Abstract
Triggered arrhythmias due to spontaneous cytoplasmic calcium oscillations occur in a variety of disease conditions; however, their cellular mechanisms in tissue are not clear. We hypothesize that spontaneous calcium oscillations in the whole heart are due to calcium release from the sarcoplasmic reticulum and are facilitated by calcium diffusion through gap junctions. Optical mapping of cytoplasmic calcium from Langendorff perfused guinea pig hearts (n = 10) was performed using oxygenated Tyrode's solution (in mM): 140 NaCl, 0.7 MgCl, 4.5 KCl, 5.5 dextrose, 5 HEPES, and 5.5 CaCl2 (pH 7.45, 34°C). Rapid pacing was used to induce diastolic calcium oscillations. In all preparations, pacing-induced multicellular diastolic calcium oscillations (m-SCR) occurred across most of the mapping field, at all pacing rates tested. Ryanodine (1 μM) eliminated all m-SCR activity. Low-dose caffeine (1 mM) increased m-SCR amplitude (+10.4 ± 4.4%, P < 0.05) and decreased m-SCR time-to-peak (−17.4 ± 6.7%, P < 0.05) and its temporal synchronization (i.e., range) across the mapping field (−26.9 ± 17.1%, P < 0.05). Surprisingly, carbenoxolone increased the amplitude of m-SCR activity (+14.8 ± 4.1%, P < 0.05) and decreased m-SCR time-to-peak (−11.3 ± 9.6%, P < 0.01) and its synchronization (−37.0 ± 19.1%, P < 0.05), similar to caffeine. In isolated myocytes, carbenoxolone (50 μM) had no effect on the frequency of aftercontractions, suggesting the effect of cell-to-cell uncoupling on m-SCR activity is tissue specific. Therefore, in the whole heart, overt m-SCR activity caused by calcium release from the SR can be induced over a broad range of pacing rates. Enhanced ryanodine receptor open probability and, surprisingly, decreased cell-to-cell coupling increased the amplitude and temporal synchronization of spontaneous calcium release in tissue.
Keywords: delayed afterdepolarization, triggered arrhythmia, calcium wave
calcium-mediated triggered arrhythmias have been shown to occur in a wide variety of conditions such as heart failure (9, 21, 27), catecholaminergic polymorphic ventricular tachycardia (29), atrial fibrillation (35), postshock arrhythmias (20), and myocardial infarction (26). Such triggered arrhythmias are due to cytoplasmic calcium oscillations during diastole that activate a transient inward current, producing a delayed afterdepolarization (DAD). If DAD voltage is sufficient to reach sodium current activation threshold, a triggered beat will ensue. When this happens within a single myocyte in situ, neighboring myocytes collectively act as a current sink and suppress DAD formation. To overcome this current sink and produce a triggered beat, spontaneous calcium oscillations during diastole must occur in multiple neighboring myocytes within a similar time frame (24).
Even though significant evidence exists demonstrating spontaneous calcium oscillations during diastole from neighboring myocytes (3, 9, 14, 23), the mechanisms that underlie this activity in situ are not well understood. Do calcium oscillations during diastole come from the sarcoplasmic reticulum (SR) through the ryanodine receptor (RyR), or from alternative sources such as myofilaments (31), or calcium entry from the sarcolemma (25)? It is also unknown what mechanisms synchronize spontaneous calcium oscillations between neighboring myocytes in situ. Synchronized activity could be explained by regional variations in calcium handling, such that neighboring myocytes share a similar propensity for calcium oscillations. We (15, 17) and others (19, 28) have shown that calcium handling properties vary both from apex to base and transmurally. An alternative mechanism for synchronizing calcium oscillations is calcium diffusion between neighboring myocytes through gap junctions (11, 16, 34). We hypothesize that spontaneous calcium oscillations during diastole in multiple neighboring myocytes (an m-SCR) are due to calcium release from SR through the RyR and are facilitated by calcium diffusion between cells through gap junctions.
METHODS
Experimental preparation.
Experiments were performed in accordance with Public Health Service guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Male guinea pigs (n = 10, 300–350 g) were anesthetized (3 ml Nembutal ip), and their hearts were rapidly removed via medial thoracotomy and Langendorff perfused during the entire experiment with oxygenated (100% O2) Tyrode's solution containing (in mM) 140 NaCl, 0.7 MgCl, 4.5 KCl, 5.5 dextrose, 5 HEPES, and 5.5 CaCl2 (pH 7.45, 34°C). Perfusion pressure was maintained between 50 and 70 mmHg by regulating coronary flow using a pulsatile flow system. Endocardial cryoablations were performed in all preparations to eliminate the Purkinje fibers and deeper layers of myocardium (8). To measure intracellular calcium transients, hearts were stained with the calcium-sensitive indicator indo 1-AM (Invitrogen) at a final concentration of 10 μM for 30 min at room temperature to minimize dye compartmentalization, followed by a 15-min washout period. In all experiments, 7 μM of cytochalasin D (Sigma-Aldrich) was used to ensure that motion artifact did not influence our result.
