Abstract
Vesicoureteral reflux (VUR) is a common pediatric anomaly linked to renal scarring and hypertension. Although there are many mouse VUR models, cystograms have previously only been performed in euthanized animals, thus preventing serial assessments for VUR in the same animal and not delineating “live” physiology. Our purpose was to develop a live murine cystogram assay that could be used serially to track reflux. We injected microbubbles via transurethral catheters into bladders of C57BL6/J and C3H/HeJ inbred mouse strains that are known to have low and high VUR rates, respectively. We performed ultrasound to visualize microbubbles in the renal pelvis to determine feasibility of the procedure. We then repeated the microbubble ultrasound using a transducer allowing for visualization of both kidneys and ureters simultaneously and for 3 dimensional (3D) reconstruction. We then performed “euthanized” cystograms on all mice for comparison. C3H/HeJ mice had a strong and persistent microbubble signal in the renal pelvis and ureters bilaterally with low-contrast infusion volumes (<100 μl) and similarly showed bilateral reflux by euthanized cystograms. With larger infused volumes (≥150 μl), C57BL6/J mice had small volumes of microbubbles in the renal pelvis that cleared quickly and did not show reflux on euthanized cystograms. Thus, using animal models of known VUR, we demonstrate the utility of contrast-enhanced ultrasound to visualize reflux in live mice.
Keywords: live cystogram, mouse
urinary backflow from the bladder into ureters, vesicoureteral reflux (VUR), affects 1–2% of the pediatric population (3, 13, 15, 24). While many children with VUR are asymptomatic, others develop reflux nephropathy (renal scarring) associated with VUR (13, 24). Reflux nephropathy causes 8% of pediatric chronic kidney disease and is the most common cause of severe hypertension in children (1, 13, 15).
Recently, several inbred and genetically altered mouse strains have been shown to have variable degrees of VUR (8, 12, 14–17). The gold-standard assay for VUR in mice is to euthanize the animal and to expose surgically the urinary tract followed by gravity filling the bladder with dye to visualize reflux into the ureters (8, 14–17). These cystograms are simple and inexpensive, but death likely perturbs many parameters that contribute to reflux in live animals. Also, serial “euthanized” cystograms on the same animals are impossible, compromising studies of the natural history of VUR and/or kidney injury.
In humans, one method for assessing VUR is a voiding cystourethrogram (VCUG), in which a catheterized bladder is filled with radiopaque contrast under fluoroscopy (22). Alternatively, a nuclear cystogram entails filling the bladder with a radioactive isotope that is tracked to look for reflux (5, 11). In mice, fluoroscopy would be technically difficult and the small volumes of contrast relatively difficult to visualize. Nuclear cystograms would require expensive radioactive isotopes and sophisticated cameras that are not typically available in animal imaging cores.
Recently, an alternative technique that has been utilized in humans is to infuse microbubbles into the bladder and then to track the bubbles in the urinary tract via ultrasound (6, 7, 9, 10, 21, 25, 26). Given that many small animal imaging facilities have access to high-resolution ultrasound equipment, we developed an in vivo technique using contrast-enhanced sonography to assess VUR in mice. We validated the technique using two inbred mouse strains with known reflux rates and by comparison with standard euthanized cystograms.
MATERIALS AND METHODS
The University of Pittsburgh Institutional Animal Care and Use Committee approved the study. C3H/HeJ and C57BL6/J inbred female mice at 6–8 wk of age and with weights ranging from 20 to 25 g were used for all experiments.
Initial cystograms with a microbubble contrast agent.
