Abstract
Endothelial cells exert an enormous influence on blood vessels throughout the circulation, but their impact is particularly pronounced in the brain. New concepts have emerged recently regarding the role of this cell type and mechanisms that contribute to endothelial dysfunction and vascular disease. Activation of the renin-angiotensin system plays a prominent role in producing these abnormalities. Both oxidative stress and local inflammation are key mechanisms that underlie vascular disease of diverse etiology. Endogenous mechanisms of vascular protection are also present, including antioxidants, anti-inflammatory molecules, and peroxisome proliferator-activated receptor-γ. Despite their clear importance, studies of mechanisms that underlie cerebrovascular disease continue to lag behind studies of vascular biology in general. Identification of endogenous molecules and pathways that protect the vasculature may result in targeted approaches to prevent or slow the progression of vascular disease that causes stroke and contributes to the vascular component of dementia and Alzheimer's disease.
Keywords: cardiovascular risk factors, angiotensin II, endothelium, oxidative stress, nitric oxide, cerebral blood flow, peroxisome proliferator-activated receptor-γ
the brain accounts for 20% of the body's oxygen consumption and 25% of total glucose utilization, despite being only 2% of body weight. Because the brain lacks energy reserves, normal cell function depends upon a level of perfusion that provides optimal nutrient delivery. Cerebral blood flow is highly regulated, involving multiple coordinated mechanisms. This regulation includes the integration of both regional and segmental changes in vascular tone, as well as major interactions between different cell types. This review focuses on the impact of endothelial cells under normal conditions and how endothelial dysfunction contributes to cerebrovascular disease.
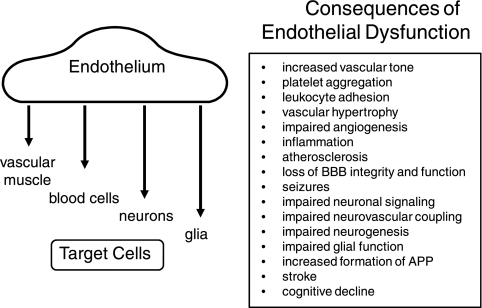
Endothelial cells exert diverse effects within the vessel wall and on nearby nonvascular target cells in the brain (Fig. 1). Endothelial dysfunction is a term commonly used to describe cell-based abnormalities that promote vasoconstriction, inflammation, increased permeability, atherosclerosis, and thrombosis. Endothelial dysfunction is perhaps the earliest event in the initiation of vascular disease (257) but continues to play a key role throughout the disease process. In brain, functional changes in endothelial cells contribute to reductions in resting blood flow (hypoperfusion), impairment of vasodilator responses, and subsequent cellular injury. Modest, but chronic reductions in cerebral blood flow (which do not produce ischemia) have functional consequences (127, 131, 141, 181). In addition to cerebrovascular disease and stroke, cardiovascular risk factors increase the likelihood of developing dementia and Alzheimer's disease (130). Evidence continues to accumulate supporting the link between vascular abnormalities and cognitive decline (130, 131, 141, 181). Despite its clear importance, studies of mechanisms that underlie cerebrovascular disease continue to lag behind studies of vascular biology in general, and the unique features of the cerebral circulation make it difficult to extrapolate findings from peripheral blood vessels.
Fig. 1.
Target cells affected by endothelium (left) and summary of the consequences of endothelial dysfunction in brain (right). See text for detailed discussion. BBB, blood-brain barrier; APP, amyloid precursor protein.
This review highlights features of cerebral endothelium and its role in regulation of cerebrovascular biology. The contributions of oxidative stress, inflammation, and angiotensin II (ANG II) in the pathogenesis of cerebrovascular disease are summarized. Finally, an overview of select mechanisms that protect against cerebrovascular disease is presented. Despite advances in prevention and treatment, the incidence of stroke and dementia continues to rise (201). Identification of endogenous molecules or pathways that protect the vasculature may lead to more specific cell-based therapeutic approaches to prevent or slow the progression of vascular disease that causes stroke and contributes to the vascular component of dementia and Alzheimer's disease.
Unique Features of Cerebral Blood Vessels
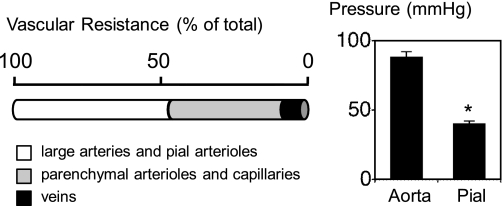
The cerebral circulation has many distinctive elements (51, 84, 131). In the context of this review, a few key features will be highlighted initially. The circulation of the brain is unique because large cerebral arteries and cerebral arterioles on the surface of the brain (pial vessels) collectively account for 50–60% of total cerebral vascular resistance (Fig. 2). Prior to the point where pial arterioles dive into the parenchyma, local intravascular pressure is approximately half of systemic blood pressure in the cerebral cortex (Fig. 2). Thus cerebral arteries and pial arterioles make major contributions to the regulation of cerebral blood flow and local microvascular perfusion pressure (93). In common experimental models as well as in humans, large cerebral arteries have substantial tone and react to diverse vasodilator stimuli including nitric oxide (NO), endothelium-dependent agonists, increases in blood flow, and hypercapnia (94, 103, 148, 248).
Fig. 2.
Left: distribution of vascular resistance in the cerebral circulation. Figure is based on measurements of intravascular pressure in multiple species and different segments of the circulation (20–24, 93, 118, 119). Right: mean arterial pressure in aorta and pial microcirculation in normal mice. Data for normal mice are from Baumbach et al. (23). *P < 0.05.
About one-third of vascular resistance resides in small blood vessels within the brain parenchyma (arterioles, capillaries, and small venules) (93, 118, 119) (Fig. 2). These vessels are components of what is often referred to as the neurovascular unit. In blood vessels both on the brain surface and within the parenchyma, multiple interactions between cell types, including endothelium, smooth muscle, and local neurons (intrinsic neurons or perivascular innervation from extrinsic nerves), occur (16, 51, 94, 107, 117, 132). In addition, astrocytes and cells comprising the glia limitans interact with nearby vessels (16, 132, 258). This segmental arrangement of vascular resistance necessitates well-coordinated integration between different vascular segments for optimal regulation of local microvascular pressure and blood flow (93, 103). Although clearly important, mechanisms that control communication along vascular segments, including between upstream and downstream blood vessels, remain poorly defined (103, 133).
The blood-brain barrier (BBB) is formed by a continuous layer of cerebral endothelial cells anchored to each other by an array of tight junction proteins that limit the entry of circulating cells and most blood-borne substances into brain (1, 51). The BBB is a unique structure with very specific functional characteristics (1). Although often discussed in relation to capillaries, the BBB is also functional in cerebral arteries and arterioles as well as cerebral venules (35, 51, 192, 205). Thus endothelial dysfunction can also involve loss of BBB integrity and function (205).
In addition to intracranial vessels, studies of the carotid artery are important in relation to defining mechanisms that underlie vascular disease and stroke. Although the carotid arteries are not major resistance vessels, these vessels are a primary site for development of atherosclerosis (carotid artery disease). Carotid artery disease is a major risk factor for ischemic stroke, being the cause of more than half of all strokes of this type (64, 172) (http://www.nhlbi.nih.gov/health/). Endothelial dysfunction is a fundamental component of mechanisms that initiate and contribute to the progression of atherosclerosis (227, 257). Carotid artery disease is a strong predictor of future vascular events, and quantification of disease in this vascular segment is used widely in both clinical and epidemiological studies to access the progression or regression of atherosclerosis (64, 172). Thus carotid arteries are studied clinically and are used in experimental models to define mechanisms that promote or protect against carotid artery disease.
Vascular Endothelium: One Cell to Rule Them All
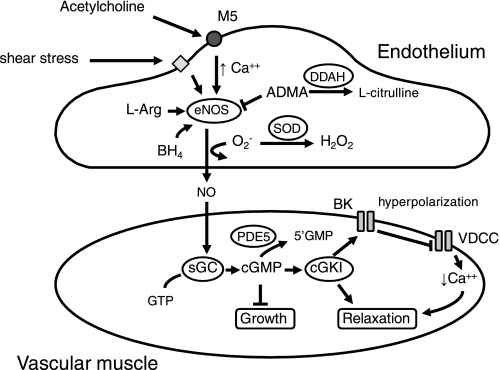
The study of endothelial cells and their impact within the vessel wall continues to occupy a major area in the study of vascular biology (100). Much of the influence of endothelium on other cells is mediated by release of diffusible factors (8, 33, 94, 269). A key mechanism by which endothelium communicates with target cells is via the production of NO by endothelial nitric oxide synthase (eNOS) (89, 94, 269) (Fig. 3). Through this mechanism, endothelium normally exerts a chronic dilator influence on vessels throughout the brain, ranging from large cerebral arteries to small arterioles within the parenchyma (3, 24, 53, 54, 94, 105, 269). A primary molecular target of NO is soluble guanylate cyclase (Fig. 3). NO binds to and activates soluble guanylate cyclase, increasing the synthesis of cGMP from GTP. Downstream effects of cGMP are largely mediated by cGMP-dependent protein kinase I, which produces reductions in intracellular calcium, decreases in vascular tone, and changes in gene expression (102). Most vascular effects of NO require activation of soluble guanylate cyclase (68, 98, 185, 217, 253, 254, 299).
Fig. 3.
Mechanisms regulating production of nitric oxide (NO) by endothelial nitric oxide synthase (eNOS) as well as signaling molecules activated by NO in vascular muscle. In response to receptor-mediated agonists such as acetylcholine acting on type 5 muscarinic receptors (M5) and other stimuli, NO is produced by eNOS from the substrate l-arginine (l-Arg). Some studies have concluded that increased shear stress also produces endothelium- and NO-dependent vasodilation. These processes require tetrahydrobiopterin (BH4). Activity of eNOS is reduced by the endogenous inhibitor asymmetric dimethylarginine (ADMA). Levels of ADMA are dependent on activity of dimethylarginine dimethylaminohydrolase (DDAH). By scavenging superoxide (O2−), superoxide dismutase (SOD) protects NO-mediated signaling. Within vascular muscle, NO activates soluble guanylate cyclase (sGC), which produces cGMP from GTP. A key molecular target for cGMP is cGMP-dependent protein kinase I (cGKI), which produces relaxation of vascular muscle via several mechanisms including activation of Ca2+-sensitive large-conductance potassium channels (BK), inhibition of rho kinase (not shown), and other mechanisms (102). Activation of BK channels produces local hyperpolarization, which inhibits voltage-dependent Ca2+ channels (VDCC). Steady-state levels of cGMP are also determined by activity of type 5 phosphodiesterase (PDE5).
In addition to influencing resting tone, endothelial cells mediate vasodilator responses to a diverse group of stimuli including neurotransmitters (e.g., acetylcholine and substance P), products released by platelets (e.g., ADP) or astrocytes, as well as mediators of injury or inflammation (e.g., bradykinin and histamine) (94, 204, 269). Vasodilator responses to many physiological stimuli as well as agents used therapeutically {e.g., corticosteroids and statins [3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors]} are mediated by endothelium-derived NO (3, 80, 82, 94, 96, 98, 126, 160, 166, 208, 219, 264, 269). Finally, neurovascular coupling in some brain regions (e.g., basal forebrain) is mediated by neuronally released acetylcholine acting on endothelium with activation of eNOS (295). The basal forebrain is a key region in relation to the development of dementia and Alzheimer's disease (234).
Substances such as acetylcholine are used commonly in both experimental models and humans as tools to examine endothelium-dependent regulation of vascular tone (94, 100). As this field of study has progressed, it has become apparent that this approach provides insight into the overall “health” of the endothelium as well. Impairment of endothelium-dependent vasodilation is predictive of clinical events and stroke, independent of differences in arterial pressure (100, 116, 276, 281). Genetic links between impaired endothelium-dependent vasodilation and stroke have been established (276). Thus, while studies of endothelium-dependent vasodilation are important in defining determinants of vascular tone, they also have broad implications for mechanisms that promote cerebrovascular disease and its contribution to stroke and dementia (Fig. 1).
