Abstract
Mice deficient in Notch3 have defects in arterial vascular smooth muscle cell (VSMC) mechanosensitivity, including impaired myogenic responses and autoregulation, and inappropriate VMSC orientation. Experiments were performed to determine if Notch3 is activated by mechanical stimulation and contributes to mechanosensitive responses of VSMCs, including cell realignment. Cyclic, uniaxial stretch (10%, 1 Hz) of human VSMCs caused Notch3 activation, demonstrated by a stretch-induced increase in hairy and enhancer of split 1/hairy-related transcription factor-1 expression, translocation of Notch3 to the nucleus, and a decrease in the Notch3 extracellular domain. These effects were prevented by inhibiting the expression [small interfering (si)RNA] or proteolytic activation of Notch3 {N-(R)-[2-(hydroxyaminocarbonyl)methyl]−4-methylpentanoyl-l-naphthylalanyl-l-alanine-2-aminoethyl amide (TAPI-1; 50 μmol/l) to inhibit TNF-α-converting enzyme (TACE) or N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT; 20 μmol/l) to inhibit γ-secretase}. Stretch increased the activity of ROS within VSMCs, determined using dichlorodihydrofluorescein fluorescence. Catalase (1,200 U/ml), which degrades H2O2, inhibited the stretch-induced activation of Notch3, whereas in nonstretched cells, increasing H2O2 activity [H2O2 or manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin] caused activation of Notch3. Stretch increased the activity of TACE, which was prevented by catalase. Stretch-induced activation of p38 MAPK in VSMCs was inhibited either by catalase or by inhibiting Notch3 expression (siRNA). Stretch caused VSMCs to realign perpendicular to the direction of the mechanical stimulus, which was significantly inhibited by catalase or by inhibiting the expression (siRNA) or activation of Notch3 (TAPI-1 or DAPT). Therefore, cyclic uniaxial stretch activates Notch3 signaling through a ROS-mediated mechanism, and the presence of Notch3 is necessary for proper stretch-induced cell alignment in VSMCs. This mechanism may contribute to the physiological role of Notch3 in mediating developmental maturation of VSMCs.
Keywords: tumor necrosis factor-α-converting enzyme, hydrogen peroxide, p38 mitogen-activated protein kinase
the notch signaling pathway is critical for cell fate determination during embryonic development, including many aspects of vascular development (1, 40). Notch receptor activity is mediated by sequential proteolysis. Full-length Notch is cleaved [site 1 (S1)] by a furin-like convertase to generate an extracellular component [Notch extracellular domain (NECD)] and a predominantly intracellular component (PIC), which remain associated by noncovalent interaction to form mature Notch receptors (9, 19). The PIC comprises a large intracellular domain, a transmembrane region, and a small extracellular sequence, which is responsible for the interaction with the NECD. Notch activation is initiated by ligand-dependent (e.g., Delta and Jagged) or independent cleavage of the PIC within the extracellular sequence [site 2 (S2)] by a disintegrin and metalloprotease (ADAM) proteases, including TNF-α-converting enzyme (TACE). S2 cleavage generates a membrane-tethered fragment of PIC termed Notch extracellular truncation (NEXT) and releases the NECD, which is then internalized and degraded (9, 19). After the subsequent cleavage of NEXT by γ-secretase [site 3 (S3)], the Notch intracellular domain (NICD) is released and translocates to the nucleus, where it forms a transcriptional activation complex with Cmp-binding factor-1/Su(H)/Lag-1 and coactivators of the mastermind-like family to regulate the expression of target genes including hairy and enhancer of split (Hes) and hairy-related transcription factor (HRT) (9, 19).
Notch3 is predominantly expressed in arterial vascular smooth muscle cells (VSMCs) (8, 35). Notch3−/− mice have defects in arterial VSMC maturation, including decreased expression of differentiation markers and inappropriate cellular orientation (8). Notch3−/− mice also display impaired cerebral autoregulation and impaired pressure-induced myogenic constriction of isolated arteries while retaining the ability to constrict normally to other stimuli (4, 8). Although these studies have suggested that Notch3 may positively regulate mechanotransduction in VSMCs (4, 8), cyclic equibiaxial mechanical stretch of cultured VSMCs (4–24 h) has been reported to inhibit Notch3 activity, based on decreased activity of Notch-3-dependent target genes (HRT and Hes) (25). However, equibiaxial strain can affect cells in an opposite manner to the more physiologically relevant uniaxial strain (17, 32). Furthermore, in response to cyclic stretch, cultured VSMCs rapidly realign perpendicular to the axis of the strain (7, 21), which decreases the mechanical stimulus and can reverse strain-dependent responses (20, 32). Therefore, the previous study may not have clearly defined the role of Notch3 signaling in VSMC mechanosensitivity. Mechanical stretch of VSMCs stimulates a rapid generation of ROS and redox-dependent activation of p38 MAPK, which then mediate stretch-induced responses including VSMC realignment (7, 30). The goal of the present study was therefore to determine whether Notch3 has a role in early responses of VSMCs to uniaxial mechanical stimulation.
MATERIALS AND METHODS
Materials and cells.
Human aortic VSMCs were obtained from Lonza (Walkersville, MD). Cells were expanded in smooth muscle growth medium (SMGM; Lonza) and used between passage 4 and 8. Before experiments, cells were made quiescent by exchanging the growth media for quiescent media [50:50 mixture of DMEM and Ham's F-12 media with glutamine (200 mg/ml), penicillin-streptomycin (100 U/ml each), and ITS] containing 0% or 0.5% serum for 16 h (34). N-(R)-[2-(hydroxyaminocarbonyl)methyl]-4-methylpentanoyl-l-naphthylalanyl-l-alanine 2-aminoethyl amide (TAPI-1) and N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT) were purchased from Calbiochem (San Diego, CA). DAPT is a selective inhibitor of γ-secretase (24), whereas TAPI-1 has been shown to inhibit both ADAM10 and TACE (38). Cells were treated with TAPI-1 (50 μmol/l) (12) or DAPT (20 μmol/l) (12) for 30 min before stimulation. Catalase (Sigma) is an antioxidant enzyme that degrades H2O2. VSMCs were pretreated with catalase (1,200 U/ml) for 4 h before treatment (34).
Jagged1-FC (Jag1-Fc, R&D Systems, Minneapolis, MN) and anti-Fc antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) were preclustered in a 1:1 combination at 4°C for 1 h (11, 22, 33). The mixture was then used to treat cells. The final concentration of Jag1-Fc was 1 μg/ml. Anti-Fc antibody alone (1 μg/ml) was used as the “control” treatment. Preliminary experiments demonstrated that it did not cause any change of Hes1 or Notch3 processing at 0.5, 1, 3, or 5 h posttreatment (data not shown).
Plasmids and small interfering RNA transfections.
Cells were transiently transfected by nucleofection with the Amaxa Nucleofector (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer's instructions (program no. CM137). Notch3 small interfering (si)RNA and negative control siRNA were purchased from Qiagen (Valencia, CA). The target sequence of Notch3 siRNA was 5′-ATGCCTAGACCTGGTGGACAA-3′. siRNA-transfected cells were cultured in SMGM for 2 days before the switch to quiescent medium. To assess Hes1 promoter activity, we used a luciferase Hes1 reporter construct, which was kindly provided by Dr. Michael Wang (University of Michigan). pRL-CMV (Promega, Madison, WI) was used as an internal control to normalize the firefly luciferase units. After transfection, cells were allowed to recover for 1 day in SMGM before the change to quiescent medium. Luciferase activity was analyzed using the dual-luciferase assay kit from Promega following the manufacturer's instructions. The final concentration of siRNA used for transfection was 0.5 μM, Hes1 reporter plasmid was 50 μg/ml, and pRL-CMV was 0.5 μg/ml.
