Abstract
Ubiquitin-mediated protein degradation is necessary for both increased ventricular mass and survival signaling for compensated hypertrophy in pressure-overloaded (PO) myocardium. Another molecular keystone involved in the hypertrophic growth process is the mammalian target of rapamycin (mTOR), which forms two distinct functional complexes: mTORC1 that activates p70S6 kinase-1 to enhance protein synthesis and mTORC2 that activates Akt to promote cell survival. Independent studies in animal models show that rapamycin treatment that alters mTOR complexes also reduces hypertrophic growth and increases lifespan by an unknown mechanism. We tested whether the ubiquitin-mediated regulation of growth and survival in hypertrophic myocardium is linked to the mTOR pathway. For in vivo studies, right ventricle PO in rats was conducted by pulmonary artery banding; the normally loaded left ventricle served as an internal control. Rapamycin (0.75 mg/kg per day) or vehicle alone was administered intraperitoneally for 3 days or 2 wk. Immunoblot and immunofluorescence imaging showed that the level of ubiquitylated proteins in cardiomyocytes that increased following 48 h of PO was enhanced by rapamycin. Rapamycin pretreatment also significantly increased PO-induced Akt phosphorylation at S473, a finding confirmed in cardiomyocytes in vitro to be downstream of mTORC2. Analysis of prosurvival signaling in vivo showed that rapamycin increased PO-induced degradation of phosphorylated inhibitor of κB, enhanced expression of cellular inhibitor of apoptosis protein 1, and decreased active caspase-3. Long-term rapamycin treatment in 2-wk PO myocardium blunted hypertrophy, improved contractile function, and reduced caspase-3 and calpain activation. These data indicate potential cardioprotective benefits of rapamycin in PO hypertrophy.
Keywords: hypertrophy, mammalian target of rapamycin, signal transduction, cell survival
the increase in cardiac mass during pressure-induced hypertrophy of terminally differentiated cardiomyocytes requires increased protein synthesis (26), activation of prosurvival pathways (1, 8), and active ubiquitin/proteasome-mediated degradation of select target proteins (10). The ubiquitin proteasome system (UPS) offers the major nonlysosomal degradation of proteins in the cell, a process that requires both polyubiquitination (Ub) of select target proteins via specific E3 ligases and subsequent degradation of polyubiquitinated proteins via the proteasome. Accumulation of ubiquitinated proteins in the heart occurs because of insufficient proteasome function and precedes heart failure (36). In line with this, our recent study linked ubiquitin-mediated degradation to activation of survival signaling in pressure-overloaded (PO) hypertrophy. In fact, ubiquitin-mediated protein degradation in cells plays at least three major roles: 1) Ub of select targets and their subsequent efficient elimination by the proteasome system contributes to cellular homeostasis. Indeed, activation of this mechanism in PO myocardium is critical for cell survival and ventricular function (18). 2) Global Ub of cellular proteins and their effective elimination as observed in muscle-cell atrophy leads to decreased cell mass and size and often results in programmed cell death (33). 3) Finally, Ub of cellular proteins and their inefficient elimination attributable to inadequate proteasome function as observed in failing myocardium can lead to autophagic cell death (20).
A key regulatory protein also governing both cell growth and survival is the highly conserved mammalian target of rapamycin (mTOR), which forms two distinct complexes: mTORC1 activates p70S6 kinase-1 (S6K1) to enhance protein synthesis (16, 31, 32) and mTORC2 activates Akt (30) to promote survival. Akt, a member of the Akt/PKG/PKC family of serine/threonine kinases, is known to function both upstream as an activator of mTOR as well as downstream as a phosphorylation target of mTOR at S473. The divergent mTOR functions are mediated by molecular interactions between mTORC1 and mTORC2 complexes, which share some common molecules yet are defined by the presence of two distinct proteins, the rapamycin-sensitive adaptor protein of mTOR (raptor) in mTORC1 and the rapamycin-insensitive companion of mTOR (rictor) in mTORC2 (28, 38). As a molecular tool, the fungal macrolide rapamycin acts by irreversibly binding the 12-kDa FK506-binding protein (FKBP12), which inhibits mTORC1 assembly, but not mTORC2 (28).
mTORC2-mediated signaling is linked to cell survival via S473 phosphorylation and activation of Akt as well as through the associated Ub and elimination of many proapoptotic proteins, including the inhibitor of κB (IκB) (35), which undergoes phosphorylation at S32 and S36 by IκB kinase for its removal via UPS-mediated degradation (35). The prosurvival transcription factor NF-κB is then released for nuclear localization. Cardiac-specific expression of constitutively active Akt has been shown to promote NF-κB transcriptional activation, where rapamycin administration partially attenuated hypertrophic growth, but not NF-κB DNA binding (12, 31). Furthermore, the absence of ubiquitin-mediated NF-κB-dependent transcription during pressure-induced hypertrophic growth corresponded with increased mortality in our previous study (18). These data support a role for mTORC2 in cell survival by activating NF-κB.
The ubiquitin and mTOR pathways have recently been linked, as proteasomal inhibition was shown to repress mTORC1 signaling and downstream protein synthesis (37). However, unlike proteasomal inhibition, which also causes programmed cell death in the heart (20), inhibition of mTORC1 signaling in either C. elegans (17) or in aging mice (13) with rapamycin was shown to increase lifespan. Interestingly, cell-culture studies also show that inhibiting mTORC1, genetically or pharmacologically, can acutely increase Akt activation through mTORC2, depending on cell type (29). Therefore, in the present study, we utilized rapamycin administration during PO hypertrophy to evaluate whether ubiquitin-mediated regulation of growth and survival in hypertrophic myocardium is linked to mTOR/Akt signaling. As such, we demonstrate that acute rapamycin administration causes an increase in targeted Ub, Akt activation, and survival signaling, indicating that inhibition of mTORC1 during hypertrophy can tilt the molecular balance toward mTORC2 survival signaling in the myocardium.
MATERIALS AND METHODS
Reagents.
All chemicals were obtained from Sigma, St. Louis, MO except the following: phorbol-12-myristate-13-acetate (Calbiochem, Darmstadt, Germany), rapamycin (LC Laboratory, Woburn, MA). Antibodies were obtained from the following companies: anti-ubiquitin (clone P4D1) for Western analysis and pIκB (Santa Cruz Biotechnology, Santa Cruz, CA), ubiquitin for microscopy (Dako, Carpinteria, CA), N-cadherin (Zymed, San Francisco, CA), α-actinin (Sigma), pS2448-mTOR, pS2481-mTOR, pS473-Akt, Akt, p389-p70S6K1, IκB, caspase, cleaved caspase-3, pS235/S236-S6 Protein (Cell Signaling, Beverly, MA), raptor, rictor (Bethyl Laboratories, Montgomery, TX), mTOR (BD Biosciences, San Jose, CA), cellular inhibitor of apoptosis-1 (cIAP1; R & D Systems, Minneapolis, MN), total actin (Sigma), GAPDH (Fitzgerald, Concord, MA), horseradish peroxidase-labeled secondary antibodies (Promega, Madison, WI), and Alexa Fluor-labeled secondary antibodies (Invitrogen, Carlsbad, CA). Primary antibodies were used at a 1:1,000 dilution for immunoblotting.
