Abstract
We previously demonstrated that myocardial p38 mitogen-activated protein kinase (MAPK) and heat shock protein 27 (HSP27) are phosphorylated following cardioplegic arrest in patients undergoing cardiac surgery and correlate with reduced cardiac function. The following studies were performed to determine whether inhibition of p38 MAPK and/or overexpression of nonphosphorylatable HSP27 improves cardiac function following cardioplegic arrest. Langendorff-perfused isolated rat hearts were subjected to 2 h of intermittent cold cardioplegia followed by 30 min of reperfusion. Hearts were treated with (CP+SB) or without (CP) the p38 MAPK inhibitor SB-203580 (5 μM) supplied in the cardioplegia. Sham-treated hearts served as controls. In separate experiments, isolated rat ventricular myocytes infected with either green fluorescent protein (GFP) or a nonphosphorylatable HSP27 mutant (3A-HSP27) were subjected to 3 h of cold hypoxic cardioplegia and simulated reperfusion (CP) followed by video microscopy and length change measurements. Baseline parameters of cardiac function were similar between groups [left ventricular developed pressure (LVDP), 119 ± 4.9 mmHg; positive and negative first derivatives of LV pressure (± dP/dt), 3,139 ± 245 and 2, 314 ± 110 mmHg/s]. CP resulted in reduced cardiac function (LVDP, 72.2 ± 5.8 mmHg; ± dP/dt, 2,076 ± 231 and −1,317 ± 156 mmHg/s) compared with baseline. Treatment with 5 μM SB-203580 significantly improved CP-induced cardiac function (LVDP, 101.9 ± 0 mmHg; ±dP/dt, 2,836 ± 163 and −2,108 ± 120 mmHg/s; P = 0.03, 0.01, and 0.04, CP+SB vs. CP). Inhibition of p38 MAPK significantly lowered CP-induced p38 MAPK, HSP27, and αB-crystallin (cryAB) phosphorylation. In vitro CP decreased myocyte length changes from 10.3 ± 1.5% (GFP) to 5.7 ± 0.8% (GFP+CP). Infection with 3A-HSP27 completely rescued CP-induced decreased myocyte contraction (11.1 ± 1.0%). However, infection with 3A-HSP27 did not block the endogenous HSP27 response. We conclude that inhibition of p38 MAPK and subsequent HSP27 and cryAB phosphorylation and/or overexpression of nonphosphorylatable HSP27 significantly improves cardiac performance following cardioplegic arrest. Modulation of HSP27 phosphorylation may improve myocardial stunning following cardiac surgery.
Keywords: ischemia, small heat shock protein, heat shock protein 27, p38 mitogen-activated protein kinase
cardiac surgery using cardioplegic arrest (CP) and cardiopulmonary bypass (CP/CPB) subjects myocardium to hypothermic reversible ischemic injury that can impair cardiac function (also known as myocardial stunning). Although protective, CP is associated with prolonged ischemic insults including myocyte hypoxia, acidosis, oxidant-dependent damage, metabolic and structural alterations, and reduced cardiac function (2, 17, 18, 29). The small heat shock proteins (sHSP) heat shock protein 27 (HSP27) and αB-crystallin (cryAB) are phosphorylated in response to all of these ischemic insults (4, 11, 30).
One pathway involved in myocardial contractile defects post-CP/CPB may be the p38 mitogen-activated protein kinase (MAPK) signaling cascade. p38 MAPK is activated by various cytokines and stress signals including ischemia-reperfusion (I/R) and subsequent oxidant stress (19). The p38 MAPK/MAPKAP-2 pathway can phosphorylate both HSP27 and cryAB (15, 20). In addition, numerous studies have demonstrated ischemia or hypoxia-dependent activation of p38 MAPK decreases contractile function of isolated perfused hearts and isolated myocyte preparations (3, 12, 21, 28). In addition, both phosphorylation of HSP27 and knockout of cryAB have been implicated in reduced cardiac function post-I/R injury (14, 24).
p38 MAPK activity is increased in human myocardial tissue post-CP/CPB (7, 26). We previously characterized the p38 MAPK, HSP27, and cryAB phosphorylation response in atrial myocardium of patients undergoing CP/CPB for coronary artery bypass graft or valve repair (7). CP/CPB results in a significant increase in phosphorylation of p38 MAPK, HSP27, and cryAB. In addition, the increased phosphorylation of HSP27 and cryAB negatively correlate with the patient cardiac index measured in the postsurgical intensive care unit.
The purpose of the following experiments was to determine whether inhibition of p38 MAPK-mediated sHSP phosphorylation or overexpression of a nonphosphorylatable HSP27 could alleviate CP-induced myocardial stunning.
METHODS
All animal protocols were approved by the Rhode Island Hospital Institutional Animal Care and Use Committee.
Isolated Langendorff-perfused model of cardioplegic arrest.