Stretch.
VSMCs were cultured on silicone chambers that had been coated with collagen type I (100 μg/ml, BD Biosciences, Bedford, MA). VSMCs were then left nonstretched or exposed to cyclic, uniaxial stretch using the STREX Cell Strain device (B-Bridge International, Mountain View, CA) with 10% elongation and 1 Hz for different time periods. For realignment experiments, cells were exposed to stretch for 24 h before analysis. The angles of nonstretched or stretched cells against the stretch direction were measured using ImageJ. The cell angles were sorted into nine groups: 0–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, and 81–90°. The total cell number was set as 100%, and the number in each group was expressed relative to this value.
Nuclei preparation.
Methods were adapted from those of Natarajan et al. (28). Briefly, confluent cell cultures were washed with cold PBS and suspended in 0.4 ml lysis buffer [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, and protease inhibitor cocktail]. Cells were allowed to swell on ice for 15 min, after which 12.5 μl of 10% Nonidet P-40 was added. The tube was then vortexed vigorously for 10 s, and the homogenate was centrifuged at 2,000 g for 30 s. The nuclear pellet was resuspended in 25 μl ice-cold nuclear extraction buffer [20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and protease inhibitor cocktail] and incubated on ice for 30 min with intermittent mixing. Samples were centrifuged at 15,000 g for 5 min, and the supernatant used as the nuclear extract. All steps were carried out at 4°C.
Membrane and cytosol preparation.
Methods were modified from those of Walker and Burgess (39). Briefly, confluent cell cultures were rinsed twice in hypotonic buffer (10 mM HEPES, 10 mM KCI, 10 mM NaCl, 1 mM KH2P04, 5 mM NaHC03, 1 mM CaC12, 0.5 mM MgC12, and protease inhibitor cocktail), scraped, and disrupted with 50 strokes in a Dounce-type homogeneizer. Lysates were centrifuged at 1,500 g to remove intact cells and nuclei. The supernatant was then centrifuged at 21,000 g for 1 h to generate cytosolic (supernatant) and membrane (pellet) fractions. The membrane pellet was resuspended in hypotonic buffer. All steps were carried out at 4°C.
Western blot analysis.
Cells were lysed in sample buffer containing 2% SDS and 60 mM Tris·HCl (pH 6.8). Cell lysates were then boiled for 5 min. The total protein concentration was determined (BCA, Thermo Scientific, Rockford, IL), and 5–10 μg protein was loaded for Western blot analysis. In brief, lysate proteins were resolved by 7% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), which was blocked with 5% milk for 1 h at room temperature. The membrane was incubated overnight at 4°C with primary antibody followed by a secondary horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibody for 1 h at room temperature (1:2,000 dilution, GE Healthcare). Mouse anti-Notch3 NECD was purchased from Novus (1:500 dilution, Littleton, CO), and rabbit anti-Notch3 was from Santa Cruz Biotechnology (1: 500 dilution, Santa Cruz, CA). This antibody recognizes Notch3 PIC, NEXT, and NICD species. Rabbit anti-Hes1 antibody was from Chemicon (1:1,000 dilution, Temecula, CA), rabbit anti-HRT1 was from Millipore (1:1,000 dilution, Temecula, CA), mouse anti-EGF receptor was from BD Biosciences (1:500 dilution, San Diego, CA), rabbit anti-lamin A/C was from Cell Signaling (1:2,000 dilution, Boston, MA), rabbit anti-p38 and rabbit anti-phospho-p38 were from Cell Signaling (1:500 dilution, Boston, MA), and mouse anti-β-actin was from Sigma (1:2,000 dilution). Blots were developed using enhanced chemiluminescence and quantified using ImageQuant TL (GE Healthcare).
Detection of ROS.
The ROS-sensitive fluorescent probe 5- (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCDHF; Invitrogen, Eugene, OR) was used to assess intracellular ROS activity (34). Cells were equilibrated in control Krebs bicarbonate solution [which contained (in mmol/l) 118.3 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 25.0 NaHCO3, and 11.1 glucose] for 1 h, and DCDHF (final concentration: 5 μg/ml) was then added to the cells 30 min before stretch (34). After 5 min of stretch (or no stimulation for nonstretched control cells), cells were rinsed with cold Krebs solution (4°C) and kept on ice. Fluorescence activity in cells was quantified immediately using a Leica AOBS-equipped SP5 laser scanning microscope. Images (512 × 512 pixels) were obtained using a ×63 dipping objective (0.9 numerical aperture), a pinhole of 1 Airy unit, scan speed of 400 Hz, and 4-line averaging. Excitation was at 488 nm, and emission was captured from 497 to 568 nm. Images are presented in their original unprocessed condition. Fluorescence intensity was determined using regions of interest (ROI) for each cell. The mean of the average ROI signal intensities in control nonstretched VSMCs was set as 100%, and all images in each experiment were expressed relative to that value.
TACE activity assay.
To assess TACE activity, cells were rinsed once with PBS and then incubated with TACE fluorogenic substrate (Abz-LAQAVRSSSR-Dpa, Calbiochem) solution [20 μmol/l, prepared in 50 mmol/l Tris·HCl (pH 7.4), 25 mmol/l NaCl, and 4% glycerol]. After 1 h of incubation at 37°C, 100 μl of the reaction solution were transferred to a 96-well plate, and fluorescence was determined using a plate reader with excitation at 320 nm and emission at 420 nm. This fluorogenic substrate is preferentially cleaved by TACE, with no substantially activity from other metalloproteinases (16, 26).
Statistics.
Statistical evaluation of the data was performed by Student's t-test for paired or unpaired observations. When more than two means were compared, ANOVA was used. If a significant F-value was found, Tukey's test for multiple comparisons was used to identify differences among groups. Unless stated otherwise, data are expressed as means ± SE for n number of experiments, where n equals the number of different cultures. Values were considered to be statistically different when P < 0.05.
RESULTS
Cyclic stretch of VSMCs activates Notch3.
