Abstract
Ischemic acute kidney injury (AKI) triggers expression of adaptive (protective) and maladaptive genes. Agents that increase expression of protective genes should provide a therapeutic benefit. We now report that bardoxolone methyl (BARD) ameliorates ischemic murine AKI as assessed by both renal function and pathology. BARD may exert its beneficial effect by increasing expression of genes previously shown to protect against ischemic AKI, NF-E2-related factor 2 (Nrf2), peroxisome proliferator-activated receptor-γ (PPARγ), and heme oxygenase 1 (HO-1). Although we found that BARD alone or ischemia-reperfusion alone increased expression of these genes, the greatest increase occurred after the combination of both ischemia-reperfusion and BARD. BARD had a different mode of action than other agents that regulate PPARγ and Nrf2. Thus we report that BARD regulates PPARγ, not by acting as a ligand but by increasing the amount of PPARγ mRNA and protein. This should increase ligand-independent effects of PPARγ. Similarly, BARD increased Nrf2 mRNA; this increased Nrf2 protein by mechanisms in addition to the prolongation of Nrf2 protein half-life previously reported. Finally, we localized expression of these protective genes after ischemia and BARD treatment. Using double-immunofluorescence staining for CD31 and Nrf2 or PPARγ, we found increased Nrf2 and PPARγ on glomerular endothelia in the cortex; Nrf2 was also present on cortical peritubular capillaries. In contrast, HO-1 was localized to different cells, i.e., tubules and interstitial leukocytes. Although Nrf2-dependent increases in HO-1 have been described, our data suggest that BARD's effects on tubular and leukocyte HO-1 during ischemic AKI may be Nrf2 independent. We also found that BARD ameliorated cisplatin nephrotoxicity.
Keywords: acute kidney injury, cisplatin, heme oxygenase 1, ischemia
despite modern medical technology, ischemic acute kidney injury (AKI) remains a major frequent clinical problem (7). Not only does AKI increase acute mortality (10, 32, 66), recent data suggest that AKI also results in permanent and progressive renal injury (17, 26, 39). New therapies for ischemic AKI are needed, not only to decrease acute mortality and morbidity but also to decrease progressive CKD.
We now report that bardoxolone methyl (BARD) ameliorates ischemic murine AKI as assessed by both renal function and pathology. We also show that BARD may exert its beneficial effect by increasing expression of genes previously shown to protect the kidney against ischemic AKI, NF-E2-related factor 2 (Nrf2), peroxisome proliferator-activated receptor-γ (PPARγ), and heme oxygenase 1 (HO-1) (9, 19, 38, 42, 45, 53).
We localized expression of these protective genes after ischemia and BARD treatment. To our knowledge, these are the first studies to localize protein expression of these genes and their relationship with one another in nonischemic and ischemic kidneys. We found Nrf2 and PPARγ on glomerular endothelia in the cortex. Nrf2 was also present on cortical peritubular capillaries. In contrast, HO-1 was localized to different cells: tubules and interstitial leukocytes. Although Nrf2-dependent increases in HO-1 have been described, our data suggest that BARD's effects on HO-1 are Nrf2 independent (2, 41, 63).
METHODS
Mice.
All animal care and procedures were approved by the UT Southwestern Medical Center Institutional Animal Care and Use Committee. Male C57BL/6 mice, aged 6∼8 wk, were supplied by the Mouse Breeding Core of the University of Texas Southwestern Medical Center. The mice were anesthetized with isoflurane and placed on a heated surgical pad. Rectal temperature was maintained at 37°C using a TR100 system with a rectal probe (Fine Science Tools, Heidelberg, Germany). After a midline abdominal incision, the renal pedicles were exposed by blunt dissection, and a microvascular clamp (Fine Science Tools) was applied for the left kidney for the indicated period of time. After 23 min, the clamp and the right kidney were removed. The incision was closed using a 5-0 suture and surgical staples. “Sham” surgery was the same as the above, but the microvascular clamp was placed underneath the renal pedicle without occluding the renal blood flow.
BARD administration.
Thirty milligrams of BARD (lot no. 520-05-11-56, Reata) was dissolved in 30 ml of sesame oil (lot no. PH05082304, Voigt Global Distributors). BARD or vehicle was administered to mice at 20 mg/kg by gavage before renal ischemia, as detailed in results.
Histopathology.
Kidneys were fixed in 10% formalin 4 h before routine processing and paraffin embedding. Kidney sections (2 μm in thickness) were stained with hematoxylin and eosin. The pathological examination of sections by light microscopy was performed. Neutrophils were stained with napthol AS-D chloracetate esterase (Sigma). A terminal transferase-dUTP nick-end labeling (TUNEL) assay was performed using the Promega DeadEnd Fluorometric TUNEL System.
Immunohistochemical analysis.
Renal tissue was fixed by 10% formalin and embedded in paraffin. The following antibodies were used: rabbit polyclonal Nrf2 (Abcam, Cambridge, MA) and rabbit polyclonal HO-1 (Abcam). Paraffin tissues were treated by microwave oven heating in 0.01 M sodium citrate buffer with pH 6.0 after gradual dewaxing. Following microwave treatment, slides were incubated with the rabbit anti-mouse Nrf2 antibody (Abcam) for 1 h at room temperature. Endogenous peroxidase was inactivated by incubation in 0.3% hydrogen peroxide in methanol, and endogenous biotin was blocked by a streptavidin-biotin block system. Sections were incubated with biotinylated secondary antibody and peroxidase-labeled streptavidin and stained with 3,3-diaminobenzidine (Santa Cruz Biotechnology, Santa Cruz, CA). Slides were then treated by another round of microwave heating as described above. Sections were incubated with the rabbit anti-mouse PPARγ antibody (Abcam) overnight at 4°C and incubated sequentially with biotinylated antibody and alkaline phosphatase-conjugated streptavidin (Abcam). Finally, the slides were developed with Fast Red (Santa Cruz Biotechnology) to produce a pink color after blockage of endogenous alkaline phosphatase by levamisole.
For HO-1 staining, paraffin tissues were treated by microwave oven heating in 0.01 M sodium citrate buffer (pH 6.0) after gradual dewaxing. Following microwave treatment, slides were incubated with the rabbit anti-mouse HO-1 antibody (Abcam) for 1 h at room temperature. Endogenous peroxidase was inactivated by incubation in 0.3% hydrogen peroxide in methanol, and endogenous biotin was blocked by the streptavidin-biotin block system (Santa Cruz Biotechnology). Sections were incubated with biotinylated link antibody and peroxidase-labeled streptavidin and stained with 3,3-diaminobenzidine (Santa Cruz Biotechnology). Finally, slides were counterstained by hematoxylin (Sigma).
Confocal microscopy.
Kidney tissues were frozen in OCT medium (Tissue-tek, Torrance, CA) and stored in liquid nitrogen. Serial sections were cut at 4 μm, placed onto slides, and cold acetone-fixed for 30 min at room temperature. Then, the slides were rinsed three times in PBS (pH 7.4) for 5 min each and blocked with 10% goat serum (Sigma) for 1 h in room temperature. For CD31 and PPARγ double staining, the slides were incubated with rat anti-mouse CD31 mAb (BD Biosciences Pharmingen) and rabbit-anti-mouse PPARγ (Abcam) overnight +4°C. For CD31 and Nrf2 Double staining, rat anti-mouse CD31 mAb (BD Biosciences Pharmingen) and rabbit anti-mouse Nrf2 mAb (Abcam) were used as the primary antibodies overnight at +4°C. After washing with PBS, the slides were incubated with both Alexa Fluor 594 goat anti-rabbit (Molecular Probes) and Alexa Fluor 488 goat anti-rat (Molecular Probes) for 2 h at room temperature in the dark. As negative controls, isotype-matched antibodies were used. The slides were mounted in fluorescent mounting medium (Dako) and sealed after washing three times in PBS. Fluorescence analysis was established with confocal laser-scanning microscopy (LSM510 META, Ziess).
Quantification of morphological data.
Slides were analyzed in a blinded fashion. The number of positive glomerular signals in at least 20 glomerular cross sections was counted, and the average number of positive signals in one glomerular cross section was calculated. The number of peritubular signals in at least 20 microscopic high-power fields (×400) of the cortical interstitium was counted, and the average number of peritubular signals per high-power field was calculated. The number of vasa recta positive signals was calculated as for the peritubular fields. Values were expressed as number of signals per glomerular cross section or per microscopic high-power field. Based on the intensity and distribution of HO-1 staining, the degrees of HO-1 staining were classified into 0–4 points as follows: point 0, no staining; point 1, weak and focal; point 2, weak and diffuse or moderate and focal; point 3, moderate and diffuse or strong and focal; and point 4, strong and diffuse.
Analysis of RNA abundance.
Total RNA was isolated from kidneys with TRIzol reagent (Invitrogen, Grand Island, NY) according to the manufacturer's recommendations. Samples were checked for degradation of total RNA on 2% agarose gels. RNA concentrations were determined by spectrophotometric measurements at wavelengths of 260/280 nm. Semiquantitative RT-PCR was performed. Primers used for GAPDH were 5′-GGA TGC AGG GAT GAT GTT C-3′ (sense primer) and 5′-TGC ACC ACC AAC TGC TTA G-3′ (antisense primer), for Nrf2 were 5′-CTC GCT GGA AAA AGA AGT G-3′ (sense primer) and 5′-CCG TCC AGG AGT TCA GAG G-3′ (antisense primer), and for HO-1 were 5′-TAA GAC CGC CTT CCT GCT CAA CAT-3′ (sense primer) and 5′-TGC TGG TTT CAA AGT TCA GGC CAC-3′ (antisense primer). Each PCR reaction mix contained 1.5 U of Taq DNA polymerase (Invitrogen), 0.1 mM dNTP, 50 mM MgCl2, 20 mM Tris·HCl (pH 8.4), 1 μl cDNA, and 50 nM of sense and antisense primers in a final volume of 50 μl. PCR conditions were as follows: for GAPDH and Nrf2, denaturation at 94°C for 50 s, annealing at 58°C for 45 s, and extension at 72°C for 1 min for 35 cycles; for HO-1 and PPARγ, denaturation at 94°C for 1 min, annealing at 53°C for 45 s, and extension at 72°C for 1 min for 35 cycles. After completion of RT-PCR, PCR products for GAPDH, Nrf2, HO-1, and PPARγ from each sample were combined and electrophoresed on a 2% gel for 1 h at 110 V. The gel was stained with ethidium bromide for 30 min, visualized with ultraviolet illumination, scanned, and analyzed using digital imaging. No PCR products were detected in the absence of RT. Assays were done in triplicate.
Statistical analysis.
Values are means ± SE calculated using SigmaPlot unless stated otherwise.