Animals were anesthetized with inhaled isoflurane, and the breathing rate (maintained between 30 and 50 breaths/min) and heart rate (maintained between 450 and 500 beats/min) were monitored (Fig. 1). Fur was removed on the abdomen over the bladder and on the back over the kidneys. PE-10 tubing was flushed with a MicroMarker Non-Targeted Contrast Agent (VisualSonics, Toronto, ON) diluted to 200,000 bubbles/μl in PBS. The MicroMarker Non-Targeted Contrast Agent consists of an external lipid bilayer that contains a mixture of nitrogen and perfluorobutane, and the average diameter of the bubbles is between 2.3 and 2.9 μm. With the mouse prone, the tubing was advanced through the urethra into the bladder. Before imaging the kidneys of each animal, we gated the breathing and heart rate to allow for accurate three-dimensional (3D) reconstruction. Background ultrasound images of kidneys and bladders were obtained with a Vevo 770 ultrasound machine (VisualSonics) in the fundamental mode at a frequency of 25 MHz. Contrast was infused at 50 μl boluses/min into the bladders, and the right renal papilla was visualized in real time using the subharmonic mode to look for presence of refluxed microbubbles. In the subharmonic mode, two ultrasound pulses are sent consecutively into tissue, with the second pulse being an inverted copy of the first. Based on the properties of linear systems, a summary of the two pulses will result in tissue being canceled (primary linear scattering) and bubble signal retained because of nonlinear oscillations. This mode is frequently used for microbubble imaging in animals (18). After a 250-μl total infused volume was reached, the bladder was imaged to confirm the presence of bubbles.
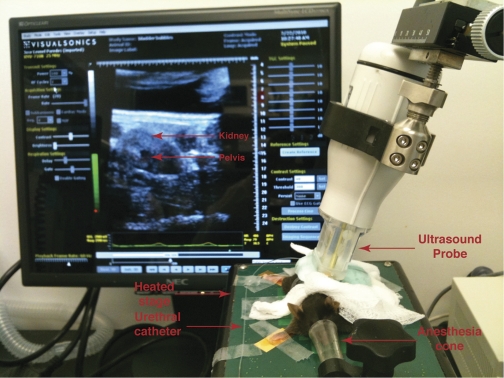
Fig. 1.
Mouse undergoing live cystogram. Representative image showing a mouse with its nose in the anesthesia cone and on a heated table that can monitor breathing and heart rate. PE-10 tubing used to catheterize the mice is visible as is the ultrasound probe that is pressed against the shaved flank. The ultrasound image of the kidney is seen on the monitor.
3D reconstructions of contrast-enhanced urinary tract.
After a 1- to 2-wk recovery period to clean out residual microbubbles and to allow recovery from bladder distension, distances that included the ureters and kidneys were measured with a 710b probe operating at 25 MHz. Thirty microliters of microbubble contrast was introduced into the bladder, followed by 10 μl/min, not to exceed a 150-μl total volume. The field containing ureters and kidneys was scanned at 1 mm/min [50 successive images were taken at intervals of 100 μm, and the cumulative contrast for all images was visualized with maximum intensity persistence software (VisualSonics)]. All layers were aligned and stacked to render a 3D image. Bladders were then imaged to confirm the presence of bubbles. Utilizing Vevo 770 software, three axes (length, width, and depth) and volumes of a renal pelvis containing bubbles was calculated (see supplemental protocol outlining each step of the microbubble procedure including the 3D rendering; supplementary material for his article is available online at the journal web site).
Euthanized cystograms.
Following 24 h of recovery from the 3D study, all mice were euthanized via CO2 inhalation and gravity cystograms were performed as previously described (8, 14–17). Briefly, after urogenital tracts were surgically exposed, a 25-gauge butterfly needle was placed in the bladder. The needle was attached via tubing to an open 50-ml syringe containing 1% methylene blue. Bladders were gravity filled with the dye (increasing syringe height by 30 cm every 5 s), and the height at which reflux occurred was recorded.
RESULTS
Determining feasibility of live cystograms via microbubble/ultrasound.
All C3H/HeJ mice (4/4) had a robust and persistent microbubble signal in the renal pelvis (4/4) after a single 50-μl infusion, whereas no C57BL6/J mice (0/4) had bubbles in the pelvis (Fig. 2). We confirmed the presence of bubbles in each mouse bladder (Fig. 2). With subsequent 50-μl infusions every minute, we noted the presence of a small volume of bubbles that cleared quickly in the renal pelvis of all C57BL6/J mice (2/4 at 150 μl, 1/4 at 200 μl, and 1/4 at 250 μl) (Fig. 3).