Male Sprague-Dawley rats (Charles River, MA) were anesthetized intraperitoneally with 80 mg/kg ketamine and 5 mg/kg xylazine, anticoagulated with heparin (2,000 U/kg iv), and the heart was rapidly exposed. The aorta was immediately cannulated and retrogradely perfused in Langendorff mode with a water-jacketed organ chamber and perfusion system (IH-SR; Harvard Apparatus, Boston, MA). The hearts were perfused in constant-pressure mode (∼70 mmHg) with a modified Krebs-Henseleit buffer (KHB; in mM: 118 NaCl, 4.7 KCl, 1.25 CaCl2, 1.66 MgSO4, 24.88 NaHCO3, 1.18 KH2PO4, 5.55 glucose, and 2.0 Na-pyruvate) for 30 min to stabilize and record baseline measurements. After cannulation, the heart was cleaned of excess tissue and vessels, the left atrium was removed, and a balloon was placed in the left ventricle. Left ventricular end-diastolic pressure was set to ∼8 mmHg at the beginning of the experiment. A temperature probe placed in the pulmonary artery monitored myocardial temperature. During baseline measurements, myocardial temperature was maintained at 37°C. Groups subjected to cold crystalloid cardioplegia solution [with (CP+SB) or without (CP) the p38 MAPK inhibitor SB-203580 (5 μM) supplied in the cardioplegia] were perfused with St. Thomas II solution (in mM: 110 NaCl, 16 KCl, 16 MgCl2, 1.5 CaCl2, and 10 NaHCO3). Myocardial cooling during CP was initiated at the onset of cardioplegia infusion by rapidly switching the Langendorff organ chamber and perfusate to a refrigerated circulator. Myocardial temperature was maintained at 10°C for the duration of CP. Cardioplegia groups were perfused initially for 2 min, followed by a 1-min infusion at 30, 60, and 90 min of arrest, respectively. After 120 min, the organ chamber and perfusate were switched back to a heating circulator and the heart was perfused at 70 mmHg with modified KHB. Myocardial temperature was subsequently maintained at 37°C. Indexes of ventricular function, perfusion pressure, myocardial temperature, and organ chamber temperature were measured continuously throughout the experiment using a LDS-Ponemah data acquisition system. At the conclusion of the experiment, the heart was removed from the perfusion apparatus and a small midtransverse slice was removed and immediately placed in 10% formalin for confocal microscopy. The remainder of the tissue was immediately place in liquid N2.
Experiments evaluating the role of SB-203580 alone.
Briefly, hearts (n = 3) were perfused for 30 min with KHB as described above to record baseline measurements. Hearts were then perfused with 200 ml of KHB + 5 μM SB-203580 for 30 min in recirculation mode at 37°C. Hemodynamic measurements were continuously recorded. For indexes of HSP27 and cryAB phosphorylation, SB-203580-treated hearts were compared with sham-perfused hearts as described above. All other parameters were the same as described above (see Supplemental Figs. S1 and S2). (Supplemental data for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.)
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting were performed using standard methodology as previously described (6). Antibodies for immunoblotting and confocal microscopy were as follows: phospho-specific and total HSP27, total HSP25, and cryAB antibodies were obtained from Stressgen (Vancouver, BC); phospho- and total p38 MAPK were obtained from Cell Signaling (Danvers, MA), and anti-MLC-2v was obtained from Synaptic Systems (Göttingen, Germany). For purposes of clarity, all blots shown are labeled with the amino acid residue corresponding to the human HSP27 numbering sequence (to which the antibodies were raised). In rats, the numbering sequence is slightly different from that in humans: Ser15 is the same in both species, the residue corresponding to Ser78 in humans is an asparagine at residue 82, and Ser82 in human HSP27 corresponds to Ser86 in rat HSP27. In addition, the rat homolog of HSP27 is termed HSP25. For purposes of clarity, we refer to total levels of rat HSP25 as HSP27, although all blots for rat total HSP27 were performed with the anti-HSP25 antibody described above.
Triton insolubility assay.
For Western blots examining soluble/insoluble fractions for HSP27 and cryAB, tissue was lysed as described above with 20 mM Tris, pH 7.4, 0.1% Triton X-100, protease, and phosphatase inhibitors. Lysates were centrifuged at 14,000 g at 4°C for 10 min. Supernatants were taken as the Triton-soluble fraction, and pellets were resuspended in an equal volume of SDS sample buffer, rehomogenized with a Polytron homogenizer, and centrifuged at 14,000 g for 10 min at 4°C. Immunoblotting and protein concentration assay with appropriate standards were performed as described above. To determine the quality of fractionation, we blotted known Triton-soluble and -insoluble proteins [phospholamban (Millipore) and MyBPC3 (Santa Cruz Biotechnology, Santa Cruz, CA), respectively (see Supplemental Materials)].