RESULTS
BARD ameliorates ischemic AKI by both functional and pathological measures.
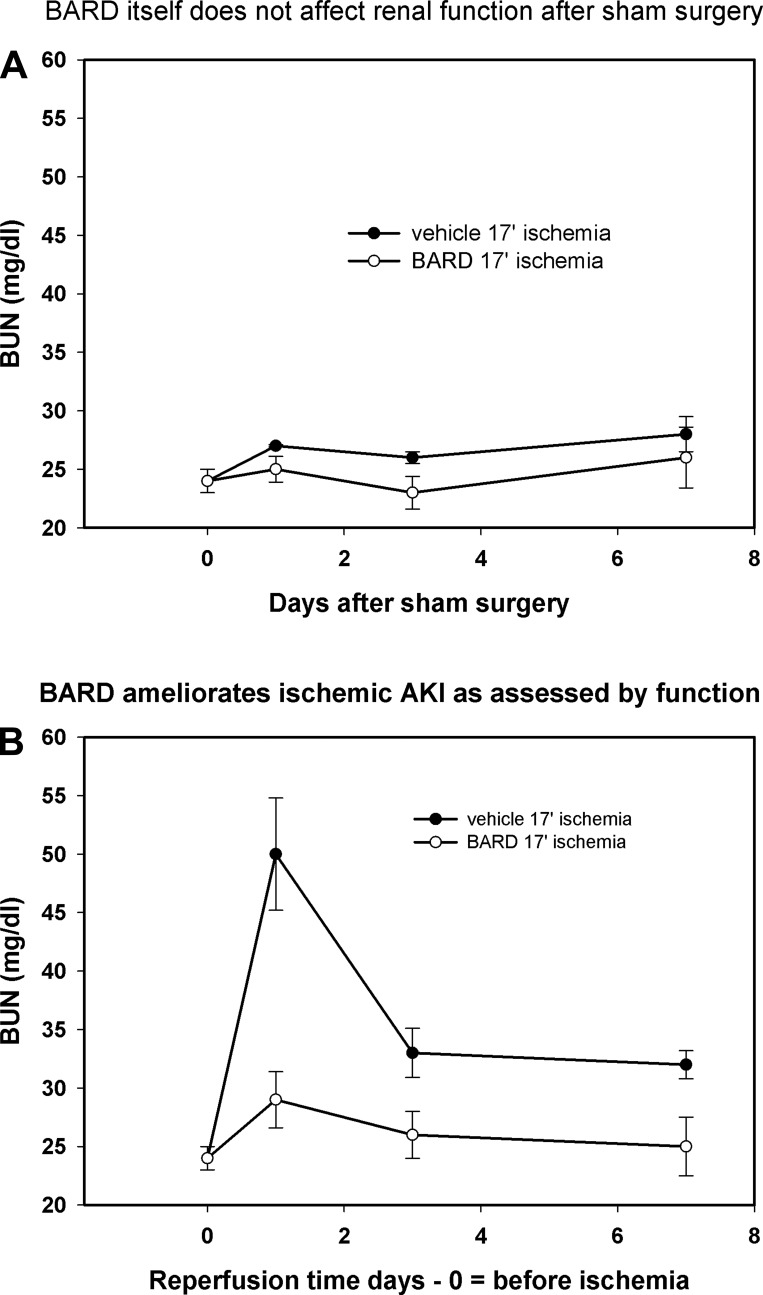
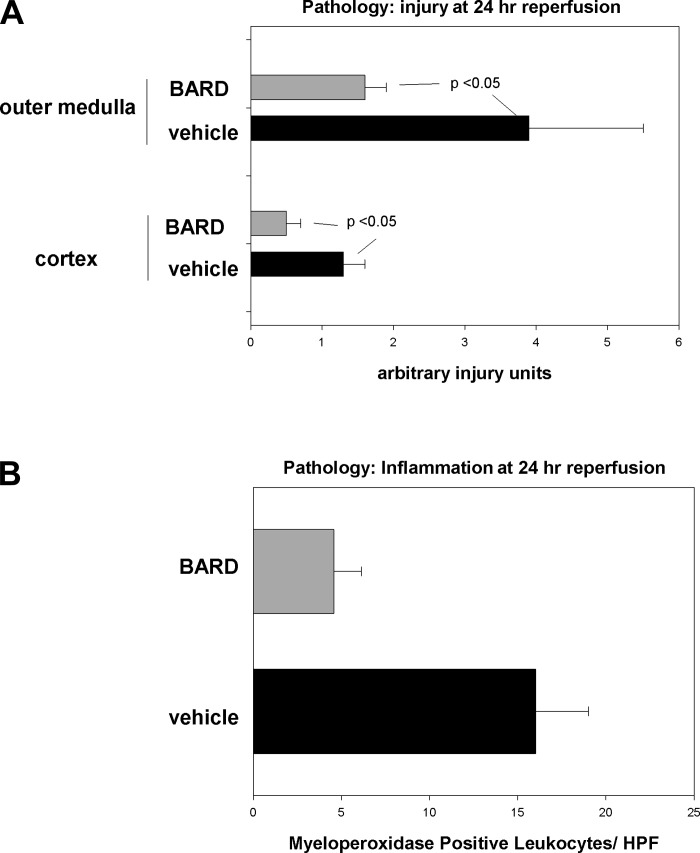
We began our studies by showing that BARD had no effect on the function of normal kidneys (Fig. 1A). However, BARD did have a major beneficial effect on ischemic AKI as assessed by both renal function and pathology. In the vehicle group, the blood urea nitrogen (BUN) increased from 24 to 50 mg/dl at day 1 of reperfusion and decreased to 33 mg/dl by day 3 of reperfusion; in contrast, mice given BARD only increased their BUN to 29 mg/dl (Fig. 1B). We also compared the pathology of BARD- and vehicle-treated kidneys, particularly because the kidney may not recover completely from any structural injury (60) and because pathology is not always correlated with renal function (59). We confirmed the beneficial functional effect of BARD on structural injury (Fig. 2A). The data are shown in two ways: morphometry that summarizes the data from five kidneys/group (Fig. 2) and representative photomicrographs (Fig. 3). Morphometry was performed on hematoxylin and eosin slides by our pathologist, X. J. Zhou, who had no knowledge of the treatment of the kidneys. We used a previously reported scoring system (58). The cortex and outer medulla were evaluated for epithelial necrosis, loss of brush border, tubular dilation, and cast formation. The slides were scored on the basis of the percentage of affected tubules: 0, none; 1, <10%; 2, 11–25%; 3, 26–50%; 4, 51–75%; and 5, >75%. At least 10 high-power fields (×400) were scored for each slide. As reported by others (8, 22, 35, 40, 46, 55), there was more ischemic injury in the outer medulla than the cortex. BARD ameliorated structural injury in both areas. Inflammation exacerbates ischemic AKI (14, 24, 25, 27, 49). We therefore also analyzed inflammation of BARD- vs. vehicle-treated ischemic kidneys. Figure 2B shows that there was less inflammation after BARD treatment.
Fig. 1.
Bardoxolone methyl (BARD) and renal function of normal vs. ischemic kidneys. A: BARD itself did not affect renal function after sham surgery. BARD, 2 mg/kg bid, was started 48 h before sham surgery (day 0) and continued for 7 days. Sham surgery was a right nephrectomy, dissection of the left renal pedicle, and placement of the clamp beneath this pedicle so that blood flow was not occluded; n = 5 mice/group. Values are means ± SE. B: BARD ameliorates ischemic AKI as assessed by function. BUN, blood urea nitrogen. BARD or vehicle was given as in A, but renal pedicles were clamped for 17 min on day 0. Values are means ± SE; n = 4 mice/group. P < 0.05 between BARD and vehicle groups at day 1 of reperfusion.
Fig. 2.
Effects of BARD on ischemic AKI at 24-h reperfusion. A: pathology: injury. The x-axis is arbitrary injury units, with 0 being the least and with 5 being maximal injury; see the text. BARD was administered as in Fig. 1; n = 5 kidneys/group. B: pathology: inflammation. The x-axis is the number of napthol-AS-d-chloracetate-esterase-positive leukocytes per high-powered field; n = 5 kidneys/group. Values are means ± SD. P values were determined by t-tests.
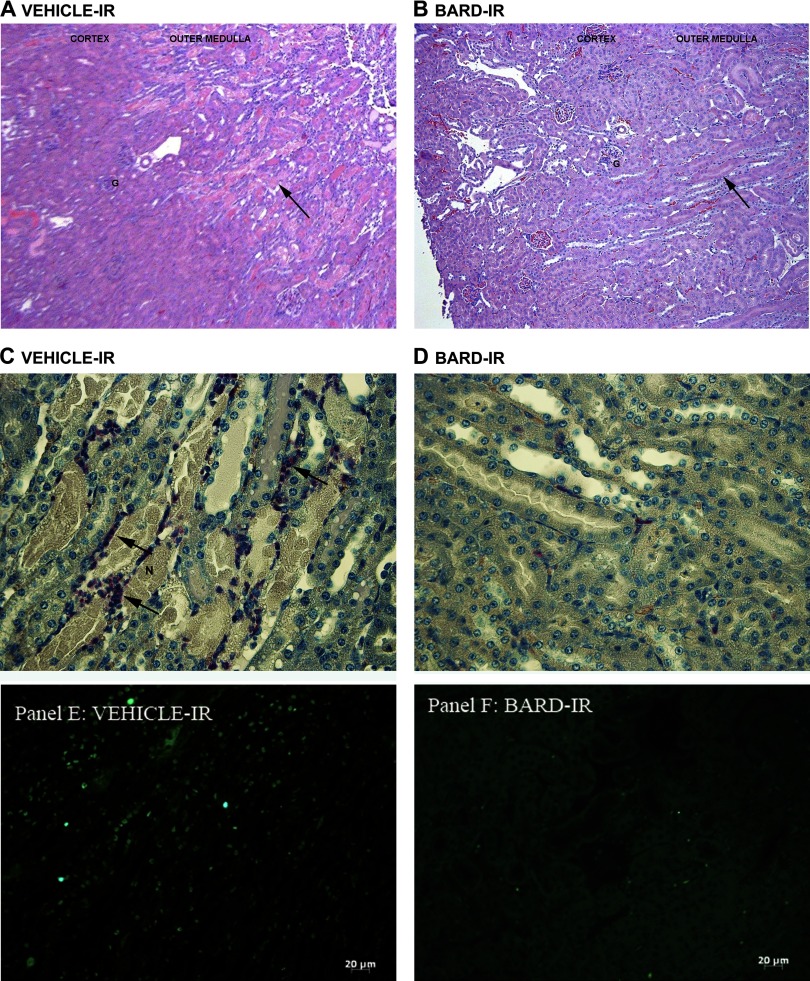
Fig. 3.