Endothelium and eNOS do much more than influence vascular tone in the brain (Fig. 1). eNOS inhibits vascular hypertrophy (increase in cross-sectional area of the vessel wall) (24), promotes maintenance of native collateral vessels during maturation (58), and is required for normal vascular remodeling in responses to changes in blood flow (236). eNOS-derived NO affects local neuronal transmission (104) and inhibits amyloidogenic processing of amyloid precursor protein (17). Endothelial cells play a supportive role in neurogenic regions by promoting neurogenesis (161, 251). Endothelial cells also promote survival and growth of oligodendrocyte precursor cells (9). eNOS-deficient mice have larger infarcts, impaired angiogenesis, as well as impaired neurogenesis and recovery of neuronal function after stroke (44, 169). Endothelial dysfunction and loss of trophic support by endothelium-derived signaling molecules may contribute to the decline in neurogenesis that occurs with aging (161) or in the presence of other cardiovascular risk factors that produce oxidative stress in endothelium. Activity of eNOS suppresses local inflammation and associated complications during meningitis (153). Thus endothelial dysfunction in brain produces deleterious vascular effects but can also have other negative consequences that promote the progression of Alzheimer's disease and impair recovery after stroke and other forms of brain injury (Fig. 1).
In addition to eNOS, other endothelium-dependent mechanisms influence vasomotor tone (8, 33, 99, 274). Activation of calcium-sensitive potassium channels or transient receptor potential channels results in the release of a diffusible endothelium-derived hyperpolarizing factor (EDHF) that relaxes underlying smooth muscle (8, 33, 81, 105, 291). The exact identity of EDHF(s) remains controversial and may consist of a family of relaxing factors. Some studies have implicated K+ and hydrogen peroxide (H2O2) in this role (8, 88, 159, 198). A key related mechanism involves endothelium-dependent hyperpolarization of vascular muscle as the result of direct intercellular communication between cells via myoendothelial gap junctions (8, 272).
While there has been little direct evidence that EDHF or an “EDHF-like” mechanism affects basal tone in cerebral arteries, recent work suggests that such a mechanism influences resting tone in parenchymal arterioles (54). In the context of endothelial dysfunction, it is noteworthy that EDHF can compensate for the loss of NO-mediated responses during disease or brain injury (8, 33, 54, 111). Because most studies of EDHF have been performed in normal blood vessels, the overall impact of EDHF or EDHF-related mechanisms during cerebrovascular disease and particularly in vivo remains poorly defined.
How Common is Endothelial Dysfunction?
Vascular disease almost invariably includes end-organ damage or dysfunction at the level of the endothelial cell. Impairment of endothelium-dependent regulation of vascular tone has been described in diverse experimental models and in cerebral arteries from humans (94) (Table 1). All major cardiovascular risk factors are associated with endothelial dysfunction in brain. Although rarely studied, it is likely that combinations of cardiovascular risk factors (hypertension with aging, for example) interact with each other and accelerate endothelium-based cerebrovascular abnormalities.
Table 1.
Disease states, conditions, or stimuli that produce endothelial dysfunction in carotid artery and cerebral circulation
| Model | References |
|---|---|
| Acute hypertension | 280 |
| Aging | 121, 187, 188, 199, 220 |
| Alcohol | 260–262 |
| Alzheimer's disease | 134, 209, 210, 221, 222 |
| Angiotensin II | 39, 47, 49, 50, 69, 72 |
| Atherosclerosis | 67, 264 |
| Ceramide | 66 |
| Chronic hypertension | 76, 95, 108, 144, 152, 276, 288 |
| Cigarette smoke | 129 |
| Cigarette smoke extract | 294 |
| Diabetes | 10, 11, 73, 150, 270 |
| Endothelin | 142 |
| High-salt diet | 263 |
| Hypercholesterolemia | 136, 151, 196, 214, 287 |
| Hyperhomocysteinemia | 60, 233 |
| Hypoxia | 286 |
| Inflammation | 70, 124 |
| Insulin resistance | 85, 86 |
| Intermittent hypoxia | 224 |
| Ischemia with reperfusion | 53, 54, 207 |
| Metabolic syndrome | 225 |
| Methionine synthase deficiency | 61 |
| Microgravity | 298 |
| Nicotine | 87, 186 |
| PPARγ interference | 26,27 |
| Sickle-cell disease | 283, |
| SOD deficiency | 22, 75, 96, 198 |
| Subarachnoid hemorrhage | 138, 218 |
| Traumatic brain injury | 157 |
PPARγ, peroxisome proliferator-activated receptor-γ; SOD, superoxide dismutase.
In some models, endothelial dysfunction occurs earlier in the disease process or is larger in magnitude in the cerebral circulation than in blood vessels outside of the brain. Examples of such augmented vascular dysfunction have been described during aging (32, 199) as well as in models of hypertension (74, 76, 95, 197), diabetes (150), hyperhomocysteinemia (60), and high-fat diet feeding (27). Thus, although endothelium-based abnormalities can develop throughout the entire vascular tree, the circulation of the brain may be particularly susceptible to this key element of vascular disease.
Oxidative Stress: Cornerstone of Vascular Dysfunction
Oxidative stress occurs as the result of a shift in balance that favors the generation of oxygen-derived free radicals or reactive oxygen species (ROS) over various antioxidant defense mechanisms (92, 279). ROS have direct effects on vascular tone but also impair vasomotor responses to other stimuli (90, 92). The effects of ROS are prominent in the cerebral circulation. Cerebral blood vessels have the capacity to generate high levels of superoxide compared with peripheral blood vessels and are particularly sensitive to the effects of ROS (22, 48, 88, 197, 213). In the majority of the studies listed in Table 1, impairment of endothelium-dependent vasodilation was mediated by ROS.
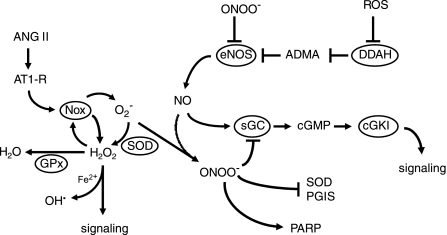
The species of ROS produced by many enzymatic sources is superoxide anion (Fig. 4). Superoxide has direct cellular effects but is also the precursor for other ROS and some reactive nitrogen species (Fig. 4). There are multiple sources of superoxide in the vasculature, including mitochondria, NADPH oxidase, cyclooxygenase (COX), and xanthine oxidase (48, 164). Mitochondria generate superoxide during oxidative phosphorylation. Cerebral endothelial cells are metabolically active, and the number of mitochondria present in cerebral endothelium is high compared with other cells (215). It is well established that COX is an important source of superoxide in the cerebral circulation (67, 156), and COX has been implicated as an important source of ROS in humans with essential hypertension (275). After oxidation of the cofactor tetrahydrobiopterin or disruption of its zinc-thiolate center, eNOS may reduce molecular oxygen, instead of l-arginine, producing superoxide instead of NO (88, 92). While a few studies provide evidence that eNOS “uncoupling” contributes to vascular dysfunction in models of disease (87, 261), the overall importance of this mechanism for cerebrovascular dysfunction remains to be defined.
Fig. 4.
Interactions between NO and reactive oxygen species (ROS). eNOS-derived NO signals via sGC, cGMP, and cGKI. By oxidizing BH4 or disrupting the zinc-thiolate center of the enzyme, peroxynitrite (ONOO−) produces uncoupling of eNOS, thus reducing NO production. Under these conditions, eNOS produces superoxide (O2−) instead of NO (not shown). Activity of eNOS can also be reduced by increased ADMA following ROS-mediated inhibition of DDAH. After activation of AT1 receptors (AT1-R) by angiotensin II (ANG II), NADPH oxidase (Nox) produces superoxide. Once formed, superoxide can directly produce injury (not shown), can be converted by SOD into H2O2, or can react with NO to form ONOO−. The NADPH oxidase containing Nox4 may produce H2O2 directly. In addition to being an important signaling molecule, H2O2 can form hydroxyl radical (OH•), a highly reactive mediator of cellular injury, in the presence of Fe2+. H2O2 can also be degraded by glutathione peroxidase (GPx). Once formed, peroxynitrite inhibits activity of SOD and prostacyclin synthase (PGIS) and activates poly(ADP-ribose) polymerases (PARP). See text for additional details.
Many studies have focused on the importance of NADPH oxidase in vascular disease. The catalytic domain of NADPH oxidase resides in its Nox subunit. Depending on species, cell type, and condition, different homologs of Nox are expressed in vascular cells, including Nox1, Nox2, Nox4, and Nox5 (48, 200). Impairment of endothelial function and neurovascular coupling is restored to normal with scavengers of superoxide, pharmacological inhibitors of NADPH oxidase, or genetic deletion of the Nox2 component of NADPH oxidase in models of aging, hypertension, hypercholesterolemia, sickle-cell disease, and Alzheimer's disease (109, 136, 144, 196, 220–222, 249, 283). Constriction in cerebral arteries in response to ANG II is dependent on expression of Nox2 (65). Collectively, these findings support the concept that NADPH oxidases are key promoters of oxidative stress. To date, most studies have focused on the role of the Nox2-containing NADPH oxidase. Cerebral arteries also express relatively high levels of Nox4 (197), which is thought to produce H2O2 rather than superoxide and thus may elicit different effects (91, 200). Other Nox-containing enzymes and Nox-dependent effects have been described (48, 200), but little is known regarding their importance in the cerebral circulation.
Once it is produced, how does superoxide affect vascular function? Superoxide can adversely affect mitochondrial and vascular function by the inactivation of proteins containing iron-sulfur centers (e.g., aconitase) (92). A key mechanism that has received considerable attention involves the interaction between NO and superoxide (Fig. 4). The first evidence that ROS impair endothelium-dependent vasodilation came from studies by Wei and Kontos and colleagues (280) demonstrating that endothelial dysfunction in pial arterioles following acute hypertension was mediated by ROS. NO reacts with superoxide at a near diffusion-limited rate. There are many examples of the interaction between these two molecules, with recent studies focusing primarily on defining the sources of superoxide, mechanisms that protect against such interactions, and the consequences of these interactions including effects on downstream targets. Several mechanisms account for ROS-induced vascular dysfunction. One such mechanism is loss of the effects of NO and cGMP-mediated signaling that would have been driven by NO (Fig. 3). This form of signalopathy alters vascular structure and function including effects on vascular cross-sectional area, resting tone, endothelium-dependent vasodilation, angiogenesis, and changes in BBB permeability (68, 98, 185, 217, 250, 253, 254, 299).
It is clear that superoxide also impairs vascular responses that occur independent of endothelial cells (12, 14, 37, 85, 86, 110, 144, 220–222, 225, 260). NO produced by neuronal NOS (nNOS) plays an important role in neurovascular coupling (16, 107). A large component of neurovascular coupling in the sensory cortex is dependent on nNOS-derived NO, while essentially the entire response is mediated by nNOS in the cerebellum (107). Like endothelium-dependent vasodilation, neurovascular coupling is impaired by superoxide (107, 144, 220–222). Reductions in resting blood flow and impairment of vasodilator responses as a result of oxidative stress may result in a mismatch between energy requirements, substrate delivery, and clearance of cellular by-products. Thus the loss of bioavailability of either eNOS- or nNOS-derived NO has far-reaching implications contributing to both short- and long-term consequences of vascular disease (48, 89, 130, 181).
Activity of rho kinase is a key determinant of vascular tone with cell-specific effects in the vasculature (170, 212, 250). In endothelium, rho kinase inhibits activity and expression of eNOS (34, 170). In vascular muscle, intracellular levels of calcium and sensitivity of contractile proteins to calcium determine the degree of actomyosin activation. Because of effects on calcium sensitivity and phosphorylation/dephosphorylation of the regulatory myosin light chain, rho kinase is a major determinant of vascular tone (34, 170). Rho kinase interacts with both NO and ROS. Via effects on cGMP-dependent protein kinase I, NO and cGMP inhibit rho kinase in vascular muscle (170, 242) (Fig. 3). As a consequence, reductions in NO increase the activity of rho kinase. Inhibition of NO production rapidly increases the influence of rho kinase on vascular tone (73).
As noted above, peroxynitrite is produced by the reaction of superoxide with NO (Fig. 4). Peroxynitrite is vasoactive (90, 178, 179) but also promotes cellular injury and impairs regulation of vascular tone via activation of poly(ADP-ribose) polymerases (PARP) and other effects (139, 178, 179) (Fig. 4). Activation of PARP contributes to endothelial dysfunction in models of disease and aging (10, 71, 110, 199). Oxidation of soluble guanylate cyclase by peroxynitrite reduces its responsiveness to NO, causing further loss of NO signaling (255) (Fig. 4). Other detrimental effects of peroxynitrite include reduced activity of prostacyclin synthase and superoxide dismutase (SOD; see below) as a result of nitration of tyrosine residues (90, 92) (Fig. 4).
