Abstract
Na+ absorption and K+ secretion in the distal segments of the nephron are modulated by the tubular flow rate. Epithelial Na+ channels (ENaC), composed of α-, β-, and γ-subunits respond to laminar shear stress (LSS) with an increase in open probability. Higher vertebrates express a δ-ENaC subunit that is functionally related to the α-subunit, while sharing only 35% of sequence identity. We investigated the response of δβγ channels to LSS. Both the time course and magnitude of activation of δβγ channels by LSS were remarkably different from those of αβγ channels. ENaC subunits have similar topology, with an extracellular region connected by two transmembrane domains with intracellular N and C termini. To identify the specific domains that are responsible for the differences in the response to flow of αβγ and δβγ channels, we generated a series of α-δ chimeras and site-specific α-subunit mutants and examined parameters of activation by LSS. We found that specific sites in the region encompassing and just preceding the second transmembrane domain were responsible for the differences in the magnitude and time course of channel activation by LSS.
Keywords: epithelial Na channel
epithelial sodium channels (ENaCs) mediate the rate-limiting step of Na+ absorption in the distal segments of the nephron and have an essential role in the regulation of volume homeostasis and blood pressure (18, 33). Net Na+ absorption and K+ secretion in the collecting ducts are modulated by tubular flow rate (13, 19, 23, 28, 29, 37). Flow-mediated K+ secretion is accomplished by large-conductance Ca2+-activated K+ channels, is largely coupled to Na+ absorption, and requires an increase in intracellular Ca2+ (22). In contrast, flow-mediated Na+ absorption reflects direct activation of ENaC, occurs in the absence of K+ secretion, and is not dependent on changes in intracellular Ca2+ (25).
Four ENaC subunits, α, β, γ, and δ, with differential tissue distribution are found in primates. The α-, β-, and γ-subunits are expressed primarily in Na+-transporting epithelia, such as the distal nephron, airway and alveoli, and distal colon (5). In contrast, the δ-subunit is expressed mainly in the human brain, ovaries, testis, and pancreas (36). Each subunit has two predicted α helical membrane-spanning domains separated by a large extracellular loop that is composed of ∼450 residues (4, 5, 35).
Genetic analysis in the nematode Caenorhabditis elegans identified a group of proteins, structurally related to ENaC, which contribute to gentle body touch sensation and locomotion (14). MEC-4 and MEC-10 apparently constitute the pore-forming subunits of a large mechanosensory channel complex expressed in touch receptor neurons. These channels are postulated to be tethered through their intracellular and extracellular regions to the cytoskeleton and a specialized extracellular matrix, respectively (9, 24). In this model, mechanical deflections generated by gentle touch stimuli are hypothesized to initiate a conformational rearrangement that triggers pore opening and Na+ and Ca2+ influx (2, 9).
Laminar shear stress (LSS) modulates the activity of ENaCs heterologously expressed in Xenopus laevis oocytes. Mutant channels that are constitutively locked in the open conformation do not respond to LSS, suggesting that LSS activates ENaC by increasing channel open probability (7). Furthermore, patch-clamp studies with an outside-out configuration confirmed this finding (1). How these channels sense changes in the magnitude of LSS, and how this results in an increase in channel open probability remain unknown.
We previously showed that mutations in the second transmembrane (TM2) segment of ENaC modify the response to LSS, suggesting that the pore region is involved in the detection or transduction of the mechanical stimulus to the gate (8). We now report that the magnitude and time course of the response of αβγ and δβγ channels to LSS are notably different. We explored the structural basis that determined those differences. We found that the time course of the response to LSS is mainly defined by residues within and just preceding the TM2 segments of the α- and δ-subunits.
MATERIALS AND METHODS
DNA constructs.