The perfused hearts were placed in a Lexan chamber. The mapping field was positioned over the left anterior descending artery just below its bifurcation with the diagonal coronary artery. To avoid epicardial surface cooling and desiccation, the heart was immersed in the coronary effluent that was maintained at a uniform temperature (34°C) equal to the perfusion temperature with a heat exchanger located in the chamber. The volume-conducted electrocardiogram (ECG) was monitored by using three Ag-AgCl disk electrodes fixed to the chamber in positions roughly corresponding to ECG limb leads I, II, and III. ECG signals were filtered (0.3–300 Hz), amplified (1,000×), and displayed on an oscilloscope. A fine-gauge (0.003 in. diameter), polytetrafluoroethylene-coated silver bipolar electrode was inserted in the left ventricular anterior wall to stimulate the ventricular endocardial surface at two times diastolic threshold current. Physiological stability of the preparation was assured by monitoring the ECG, coronary pressure, coronary flow, and perfusion temperature continuously throughout each experiment. Preparations remain viable for 4–5 h, but the entire experimental protocol typically lasted 1–2 h.
Excitation light was obtained from a 365-nM, 500-mW LED (Nichea) and directed through a flexible liquid light guide (Thermo-Oriel) to the preparation. Fluorescent light from the preparation was collected with two high-numerical aperture complex Nikon photographic lenses placed facing each other. To measure high-fidelity calcium signals, a 445-nm long-pass filter (Chroma Technology) was positioned to transmit the indo-1 AM fluorescence to a 16 × 16 element photodiode array (Hamamatsu). For the present study, an optical magnification of ×1.24 was used, resulting in a total mapping field of 14.2 × 14.2 mm, with 0.9-mm spatial resolution and 0.81-mm2 pixel size. All recordings made after indo 1 loading were AC coupled, which eliminated background fluorescence originating from the dye and the tissue (background fluorescence) to maximize the dynamic range of the photodiode array to image very small changes in intracellular calcium. To view the preparation, a mirror was positioned to reflect visible light to a charge-coupled device video camera (MiCam02-HR; SciMedia). Ratiometric calcium (15) and dual calcium-voltage imaging (18) were performed in two separate subsets of experiments, which enabled direct comparison of intracellular calcium levels over time and concomitant DAD activity, respectively.
Isolated myocytes.
Myocytes were isolated enzymatically from guinea pig hearts using the enzymatic dispersion technique described previously (32). Sarcomere shortening was assessed using a video-based sarcomere length detection system (IonOptix) at room temperature. Aftercontractions were initiated by 20 s of 6 Hz field stimulation.
Experimental protocol.
All preparations were electrically quiescent or had slow automatic rhythms (30–60 beats/min). Hearts were rapid paced (170–400 beats/min) with one-to-one capture for 15 s followed by a halt in pacing to elicit spontaneous calcium oscillations during diastole. To determine if spontaneous calcium oscillations were due to calcium release from the SR, recordings were also performed during the administration of the RyR agonist caffeine (CAFF, 1 mM; Sigma-Aldrich) or the RyR antagonist ryanodine (1 μM; Sigma-Aldrich). To examine the role of gap junctions, recordings were also performed during administration of the gap junction antagonist carbenoxolone (CBX, 50 μM; Sigma-Aldrich).
Data analysis.
Calcium oscillation amplitude was measured as a percent of the fluorescence intensity of the last paced beat within each optical channel, unless otherwise noted. The time-to-peak (Tp) of calcium oscillations was measured as the time between the maximum dCa/dt of the last paced beat and the peak of the largest calcium oscillation. For each recording, amplitude and Tp were measured from all 256 channels within the mapping array. The range of Tp was measured as the difference between the longest and the shortest Tp from each of 256 channels within the mapping field. Student's paired t-test was considered significant for P values <0.05.
RESULTS
Pacing-induced diastolic calcium oscillations in the intact guinea pig heart.