A and B: effects of BARD on pathology and inflammation with representative photomicrographs. Representative low-power views of hematoxylin- and eosin-stained sections of vehicle-treated (A) vs. BARD-treated (B) ischemic kidneys are shown. IR, ischemia-reperfusion. G indicates a few of many glomeruli. Arrow shows outer medulla, where there are many necrotic tubules in A but very few necrotic tubules in B. C and D: effects of BARD on pathology and inflammation with representative photomicrographs. Representative high-power views of kidneys from vehicle-treated (C) vs. BARD-treated (D) mice are shown. N indicates several of many necrotic tubules, and arrows indicate some of many esterase-positive neutrophils (see methods) in the vehicle-treated outer medulla (C). E and F: effects of BARD on apoptosis as assessed by terminal transferase-dUTP nick-end labeling (TUNEL) assay. The vehicle-treated ischemic kidney has many TUNEL-positive cells (E), whereas the BARD-treated ischemic kidney has few TUNEL-positive cells (F).
Representative photomicrographs of kidneys from BARD- vs. vehicle-treated mice are shown in Fig. 3. The low-power views (Fig. 3, A and B) show greater injury in the kidneys from vehicle-treated mice. The high-power views (Fig. 3, C and D) show tubular necrosis, designated by “N,” and polymorphonuclear leukocytes, only in the vehicle-treated kidneys (Fig. 3C). Neutrophils were identified by esterase staining. Figure 3, E and F, shows greater apoptosis, defined by TUNEL staining, in the vehicle-treated kidneys. Although the TUNEL assay is widely used to detect apoptotic cells, some necrotic cells may also be detected. Our data are compatible with previous reports that cell death during ischemic AKI occurs by both necrosis and apoptosis (23, 47) and that cell death in general is decreased by BARD.
We also found that a single dose of BARD, 2 mg/kg given 3 h before renal ischemia, ameliorated ischemic AKI. At 24-h reperfusion, the serum creatinine was 2.15 ± 0.41 mg/dl in vehicle-treated and 0.97 ± 0.11 mg/dl in BARD-treated mice; n = 5 in each group, P < 0.04 by t-test between the vehicle and BARD groups. We used this single-dose regimen in the experiments below.
BARD increases Nrf2 on glomerular and peritubular capillaries during ischemic AKI.
Having shown a beneficial effect of BARD on ischemic AKI, we now investigated potential mechanisms. We chose to start by investigating the effect of BARD on the putative protective gene Nrf2 for several reasons. First, Nrf2 is a transcription factor that activates genes that protect cells from oxidant stress (44) such as that occurring during ischemic AKI (43). Second, although Nrf2 has been shown to ameliorate ischemic AKI through the use of transgenic Nrf2 knockout mice and pharmacological manipulations (31, 38, 67), the effects of BARD and/or renal ischemia-reperfusion (IR) on the kinetics of gene expression and on the localization of the protein have not, to our knowledge, previously been investigated.
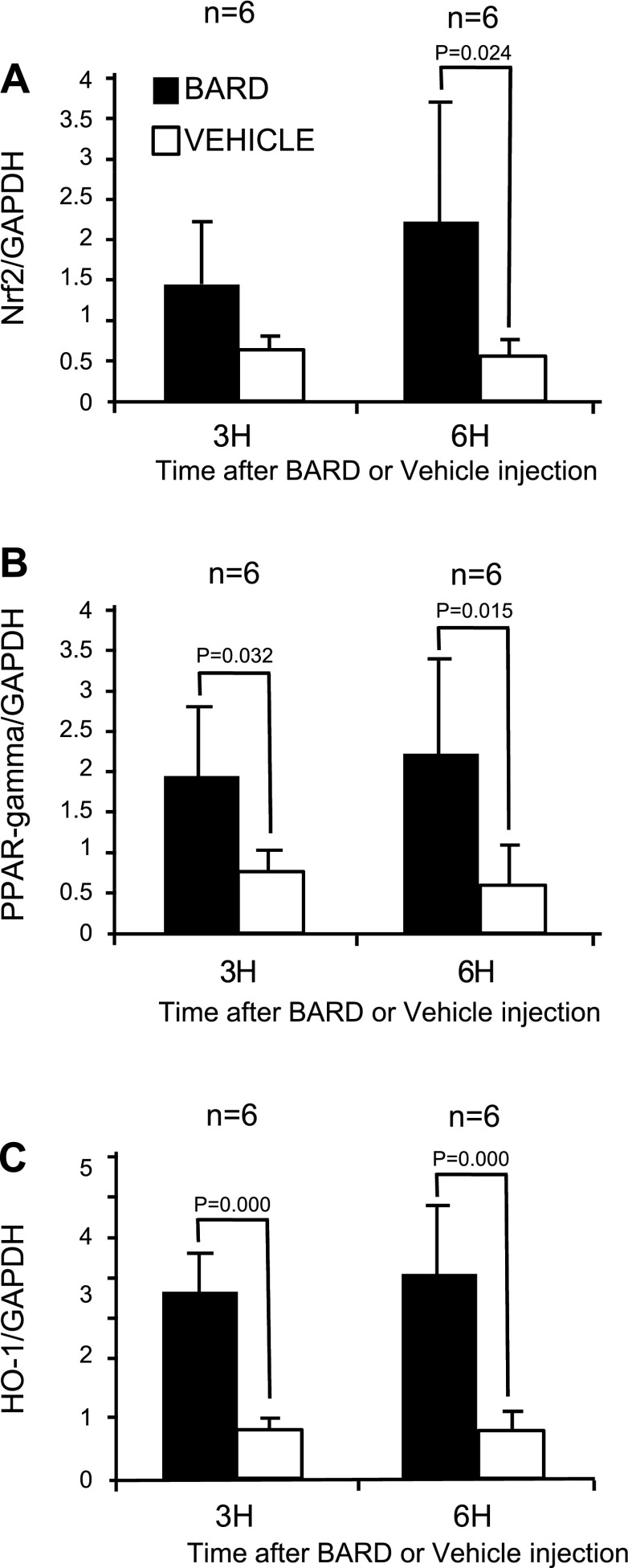
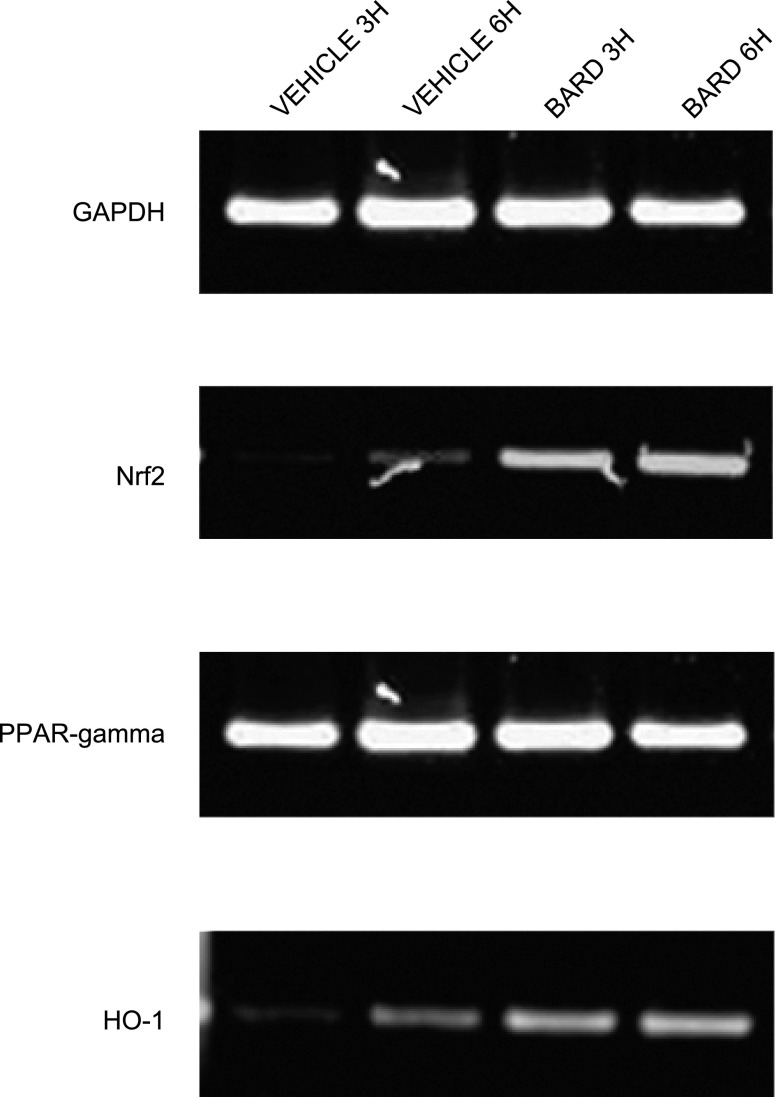
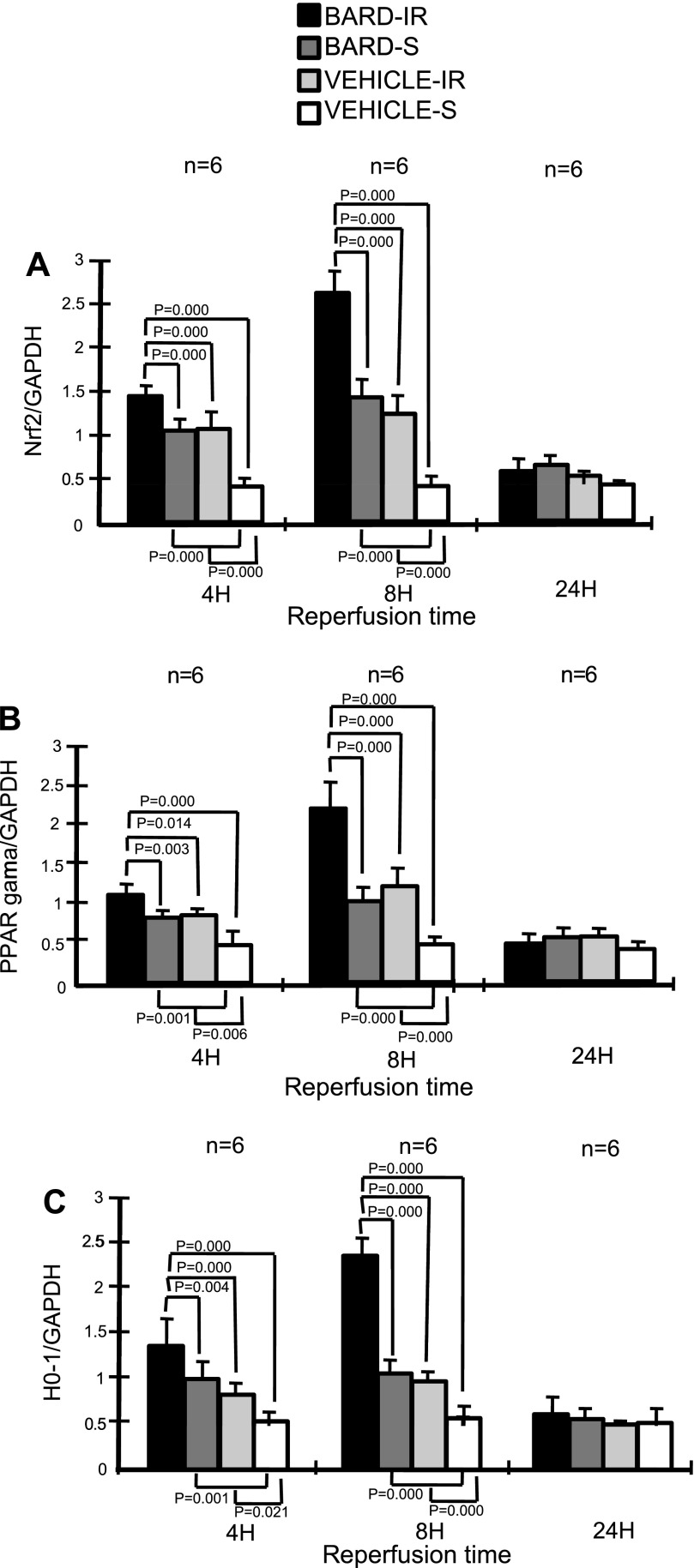
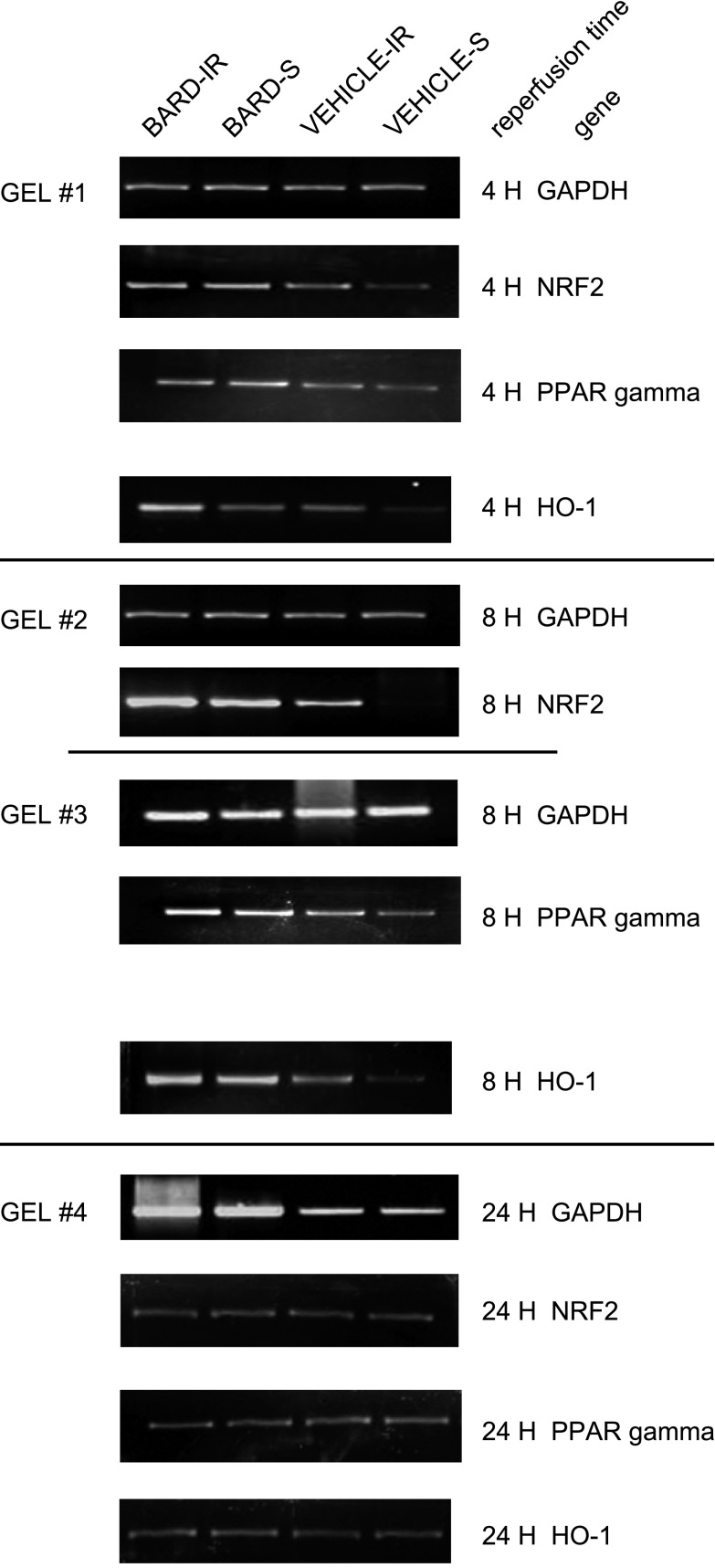