Human α- and δ-ENaC subunits were subcloned in the vector pSP64 Poly(A) (Promega). To generate α-δ chimeras, unique restriction sites were introduced into human α- and δ-ENaC subunits. The restriction sites for NsiI and XhoI were introduced at Ile118-Asn119 and Asn536-Ser537 in the α-subunit. As for δ, we introduced the restriction sites for ClaI and XhoI at Val119-Ser120 and Val513-Glu514, respectively. Using specific primers containing the proper restriction sites, we amplified three regions from α- and δ-subunits: 1) the region extending from the N terminus to the end of the first transmembrane segment (N-TM1), 2) the extracellular region (or loop) connecting the transmembrane segments (ECL), and 3) the region extending from the distal part of the ECL to the C terminus (TM2-C). PCR products were digested with the appropriate restriction enzymes and ligated into the αNsiI-XhoI- or δClaI-XhoI-digested constructs, respectively. The chimeras generated are as follow: αN-TM1-δ (α1-117, δ119-638); α-ECL-δ (δ1-120, α121-536, δ513-638); αTM2-C-δ (δ1-514, α538-669); δN-TM1-α (δ1-118, α118-669); δECL-α (α1-119, δ121-546, α571-669); δTM2-C-α (α1-537, δ515-638); αTM2-δ (δ1-514, α538-578, δ556-638); αC-δ (δ1-555, α579-669); δTM2-α (α1-537, δ515-555, α579-669); and δC-α (α1-578, δ556-638). TM2 constructs also included a six-residue linker region immediately preceding TM2, termed the wrist domain in the resolved structure of the acid-sensing ion channel 1 (ASIC1) (15). Human wild-type α-subunit cDNA was used as a template to generate specific point mutations with a QuikChange II XL site-directed mutagenesis kit (Stratagene). All constructs were confirmed by DNA sequencing.
Oocytes expressing αNsiI-XhoIβγ or δClaI-XhoIβγ with the introduced restriction sites responded to LSS with time constants of activation (τ) that were similar to wild-type αβγ and δβγ, respectively (n = 12–14; data not shown). Oocytes expressing δClaI-XhoIβγ or δβγ, exhibited a similar relative increase in whole-cell currents in response to LSS [relative response of 1.69 ± 0.26 (δβγ) and 1.68 ± 0.22 (δNsiI-XhoIβγ)], P = not significant, n = 13–14 [the relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal)]. Oocytes expressing αNsiI-XhoIβγ or αβγ exhibited a modest, but significant difference in the increase in whole-cell current in response to LSS [relative response of 2.13 ± 0.42 (αβγ) and 2.55 ± 0.50 (αNsiI-XhoIβγ), P < 0.05, n = 12–14].
Oocyte expression.
Stage V-VI oocytes were harvested from X. laevis using a protocol approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. cRNAs for human α, β, γ, δ, and α-δ chimeric subunits were synthesized with mMessage mMachine (Ambion, Austin, TX). X. laevis oocytes were injected with 2 ng/subunit of cRNA encoding ENaC subunits. Injected oocytes were maintained at 18°C in modified Barth's saline containing (in mM) 88 NaCl, 1 KCl, 2.4 NaHCO3, 15 HEPES, 0.3 Ca(NO3)2, 0.41 CaCl2, and 0.82 MgSO4, pH 7.4, supplemented with 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulfate, and 100 μg/ml gentamycin sulfate.
Two-electrode voltage clamp.
Two-electrode voltage clamp (TEV) was performed at room temperature (20–25°C) using a GeneClamp 500B amplifier (Axon Instruments, Union City, CA). Data were acquired with Clampex 10.1 using a Digidata 1320 interface (Axon Instruments). Pipettes filled with 3 M KCl had resistances of 0.5–5 MΩ. The extracellular solution (TEV solution) contained (in mM) 110 NaCl, 1 KCl, 1.54 CaCl2, and 10 HEPES, pH 7.4. The recording chamber was perfused at a rate of 3.5 ml/min. LSS was applied by perfusing TEV solution through a vertical pipette (1.8-mm internal diameter) that was placed near the surface of the oocyte as previously described (7). Bath perfusion was stopped during the application of LSS. The vertical pipette perfusion rate was 1.9 ml/min, corresponding to 0.137 dynes/cm2 of shear stress. Whole-cell currents were recorded at −60 mV. ENaC-mediated whole-cell Na+ currents were defined as the benzamil-sensitive component of the current. Benzamil was used at a concentration of 5 or 50 μM as indicated. The relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal).
Data and statistical analyses.