Pleiotropic Effects of Angiotensin II
The renin-angiotensin system (RAS) plays a major role in vascular biology, particularly in relation to vascular disease (175, 180, 194, 230, 278). This system is a key contributor to pathophysiology in many models of hypertension and is a primary therapeutic target in patients with essential hypertension (2, 230). Chronic hypertension with activation of the RAS is associated with more cardiovascular events than other forms of hypertension (4).
The cerebral circulation is particularly sensitive to ANG II. Cerebral blood vessels are exposed to ANG II from both circulating and local (ANG II formed locally within the vessel wall and brain) sources. ANG II-mediated effects occur as a result of activation of specific receptors, and most of the detrimental effects of the peptide are mediated by AT1 receptors (Fig. 5) (11, 95, 109, 144, 175, 199, 238, 278).
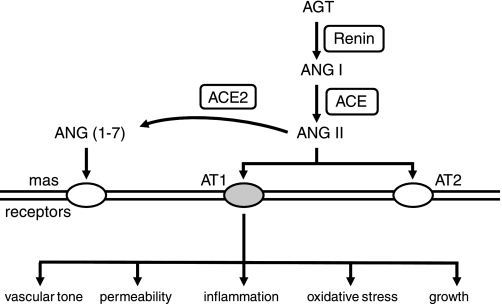
Fig. 5.
Components of the renin-angiotensin system. Receptors mediating responses to ANG II and angiotensin 1–7 (ANG 1–7) are shown, as well as consequences of activation of AT1 receptors. Both AT2 and mas receptors generally antagonize effects of activation of AT1 receptors. ACE, angiotensin-converting enzyme; AGT, angiotensinogen.
ANG II produces diverse effects including vasoconstriction, oxidative stress, inflammation, endothelial dysfunction, increases in permeability, impairment of neurovascular coupling, and altered vascular structure (5, 7, 23, 25, 37–39, 47, 49, 50, 59, 69, 72, 74, 76, 95, 101, 144, 158, 175, 240, 249, 266, 278, 296) (Fig. 5). In addition to its effects on upstream resistance vessels, ANG II affects the contractile state of pericytes (143) and thus may regulate local capillary blood flow in vivo. Effects of ANG II on superoxide formation, vasomotor tone, endothelial function, and neurovascular coupling are sex dependent (48, 65, 95, 108). Vascular effects of ANG II are much larger in male compared with female mice and are dependent on the phase of the estrus cycle in female mice (37, 49, 65, 95).
In addition to hypertension, ANG II and activation of AT1 receptors contribute to vascular disease under conditions without coexisting hypertension, including aging (199), diabetes (11, 270), atherosclerosis (59, 227), and in response to cigarette smoking (129). Increases in superoxide as well as impairment of neurovascular coupling and autoregulation are reduced by AT1 receptor inhibition in a model of Alzheimer's disease (266). This same study suggests that cognitive impairment in Alzheimer's disease may be mediated in part by ANG II (266). Similarly, loss of autoregulation following fluid-percussion injury in brain is restored toward normal after inhibition of AT1 receptors (18). Subarachnoid hemorrhage and focal cerebral ischemia increase both expression and functional effects of AT1 receptors in the vasculature (83). Inhibition of AT1 receptors improves local cerebral perfusion after cerebral ischemia (83, 137, 211). Autoantibodies against the AT1 receptor are thought to contribute to the pathogenesis of preeclampsia (125). After isolation from preeclamptic patients, these antibodies contract cerebral arteries via activation of AT1 receptors (289).
Because ANG II is a key contributor to vascular disease of diverse etiology, studies have focused on defining mechanisms that promote vascular dysfunction as well as endogenous mechanisms that protect against vascular abnormalities in ANG II-dependent models (Fig. 6). Experimental models used for these purposes include 1) testing direct effects of ANG II on isolated vessels or vascular cells, 2) testing effects of chronic administration of nonpressor or pressor doses of ANG II (administered systemically with osmotic minipumps), 3) studies of transgenic animals expressing components of the RAS, and 4) examining the role of endogenously produced ANG II in models of disease (e.g., spontaneously hypertensive rats).
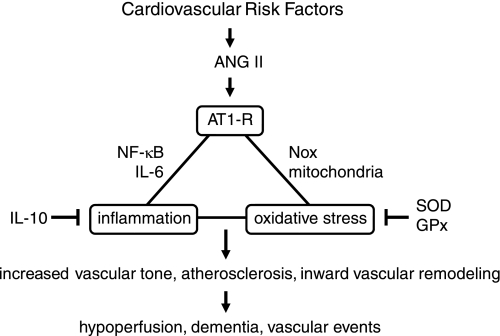
Fig. 6.
Mechanisms that promote ANG II-induced vascular disease. ANG II activates AT1 receptors, producing oxidative stress and activating components of the inflammatory response. Mechanisms that promote these cascades interact. Endogenous inhibitory molecules can also suppress effects of ANG II on oxidative stress and inflammation. Key consequences of vascular disease are shown at bottom. IL, interleukin. See text for additional details.
The ability of ANG II to promote oxidative stress has received considerable study in blood vessels outside the brain (194, 230, 278). In relation to the brain, increases in superoxide in response to ANG II are much larger in cerebral arteries compared with extracranial vessels (197). ANG II-induced oxidative stress produces endothelial dysfunction (47, 50, 72, 108–110, 249), loss of BBB integrity (101, 296), and impairment of neurovascular coupling (110, 144) as well as inward vascular remodeling (23). Constriction of cerebral arteries in response to ANG II is mediated by superoxide and rho kinase and is inhibited in Nox2-deficient mice (65, 95). Most of these effects occur independent of the effects of ANG II on arterial pressure (39, 49, 72, 249). In transgenic mice expressing human renin and angiotensinogen, impairment of endothelial function is greater in cerebral arteries than in carotid arteries or aorta (74, 76, 95). In genetically hypertensive rats, inhibition of angiotensin-converting enzyme or AT1 receptors reduces the levels of superoxide and inflammation, restores endothelium-dependent responses, and improves autoregulation (7, 158, 240, 288).
Significant progress has been made in relation to defining how ANG II contributes to vascular disease (Fig. 6). Contributions of ANG II to oxidative stress are due in large part to activation of NADPH oxidase. ANG II increases vascular expression of components of NADPH oxidase and levels of superoxide (72). ANG II-induced endothelial dysfunction is prevented by inhibition or genetic deletion of AT1 receptors (109, 238), pharmacological scavengers of superoxide (49, 72, 95, 144), or genetic deletion of Nox2 (65, 109, 144, 249).
Members of the calcitonin family (calcitonin gene-related peptide, intermedin, and adrenomedullin) exert antioxidant effects on vascular cells in culture including inhibitory effects on NADPH oxidase (168, 246, 290). Signaling by these peptides depends upon the expression of receptor activity-modifying proteins (RAMPs) (226). Overexpression of RAMP2 protects against vascular inflammation and hypertrophy in a model of ANG II-dependent hypertension (165), while overexpression of RAMP1 protects against ANG II-induced oxidative stress and vascular dysfunction (50). Thus, although details of their interactions are unclear, other peptide and protein families modulate oxidative stress following activation of AT1 receptors.
A key feature of oxidative stress is that once it is initiated, ROS or peroxynitrite can feed forward, promoting additional oxidative stress. Several mechanisms may contribute to these effects (Fig. 4). First, angiotensinogen is cleaved by renin to produce ANG I (the precursor to ANG II). Oxidized angiotensinogen is more readily cleaved than the nonoxidized form (300). Thus, in an oxidative environment, the oxidized form of angiotensinogen may be more prevalent so ANG II-induced oxidative stress may feed forward, promoting further production of ANG II. Through posttranslational modification of cysteine thiols in angiotensinogen, NO may protect against these effects (300). Second, once produced, superoxide can be converted by SODs to H2O2, which then activates NADPH oxidase in vascular cells (88). Thus H2O2 can stimulate formation of additional superoxide (Fig. 4). Third, as noted above, peroxynitrite can uncouple eNOS (92, 203) (Fig. 4). Fourth, oxidative and nitrosative stress can reduce activity of SOD through nitrosylation or other effects (92) (Fig. 4).
Additional mechanisms may contribute to ANG II-induced vascular dysfunction. ANG II can reduce synthesis of NO by causing mislocalization and tyrosine phosphorylation of eNOS (106, 171). Formation of endothelium-derived contracting factors (EDCFs) has been described in cerebral arteries in response to ANG II, in models of vascular disease, and in hypertensive humans (76, 94, 177, 191, 275). In many cases, EDCF has been suggested to be a prostanoid produced by COX (274). Consistent with this concept, a COX-derived EDCF and activation of thromboxane receptors contribute to impaired endothelial function in spontaneously hypertensive rats and a genetic model of ANG II hypertension (76, 94, 191). In humans with essential hypertension, COX-derived prostanoids and ROS contribute to endothelial dysfunction (275). An EDCF appears to be functionally important in vertebral arteries from hypertensive humans (43). The powerful vasoconstrictor endothelin-1 is another EDCF that impairs endothelial function and produces marked reductions in blood flow and vasospasm (13, 56, 94, 142, 275). Interactions between endothelin and ANG II have been described (154). For example, ANG II stimulates endothelin-1 production, and some effects of ANG II can be mediated by endothelin-1 (154). Interactions between formation of EDCF and oxidative stress exist. Both increased levels of ROS and reductions in NO bioavailability enhance EDCF-mediated responses (274). Activation of thromboxane receptors can increase endothelial production of superoxide and peroxynitrite through stimulation of NADPH oxidase and uncoupling of eNOS (297). In addition, COX-1 and prostaglandin E2 EP1 receptors can also contribute to ANG II-induced cerebrovascular dysfunction (38).
Impact of Angiotensin II on Vascular Structure
Hypertension is associated with hypertrophy in aorta and large conduit arteries but inward remodeling (with or without increases in vessel cross-sectional area) in smaller resistance vessels (20, 23). Inward remodeling represents a rearrangement of the vessel wall around a smaller lumen (20, 23). This form of remodeling has been described in cerebral vessels in some models of hypertension and in hypertensive people (20, 23, 231). Both inward remodeling and vascular hypertrophy (if it encroaches on the vessel lumen) impair vasodilator capacity. Of these two changes, inward remodeling has the greatest impact on lumen diameter (20). Inward vascular remodeling can have either detrimental or protective effects, depending upon the circumstances (Fig. 7). During hypertension, inward remodeling of upstream vessels protects the distal microcirculation from full transmission of pressure, thus attenuating vascular dysfunction when local pressure rises (Fig. 7). Such a mechanism protects the BBB during acute hypertension in chronically hypertensive animals (190). In contrast, inward vascular remodeling increases minimal resistance, resulting in reduced vasodilator responses, lower levels of perfusion pressure, and impaired blood flow in collateral-dependent regions (Fig. 7). For these reasons, inward vascular remodeling increases the susceptibility to focal ischemia. Importantly, inward remodeling has emerged as a potential risk factor for cardiovascular events and cerebrovascular disease (122, 202).
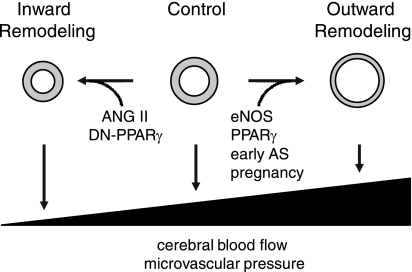
Fig. 7.
Blood vessels (shown in cross section) under control conditions and after inward and outward remodeling. Both ANG II and dominant-negative (DN) forms of PPARγ produce inward remodeling. Activation of eNOS, PPARγ, pregnancy, and early atherosclerosis (AS) produce outward remodeling. Inward remodeling increases vascular resistance and impairs vasodilator capacity but can protect the distal microcirculation during acute hypertension by reducing the transmission of pressure. Outward remodeling reduces vascular resistance and can augment blood flow. In contrast to inward remodeling, outward remodeling increases the transmission of pressure to the distal microcirculation and capillaries. Bottom: ramp illustrates conceptually how vascular remodeling affects maximal dilator capacity (and thus cerebral blood flow) and local microvascular pressure. See text for additional details.