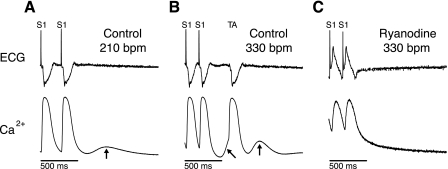
Shown in Fig. 1A is the ECG and intracellular calcium recorded upon termination of rapid pacing at a rate of 210 beats/min under control conditions in the whole heart. The calcium trace from a single photodiode element represents average cellular activity from a 0.81-mm2 region of roughly 25,000 cells. Immediately after the last paced beat, during diastole, an oscillation of calcium is observed (Fig. 1A, arrow). There is no evidence of electrical activity on the ECG, suggesting that this oscillation is not driven by action potential activity. Moreover, the oscillation has a relatively slow rise time and is low in amplitude compared with the pacing-induced calcium transients. Figure 1B shows another example following cessation of pacing at a faster rate (330 beats/min). However, in this example, a triggered beat is initiated. Immediately preceding, and then following the triggered beat is evidence of calcium oscillations. Finally, the application of ryanodine (Fig. 1C) abolished all calcium oscillations and suppressed the formation of triggered activity over the same range of pacing rates, suggesting that the calcium oscillations are driven by release of calcium from the SR. We refer to multicellular spontaneous calcium release in tissue as an m-SCR.
Fig. 1.
Spontaneous diastolic calcium oscillations were induced by 15 s of rapid pacing followed by a pause. Shown in each panel are the electrocardiogram (ECG, top) and intracellular calcium (bottom) recorded from a single site on the epicardium under control conditions (A), control at a faster pacing rate (B), and with ryanodine (C). Under control conditions at 210 beats/min (A), a spontaneous diastolic calcium oscillation was observed (arrow) after termination of rapid pacing. S1, rapid pacing. A more rapid heart rate [330 beats/min (bpm)] resulted in a triggered beat (TA, B) with multiple oscillations of diastolic calcium (arrows). Ryanodine (1 μM, C) abolished TA and any spontaneous calcium oscillations during diastole.
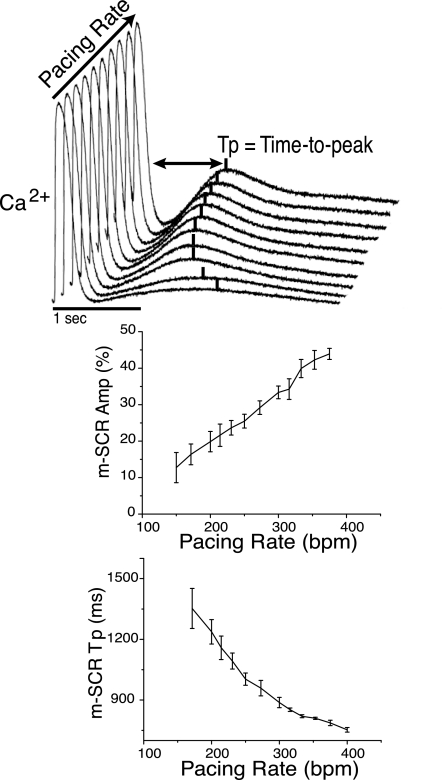
Shown in Fig. 2 is the rate dependence of m-SCR activity in the intact guinea pig heart. Figure 2, top, shows representative traces of m-SCRs from the same recording site over a broad range of pacing rates (150–380 beats/min). For all pacing rates tested, m-SCR activity was observed. As pacing rate increased, m-SCR amplitude increased and the Tp relative to the last paced beat shortened (occurred earlier). For comparison across preparations, m-SCR amplitude was quantified as a percentage of the last paced calcium transient amplitude. Over all experiments (n = 5), m-SCR amplitude increased, and Tp decreased as a function of increasing pacing rate (Fig. 2, bottom).
Fig. 2.
Shown at the top are superimposed intracellular calcium traces measured from a single recording site during the termination of pacing at increasing rates. As pacing rate increases (400–160 beats/min), multicellular spontaneous calcium release (m-SCR) amplitude increases and the time-to-peak (Tp) occurs earlier. Summary data show that faster pacing rates increase m-SCR amplitude (bottom left) and decrease m-SCR Tp (bottom right).
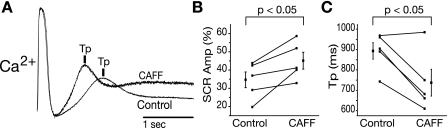
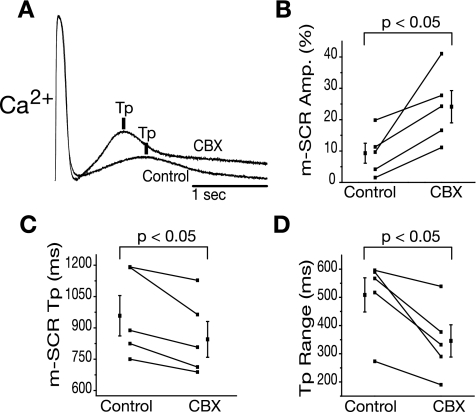
To test the sensitivity of m-SCR activity to increased RyR open probability (Po), a low concentration of CAFF (1 mM) was administered. Figure 3A shows representative m-SCR activity measured from the same recording site before (control) and after CAFF that is normalized to the peak transient amplitude during the last paced beat. Following the application of CAFF, m-SCR activity occurred earlier (Tp shorter) and was greater in amplitude, for the same pacing rate. Summary data (Fig. 3, B and C) show that, over all experiments, CAFF increased the amplitude of m-SCR activity (+10.4 ± 4.4%, P < 0.05) and shortened Tp (−17.4 ± 6.7%, P < 0.05). Because CAFF also decreases calcium transients during pacing, ratiotmetric intracellular calcium imaging was performed in additional experiments (n = 2) to determine the absolute change in m-SCR amplitude. Ratiometric recordings indicated that m-SCR amplitude increased following CAFF (30.3 ± 12.0%), similar to nonratiotmetric recordings. Time control experiments showed no change in m-SCR amplitude or Tp over 2 h. Additionally, CAFF washout showed a reversal of effect (results not shown). Taken together, these data provide further evidence that m-SCR activity is the result of multicellular spontaneous calcium release from the SR.