Data are expressed as means ± SE, where n represents the number of independent experiments analyzed. Experiments were repeated with a minimum of two batches of oocytes obtained from different frogs. Electrophysiological data were analyzed with Clampfit 10.1 (Axon Instruments), SigmaPlot 11.0 (SPSS, Chicago, IL), and GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA). The time constant of activation (τ) was determined by fitting experimental data to a first-order exponential function. IC50 values for benzamil are expressed as means with 95% confidence intervals. IC50 values were estimated using normalized benzamil-sensitive currents and plotted as a function of the benzamil concentration fitted by the equation
where X is the concentration of benzamil, and IC50 is the concentration of benzamil that inhibits currents (y) halfway between the baseline and the maximal response.
RESULTS
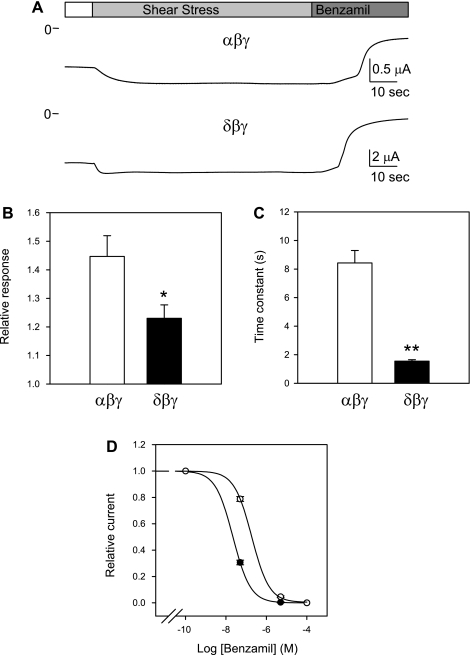
The structural characteristics that enable ENaC to respond to flow have not been clearly determined. We found that the time course and magnitude of activation by LSS of αβγ and δβγ channels were remarkably different (Fig. 1). LSS increased benzamil-sensitive currents in αβγ-injected oocytes by 0.45 ± 0.07-fold, whereas δβγ-injected oocytes responded to flow with a 0.23 ± 0.05-fold increase (P < 0.05, n = 17–19). δβγ Channels exhibit significantly faster activation in response to LSS, with an average time constant (τ) of 1.5 ± 0.1 s compared with 8.4 ± 0.9 s for αβγ channels (P < 0.01, n = 16–18). α- and δ-Subunits share ∼35% amino acid sequence identity. To elucidate the contribution of the different ENaC domains to the response to flow, we generated a series of α-δ chimeras and assessed the response of these channels to LSS. ENaCs are inhibited by the potassium-sparing diuretics amiloride and benzamil, and the apparent affinity for inhibition by these compounds depends of the subunits forming the channel complex. These compounds interact with a putative binding site in the pore of the channel, and sites in the TM2 segments of the ENaC subunits contribute to this interaction (17, 30). Benzamil inhibited αβγ and δβγ with IC50 of 22 nM (CI 20–25 nM) and 187 nM (CI 159–219 nM) (n = 5–8), respectively (Fig. 1D). In our studies, benzamil at a 5 μM concentration was used to inhibit αβγ and chimeras bearing the TM2 segment of the α-subunit, whereas 50 μM was used to inhibit δβγ and chimeras bearing the TM2 segment of the δ-subunit.
Fig. 1.
Activation of αβγ and δβγ channels by laminar shear stress (LSS). A: representative recordings from oocytes expressing αβγ and δβγ channels. The membrane potential was held at −60 mV. LSS was applied by perfusing through a vertical pipette that was placed near the surface of the oocyte as previously described (7). Benzamil (5 and 50 μM) was used to inhibit αβγ and δβγ channels, respectively. B: relative response to LSS of oocytes expressing αβγ and δβγ channels. The relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal). C: time constants (τ) of activation of αβγ and δβγ channels by LSS. The time constant was estimated by fitting the time course of activation by LSS to a first-order exponential function. Statistically significant differences are indicated as *P < 0.05 and **P < 0.01 (n = 16–19) by a Mann-Whitney nonparametric 2-tailed test. D: dose-response curves of inhibition of αβγ and δβγ channels by benzamil. Relative current represents the ratio of the current after benzamil addition to the basal benzamil-sensitive current. Data were fitted to a 3-parameter sigmoid equation as described in materials and methods. Values are means ± SE of 5–8 independent determinations. Benzamil inhibited αβγ (●) and δβγ (○) with IC50 of 22 (CI 20–25 nM) and 187 nM (CI 159–219 nM; n = 5–8), respectively.