ANG II produces inward remodeling independent of changes in blood pressure (23, 42) (Fig. 7). Despite similar levels of hypertension, cerebral arterioles undergo hypertrophy with inward remodeling during ANG II-dependent hypertension but only hypertrophy in ANG II-independent hypertension (23). In preliminary studies, chronic treatment with a nonpressor dose of ANG II caused inward remodeling of cerebral arterioles without a change in vessel cross-sectional area, while infusion of a pressor dose of ANG II produced inward remodeling with vascular hypertrophy (42). These changes did not occur in Nox2-deficient mice, indicating a critical role for NADPH oxidase (42). Thus inward remodeling is a unique response to ANG II. Inward vascular remodeling is present in a genetic model of ANG II-dependent hypertension (23) and, as would be expected, is accompanied by greater susceptibility to focal cerebral ischemia (45).
Endogenous Antioxidants
An array of antioxidant enzymes are expressed in vascular cells. These enzymes reside in different subcellular compartments and include SODs and glutathione peroxidases (GPx) (92, 173, 279). One of the simplest approaches to examine the impact of oxidative stress involves testing effects of genetic deletion of individual antioxidant genes. Studies utilizing mice with genetic alterations of specific isoforms of SOD or GPx have revealed key roles for these enzymes in protecting the vasculature under normal conditions as well as in models of disease and aging (Fig. 6).
SOD1 (CuZn-SOD) is the predominant form of SOD in the vessel wall, accounting for approximately two-thirds of total SOD activity (75, 92). SOD1 is localized in the cytosol and portions of the mitochondria and within the cell nucleus (92). Genetic deficiency in SOD1 increases vascular superoxide, augments vasoconstrictor responses, and impairs endothelium-dependent dilation in cerebral arteries and in the brain microcirculation (22, 75, 96, 198). Because they convert superoxide to H2O2, SODs are a major source of H2O2 (88, 92) (Fig. 4). Effects of arachidonic acid on vascular tone are greatly impaired in animals lacking SOD1 (198), consistent with the concept that ROS, and more specifically H2O2, mediates this response. Increased expression of SOD1 protects against endothelial dysfunction in response to ANG II and ceramide (a sphingolipid signaling molecule) and in models of inflammation and Alzheimer's disease (66, 69, 70, 134), while decreased expression of SOD1 augments loss of BBB integrity following cerebral ischemia (155).
ROS have important effects on the growth of vascular cells and the structure of blood vessels. Deficiencies in SOD1 increase superoxide and produce marked hypertrophy in cerebral arterioles (22), demonstrating that a key role for SOD1 is normally to inhibit this form of vascular growth. This function of SOD1 is particularly prominent, as the magnitude of hypertrophy in cerebral arterioles in SOD1-deficient animals is greater than that seen in other models in which vascular hypertrophy occurs, including models with lifelong hypertension (20–23).
Mitochondria are a key source of ROS that affect the vasculature (282). Complete deficiency in the mitochondrial form of SOD (SOD2, manganese SOD) is lethal, so genetic studies of SOD2 have focused on phenotypes present in heterozygous deficient mice (92). Partial deficiency in SOD2 enhances vasoconstrictor responses and impairs endothelium-dependent dilation in the microcirculation under baseline conditions (96). In addition, deficiency in SOD2 predisposes to increased oxidative stress and endothelial dysfunction in response to ANG II as well as during aging and hypercholesterolemia (32, 49, 214). In addition, interactions or cross talk between mitochondria, mitochondria-derived ROS, and other sources of superoxide including NADPH oxidase have emerged (78, 147, 284).
Studies on the impact of antioxidants and eNOS highlight an important issue regarding the use of genetically altered mice (22, 47, 49, 69, 71, 96, 155, 160, 214). When defining mechanisms, an extremely valuable approach is to use animals in which both copies of a gene have been deleted through gene targeting. However, in relation to understanding disease, models in which only one gene copy is absent (heterozygotes) can also be very insightful (265). Partial reductions in the expression or activity of a protein during disease or as the result of a genetic polymorphism are more likely to occur than complete loss of function of that same protein. It is noteworthy that multiple phenotypes have been described in both SOD1 and SOD2 heterozygote mice (22, 32, 49, 69, 71, 96, 155, 214). For example, while effects on endothelial function at baseline are not detectable, partial SOD1 deficiency accelerates oxidative stress and vascular dysfunction with aging and in response to ANG II (49, 69, 71). Similarly, complete loss of GPx1 expression produces marked endothelial dysfunction (47). In contrast, a partial deficiency in GPx1 does not affect vascular function under control conditions but increases sensitivity to ANG II-induced vascular dysfunction by ∼10-fold (47). Thus partial reductions in expression of GPx-1 or SOD1 or SOD2 predispose to ANG II-induced vascular dysfunction (Fig. 6). Consistent with these findings, activity of GPx is inversely related to the risk for cardiovascular events in people (28, 173), supporting the concept that H2O2 plays a key role in vascular disease.
In relation to translational approaches that may protect the vasculature from oxidative stress, it is possible to enhance expression of endogenous antioxidants within the vessel wall. Expression of SOD1 can be increased by virus-based gene transfer (292) and exercise (237) and with pharmacological approaches including treatment with erythropoietin or exogenous activators of peroxisome proliferator-activated receptor-γ (PPARγ) (79, 135).
Putting the Brakes on Vascular Inflammation
Components of the inflammatory response are activated within the vasculature during aging and in many diseases including atherosclerosis, hypertension, and diabetes (57, 72, 180, 243, 257). Nuclear factor-κB (NF-κB) is a key transcription factor that promotes inflammation (57, 180). Through activation of NF-κB and other mechanisms, ANG II and ROS increase expression of proinflammatory cytokines, adhesion molecules, chemokines, toll-like receptors, inducible NOS (iNOS), and matrix metalloproteinases (31, 57, 72, 140, 175, 180, 296), all of which are known contributors to vascular disease. ANG II-dependent hypertension increases vascular expression of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and p22phox (a component of NADPH oxidase) (73). Increases in superoxide, vascular dysfunction, and vascular hypertrophy in response to ANG II are greatly reduced in IL-6-deficient mice (249). Overall, these studies and others support the concept that after activation of AT1 receptors, inflammation-related mechanisms act in synergy with oxidative stress to promote vascular disease (Fig. 6) (175, 180, 278).
While proinflammatory mechanisms promote the progression of vascular disease, endogenous mechanisms are also present to limit the impact of vascular inflammation. The anti-inflammatory cytokine IL-10 plays a key role in this regard (36, 112–114, 176, 267). Through effects on IκB kinase activity, NF-κB degradation, and DNA-binding activity, IL-10 is a potent inhibitor of NF-κB-mediated effects (15) (Fig. 6). Many of the protective effects of IL-10 may be through this mechanism (15, 228). Endogenous IL-10 attenuates increases in superoxide, expression of iNOS, and vascular dysfunction in response to lipopolysaccharide and during diabetes (36, 112–114, 176). IL-10 also protects against thrombosis and progression of atherosclerosis (36, 176).
In relation to ANG II, treatment with exogenous IL-10 protects against ANG II-induced endothelial dysfunction (293). Genetic deletion of IL-10 greatly increases vascular sensitivity to ANG II (72). Endogenous IL-10 limits the expression of IL-6 and p22phox and increases in superoxide and protects against endothelial dysfunction in models of ANG II-induced vascular dysfunction (72) (Fig. 6). These findings provide additional support for the concept that ANG II is proinflammatory and that IL-10 is a key protective molecule in vascular disease. These findings are also consistent with the work of Harrison and others implicating a key role for the immune system and T cells in contributing to the pathogenesis of hypertension (120). In this regard, it is noteworthy that IL-10 strongly inhibits T-cell function (15).
Other mechanisms can suppress vascular inflammation. For example, eNOS-derived NO decreases activation of NF-κB and expression of proinflammatory genes (63). Thus reductions in the bioavailability of NO during oxidative stress promote vascular inflammation. IL-10 may contribute to this mechanism as well, as the cytokine increases eNOS expression following activation of the transcription factor signal transducer and activator of transcription-3 (41).
Dimethylarginine Dimethylaminohydrolase: Protecting eNOS
Oxidative stress can also produce vascular dysfunction as a result of oxidant-induced inactivation of dimethylarginine dimethylaminohydrolase (DDAH), the enzyme that degrades asymmetric dimethylarginine (ADMA) (Figs. 3 and 4). ADMA is an endogenously produced inhibitor of eNOS, and circulating levels of ADMA increase in many disease states including hypertension (29, 273). ADMA is taken up and accumulates in endothelial cells, so intracellular concentrations may be much higher than circulating levels (40).
A major determinant of ADMA levels is the activity of DDAH, which accounts for most of the clearance of ADMA (29, 273). Thus DDAH protects the vasculature by preventing increases in local ADMA concentrations. Importantly, DDAH is an oxidant-sensitive enzyme whose activity is reduced during oxidative stress (167, 244, 245). In addition, ADMA may contribute to the uncoupling of eNOS (273). Thus reductions in the activity of DDAH as well as increased levels of ADMA may contribute to oxidative stress-induced vascular disease (Fig. 4).
Physiologically relevant concentrations of ADMA inhibit activity of NOS, increase vascular tone (via inhibition of basal production of NO), and impair endothelial function in the cerebral circulation (91). ADMA reduces cerebral perfusion in humans (149). Overexpression of DDAH in transgenic mice reduces plasma levels of ADMA, increases basal levels of vascular NO, and protects against ADMA-induced endothelial dysfunction in brain (62). Hypertrophy of cerebral arterioles during hyperhomocysteinemia is prevented in DDAH transgenic mice (233).
Peroxisome Proliferator-Activated Receptor-γ
PPARγ is a member of the nuclear hormone receptor superfamily that forms heterodimers with retinoid X receptors and functions as a ligand-activated transcription factor (146, 247, 252) (Fig. 8). Although it was initially thought that key roles for PPARγ were limited to adipocyte function, as well as glucose and lipid metabolism (247), it is now clear that PPARγ is expressed and is functional in many cell types including many within the vasculature. PPARγ regulates the expression of clusters of target genes through several mechanisms, including binding to PPAR-response elements and protein-protein interactions with other transcription factors including NF-κB (247, 252).
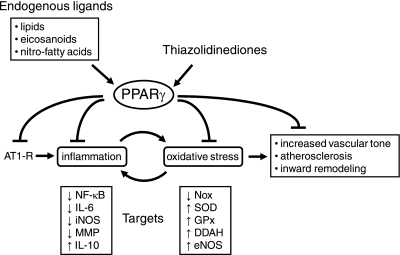
Fig. 8.
Effects of PPARγ on vascular cells. PPARγ exerts inhibitory effects on AT1 receptors, oxidative stress, and inflammation. After activation, PPARγ functions as a ligand-activated transcription factor that affects many targets. Endogenous ligands for PPARγ include lipids, eicosanoids, and nitro-fatty acids. Thiazolidinediones (TZDs) are exogenous ligands used clinically to treat type 2 diabetes. The select targets of PPARγ listed here are based on studies using TZDs. MMP, matrix metalloproteinases.
Endogenous ligands that activate PPARγ include lipids, eicosanoids, and nitro-fatty acids (247, 277) (Fig. 8). Thiazolidinediones (TZDs)(e.g., rosiglitazone and pioglitazone) are synthetic activators of PPARγ used to treat type 2 diabetes (162). Studies using vascular cells in culture or extracranial blood vessels have described numerous protective effects of TZDs (146, 239, 252) (Fig. 8). In relation to effects of ANG II on the vasculature, both antioxidant and anti-inflammatory effects of TZDs have been observed, including reductions in the expression of AT1 receptors, components of NADPH oxidase (Nox1, Nox2, and Nox4), and IL-6 (77, 128, 135, 140, 146, 259). In contrast, TZDs increase expression of SOD1 and IL-10 (135, 268) and the activity of eNOS (146) (Fig. 8). Expression of PPARγ in atherosclerotic carotid arteries highly correlates with expression of IL-10 (30). While TZDs can activate PPARγ, ANG II may inhibit PPARγ-mediated effects by increasing activity of Brc kinase, resulting in phosphorylation and reductions in PPARγ transcriptional activity (5). The consequence of reduced transcriptional activity of PPARγ is increased activation of NF-κB and local inflammation (5).
