis library of canonical pathways that were most significant to the data set.
Western blot analysis.
Cilia (+) and cilia (−) cells were maintained as described and were incubated in a stationary state or rotated at 1 Hz for 2 or 12 h. Cells were collected in ice-cold RIPA buffer with 1× protease/phosphatase inhibitors (Thermo Scientific). Mouse kidney tissue was homogenized, and proteins were extracted using Qiagen's AllPrep DNA/RNA/Protein Mini Kit (Valencia, CA). Protease and phosphatase inhibitors were added to each sample. Samples (∼40 ug protein) were reduced with Tris(2-carboxyethyl)phosphine (Thermo Scientific), and proteins were separated by SDS-PAGE on Tris-glycine gels (Bio-Rad). Proteins were transferred to nitrocellulose and immunoblotted with anti-PBP (RKIP; Abcam, Cambridge, MA), anti-phospho-p44/42 MAPK (Thr202/Tyr204; Cell Signaling, Danvers, MA), or anti-ERK1+ERK2 (Abcam). All membranes were blocked in 5% BSA. Immunoblots were visualized using ECL plus (GE Healthcare, Piscataway, NJ) on a Typhoon Trio instrument (GE Healthcare).
Proliferation assays.
Cilia (+) and cilia (−) cells were maintained as described in media without phenol red. Following differentiation, cells were collected and plated at 10,000 cells/well in 96-well plates and allowed to adhere overnight. Plates were incubated in a stationary state or rotated at 1 Hz for 0, 12, 24, or 48 h. Fresh media was added every other day. Cell proliferation as measured by DNA content was determined using a CyQUANT cell proliferation assay (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. DNA content was determined by comparing to a standard curve.
Immunohistochemistry.
Paraffin-embedded 5-μm sections of mouse kidney tissue were stained using an immunoperoxidase method (Vectastain Elite ABC System, Vector Labs, Burlingame, CA) for RKIP (1:250 anti-PBP, Abcam) according to the protocol. ImmPACT diaminobenzidine (DAB; Vector Labs) was applied for color development, and sections were counterstained with hematoxylin. RKIP staining in 10 fields/section at ×40 magnification (Leica DMI 4000 B, Leica Microsystems, Bannockburn, IL) was determined by densitometry using Scion Image 4.0.3.2. Sections incubated without primary antibody were used as a negative control.
PKA, PKC, and calcium inhibition.
Cilia (+) and cilia (−) cells were maintained as described. Following differentiation, inhibitors/chelators of PKA (50 μm Rp-8-Cl-cAMPS, Na; EMD Biosciences, San Diego, CA), PKC (20 nM bisindolylmaleimide I, EMD Biosciences), and calcium (20 μM BAPTA-AM, Invitrogen) were added in fresh media immediately before incubation in a stationary state or rotation for 12 h.
Statistics.
Protein spots determined significantly different in abundance by ANOVA corrected for false discovery rate (q-value) were submitted for post hoc analysis. Pairwise comparisons were made by the Holms-Sidak test to determine differences between individual groups using SigmaStat (Aspire Software International, Ashburn, VA).
Data are expressed as means ± SE, and the statistical significance of differences in mean values was assessed by Student's t-test or one-way ANOVA with Tukey's post hoc test, as appropriate, using GraphPad Prism (La Jolla, CA). Differences between means were considered significant at values of P < 0.05.
RESULTS
Proteomic identification of differentially expressed proteins following cilia bending.
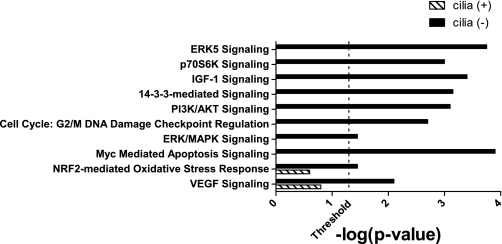
Forty-eight protein spots were differentially abundant between the four groups of cells (q < 0.05), and 46 were identified by tandem mass spectrometry. Proteins that were statistically different between stationary and rotated cilia (+) and cilia (−) cells are listed in Supplemental Table S1 (supplementary material for this article is available online at the journal web site). Proteins whose expression changed in either stationary or rotated cells are listed in Table 1. Phosphatidylethanolamine binding protein 1 (RKIP) abundance was not different between stationary cilia (+) and cilia (−) cells; however, RKIP was elevated 1.30 ± 0.04-fold in cilia (+) cells that were rotated compared with stationary cilia (+) cells. RKIP abundance did not change in cilia (−) cells when shear stress was applied. Only RKIP exhibited this pattern of differential abundance. All identified proteins were analyzed using Ingenuity Pathway Analysis software to determine canonical pathways represented by the differentially abundant proteins. Several pathways were altered in rotated cilia (−) cells that are involved in cell proliferation (Fig. 1).