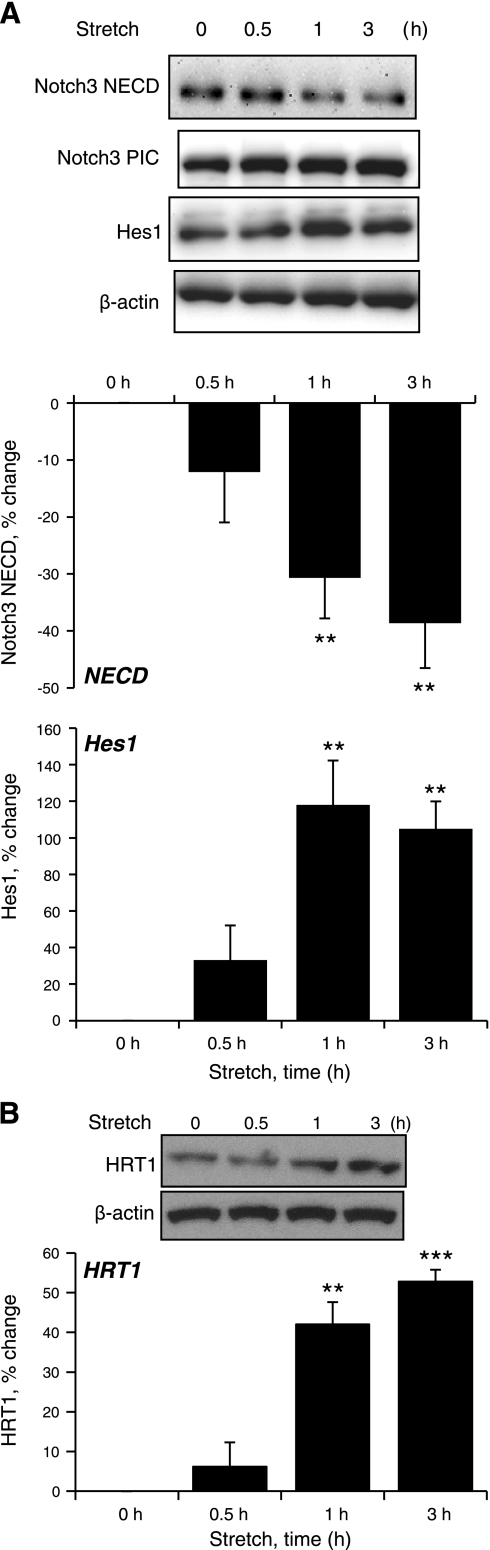
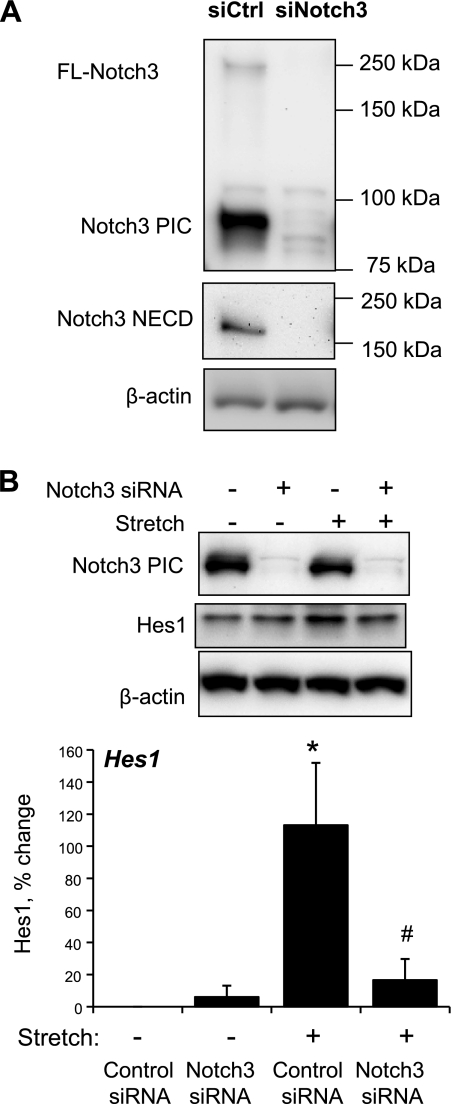
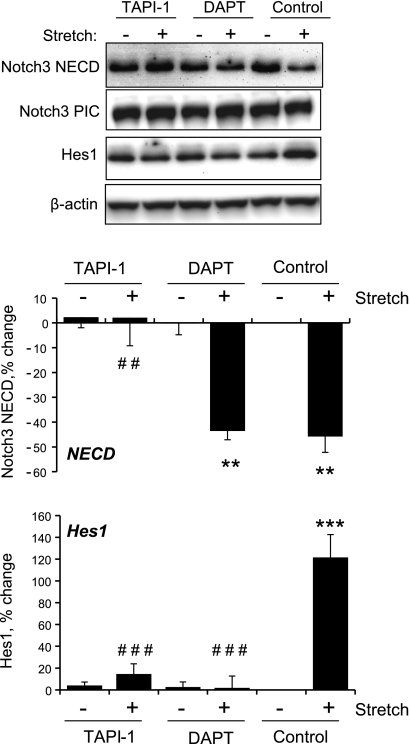
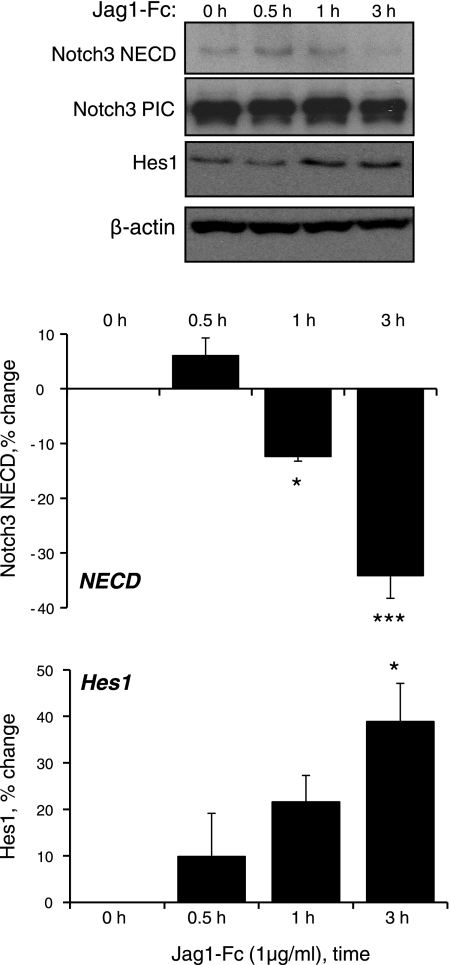
Cyclic stretch of VSMCs increased expression of the Notch3 target proteins Hes1 and HRT1 (Fig. 1) and increased Hes1 reporter activity (data not shown). The stretch-induced increase in Hes1 expression was abolished by siRNA-mediated knockdown of Notch3 (Fig. 2) or by selective inhibition of ADAM/TACE (TAPI-1, 50 μmol/l) or γ-secretase (DAPT, 20 μmol/l), which inhibit the S2 and S3 activation steps of Notch3, respectively (Fig. 3). Direct stimulation of Notch3 by the exogenous Notch activator Jag1-Fc (1 μg/ml) also increased the expression of Hes1 (Fig. 4).
Fig. 1.
Activation of Notch3 signaling by cyclic stretch. Cultured vascular smooth muscle cells (VSMCs) were exposed to cyclic uniaxial stretch (10%, 1 Hz) for up to 3 h, and the expression levels of Notch3 extracellular domain (NECD), Notch3 predominantly intracellular component (PIC), hairy and enhancer of split-1 (Hes1; A), and hairy-related transcription factor-1 (HRT1; B), and β-actin were determined by Western blot. siRNA, small interfering RNA; siCtrl, control siRNA; siNotch3, Notch3 siRNA. Data are expressed as percent changes in protein level from 0 h (normalized to β-actin) and are presented as means ± SE; n = 5 (A) or 3 (B). Statistically significant differences from time 0 (**P < 0.01; ***P < 0.001) are indicated.
Fig. 2.