In relation to the cerebral circulation, PPARγ is expressed in endothelium in the carotid artery and brain vessels of humans and other species (182, 229). In the carotid artery and cerebral vasculature, TZDs have beneficial effects on both endothelial function and regulation of vascular tone, as well as on vascular permeability in models of Alzheimer's disease and hypertension (52, 206, 209, 210, 232, 239). TZDs prevent inward vascular remodeling during hypertension (52) and produce outward remodeling of cerebral arterioles in nonpregnant animals, thus mimicking changes that occur during pregnancy (55) (Fig. 7). In contrast, treatment with an inhibitor of PPARγ in pregnant animals prevents outward vascular remodeling (55). In spontaneously hypertensive rats, pioglitazone reduced superoxide and both the expression and activity of NADPH oxidase, improved endothelial function, and reversed vascular remodeling in cerebral arteries and arterioles (206). Vascular dysfunction in the brain in aged mice overexpressing amyloid precursor protein, a model of Alzheimer's disease, was restored toward normal by TZD treatment (209, 210). These findings suggest that pharmacological activation of PPARγ has protective effects on cerebrovascular structure and function.
An underlying assumption of TZD-based studies is that the effects observed are mediated by PPARγ. However, TZDs can have off-target or PPARγ-independent effects as well (46, 223, 235). Because systemic administration of TZDs affects cellular metabolism, it is not always clear whether phenotypes produced are due to direct vascular effects of PPARγ or indirect changes that result from other effects of whole body TZD treatment.
Patients with dominant-negative mutations (V290M or P467L) in the ligand-binding domain of PPARγ exhibit early-onset hypertension (19). These mutations inhibit transcriptional activity of wild-type PPARγ (19, 145) and exert effects on vascular gene expression that are the opposite of those produced by TZDs (19, 145). Mice that express dominant-negative forms of PPARγ provide a novel approach to interference with wild-type PPARγ and thus allow insight into the importance of endogenous PPARγ without TZD treatment. In addition to being clinically relevant mutations, these models mimic reductions in activity of PPARγ described in disease states and with other genetic polymorphisms (123). In heterozygous knockin mice with an equivalent PPARγ mutation (P465L, designated L/+) (271), oxidative stress and endothelial dysfunction occur in cerebral arteries and arterioles, but not in the aorta or carotid artery (Ref. 26; unpublished observations). Cerebral arterioles in L/+ mice exhibit hypertrophy with inward remodeling (26). As expected, these vascular changes are accompanied by greater susceptibility to ischemia (285). Consistent with these findings, neointimal proliferation in the carotid artery following mechanical injury is augmented in L/+ mice (195). Thus interference with PPARγ has pronounced effects in the cerebral circulation that mimic effects of ANG II including oxidative stress, endothelial dysfunction, and inward remodeling. Collectively, these findings suggest that the balance between the activity of PPARγ and effects of ANG II may be a key determinant of the progression of cerebrovascular disease (Fig. 8).
Studies to this point had not examined cell-specific effects of PPARγ in the vasculature in vivo. Cell-specific alterations in PPARγ avoid potential metabolic effects seen with global genetic manipulation of PPARγ or whole body TZD treatment. To advance beyond this limitation, mice expressing dominant-negative mutations in PPARγ in either smooth muscle or endothelium have been developed (27, 115). In mice expressing V290M under control of the endothelium-specific vascular cadherin promoter, expression of NADPH oxidase and SOD1 were increased and decreased, respectively (27). As shown by responses to acetylcholine, endothelial function was not altered under baseline conditions in these mice (27). In contrast, feeding of a high-fat diet for 12 wk produced superoxide-mediated reductions in responses to acetylcholine in the basilar artery but not in the aorta (27). Thus interference with PPARγ in endothelium predisposes the cerebrovasculature to oxidative stress and endothelial dysfunction in a model of diet-induced obesity.
To examine the role of PPARγ in vascular muscle, transgenic mice expressing a dominant-negative form of PPARγ under the control of the smooth muscle myosin heavy chain promoter were developed (115). Cerebral arterioles in these mice underwent hypertrophy with increased distensibility and inward remodeling (115). Preliminary studies indicate that cerebrovascular function is markedly impaired after genetic interference with PPARγ in vascular muscle (97). Thus studies of several mouse models expressing dominant-negative mutations suggest that PPARγ is active under basal conditions (in the absence of TZD treatment), exerting a particularly profound influence on the cerebral circulation.
In relation to therapy, PPARγ is particularly attractive because rather than targeting a single molecule or pathway, PPARγ affects many target genes and ultimately may mediate protective effects at multiple levels (Fig. 8). Genetic variations in PPARγ genotypes are associated with carotid artery disease (6). Thus cell-based strategies to suppress the progression of cerebrovascular disease using approaches that retain beneficial but reduce detrimental or off-target effects of current pharmacological activators of PPARγ may have substantial potential.
Conclusions
Vascular endothelium plays a major role in the cerebral circulation in both health and disease. The impact of endothelium on its multiple target cells is particularly prominent and quite diverse in the brain. All major cardiovascular risk factors are associated with endothelial dysfunction. Both oxidative stress and local inflammation are key mechanisms that promote vascular disease. Activation of the RAS and AT1 receptors plays a prominent role in producing these abnormalities. In contrast, endogenous molecules, including antioxidants, IL-10, DDAH, and PPARγ, protect vascular cells by limiting these changes. A more precise understanding of mechanisms that promote or protect against cell-based vascular dysfunction may result in targeted approaches that prevent or delay the progression of cerebrovascular disease.
GRANTS
Work summarized here from the author's laboratory was supported by National Institutes of Health Grants HL-38901, HL-62984, and NS-24621 and by a Bugher Foundation Award in Stroke from the American Heart Association (0575092N).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The author is indebted to the students, postdoctoral fellows, and research assistants who contributed to these studies. He also thanks his mentors and collaborators for their many contributions to these efforts. In particular, the author acknowledges Dr. Curt Sigmund for his part in many collaborative studies including recent work on PPARγ. Finally, thanks to Dr. Marilyn Cipolla for all her help including critical evaluation of this manuscript.
REFERENCES
- 1. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 37: 13–25, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: role of Ang II. Semin Nephrol 24: 101–114, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Ahmed E, Hamel E. Muscarinic—but not nicotinic—acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab 20: 298–305, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 324: 1098–1104, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Alexis JD, Wang N, Che W, Lerner-Marmarosh N, Sahni A, Korshunov VA, Zou Y, Ding B, Yan C, Berk BC, Abe J. Bcr kinase activation by angiotensin II inhibits peroxisome proliferator-activated receptor γ transcriptional activity in vascular smooth muscle cells. Circ Res 104: 69–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Shali KZ, House AA, Hanley AJG, Khan HMR, Harris SB, Zinman B, Mamakeesick M, Fenster A, Spence JD, Hegele RA. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor γ associated with carotid atherosclerosis. Stroke 35: 2036–2040, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Ando H, Zhou J, Macova M, Imboden H, Saavedra JM. Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke 35: 1726–1731, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Andresen JJ, Shafi NI, Bryan RM. Endothelial influences on cerebrovascular tone. J Appl Physiol 100: 318–327, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci 29: 4351–4355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arrick DM, Sharp GM, Sun H, Mayhan WG. Diabetes-induced cerebrovascular dysfunction: role of poly(ADP-ribose) polymerase. Microvasc Res 73: 1–6, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Arrick DM, Sharp GM, Sun H, Mayhan WG. Losartan improves impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type 1 diabetic rats. Brain Res 1209: 128–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arrick DM, Mayhan WG. Acute infusion of nicotine impairs nNOS-dependent reactivity of cerebral arterioles via an increase in oxidative stress. J Appl Physiol 103: 2062–2067, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Arrick DM, Mayhan WG. Inhibition of endothelin-1 receptors improves impaired nitric oxide synthase-dependent dilation of cerebral arterioles in type-1 diabetic rats. Microcirculation 17: 439–446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res 1184: 365–371, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—a review of a new approach. Pharmacol Rev 55: 241–269, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 107: 1498–1502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baranov D, Armstead WM. Selective blockade of AT1 receptor attenuates impairment of hypotensive autoregulation and improves cerebral blood flow after brain injury in the newborn pig. Anesthesiology 99: 1118–1124, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402: 880–883, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13: 968–972, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Baumbach GL, Sigmund CD, Bottiglieri T, Lentz SR. Structure of cerebral arterioles in cystathione β-synthase deficient mice. Circ Res 91: 931–937, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Baumbach GL, Sigmund CD, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke 37: 1850–1855, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension 41: 50–55, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Baumbach GL, Sigmund CD, Faraci FM. Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ Res 95: 822–829, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2: 247–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange W, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension 51: 867–871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with PPARγ causes cerebral vascular dysfunction in response to a high fat diet. Circ Res 103: 654–661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 349: 1605–1613, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Boger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med 38: 126–136, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadski C, Jude B, Torpler G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARγ activation primes human monocytes into alternative M2 monocytes with anti-inflammatory properties. Cell Metab 6: 137–143, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Brasier AR. The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86: 211–218, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Bryan RM, Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology 102: 1261–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci 27: 97–104, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anesthetized rats: a developmental study. J Physiol 429: 47–62, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med 9: 10–17, 2003 [PMC free article] [PubMed] [Google Scholar]
- 37. Capone C, Anrather J, Milner TA, Iadecola C. Estrous cycle-dependent neurovascular dysfunction induced by angiotensin II in the mouse neocortex. Hypertension 54: 302–307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension 55: 911–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol 300: H397–H407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Cattaruzza M, Slodowski W, Stojakovic M, Krzesz R, Hecker M. Interleukin-10 induction of nitric-oxide synthase expression attenuates CD40-mediated interleukin-12 synthesis in human endothelial cells. J Biol Chem 278: 37874–37880, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Chan SL, Baumbach GL. Nox2 deficiency prevents angiotensin II-induced hypertrophy and inward remodeling in cerebral arterioles (Abstract). FASEB J 23: 613.11, 2009. 18824519 [Google Scholar]