Confocal immunofluorescent microscopy.
Tissue was fixed overnight, embedded in paraffin blocks, and cut into 4-μm sections. Deparaffinized slides were boiled in 10 mM Na-citrate (pH 6.0) for 10 min. Blocking and overnight primary incubation (4°C) were performed with 2% bovine serum albumin (BSA) in Tris-buffered saline (TBS). Slides were washed three times with TBS and incubated with the appropriate Alexa Fluor secondary antibodies and ToPro-3 (Invitrogen, San Diego, CA) followed by mounting with fluorescent mounting medium (Vector Laboratories, Burlingame, CA). Tissue was visualized using a Zeiss LSM-510 meta confocal microscope system (Stuttgart, Germany). Tissue labeled with secondary antibody only was used as a negative control.
Adenoviral expression of recombinant proteins.
Green fluorescent protein (GFP; a gift of P. A. Vincent, Albany, NY) and human HSP27-3A (a gift of W. Gerthoffer, as described in Ref. 13) were used. The human HSP27-3A construct has the serine residues at Ser15, Ser78, and Ser82 mutated to alanine. Serine 82 corresponds to Ser86 in the rat numbering sequence. The rat residue corresponding to Ser78 is an asparagine. Acquired recombinant adenoviruses were amplified in QBI-293A cells and purified using cesium chloride gradients as previously described (5). All infections of mutant sHSP proteins were accompanied by control infection with GFP by using a multiplicity of infection greater than or equal to the greatest viral number used in the experimental groups. All infections were for 24 h.
Culture and purification of adult rat cardiomyocytes.
Adult rat cardiomyocyte cultures were obtained from hearts according to a previously published protocol with modifications (16). Briefly, hearts were excised from anesthetized adult rats, the aorta was cannulated, and hearts were perfused with 0.3% collagenase solution in perfusion buffer consisting of MEM (Joklik modification) supplemented with creatine, 2,3-butanedione monoximine, taurine, and insulin for 45 min. After perfusion, ventricles were removed and minced in Ca2+-free collagenase solution for 3–5 min. Chunks were then incubated in 10 ml of perfusion buffer supplemented with BSA and 0.3 mM CaCl2. Cells were washed in collagenase-free perfusion buffer three times with centrifugation. Myocytes were resuspended in DMEM culture medium supplemented with creatine, carnitine, taurine, penicillin/streptomycin, and insulin. Myocytes were plated at a density of 2 × 104 cells/cm2 on 4 μg/ml laminin-coated dishes (Western blots) or microscope slides (cell length recording) for adenoviral infection the following day.
In vitro CP and myocyte length recordings.
Cultured rat myocytes were switched to crystalloid cardioplegia solution bubbled with 5% CO2-95% N2 under anoxic conditions. Cells were placed in a hypoxia chamber evacuated with 5% CO2-95% N2 for the indicated times and retained at room temperature. For simulated reperfusion, CP-treated cells were removed from the hypoxic chamber and returned to the cell culture incubator, and cardioplegia solution was replaced with a modified Tyrode buffer containing (in mM) 118 NaCl, 25 KCl, 1.25 CaCl2, 16 MgCl2, 10 NaHCO3, and 11 glucose. Control cells were switched from medium and maintained in Tyrode buffer in a standard cell culture incubator for the duration of CP. For length measurement, cells were moved to a heated stage with temperature-controlled perfusion and field stimulation capable of accepting 35-mm culture dishes (Harvard Apparatus). The cells were imaged via a charge-coupled device camera-equipped inverted microscope attached to a raster line video edge detector (Living Systems Instrumentation, Burlington, VT). Voltage signals were captured via a data acquisition unit attached to a computer running Ponemah physiology platform software. The recorded length data can be visualized in real time at 60 Hz via a computer monitor and stored for subsequent analysis of percent length change and rate of change in length over time. The video edge detector and data capture software were calibrated using voltage signals from a slide micrometer before each experiment. Only rod-shaped viable cells that responded appropriately to field stimulation were used.
RESULTS
p38 MAPK mediates CP-induced myocardial stunning in the isolated heart.