Table 1.
Differentially abundant proteins in stationary or rotated cells by 2-dimensional gel analysis
| Stationary Cilia (+) vs. Cilia (−) |
Rotated Cilia (+) vs. Cilia (−) |
||
|---|---|---|---|
| Protein name | Fold-change | Protein name | Fold-change |
| Enolase | 1.3 | Keratin Hb5 | 1.9 |
| Heat shock protein 65 | 1.2 | Cyclophilin A | 1.5 |
| Cytosolic malate dehydrogenase | 1.2 | Raf-1 kinase inhibitor protein | 1.3 |
| Heterogeneous nuclear ribonucleoprotein H1 | 1.2 | EndoA cytokeratin | −1.5 |
| Tropomyosin 3-γ | −1.3 | Ezrin | −1.4 |
| γ -Actin | −1.2 | Annexin A5 | −1.2 |
| 14-3-3-ζ | −1.1 | Proliferating cell nuclear antigen | −1.2 |
Shown are proteins identified by 2-dimensional gel analysis as being significantly different in either stationary or rotated cells when protein abundance in cilia (+) is compared with cilia (−) cells. Fold-change indicated represents whether protein level is higher or lower in cilia (+) cells.
Fig. 1.
Canonical pathways altered by rotation in cilia (+) or cilia (−) cells. Significantly different proteins identified following 2-dimensional gel electrophoresis of stationary and rotated cilia (+) and cilia (−) cells were analyzed using Ingenuity Pathway Analysis. The threshold indicates the point at which the probability that the association between proteins in the data set and the canonical pathway is not explained by chance alone. Only pathways crossing the threshold for 1 group [cilia (+) or cilia (−)] are shown.
Cilia bending increases levels of RKIP.
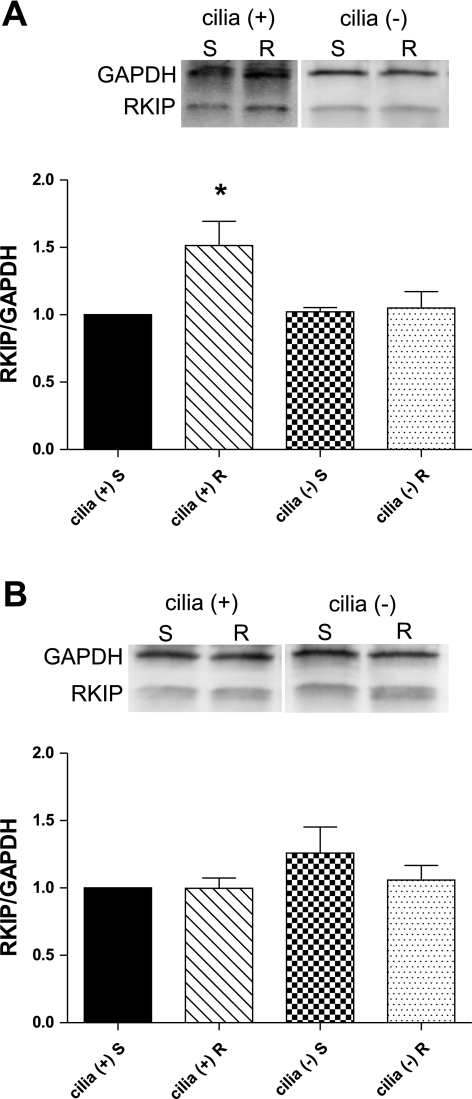
To verify the results of the proteomic experiments, differentiated cilia (+) and cilia (−) cells were rotated at 1 Hz to stimulate cilia bending or placed in a stationary state for 2 or 12 h. Western blot analysis revealed that RKIP levels were statistically higher in cilia (+) cells rotated for 12 h (1.51 ± 0.18), but not for 2 h (1.00 ± 0.08) (Fig. 2). RKIP levels in cilia (−) cells did not differ significantly from stationary cilia (+) cells at 12 h (1.02 ± 0.03 stationary, 1.05 ± 0.12 rotating) or at 2 h (1.26 ± 0.19 stationary, 1.06 ± 0.11 rotating). These results confirmed that RKIP levels are increased following bending of cilia.