Previous studies of Nrf2 have focused on its posttranslational regulation by oxidative stress (44). We now show that Nrf2 mRNA abundance is also regulated. In the absence of ischemic injury, we found that BARD increased Nrf2 mRNA abundance. The results from six kidneys/group are summarized by the densitometry in Fig. 4, and a representative gel is shown in Fig. 5. Both figures indicate that at 3 and 6 h after a single oral dose, BARD increased the renal mRNA abundance of Nrf2. We also examined renal Nrf2 mRNA abundance at 4-, 8-, and 24-h reperfusion. Figure 6 summaries the densitometry of six kidneys/group, and Fig. 7 shows the results of a representative RT-PCR gel. At 4 h after surgery, both densitometry (Fig. 6) and visual examination (Fig. 7) showed that BARD or ischemia increased NRF2 mRNA. The small, but statistically significant differences by densitometry between BARD-IR vs. BARD-sham (S) vs. vehicle-IR are not seen by visual inspection and were so small that they are unlikely to be biologically important. At 8 h after surgery, both densitometry and visual examination showed vehicle-IR had greater Nrf2 mRNA than vehicle-S, that BARD-S Nrf2 was at least equal to vehicle-IR Nrf2 mRNA, and that BARD-IR had the greatest Nrf2 mRNA of all groups. At 24 h after surgery, all groups had low baseline levels of Nrf2 mRNA. The greatest differences between the various groups occurred at 8 h after surgery. Note that the renal artery clamping was always performed after BARD administration, when expression of the protective genes was already occurring.
Fig. 4.
BARD increases renal NF-E2-related factor 2 (Nrf2), peroxisome proliferator-activated receptor-γ (PPARγ), and heme oxygenase-1 (HO-1) mRNA abundance in surgically unmanipulated kidneys: densitometries. Kidneys were harvested 3 or 6 h after a single injection of BARD; n = 6/group. Average and standard ratios of the RT-PCR are shown. P values are comparisons between the indicated groups by t-test.
Fig. 5.
BARD increases renal Nrf2, PPARγ, and HO-1 mRNA abundance in surgically unmanipulated kidneys. Shown is a representative RT-PCR gel. 3H and 6H indicate 3 and 6 h after BARD or vehicle administration, respectively.
Fig. 6.
BARD increases renal Nrf2 (A), PPARγ (B), and HO-1 (C) mRNA abundance after IR: densitometries. The y-axis shows the ratio of densitometry of the indicated gene to the densitometry of GAPDH. IR, ischemia caused by clamping the renal arteries, followed by the reperfusion times shown on the x-axis; S, sham surgery where a clamp was placed under the renal arteries and no occlusion of the renal arteries occurred. P values are by 1-way ANOVA of all treatment groups at a given reperfusion time, followed by the Student-Newman-Keuls method applied to the indicated groups.
Fig. 7.
BARD increases renal Nrf3, PPARγ, and HO-1 mRNA abundance after IR: representative gels. Gel 1 compares the RT-PCR for the above 3 genes compared with GAPDH at 4-h reperfusion in kidneys; gels 2 and 3 show the results at 8-h reperfusion; gel 4 shows the results at 24-h reperfusion.
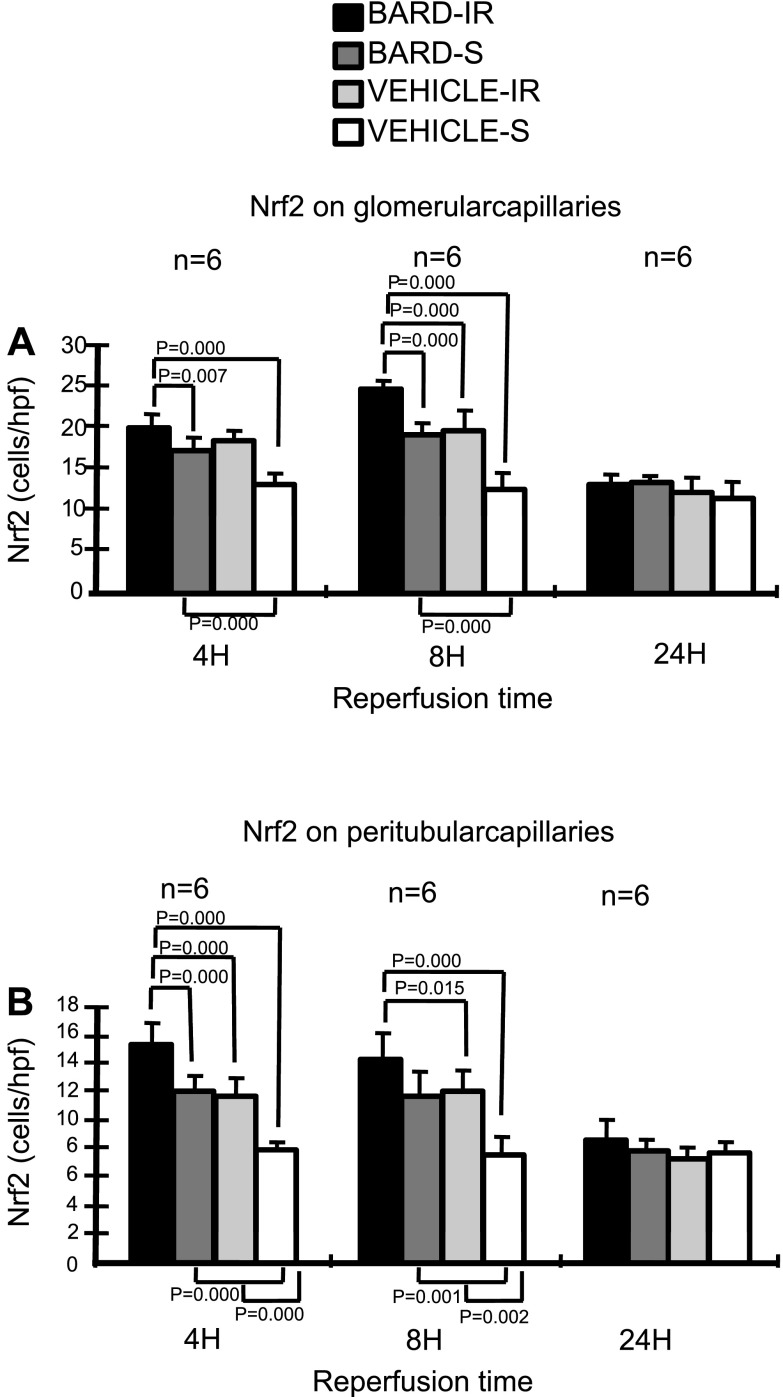
In addition to assaying mRNA abundance, we used immunohistology to both assess protein abundance and also protein localization. Figure 8 shows the semiquantitative analysis of Nrf2 protein determined by immunohistology. Six kidneys per group were immunostained for Nrf2, and the slides were examined and scored for the number of positive endothelial cells in the glomeruli and peritubular areas. This figure shows that 1) IR increased protein expression of Nrf2 above that of sham-operated kidneys, 2) BARD increased protein expression above that of vehicle-treated sham-operated kidneys, and 3) maximal protein expression was found after both BARD and IR. Figure 9, A and B, shows representative photomicrographs of BARD-induced increases in Nrf2 protein after IR. Figure 10 shows that the increased Nrf2 was on glomerular endothelial cells. Double immunofluorescence staining shows colocalization of Nrf2 and the endothelial marker CD31 in glomeruli. Figure 11 shows the increased Nrf2 on cortical peritubular capillaries.