Fig. 3.
A: representative calcium traces with caffeine (CAFF) and without (control) upon the termination of rapid pacing (330 beats/min). Increasing ryanodine receptor (RyR) open probability (Po) with CAFF increases m-SCR amplitude (Amp) and decreases Tp. Summary data show that, over all experiments, CAFF increases m-SCR amplitude (B) and decreases Tp (C).
Spatial heterogeneity of m-SCR and DAD activity.
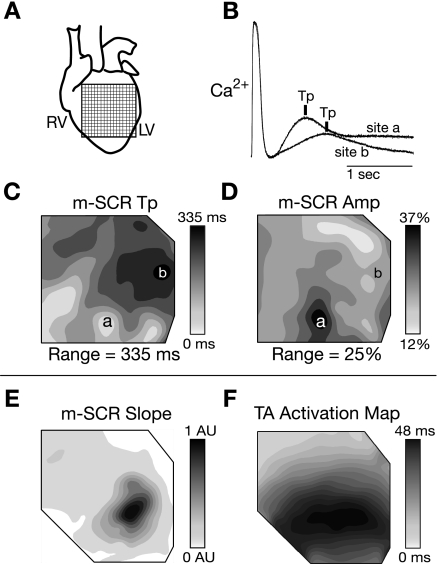
In every experiment, m-SCR activity was observed over the majority of the mapping field (14.2 × 14.2 mm; Fig. 4A), even at slow pacing rates (150 beats/min). However, Tp and m-SCR amplitude were heterogeneously distributed. Figure 4B shows intracellular calcium measured at two separate sites (Fig. 4B, sites a and b) during the same recording under control conditions. The m-SCR amplitude at locations a and b were 28% and 16%, respectively, measured as a percentage of the last paced beat at each site. In addition, Tp occurred earliest at site a and 355 ms later at site b. Figure 4, C and D, shows contour maps of Tp and m-SCR amplitude across the mapping field. Both Tp and m-SCR amplitude were heterogeneously distributed across the mapping field. Interestingly, the contour maps of Tp and m-SCR amplitude are similar but not identical. Site a was the location of both the earliest and largest m-SCR while site b was the latest but not the smallest m-SCR. Within individual preparations, the region of largest m-SCR amplitude was always consistent between sequential recordings, and, in general, m-SCR activity was largest near the apical region (bottom of the contour map) in 8 out of 10 experiments. In a separate experiment, the initial slope of m-SCR activity (i.e., dCa2+/dt before the full calcium transient onset) was compared with the origin of triggered activity. The location of maximum positive dCa2+/dt during the m-SCR (Fig. 4E) corresponded exactly to the origin of the associated triggered beat (Fig. 4F). Similar results were observed in all experiments when triggered activity occurred in the mapping field.
Fig. 4.
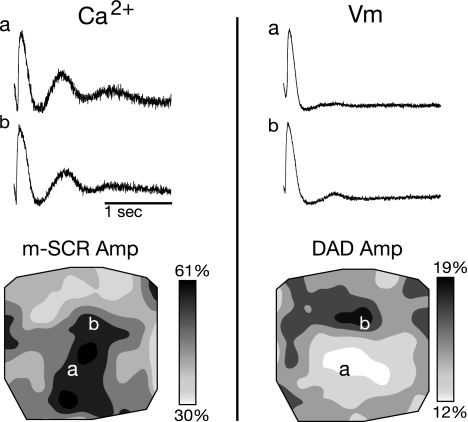
A: the location of the photodiode array on the anterior surface of the heart. RV, right ventricle; LV, left ventricle. B: two calcium traces recorded at the same time from different sites on the epicardial surface (sites a and b in C and D) during the termination of rapid pacing (250 beats/min). The m-SCR at site a is larger and occurs earlier than the m-SCR at site b. C and D: spatial distribution of m-SCR Tp and amplitude, respectively, across the mapping field. From a different experiment, E and F show the distribution of dCa2+/dt before the full calcium transient onset and activation time of the associated triggered beat, respectively. AU, arbitrary units. The location of maximum dCa2+/dt is the same as the triggered beat origin.