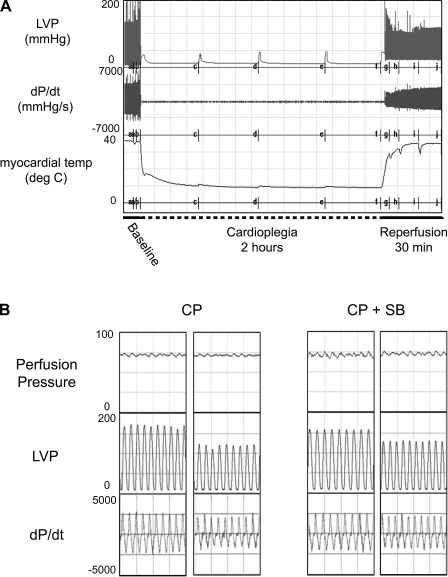
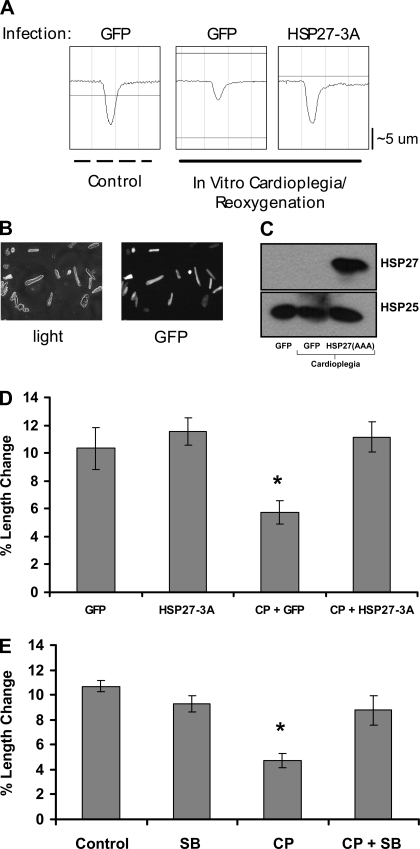
Cold crystalloid CP for 2 h in the isolated rat heart causes depressed cardiac contractile function on reperfusion. Figure 1A displays the tracings for a representative experiment with CP delivery. During CP, myocardial temperature was maintained at ∼10°C, followed by rewarming to 37°C during reperfusion (Fig. 1A, bottom tracing). On reperfusion there were significant reductions in systolic pressure, developed pressure (LVP; Fig. 1A, top tracing), and positive and negative first derivatives of LVP (±dP/dt; Fig. 1A, middle tracing). Inclusion of the p38 MAPK inhibitor SB-203580 in the cardioplegic solution improved the CP-induced depressed cardiac function relative to baseline (Fig. 1B, middle and bottom traces).
Fig. 1.
Cold crystalloid cardioplegia reduces cardiac performance. A: representative experiment of cardioplegia followed by reperfusion (CP/R) in isolated rat hearts: a, baseline measurement; b, induction of cardioplegic arrest (CP; 2-min delivery); c, 30 min of CP (1-min CP delivery); d, 60 min of CP; e, 90 min of CP; f, start of reperfusion; g to h, 5, 10, 20, and 30 min of reperfusion. B: tracings from individual hearts subjected to CP and reperfusion with (CP+SB) or without (CP) the p38 MAPK inhibitor SB-203580. LVP, left ventricular developed pressure; ±dP/dt, positive and negative first derivative of LVP; temp, temperature.
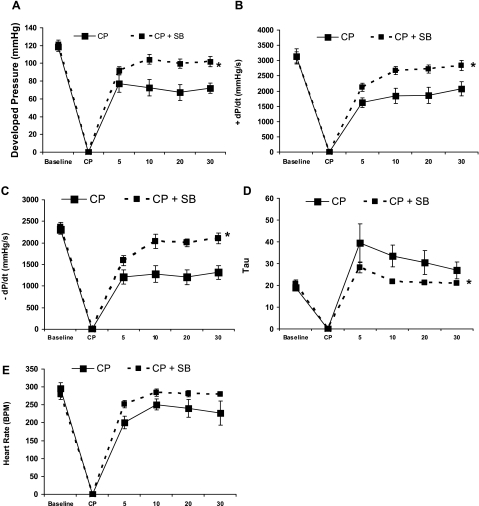
Postreperfusion, inhibition of p38 MAPK significantly improved indexes of cardiac function, including developed pressure (Fig. 2A), ±dP/dt (Fig. 2, B and C), and the relaxation time constant τ (Fig. 2D). There were no significant changes in heart rate between CP and CP+SB groups (Fig. 2E). A separate study evaluating SB-203580 treatment alone showed no changes in cardiac function parameters, with the exception of a small increase in +dP/dt after 30 min of 5 μM SB-203580. These results indicate that improved cardiac function post-CP is not a generalized effect of SB-203580 independent of injury (see Supplemental Fig. 1).
Fig. 2.
p38 MAPK mediates CP-induced depressed contractile function. A: left ventricular (LV) developed pressure. B: positive first derivative of LV pressure (+dP/dt). C: negative first derivative of LV pressure (−dP/dt). D: relaxation time constant τ. E: heart rate. The x-axis represents time (baseline, pre-CP/R function; CP, cardioplegia; 5–30 min, reperfusion). Values are means ± SE; n = minimum of 6 per group. *P < 0.05 (2-way repeated-measures ANOVA).
Inhibition of p38 MAPK blocks CP-induced phosphorylation of p38 MAPK, HSP27, and cryAB phosphorylation.