Effects of molecular inhibition of Notch3 (siRNA) on the expression of Notch3 (A) and the stretch-induced activation of Notch3 signaling (B). Expression levels of full-length Notch3 (FL-Notch3), NECD, PIC, and Hes1 were determined by Western blot analysis. A: cells were transfected with control or Notch3 siRNA as described in materials and methods. B: cells were exposed to cyclic uniaxial stretch (10%, 1 Hz) or left nonstretched for 60 mins. Hes1 data are expressed as percent changes of protein level from nonstretched control siRNA-transfected cells (normalized to β-actin) and are presented as means ± SE; n = 3. Statistically significant differences from nonstretched control siRNA-transfected cells (*P < 0.05) and from stretched control siRNA-transfected cells (#P < 0.05; not shown for comparisons with nonstretched cells) are indicated.
Fig. 3.
Effects of pharmacological inhibition of Notch on the stretch-induced activation of Notch3 signaling. Cells in the absence and presence of a TNF-α-converting enzyme (TACE) inhibitor, N-(R)-[2-(hydroxyaminocarbonyl)methyl]−4-methylpentanoyl-l-naphthylalanyl-l-alanine-2-aminoethyl amide (TAPI-1; 50 μmol/l), or a γ-secretase inhibitor, N-[N-(3,5-difluorophenacetyl-l-alanyl)]-S-phenylglycine t-butyl ester (DAPT; 20 μmol/l), were exposed to cyclic uniaxial stretch (10%, 1 Hz) or not stretched for 60 min. Expression levels of NECD, PIC, Hes1, and β-actin were determined by Western blot analysis. Data are expressed as percent changes in protein level from nonstretched control cells (normalized to β-actin) and are presented as means ± SE; n = 3. Statistically significant differences from nonstretched control cells (**P < 0.01; ***P < 0.001) and from stretched control cells (##P < 0.01; ###P < 0.001; not shown for comparisons with nonstretched cells) are indicated.
Fig. 4.
Activation of Notch3 signaling by the exogenous agonist Jagged1 (Jag1)-Fc (1 μg/ml). Cultured VSMCs were exposed to Jag1-Fc (1 μg/ml) for up to 3 h, and the expression levels of NECD, PIC, Hes1, and β-actin were determined by Western blot analysis. Data are expressed as percent changes in protein level from 0 h (normalized to β-actin) and are presented as means ± SE; n = 3. Statistically significant differences from time 0 (*P < 0.05; ***P < 0.001) are indicated.
As observed in other systems (15), activation of Notch3 could not be discerned as changes in molecular weight or cleavage of Notch3 PIC (either to NEXT or NICD), which remained constant during the experiments, including after pharmacological inhibition of Notch3 activation (e.g., Fig. 3). As with Notch3 PIC, no changes were observed in the expression of the full-length protein (data not shown). However, levels of Notch3 NECD decreased in response to cyclic stretch (Fig. 1). This decrease in NECD was prevented by inhibition of ADAM/TACE (TAPI-1, 50 μmol/l) but not by inhibition of γ-secretase (DAPT, 20 μmol/l; Fig. 3), confirming that it reflects S2 activation of Notch3, which releases NECD. As observed with cyclic stretch, activation of Notch3 by Jag1-Fc (1 μg/ml) caused a time-dependent decrease in Notch3 NECD (Fig. 4).
Role of ROS in stretch-induced activation of Notch3.
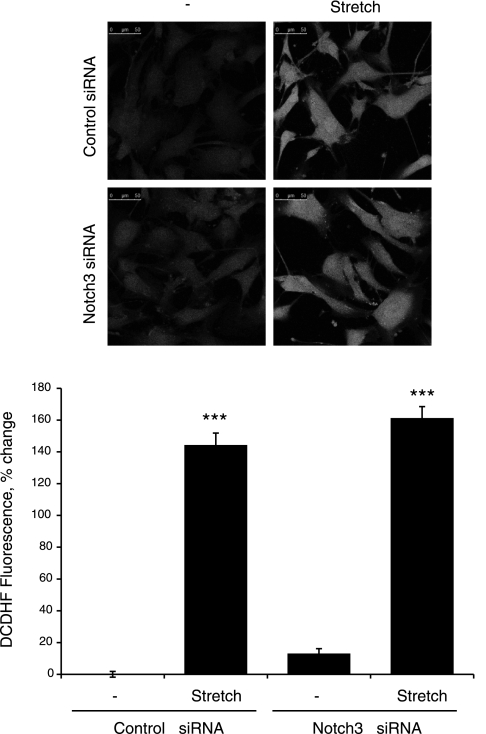
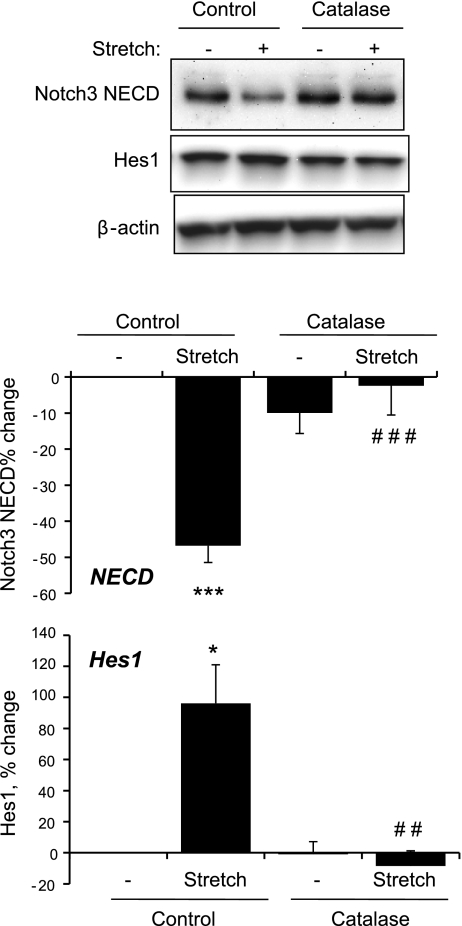
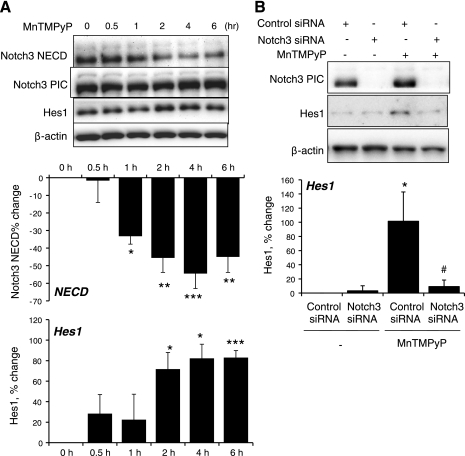
The ability of stretch to increase the generation of ROS in VSMCs (7, 30) was confirmed using the fluorescent probe DCDHF (Fig. 5) (30). The stretch-induced increase in DCDHF fluorescence was not significantly affected by siRNA-mediated downregulation of Notch3 expression (Fig. 5). Inhibition of H2O2 by catalase (1,200 U/ml) abolished the effect of stretch to stimulate the Notch3-dependent expression of Hes1 and the S2 cleavage-dependent decrease in Notch3 NECD (Fig. 6). In nonstretched cells, a cell-permeable mimic of SOD, manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 25 μmol/l), which increases the activity of H2O2 in VSMCs (34), increased the expression of Hes1 and decreased levels of Notch3 NECD (Fig. 7). The effect of MnTMPyP to increase Hes1 expression was markedly reduced by siRNA-mediated suppression of Notch3 expression (Fig. 7B).
Fig. 5.