Fig. 2.
Western blot analysis for Raf-1 kinase inhibitor protein (RKIP) in cilia (+) and cilia (−) cells at 12 (A) or 2 h (B). Cells were incubated stationary (S) or rotated (R) at 1 Hz to stimulate cilia bending. RKIP levels were normalized to GAPDH, and relative band density is presented compared with RKIP levels in stationary cilia (+) cells. *P < 0.05; n = 5.
Cilia bending correlates with reduced levels of pERK.
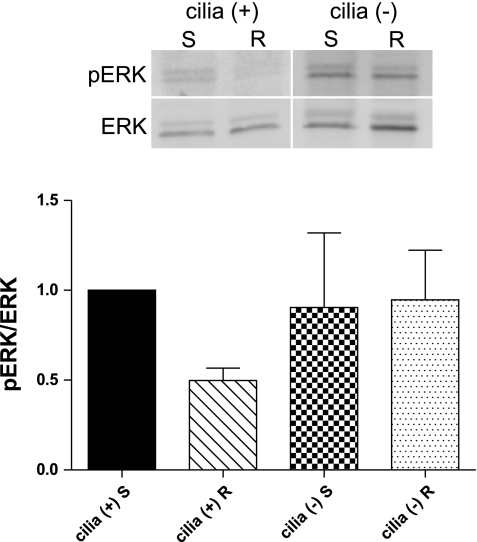
Differentiated cilia (+) and cilia (−) cells were rotated at 1 Hz to stimulate cilia bending or placed in a stationary state for 12 h. Western blot analysis revealed that pERK levels were lower in rotated cilia (+) cells compared with stationary cilia (+) cells (0.50 ± 0.07), whereas pERK levels in cilia (−) cells were not significantly different from stationary cilia (+) cells (0.90 ± 0.42 stationary, 0.95 ± 0.28 rotating) (Fig. 3). This finding suggests that the increased levels of RKIP function to suppress signaling through the MAPK pathway.
Fig. 3.
Western blot analysis for phosphorylated ERK (pERK) in cilia (+) and cilia (−) cells at 12 h. Cells were incubated while stationary (S) or rotated (R) at 1 Hz to stimulate cilia bending. pERK levels were compared with total ERK levels, and relative band density is presented compared with stationary cilia (+) cells. Although not significant by 1-way ANOVA, there was significance between stationary and rotating conditions in cilia (+) cells when examined by t-test; n = 5.
Cellular proliferation rates and cilia bending.
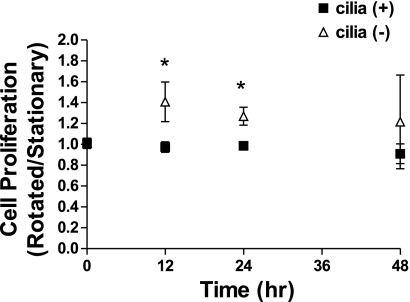
Differentiated cilia (+) and cilia (−) cells were rotated at 1 Hz to stimulate cilia bending or placed in a stationary state for 0, 12, 24, or 48 h, and cell proliferation was determined at each time point by measuring the total amount of DNA. When the proliferation of rotated cells compared with stationary cells was examined, rotated cilia (−) cells had higher rates of proliferation at 12 (1.41 ± 0.19) and 24 h (1.27 ± 0.09) while cilia (+) cells had similar rates between conditions (0.97 ± 0.05 12 h, 0.99 ± 0.04 24 h) (Fig. 4), suggesting that ciliary movement helps suppress proliferation. This finding also supports the suggestion that enhanced RKIP expression upon cilia bending helps to suppress signaling through the MAPK pathway, one of the primary pathways controlling cellular proliferation.
Fig. 4.
Cellular proliferation as determined by DNA content for cilia (+) and cilia (−) cells. Cells were incubated stationary or rotated at 1 Hz to stimulate cilia bending for 0, 12, 24, or 48 h. DNA content was determined and is presented as amount in rotated cells compared with amount in stationary cells at each time point. *P < 0.05 between cilia (+) and cilia (−); n = 3 (0 h) and n = 4 (12, 24, and 48 h).