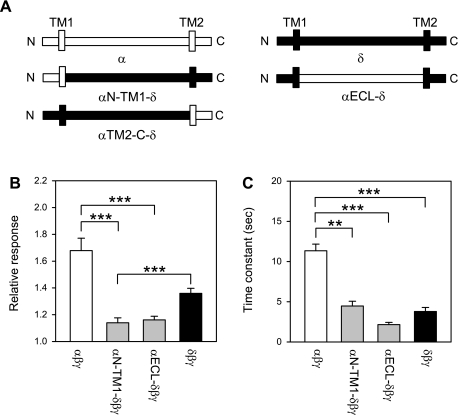
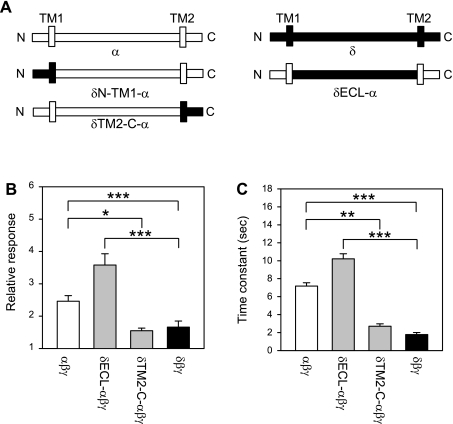
We compared the flow responses in oocytes expressing αβγ, δβγ, as well as channels in which a part of the α-subunit (αN-TM1-δβγ, αECL-δβγ, αTM2-C-δβγ) was inserted into the δ-subunit. The residues contributed by the α- and δ-subunit for each α-δ chimera are indicated in materials and methods, and schematic representations of the constructs are shown in Fig. 2. Oocytes expressing αTM2-C-δβγ did not exhibit ENaC currents. The magnitude and time course of the response elicited by LSS in oocytes expressing αN-TM1-δβγ and αECL-δβγ channels were similar to the response of δβγ-expressing oocytes (Fig. 2). These results suggest that the region extending from the N terminus to the distal part of the ECL does not have a significant contribution to the differences in the magnitude or time course of the response to LSS between αβγ and δβγ. To corroborate our finding, we studied the response to LSS of channels in which a part of the δ-subunit (δN-TM1-αβγ, δECL-αβγ, δTM2-C-αβγ) was inserted into the α-subunit. As above, αβγ and δβγ were used as controls. Oocytes expressing δN-TM1-αβγ channels did not exhibit ENaC currents. The magnitude and time course of the response of δECL-αβγ and δTM2-C-αβγ were comparable to those of αβγ and δβγ channels, respectively (Fig. 3). Our results indicate that the region extending from the TM2 segment to the C terminus defines the magnitude and time course of the response to LSS.
Fig. 2.
Flow response of α-δ chimeras. A: schematic representation of the wild-type α-, αN-TM1-δ, αECL-δ, αTM2-C-δ, and δ-constructs. B: relative response to LSS of wild-type channels and α-δ chimeras. Experiments were performed with oocytes expressing αβγ, αN-TM1-δβγ, αECL-δβγ, and δClaI-XhoIβγ (δβγ channels). The relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal). C: time constants (τ) of activation by LSS of wild-type channels and α-δ chimeras. Statistically significant differences are indicated as **P < 0.01 and ***P < 0.001 (n = 4–27) by a Kruskal-Wallis test following by Dunn's multiple comparisons test. ENaC currents were not observed in oocytes expressing αTM2-C-δ channels.
Fig. 3.
Flow response of δ-α chimeras. A: schematic representation of the wild-type δ, δN-TM1-α, δECL-α, δTM2-C-α, and α-constructs. B: relative response to LSS of wild-type and δ-α chimeras. Experiments were performed with oocytes expressing δβγ, δECL-αβγ, δTM2-C-αβγ, and αNsiI-XhoIβγ (αβγ channels). The relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal). C: time constants (τ) of activation by LSS of wild-type channels and δ-α chimeras. ENaC currents were not observed in oocytes expressing δN-TM1-α channels. Statistically significant differences are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 (n = 15–29) by a Kruskal-Wallis test following by Dunn's multiple comparisons test.