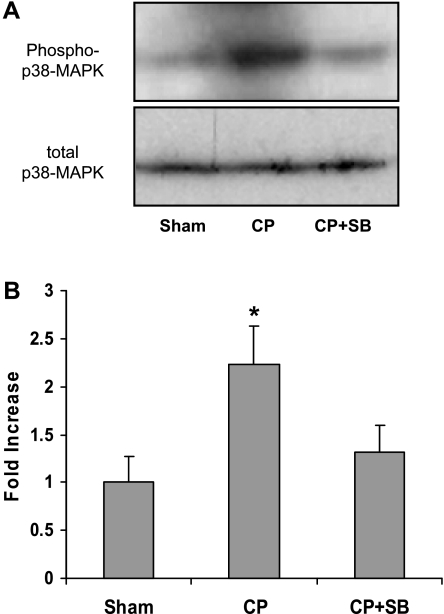
CP significantly elevated phosphorylation of p38 MAPK relative to 150 min of sham perfusion. Phosphorylation of p38 MAPK was blocked with 5 μM SB-203580 supplied in the cardioplegic solution (Fig. 3).
Fig. 3.
p38 MAPK is activated by CP/R. A: immunoblot analysis of isolated heart LV tissue probed with anti-phospho-p38 MAPK and total p38 MAPK. B: normalized fold increase in phosphorylation over sham. Values are means ± SE; n = minimum of 6 per group. *P < .05 vs. sham (1-way ANOVA, Holm-Sidak). S, sham; CP, 2 h of cardioplegia + 30 min of reperfusion; CP +SB, 2 h of cardioplegia with 5 μM SB-203580 + 30 min of reperfusion.
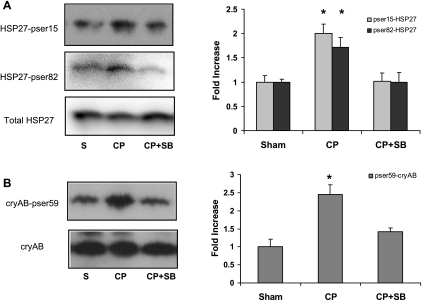
CP significantly increased the phosphorylation of HSP27 on both Ser15 (Fig. 4A, top) and Ser82 (Fig. 4A, middle) as well as increased phosphorylation of cryAB on Ser59 (Fig. 4B). Inhibition of p38 MAPK was associated with significantly decreased phosphorylation of both HSP27 and cryAB (Fig. 4, right). SB-203580 treatment alone decreased basal HSP27 and cryAB phosphorylation compared with sham-perfused hearts, indicating that modulation of HSP phosphorylation does not affect cardiac function under basal conditions (Supplemental Fig. S1B).
Fig. 4.
p38 MAPK mediates CP-induced heat shock protein 27 (HSP27) and αB-crystallin (cryAB) phosphorylation. A: immunoblot analysis of isolated heart LV tissue probed with anti-phospho (p)-Ser15-HSP27, anti-pSer86-HSP27 and total HSP27. Graph displays normalized fold increase in phosphorylation over sham. B: immunoblot analysis of isolated heart LV tissue probed with anti-pSer59-cryAB and total cryAB. Graphs display normalized (to nonphosphorylated proteins) fold increase in phosphorylation over sham. Values are means ± SE; n = minimum of 6 per group. *P < .05 vs. sham (1-way ANOVA, Holm-Sidak).
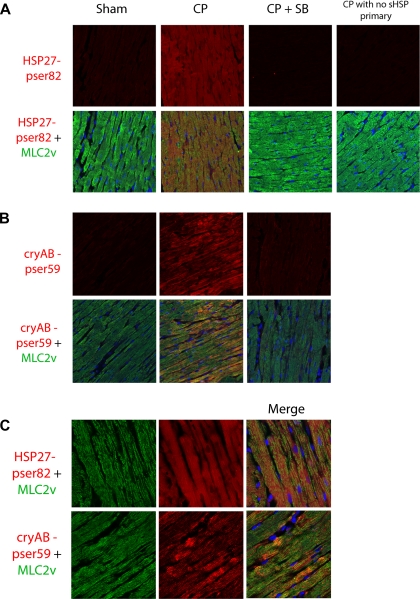
Confocal microscopy revealed that CP induced increases in phosphorylation of both HSP27 (Fig. 5A, red) and cryAB (Fig. 5B, red) in cardiomyocytes as revealed by costaining with MLC-2v (Fig. 5, A and B, green). Higher magnification images (Fig. 5C, top) revealed staining of phosphorylation of HSP27 on Ser82 was more diffuse than did our earlier investigations in human myocardium demonstrating translocation to z lines (7). However, phosphorylation of cryAB was localized to z lines of cardiomyocytes as demonstrated by alternating staining with MLC-2v (Fig. 5C, bottom). In contrast to human myocardium (7), there were no differences in the ratio of soluble to insoluble total HSP27 and cryAB, indicating an absence of translocation (see Supplemental Fig. S2). Inhibition of p38 MAPK displayed a significant decrease in phosphorylation of both HSP27 and cryAB. There was no significant signal with secondary antibody alone (Fig. 5A, far right).