RVPO rat model.
Three-month-old male Fischer-F344 rats (Charles River, Wilmington, MA) weighing ∼350 g were subjected to right ventricular PO (RVPO), as described for the feline model (6). RVPO was created by placing a constrictive band around the pulmonary artery of adult rats. Rats were intubated and placed on a biofeedback warming table. A rodent respirator supplanted 100% oxygen containing a mixture of 3% isoflurane. Left thoracotomy was performed under sterile surgical conditions, pulmonary artery was isolated, and a 17-gauge needle was placed along the artery. A sterile band of 3.0 silk suture was secured around the needle and artery, constricting its diameter to approximately 50% of its original diameter. The needle was then removed. The chest incision was repaired, buprenorphine was given for analgesia, and the animal was allowed to recover under close supervision. Four weeks after pulmonary artery banding (PAB) or sham operation, catheterization was performed similar to previously described methods (6). In this PAB model, the pulmonary arterial pressure at least doubles while the systemic arterial pressure remains the same. The left ventricle (LV) then serves as a same-animal internal control for RVPO. These rats were euthanized at the specified times.
All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996) and were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Cell isolation.
Adult ventricular feline and rat cardiomyocytes regularly isolated in our core facilities were obtained via a hanging-heart preparation using enzymatic digestion and cultured by the protocols described previously (19). Freshly isolated rat cardiomyocytes were plated on laminin-coated tissue culture plates at a density of (7.5 × 104 cells/ml media). After allowing 4 h for attachment, media was changed with serum-free Medium 199 (M199; GIBCO-BRL, Grand Island, NY) containing 200 U/ml penicillin and 200 μg/ml streptomycin (GIBCO-BRL).
Adenoviral construct for rapamycin-resistant S6K1.
Plasmid carrying rapamycin-resistant S6K1 (RR-S6K1) mutant (9) with NH2- and COOH-terminal deletions (53 and 103 residues, respectively) was obtained from Dr. George Thomas (Basel, Switzerland) and used for subcloning into adenovirus shuttle plasmid pAd CMV Link.1 for the construction of adenovirus, as described previously (16).
Rapamycin delivery.
For short-term treatment, rapamycin (0.75 mg/kg per day) or vehicle alone was administered via intraperitoneal injection three times: 24 h before surgery, during surgery, and 24 h postsurgery. The vehicle consisted of 0.2% high-viscosity carboxymethyl cellulose (C5013, Sigma) and 0.25% Tween 20 (Sigma) (4). A 20 mM stock of rapamycin was prepared in ethanol and then diluted in the vehicle. Injection volume was maintained at 150 μl each time for each animal, so the appropriate proportion of rapamycin plus vehicle was calculated according to the weight of each animal (∼350 g). For long-term studies, the daily injection of rapamycin or vehicle alone was extended for 2 wk until euthanasia.
Confocal microscopy.
Fresh frozen rat tissue samples of the RV and LV free walls were used for confocal studies as described previously (2). Tissue sections (10 μM thick) were fixed with 2% paraformaldehyde, permeabilized in 2% SDS for 5 min at room temperature, and blocked with 10% donkey serum for 1 h at room temperature. Primary antibodies, anti-ubiquitin antibody (1:100, Dako), anti-N-cadherin (1:150, Zymed), anti-α-actinin (1:200, Sigma), anti-active caspase-3 (1:100, Sigma), and anti-μ-calpain (1:100, Sigma) were added overnight at 4°C. Sections were washed in PBS and incubated with Alexa Fluor secondary antibodies for 2 h and imaged with laser-scanning confocal microscopy (Zeiss LSM 510 META).
Preparation of cell and tissue lysates and Western blot analysis.
Triton X-100-soluble and -insoluble fractions were prepared from fresh tissue and cell samples as established in our laboratory (21, 22). For cell-culture studies, isolated rat cardiomyocytes (7.5 × 105) were incubated overnight in serum-free M199 media. Agonist stimulation and rapamycin treatment were administered to cells at the concentrations and time periods indicated. Briefly, both soluble and insoluble proteins were prepared by extracting tissue or rat cardiomyocytes with Triton X-100 buffer and subjecting them to low- and high-speed (only the low-speed spin is necessary for cells) centrifugation. The Triton-insoluble material from the low-spin consists primarily of cytoskeletal-associated proteins. The insoluble proteins from the high-speed spin are made of membrane skeleton proteins. The soluble and insoluble fractions (cells) or soluble and two insoluble fractions (tissue) were boiled in SDS sample buffer used for gel electrophoresis, transferred to Immobilon-P membranes (Millipore, Bedford, MA) and analyzed by immunoblotting with the indicated antibodies. Densitometry was performed using NIH ImageJ. Quantification for each protein of interest was accomplished by first normalizing the protein band in each ventricle to a control endogenous protein (either GAPDH or actin).
Assessment of myocardial contractile function using isolated RV papillary muscle ex vivo.
After initial standard gravimetric and hemodynamic measurements, the heart was extracted, and the RV papillary muscle was isolated. The muscle was then placed in a custom-made study chamber and superfused with Krebs-Henseleit “mechanical testing buffer” consisting of (in mM): 98 NaCl, 4.7 KCl, 1.2 MgSO4, 1.1 KH2PO4, 24 NaHCO3, 20 NaAc, 2.5 CaCl2, 11.2 glucose, and 10 U/l insulin (95% O2-5% CO2, pH 7.38, 29°C) to perform mechanical testing and characterize myocardial performance (42). A computer-controlled dual-mode (length and force) servo system (Aurora Scientific, Aurora, ON, Canada) was used to mechanically test the papillary muscles. Myocardial systolic performance was examined in ventricular papillary muscles by measuring 1) extent and velocity of isotonic shortening, normalized to resting length, and 2) extent and rate of isometric force development, normalized to muscle cross-sectional area.
Statistics.
Differences were determined by one-way ANOVA followed by Tukey test for multiple comparisons. Statistical significance was defined as P < 0.05.
RESULTS
Rapamycin administration blunts hypertrophic growth in RVPO in rats.