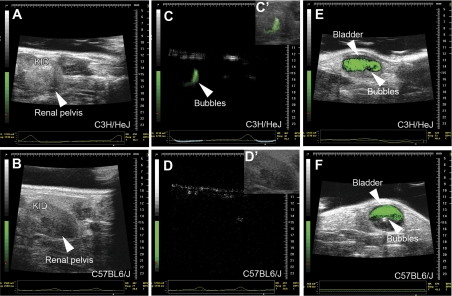
Fig. 2.
Still images of live cystograms in C3H/HeJ and C57BL6/J mice. A and B: fundamental mode ultrasound images showing the kidney (kid) and renal pelvis of C3H/HeJ (A) and C57BL6/J (B) mice at baseline. C and D: subharmonic mode ultrasound images of C3H/HeJ (C) and C57BL/6J (D) mice, depicting reflux of green colorized bubbles into the renal pelvis of the C3H/HeJ mice. C′ and D′: overlay of fundamental and subharmonic mode images confirm that the bubbles are in renal pelvis of C3H/HeJ mice (green) and are absent in the C57BL6/J mice. E and F: overlay of fundamental and subharmonic images confirming the presence of green bubbles in the bladders of both mouse strains. Note the scale bar on the right-hand side of each image.
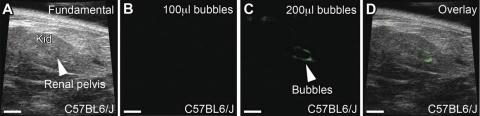
Fig. 3.
Still images showing renal pelvis of C57BL6/J mice given variable amounts of bubbles. A: fundamental mode of C57BL6/J mouse depicting kidney (kid) and renal pelvis at baseline. B: subharmonic ultrasound mode image following infusion of 100 μl of contrast into the bladder shows no bubbles in the renal pelvis. C: subharmonic ultrasound mode image following 200 μl of bubbles injected into the bladder shows trace amounts of contrast in the pelvis (arrowhead). D: overlay of fundamental and subharmonic modes confirms bubbles (green) within the renal pelvis. Scale bars = 2.5 mm.
3D reconstruction of microbubbles shows bilateral reflux in C3H/HeJ mice.
To visualize both kidneys and ureters of each animal simultaneously, we performed 3D reconstructions of contrast-enhanced ultrasound images within kidneys and proximal ureters. The length encompassing kidneys and ureters ranged from 10 to 14 mm. We then slowly infused microbubbles to a total volume of 150 μl (given that C57BL6/J mice had a transient signal in the renal pelvis at volumes ≥150 μl) and imaged the length of kidneys and ureters from the dorsal, midline surface of the mice. Using this technique, we detected a robust presence of bubbles in the proximal ureters and the renal pelvis bilaterally in all C3H/HeJ mice (4/4) and none in any C57BL6/J mice (0/4) (Fig. 4). From these images, we are able to measure the three axes (length, width, and depth) and volume of a renal pelvis that contains bubbles (Supplemental Fig. S1).
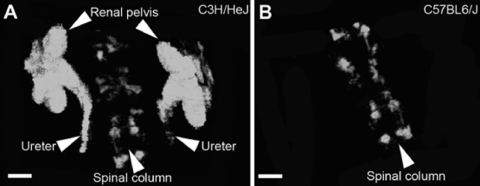
Fig. 4.
Three-dimensional (3D) reconstruction of live cystograms in C3H/HeJ and C57BL6/J mice. A: subharmonic mode revealing 3D reconstruction of bilateral reflux into ureters and renal pelvis of a C3H/HeJ mouse. The spinal column was used as a reference point. B: subharmonic mode 3D reconstruction of C57BL6/J mice shows no reflux into the ureters or kidneys although the spinal column is visible. Scale bars = 2 mm.
Euthanized cystograms revealed reflux in C3H/HeJ mice but not C57BL6/J mice.