Fig. 5.
p38 MAPK increases HSP27 and cryAB phosphorylation in cardiomyocytes. A: paraffin sections of isolated heart LV were stained with anti-pSer86-HSP27 (red) and visualized with confocal microscopy. No primary CP group received both secondary antibodies and anti-MLC-2v. sHSP, small heat shock protein. B: sections were stained with anti-pSer59-cryAB (red), MLC-2v (green), and ToPro3 (nuclei, blue) and visualized as in A. Images are representative of at least 6 animals per group. Inclusion of SB-203580 (5 μM) in the cardioplegia solution completely blocked CP/R-induced changes in phosphorylation. C: high-magnification images of CP-treated samples stained with anti-pSer82-HSP27 (red) or pSer59-cryAB (red), as well as MLC-2v (green) and ToPro-3 (nuclei, blue).
Overexpression of nonphosphorylatable HSP27 or p38 MAPK inhibition alleviates CP-impaired contractility in vitro.
To further explore the role of sHSP phosphorylation in CP-induced depressed contractile function, we subjected isolated rat cardiomyocytes to in vitro CP and simulated reperfusion with reoxygenation and rewarming. In vitro CP resulted in depressed myocyte contraction as determined by decreased myocyte length shortening (Fig. 6A). Efficient overexpression of GFP was determined via fluorescent microscopy of isolated myocytes (Fig. 6B), and overexpression of mutant HSP27 was verified using a human specific HSP27 antibody (Fig. 6C, top). Adenoviral overexpression of a nonphosphorylatable human HSP27 mutant (HSP27-3A) completely rescued in vitro CP-induced deficits in myocyte contractility (Fig. 6, A and D). In addition, inhibition of p38 MAPK with 5 μM SB-203580 also blocked CP-induced decreases in contraction but had no effect on baseline contractility (Fig. 6E).
Fig. 6.
Overexpression of a nonphosphorylatable HSP27 mutant rescues CP-induced myocyte contractile deficits in vitro. A: representative tracings of isolated rat myocyte length changes following 3-h hypoxic hypothermic crystalloid CP and normothermic reoxygenation. Human HSP27-3A virus has serines at 15, 78, and 82 mutated to alanines. GFP, green fluorescent protein. B: images show high-efficiency viral expression of GFP. C: expression and detection of the mutant human HSP27 in rat myocytes that express endogenous rat HSP27 (species-specific antibodies to rat and human HSP27: anti-HSP25 and HSP27, respectively; see methods). D: quantitative analysis of data in A. Myocytes subjected to in vitro hypoxic, hypothermic CP and rewarming/oxygenation displayed reduced contractile activity that was rescued by HSP27-3A overexpression. E: myocytes subjected to CP or CP+SB. Inhibition of p38 MAPK rescued CP-induced contractile deficits. Values are means ± SE; n = minimum of 15 myocytes per group. *P < .05 (1-way ANOVA).
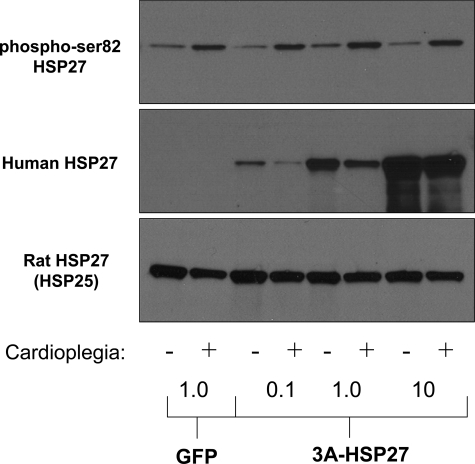
Isolated rat cardiomyocytes were infected with GFP or increasing doses of HSP27-3A and subjected to in vitro CP. Simulated CP and reperfusion increased the phosphorylation of endogenous rat HSP27 (Fig. 7, top). Increased expression of the nonphosphorylatable HSP27 (Fig. 7, middle) did not block the phosphorylation of endogenous nonmutant rat HSP27 (HSP25). In conjunction with Fig. 6, these results suggest the possibility that phosphorylation of HSP27 does not cause depressed myocardial contraction, per se, but that maintaining a pool of unphosphorylated HSP27 promotes improved cardiomyocyte contraction.
Fig. 7.
Overexpression of nonphosphorylatable HSP27 does not block endogenous HSP27 phosphorylation. Hypoxic, hypothermic CP increased the phosphorylation of endogenous rat HSP27 in isolated cardiomyocytes (top blot). Phosphorylation of rat HSP27 was not blocked by increasing overexpression of the nonphosphorylatable HSP27 mutant (middle blot; human-specific virus/antibody). Endogenous levels of rat HSP27 (HSP25) are shown (bottom blot). Concentrations at bottom indicate μl of virus.