For PO studies, two separate sets of rats were used to evaluate both short-term (48 h) and long-term (2 wk) effects of rapamycin. Each set of rats consists of four groups: sham control with vehicle, sham control with rapamycin, PO with vehicle, and PO with rapamycin. For short-term PO studies the body weights (BW) for the four treatment groups were 356 ± 20 (n = 4), 346 ± 4 (n = 4), 312 ± 9.1 (n = 5), and 327 ± 10 (n = 5), respectively. Measurements of pressure gradient showed that, whereas LV systolic pressures (SP) were unaltered, the RV SP was doubled in PO groups. That is, the RV SP for these four groups of rats were 26.1 ± 1.1, 27.2 ± 1.3, 55.9 ± 1.5, and 56.8 ± 2.1, respectively. The increased RV SP in PO rats given vehicle only was accompanied by a 43% increase in RV/BW ratio compared with sham control rats. Importantly, this increase was significantly blunted to 18.5% in the PO rats given rapamycin when compared with sham rats with rapamycin. The short-term PO rats were used for the following biochemical studies. The 48-h PO time point for these studies was used according to our previous work showing activation of tyrosine kinases and initiation of growth signaling at 48 h of PO feline RV (21). Furthermore, our studies using both feline and murine models of PO showed robust Ub of proteins in the Triton X-100-insoluble fractions following 24–48 h of PO that returned to baseline conditions after 1 wk of PO (2, 18). Therefore, 48-h PO was chosen as an ideal time point to study the molecular mechanisms that initiate growth and the immediate induction of prosurvival signaling to counteract the stress created by PO.
PO was induced in rats for 48 h by PAB to overload the RV, allowing the LV to remain a normotensive internal control. We preferred the RVPO model primarily to have a same animal LV control for the PO RV. Using this internal control eliminates the effects of neurohumoral factors (if any) that are common to both the LV and RV during RVPO from analysis of signaling of rapamycin-mediated effects. Furthermore, the rat model was chosen to minimize drug volume and trauma with intraperitoneal injection instead of using the feline model (5). For rapamycin studies, although several different doses have been used in animal models (4, 11, 25), a relatively low dose of rapamycin (0.75 mg/kg) was administered every 24 h to block mTORC1 signaling while maintaining enhanced Akt activation throughout the experiment.
Rapamycin or vehicle alone was administered to rats intraperitoneally three times: 24 h before surgery, at the time of surgery, and 24 h after surgery. Sham-operated rats with and without drug served as baseline controls. In sham rats, treatment of rapamycin did not cause any change in the RV-to-BW ratio (data not shown). As expected, 48-h PO rats treated with vehicle alone (vehicle + PO) showed a significant increase in RV but not LV mass and thus the RV/BW ratio. Compared with vehicle + PO rats, rapamycin administration (Rapa + PO) significantly blunted RV/BW although, compared with sham rats with rapamycin, significant hypertrophic growth at the 48-h PO time period still occurred (data not shown).
Ub is increased with rapamycin treatment in vivo during PO hypertrophy.
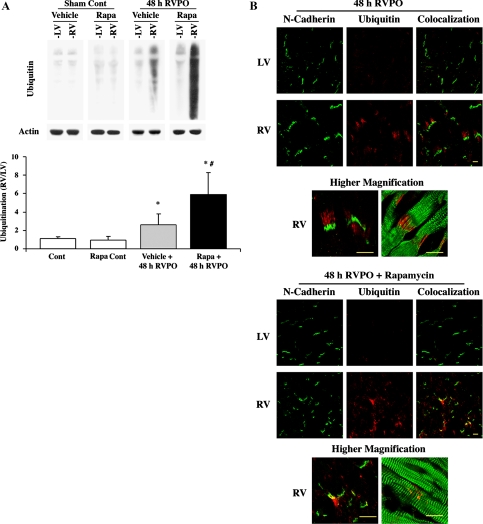
We next tested the effect of rapamycin on Ub at 48 h PO. Our previous studies both in feline RVPO and mouse transverse-aortic constriction models (2, 18) clearly demonstrate that an increased level of Ub at 24 and 48 h of PO occurs in the Triton X-100-insoluble fraction. Similarly, in the present study, analyses of protein Ub in the Triton X-100-insoluble fraction of sham control rats show low levels of ubiquitinated proteins that were similar to both LV and RV (Fig. 1A). However, in 48-h PO rats, although the LV showed low levels of Ub similar to sham controls (LV or RV), there was over a 2.5-fold increase in Ub in the RV with 48-h PO, and this is consistent with our previous studies using other animal models (2, 18). Rapamycin administration in sham control rats showed no changes in Ub in either LV or RV. Furthermore, with rapamycin administration in 48-h RVPO rats, the normally loaded control LV did not exhibit any appreciable change in the level of Ub compared with sham control animals. These data indicate that the release of neurohumoral factors alone (if any) during RVPO was not sufficient to cause changes in Ub during rapamycin treatment. However, rapamycin treatment in RVPO showed a threefold further increase in Ub, indicating substantiation of Ub attributable to rapamycin treatment. On the basis of the fact that tissue samples from either sham control rats (LV or RV) or same-animal control LV from 48-h RVPO rats showed no obvious changes in Ub during rapamycin treatment, for subsequent analyses, only the same animal LV was used as control.
Fig. 1.
Localized ubiquitination (Ub) at the intercalated disc is enhanced by rapamycin treatment during pressure overload (PO). A: Triton-insoluble proteins were normalized to actin and analyzed by immunoblotting with anti-ubiquitin antibody. The summary data for quantification conducted using ImageJ is represented in the graph as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. PO vehicle. (n = 4). RV, right ventricle; LV, left ventricle; Rapa, rapamycin; Cont, control. B: confocal microscopic analysis was performed on 10-μm sections of PO ventricles from vehicle (top) or rapamycin-treated (bottom) rats stained with anti-N-cadherin (green) and anti-ubiquitin (red). The higher-magnification images below each treatment group show anti-N-cadherin (green) and anti-ubiquitin (red) on the left and anti-α-actinin (green) and anti-ubiquitin (red) on the right. Scale = 10 μm.
To ensure that the rapamycin-enhanced Ub observed during 48-h PO occurred in cardiomyocytes, immunofluorescence was performed to determine localization of Ub. Consistent with our previous data (2, 18), in vehicle-treated rats, 48-h PO RV showed enhanced Ub compared with normally loaded same-animal control LV, and this increase was observed near the intercalated discs of cardiomyocytes as evidenced by the intercalated disc marker N-cadherin (Fig. 1B, top). The higher magnification micrographs show ubiquitin (red) localized near N-cadherin (left, green), radiating out from the intercalated discs to fill the space between α-actinin structures (right, green). Rapamycin administration results in a more punctuated pattern of localized Ub within cardiomyocytes near intercalated discs (Fig. 1B, bottom). Rapamycin administration alone did not cause an increase in Ub as evidenced by the normally loaded same-animal control LV. The higher-magnification micrographs show that ubiquitinated proteins (red) are present predominantly near the intercalated disc, as evidenced by costaining with N-cadherin (left, green) or α-actinin (right, green).