To determine if DAD activity is concomitant with m-SCR activity, dual calcium-voltage recordings were performed (Fig. 5). Shown are calcium traces during the termination of rapid pacing (Fig. 5, top left) and a contour map of m-SCR amplitude (Fig. 5, bottom). SCR amplitude is similar at both locations shown (Fig. 5, sites a and b). Evidence of concomitant DAD activity is present on the epicardial surface (Fig. 5, right). However, the voltage traces (Fig. 5, top) and contour map of DAD amplitude (Fig. 5, bottom) revel that there is not a one-to-one correspondence between m-SCR and DAD amplitude.
Fig. 5.
Dual calcium and voltage recordings during m-SCR activity. Left, calcium traces recorded during the termination of rapid pacing (top) and a contour map of m-SCR amplitude (bottom). From the same recording, voltage traces (top) and contour map of delayed afterdepolarization (DAD) amplitude (bottom) are shown on right. Evidence of DAD activity is present on the epicardial surface; however, the spatial distribution DAD amplitude is not identical to m-SCR amplitude. Vm, membrane potential.
Heterogeneity of m-SCR activity during enhanced RyR Po and decreased gap junction coupling.
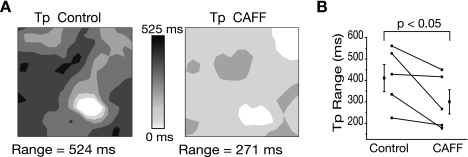
The effects of enhanced RyR Po on the spatial distribution of m-SCRs are shown in Fig. 6. Figure 6A shows contour maps of Tp before and after CAFF administration. Under control conditions, Tp was heterogeneously distributed with a range of 524 ms. Following the administration of CAFF, the range of Tp decreased to 271 ms. The range of Tp across the mapping field was used as a measure of m-SCR temporal synchronization. After the application of CAFF (Fig. 6A, left), the range of Tp decreased, suggesting that increased RyR Po increased synchronization of m-SCR activity. Over all experiments (n = 5), Tp range decreased by −26.9 ± 17.1% (P < 0.05) following the application of CAFF (Fig. 6B).
Fig. 6.
A: contour maps of Tp before (control) and during CAFF administration. Under control conditions, Tp was heterogeneous and occurred over a range of 524 ms. CAFF decreased the range of Tp to 271 ms, suggesting that local m-SCR activity became more synchronized with increased RyR Po. Summary data (B) show that, over all experiments, CAFF significantly decreased Tp range.
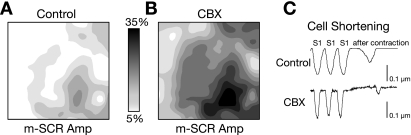
Because m-SCR activity occurs in many cells over a similar time frame, it is possible that calcium diffuses through gap junctions, triggering calcium release in neighboring cells, or calcium release is an intrinsic property of neighboring cells, independent of gap junctions. To address this, we used CBX (50 μM, n = 5) to inhibit gap junction communication between cells. Following CBX application, conduction velocity, as estimated by the time of calcium release during pacing, decreased from 41 to 18 cm/s (P < 0.01) and QRS width increased from 57 to 92 ms (P < 0.01). Figure 7A shows representative m-SCR activity measured from the same recording site before and after CBX following 15 s of rapid pacing at 215 beats/min. The m-SCR was not abolished with CBX, indicating that multicellular spontaneous calcium release does not require calcium signaling through gap junctions. Surprisingly, CBX increased m-SCR amplitude and decreased Tp. Summary data show that CBX significantly increased m-SCR amplitude (+14.8 ± 4.1%, P < 0.05; Fig. 7B), decreased Tp (−11.3 ± 9.6%, P < 0.05; Fig. 7C), and decreased Tp range (−37.0 ± 19.1%, P < 0.05; Fig. 7D), similar to the effect that increased RyR Po (CAFF) had on m-SCRs (Fig. 6). Shown in Fig. 8 are contour maps depicting the spatial heterogeneity of m-SCR amplitude before (Fig. 8A) and after (Fig. 8B) CBX. The amplitude of m-SCR activity increased with CBX, but the pattern of m-SCR amplitude remained the same (greatest near the apex), where we have previously shown calcium transients are largest (15). These data suggest that similar intrinsic properties between neighboring sites is a mechanism of synchronizing regional m-SCR activity, rather than calcium diffusion through gap junctions. It is possible that nonspecific effects of CBX increase spontaneous calcium release from the SR. However, experiments in isolated guinea pig myocytes with and without CBX showed that aftercontractions (slow and small amplitude response) were not altered by CBX (Fig. 8C). For control, two of seven cells had one aftercontraction. For CBX, one of eight cells had one aftercontraction.