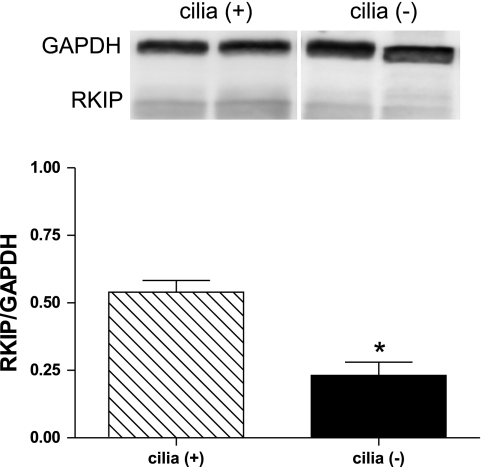
RKIP levels are significantly reduced in mice lacking cilia.
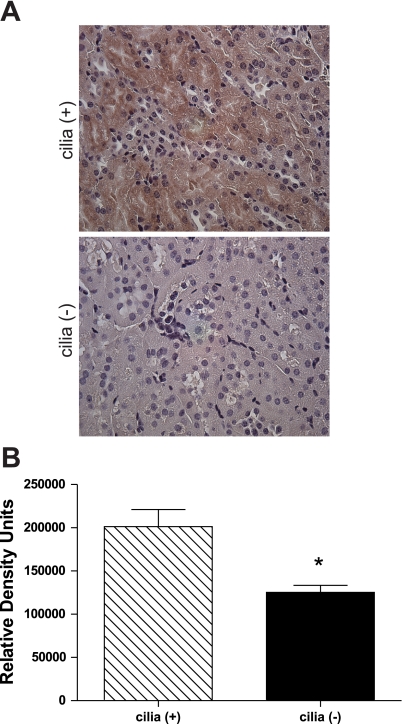
Mice with a conditional floxed allele to the ift88 gene were given tamoxifen to knockout the IFT protein polaris. After 3 wk, >80% of polaris and cilia were absent from epithelial cells in the kidney (16). RKIP levels from mouse kidney tissue lysates were determined by Western blot analysis. Mice in which cilia had been depleted expressed significantly lower levels of RKIP (0.23 ± 0.05) than mice with cilia (0.54 ± 0.04) (Fig. 5). Additionally, staining for RKIP in mouse kidney sections identified that not only are RKIP levels significantly lower in cilia (−) mice but also RKIP staining is most predominate in proximal convoluted tubules of cilia (+) mice (Fig. 6). These findings support the in vitro results, suggesting that cilia expression in vivo does correlate with RKIP levels.
Fig. 5.
Western blot analysis for RKIP in total kidney lysate from mice with or without cilia. RKIP levels were normalized to GAPDH, and relative band density is shown. *P < 0.05; n = 6 [cilia (+)] and n = 8 [cilia (−)].
Fig. 6.
Immunohistochemical analysis of RKIP in mouse kidney sections. A: kidney sections from cilia (+) and cilia (−) mice were stained for RKIP and imaged at ×40 magnification in a random order. B: densitometry analysis determined the relative density of staining. *P < 0.05; n = 3 [cilia (+)] and n = 4 [cilia (−)], 1 slide/mouse, 10 fields/slide.
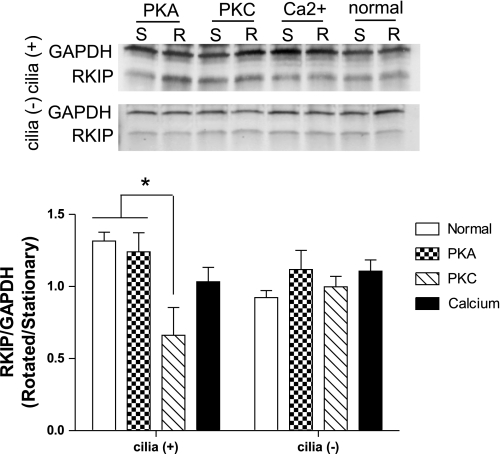
RKIP levels with cilia bending are diminished by a PKC inhibitor.