Stretch-induced increase in ROS activity in VSMCs using laser scanning microscopy analysis of 5- (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCDHF) fluorescence. Cells were exposed to cyclic uniaxial stretch (10%, 1 Hz) or left nonstretched for 5 min, and fluorescence intensity was then determined as described in materials and methods. Data are expressed as percent changes of fluorescence intensity from nonstretched control siRNA-transfected cells and are presented as means ± SE; n = 3. Statistically significant differences from nonstretched control siRNA-transfected cells (***P < 0.001) are indicated.
Fig. 6.
Effects of inhibition of H2O2 by catalase on the stretch-induced increase in Notch3 signaling. Cells were incubated in the absence and presence of catalase (1,200 U/ml, 4 h) and then exposed to cyclic uniaxial stretch (10%, 1 Hz) or left nonstretched for 60 min. Expression levels of NECD, Hes1, and β-actin were determined by Western blot analysis. Data are expressed as percent changes of protein level from nonstretched control cells (normalized to β-actin) and are presented as means ± SE; n = 3. Statistically significant differences from nonstretched control cells (*P < 0.05; ***P < 0.001) and from stretched control cells (##P < 0.01; ###P < 0.001; not shown for comparisons with nonstretched cells) are indicated.
Fig. 7.
Effects of a cell-permeable SOD mimic, manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP; 25 μM), on Notch3 signaling. A: cells were incubated with MnTMPyP (25 μM) for up to 6 h, and the expression levels of NECD, PIC, Hes1, and β-actin were determined by Western blot analysis. Data are expressed as percent changes of protein level from 0 h (normalized to β-actin) and are presented as means ± SE; n = 4–5. Statistically significant differences from 0 h (*P < 0.05; **P < 0.01; ***P < 0.001) are indicated. B: effect of siRNA knockdown of Notch3 on the MnTMPyP-induced increase in Hes1 expression. The expression levels of PIC, Hes1, and β-actin were determined by Western blot analysis. Cells were exposed to MnTMPyP (25 μM) for 2 h. Hes1 data are expressed as percent changes of protein level from nontreated control siRNA-transfected cells (normalized to β-actin) and are presented as means ± SE; n = 3–4. Statistically significant differences from nontreated control siRNA-transfected cells (*P < 0.05) and from MnTMPyP-treated control siRNA-transfected cells (#P < 0.05; not shown for comparisons with nontreated cells) are indicated.
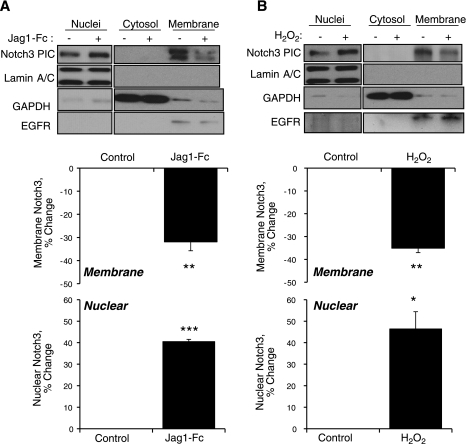
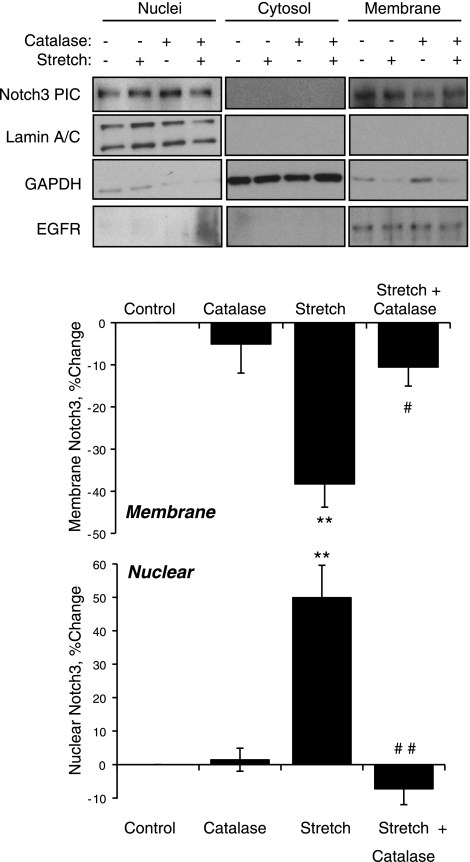
To confirm the role of H2O2 in cyclic stretch-induced activation of Notch3 in VSMCs, experiments evaluated the translocation of Notch3 from the plasma membrane to the nucleus. Exogenous H2O2 (50 μmol/l) acted in a similar manner to the exogenous Notch agonist Jag1-Fc (1 μg/ml) and caused significant translocation of Notch3 from the membrane to the nuclear fraction of VSMCs (Fig. 8). Cyclic stretch also caused translocation of Notch3 from the membrane to the nuclear fraction, which was abolished by catalase (1,200 U/ml; Fig. 9).
Fig. 8.
Effects of the exogenous Notch agonist Jag1-Fc (1 μg/ml, 2 h; A) or of H2O2 (50 μM, 1 h; B) on the activity of Notch3 as assessed by the translocation of Notch3 from the membrane to the nucleus. Cells that had been incubated in the absence and presence of the stimuli were processed to separate nuclear, cytosolic, and membrane fractions as described in materials and methods. Effectiveness of the fractionation was assessed by analyzing the expression by Western blot analysis of lamin A/C (for nuclei), GAPDH (for the cytosol), and EGF receptor (EGFR; for membranes). Notch3 expression was also assessed by Western blot analysis (PIC). Membrane and nuclear Notch3 were expressed as percent changes in the levels of nontreated cells (normalized to EGFR and lamin A/C expression, respectively) and are presented as means ± SE; n = 3. Statistically significant differences from nontreated control cells (*P < 0.05; **P < 0.01; ***P < 0.001) are indicated.
Fig. 9.
Effects of cyclic uniaxial stretch (10%, 1 Hz) and/or catalase (1,200 U/ml) on the activity of Notch3 as assessed by the translocation of Notch3 from the membrane to the nucleus. Cells that had been incubated in the absence and presence of catalase (1,200 U/ml, 4 h) were then exposed to cyclic uniaxial stretch (10%, 1 Hz) or left nonstretched for 1 h. Samples were processed to separate nuclear, cytosolic, and membrane fractions as described in materials and methods. Effectiveness of the fractionation was assessed by analyzing the expression by Western blot analysis of lamin A/C (for nuclei), GAPDH (for the cytosol), and EGFR (for membranes). Notch3 expression was also assessed by Western blot analysis (Notch3 PIC, predominantly intracellular component). Notch3 PIC was spliced between cytosol and membrane fractions to match the order of lamin A/C, GAPDH, and EGFR, and white spaces were inserted between images of these two fractions. Membrane and nuclear Notch3 were expressed as percent changes in the levels in nonstretched control cells (normalized to EGFR and lamin A/C expression, respectively) and are presented as means ± SE; n = 3. Statistically significant difference from nonstretched control cells (**P < 0.01) and from untreated stretched cells (#P < 0.05; ##P < 0.01; not shown for comparisons with nonstretched cells) are indicated.