Fig. 8.
BARD increases renal Nrf2 protein. Sections were immunostained for Nrf2 protein. A: semiquantitative analysis of the glomeruli. B: semiquantitative analysis of the peritubular capillaries of the cortex. The y-axis shows the number of Nrf2-positive cells per high-power field (hpf). The statistical analyses for each time point were by ANOVA, and the comparisons between the indicated groups were then performed by the Student-Newman-Keuls method.
Fig. 9.
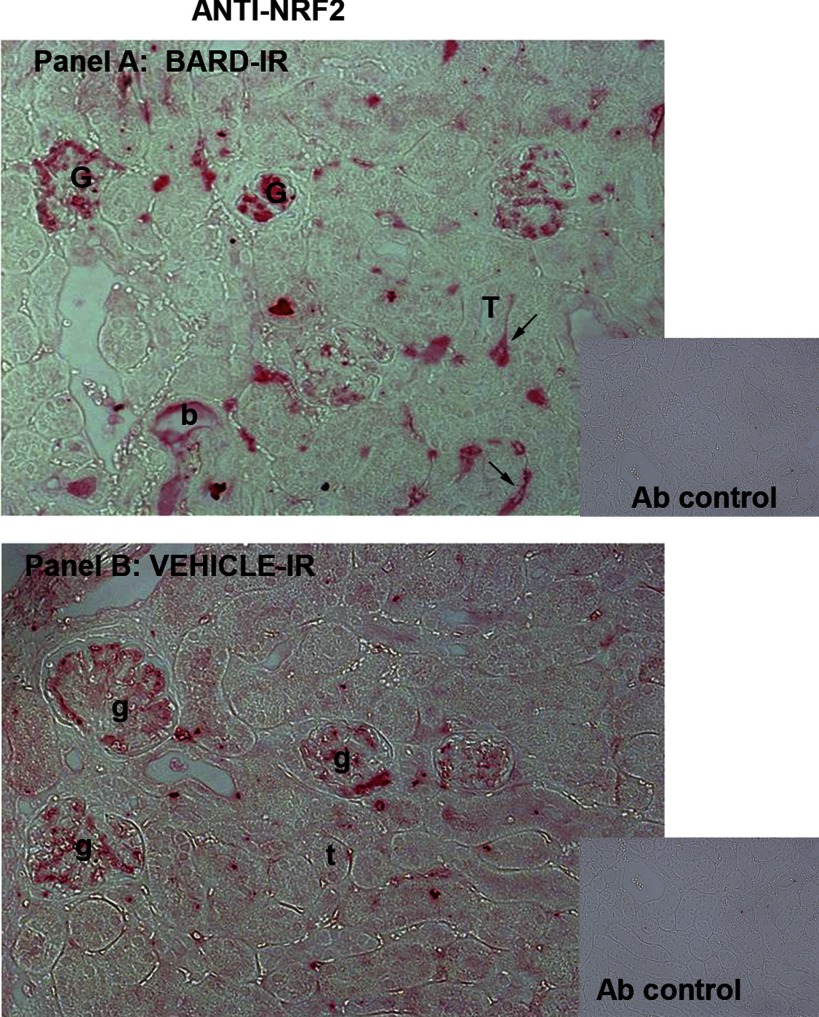
Increased Nrf2 in BARD- vs. vehicle-treated ischemic renal cortex. A: anti-Nrf2 immunoperoxidase staining of BARD-treated ischemic kidneys at 8-h reperfusion. G, intensely Nrf2-positive glomeruli; T, one of many Nrf2-negative tubules; b, one rare Nrf2-positive tubule. Black arrows indicate peritubular Nrf2-positive “capillaries.” Capillaries are provisionally identified in this figure, and definitively identified in Figs. 11 and 12 that show double staining of CD31 and Nrf2. Inset: control antibody. B: anti-Nrf2 immunoperoxidase in vehicle-treated ischemic kidneys at 8-h reperfusion. Glomeruli that are less intensely stained than in the BARD-treated kidney in A are indicated as g, and t indicates one of many tubules. Inset: control antibody.
Fig. 10.
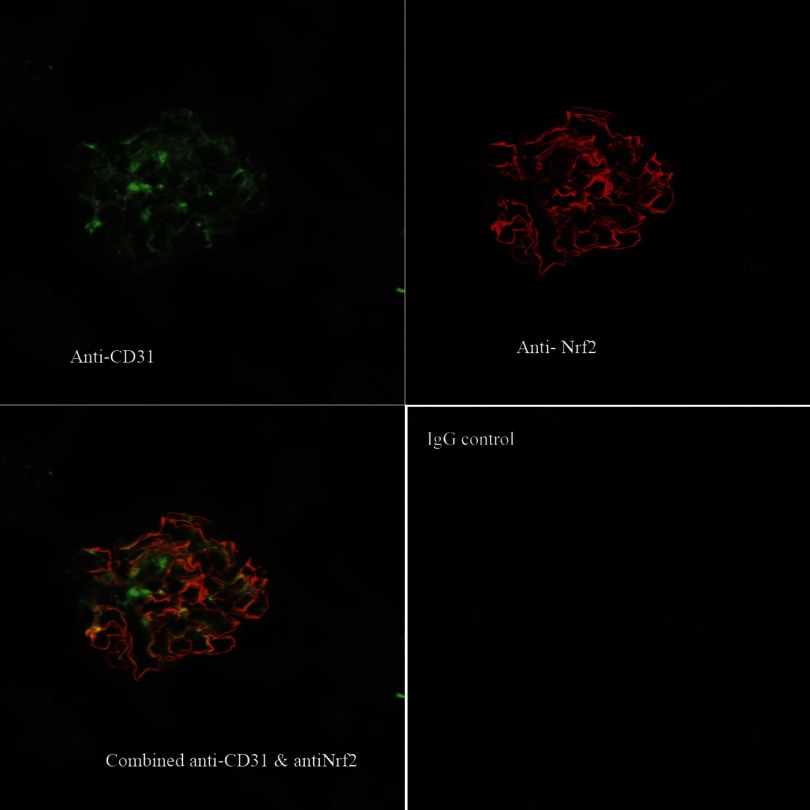
Localization of Nrf2 to glomerular endothelia of BARD-treated ischemic kidneys at 8-h reperfusion. Anti-CD31 is shown in green, anti-Nrf2 in red, and the overlap of both antibodies in yellow.
Fig. 11.
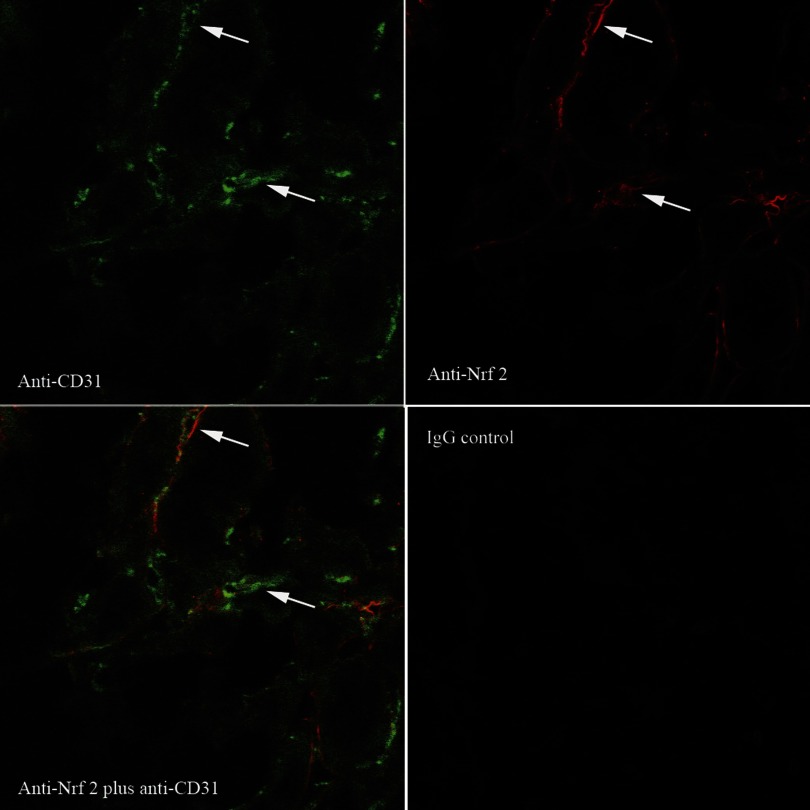
Localization of Nrf2 to cortical peritubular endothelia of BARD-treated ischemic kidneys at 8-h reperfusion. White arrows show a few of the many endothelia stained with anti-CD31 (green), anti-Nrf2 (red), and both antibodies (yellow).
BARD increases PPARγ on glomerular capillaries during ischemic AKI.