- 43. Charpie JR, Schreur KD, Papadopoulos SM, Webb RC. Acetylcholine induces contraction in vertebral arteries from treated hypertensive patients. Clin Exp Hypertens 18: 87–99, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci 25: 2366–2375, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen S, Li G, Zhang W, Wang J, Sigmund CD, Olson JE, Chen Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am J Physiol Regul Integr Comp Physiol 297: R1526–R1531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric oxide synthase phosphorylation by prolonged treatment with troglitizone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPAR gamma-independent signaling pathways. J Biol Chem 279: 2499–2506, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension 51: 872–877, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med 14: 495–502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chrissobolis S, Faraci FM. Sex differences in protection against angiotensin II-induced endothelial dysfunction by manganese superoxide dismutase in the cerebral circulation. Hypertension 55: 905–910, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chrissobolis S, Zhang Z, Kinzenbaw DA, Lynch CM, Russo AF, Faraci FM. Receptor activity-modifying protein-1 augments cerebrovascular responses to calcitonin gene-related peptide and inhibits angiotensin II-induced vascular dysfunction. Stroke 41: 2329–2334, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cipolla MJ. The cerebral circulation. In: Integrated Systems Physiology: From Molecule to Function, edited by Granger DN, Granger J. San Rafael, CA: Morgan & Claypool Life Sciences, 2010, p. 1–59 [Google Scholar]
- 52. Cipolla MJ, Bishop N, Vinke RS, Godfrey JA. PPARγ activation prevents hypertensive remodeling of cerebral arteries and improves vascular function in female rats. Stroke 41: 1266–1270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation 15: 495–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40: 1451–1457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol 110: 329–339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crowley RW, Medel R, Kassell NF, Dumont AS. New insights into the causes and therapy of cerebral vasospasm following subarachnoid hemorrhage. Drug Discov Today 13: 254–260, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Inflammation and endothelial dysfunction during aging: role of NF-κB. J Appl Physiol 105: 1333–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res 106: 1870–1881, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1a receptor. Circulation 110: 3849–3857, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 35: 1957–1962, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Dayal S, Devlin AM, McCaw RB, Liu ML, Arning E, Bottiglieri T, Shane B, Faraci FM, Lentz SR. Cerebral vascular dysfunction in methionine synthase-deficient mice. Circulation 112: 737–744, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Dayoub H, Radionov RN, Lynch C, Cooke JP, Arning E, Bottiglieri T, Lentz SR, Faraci FM. Overexpression of dimethylarginine dimethylaminohydrolase inhibits asymmetric dimethylarginine-induced endothelial dysfunction in the cerebral circulation. Stroke 39: 180–184, 2008 [DOI] [PubMed] [Google Scholar]
- 63. De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Groot E, van Leuven SI, Duivenvoorden R, Meuwese MC, Akdim F, Bots ML, Kastelein JJ. Measurement of carotid intima-media thickness to assess progression and regression of atherosclerosis. Nat Rev Cardiol 5: 280–288, 2008 [DOI] [PubMed] [Google Scholar]
- 65. De Silva TM, Broughton BR, Drummond GR, Sobey CG, Miller AA. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke 40: 1091–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Didion SP, Faraci FM. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler Thromb Vasc Biol 25: 90–95, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol 281: H1697–H1703, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Didion SP, Heistad DD, Faraci FM. Mechanisms that produce nitric oxide-mediated relaxation of cerebral arteries during atherosclerosis. Stroke 32: 761–766, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Didion SP, Kinzenbaw DA, Faraci FM. Genetically-altered mice reveal a critical role for CuZnSOD in preventing angiotensin-II-induced endothelial dysfunction. Hypertension 46: 1147–1153, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Didion SP, Kinzenbaw DA, Fegan PR, Didion LA, Faraci FM. Overexpression of CuZn-SOD prevents lipopolysaccharide-induced endothelial dysfunction. Stroke 35: 1963–1967, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 48: 1072–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 54: 619–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Didion SP, Lynch C, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of rho-kinase in cerebral arterioles in type II diabetes. Stroke 36: 342–347, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Didion SP, Ryan MJ, Baumbach GL, Sigmund CD, Faraci FM. Superoxide contributes to vascular dysfunction in mice that express human renin and angiotensinogen. Am J Physiol Heart Circ Physiol 283: H1569–H1576, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res 91: 938–944, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Didion SP, Sigmund CD, Faraci FM. Impaired endothelial function in transgenic mice expressing both human renin and human angiotensinogen. Stroke 31: 760–764, 2000 [DOI] [PubMed] [Google Scholar]
- 77. Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-γ. Circulation 105: 2296–302, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. d'Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase. Hypertension 55: 998–1004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. d'Uscio LV, Smith LA, Santhanam AV, Richardson D, Nath KA, Katusic ZS. Essential role of endothelial nitric oxide synthase in vascular effects of erythropoietin. Hypertension 49: 1142–1148, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Earley S, Gonzalez AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res 104: 987–994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 95: 8880–8885, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Edvinsson L. Cerebrovascular angiotensin AT1 receptor regulation in cerebral ischemia. Trends Cardiovasc Med 18: 98–103, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Edvinsson L, Krause D. Cerebral Blood Flow and Metabolism (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins, 2001 [Google Scholar]
- 85. Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes 53: 1352–1359, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol 290: H1264–H1270, 2006 [DOI] [PubMed] [Google Scholar]
- 87. Fang Q, Sun H, Mayhan WG. Impairment of nitric oxide synthase-dependent dilatation of cerebral arterioles during infusion of nicotine. Am J Physiol Heart Circ Physiol 284: H528–H534, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Faraci FM. Hydrogen peroxide: watery fuel for change in vascular biology. Arterioscler Thromb Vasc Biol 26: 1931–1933, 2006 [DOI] [PubMed] [Google Scholar]
- 89. Faraci FM. Protecting the brain with eNOS: run for your life. Circ Res 99: 1029–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 90. Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol 100: 739–743, 2006 [DOI] [PubMed] [Google Scholar]
- 91. Faraci FM, Brian JE, Heistad DD. Response of cerebral blood vessels to an endogenous inhibitor of nitric oxide synthase. Am J Physiol Heart Circ Physiol 269: H1522–H1527, 1995 [DOI] [PubMed] [Google Scholar]
- 92. Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 66: 8–17, 1990 [DOI] [PubMed] [Google Scholar]
- 94. Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev 78: 53–97, 1998 [DOI] [PubMed] [Google Scholar]
- 95. Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: new insights from genetic models. J Cereb Blood Flow Metab 26: 449–455, 2006 [DOI] [PubMed] [Google Scholar]
- 96. Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol 100: 2089–2093, 2006 [DOI] [PubMed] [Google Scholar]
- 97. Faraci FM, Modrick ML, Lynch CM, Pelham CJ, Ketsawatsomkron P, Sigmund CD. Vasodilation to nitric oxide is markedly impaired following selective interference with PPARγ in vascular muscle (Abstract). Stroke 42: e42, 2011 [Google Scholar]
- 98. Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol Heart Circ Physiol 274: H564–H570, 1998 [DOI] [PubMed] [Google Scholar]
- 99. Feletou M, Kohler R, Vanhoutte PM. Endothelium-derived vasoactive factors and hypertension: possible roles in pathogenesis and as treatment targets. Curr Hypertens Rep 12: 267–275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Flammer AJ, Luscher TF. Three decades of endothelium research. From the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular disease. Swiss Med Wkly 140: w13122, 2010 [DOI] [PubMed] [Google Scholar]
- 101. Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT1 receptor in brain endothelial cells. J Cereb Blood Flow Metab 29: 640–647, 2009 [DOI] [PubMed] [Google Scholar]
- 102. Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res 69: 697–705, 1991 [DOI] [PubMed] [Google Scholar]
- 104. Garthwaite G, Bartus K, Malcolm D, Goodwin D, Kollb-Sielecka M, Dooldeniya C, Garthwaite J. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci 26: 7730–7740, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Geddawy A, Shimosato T, Tawa M, Imamura T, Okamura T. Comparison of endothelium-related responses to nucleotides of dog and monkey cerebral arteries. J Pharmacol Sci 112: 378–381, 2010 [DOI] [PubMed] [Google Scholar]
- 106. Gerzanich V, Ivanova S, Zhou H, Simard JM. Mislocalization of eNOS and upregulation of cerebral vascular Ca2+ channel activity in angiotensin-hypertension. Hypertension 41: 1124–1130, 2003 [DOI] [PubMed] [Google Scholar]
- 107. Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am J Physiol Heart Circ Physiol 294: H156–H163, 2008 [DOI] [PubMed] [Google Scholar]
- 109. Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2-derived radicals. Arterioscler Thromb Vasc Biol 26: 826–832, 2006 [DOI] [PubMed] [Google Scholar]
- 110. Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27: 303–309, 2007 [DOI] [PubMed] [Google Scholar]
- 111. Golding EM, You J, Robertson CS, Bryan RM., Jr Potentiated endothelium-derived hyperpolarizing factor-mediated dilations in cerebral arteries following mild head injury. J Neurotrauma 18: 691–697, 2001 [DOI] [PubMed] [Google Scholar]
- 112. Gunnett CA, Berg DJ, Faraci FM. Vascular effects of lipopolysaccharide are enhanced in interleukin-10-deficient mice. Stroke 30: 2191–2196, 1999 [DOI] [PubMed] [Google Scholar]
- 113. Gunnett CA, Heistad DD, Berg DJ, Faraci FM. Interleukin-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol 279: H1555–H1562, 2000 [DOI] [PubMed] [Google Scholar]
- 114. Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects endothelium-dependent relaxation during diabetes: role of superoxide. Diabetes 51: 1931–1937, 2002 [DOI] [PubMed] [Google Scholar]
- 115. Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ in smooth muscle causes vascular dysfunction and hypertension. Cell Metab 7: 215–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 118. Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension 6: 408–419, 1984 [DOI] [PubMed] [Google Scholar]
- 119. Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulaton in rats. Am J Physiol Heart Circ Physiol 246: H17–H24, 1984 [DOI] [PubMed] [Google Scholar]
- 120. Harrison DG, Guzik TJ, Lob H, Madhur M, Marvar PJ, Thabet S, Vinh A, Weyand C. Inflammation, immunity, hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hatake K, Kakishita E, Wakabayashi I, Sakiyama N, Hishida S. Effect of aging on endothelium-dependent vascular relaxation of isolated human basilar artery to thrombin and bradykinin. Stroke 21: 1039–1043, 1990 [DOI] [PubMed] [Google Scholar]
- 122. Heagerty AM. Predicting hypertension complications from small artery structure. J Hypertens 25: 939–940, 2007 [DOI] [PubMed] [Google Scholar]
- 123. Heikkinen S, Auerx J, Argmann CA. PPARγ in human and mouse physiology. Biochim Biophys Acta 1771: 999–1013, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hernanz R, Briones AM, Alonso MJ, Vila E, Salaices M. Hypertension alters role of iNOS, COX-2, and oxidative stress in bradykinin impairment after LPS in rat cerebral arteries. Am J Physiol Heart Circ Physiol 287: H225–H234, 2004 [DOI] [PubMed] [Google Scholar]
- 125. Herse F, Staff AC, Hering L, Muller DN, Luft FC, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med 86: 697–703, 2008 [DOI] [PubMed] [Google Scholar]
- 126. Hiroi Y, Kim HH, Ying H, Furuya F, Huang Z, Simoncini T, Noma K, Ueki K, Nguyen NH, Scanlan TS, Moskowitz MA, Cheng SY, Liao JK. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA 103: 14104–14109, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hossmann KA. Variability thresholds and the penumbra of focal ischemia. Ann Neurol 36: 557–565, 1994 [DOI] [PubMed] [Google Scholar]
- 128. Hwang J, Kleinhenz DJ, Rupnow HL, Campbell AG, Thule PM, Sutliff RL, Hart CM. The PPAR ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vascul Pharmacol 46: 456–462, 2007 [DOI] [PubMed] [Google Scholar]
- 129. Iida H, Iida M, Takenaka M, Fujiwara H, Dohi S. Angiotensin type 1 (AT1)-receptor blocker prevents impairment of endothelium-dependent cerebral vasodilation by acute cigarette smoking in rats. Life Sci 78: 1310–1316, 2006 [DOI] [PubMed] [Google Scholar]
- 130. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 120: 287–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab 7: 476–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Iadecola C, Nedergaard M. Glia regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007 [DOI] [PubMed] [Google Scholar]
- 133. Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol 78: 651–659, 1997 [DOI] [PubMed] [Google Scholar]