DISCUSSION
The principle findings of this study were that 1) myocardial stunning following CP and reperfusion associates with increased p38 MAPK activation and HSP27 and cryAB phosphorylation, 2) supplementation of cardioplegic solution with the p38 MAPK inhibitor SB-203580 blocks CP-induced contractile deficits and phosphorylation of both HSP27 and cryAB, and 3) overexpression of nonphosphorylatable HSP27 or blockade of HSP27 phosphorylation with SB-203580 rescues CP-induced contractile deficits in isolated myocytes. However, the effects of HSP27-3A are independent of endogenous HSP27 phosphorylation.
The significance of phosphorylation of HSP27 and cryAB in cardiac function is controversial. Phosphorylation of specific residues on each protein is implicated in positive and negative regulation of a number of processes relevant to ischemic injury, including protein chaperone function and apoptosis (25, 27). However, a number of recent animal studies have indicated that cryAB and HSP27 may be key modulators of myocardial contractile function. The fact that phosphorylation of HSP27 and cryAB causes their translocation to cytoskeletal constituents of the sarcomere in other models suggests the hypothesis that HSP27 and cryAB may directly influence mechanics of the contractile apparatus (7, 10, 31, 32). However, our current data and data presented in the literature suggest that preservation of nonphosphorylated HSP27 and cryAB may promote cardiac contractile function. The following evidence is consistent with this view. First, ischemia-induced activation of p38 MAPK is associated with depressed cardiomyocyte contractile function, and inhibition of p38 MAPK elicits a positive inotropic effect in isolated myocytes (3, 21). Second, overexpression of either wild type or a nonphosphorylatable mutant of HSP27 partially blocks ischemia-induced contractile deficits in mouse hearts (14). Third, transgenic mice with a double knockout of cryAB and the related myotonic dystrophy protein kinase-binding protein (MKBP) have impaired contractile activity following ischemic injury (24).
We have demonstrated that CP-induced contractile deficits and the associated phosphorylation of HSP27 and cryAB are mediated by p38 MAPK. Inhibition of both HSP27 and cryAB phosphorylation is associated with improvements in cardiac function, including increased LV developed and systolic pressure and ±dP/dt. However, experiments with isolated myocytes demonstrated that overexpression of a nonphosphorylatable HSP27 improved contractile performance in the absence of inhibiting HSP27 phosphorylation. These results indicate that phosphorylation and subsequent effects of phosphorylated sHSPs do not negatively affect contractile function. Therefore, the presence of nonphosphorylated sHSPs may enhance contractile function. The phosphorylation of sHSPs following CP may remove or reduce the nonphosphorylated sHSP inotropic effect.
Indeed, phosphorylation of HSP27 and cryAB have been implicated in protective functions following myocardial I/R injury including stabilization of cytoskeletal elements of the cardiomyocyte, including actin, troponin, desmin, and titin (8, 9, 22, 27). Potentially, CP-induced phosphorylation of sHSPs may represent a protective response to preserve myocardial integrity at the expense of normal cardiac function. Therefore, from a therapeutic standpoint (although less feasible), overexpression of nonphosphorylatable sHSPs may provide the best mechanism of treatment for myocardial stunning, because it does not block the endogenous phosphorylation response. However, pharmacological inhibition of p38 MAPK was not associated with any deleterious effects in our studies and may be a potential therapeutic regardless of reduced sHSP phosphorylation. Future studies examining later time points following reperfusion may help in identifying whether any deleterious effects are associated with inhibition of the sHSP phosphorylation response.
Finally, SB-203580 is an inhibitor of both p38 MAPKα and p38 MAPKβ. The general consensus in the literature is that activation of p38 MAPKα promotes reduced function and apoptotic signals. In contrast, activation of p38 MAPKβ may be associated with cardioprotective signals. Regarding ischemic insults, data indicate that inhibition of p38 MAPKα and activation of p38 MAPKβ may be beneficial for ischemic injury (reviewed in Refs. 1, 23). It is unclear which isoform(s) is(are) activated or inhibited in our rat model of cardioplegic arrest. Targeted molecular approaches and or specific inhibitors are required to clearly resolve this issue.
Limitations.