Differentiated cilia (+) and cilia (−) cells were rotated at 1 Hz to stimulate cilia bending or placed in a stationary state for 12 h following the addition of inhibitors for PKA, PKC, or the calcium chelator BAPTA. Western blot analysis revealed that the inhibitors had no significant effect on RKIP levels in cells without cilia; however, the PKC inhibitor significantly reduced the levels of RKIP in rotated cilia (+) cells (Fig. 7). The reduction of RKIP back to near baseline levels in cilia (+) cells suggests that PKC has a role in RKIP regulation in response to cilia bending.
Fig. 7.
Western blot analysis for RKIP in cilia (+) and cilia (−) cells following incubation with inhibitors for PKA, PKC, or a calcium chelator for 12 h. Cells were incubated while stationary (S) or rotated (R) at 1 Hz immediately after addition of treatment. RKIP levels were normalized to GAPDH, and relative band density is presented as amount in rotated cells compared with amount in stationary cells. *P < 0.05; n = 3.
DISCUSSION
Primary cilia at the apical membrane of renal epithelial cells act as mechanosensors by sensing the external environment and transmitting this information into the cells (31). One consequence of this information transfer is to alter the expression pattern and function of cellular proteins. A goal of this study was to identify differentially expressed proteins in stationary kidney collecting duct cell lines with or without cilia and then compare this to the pattern of protein expression in rotated cells, which would induce cilia bending. One protein was identified that had significantly elevated expression in cilia (+) cells upon cilia bending: RKIP.
The MAPK signaling pathway involves signaling through the Ras/Raf/MEK/ERK cascade. This pathway is important for various cellular functions including growth and differentiation. Since this pathway is critical for a number of cellular processes, its expression can be activated/repressed by numerous receptors and control of this pathway can occur at multiple points, especially at the level of Raf-1 (22). RKIP has been found to inhibit Raf-1 activity and therefore keep the MAPK pathway in check (46, 51). RKIP also inhibits transcription of NF-κB (44, 52) as well as inhibiting G protein-coupled receptor (GPCR) signaling by regulating G protein-coupled receptor kinase-2 (GRK2) (21). Since RKIP is a regulator of intracellular signaling pathways, especially inhibition of Raf-1 kinase, this protein has been studied in cancer metastasis. Reduced RKIP levels have been linked to human metastatic tumors from prostate (13, 18), breast (8, 15), colorectal (2), and skin (37), while RKIP overexpression in prostate (12, 13), liver (19), and skin (37) metastatic cell lines resulted in decreased tumor invasiveness. Additionally, RKIP has been shown to regulate the mitotic spindle checkpoint and has also been linked to chromosomal instabilities (1, 11, 46). The fact that RKIP expression appears to be affected by ciliary bending is intriguing in light of the fact that PKD is considered to be a ciliopathic disease. This prompted us to further assess the role of cilia bending in controlling RKIP expression.
To specifically examine RKIP expression, collecting duct cilia (+) and cilia (−) cells were grown stationary or were rotated to stimulate cilia bending. Due to the rotation method used, it is possible that all cells were not exposed to the same amount of shear stress; however, the centripetal force generated by rotation was satisfactory for cilia bending. Western blot analysis of cellular lysate revealed that RKIP expression was the same in cilia (+) and cilia (−) cells that were stationary and was similar to cilia (−) cells that were rotated. Only in cilia (+) cells subjected to rotation to stimulate cilia bending was there an elevation in RKIP levels. This elevation was seen after 12 h of rotation but not after 2 h. This suggests that cilia bending may be inducing RKIP synthesis and is consistent with the data obtained by proteomic analysis. To determine whether there were actual differences in downstream MAPK signaling, we measured pERK abundance in cilia (+) and cilia (−) cells with and without rotation. pERK was only suppressed in cilia (+) cells that were rotated, suggesting that this was the result of the elevated RKIP levels.