- 134. Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fisher E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2: 157–161, 1999 [DOI] [PubMed] [Google Scholar]
- 135. Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T, Katayama S. The ligands/activators for peroxisome proliferator-activated receptor α (PPARα) and PPARγ increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism 50: 3–11, 2001 [DOI] [PubMed] [Google Scholar]
- 136. Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia. Roles of NADPH oxidase and P-selectin. Circ Res 94: 239–244, 2004 [DOI] [PubMed] [Google Scholar]
- 137. Ito T, Yamakawa H, Bregonzio C, Terron JA, Falcon-Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke 33: 2297–2303, 2002 [DOI] [PubMed] [Google Scholar]
- 138. Iuliano BA, Pluta RM, Jung C, Oldfield EH. Endothelial dysfunction in a primate model of cerebral vasospasm. J Neurosurg 100: 287–294, 2004 [DOI] [PubMed] [Google Scholar]
- 139. Jagtop P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4: 421–440, 2005 [DOI] [PubMed] [Google Scholar]
- 140. Jones A, Deb R, Torsney E, Howe F, Dunkley M, Gnaneswaran Y, Gaze D, Nasr H, Loftus IM. Rosiglitazone reduces the development and rupture of experimental aortic aneurysm. Circulation 119: 3125–3132, 2009 [DOI] [PubMed] [Google Scholar]
- 141. Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrett B, Domenga V, Schedl A, Lacombe P, Hubner N. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 120: 433–445, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kaito N, Onoue H, Abe I. Suppression of cerebral vasodilation with endothelin-1. Peptides 16: 1127–1132, 1995 [DOI] [PubMed] [Google Scholar]
- 143. Kamouchi M, Kitazono T, Ago T, Wakisaka M, Ooboshi H, Ibayashi S, Iida M. Calcium influx pathways in rat CNS pericytes. Mol Brain Res 126: 114–120, 2004 [DOI] [PubMed] [Google Scholar]
- 144. Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 145. Keen HL, Halabi CM, Beyer AM, de Lange WJ, Liu X, Maeda N, Faraci FM, Casavant TL, Sigmund CD. Bioinformatic analysis of gene sets regulated by ligand-activated and dominant-negative PPARγ in mouse aorta. Arterioscler Thromb Vasc Biol 30: 518–525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ketsawatsomkron P, Pelham CJ, Groh S, Keen HL, Faraci FM, Sigmund CD. Does peroxisome proliferator-activated receptor γ (PPARγ) protect from hypertension directly through effects in the vasculature? J Biol Chem 285: 9311–9316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kidoguchi K, Tamaki M, Mizobe T, Koyama J, Kondoh T, Kohmura E, Sakurai T, Yokono K, Umetani K. In vivo X-ray angiography in the mouse brain using synchrotron radiation. Stroke 37: 1856–1861, 2006 [DOI] [PubMed] [Google Scholar]
- 149. Kielstein JT, Donnerstag F, Gasper S, Menne J, Kielstein A, Martens-Lobenhoffer J, Scalera F, Cooke JP, Fliser D, Bode-Boger SM. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke 37: 2024–2029, 2006 [DOI] [PubMed] [Google Scholar]
- 150. Kitayama J, Faraci FM, Gunnett CA, Heistad DD. Impairment of dilator responses of cerebral arterioles during diabetes mellitus: role of inducible NO synthase. Stroke 37: 2129–2133, 2006 [DOI] [PubMed] [Google Scholar]
- 151. Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke 38: 2136–2141, 2007 [DOI] [PubMed] [Google Scholar]
- 152. Kitazono T, Heistad DD, Faraci FM. Dilatation of the basilar artery in response to selective activation of endothelin B receptors in vivo. J Pharmacol Exp Ther 273: 1–6, 1995 [PubMed] [Google Scholar]
- 153. Koedel U, Paul R, Winkler F, Kastenbauer S, Huang PL, Pfister HW. Lack of endothelial nitric oxide synthase aggravates murine pneumococcal meningitis. J Neuropathol Exp Neurol 60: 1041–1050, 2001 [DOI] [PubMed] [Google Scholar]
- 154. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci 17: 4180–4189, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kontos HA. Role of products of univalent reduction of oxygen in hypertensive vascular injury. In: Hypertension: Pathophysiology, Diagnosis, and Management, edited by Laragh JH, Brenner BM. New York: Raven, 1990, chapt. 44, p. 667–675 [Google Scholar]
- 157. Kontos HA, Wei EP. Endothelium-dependent responses after experimental brain injury. J Neurotrauma 9: 349–354, 1992 [DOI] [PubMed] [Google Scholar]
- 158. Kumai Y, Ooboshi H, Ago T, Ishikawa E, Takada J, Kamouchi M, Kitazono T, Ibayashi S, Iida M. Protective effect of angiotensin II type 1 receptor blocker on cerebral circulation independent of blood pressure. Exp Neurol 210: 441–448, 2008 [DOI] [PubMed] [Google Scholar]
- 159. Lacza Z, Puskar M, Kis B, Perciaccante JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol 283: H406–H411, 2002 [DOI] [PubMed] [Google Scholar]
- 160. Lamping KG, Faraci FM. Role of sex differences and effects of endothelial NO synthase deficiency in responses of carotid arteries to serotonin. Arterioscler Thromb Vasc Biol 21: 523–528, 2001 [DOI] [PubMed] [Google Scholar]
- 161. Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci 33: 569–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An anti-diabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma. J Biol Chem 270: 12953–12956, 1995 [DOI] [PubMed] [Google Scholar]
- 163. Leiper JM, Vallance P. The synthesis and metabolism of asymmetric dimethylarginine (ADMA). Eur J Clin Pharmacol 62, Suppl 13: 33–38, 2006 [Google Scholar]
- 164. Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med 47: 1673–1706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Liang L, Tam CW, Pozsgai G, Siow R, Clark N, Keeble J, Husmann K, Born W, Fischer JA, Poston R, Shah A, Brain SD. Protection of angiotensin II-induced vascular hypertrophy in vascular smooth muscle-targeted receptor activity-modifying protein 2 transgenic mice. Hypertension 54: 1254–1261, 2009 [DOI] [PubMed] [Google Scholar]
- 166. Limbourg FP, Huang Z, Plumier JC, Simoncini T, Fujioka M, Tuckermann J, Schutz G, Moskowitz MA, Liao JK. Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J Clin Invest 110: 1729–1738, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106: 987–992, 2002 [DOI] [PubMed] [Google Scholar]
- 168. Liu J, Shimosawa T, Matsui H, Meng F, Supowit SC, DiPette DJ, Ando K, Fujita T. Adrenomedullin inhibits angiotensin II-induced oxidative stress via Csk-mediated inhibition of Src activity. Am J Physiol Heart Circ Physiol 292: H1714–H1721, 2007 [DOI] [PubMed] [Google Scholar]
- 169. Lo EH, Hara H, Rogowska J, Trocha M, Pierce AR, Huang PL, Fishman MC, Wolf GL, Moskowitz MA. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke 27: 1381–1385, 1996 [DOI] [PubMed] [Google Scholar]
- 170. Loirand G, Pacuad P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol 7:637–647, 2010 [DOI] [PubMed] [Google Scholar]
- 171. Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med 206: 2889–2896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115: 459–467, 2007 [DOI] [PubMed] [Google Scholar]
- 173. Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal (November 18, 2010). doi: 10.1089/ars.2010.3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lund DD, Brooks RM, Faraci FM, Heistad DD. Role of angiotensin II in endothelial dysfunction induced by lipopolysaccharide in mice. Am J Physiol Heart Circ Physiol 293: H3726–H3731, 2007 [DOI] [PubMed] [Google Scholar]
- 175. MacKenzie A. Endothelium-derived vasoactive agents, AT1 receptors and inflammation. Pharmacol Ther (November 27, 2010). doi: 10.1016/j.pharmthera.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 176. Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res 85: e17–e24, 1999 [DOI] [PubMed] [Google Scholar]
- 177. Manabe K, Shirahase H, Usui H, Kurahasi K, Fujiwara M. Endothelium-dependent contractions induced by angiotensin I and angiotensin II in canine cerebral artery. J Pharmacol Exp Ther 251: 317–320, 1989 [PubMed] [Google Scholar]
- 178. Maneen MJ, Cipolla MJ. Peroxynitrite diminishes myogenic tone in cerebral arteries: role of nitrotyrosine and F-actin. Stroke 37: 894–899, 2006 [DOI] [PubMed] [Google Scholar]
- 179. Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1042–H1050, 2007. 17040976 [Google Scholar]
- 180. Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29: 367–374, 2008 [DOI] [PubMed] [Google Scholar]
- 181. Marshall RS, Lazar RM. Pumps, aqueducts, and drought management. Vascular physiology in vascular cognitive impairment. Stroke 42: 221–226, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPAR gamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPAR gamma as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol 19: 546–551, 1999 [DOI] [PubMed] [Google Scholar]
- 183. Matsumoto T, Kobayahi T, Wachi H, Seyama Y, Kamata K. Vascular NAD(P)H oxidase mediates endothelial dysfunction in basilar arteries from Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Atherosclerosis 192: 15–24, 2007 [DOI] [PubMed] [Google Scholar]
- 184. Mayhan WG. Role of prostaglandin H2-thromboxane A2 in responses of cerebral arterioles during chronic hypertension. Am J Physiol Heart Circ Physiol 262: H539–H543, 1992 [DOI] [PubMed] [Google Scholar]
- 185. Mayhan WG. VEGF increases permeability of the blood-brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am J Physiol Cell Physiol 276: C1148–C1153, 1999 [DOI] [PubMed] [Google Scholar]
- 186. Mayhan WG, Arrick DM, Sharpe GM, Sun H. Nitric oxide synthase-dependent responses of the basilar artery during acute infusion of nicotine. Nicotine Tob Res 11: 270–2277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation 15: 225–236, 2008 [DOI] [PubMed] [Google Scholar]
- 188. Mayhan WG, Faraci FM, Baumbach GL, Heistad DD. Effects of aging on response of cerebral arterioles. Am J Physiol Heart Circ Physiol 258: H1138–H1143, 1990 [DOI] [PubMed] [Google Scholar]
- 189. Mayhan WG, Faraci FM, Heistad DD. Impairment of endothelium-dependent responses of cerebral arterioles in chronic hypertension. Am J Physiol Heart Circ Physiol 253: H1435–H1440, 1987 [DOI] [PubMed] [Google Scholar]
- 190. Mayhan WG, Faraci FM, Heistad DD. Mechanisms of protection of the blood-brain barrier during acute hypertension in chronically hypertensive rats. Hypertension 9: III101–III105, 1987 [DOI] [PubMed] [Google Scholar]
- 191. Mayhan WG, Faraci FM, Heistad DD. Responses of cerebral arterioles to adenosine 5′-diphosphate, serotonin, and the thromboxane analogue U-46619 during chronic hypertension. Hypertension 12: 556–561, 1988 [DOI] [PubMed] [Google Scholar]
- 192. Mayhan WG, Heistad DD. Role of veins and cerebral venous pressure in disruption of the blood-brain barrier. Circ Res 59: 216–220, 1986 [DOI] [PubMed] [Google Scholar]
- 193. McNeish AJ, Dora KA, Garland CJ. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke 36: 1526–1532, 2005 [DOI] [PubMed] [Google Scholar]
- 194. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007 [DOI] [PubMed] [Google Scholar]
- 195. Meredith D, Panchatcharam M, Miriyala S, Tsai YS, Morris AJ, Maeda N, Stouffer GA, Smyth SS. Dominant-negative loss of PPARγ function enhances smooth muscle cell proliferation, migration, and vascular remodeling. Arterioscler Thromb Vasc Biol 29: 465–471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Miller AA, De Silva TM, Judkins CP, Drummond GR, Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke 41: 784–789, 2010 [DOI] [PubMed] [Google Scholar]
- 197. Miller AA, Drummond GR, Schmidt HH HW, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res 97: 1055–1062, 2005 [DOI] [PubMed] [Google Scholar]
- 198. Modrick ML, Didion SP, Lynch CM, Dayal S, Lentz SR, Faraci FM. Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J Cereb Blood Flow Metab 29: 1130–1137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 296: H1914–H1919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Montezano AC, Burger D, Ceravolo GS, Yusaf H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120: 131–141, 2011 [DOI] [PubMed] [Google Scholar]
- 201. Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 67: 181–198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Mulvany MJ. Small artery structure: time to take note? Am J Hypertens 20: 853–854, 2007 [DOI] [PubMed] [Google Scholar]
- 203. Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J 31: 2741–2749, 2010 [DOI] [PubMed] [Google Scholar]
- 204. Murphy S, Rich G, Orgren KI, Moore SA, Faraci FM. Astrocyte-derived lipoxygenase product evokes endothelium-dependent relaxation of the basilar artery. J Neurosci Res 38: 314–318, 1994 [DOI] [PubMed] [Google Scholar]
- 205. Nag S, Kapadia A, Stewart DJ. Molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol 37: 3–23, 2011 [DOI] [PubMed] [Google Scholar]
- 206. Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke 38: 3016–3022, 2007 [DOI] [PubMed] [Google Scholar]
- 207. Nelson CW, Wei EP, Povlishock JT, Kontos HA, Moskowitz MA. Oxygen radicals in cerebral ischemia. Am J Physiol Heart Circ Physiol 263: H1356–H1362, 1992 [DOI] [PubMed] [Google Scholar]
- 208. Ngai AC, Winn HR. Modulation of cerebral arteriolar diameter by intraluminal flow and pressure. Circ Res 77: 832–840, 1995 [DOI] [PubMed] [Google Scholar]
- 209. Nicolakakis N, Aboulkassim T, Aliaga A, Tong XK, Rosa-Neto P, Hamel E. Intact memory in TGF-β1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazone. J Cereb Blood Flow Metab 31: 200–211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci 28: 9287–9296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 31: 2478–2486, 2000 [DOI] [PubMed] [Google Scholar]