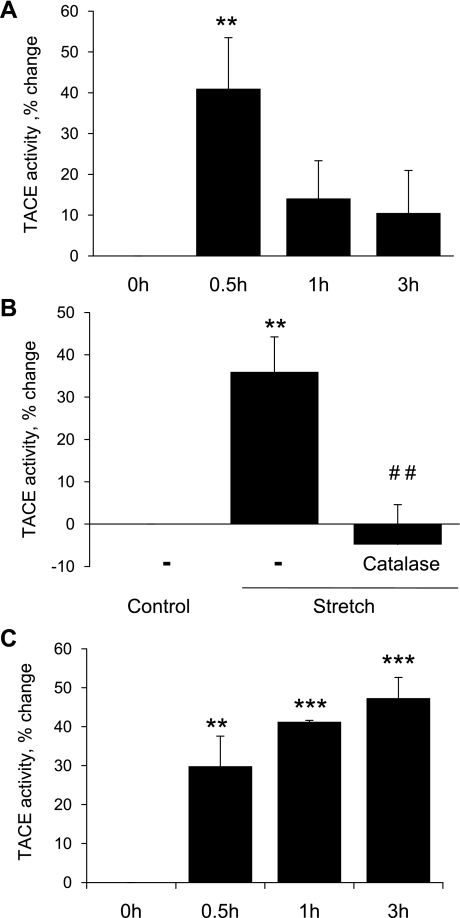
The activity of ADAM/TACE, which mediates S2 activation of Notch3, was increased in response to cyclic stretch (Fig. 10A). This stretch-induced increase was abolished by catalase (1,200 U/ml; Fig. 10B). Direct elevation of H2O2 activity using MnTMpyP (25 μmol/l) also increased ADAM/TACE activity (Fig. 10C).
Fig. 10.
Regulation of TACE activity by cyclic uniaxial stretch (A and B) or the cell permeable SOD mimic MnTMPyP (25 μM; C). A: cells were exposed to increasing periods of cyclic uniaxial stretch (10%, 1 Hz, up to 3 h). TACE activity was expressed as the percent change from 0 h and is presented as means ± SE; n = 4. Statistically significant differences compared with the 0-h time point (**P < 0.01) are indicated. B: cells were incubated in the absence or presence of catalase (1,200 U/ml, 4 h) before being exposed to cyclic uniaxial stretch (10%, 1 Hz) or left nonstretched for 30 min. TACE activity was expressed as the percent change from untreated nonstretched control cells and is presented as means ± SE; n = 4. Statistically significant differences compared with untreated nonstretched control cells (**P < 0.01) and compared with untreated stretched cells (##P < 0.01) are indicated. C: cells were exposed to MnTMPyP (25 μM) for up to 3 h. TACE activity was expressed as the percent change from the 0-h time point and is presented as means ± SE; n = 3. Statistically significant differences compared with the 0-h time point (**P < 0.01; ***P < 0.001) are indicated.
Role of Notch3 in stretch-induced realignment of VSMCs.
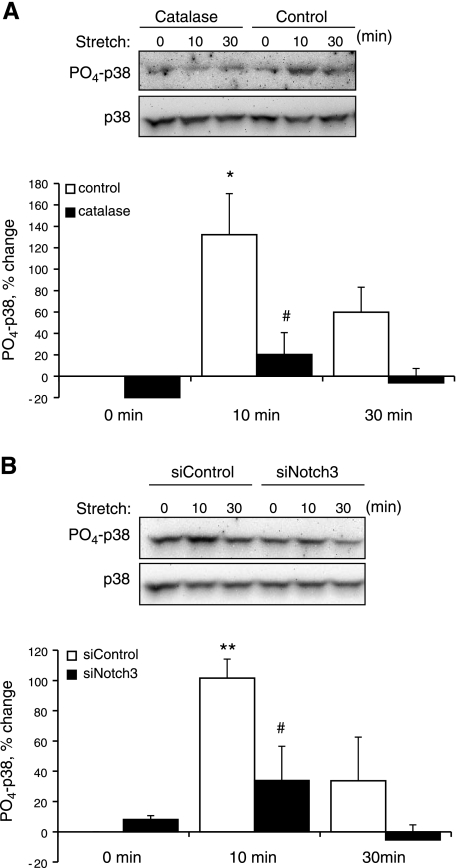
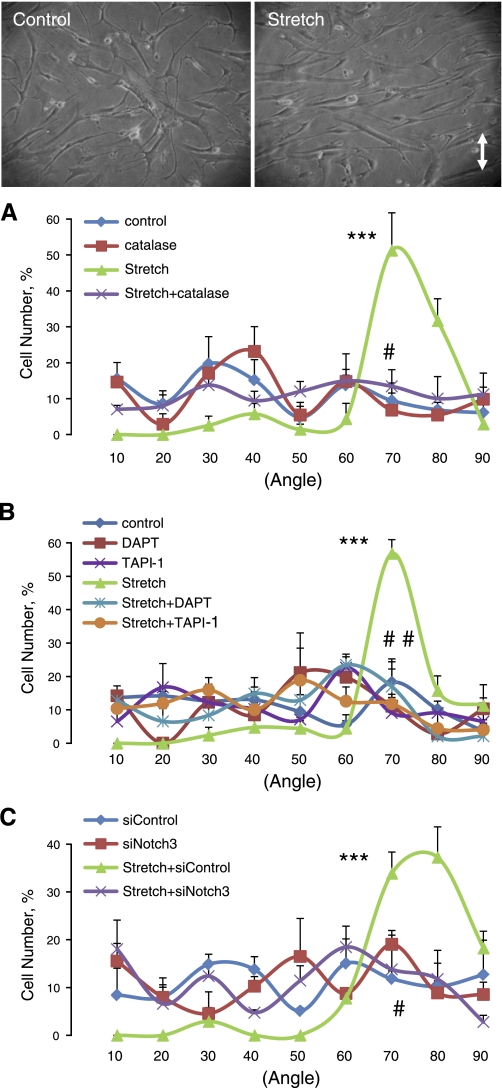
Stretch causes the realignment of VSMCs, which is dependent on stretch-induced generation of ROS and activation of p38 MAPK (7). In the present study, cyclic stretch increased levels of phosphorylated p38 MAPK (PO4-p38 MAPK) but not total p38 MAPK, and this effect was significantly reduced by catalase (1,200 U/ml) or by siRNA-mediated suppression of Notch3 expression (Fig. 11). The exogenous Notch agonist Jag1-Fc (1 μg/ml) did not cause activation of p38 MAPK (data not shown). Cyclic stretch also caused realignment of VSMCs (∼70° relative to the stretch direction), which was prevented by catalase (1,200 U/ml; Fig. 12A) or by the p38 MAPK inhibitor SB-202190 (10 μmol/l; data not shown). Furthermore, the stretch-induced realignment of VSMCs was dramatically reduced by inhibiting the S2 (TAPI-1, 50 μmol/l)- or S3 (DAPT, 20 μmol/l)-dependent activation of Notch3 or by inhibiting Notch3 expression (siRNA) (Fig. 12, B and C).
Fig. 11.
Role of oxidants and Notch3 in regulating stretch-induced activation of p38 MAPK. p38 MAPK activity was assessed by Western blot analysis. PO4-p38 MAPK was expressed as the percent change of protein level from 0 min (normalized to total p38 protein) and is presented as means ± SE; n = 3. Statistically significant differences compared with the corresponding 0-min time point (*P < 0.05; **P < 0.01) and from control cells at the same time point (#P < 0.05) are indicated. A: effect of catalase (1,200 U/ml, 4 h) on stretch-induced p38 MAPK activity. B: effect of siRNA knockdown of Notch3 on stretch-induced p38 MAPK activity.
Fig. 12.