Rapamycin treatment augments Akt activation in vivo during PO.
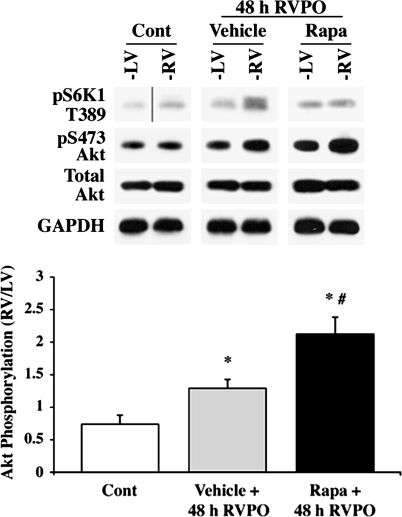
Because lack of ubiquitin signaling is associated with decompensated hypertrophy, decreased survival signaling, and increased mortality (18), we tested whether enhanced Ub correlated with enhanced Akt activation, a key survival kinase. Because transgenic mice with cardiac-restricted Akt1 knockout mice demonstrate that Akt1 is a key regulator of physiological growth and cardiomyocyte survival (8), our studies were directed toward augmenting the hypertrophic stimulant-induced activation of this isoform by rapamycin. The effect of rapamycin on pS473-Akt was first measured in the PO samples. Tissue lysates from each ventricle were subjected to immunoblotting for analyses of pT389-S6K1 and pS473-Akt to measure mTORC1 and mTORC2 activation, respectively. The basal level of pT389-S6K1 was very low in the control animal, but 48-h PO showed an enhanced level of pT389-S6K1 in the vehicle-treated PO RV vs. its control LV (Fig. 2). This activation was absent in the RV of rapamycin-treated PO animals, showing that this dose of rapamycin effectively inhibited pressure-induced mTORC1 activation. Akt activation was next analyzed by measuring pS473-Akt. Although 48-h PO caused a small increase in pS473-Akt compared with controls, rapamycin administration at 48 h PO caused over a twofold increase in pS473-Akt in the pressure-overloaded RV compared with the normally loaded same-animal LV; the LV of rapamycin-treated RVPO rats did not show any significant changes in the basal levels of T389 phosphorylation of S6K1 or S473 phosphorylation of Akt.
Fig. 2.
Rapamycin augments Akt activity induced by PO in vivo. Triton-soluble proteins were normalized to GAPDH and analyzed by immunoblotting with anti-pS473-Akt, Akt, and p389-p70S6 kinase-1 (S6K1) antibodies. Control LV and RV samples for pT389-S6K1 were run on the same gel, but they were noncontiguous as indicated by the vertical line. The summary data for quantification conducted using ImageJ for pS473-Akt are represented in the graph as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. PO vehicle (n = 4).
IκB degradation is increased with rapamycin treatment in vivo during PO hypertrophy.
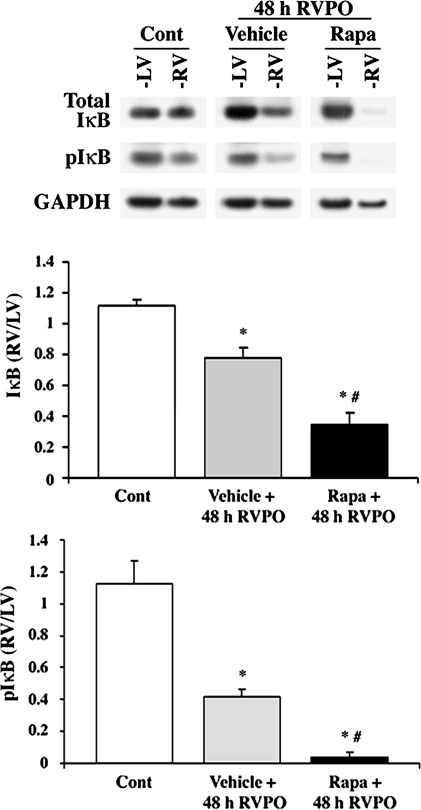
Downstream signaling of the Akt target, IκB, was measured during enhanced Akt activation caused by rapamycin administration in vivo. Ubiquitin-mediated degradation of pIκB and subsequent NF-κB transcriptional activation is activated in PO myocardium (18). For degradation, IκB is phosphorylated on S32 and S36 (35) downstream of Akt and then ubiquitinated and degraded. Predictably IκB/pIκB was decreased in 48-h PO RV in vehicle-treated rats; however, rapamycin administration further enhanced the degradation of IκB compared with vehicle PO (Fig. 3). Rapamycin alone in the normally loaded control ventricles (LV samples of 48-h RVPO rats treated with rapamycin) did not cause IκB degradation. Together, these data indicate that rapamycin promotes PO-induced pIκB degradation.
Fig. 3.
Rapamycin augments PO-induced changes in inhibitor of κB (IκB). Ventricles of control and PO rats with or without rapamycin were lysed in Triton buffer, and soluble proteins were obtained by centrifugation. Triton-soluble proteins were normalized to GAPDH and analyzed by immunoblotting with anti-IκB and pIκB antibodies. The summary data of quantification conducted using ImageJ are represented in both graphs as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. PO vehicle (n = 4).
Rapamycin increases PO-induced cIAP1 expression and inhibits caspase-3 activation.
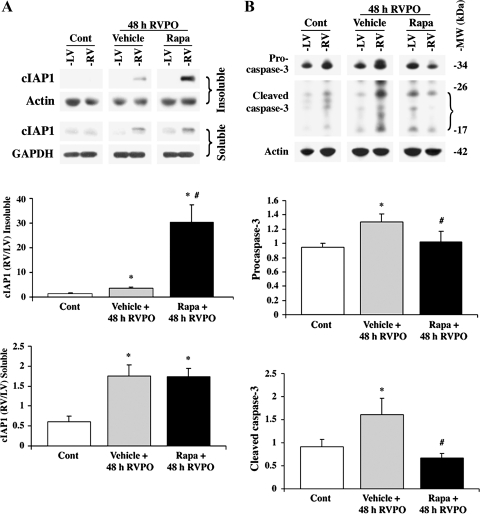
PO induces NF-κB-regulated transcription of cIAP1 (18), which is an E3 ligase targeting several molecules of the caspase-8 death pathway for ubiquitin-mediated degradation (15). If allowed to progress, the caspase-8 pathway activates the effector caspase-3 for induction of cell death. Because rapamycin increased pIκB degradation during PO, the effect of rapamycin treatment on cIAP1 protein expression was analyzed by immunoblotting. In both the Triton-insoluble and -soluble ventricular fractions, cIAP1 was increased at 48-h PO, as evidenced by cIAP1 in the RV of vehicle-treated 48-h PO compared with the normotensive LV and control animals (Fig. 4A). Significantly, rapamycin administration caused a threefold increase in cIAP1 in the Triton-insoluble fraction, compared with cIAP1 induced by 48-h PO vehicle alone. The expression of cIAP1 in the Triton-soluble fraction was comparably enhanced in both vehicle- and rapamycin-treated rats, indicating that rapamycin treatment had a strong effect on the recruitment of cIAP1 to the Triton-insoluble fraction. However, in the normally loaded control ventricles (LV samples of 48-h RVPO rats treated with rapamycin) rapamycin alone did not cause increased levels of cIAP1 in either Triton X-100-soluble or -insoluble fractions.