Fig. 7.
A: representative calcium traces with carbenoxolone (CBX) and without (control) upon termination of rapid pacing (215 beats/min). CBX does not suppress m-SCR activity but, surprisingly, increases m-SCR amplitude and decreases m-SCR Tp. Similar results were observed over all experiments (B and C). CBX also decreases the Tp range (D) similar to the effect of CAFF (Fig. 6B).
Fig. 8.
Cell-to-cell uncoupling with CBX maintains the spatial pattern but increases the amplitude of m-SCR activity. Shown are the contour plots of m-SCR amplitude measured as a percentage of the last paced beat across the epicardial surface under control conditions (A) and following CBX administration (B). C: cell shortening during the termination of rapid pacing (S1) in isolated guinea pig myocytes with CBX and without control.
DISCUSSION
In the present study, we demonstrate nonelectrically driven multicellular spontaneous calcium release (an m-SCR) in the whole Langendorff perfused heart. The primary findings of this report are: 1) overt m-SCR activity occupies a large portion of the heart and can be routinely induced over a broad range of pacing rates, 2) enhanced RyR Po increases temporal synchronization of m-SCR activity, and 3) decreased cell-to-cell coupling does not decrease m-SCR activity but, surprisingly, has an enhancing effect similar to increased RyR Po. This study is the first to report that increased RyR Po can temporally synchronize m-SCR activity across the surface of the heart. Additionally, we are the first to look at the role gap junctions play in spontaneous calcium release from the SR at the tissue level. These findings will help shed light on how diseases that increase RyR Po and decrease cell-to-cell coupling can impact calcium-mediated arrhythmogenesis in the whole heart.
Spontaneous calcium release from multiple myocytes in situ (m-SCR).
The m-SCR activity we report in the present study represents nonelectrically driven multicellular spontaneous release of calcium from the SR. Previously, we (9, 13, 15) and others (4, 20) have also shown nonelectrically driven calcium release from the SR in a variety of cardiac tissue and disease conditions. At the subcellular level, Fujiwara et al. (6) have demonstrated with confocal microscopy in the Langendorff-perfused rat heart that spontaneous calcium release occurs at the myocyte level and can be completely blocked by ryanodine. Alternatively, Housmans et al. (10) and Miura et al. (22) have shown in tissue that nonelectrically driven calcium oscillations can also be induced by nonuniform stretch, known as a triggered propagated contraction (TPC). It is unlikely that m-SCR activity measured in the present study is the result of TPCs because motion and, thus nonuniform stretch, was absent because of electromechanical uncoupling. One of the surprising findings of our study was that m-SCR activity could be induced with relatively slow pacing rates (380 beats/min) and encompassed a large region of the heart in the absence of disease. These data suggest that multicellular spontaneous calcium release may be more common than previously thought. We also observed m-SCR activity in hearts that were not endocardially cryoablated; however, these data were difficult to analyze because of intrinsic activity of the ventricles.
Mechanism of m-SCR synchronization.
Spontaneous calcium release, in the form of calcium sparks and waves, is well described at the single myocyte level, but it is unclear how spontaneous calcium release in neighboring myocytes coalesce to produce an arrhythmia. Two potential mechanisms that may play a role in synchronization of spontaneous calcium release in tissue are: 1) regional similarities in intrinsic calcium handling properties and 2) intercellular communication through gap junctions. Regional similarities in calcium handling, such as increased calcium reuptake (17) or increased diastolic calcium (13), could account for regions of increased RyR Po and subsequent increased propensity for m-SCR activity. In the guinea pig heart, calcium transients are larger and have a faster reuptake rate at the apex compared with the base (15), and endocardial layers have 22% less RyR and 44% less SR Ca2+-ATPase (33). Such intrinsic heterogeneities in calcium-handling proteins and properties could account for the regional distribution of m-SCR amplitudes that we observed between the apex and base on the epicardial surface of the guinea pig heart. A recent study by Maruyama et al. (20) suggests that difference in spontaneous calcium release between the endocardium and the epicardium may account for transmural difference in DAD amplitude, but the mechanisms that contribute to differences in calcium release are unknown.