Regulation of VSMC realignment in response to cyclic uniaxial stretch. Effects of inhibition of H2O2 (A; 1,200 U/ml catalase), inhibition of Notch3 activation (B; 50 μmol/l TAPI-1 or 20 μmol/l DAPT), or reduction of Notch3 expression (C; control or Notch3 siRNA) on stretch-induced realignment of VSMCs. Cell angles, relative to the stretch direction, were determined and sorted into the following groups: 0–10,11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, and 81–90°. Data are expressed as percentages of the total cell number for each experiment and are presented as means ± SE; n = 3. Statistically significance differences compared with other cell angles within that group (***P < 0.001) and between control untreated stretched cells and all other groups (#P < 0.05; ##P < 0.01) are indicated.
DISCUSSION
Arterial VSMCs of Notch3−/− mice display selective functional defects in cerebral autoregulation and pressure-induced myogenic constriction while retaining the ability to constrict normally to other stimuli (4, 8). They also fail to mature properly during postnatal development, which is highlighted by the decreased expression of differentiation markers and incorrect orientation around the vessel lumen (4, 8). These VSMC defects are consistent with impaired mechanosensitivity of VSMC to acute (e.g., autoregulation and myogenic response) or chronic (e.g., postnatal maturation) exposure to increases in blood (or luminal) pressure (4, 8). The present study provides the first direct demonstration that Notch3 contributes to the mechanosensitivity of VSMCs. Cyclic uniaxial stretch of human arterial VSMCs caused activation of Notch3, which was mediated by redox signaling and contributed to stretch-induced orientation of the VSMCs. Hemodynamic forces were thought to be a primary mediator in promoting postnatal maturation of arteries and the distinct differentiation program of arteries and veins (35). However, the location-dependent expression and activity of signaling molecules like Notch suggested that these vascular structures are genetically preprogrammed (35). The results of the present study suggest that genetic preprogramming and hemodynamic forces can act in concert during postnatal maturation of the arterial system through the mechanosensitive activation of VSMC Notch3.
Activation of Notch3 signaling by cyclic uniaxial stretch of VSMCs was evident from the stretch-induced increase in the translocation of Notch3 from the plasma membrane to the nucleus and stretch-induced expression of the Notch3 target proteins HRT1 and Hes1, which was prevented by inhibiting Notch3 expression (siRNA) or activation (S2 or S3 cleavage). Interestingly, mechanical strain generated by receptor endocytosis has been proposed to contribute to ligand-dependent activation of Notch (9, 19). Activation of cell surface Notch3 is dependent on sequential proteolysis. ADAM/TACE metalloproteases cleave Notch PIC [also termed Notch transmembrane and intracellular domain (NTMICD)] at S2, terminating its interaction with the extracellular domain (NECD), which is then internalized and degraded (29). The S2-cleaved membrane-tethered fragment of PIC (NEXT) is then processed by γ-secretase (at S3), releasing NICD, which translocates to the nucleus and regulates gene transcription (9, 19). Although we observed stretch-induced translocation of Notch3 from the membrane to the nucleus, we were not able to discern the small changes in molecular weight associated with the S2 and S3 cleavage of Notch3 PIC, in agreement with a previous study (15). Neither activation nor inhibition of Notch3 caused any changes in the molecular size of the PIC band. Furthermore, the translocation of this species to the nucleus indicates that the PIC band actually represents the NTMICD, NEXT, and NICD Notch3 species. Despite significant translocation between different cellular domains, the combined total of these proteins remained relatively constant during the course of the present study. In contrast, after activation of Notch3 by stretch or by the exogenous agonist Jag1-Fc, we observed significant loss of the Notch3 NECD species. Release of this species occurs during the S2 cleavage step. Indeed, the reduction in NECD was prevented by inhibiting S2 but not S3 processing of Notch3. Therefore, the S2-dependent loss of NECD provides a novel assay for monitoring a key proximal step in Notch3 activation within VSMCs. Although loss of NECD was not dependent on S3 cleavage, functional responses to Notch3 activation were highly dependent on this distal step in Notch3 activation and were blocked by inhibiting γ-secretase.
Stretch-induced mechanotransduction in VSMCs is known to be regulated by redox signaling initiated by stretch-induced activation of NADPH oxidase and increased ROS activity (7, 23, 30, 31). ROS, in particular H2O2, contribute to the activation of downstream signaling (e.g., p38 MAPK) (3, 7, 34, 37) and VSMC responses including contraction (arteriolar myogenic response) (30) and reorientation (7) in response to stretch. The results of the present study confirm that uniaxial stretch of VSMCs generates a burst in ROS activity, which contributes to the activation of p38 MAPK and subsequent cellular reorientation. They further demonstrate a novel role for Notch3 in this VSMC mechanotransduction. Indeed, as was observed with inhibition of H2O2 (catalase) or p38 MAPK (SB-202190), the molecular (siRNA) or pharmacological inhibition (inhibiting S2 or S3 cleavage) of Notch3 abolished the stretch-induced reorientation of VSMCs in response to uniaxial stretch.
The stretch-induced activation of Notch3 was prevented by catalase, which specifically degrades H2O2, indicating that it was mediated by the stretch-induced increase in H2O2. Indeed, elevation in H2O2 levels, either by providing exogenous H2O2 or increasing endogenous levels [using the SOD mimic MnTMPyP (34)], was sufficient to activate Notch3, causing nuclear translocation of Notch3, an elevation in Hes1 expression, or decreased levels of Notch3 NECD. Catalase prevented the stretch-induced translocation of Notch3 to the nucleus, the stretch-induced increase in Hes1, and the stretch-induced decrease in Notch3 NECD. Because this loss of NECD reflects a proximal step in Notch3 signaling, its modulation by catalase confirms that endogenous H2O2 is involved in a proximal step of Notch3 activation. Indeed, TACE, which mediates the S2 cleavage-dependent release and loss of NECD, has been demonstrated to be activated by H2O2 in numerous studies, which may result from reversible oxidation of critical thiols in the inhibitory prodomain (43) or the mature enzyme (41). In the present study, cyclic uniaxial stretch increased TACE activity in VSMCs, and this was prevented by catalase, suggesting it was mediated by the stretch-induced increase in H2O2. Indeed, in nonstretched cells, TACE activity was increased by directly elevating endogenous H2O2 levels using MnTMPyP. Therefore, the cyclic stretch-induced and ROS-dependent increase in TACE activity likely contributes to the activation of Notch3. This is the first demonstration of redox-dependent modulation of Notch3 activity. A previous study (5) reported that Notch1 can be activated by exogenous glucose oxidase, which was proposed to be mediated by ROS-dependent stimulation of TACE, although the role of ROS was not directly assessed.
MAPK signaling pathways, particularly p38 MAPK, have been previously demonstrated to be oxidant sensitive and activated by cyclic stretch in VSMCs. Furthermore, inhibition of p38 MAPK, but not ERK1/2, has been shown to abolish cyclic stretch-induced VSMC realignment and shear stress-induced endothelial cell realignment (2, 7), which may reflect a role for p38 MAPK signaling in actin reorganization and cell movement (13, 14). In the present study, cyclic stretch increased the activation of p38 MAPK, which was abolished by catalase, consistent with previous reports. Cross-talk has been demonstrated between Notch and p38 MAPK signaling pathways. Notch activity is associated with increased p38 MAPK signaling in macrophages (27) and glia (10). However, p38 MAPK has also been reported to regulate Notch3 activity in a negative manner in fibroblasts (18) and Notch1/2 in a positive manner in Ras-transformed cells (42) and skeletal muscle (6). Therefore, the cross-talk between Notch and p38 MAPK appears to be determined by cell type and context. In the present study, we observed that Notch3 was necessary for cyclic stretch-induced p38 MAPK activation in VSMCs. However, the exogenous Notch agonist Jag1-Fc, which activated Notch3 in VSMCs, did not increase p38 MAPK activity. Therefore, these results suggest that Notch3 may not directly activate p38 MAPK in VSMCs but that it plays a permissive role to amplify stretch-induced activation of the signaling pathway. The mechanism(s) underlying this permissive activity was not defined in the present study.