Fig. 4.
Rapamycin alters PO-induced changes in cIAP1 and caspase-3. Ventricles of control and PO rats with and without rapamycin were lysed in Triton buffer, and insoluble and soluble proteins were separated by centrifugation. A: insoluble and soluble proteins were normalized to actin and GAPDH, respectively, and analyzed by immunoblotting with anti-cIAP1 antibody. The summary data of quantification conducted using ImageJ are represented in the graph as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. PO vehicle, (n ≥ 4/group). B: insoluble proteins, normalized to actin, were analyzed by immunoblotting with anti-procaspase-3 (full-length caspase-3) and anti-active caspase-3 (cleaved caspase-3) antibodies. The active caspase-3 antibody recognizes cleaved active fragments of caspase-3 (17–20 kDa) that are indicated by the bracket. The summary data of quantification conducted using ImageJ are represented in the graph as means ± SEM. *P < 0.05 vs. control; #P < 0.05 vs. PO vehicle, (n = 4). All caspase-3 fragments were used for calculations of active caspase-3.
To evaluate whether increased cIAP1 expression and recruitment accompanies cell survival, the level of active caspase-3 was measured in 48-h PO vehicle and rapamycin-treated rats. Caspase-3 is expressed as a precursor, which must be cleaved to become active. In vehicle-treated 48-h PO rats, the full-length caspase-3 (procaspase-3) level increased, and more caspase-3 was activated (cleaved caspase-3) in the RV compared with the LV (Fig. 4B). Rapamycin administration blunted the caspase-3-associated changes, and the activation/cleavage of caspase-3 was similar to that of the basal level of sham animals.
Rapamycin effect in long-term PO rats.
To explore whether long term treatment of rapamycin improves ventricular function of PO myocardium, we extended PO studies for 2 wk in the presence or absence of rapamycin administration. Morphometric data are presented in Table. 1. Although there was no significant change in the animal BW, PO for 2 wk caused a significant increase (80%) in RV/BW ratio, which was blunted significantly to 33% in the rapamycin-treated group. In sham animals, rapamycin did not affect the RV/BW ratio. As expected, LV/BW ratio was unaffected either during 2-wk PO or with rapamycin administration. Similarly, RV SP and RV end-diastolic pressure (EDP), which were significantly increased during 2-wk PO, were also unaffected with rapamycin treatment.
Table 1.
Morphometric data for PO studies
| Group | Treatment | n | RV Mass, mg | RV/BW, mg/g | LV Mass, mg | LV/BW, mg/g | BW, g | RV SP, mmHg | RV EDP, mmHg |
|---|---|---|---|---|---|---|---|---|---|
| No PAB | Vehicle | 6 | 133.8 ± 4.7 | 0.44 ± 0.01 | 560.2 ± 5.1 | 1.86 ± 0.01 | 301.1 ± 2.3 | 25.7 ± 0.7 | 5.4 ± 0.4 |
| No PAB | Rapamycin | 6 | 134.0 ± 1.4 | 0.45 ± 0.01 | 561.3 ± 6.9 | 1.90 ± 0.02 | 296.2 ± 1.4 | 26.5 ± 0.9 | 5.9 ± 0.3 |
| PAB | Vehicle | 5 | 201.0 ± 9.9* | 0.80 ± 0.04* | 561.9 ± 5.3 | 1.94 ± 0.01 | 259.1 ± 3.7 | 50.5 ± 1.7* | 7.4 ± 0.7* |
| PAB | Rapamycin | 6 | 168.9 ± 5.5*† | 0.60 ± 0.02*† | 514.8 ± 8.5 | 1.94 ± 0.03 | 266.1 ± 2.9 | 51.6 ± 1.3* | 7.6 ± 0.4* |
Values are means ± SE.
P < 0.05 vs. corresponding No pulmonary artery banding (PAB) group;
P < 0.05 vs. corresponding PAB + vehicle group. PO, pressure overload; RV, right ventricle; BW, body weight; LV, left ventricle; SP, systolic pressure; EDP, end-diastolic pressure.
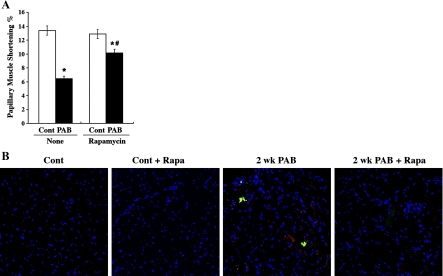
To measure myocardial function, we studied papillary muscle function in vitro with isolated papillary muscle from RV of control and PO rats, as described previously (42). Figure 5A shows papillary muscle function studies where isotonic shortening was determined. Papillary muscle shortening decreased by 51% after 2 wk of PO. However, treatment with rapamycin reduced the drop off to 21% (Fig. 5A). Also, the developed force was measured and normalized to cross-sectional area. The resultant developed stress decreased by 68% after 2 wk of PO (12.9 ± 3.5 mN/mm2 vs. 42.7 ± 7.6 mN/mm2 in normal control). However, treatment with rapamycin reduced the drop off to 27% (27.7 ± 4.9 mN/mm2 PO with Rap vs. 38.3 ± 2.7 mN/mm2 in control with rapamycin).
Fig. 5.
Effect of long-term (2 wk) rapamycin treatment in PO myocardium. Rats that underwent sham surgery or PO were treated with a daily dose of vehicle alone or rapamycin for 14 days. The animals were euthanized, and the hearts were used for the isolation of either RV papillary muscle or free wall. A: shortening percent was determined from the isolated RV papillary muscle. *P < 0.05 vs. corresponding control; #P < 0.05 vs. control + vehicle. PAB, pulmonary artery banding. B: RV tissue sections were fresh frozen and cryosectioned (12-μm sections). The sections were stained for active caspase-3 (red), μ-calpain (green), and DAPI (blue) and analyzed by using confocal microscopy.