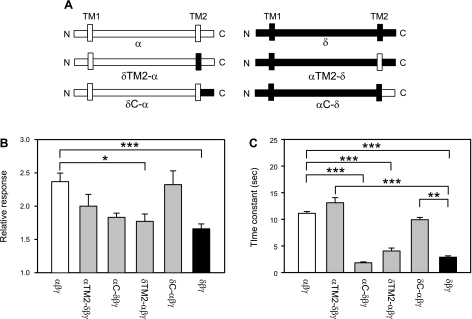
We generated four additional constructs to define the contributions of the TM2 and cytoplasmic C terminus to the response elicited by flow. Figure 4 shows the magnitude of the response and time constant of activation by LSS of αβγ, αTM2-δβγ, αC-δβγ, δTM2-αβγ, δC-αβγ, and δβγ channels. We found that both the magnitude and time course of the response to LSS were largely defined by the TM2 segment. For example, αC-δβγ and δTM2-αβγ channels had a response comparable to δβγ channels (Fig. 4). The response of αTM2-δβγ and δC-αβγ channels was similar to that of αβγ channels, particularly with regard to the time constant for channel activation (Fig. 4).
Fig. 4.
TM2 segment determines the time course of activation by LSS. A: schematic representation of wild-type α-, δTM2-α, δC-α, αTM2-δ, αC-δ, and δ-constructs. B: relative response to LSS of wild-type channels and chimeras. Experiments were performed with oocytes expressing αNsiI-XhoIβγ (αβγ), δClaI-XhoIβγ (δβγ), or the chimeras listed above. The relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal). C: time constants (τ) of activation by LSS of wild-type and chimeras. Statistically significant differences are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 (n = 15–29) by a Kruskal-Wallis test following by Dunn's multiple comparisons test.
While the primary amino acid sequences in the TM2 regions of the α- and δ-subunits are fairly well conserved (55% identity), there are differences that may account for the differential response of αβγ and δβγ channels to LSS (Fig. 5). We previously observed that selected mutations in the tract Gln581-Ser589 within the TM2 segment of the mouse α-subunit (corresponding to human Gln554-Ser562) have profound effects on the magnitude as well as the time course of the response to LSS (8). It is interesting to note that residues Gln581, Trp582, and Ser589 are not conserved among the α-and δ-subunits, and Cys mutations at these positions altered the response to LSS. Furthermore, residues in the region immediately preceding the TM2 segment are not conserved among α- and δ-subunits (Fig. 5).
Fig. 5.
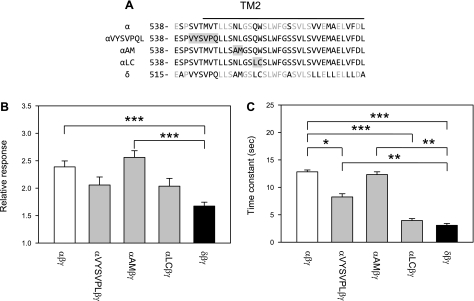
Specific sites within the region encompassing and immediately preceding TM2 contribute to the time course and magnitude of channel activation by LSS. A: alignment of α and δ TM2 regions and the 6 residue linker region immediately preceding TM2. The sequences of constructs with point mutations at specific sites are indicated. The first residue number in each sequence is indicated. Identical residues are shown in grey. The TM2 region is indicated by the line. Grey boxed regions indicate sites where residues within the α-subunit were substituted with the corresponding residues from the δ-subunit. B: relative response to LSS of wild-type channels and mutant channels. Experiments were performed with oocytes expressing wild-type αβγ, δβγ, or channels carrying mutations at specific sites. αVYSVPL refers to an α-subunit where the tract encompassing residues 541–546 was replaced with the corresponding VYSVPL tract from the δ-subunit. αAM refers to an α-subunit where the tract encompassing residues 550–551 was replaced with the corresponding AM tract from the δ-subunit. αLC refers to an α-subunit where the tract encompassing residues 554–555 was replaced with the corresponding LC tract from the δ-subunit. The relative response represents the ratio of the peak benzamil-sensitive current following vertical perfusion to the basal benzamil-sensitive current (Ipeak/Ibasal). C: time constants (τ) of activation by LSS of wild-type and mutant channels. Statistically significant differences are indicated as *P < 0.05, **P < 0.01 and ***P < 0.001 (n = 14–22) by the Kruskal-Wallis test following by Dunn's multiple comparisons test.