A main limitation of the current study is the use of a small animal model to study cardioplegic arrest. To reliably induce myocardial stunning in this model, pilot experiments demonstrated a requirement for CP duration longer than 60–90 min. In our own experience and in published human studies, stunning can be detected over considerably shorter durations of CP (29). Another limitation was the use of crystalloid cardioplegia to test the efficacy of SB-203580; although crystalloid cardioplegia is still routinely used, blood crystalloid cardioplegia is more common. Future studies are required to determine whether inhibition of p38 MAPK is effective in enhancing cardiac function following blood crystalloid cardioplegia and reperfusion. Finally, deleterious effects associated with cardiopulmonary bypass were not assessed in this model, so a model of in vivo CP and reperfusion is required to determine whether SB-203580 is as effective in alleviating contractile deficits following any injury associated with extracorporeal circulation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 5K99 HL093352-02 to R. T. Clements and 5R01 HL046716-17 to F. W. Sellke.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Bassi R, Heads R, Marber MS, Clark JE. Targeting p38-MAPK in the ischaemic heart: kill or cure? Curr Opin Pharmacol 8: 141–146, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Chen Y, Rajashree R, Liu Q, Hofmann P. Acute p38 MAPK activation decreases force development in ventricular myocytes. Am J Physiol Heart Circ Physiol 285: H2578–H2586, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Chiesi M, Longoni S, Limbruno U. Cardiac alpha-crystallin. III. Involvement during heart ischemia. Mol Cell Biochem 97: 129–136, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-β-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol 288: L294–L306, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Clements RT, Smejkal G, Sodha NR, Ivanov AR, Asara JM, Feng J, Lazarev A, Gautam S, Senthilnathan V, Khabbaz KR, Bianchi C, Sellke FW. Pilot proteomic profile of differentially regulated proteins in right atrial appendage before and after cardiac surgery using cardioplegia and cardiopulmonary bypass. Circulation 118: S24–S31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clements RT, Sodha NR, Feng J, Mieno S, Boodhwani M, Ramlawi B, Bianchi C, Sellke FW. Phosphorylation and translocation of heat shock protein 27 and alphaB-crystallin in human myocardium after cardioplegia and cardiopulmonary bypass. J Thorac Cardiovasc Surg 134: 1461–1470, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol 91: 963–972, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Binding of the stress protein alpha B-crystallin to cardiac myofibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo. J Mol Cell Cardiol 31: 569–580, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D. Ischemia-induced phosphorylation and translocation of stress protein αB-crystallin to Z lines of myocardium. Am J Physiol Heart Circ Physiol 274: H1457–H1464, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Golenhofen N, Perng MD, Quinlan RA, Drenckhahn D. Comparison of the small heat shock proteins alphaB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem Cell Biol 122: 415–425, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Gorog DA, Tanno M, Cao X, Bellahcene M, Bassi R, Kabir AM, Dighe K, Quinlan RA, Marber MS. Inhibition of p38 MAPK activity fails to attenuate contractile dysfunction in a mouse model of low-flow ischemia. Cardiovasc Res 61: 123–131, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 274: 24211–24219, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation 110: 3544–3552, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of alpha B-crystallin in response to various types of stress. J Biol Chem 272: 29934–29941, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res 87: 118–125, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation 104: 2981–2989, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation 104: 3158–3167, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem 267: 794–803, 1992 [PubMed] [Google Scholar]
- 21. Liao P, Wang SQ, Wang S, Zheng M, Zheng M, Zhang SJ, Cheng H, Wang Y, Xiao RP. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res 90: 190–196, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu XY, Chen L, Cai XL, Yang HT. Overexpression of heat shock protein 27 protects against ischaemia/reperfusion-induced cardiac dysfunction via stabilization of troponin I and T. Cardiovasc Res 79: 500–508, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Marber MS, Rose B, Wang Y. The p38 mitogen-activated protein kinase pathway—a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for αB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol 286: H847–H855, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Sun Y, MacRae TH. The small heat shock proteins and their role in human disease. FEBS J 272: 2613–2627, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Talmor D, Applebaum A, Rudich A, Shapira Y, Tirosh A. Activation of mitogen-activated protein kinases in human heart during cardiopulmonary bypass. Circ Res 86: 1004–1007, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol 38: 433–444, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Sankula R, Tsai BM, Meldrum KK, Turrentine M, March KL, Brown JW, Dinarello CA, Meldrum DR. p38 MAPK mediates myocardial proinflammatory cytokine production and endotoxin-induced contractile suppression. Shock 21: 170–174, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Weisel RD. Myocardial stunning after coronary bypass surgery. J Card Surg 8: 242–244, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Welsh MJ, Gaestel M. Small heat-shock protein family: function in health and disease. Ann NY Acad Sci 851: 28–35, 1998 [DOI] [PubMed] [Google Scholar]
- 31. White MY, Hambly BD, Jeremy RW, Cordwell SJ. Ischemia-specific phosphorylation and myofilament translocation of heat shock protein 27 precedes alpha B-crystallin and occurs independently of reactive oxygen species in rabbit myocardium. J Mol Cell Cardiol 40: 761–774, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Yoshida K, Aki T, Harada K, Shama KM, Kamoda Y, Suzuki A, Ohno S. Translocation of HSP27 and MKBP in ischemic heart. Cell Struct Funct 24: 181–185, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.