We next performed several biochemical studies in the tissue samples obtained from these long-term PO rats. Western blot analyses on protein Ub revealed that the PO-induced increase in Ub and its further augmentation by rapamycin in 48-h PO myocardium (Fig. 1A) returned to basal condition as observed in sham control treated with or without rapamycin (data not shown). We also analyzed whether long-term PO that could trigger programmed cell death is prevented by rapamycin treatment. Our earlier immunohistochemical studies reveal that PO causes activation of both calpain and caspases and that their activation, especially calpain, was responsible for the associated programmed cell death of cardiomyocytes (24). Our present studies in rat ventricular samples also show the activation of both these proteases in RV samples of 2-wk PO rats (Fig. 5B). Importantly, the activation of these two proteases was not observed in rapamycin-treated control or 2-wk PO RV samples.
Rapamycin treatment augments Akt activation in vitro in agonist-stimulated rat cardiomyocytes.
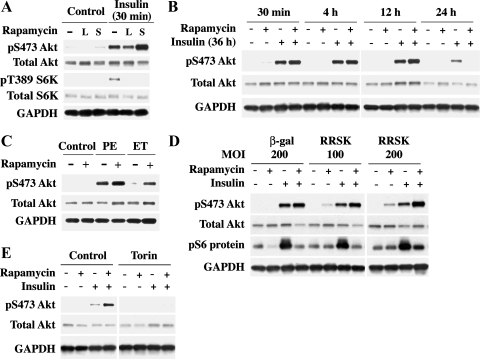
To confirm that rapamycin administration affects the balance between mTORC1 and mTORC2 resulting in Akt activation in cardiomyocytes, cell culture experiments were performed using rat cardiomyocytes. Rapamycin was used in conjunction with insulin stimulation in isolated cardiomyocytes. The insulin pathway provides a well-characterized system where the insulin receptor activates phosphatidylinositol 3-kinase (PI3K) through the insulin receptor substrate protein (IRS1) causing subsequent mTORC1 and mTORC2 activation (3). Cells were pretreated with rapamycin (2 nM) for 30 min (S) or 24 h (L) and then, in the continued presence of rapamycin, were stimulated with insulin (100 nM) for 30 min (Fig. 6A). Our previous studies show that 2 nM rapamycin is sufficient to block mTORC1-mediated S6K1 activation (16). mTORC1 and mTORC2 activation was analyzed by immunoblotting for pT389-S6K1 and pS473-Akt (Fig. 6), respectively. Both sites were phosphorylated following stimulation with insulin alone for 30 min. With rapamycin administration (30 min and 24 h) S6K1 phosphorylation was inhibited as we observed previously (16), whereas 30-min insulin-stimulated pS473-Akt was further enhanced in short-term rapamycin-treated cells (Fig. 6A). These results indicate that insulin-stimulated mTORC2 signaling can be enhanced when mTORC1 is inhibited in cardiomyocytes.
Fig. 6.
Rapamycin augments Akt activation in agonist-stimulated adult rat cardiomyocytes via mammalian target of rapamycin (mTOR)-mediated signaling. Triton-soluble proteins were extracted from isolated rat cardiomyocytes and mTOR complex 1 (mTORC1) and mTORC2 activation was analyzed though immunoblotting with anti-pT389-S6K1 and anti-pS473-Akt antibodies, respectively. Vehicle-treated cells were controls in each experiment. Total protein was normalized to GAPDH. A: cardiomyocytes were pretreated with rapamycin (2 nM, long is L = 24 h, short is S = 30 min) before insulin treatment (100 nM, 30 min). B: cardiomyocytes were supplemented with insulin (100 nM) and treated with rapamycin (2 nM) for the indicated times. C: cardiomyocytes were pretreated with rapamycin (2 nM, 30 min) before 1-h stimulation with either phenylephrine (PE) (100 μM) or endothelin (ET) (400 nM). D: cardiomyocytes were exposed to β-galactosidase (β-gal) 200 multiplicity of infection (MOI) and rapamycin-resistant S6K1 adenovirus (RR-S6K1) at 100 and 200 MOI for 48 h. Cells were then pretreated with rapamycin (2 nM, 30 min) before insulin treatment (100 nM, 30 min). Soluble proteins were analyzed by immunoblotting with anti-pS473-Akt, and insoluble proteins were analyzed with anti-pS235/S236-S6 Protein antibody. E: cardiomyocytes were pretreated with torin (100 nM, 1 h) then treated with rapamycin (2 nM, 30 min) before insulin stimulation (100 nM, 30 min). Results were confirmed by performing 2 additional experiments.
We next tested whether the potentiation effect of rapamycin on Akt is sustainable during a prolonged period of insulin treatment because earlier studies indicated that prolonged rapamycin treatment affects both mTOR complexes in a cell-type-specific manner (29). Isolated cardiomyocytes in serum-free media supplemented with insulin (100 nM) for 36 h were subjected to increasing durations of rapamycin treatment (2 nM) from 30 min up to 24 h. Immunoblotting with pS473-Akt showed that rapamycin augments the insulin effect on pS473-Akt for at least 12 h (Fig. 6B). Only with 24 h of rapamycin treatment with continuous insulin receptor stimulation was pS473-Akt augmentation inhibited. To establish that rapamycin-induced pS473-Akt augmentation is not exclusive to insulin receptor signaling, phenylephrine (PE) and endothelin (ET), which do not utilize IRS1, were used to stimulate cardiomyocytes after 30-min rapamycin (2 nM) pretreatment. Similar to insulin, 1 h of PE (100 μM) or ET (400 nM) stimulation caused an increase in pS473-Akt, which was further enhanced in the presence of rapamycin (Fig. 6C).
The mTORC1/S6K1 pathway has been shown to cause insulin resistance via mediating a negative regulation on IRS1 and IRS2 (39). This mechanism was tested as the cause for enhanced pS473-Akt with rapamycin. An adenoviral construct mediating the expression of a RR-S6K1 was generated. Expression of RR-S6K1 did not affect the basal or insulin-stimulated S6 protein phosphorylation. Additionally, pretreatment with 2 nM rapamycin blunted insulin-stimulated S6K1 activation in β-galactosidase [200 multiplicity of infection (MOI)]. However, RR-S6K1 expressing cardiomyocytes at 200 MOI demonstrated a rescue of phosphorylated S6 protein levels, indicating RR-S6K1 activity (Fig. 6D). Notably, expression of RR-S6K1, even at 200 MOI, did not block the potentiation effect of rapamycin on insulin-stimulated Akt activation. In fact, under the condition of RR-S6K1 expression, rapamycin was found to exhibit an added effect in augmenting the insulin-stimulated Akt activation.