Additional studies were performed to measure cell proliferation in stationary and rotated cilia (+) and cilia (−) cells. We found that rotated cilia (−) cells had elevated rates of cell proliferation compared with stationary cells. This was also seen in the proteomic experiments, as pathways involved in cell proliferation were altered in rotating cilia (−) cells (Fig. 1) and levels of proliferating cell nuclear antigen were increased in rotated cilia (−) cells compared with all other groups (Supplemental Table S1). It is possible that rotation would lead to enhanced mixing of cell culture media and would increase the availability of nutrients and oxygen to growing epithelial cells. Presumably, this same effect should have occurred in the cilia (+) cells. Regardless, the difference in proliferation rates between rotated cilia (+) and cilia (−) cells supports the finding of RKIP upregulation in rotated cilia (+) cells and the idea that RKIP may be functionally important in limiting proliferation. Unfortunately, there is a lack of pharmacological tools that specifically inhibit RKIP. In addition, there are at least five RKIP-like sequences in the mouse genome, with data on renal expression being scarce (45). As small-molecule drug design and use were beyond the scope of this study, we were unable to verify that the changes in the rate of cell proliferation were unequivocally due to the changes in RKIP expression. However, RKIP helps regulate the mitotic spindle checkpoint, and loss of RKIP has been shown to increase the rate at which cells progress through mitosis (11). Therefore, an increase in RKIP should control the rate of cell proliferation, such as seen here.
RKIP acts to reduce the MAPK signaling response to stimuli such as EGF (14, 46). This is of interest in PKD research since cystic fluid has been shown to contain a higher concentration of EGF receptor (EGFR) ligands (35, 42, 50, 55). In addition, EGFR mislocalizes to the apical membrane in several models of PKD so that EGFRs are exposed to cystic fluid and thus high levels of EGFR ligands (26, 34). Therefore, RKIP diminution in cystic cells may be even more severe as EGF signaling is increased, leading to increased MAPK signaling. Additionally, RKIP has been shown to regulate B-Raf activity in addition to Raf-1 kinase activity (28). This is interesting as cAMP activation of B-Raf is believed to be one of the main mechanisms of ERK activation in PKD (27, 47, 49). It is important to note that our studies do not provide a direct link between RKIP and cystogenesis. We present the important finding that RKIP is regulated by cilia.
To confirm that RKIP levels are regulated by cilia in vivo, mice containing a conditional floxed allele for the ift88 gene were used to generate cilia (+) and cilia (−) mice. RKIP levels were measured 3 wk after tamoxifen treatment, which is well before the presence of discernable cysts in the cilia (−) mice. RKIP levels were significantly decreased in the kidneys of cilia (−) mice, confirming that cilia are important for RKIP expression. When examining RKIP expression in the kidney by immunohistochemical staining, it appeared that RKIP levels were highest in the proximal tubules and appeared to be lower in distal tubular segments in the cilia (+) kidneys. Cysts predominately form in the collecting ducts or distal tubules (27, 29, 39, 43), leading us to speculate that perhaps the lower levels of RKIP in collecting duct cells make this segment more susceptible to cystic formation.
To examine potential regulators of RKIP expression in cilia (+) and cilia (−) cells, we utilized inhibitors of PKA and PKC, as well as the calcium chelator BAPTA. RKIP expression was decreased upon cilia bending with the PKC inhibitor, suggesting that PKC is involved in the regulation of RKIP expression by cilia. This was an interesting finding since PKC is known to phosphorylate RKIP, leading to RKIP dissociation from Raf-1 and subsequent inhibition of GRK2 (9, 21). Although PKC is known to play a role in the modulation of RKIP function (MAPK vs. GPCR), it is somewhat surprising that it may play a role in RKIP expression. One scenario is that cilium bending increases cytosolic calcium concentrations, leading to the activation of a calcium-sensitive isoform of PKC, which then stimulates RKIP expression. In this regard, the calcium chelator BAPTA tended to decrease RKIP levels, although not significantly. However, it is very difficult to know how effectively BAPTA is influencing cell calcium levels over the course of hours. The expression of RKIP in metastatic cell lines is repressed by the zinc finger transcription factor SNAIL, which is also known to modulate epithelial-mesenchymal transition (5). SNAIL expression is reduced upon inhibition of NF-κB (4), suggesting that NF-κB activation leads to the induction of SNAIL and reduction of RKIP. Whether a similar relationship among NF-κB, SNAIL, and RKIP exists in cilia (−) cells as well as whether PKC inactivation leads to changes in SNAIL expression in cilia (+) cells remains to be investigated.