We examined whether αβγ channels bearing mutations in the region encompassing and immediately preceding TM2 in the α-subunit exhibited a response similar to δβγ channels. Specifically, residues in the tracts 541-SVTMVT, 550-NL, and 554-QW of the α-subunit were substituted with the corresponding residues of the δ-subunit (VYSVPQ, AM and LC, respectively) (Fig. 5). Channels with the AM substitution at position 550–551 exhibited a flow response similar to wild-type αβγ channels. Interestingly, the time course of the response of channels with the VYSVPQ substitution was significantly faster than that observed for αβγ channels, but significantly slower than δβγ channels (Fig. 5). Channels with the LC substitution at position 554–555 exhibited a flow response with a time course similar to wild-type δβγ channels. The magnitude of the flow response of channels with the VYSVPQ and LC substitutions was intermediate to that of αβγ and δβγ channels.
DISCUSSION
Studies in microperfused cortical collecting ducts and X. laevis oocytes indicated that laminar flow activates ENaC by increasing channel open probability (1, 7, 25). Four ENaC subunits α, β, γ and δ, are expressed in primates. αβγ Channels represent the archetypical epithelial sodium channel found in Na+-transporting epithelia (18, 33). The δ-subunit apparently constitutes a pseudogene in mouse and rat, but is expressed in the brain, pancreas, ovary, and testis of primates (12, 36). While α- and δ-subunits form functional amiloride-sensitive channels when expressed alone in heterologous systems, their association with β- and γ-subunits dramatically increases their functional expression (5, 36). The β- and γ-subunits on their own or together do not constitute functional channels. We exploited the similar functional roles of the α- and δ-subunits to gain insight into the structural features that allow ENaC to respond to mechanical stimuli.
The magnitude of the response to flow of δβγ channels was significantly lower than the response of αβγ channels. Our observed differences in the response to flow of αβγ and δβγ may reflect differences in baseline open probability. However, the time course of the response to flow will be dependent on the ability of the channel to couple the changes evoked by the stimulus to the pore, and hence should be independent of the open probability. We hypothesize that the differences in the time course of the response to flow of αβγ and δβγ channels reflect differences in gating in response to LSS.
We identified the TM2 segment and the short linker region immediately preceding TM2 as a key region within the channel responsible for the differences in the response of αβγ and δβγ channels to LSS. Of the varying chimeric α-δ constructs that we analyzed, the time course and, to some extent, the magnitude of the response to LSS were defined by the subunit contributing the residues to the TM2 segment and the few residues immediately preceding TM2 (Fig. 5). This short region immediately preceding the TM2 segment links the highly structured extracellular region to the α-helical TM2 segment in the resolved structure of ASIC1, an ion channel that is a member of the ENaC/degenerin family (15). Interestingly, oocytes expressing the chimeras αTM2-C-δβγ and δN-TM1-αβγ did not exhibit benzamil-sensitive currents. This may reflect a lack of expression of the mutant channel at the plasma membrane, and/or a marked reduction in channel open probability.
Additional mutagenesis studies identified two tracts within the region encompassing and immediately preceding TM2 of the α-subunit that have a role in determining the time course of the response to LSS. One tract extends from the wrist domain to the initial part of TM2 (residues 541–546), while the other tract is immediately adjacent to the putative amiloride-binding site (residues 554–555) (Fig. 5). These results suggest that multiple sites within this region contribute to the ENaC response to LSS.
The resolved structure of the extracellular region of ASIC1 is composed of peripheral α-helical domains (termed finger, thumb, and knuckle) surrounding core β-sheet domains (termed palm and β-ball) (15). Short wrist domains link the extracellular region to the TM helices. Based on the overall homology between ASIC1 and α-ENaC, 25% amino acid identity excluding the poorly conserved finger domain, it is likely that these polypeptides have common structural features in their extracellular regions (16).