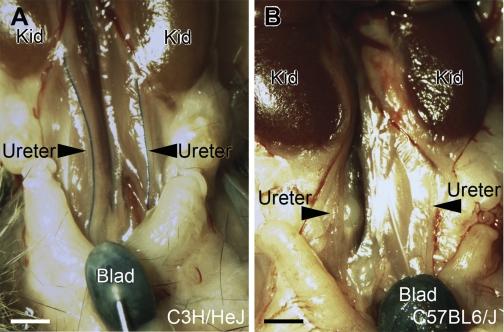
C57BL6/J mice demonstrated no reflux (0/4), even when the open syringe was raised to the maximum height of 150 cm, whereas all C3H/HeJ mice (4/4) had bilateral VUR at low syringe heights (range 0–30 cm) (Fig. 5).
Fig. 5.
Euthanized gravity cystograms in C3H/HeJ and C57BL6/J mice. A and B: representative images show reflux of dye into both ureters of the C3H/HeJ mice (A) but no reflux in the C57BL6/J mice. Kid, kidney; Blad, bladder. Scale bars = 2 mm.
DISCUSSION
Using inbred mouse strains with known rates of VUR by euthanized cystograms, we show the utility of performing contrast-enhanced ultrasound cystograms in live mice. Furthermore, we demonstrate the feasibility of performing repeat studies over time, as well as evidence that the results of cystograms using this technique are reproducible. A major advantage of live cystograms over euthanized cystograms is physiological relevance. While VUR is dependent upon where the ureter inserts into the bladder and intravesicular tunnel length, other potentially important dynamic factors such as ureteral peristaltic waves, muscular tone of the ureter and bladder, and local perfusion and innervation are altered or lost in euthanized animals (19, 20, 23). Another advantage is that serial cystograms could be performed in a minimally invasive manner (no percutaneous catheter placement) and without exposure to radiation. The volume and axes (length, width, and depth) of a microbubble-filled renal pelvis can also be directly quantitated which could potentially correlate with changes in VUR over time and could lead to more accurate grading of VUR (although the reproducibility and correlation with VUR grade have yet to be validated). Another advantage is that small-animal ultrasound machines are increasingly available in research facilities.
Among the disadvantages of this technique are that transurethral catheters only work for female mice as the male urethra is too tortuous in mice; however, one could perform ultrasound-guided percutaneous placement of a catheter in males. Since male studies would require percutaneous catheter placement, this would increase the risks of the procedures and reduce the ability to perform longitudinal studies. Also, with very high microbubble infusion volumes (and presumably pressures) in the bladder, even “nonrefluxing” C57BL6/J mice had small volumes of transient microbubbles in the renal pelvis. Given that the normal bladder capacity for young adult mice is 150–300 μl (and that we did not see VUR in the C57BL6/J until we reached those volumes), we would propose limiting the bladder infusions to 150-μl total volumes (2, 4).
In summary, the technique described in this study offers a significant advance in the study of VUR in mammalian models. Performing microbubble ultrasound cystograms in genetic mouse models will provide valuable insights to the etiology and pathogenesis of VUR and reflux nephropathy.
GRANTS
The study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1R01DK081128 (C. M. Bates) and 5R01DK078226 (W. Lu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Anonymous. North American Pediatric Renal Trials and Collaborative Studies: 2010 Annual Report. Rockville, MD: EMMES, 2010, p. 1–100 [Google Scholar]
- 2. Bhattacharya A, Dang H, Zhu QM, Schnegelsberg B, Rozengurt N, Cain G, Prantil R, Vorp DA, Guy N, Julius D, Ford AP, Lester HA, Cockayne DA. Uropathic observations in mice expressing a constitutively active point mutation in the 5-HT3A receptor subunit. J Neurosci 24: 5537–5548, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chand DH, Rhoades T, Poe SA, Kraus S, Strife CF. Incidence and severity of vesicoureteral reflux in children related to age, gender, race and diagnosis. J Urol 170: 1548–1550, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407: 1011–1015, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Cooper CS. Diagnosis and management of vesicoureteral reflux in children. Nat Rev Urol 6: 481–489, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Darge K. Voiding urosonography with US contrast agent for the diagnosis of vesicoureteric reflux in children: an update. Pediatr Radiol 40: 956–962, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Darge K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children. II. Comparison with radiological examinations. Pediatr Radiol 38: 54–63, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Hains DS, Sims-Lucas S, Carpenter A, Saha M, Murawski I, Kish K, Gupta I, McHugh K, Bates CM. High incidence of vesicoureteral reflux in mice with Fgfr2 deletion in kidney mesenchyma. J Urol 183: 2077–2084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kessler RM, Altman DH. Real-time sonographic detection of vesicoureteral reflux in children. AJR Am J Roentgenol 138: 1033–1036, 1982 [DOI] [PubMed] [Google Scholar]