Intercellular calcium diffusion through gap junctions may be an alternative mechanism by which spontaneous calcium release occurs in multiple neighboring cells. For example, m-SCR activity may arise from a calcium wave in a single myocyte, which then “diffuses” to neighboring myocytes. However, the data supporting whether or not calcium can diffuse through gap junctions are not clear. Some reports suggest that calcium waves have a low probability of propagating through gap junctions (11, 16). However, Baader et al. (1) showed that, in the whole newborn rat heart, spontaneous calcium waves readily propagated between neighboring cells. Moreover, Zhang et al. (34) showed that gap junctions are vital for the propagation of TPCs along the length of a rat trabecula and that gap junction inhibition prevents TPC formation. We show that m-SCR activity in our model is preserved during gap junction inhibition, suggesting that calcium diffusion to neighboring myocytes is not required. In fact, gap junction uncoupling increased m-SCR activity (see Fig. 7).
The mechanisms responsible for increased m-SCR activity associated with gap junction uncoupling are difficult to ascertain from our results. One possibility is that normally a small portion of cytoplasmic calcium during a local release “leaks” to neighboring myocytes, but this is not sufficient to trigger release. When gap junctions are inhibited, calcium is confined to a single cell, thus increasing local calcium release, analogous to the effect reduced electrical load has on action potential upstroke velocity. Gap junction uncoupling may also unmask underlying intrinsic heterogeneities that are voltage dependent, as demonstrated in Fig. 8. Alternatively, reduced gap junction coupling could influence intracellular calcium handling indirectly through source-sink changes in membrane potential either by 1) increasing peak L-type calcium current due to a more rapid action potential upstroke (30) or 2) a membrane voltage-dependent feedback mechanism that modulates RyR Po similar to that in skeletal muscle (12).
Clinical implications.
Calcium-mediated triggered arrhythmias have been implicated in a wide variety of disease states such as heart failure, CPVT, diabetes, and the postmyocardial infarction border zone. Our observations show that spontaneous calcium release in multiple neighboring cells (an m-SCR) occurs in ventricular muscle, a potential source of DADs in addition to Purkinje fibers (2). In disease when RyR is leaky, calcium sparks and waves may be enhanced; however, if gap junction coupling is reduced as well, then m-SCR activity will be further enhanced (Fig. 7). In addition, the change in membrane potential per m-SCR (i.e., DAD) will be enhanced because of reduced electrotonic load, creating a potent substrate for triggered arrhythmias.
We show that spontaneous calcium release from the SR occurs at slow pacing rates (Fig. 2), even under normal extracellular calcium concentrations (results not shown). However, triggered activity was never observed at slow pacing rates at any calcium concentration. This suggests that m-SCR activity, much like calcium waves in isolated myocytes, may be a natural way of unloading the SR to prevent intracellular calcium overload under physiological conditions (5). It is likely that m-SCRs, much like calcium waves, only become deleterious under pathophysiological conditions, such as heart failure, where multiple calcium-handling defects and cellular uncoupling result in the generation of triggered arrhythmias.
Study limitations.
The optical methods used in this study cannot resolve differences in intracellular calcium release between individual cells, making it difficult to know if changes in m-SCR amplitude are the result of more calcium being released or more cells releasing calcium. Confocal microscopy in the whole heart may be required to address this limitation (6). The experiments reported in this study were performed using higher than normal extracellular calcium (5.5 mM) to augment spontaneous calcium release. Experiments were also performed at normal calcium concentrations (2.5 mM), and m-SCRs were observed and behaved similar as that under high calcium concentrations; however, m-SCR amplitude was lower. We used the paralytic cytochalasin D in all the experiments to eliminate contraction and resultant motion artifact from our optically recorded calcium signals. However, by inhibiting motion, we were unable to measure diastolic calcium oscillations that could be the result of nonuniform contraction (31) and stretch-activated membrane channels (7), which may also play an important role in calcium-mediated arrhythmogenesis.
GRANTS
This material is based upon work supported under National Heart, Lung, and Blood Institute (NHLBI) Fellowship HL-090350 (M. J. Cutler) and NHLBI Grants HL-084142 and HL-100105 (K. R. Laurita).
DISCLOSURES
None.