ENaCs and other members of the ENaC degenerin family are ion channels that respond to factors in the external environment, presumably via interactions of these factors with residues in the extracellular regions (10, 11, 15, 21, 27, 31, 32, 34). These interactions likely result in conformational changes that are transmitted to the gate within the pore region (26). For example, proton binding sites have been identified within the extracellular region of ASIC subunits (15, 21, 27). ENaC gating is modified by proteases that cleave out inhibitory fragments imbedded within the extracellular regions of the α- and γ-subunits (3, 6, 20). Mutations within the extracellular regions of the α- and γ-subunit units affect ENaC gating by external Na+, a process referred to as Na+ self-inhibition (11, 31, 32, 34). Based on these observations, we speculate that the extracellular domain functions as a flow sensor that undergoes conformational changes in response to LSS. This signal is then transmitted to the channel pore composed of the TM segments. Our experiments do not shed light on the mechanism behind flow detection, as αβγ and δβγ likely share sufficient similarities in their extracellular regions that the flow-sensing component is not disrupted in the chimeras we analyzed.
In summary, our studies provide insight into the mechanism by which mechanical forces, such as LSS, modulate the activity of ENaC. Functional changes triggered by LSS are defined, in part, by residues within and just preceding the TM2 segment of the α- and δ-subunits.
GRANTS
This work was supported by grants from the National Institutes of Health (R01 DK051391 and P30 DK079307). T. Abi-Antoun was supported by a postdoctoral fellowship award from the National Kidney Foundation. S. Shi was supported by a postdoctoral fellowship award from the American Heart Association. M. D. Carattino was supported by a Carl W. Gottschalk Award from the American Society of Nephrology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J 21: 2389–2399, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 7: 1337–1344, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol Cell Physiol 267: C1682–C1690, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its alpha subunit. J Biol Chem 281: 18901–18907, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Carattino MD, Sheng S, Kleyman TR. Epithelial Na+ channels are activated by laminar shear stress. J Biol Chem 279: 4120–4126, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Carattino MD, Sheng S, Kleyman TR. Mutations in the pore region modify epithelial sodium channel gating by shear stress. J Biol Chem 280: 4393–4401, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Chalfie M. Neurosensory mechanotransduction. Nat Rev Mol Cell Biol 10: 44–52, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 284: 29320–29325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 284: 792–798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giraldez T, Afonso-Oramas D, Cruz-Muros I, Garcia-Marin V, Pagel P, Gonzalez-Hernandez T, Alvarez de la Rosa D. Cloning and functional expression of a new epithelial sodium channel delta subunit isoform differentially expressed in neurons of the human and monkey telencephalon. J Neurochem 102: 1304–1315, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192–F205, 1979 [DOI] [PubMed] [Google Scholar]
- 14. Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367: 467–470, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel alpha subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286: 649–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kashlan OB, Sheng S, Kleyman TR. On the interaction between amiloride and its putative alpha-subunit epithelial Na+ channel binding site. J Biol Chem 280: 26206–26215, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Khuri RN, Strieder WN, Giebisch G. Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975 [DOI] [PubMed] [Google Scholar]
- 20. Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liechti LA, Berneche S, Bargeton B, Iwaszkiewicz J, Roy S, Michielin O, Kellenberger S. A combined computational and functional approach identifies new residues involved in pH-dependent gating of ASIC1a. J Biol Chem 285: 16315–16329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 256: F932–F941, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Mano I, Driscoll M. DEG/ENaC channels: a touchy superfamily that watches its salt. Bioessays 21: 568–578, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Passero CJ, Okumura S, Carattino MD. Conformational changes associated with proton-dependent gating of ASIC1a. J Biol Chem 284: 36473–36481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paukert M, Chen X, Polleichtner G, Schindelin H, Grunder S. Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem 283: 572–581, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 109: 15–26, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheng S, Bruns JB, Kleyman TR. Extracellular histidine residues crucial for Na+ self-inhibition of epithelial Na+ channels. J Biol Chem 279: 9743–9749, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channels by relieving Na+ self-inhibition. Am J Physiol Renal Physiol 290: F1488–F1496, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Sheng S, Johnson JP, Kleyman TR. Epithelial sodium channels. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. Philadelphia, PA: Elsevier, 2008, p. 743–768 [Google Scholar]
- 34. Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of the epithelial Na+ channel in Na+ self-inhibition. J Biol Chem 282: 20180–20190, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem 269: 24379–24383, 1994 [PubMed] [Google Scholar]
- 36. Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem 270: 27411–27414, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]