- 10. Kis E, Nyitrai A, Varkonyi I, Mattyus I, Cseprekal O, Reusz G, Szabo A. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol 25: 2289–2293, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Lebowitz RL. The detection and characterization of vesicoureteral reflux in the child. J Urol 148: 1640–1642, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, Sanlaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay JC, Wijmenga C, de Jong TP, Feather SA, Woolf AS, Rao Y, Lupski JR, Eccles MR, Quade BJ, Gusella JF, Morton CC, Maas RL. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet 80: 616–632, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mak RH, Kuo HJ. Primary ureteral reflux: emerging insights from molecular and genetic studies. Curr Opin Pediatr 15: 181–185, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Murawski IJ, Gupta IR. Gene discovery and vesicoureteric reflux. Pediatr Nephrol 23: 1021–1027, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Murawski IJ, Gupta IR. Vesicoureteric reflux and renal malformations: a developmental problem. Clin Genet 69: 105–117, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Murawski IJ, Maina RW, Malo D, Guay-Woodford LM, Gros P, Fujiwara M, Morgan K, Gupta IR. The C3H/HeJ inbred mouse is a model of vesico-ureteric reflux with a susceptibility locus on chromosome 12. Kidney Int 78: 269–278, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Murawski IJ, Myburgh DB, Favor J, Gupta IR. Vesicoureteric reflux and urinary tract development in the Pax2 1Neu+/− mouse. Am J Physiol Renal Physiol 293: F1736–F1745, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Needles A, Arditi M, Rognin NG, Mehi J, Coulthard T, Bilan-Tracey C, Gaud E, Frinking P, Hirson D, Foster FS. Nonlinear contrast imaging with an array-based micro-ultrasound system. Ultrasound Med Biol 36: 2097–2106, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Oswald J, Brenner E, Schwentner C, Deibl M, Bartsch G, Fritsch H, Radmayr C. The intravesical ureter in children with vesicoureteral reflux: a morphological and immunohistochemical characterization. J Urol 170: 2423–2427, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Oswald J, Schwentner C, Brenner E, Deibl M, Fritsch H, Bartsch G, Radmayr C. Extracellular matrix degradation and reduced nerve supply in refluxing ureteral endings. J Urol 172: 1099–1102, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Riccabona M, Mache CJ, Lindbichler F. Echo-enhanced color Doppler cystosonography of vesicoureteral reflux in children. Improvement by stimulated acoustic emission. Acta Radiol 44: 18–23, 2003 [PubMed] [Google Scholar]
- 22. Rossleigh MA. Renal infection and vesico-ureteric reflux. Semin Nucl Med 37: 261–268, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Schwentner C, Oswald J, Lunacek A, Fritsch H, Deibl M, Bartsch G, Radmayr C. Loss of interstitial cells of Cajal and gap junction protein connexin 43 at the vesicoureteral junction in children with vesicoureteral reflux. J Urol 174: 1981–1986, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Silva JM, Oliveira EA, Diniz JS, Cardoso LS, Vergara RM, Vasconcelos MA, Santo DE. Gender and vesico-ureteral reflux: a multivariate analysis. Pediatr Nephrol 21: 510–516, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Valentini AL, De Gaetano AM, Minordi LM, Nanni G, Citterio F, Viggiano AM, Tancioni V, Destito C. Contrast-enhanced voiding US for grading of reflux in adult patients prior to antireflux ureteral implantation. Radiology 233: 35–39, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Zimbaro G, Ascenti G, Visalli C, Bottari A, Zimbaro F, Martino N, Mazziotti S. Contrast-enhanced ultrasonography (voiding urosonography) of vesicoureteral reflux: state of the art. Radiol Med 112: 1211–1224, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.