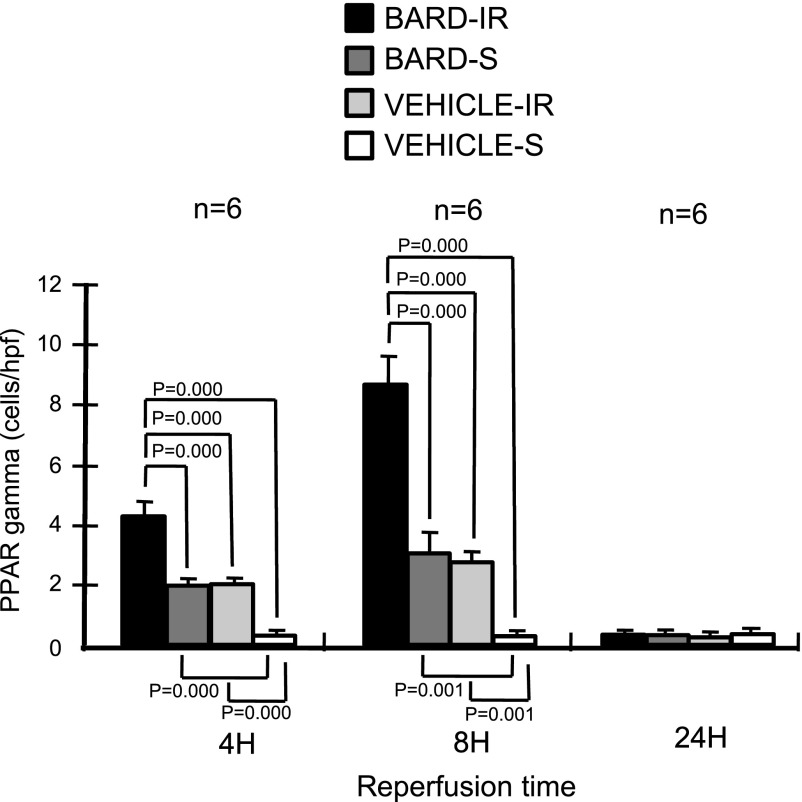
BARD might also ameliorate ischemic AKI by its effect on PPARγ, another putative protective gene, during ischemic AKI. Previous studies have focused on the posttranslational regulation of PPARγ, which is its activity as a transcription factor after it is bound by ligands such as thiaglitazones, but the effect of IR and pharmacological agents on the abundance of PPARγ mRNA and protein was not explored. We now show that BARD increased mRNA abundance in the absence of IR (Figs. 4 and 5). Furthermore, at 4 h of IR, both densitometry (Fig. 6) and the visual inspection (Fig. 7) show increased PPARγ expression in both BARD-IR and BARD-S groups compared with the vehicle-IR and vehicle-S groups. Although densitometry showed a further increase in the BARD-IR group compared with the BARD-S group, this was not apparent on visual inspection. In addition, both densitometry and visual inspection show that at 8-h reperfusion, the vehicle-S group had the least PPARγ mRNA, BARD-S and vehicle-IR had similar increased PPARγ expression, and BARD-IR had the greatest expression of all. Maximum PPARγ mRNA occurred at 8-h reperfusion. We also found that either BARD or IR increases PPARγ protein and that maximal protein expression occurred after the combination of both BARD and IR (Figs. 12 and 13). Double-staining immunofluorescence using anti-PPARγ and anti-CD31, an endothelial marker, showed that BARD was localized to glomerular endothelium (Fig. 14). Overall, the increased PPARγ expression and localization after BARD was similar to that of Nrf2 discussed in the previous section.
Fig. 12.
Semiquantitative analysis of renal PPARγ protein in glomeruli. Sections were immunostained for PPARγ protein. The y-axis shows the number of Nrf2-positive cells per hpf. The statistical analyses for each time point were by ANOVA, and the comparisons between the indicated groups were then performed by the Student-Newman-Keuls method.
Fig. 13.
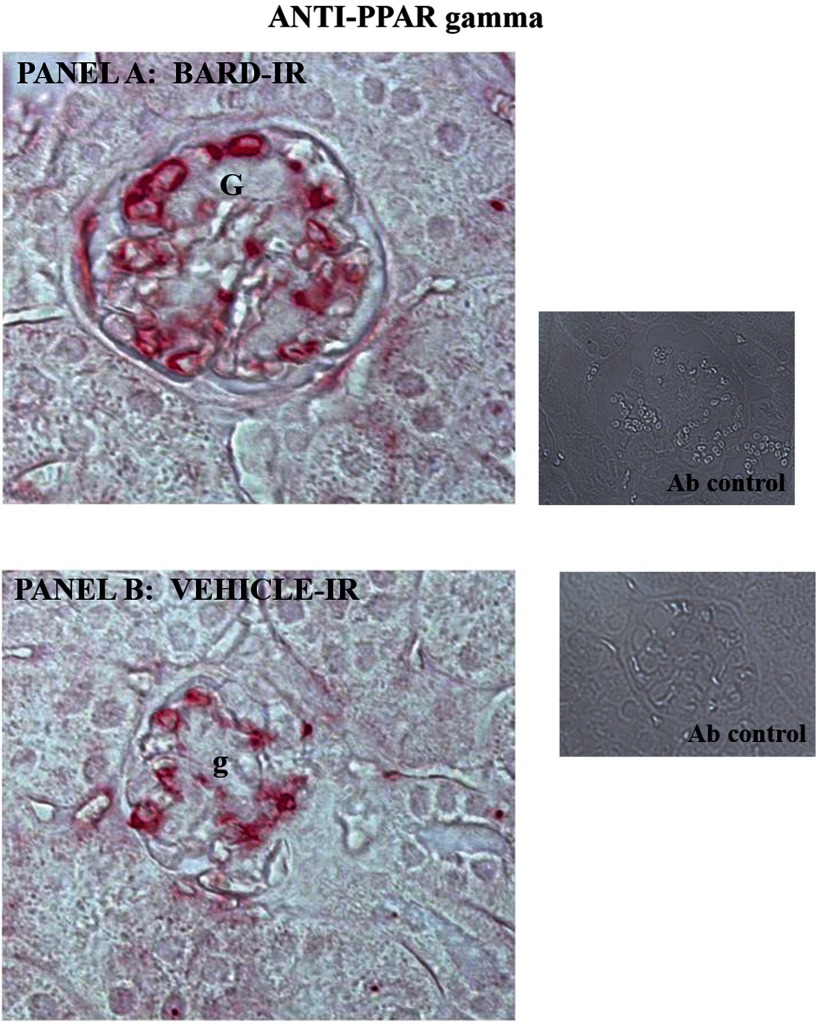
Increased PPARγ in BARD- vs. vehicle-treated ischemic renal cortex. A: anti-PPARγ immunoperoxidase staining of BARD-treated ischemic kidneys at 8-h reperfusion. G, intensely PPARγ-positive glomeruli. Inset: control antibody. B: anti-PPARγ immunoperoxidase in vehicle-treated ischemic kidneys at 8-h reperfusion. Glomeruli that are less intensely stained than in the BARD-treated kidney in A are indicated as g. Inset: control antibody.
Fig. 14.
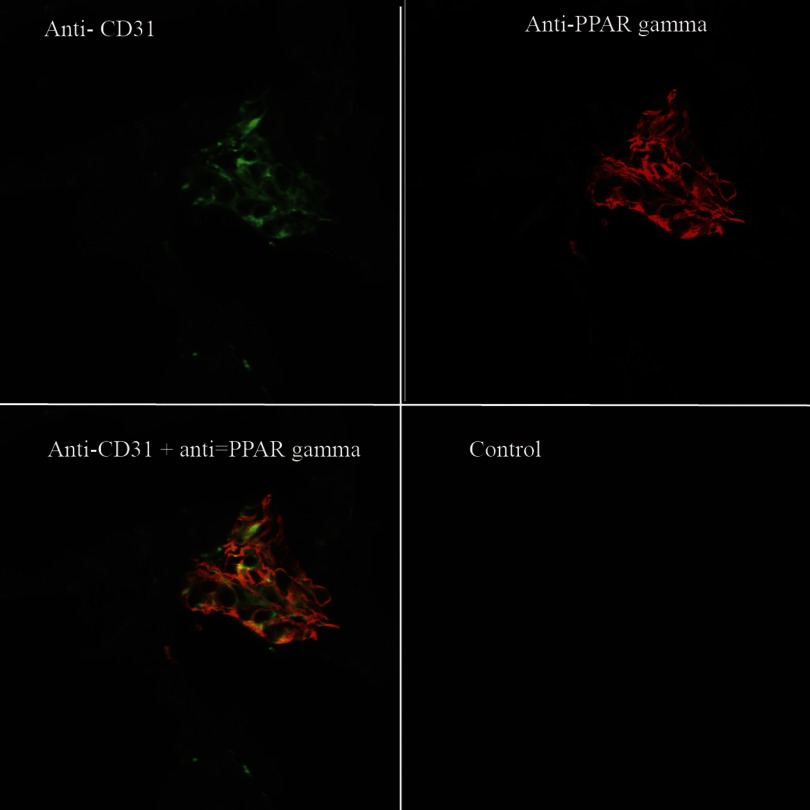
Localization of PPARγ to glomerular endothelia of BARD-treated ischemic kidneys at 8-h reperfusion. Anti-CD31 is shown in green, anti-PPARγ in red, and the overlap of both antibodies in yellow.
BARD and IR increase HO-1 protein in tubules and leukocytes.
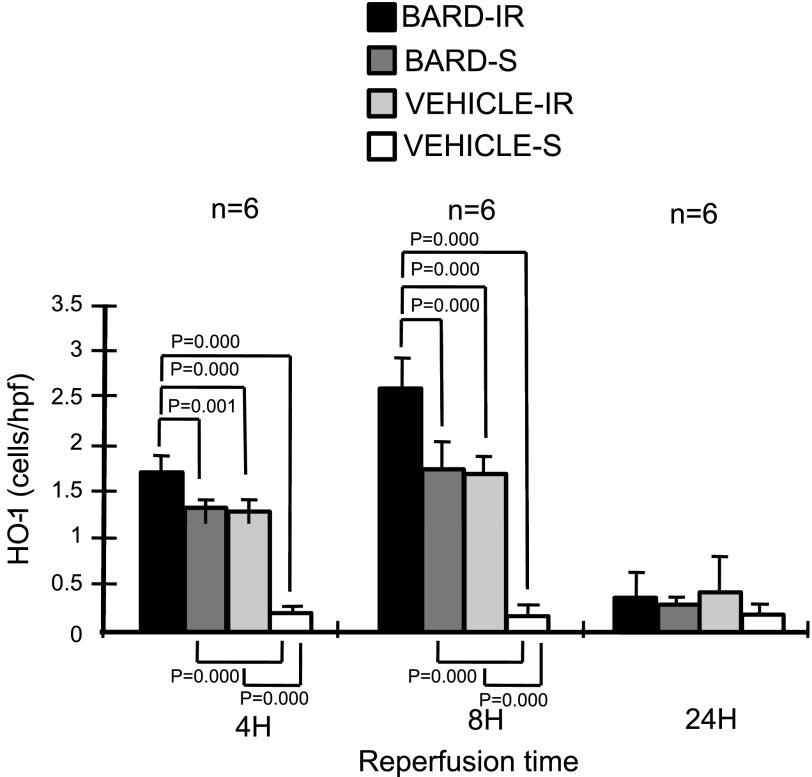
Finally, BARD might ameliorate ischemic AKI by its effect on HO-1. In many studies, increasing HO-1 expression ameliorated ischemic AKI (reviewed in Ref. 42). Similar to Nrf2 and PPARγ, we found that BARD and IR increased HO-1 mRNA and protein (Figs. 4, 5, 6, 7, and 15). In contrast to Nrf2 and PPARγ, we found localization of HO-1 protein on tubules and interstitial cells instead of endothelia at 8-h reperfusion. Figure 16 shows the prominent HO-1 immunostaining in BARD- compared with vehicle-ischemic kidneys (Fig. 16, A and B). HO-1, shown by arrows, is mostly localized to tubules. Figure 16C shows a high-power view of HO-1 protein on tubules and interstitial leukocytes.