REFERENCES
- 1. Baader AP, Buchler L, Bircher-Lehmann L, Kleber AG. Real time, confocal imaging of Ca2+ waves in arterially perfused rat hearts. Cardiovasc Res 53: 105– 115, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Boyden PA, Pu J, Pinto J, Keurs HE. Ca(2+) transients and Ca(2+) waves in purkinje cells: role in action potential initiation. Circ Res 86: 448– 455, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou CC, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, Fishbein MC, Lin SF, Chen PS. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation 111: 2889– 2897, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Chou CC, Zhou S, Tan AY, Hayashi H, Nihei M, Chen PS. High-density mapping of pulmonary veins and left atrium during ibutilide administration in a canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol 289: H2704– H2713, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Diaz ME, Trafford AW, O'Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol Online 501: 3– 16, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res 103: 509– 518, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Gannier F, White E, Garnier A, Le Guennec JY. A possible mechanism for large stretch-induced increase in [Ca2+]i in isolated guinea-pig ventricular myocytes. Cardiovasc Res 32: 158– 167, 1996 [PubMed] [Google Scholar]
- 8. Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol 7: 1024– 1038, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol 297: H1235– H1242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Housmans PR, Lee NK, Blinks JR. Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle. Science New York 221: 159– 161, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Kaneko T, Tanaka H, Oyamada M, Kawata S, Takamatsu T. Three distinct types of Ca(2+) waves in Langendorff-perfused rat heart revealed by real-time confocal microscopy. Circ Res 86: 1093– 1099, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Katoh H, Schlotthauer K, Bers DM. Transmission of information from cardiac dihydropyridine receptor to ryanodine receptor: evidence from BayK 8644 effects on resting Ca(2+) sparks. Circ Res 87: 106– 111, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Katra RP, Laurita KR. Cellular mechanism of calcium-mediated triggered activity in the heart. Circ Res 96: 535– 542, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Katra RP, Oya T, Hoeker GS, Laurita KR. Ryanodine receptor dysfunction and triggered activity in the heart. Am J Physiol Heart Circ Physiol 292: H2144– H2151, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Katra RP, Pruvot E, Laurita KR. Intracellular calcium handling heterogeneities in intact guinea pig hearts. Am J Physiol Heart Circ Physiol 286: H648– H656, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Lamont C, Luther PW, Balke CW, Wier WG. Intercellular Ca2+ waves in rat heart muscle. J Physiol 512: 669– 676, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res 92: 668– 675, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Laurita KR, Singal A. Mapping action potentials and calcium transients simultaneously from the intact heart. Am J Physiol Heart Circ Physiol 280: H2053– H2060, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Lou Q, Fedorov VV, Glukhov AV, Fast VG, Moazami N, Efimov IR. Abstract 2626: simultaneous transmural mapping of voltage and calcium in the human heart. Circulation 120: S667, 2009 [Google Scholar]
- 20. Maruyama M, Joung B, Tang L, Shinohara T, On YK, Han S, Choi EK, Kim DH, Shen MJ, Weiss JN, Lin SF, Chen PS. Diastolic intracellular calcium-membrane voltage coupling gain and postshock arrhythmias: role of purkinje fibers and triggered activity. Circ Res 106: 399– 408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365– 376, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Miura M, Boyden PA, ter Keurs HE. Ca2+ waves during triggered propagated contractions in intact trabeculae. Am J Physiol Heart Circ Physiol 274: H266– H276, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Mulder BJ, de Tombe PP, ter Keurs HE. Spontaneous and propagated contractions in rat cardiac trabeculae. J Gen Physiol 93: 943– 961, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen TP, Weiss JN. Coupling of isolated adult rabbit ventricular myocytes to fibroblasts under stress induces afterdepolarizations (Abstract). Heart Rhythm 6: 1693, 2009 [Google Scholar]
- 25. Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature 304: 735– 738, 1983 [DOI] [PubMed] [Google Scholar]
- 26. Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL, Cain ME. Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation 86: 1872– 1887, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation 98: 2404– 2414, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Prestle J, Dieterich S, Preuss M, Bieligk U, Hasenfuss G. Heterogeneous transmural gene expression of calcium-handling proteins and natriuretic peptides in the failing human heart. Cardiovasc Res 43: 323– 331, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103: 196– 200, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Wagner MB, Wang YG, Kumar R, Golod DA, Goolsby WN, Joyner RW. Measurements of calcium transients in ventricular cells during discontinuous action potential conduction. Am J Physiol Heart Circ Physiol 278: H444– H451, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Wakayama Y, Miura M, Sugai Y, Kagaya Y, Watanabe J, ter Keurs HE, Shirato K. Stretch and quick release of rat cardiac trabeculae accelerates Ca2+ waves and triggered propagated contractions. Am J Physiol Heart Circ Physiol 281: H2133– H2142, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Walker ML, Wan X, Kirsch GE, Rosenbaum DS. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation 108: 2704– 2709, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol 39: 419– 428, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Zhang YM, Miura M, ter Keurs HE. Triggered propagated contractions in rat cardiac trabeculae Inhibition by octanol and heptanol. Circ Res 79: 1077– 1085, 1996 [DOI] [PubMed] [Google Scholar]
- 35. Zhou S, Chang CM, Wu TJ, Miyauchi Y, Okuyama Y, Park AM, Hamabe A, Omichi C, Hayashi H, Brodsky LA, Mandel WJ, Ting CT, Fishbein MC, Karagueuzian HS, Chen PS. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol 283: H1244– H1252, 2002 [DOI] [PubMed] [Google Scholar]