Although mechanosensitive activation of Notch3 by cyclic uniaxial stretch is in agreement with the VSMC dysfunctions observed in Notch3−/− mice (4, 8), a previous study (25) observed that equibiaxial stretch of rat VSMCs decreased Notch3 activity, which was based on decreased expression of Notch-3-dependent target genes (HRT and Hes). This likely reflects the ability of equibiaxial stretch to produce opposite responses in VSMCs compared with the more physiologically relevant uniaxial stretch (32). In addition to Notch3, the human VSMCs used in the present study express Notch1 and Notch2 proteins (36). Therefore, other Notch receptors could be modulated by the pharmacological inhibitors used in the present study, and these receptors as well as Notch-independent mechanisms may contribute to the expression of the Notch target proteins Hes1 and HRT1. However, our results clearly demonstrate a key role for Notch3 in the responses to cyclic stretch. Cyclic stretch caused the activation of Notch3, demonstrated by the nuclear translocation of Notch3 and loss of Notch3 NECD. Furthermore, the stretch-induced expression of Hes1 and stretch-induced realignment of VSMCs were abolished by the specific molecular knockdown of Notch3 (siRNA) as well as by inhibitors of Notch activation.
In conclusion, we demonstrated that cyclic uniaxial stretch causes a burst in ROS activity in human VSMCs, resulting in redox activation of TACE and Notch3, and that this mechanosensitive activation of Notch3 contributes to stretch-induced signaling and alignment of VSMCs. The mechanosensitive activity of VSMC Notch3 explains the vascular defects observed in Notch3−/− mice and may contribute to the activity of Notch3 during vascular development and the vascular dysfunction associated with Notch3 mutations in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (4, 8, 9).
GRANTS
This work was supported by National Institute for Occupational Safety and Health Grant OH-8531 (to N. A. Flavahan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Michael Wang (University of Michigan) for the Hes1 plasmid.
REFERENCES
- 1. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 284: 770– 776, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Azuma N, Akasaka N, Kito H, Ikeda M, Gahtan V, Sasajima T, Sumpio BE. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol 280: H189– H197, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Baas AS, Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2− in vascular smooth muscle cells. Circ Res 77: 29– 36, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Belin de Chantemele EJ, Retailleau K, Pinaud F, Vessieres E, Bocquet A, Guihot AL, Lemaire B, Domenga V, Baufreton C, Loufrani L, Joutel A, Henrion D. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol 28: 2216– 2224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5: 207– 216, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Brown D, Hikim AP, Kovacheva EL, Sinha-Hikim I. Mouse model of testosterone-induced muscle fiber hypertrophy: involvement of p38 mitogen-activated protein kinase-mediated Notch signaling. J Endocrinol 201: 129– 139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Q, Li W, Quan Z, Sumpio BE. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and p38 mitogen-activated protein kinase. J Vasc Surg 37: 660– 668, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 18: 2730– 2735, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16: 633– 647, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci 30: 3101– 3112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashimi ST, Fulcher JA, Chang MH, Gov L, Wang S, Lee B. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood 114: 404– 414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol 316: 87– 99, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 274: 24211– 24219, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res 80: 383– 392, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG, Israël A. Delta-1 activation of notch-1 signaling results in HES-1 transactivation. Mol Cell Biol 18: 7423– 7431, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin G, Huang X, Black R, Wolfson M, Rauch C, McGregor H, Ellestad G, Cowling R. A continuous fluorimetric assay for tumor necrosis factor-α converting enzyme. Anal Biochem 302: 269– 275, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell Signal 18: 1924– 1931, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kennard S, Liu H, Lilly B. Transforming growth factor-beta (TGF-β1) down-regulates Notch3 in fibroblasts to promote smooth muscle gene expression. J Biol Chem 283: 1324– 1333, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216– 233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc Natl Acad Sci USA 103: 16095– 16100, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu B, Qu MJ, Qin KR, Li H, Li ZK, Shen BR, Jiang ZL. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys J 94: 1497– 1507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu R, Li X, Tulpule A, Zhou Y, Scehnet JS, Zhang S, Lee JS, Chaudhary PM, Jung J, Gill PS. KSHV-induced notch components render endothelial and mural cell characteristics and cell survival. Blood 115: 887– 895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mata-Greenwood E, Grobe A, Kumar S, Noskina Y, Black SM. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-β1 and reactive oxygen species: a requirement for NAD(P)H oxidase. Am J Physiol Lung Cell Mol Physiol 289: L288– L298, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, Tomita T. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester). J Biol Chem 281: 14670– 14676, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res 96: 567– 575, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Moss ML, Rasmussen FH. Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal Biochem 366: 144– 148, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Narayana Y, Balaji KN. NOTCH1 up-regulation and signaling involved in Mycobacterium bovis BCG-induced SOCS3 expression in macrophages. J Biol Chem 283: 12501– 12511, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA 93: 9090– 9095, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nichols JT, Miyamoto A, Weinmaster G. Notch signaling–constantly on the move. Traffic 8: 959– 969, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, Flavahan NA. Redox signaling of the arteriolar myogenic response. Circ Res 89: 114– 116, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Ohmine T, Miwa Y, Takahashi-Yanaga F, Morimoto S, Maehara Y, Sasaguri T. The involvement of aldosterone in cyclic stretch-mediated activation of NADPH oxidase in vascular smooth muscle cells. Hypertens Res 32: 690– 699, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng 88: 359– 368, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Shimizu K, Chiba S, Saito T, Takahashi T, Kumano K, Hamada Y, Hirai H. Integrity of intracellular domain of Notch ligand is indispensable for cleavage required for release of the Notch2 intracellular domain. EMBO J 21: 294– 302, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res 89: 39– 46, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res 104: 576– 588, 2009. [DOI] [PubMed] [Google Scholar]
- 36. Tang Y, Urs S, Boucher J, Bernaiche T, Venkatesh D, Spicer DB, Vary CP, Liaw L. Notch and transforming growth factor-beta (TGFβ) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J Biol Chem 285: 17556– 17563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin. II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 273: 15022– 15029, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, Grassi J, Lopez-Perez E, Checler F. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J Biol Chem 276: 37743– 37746, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Walker F, Burgess AW. Reconstitution of the high affinity epidermal growth factor receptor on cell-free membranes after transmodulation by platelet-derived growth factor. J Biol Chem 266: 2746– 2752, 1991 [PubMed] [Google Scholar]
- 40. Wang T, Baron M, Trump D. An overview of Notch3 function in vascular smooth muscle cells. Prog Biophys Mol Biol 96: 499– 509, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol 182: 2449– 2457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med 8: 979– 986, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Z, Oliver P, Lancaster JJ, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor-α-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J 15: 303– 305, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.