Fig. 15.
Semiquantitative analysis of renal HO-1 protein. Sections were immunostained for HO-1. The y-axis shows the number of Nrf2-positive cells per hpf. The statistical analyses for each time point were by ANOVA, and the comparisons between the indicated groups were then performed by the Student-Newman-Keuls method.
Fig. 16.
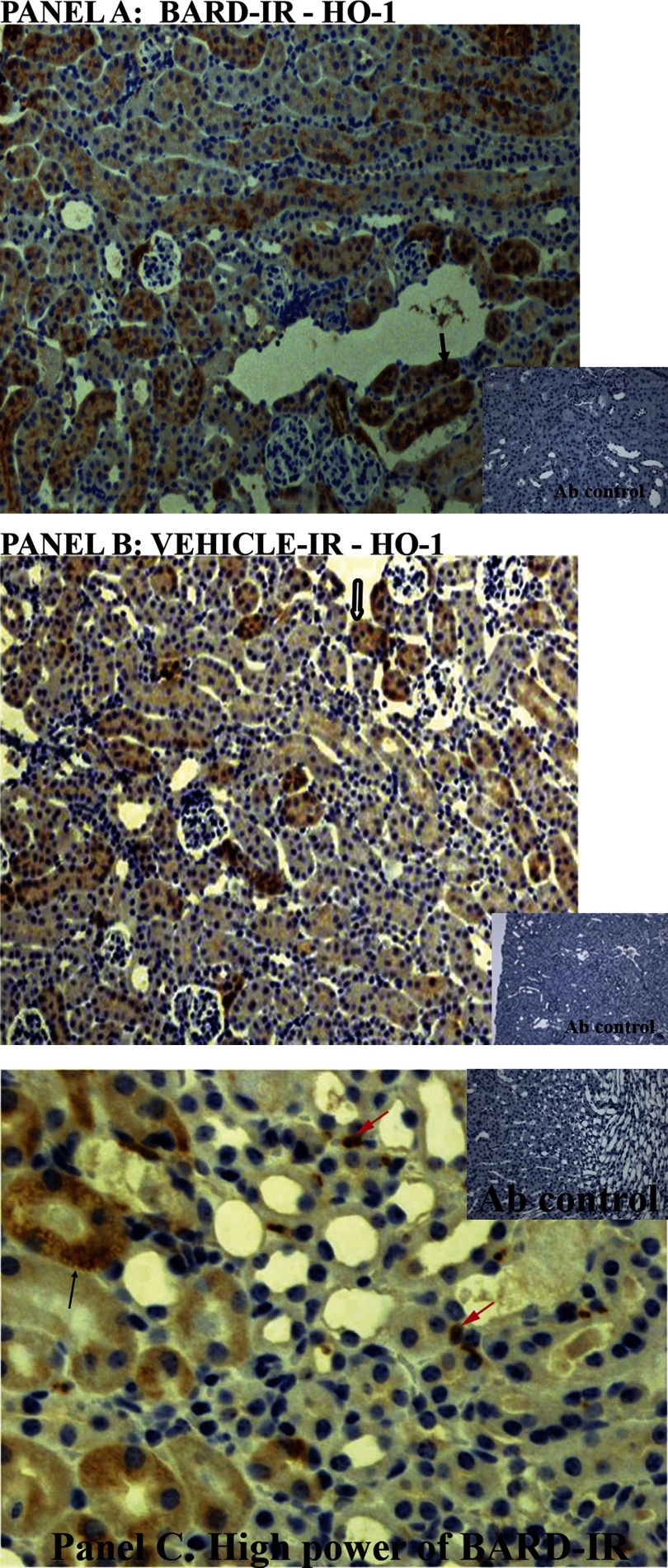
A and B: localization of increased HO-1 in BARD-treated ischemic kidneys. A: BARD-treated ischemic kidney. Black arrow indicates one of many tubules prominently stained for HO-1. B: vehicle-treated ischemic kidney. Hollow black arrow indicates one of many tubules less prominently stained for HO-1 compared with A. C: localization of increased HO-1 in BARD-treated ischemic kidneys. C: high power of BARD-treated ischemic kidney. Black arrow indicates one of many HO-1 positive tubules. Red arrows indicate some interstitial cells that may be leukocytes by virtue of their morphology.
DISCUSSION
Our data show that BARD ameliorates ischemic AKI by both functional and pathological measurements. We also show that BARD may exert its salutary effect by increasing the expression of three protective genes: Nrf2, HO-1, and PPARγ.
One protective gene is Nrf2. In response to oxidative stress, such as that occurring during ischemic AKI (reviewed in Ref. 42), this transcription factor activates antioxidant genes (44) and ameliorates ischemic AKI. This protective role for Nrf2 is based on the following observations. Nrf2 is activated in wild-type kidneys during IR (31). Pharmacological activation of Nrf2 by sulforaphane ameliorated ischemic AKI (67). Inactivation of Nrf2 by transgenic knockout decreased expression of adaptive genes (38), increased oxidative stress during ischemic AKI (15), and exacerbated ischemic AKI. Despite these published data, details of how Nrf2 is regulated, the kinetics of Nrf2 expression, and the precise localization of Nrf2 in the ischemic kidney had not previously been examined.
We addressed three previously unexplored issues in the course of showing that BARD increased Nrf2.
Regulation.
Most previous studies of Nrf2 regulation have focused on its posttranslational regulation by KEAP (11, 31). Both ROS and BARD have been reported in other models to inactivate KEAP and thus prevent degradation of Nrf2; the resulting increased Nrf2 protein acts as a transcription factor to activate downstream adaptive antioxidant genes (30, 44, 64). We now show that IR also increases Nrf2 mRNA abundance; this is an additional regulatory mechanism.
Kinetics.
We also showed that maximal Nrf2 expression occurred at 8-h reperfusion.
Localization.
We show Nrf2 protein expression on endothelia of the glomeruli and cortical peritubular capillaries at 8-h reperfusion.
Although the many studies of BARD have emphasized its action via stabilization of Nrf2, comparison of microarray studies on KEAP conditional knockout mice which overexpress Nrf2 vs. administration of a BARD-like compound indicate that there are Nrf2-independent beneficial effects of the drug (65). This is consistent with the additional beneficial effects of BARD on PPARγ and HO-1 production that we describe below.
The second renal-protective gene we examined was PPARγ. Most previous studies have concentrated on the effect of ligands, such as thioglitazones, that bind to PPARγ and cause this transcription factor to then activate beneficial downstream genes. Such studies have shown a beneficial effect of PPARγ on ischemic AKI (9, 45, 53, 69) and chronic kidney disease (6, 18, 21, 50). In contrast to these studies, we found that BARD and IR increased the mRNA and protein abundance of PPARγ. This is an important distinction because the downstream effects of PPARγ are complex. They include not only the above ligand-dependent effects but also ligand-independent activation of downstream genes (16, 61, 72). The latter effects may be independent of pharmacological agonists, but may be altered by the increased abundance of PPARγ mRNA and protein that we found after BARD and IR. Although a beneficial role for PPARγ has been suggested, the protein has not previously been localized during ischemic AKI. We now demonstrate PPARγ on glomerular endothelia by immunohistology. This is a similar localization to Nrf2, as discussed above, but different from HO-1, discussed below.
Finally, we examined the effects of BARD on HO-1. HO-1 has previously been proposed as a protective gene during ischemic AKI. On the one hand, the HO-1 knockout mouse has increased ischemic AKI, but on the other hand, other manipulations of HO-1 have variable effects on injury. In some circumstances, too much HO-1 has been harmful; apparently HO-1 is protective if expressed in the right amounts at the right time by the right cells (42). If BARD ameliorates ischemic AKI by increasing HO-1, it may do so by satisfying these requirements. We found that BARD and IR increased HO-1 mRNA and protein abundance. Although it may be difficult to localize HO-1 (42, 71) during ischemia, we were able to demonstrate this protein on renal tubules and leukocytes. The benefit of increased HO-1 on tubules may be decreased apoptosis of these cells during ischemic AKI (13). The benefit of HO-1 on leukocytes may be its anti-inflammatory actions (54).
Both PPARγ (4, 5, 12, 28, 29, 36, 48, 51, 62, 70) and Nrf2 have been proposed as transcription factors that activate the HO-1 gene. However, we found that PPARγ and Nrf2 were localized to endothelia, while HO-1 was localized to tubules and leukocytes. This suggests that other transcription factors regulate HO-1 during ischemic AKI (2, 19, 52).
How can BARD have such multiple effects, on Nrf2, PPARγ, and HO-1 in endothelia, epithelia, and also leukocytes? Indeed, such multiple effects were the goal of the original producers of BARD, who were aiming to develop a new class of pharmaceuticals that modulate regulatory physiological networks, rather than specific enzymes or cell surface receptors in the manner of classic drugs (34, 56). Although the precise mechanisms of BARD are unknown, several have been proposed that account for the multiplicity of beneficial effects. BARD may form Michael adducts with only a few specific cysteine, lysine, arginine, and histadine residues on specific proteins (56). If these are key regulatory proteins, then multiple effects may result. Examples follow. BARD may bind to and inhibit IκB kinase β (IKKβ) and thus prevent activation of NF-κB and the NF-κB-regulated proteins (1, 68). BARD may alter Smad and ERK signaling (20, 57) or STAT signaling (33, 37) and thus other fundamental cellular responses to stress. By identifying which cells express putative protective genes after BARD treatment, we have made an important forward step in understanding the mechanism of this agent in ischemic AKI. Future studies, including those using BARD simultaneously with conditional knockout of the putative protective genes on specific cells, are needed to fully understand this agent.