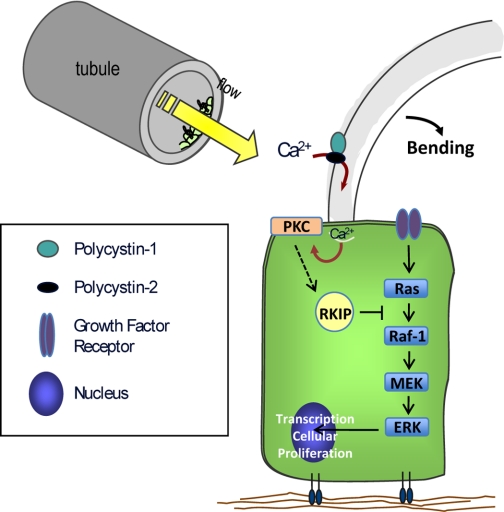
Although this is the first known work examining proteomic alterations following cilium bending, widespread gene expression analysis has been performed in renal cystic tissues (41). While several genes involved in MAPK signaling were upregulated (including Raf-1 and ERK1), RKIP gene expression was downregulated 2.4-fold. This downregulation of gene expression in tissue derived from human autosomal dominant PKD cysts supports our findings of decreased RKIP protein expression in cells that do not have cilia. We believe therefore that bending of the primary cilium leads to enhanced RKIP expression, potentially downstream of PKC activation, which then diminishes MAPK signaling and helps to control cellular proliferation (Fig. 8). When the cilia are lost or function is inhibited, as in PKD, RKIP expression is decreased, leading to enhanced MAPK signaling and cellular proliferation.
Fig. 8.
Schematic of proposed signaling pathway in normal kidney cells. Cilium bending in response to tubular flow allows for calcium entry into the cell. This results in the translocation of PKC to the cell membrane, where it leads to an increase in RKIP. RKIP then suppresses signaling through the MAPK/ERK pathway, leading to control of cellular proliferation and maintenance of cell differentiation.
In summary, we have identified through proteomic analysis that expression of the Raf-1 kinase inhibitor protein is significantly increased upon cilia bending, and this correlates with decreased levels of pERK and therefore inhibition of the MAPK pathway. Cilia movement may modify RKIP levels through the activation of PKC. These findings suggest that it may not be just the presence of cilia but rather ciliary movement that is essential for the maintenance of cell differentiation and suppression of cell proliferation and that this occurs, at least in part, through regulation of the Raf-1 kinase inhibitor protein.
GRANTS
This work was supported by Veterans Affairs (VA) Merit Awards (P. D. Bell, J. M. Arthur), a VA Career Development Award (M. G. Janech), National Institutes of Health (NIH) Grants DK32032 (P. D. Bell), P30 DK074038 (P. D. Bell; UAB Core Center, Lisa Guay-Woodford, PI) and T32 DK007752 (K. M. Sas; P. D. Bell, PI). Support was also provided by Dialysis Clinic, Inc., and a Nephcure Foundation Young Investigator Award (M. G. Janech). Foundation Young Investigator Award (M. G. Janech). This work was conducted in a facility constructed with support from NIH Grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Alison Bland and May Amira for technical assistance and B. J. Harris for administrative support.
REFERENCES
- 1. Al-Mulla F, Hagan S, Al-Ali W, Jacob SP, Behbehani AI, Bitar MS, Dallol A, Kolch W. Raf kinase inhibitor protein: mechanism of loss of expression and association with genomic instability. J Clin Pathol 61: 524–529, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Al-Mulla F, Hagan S, Behbehani AI, Bitar MS, George SS, Going JJ, Garcia JJ, Scott L, Fyfe N, Murray GI, Kolch W. Raf kinase inhibitor protein expression in a survival analysis of colorectal cancer patients. J Clin Oncol 24: 5672–5679, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet 75: 107–117, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Baritaki S, Yeung K, Palladino M, Berenson J, Bonavida B. Pivotal roles of snail inhibition and RKIP induction by the proteasome inhibitor NPI-0052 in tumor cell chemoimmunosensitization. Cancer Res 69: 8376–8385, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene 27: 2243–2248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet 151: 263–280, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Charron AJ, Nakamura S, Bacallao R, Wandinger-Ness A. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J Cell Biol 149: 111–124, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R, Braastad C, Sun Y, Mukhopadhyay A, Aggarwal BB, Darnowski J, Pantazis P, Wyche J, Fu Z, Kitagwa Y, Keller ET, Sedivy JM, Yeung KC. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem 279: 17515–17523, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem 278: 13061–13068, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 17: 1586–1594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eves EM, Shapiro P, Naik K, Klein UR, Trakul N, Rosner MR. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol Cell 23: 561–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate 66: 248–256, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst 95: 878–889, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Granovsky AE, Rosner MR. Raf kinase inhibitory protein: a signal transduction modulator and metastasis suppressor. Cell Res 18: 452–457, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Hagan S, Al-Mulla F, Mallon E, Oien K, Ferrier R, Gusterson B, Garcia JJ, Kolch W. Reduction of Raf-1 kinase inhibitor protein expression correlates with breast cancer metastasis. Clin Cancer Res 11: 7392–7397, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development 134: 307–316, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Ibrahim S. Increased apoptosis and proliferative capacity are early events in cyst formation in autosomal-dominant, polycystic kidney disease. ScientificWorldJournal 7: 1757–1767, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller ET, Fu Z, Brennan M. The biology of a prostate cancer metastasis suppressor protein: Raf kinase inhibitor protein. J Cell Biochem 94: 273–278, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology 131: 1208–1217, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 426: 574–579, 2003 [DOI] [PubMed] [Google Scholar]
- 22. McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773: 1263–1284, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays 26: 844–856, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Nazeer K, Janech MG, Lin JJ, Ryan KJ, Arthur JM, Budisavljevic MN. Changes in protein profiles during course of experimental glomerulonephritis. Am J Physiol Renal Physiol 296: F186–F193, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Omori S, Hida M, Fujita H, Takahashi H, Tanimura S, Kohno M, Awazu M. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol 17: 1604–1614, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int 47: 490–499, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Park EY, Sung YH, Yang MH, Noh JY, Park SY, Lee TY, Yook YJ, Yoo KH, Roh KJ, Kim I, Hwang YH, Oh GT, Seong JK, Ahn C, Lee HW, Park JH. Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem 284: 7214–7222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park S, Yeung ML, Beach S, Shields JM, Yeung KC. RKIP downregulates B-Raf kinase activity in melanoma cancer cells. Oncogene 24: 3535–3540, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol 67: 515–529, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Resnick A, Hopfer U. Force-response considerations in ciliary mechanosensation. Biophys J 93: 1380–1390, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest 101: 935–939, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rohatgi R, Zavilowitz B, Vergara M, Woda C, Kim P, Satlin LM. Cyst fluid composition in human autosomal recessive polycystic kidney disease. Pediatr Nephrol 20: 552–553, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298: F1096–F1102, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Schuierer MM, Bataille F, Hagan S, Kolch W, Bosserhoff AK. Reduction in Raf kinase inhibitor protein expression is associated with increased Ras-extracellular signal-regulated kinase signaling in melanoma cell lines. Cancer Res 64: 5186–5192, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol Renal Physiol 272: F132–F138, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290: F1320–F1328, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18: 2328–2343, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Sullivan LP, Wallace DP, Grantham JJ. Chloride and fluid secretion in polycystic kidney disease. J Am Soc Nephrol 9: 903–916, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Takakura A, Contrino L, Beck AW, Zhou J. Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol 19: 2351–2363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang H, Park S, Sun SC, Trumbly R, Ren G, Tsung E, Yeung KC. RKIP inhibits NF-kappaB in cancer cells by regulating upstream signaling components of the IkappaB kinase complex. FEBS Lett 584: 662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Theroux S, Pereira M, Casten KS, Burwell RD, Yeung KC, Sedivy JM, Klysik J. Raf kinase inhibitory protein knockout mice: expression in the brain and olfaction deficit. Brain Res Bull 71: 559–567, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trakul N, Rosner MR. Modulation of the MAP kinase signaling cascade by Raf kinase inhibitory protein. Cell Res 15: 19–23, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi T, Pelling JC, Ramaswamy NT, Eppler JW, Wallace DP, Nagao S, Rome LA, Sullivan LP, Grantham JJ. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int 57: 1460–1471, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Ye M, Grant M, Sharma M, Elzinga L, Swan S, Torres VE, Grantham JJ. Cyst fluid from human autosomal dominant polycystic kidneys promotes cyst formation and expansion by renal epithelial cells in vitro. J Am Soc Nephrol 3: 984–994, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401: 173–177, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Yeung KC, Rose DW, Dhillon AS, Yaros D, Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol 21: 7207–7217, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol 18: 1381–1388, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res 315: 602–610, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]