Finally, to explore whether the augmentation of insulin-stimulated Akt activation by rapamycin is mediated via mTORC2, we used torin, a newly characterized mTOR inhibitor (34). Unlike rapamycin that blocks only mTORC1 but not mTORC2, torin has been found to block both mTORC1 and mTORC2 by blocking mTOR kinase activity. Pretreatment of cardiomyocytes with torin abolished both insulin-stimulated pS473-Akt and its further potentiation by rapamycin (Fig. 6E), indicating that the potentiation effect of rapamycin is mediated via mTORC2.
DISCUSSION
The present study tested the effect of rapamycin on protein Ub and S473-Akt phosphorylation in PO myocardium and in isolated cardiomyocytes. Because our earlier work showed enhanced protein Ub and NF-κB-mediated survival signaling in cardiomyocytes of PO myocardium (18), we hypothesized that potentiation of these changes would benefit the hypertrophying heart via cardiomyocyte survival. We show here that rapamycin treatment potentiates both targeted Ub of cellular proteins and phosphorylation of S473-Akt. Furthermore, these augmented changes are accompanied by the alleviation of both pressure-induced caspase-3 activation and heart-muscle-function declination. Thus upregulating targeted Ub with rapamycin may be useful for prevention of accumulation of apoptotic proteins, which can contribute to heart failure. In decompensating mouse hearts, rapamycin treatment significantly improved ventricular function, fractional shortening, and ejection fraction (25) and decreased PO-induced hypertrophic growth without compromising ventricular function when administered during the first week of PO (32). These data indicate that rapamycin regulates pathological remodeling and promotes physiological growth by enhancing survival signaling in stressed myocardium.
Our previous studies demonstrate that inhibiting Ub during PO hypertrophy correlates with ventricular dysfunction, cell death, and decreased survival rates (18). Other studies report that proteasomal inhibition can activate caspase-induced cell death (14). Because hypertrophic growth is partially limited by inhibiting mTORC1 signaling with rapamycin treatment (12, 32), the targeted Ub found in the present study to associate with the intercalated discs, especially with rapamycin treatment (Fig. 1B), may be involved in the regulation of growth-independent survival signaling. In this context, Akt is known to phosphorylate several proapoptotic substrates for subsequent UPS-mediated elimination (36), and no UPS activity was observed in the absence of pS473. Therefore, a mechanism underlying increased lifespan (13) as well as improved ventricular function with rapamycin treatment (25) (Fig. 5A) could be through enhanced survival signaling involving Akt phosphorylation and the subsequent ubiquitin-mediated clearance of detrimental proteins. In support of this view, our studies also show that rapamycin treatment abolishes the levels of active calpain and caspase-3 in long-term PO-induced rats (Fig. 5B). Furthermore, cIAP1, an E3 ligase that ubiquitinates caspases, was enhanced with rapamycin in short-term PO myocardium, corresponding with enhanced mTORC2 activation and elimination of active caspase-3 (Fig. 4). Therefore, downstream of Akt, the enhanced expression of cIAP1, a known target gene of NF-κB, is a potential mechanism for the increased Ub and elimination of deleterious proteins in cardiomyocytes of PO myocardium. In support of this view, a number of studies demonstrate that Akt activation leads to expression/activation of several E3 ligases for protein ubiquitination. For example, expression of either cIAP1 (7) and Mdm2 (41) are under the control of Akt, and their expression is linked to cell survival. Our previous work also shows activation of these E3 ligases in PO myocardium (2). Therefore, increased activities of E3 ligases by rapamycin could be one key mechanism for the increased Ub in PO myocardium.
Previous studies revealed a transient enhancement of pS473-Akt by rapamycin occurring in a cell-type-specific manner (29) although it had not been evaluated in cardiomyocytes. Our in vitro studies using adult cardiomyocytes demonstrated potentiation of pS473-Akt by rapamycin when stimulated with insulin, PE, or ET (Fig. 6). We tested the role of a potential mechanism involving mTORC1/S6K1-mediated negative regulation on the insulin receptor/PI3K signaling module via IRS1 phosphorylation. Earlier studies showed that phosphorylation of IRS1 by mTORC1/S6K1 leads to inactivation of IRS1 signaling (39), resulting in the loss of mTORC2/Akt activation. Because pS473Akt is not affected by expression of a constitutively active rapamycin-insensitive S6K1 (Fig. 6), the rapamycin-induced potentiation of pS473Akt is not downstream of IRS1 signaling. However, it is possible that mTORC1 may have additional cellular targets other than S6K1 that might negatively regulate mTORC2/Akt, and rapamycin relieves these negative feedback mechanism(s) for Akt activation. The other possibility is that rapamycin may promote the association and/or activation of mTORC2 that augments pS473Akt. Finally, our studies with torin, known to block both mTOR complexes (34), indicate that the augmented effect of agonist-stimulated Akt activation by rapamycin is primarily mediated via mTORC2. Of note, rapamycin treatment alone is insufficient for the potentiation of Akt phosphorylation, indicating that additional changes in mTORC2/Akt module during the anabolic stimulation are necessary for the overall Akt S473 phosphorylation.
As a precaution, rapamycin administration was evaluated for activation of autophagic pathways in the myocardium because mTOR inhibits autophagy downstream of PI3K (27). Autophagic cell death in heart failure is characterized by decreased proteasomal degradation, resulting in sequestration of ubiquitinated proteins in autophagic vacuoles (20). We have shown that proteasomal inhibition causes the accumulation of pIκB in cardiomyocytes (18). The present work shows that elimination of pIκB from the cell was instead increased with rapamycin administration during 48-h PO (Fig. 3), suggesting that ubiquitinated IκB is effectively degraded by the proteasome. In support of this data, the enhanced levels of Ub in rapamycin-treated PO myocardium were found to return to baseline levels in long-term PO myocardium (data not shown), and rapamycin treatment without PO does not cause enhanced Ub on its own (Fig. 1, A and B). These data indicate that the enhanced level of ubiquitinated proteins is not attributable to rapamycin inhibiting proteasomal degradation. Furthermore, our immunohistochemical studies reveal that the increased Ub and its potentiation by rapamycin in PO myocardium occur at the level of cardiomyocytes, suggesting that cardiomyocytes are the cells primarily benefitting from increased Ub. Additionally, increased Ub in rapamycin-treated PO myocardium near intercalated discs (Fig. 2B) and not in autophagic vacuoles in the cytoplasm indicates that rapamycin treatment did not cause increased Ub by activation of autophagy in PO myocardium, in accordance with a recent study in skeletal muscle demonstrating that mTORC2 inhibits autophagy (23).