Although we now report that BARD was effective in preventing ischemic AKI when given before the injury, we also found that giving BARD 4 h after ischemic injury did not ameliorate disease. Thus BARD may be effective as a therapeutic agent given before expected renal ischemia, for example, before cardiac surgery. Furthermore, progressive chronic kidney disease may result from repeated subclinical episodes of ischemic AKI (60), and chronic administration of BARD, before such episodes, might ameliorate progression to end-stage renal disease. Recently, CDDO-IM, a compound similar to BARD, has been reported to ameliorate cisplatin AKI (3). We also found that BARD ameliorates this disease (see Supplemental Fig. S1, and Supplemental Methods and Results; all supplementary material for this article is available online at the journal web site).
In conclusion, we found that BARD ameliorated murine ischemic AKI as measured by function and pathology. In addition, we found that BARD increased mRNA and protein expression of the renal-protective genes Nrf2, PPARγ, and HO-1, the former two genes on endothelia and the latter gene on tubules and leukocytes. Altogether, our data suggest that BARD may be a useful tool both for treating renal disease and for understanding the mechanisms that protect the kidney from injury.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants RO-1 DK069633 (to C. Y. Lu), F32DK084701 and T32DK07257 (to J. Hartono), and DK079328 (UT Southwesterrn O'Brien Kidney Research Core Center) and a Beecherl grant (to C. Y. Lu). This work was also supported in part by a research grant from Reata Pharmaceuticals, Inc.
DISCLOSURES
Drs. Meyer, Wigley, Ferguson, and Grossman are employed by Reata Pharmaceuticals, Inc., which manufactures BARD.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Kathy Trueman for assistance in preparing the manuscript and figures.
REFERENCES
- 1.Ahmad R, Raina D, Meyer C, Kharbanda S, Kufe D. Triterpenoid CDDO-Me blocks the NF-kappa B pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem 281: 35764–35769, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, Klaassen CD. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335: 2–12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Maqueda M, El Bekay R, Alba G, Monteseirin J, Chacon P, Vega A, Martin-Nieto J, Bedoya FJ, Pintado E, Sobrino F. 15-Deoxy-delta 12,14-prostaglandin J2 induces heme oxygenase-1 gene expression in a reactive oxygen species-dependent manner in human lymphocytes. J Biol Chem 279: 21929–21937, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension 43: 993–1002, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Boulanger H, Mansouri R, Gautier JF, Glotz D. Are peroxisome proliferator-activated receptors new therapeutic targets in diabetic and non-diabetic nephropathies? Nephrol Dial Transplant 21: 2696–2702, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brady HR, Clarkson MR, Lieberthal W, Brenner BM, Rector F. Acute renal failure. In: The Kidney. St. Louis, MO: Saunders, 2004, p. 1215–1270. [Google Scholar]
- 8.Brezis M, Rosen S, Silva P, Epstein FH. Renal ischemia: a new perspective. Kidney Int 26: 375–383, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee PK, Patel NS, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Britti D, Eberhardt W, Pfeilschifter J, Thiemermann C. The cyclopentenone prostaglandin 15-deoxy-Delta(12,14)-prostaglandin J2 ameliorates ischemic acute renal failure. Cardiovasc Res 61: 630–643, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28: 1339–1346, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Gong P, Stewart D, Hu B, Li N, Cook J, Nel A, Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J2 is mediated by the stress response elements and transcription factor Nrf2. Antioxid Redox Signal 4: 249–257, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323–354, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Haller H, Dragun D, Miethke A, Park JK, Weis A, Lippoldt A, Gross V, Lust FC. Antisense oligonucleotides for ICAM 1 attenuate reperfusion injury and renal failure in the rat. Kidney Int 50: 473–480, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Hirayama A, Nagase S. Electron paramagnetic resonance imaging of oxidative stress in renal disease. Nephron Clin Pract 103: c71–e76, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev 18: 461–467, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iglesias P, Diez JJ. Peroxisome proliferator-activated receptor gamma agonists in renal disease. Eur J Endocrinol 154: 613–621, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep 11: 56–62, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y, Lee HJ, Goodman C, Uskokovic M, Liby K, Sporn M, Suh N. The synthetic triterpenoid CDDO-imidazolide induces monocytic differentiation by activating the Smad and ERK signaling pathways in HL60 leukemia cells. Mol Cancer Ther 5: 1452–1458, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kanjanabuch T, Ma LJ, Chen J, Pozzi A, Guan Y, Mundel P, Fogo AB. PPAR-gamma agonist protects podocytes from injury. Kidney Int 71: 1232–1239, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kashgarian M. Acute tubular necrosis an ischemic renal injury. In: Heptinstall's Pathology of the Kidney (5th ed.), edited by Jennette JC, Olsen JC, Schwartz MM, Silva FG. Philadelphia, PA: Lippincott-Raven, 1999, p. 863–889. [Google Scholar]
- 23.Kelly KJ, Sandoval RM, Dunn KW, Molitoris BA, Dagher PC. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. Am J Physiol Cell Physiol 284: C1309–C1318, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA 91: 812–816, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khosla N, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini E, Mehta RL. Preexisting chronic kidney disease: a potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 4: 1914–1919, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiew LV, Munavvar AS, Law CH, Azizan AN, Nazarina AR, Sidik K, Johns EJ. Effect of antisense oligodeoxynucleotides for ICAM-1 on renal ischaemia-reperfusion injury in the anaesthetised rat. J Physiol 557: 981–989, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EH, Na HK, Surh YJ. Upregulation of VEGF by 15-deoxy-Delta12,14-prostaglandin J2 via heme oxygenase-1 and ERK1/2 signaling in MCF-7 cells. Ann NY Acad Sci 1090: 375–384, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 27: 1276–1282, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J 19: 1061–1066, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J 20: 2624–2626, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996. [PubMed] [Google Scholar]
- 33.Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, Gribble GW, Sporn MB, Letterio JJ. The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res 12: 4288–4293, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer 7: 357–369, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Lieberthal W, Nigam SK, Bonventre JV, Brezis M, Siegel N, Rosen S, Portilla D, Venkatachalam M. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol Renal Physiol 275: F623–F631, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Liou JY, Shyue SK, Wu KK. 15d-Prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 26: 481–487, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Ling X, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, McQueen T, Dietrich M, Madden TL, Andreeff M. The novel triterpenoid C-28 methyl ester of 2-cyano-3, 12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res 67: 4210–4218, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76: 277–285, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Lo L, Liu KD, Hsu CY. Long-term outcomes after acute kidney injury: where we stand and how we can move forward. Am J Kidney Dis 53: 928–931, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Lucke B. Lower nephron nephrosis. Mil Surg 99: 371–396, 1946. [PubMed] [Google Scholar]
- 41.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med 47: 1–12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada M, Yan SF, Pinsky DJ. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J 16: 1861–1868, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Oliver J, Mac DM, Tracy A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest 30: 1307–1439, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284: F608–F627, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Park MK, Kim CH, Kim YM, Kang YJ, Kim HJ, Seo HG, Lee JH, Chang KC. Akt-dependent heme oxygenase-1 induction by NS-398 in C6 glial cells: a potential role for CO in prevention of oxidative damage from hypoxia. Neuropharmacology 53: 542–551, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Rabb H, Mendiola CC, Dietz J, Saba SR, Issekutz TB, Abanilla F, Bonventre JV, Ramirez G. Role of CD11a and CD11b in ischemic acute renal failure in rats. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1052–F1058, 1994. [DOI] [PubMed] [Google Scholar]
- 50.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int 70: 1223–1233, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sato N, Moore FA, Kone BC, Zou L, Smith MA, Childs MA, Moore-Olufemi S, Schultz SG, Kozar RA. Differential induction of PPAR-γ by luminal glutamine and iNOS by luminal arginine in the rodent postischemic small bowel. Am J Physiol Gastrointest Liver Physiol 290: G616–G623, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Sikorski EM, Hock T, Hill-Kapturczak N, Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol 286: F425–F441, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Sivarajah A, Chatterjee PK, Patel NS, Todorovic Z, Hattori Y, Brown PA, Stewart KN, Mota-Filipe H, Cuzzocrea S, Thiemermann C. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol 23: 267–276, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Soares MP, Marguti I, Cunha A, Larsen R. Immunoregulatory effects of HO-1: how does it work? Curr Opin Pharmacol 9: 482–489, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Solez K. Pathogenesis of acute renal failure. Int Rev Exp Pathol 24: 277–333, 1983. [PubMed] [Google Scholar]
- 56.Sporn MB, Liby K, Yore MM, Suh N, Albini A, Honda T, Sundararajan C, Gribble GW. Platforms and networks in triterpenoid pharmacology. Drug Dev Res 68: 174–182, 2007. [Google Scholar]
- 57.Suh N, Roberts AB, Birkey Reffey S, Miyazono K, Itoh S, ten Dijke P, Heiss EH, Place AE, Risingsong R, Williams CR, Honda T, Gribble GW, Sporn MB. Synthetic triterpenoids enhance transforming growth factor beta/Smad signaling. Cancer Res 63: 1371–1376, 2003. [PubMed] [Google Scholar]
- 58.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol 170: 1517–1523, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28: 478–485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villacorta L, Schopfer FJ, Zhang J, Freeman BA, Chen YE. PPARgamma and its ligands: therapeutic implications in cardiovascular disease. Clin Sci (Lond) 116: 205–218, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wayman NS, Hattori Y, McDonald MC, Mota-Filipe H, Cuzzocrea S, Pisano B, Chatterjee PK, Thiemermann C. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J 16: 1027–1040, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Wright MM, Kim J, Hock TD, Leitinger N, Freeman BA, Agarwal A. Human heme oxygenase-1 induction by nitro-linoleic acid is mediated by cyclic AMP, AP-1, and E-box response element interactions. Biochem J 422: 353–361, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther 6: 154–162, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, Liby KT, Sporn MB, Sutter TR, Kensler TW. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis 30: 1024–1031, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ympa YP, Sakr Y, Reinhart K, Vincent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med 118: 827–832, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol 75: 2214–2223, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther 5: 3232–3239, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Yoshimura R, Matsuyama M, Segawa Y, Tsuchida K, Takemoto Y, Kuratsukuri K, Kawahito Y, Shinka T, Sano H, Nakatani T. Study of peroxisome proliferator-activated receptor (PPAR)-gamma in renal ischemia-reperfusion injury. Transplant Proc 36: 1946–1948, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Yu X, Shao XG, Sun H, Li YN, Yang J, Deng YC, Huang YG. Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus. Brain Res 1200: 146–158, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics 25: 375–386, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta 1771: 915–925, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.