Akt is a prosurvival molecule necessary for physiological growth and is able to suppress pathological growth (8). Thus, whereas Akt is active during initial PO-mediating hypertrophic growth and survival (40), augmented Akt activation by rapamycin may reduce the progression of the pathological state. Both the Akt/mTOR pathway and the ubiquitin degradation system are highly conserved across species. The increased lifespan attributable to rapamycin treatment (13) points to an evolutionarily conserved pathway as the mechanism of action. Indeed, ubiquitin obtained its name because of its presence in all tissues of all eukaryotic species. Thus it is possible that the UPS is involved in the mechanism of enhanced survival because of mTORC1 inhibition. By prioritizing mTORC2 signaling for pS473-Akt activation in cardiomyocytes, administration of rapamycin has a modality for therapeutic potential through promotion of physiological growth as a means to preclude ventricular decompensation frequently occurring with hypertension.
GRANTS
This study was supported by the National Institutes of Health (RHL092124A to D. Kuppuswamy), and by Merit Award from the Research Service of the Department of Veterans Affairs (to M. Zile), by AHA predoctoral fellowship 0615468U (to R. K. Harston), and by NIH predoctoral fellowship NIH T32HL07260 (to R. K. Harston).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Amy Bradshaw for critical reading of the manuscript.
REFERENCES
- 1. Balasubramanian S, Johnston RK, Moschella PC, Mani SK, Tuxworth WJ, Jr, Kuppuswamy D. mTOR in growth and protection of hypertrophying myocardium. Cardiovasc Hematol Agents Med Chem 7: 52–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balasubramanian S, Mani S, Shiraishi H, Johnston RK, Yamane K, Willey CD, Cooper GT, Tuxworth WJ, Kuppuswamy D. Enhanced ubiquitination of cytoskeletal proteins in pressure overloaded myocardium is accompanied by changes in specific E3 ligases. J Mol Cell Cardiol 41: 669–679, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell 12: 487–502, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Boluyt MO, Li ZB, Loyd AM, Scalia AF, Cirrincione GM, Jackson RR. The mTOR/p70S6K signal transduction pathway plays a role in cardiac hypertrophy and influences expression of myosin heavy chain genes in vivo. Cardiovasc Drugs Ther 18: 257–267, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cooper G, Satava RM. A method for producing reversible long-term pressure overload of the cat right ventricle. J Appl Physiol 37: 762–764, 1974 [DOI] [PubMed] [Google Scholar]
- 6. Cooper GT, Satava RM, Jr, Harrison CE, Coleman HN., 3rd Mechanisms for the abnormal energetics of pressure-induced hypertrophy of cat myocardium. Circ Res 33: 213–223, 1973 [DOI] [PubMed] [Google Scholar]
- 7. Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-[kappa]B is controlled by mTOR and Raptor in association with IKK. Genes Dev 22: 1490–1500, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol 16: 6242–6251, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation 114: 1821–1828, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens 24: 1663–1670, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Ha T, Li Y, Gao X, McMullen JR, Shioi T, Izumo S, Kelley JL, Zhao A, Haddad GE, Williams DL, Browder IW, Kao RL, Li C. Attenuation of cardiac hypertrophy by inhibiting both mTOR and NFkappaB activation in vivo. Free Radic Biol Med 39: 1570–1580, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henderson CJ, Aleo E, Fontanini A, Maestro R, Paroni G, Brancolini C. Caspase activation and apoptosis in response to proteasome inhibitors. Cell Death Differ 12: 1240–1254, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem 278: 10055–10060, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, McDermott PJ, Kuppuswamy D. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J Biol Chem 277: 23065–23075, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131: 3897–3906, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Johnston RK, Balasubramanian S, Kasiganesan H, Baicu CF, Zile MR, Kuppuswamy D. β3-Integrin-mediated ubiquitination activates survival signaling during myocardial hypertrophy. FASEB J 23: 2759–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kent RL, Mann DL, Urabe Y, Hisano R, Hewett KW, Loughnane M, Cooper GT. Contractile function of isolated feline cardiocytes in response to viscous loading. Am J Physiol Heart Circ Physiol 257: H1717–H1727, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res 92: 715–724, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kuppuswamy D, Kerr C, Narishige T, Kasi VS, Menick DR, Cooper G. Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. J Biol Chem 272: 4500–4508, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Laser M, Willey CD, Jiang W, Cooper Gt, Menick DR, Zile MR, Kuppuswamy D. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem 275: 35624–35630, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Mammucari C, Schiaffino S, Sandri M. Downstream of Akt: FoxO3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 4: 524–526, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Mani SK, Shiraishi H, Balasubramanian S, Yamane K, Chellaiah M, Cooper G, Banik N, Zile MR, Kuppuswamy D. In vivo administration of calpeptin attenuates calpain activation and cardiomyocyte loss in pressure-overloaded feline myocardium. Am J Physiol Heart Circ Physiol 295: H314–H326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 109: 3050–3055, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Nagatomo Y, Carabello BA, Hamawaki M, Nemoto S, Matsuo T, McDermott PJ. Translational mechanisms accelerate the rate of protein synthesis during canine pressure-overload hypertrophy. Am J Physiol Heart Circ Physiol 277: H2176–H2184, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36: 585–595, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol 22: 2799–2809, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 107: 1664–1670, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Siu PM, Alway SE. Response and adaptation of skeletal muscle to denervation stress: the role of apoptosis in muscle loss. Front Biosci 14: 432–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J 14: 2876–2883, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsukamoto O, Minamino T, Okada K, Shintani Y, Takashima S, Kato H, Liao Y, Okazaki H, Asai M, Hirata A, Fujita M, Asano Y, Yamazaki S, Asanuma H, Hori M, Kitakaze M. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun 340: 1125–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Wu WK, Volta V, Cho CH, Wu YC, Li HT, Yu L, Li ZJ, Sung JJ. Repression of protein translation and mTOR signaling by proteasome inhibitor in colon cancer cells. Biochem Biophys Res Commun 386: 598–601, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J Biol Chem 283: 35375–35382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang P, Hu X, Xu X, Fassett J, Zhu G, Viollet B, Xu W, Wiczer B, Bernlohr DA, Bache RJ, Chen Y. AMP activated protein kinase-[alpha]2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension 52: 918–924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol 3: 973–982, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Zile MR, Koide M, Sato H, Ishiguro Y, Conrad CH, Buckley JM, Morgan JP, Cooper GT. Role of microtubules in the contractile dysfunction of hypertrophied myocardium. J Am Coll Cardiol 33: 250–260, 1999 [DOI] [PubMed] [Google Scholar]