- 212. Nunes KP, Rigsby CS, Webb RC. RhoA/rho-kinase and vascular diseases: what is the link? Cell Mol Life Sci 67: 3823–3836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ogawa K, Tokinaga Y, Iwahashi S, Mizumoto K, Hatano Y. Halothane does not protect against vascular injury in isolated cerebral and mesenteric arteries. Can J Anaesth 52: 870–877, 2005 [DOI] [PubMed] [Google Scholar]
- 214. Ohashi M, Runge MS, Faraci FM, Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 26: 2331–2336, 2006 [DOI] [PubMed] [Google Scholar]
- 215. Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1: 409–417, 1977 [DOI] [PubMed] [Google Scholar]
- 216. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 340: 14–22, 1999 [DOI] [PubMed] [Google Scholar]
- 217. Onoue H, Katusic ZS. The effect of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and charybdotoxin (CTX) on relaxations of isolated cerebral arteries to nitric oxide. Brain Res 785: 107–113, 1998 [DOI] [PubMed] [Google Scholar]
- 218. Onoue H, Tsutsui M, Smith L, Stelter A, O'Brien T, Katusic ZS. Expression and function of recombinant endothelial nitric oxide synthase gene in canine basilar artery after experimental subarachnoid hemorrhage. Stroke 29: 1959–1966, 1998 [DOI] [PubMed] [Google Scholar]
- 219. Paravicini TM, Miller AA, Drummond GR, Sobey CG. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3 kinase, NADPH oxidase, and nitric oxide synthase. J Cereb Blood Flow Metab 26: 836–845, 2006 [DOI] [PubMed] [Google Scholar]
- 220. Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab 27: 1908–1918, 2007 [DOI] [PubMed] [Google Scholar]
- 221. Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid β peptide. J Neurosci 25: 1769–1777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105: 1347–1352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Peraza M, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activator receptors (PPAR). Toxicol Sci 90: 269–295, 2006 [DOI] [PubMed] [Google Scholar]
- 224. Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 286: H388–H393, 2004 [DOI] [PubMed] [Google Scholar]
- 225. Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R522–R530, 2005 [DOI] [PubMed] [Google Scholar]
- 226. Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fisher JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 54: 233–246, 2002 [DOI] [PubMed] [Google Scholar]
- 227. Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature 451: 904–913, 2008 [DOI] [PubMed] [Google Scholar]
- 228. Raines EW, Garton KJ, Ferri N. Beyond the endothelium: NF-κB regulation of smooth muscle function. Circ Res 94: 706–708, 2004 [DOI] [PubMed] [Google Scholar]
- 229. Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor gamma (PPAR gamma) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. J Immunol 180: 1854–1865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol 284: R893–R912, 2003 [DOI] [PubMed] [Google Scholar]
- 231. Rizzoni D, De Ciuceis C, Porteri E, Paiardi S, Boari GEM, Mortini P, Cornali C, Cenzato M, Rodella LF, Borsani E, Rizzardi N, Platto C, Rezzani R, Rosei EA. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens 27: 838–845, 2009 [DOI] [PubMed] [Google Scholar]
- 232. Roberts TJ, Chapman AC, Cipolla MJ. PPAR-gamma agonist rosiglitazone reverses increased cerebral venous hydraulic conductivity during hypertension. Am J Physiol Heart Circ Physiol 297: H1347–H1353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Rodionov RN, Dayoub H, Lynch CM, Wilson KM, Stevens JW, Murry DJ, Kimoto M, Arning E, Bottiglieri T, Cooke JP, Baumbach GL, Faraci FM, Lentz SR. Overexpression of dimethylarginine dimethylaminohydrolase protects against cerebral vascular effects of hyperhomocysteinemia. Circ Res 106: 551–558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Roman GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging 27: 1769–1785, 2006 [DOI] [PubMed] [Google Scholar]
- 235. Roszer T, Ricote M. PPARs in the renal regulation of systemic blood pressure. PPAR Res 2010: 698730, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Rush JWE, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 238. Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension 43: 1074–1079, 2004 [DOI] [PubMed] [Google Scholar]
- 239. Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. The PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension 43: 661–666, 2004 [DOI] [PubMed] [Google Scholar]
- 240. Saavedra JM, Sanchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology 36: 1–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Santhanam AVR, Smith LA, Nath KA, Katusic ZS. In vivo stimulatory effect of erythropoietin on endothelial nitric oxide synthase in cerebral arteries. Am J Physiol Heart Circ Physiol 291: H781–H786, 2006 [DOI] [PubMed] [Google Scholar]
- 242. Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohman SM, Berotglio J, Chardin P, Pacuad P. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 275: 21722–21729, 2000 [DOI] [PubMed] [Google Scholar]
- 243. Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 112: 375–384, 2007 [DOI] [PubMed] [Google Scholar]
- 244. Scalera F, Martens-Lobenhoffer J, Bukowska A, Lendeckel U, Tager M, Bode-Boger SM. Effect of telmisartan on nitric oxide-asymmetrical dimethylarginine system. Role of angiotensin II type 1 receptor and peroxisome proliferator activated receptor γ signaling during endothelial aging. Hypertension 51: 696–703, 2008 [DOI] [PubMed] [Google Scholar]
- 245. Scalera F, Borlak J, Beckmann B, Martens-Lobenhoffer J, Thum T, Tager M, Bode-Boger SM. Endogenous nitric oxide synthesis inhibitor asymmetric dimethyl l-arginine accelerates endothelial cell senescence. Arterioscler Thromb Vasc Biol 24: 1816–1822, 2004 [DOI] [PubMed] [Google Scholar]
- 246. Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim Biophys Acta 1643: 65–73, 2003 [DOI] [PubMed] [Google Scholar]
- 247. Schmidt MV, Bernhard B, Von Knethen A. The nuclear hormone receptor PPARγ as a therapeutic target in major diseases. ScientificWorldJournal 10: 2181–2197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Schoonman GG, Van der Grond J, Kortmann C, Van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation—a 3T magnetic resonance angiography study. Brain 131: 2192–2200, 2008 [DOI] [PubMed] [Google Scholar]
- 249. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II-induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol 27: 2576–2581, 2007 [DOI] [PubMed] [Google Scholar]
- 250. Schreibelt G, Kooij G, Reijerkerk A, Van Doorn R, Gringhuis SI, Van der Pol S, Weksler BB, Romera IA, Courand PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21: 3666–3676, 2007 [DOI] [PubMed] [Google Scholar]
- 251. Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304: 1338–1340, 2004 [DOI] [PubMed] [Google Scholar]
- 252. Sigmund CD. Endothelial and vascular muscle PPARγ in arterial pressure regulation. Lessons from genetic interference and deficiency. Hypertension 55: 437–444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Sobey CG, Cocks TM. Activation of protease-activated receptor-2 (PAR-2) elicits nitric oxide-dependent dilatation of the basilar artery in vivo. Stroke 29: 1439–1444, 1998 [DOI] [PubMed] [Google Scholar]
- 254. Sobey CG, Faraci FM. Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles. Stroke 28: 837–843, 1997 [DOI] [PubMed] [Google Scholar]
- 255. Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, Kumar A, Meurer S, Deile M, Taye A, Knorr A, Lapp H, Muller H, Turgay Y, Rothkegel C, Tersteegan A, Kemp-Harper B, Muller-Esterl W, Schmidt HHHW. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116: 2552–2561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Stenman E, Edvinsson L. Cerebral ischemia enhances vascular angiotensin AT1 receptor-mediated contraction in rats. Stroke 35: 970–974, 2004 [DOI] [PubMed] [Google Scholar]
- 257. Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 258. Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med 17: 183–190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, Sato K, Taniyama Y, Ito S. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology 142: 3125–3134, 2001 [DOI] [PubMed] [Google Scholar]
- 260. Sun H, Molacek E, Zheng H, Fang Q, Patel KP, Mayhan WG. Alcohol-induced impairment of neuronal nitric oxide synthase (nNOS)-dependent dilation of cerebral arterioles: role of NAD(P)H oxidase. J Mol Cell Cardiol 40: 321–328, 2006 [DOI] [PubMed] [Google Scholar]
- 261. Sun H, Patel KP, Mayhan WG. Tetrahydrobiopterin, a cofactor for NOS, improves endothelial dysfunction during chronic alcohol consumption. Am J Physiol Heart Circ Physiol 281: H1863–H1869, 2001 [DOI] [PubMed] [Google Scholar]
- 262. Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG. Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke 37: 495–500, 2006 [DOI] [PubMed] [Google Scholar]
- 263. Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol 283: H353–H363, 2002 [DOI] [PubMed] [Google Scholar]
- 264. Taguchi H, Faraci FM, Kitazono T, Heistad DD. Relaxation of the carotid artery during hypoxia is impaired in Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol 15: 1641–1645, 1995 [DOI] [PubMed] [Google Scholar]
- 265. Takahashi N, Smithies O. Human genetics, animal models and computer simulations for studying hypertension. Trends Genet 20: 136–145, 2004 [DOI] [PubMed] [Google Scholar]
- 266. Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, Morishita R. Angiotensin receptor blocker prevented β-amyloid induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 54: 1345–1352, 2009 [DOI] [PubMed] [Google Scholar]
- 267. Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res 88: 877–887, 2001 [DOI] [PubMed] [Google Scholar]
- 268. Thompson PW, Bayliffe Warren AP, Lamb JR. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine 39: 184–191, 2007 [DOI] [PubMed] [Google Scholar]
- 269. Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 61: 62–97, 2009 [DOI] [PubMed] [Google Scholar]
- 270. Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke 34: 2698–2703, 2003 [DOI] [PubMed] [Google Scholar]
- 271. Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest 114: 240–249, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Ujiie H, Chaytor AT, Bakker LM, Griffith TM. Essential role of gap junctions in NO- and prostanoid-independent relaxations evoked by acetylcholine in rabbit intracerebral arteries. Stroke 34: 544–550, 2003 [DOI] [PubMed] [Google Scholar]
- 273. Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 24: 1023–1030, 2004 [DOI] [PubMed] [Google Scholar]
- 274. Vanhoutte PM, Shimokawa J, Tang EHC, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196: 193–222, 2009 [DOI] [PubMed] [Google Scholar]
- 275. Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflügers Arch 459: 1015–1023, 2010 [DOI] [PubMed] [Google Scholar]
- 276. Volpe M, Iaccarino G, Cecchione C, Rizzoni D, Russo R, Rubattu S, Condorelli G, Ganten U, Ganten D, Trimarco B, Lindpainter K. Association and cosegregation of stroke with impaired endothelium-dependent vasorelaxation in stroke prone, spontaneously hypertensive rats. J Clin Invest 98: 256–261, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res 20: 124–137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Wassman S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens 24, Suppl 1: S15–S21, 2006 [DOI] [PubMed] [Google Scholar]
- 279. Wassman S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 280. Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res 57: 781–787, 1985 [DOI] [PubMed] [Google Scholar]
- 281. Widlandsky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 282. Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal (January 2, 2011). doi: 10.1089/ars.2010.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J 19: 989–991, 2005 [DOI] [PubMed] [Google Scholar]
- 284. Wosniak J, Santos CXC, Kowaltowski AJ, Laurindo FRM. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal 11: 1265–1278, 2009 [DOI] [PubMed] [Google Scholar]
- 285. Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-activated peroxisome proliferator-activated receptor-γ protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3ε upregulation. Circulation 119: 1124–1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Xie H, Ray PE, Short BL. NF-κB activation plays a role in superoxide-mediated cerebral endothelial dysfunction after hypoxia/reoxygenation. Stroke 36: 1047–1052, 2005 [DOI] [PubMed] [Google Scholar]
- 287. Yamashiro K, Milsom AB, Duchene J, Panayiotou C, Urabe T, Hattori N, Ahluwalia A. Alterations in nitric oxide and endothelin-1 bioactivity underlie cerebrovascular dysfunction in ApoE-deficient mice. J Cereb Blood Flow Metab 30: 1494–1503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Yang ST, Faraci FM, Heistad DD. Effects of cilazapril on cerebral vasodilatation in hypertensive rats. Hypertension 22: 150–155, 1993 [DOI] [PubMed] [Google Scholar]
- 289. Yang X, Wang F, Chang H, Zhang S, Yang L, Wang X, Cheng X, Zhang M, Ma XL, Liu H. Autoantibody against AT1 receptor from preelamptic patients induces vasoconstriction through angiotensin receptor activation. J Hypertens 26: 1629–1635, 2008 [DOI] [PubMed] [Google Scholar]
- 290. Yoshimoto T, Gochou N, Fukai N, Sugiyama T, Shichiri M, Hirata Y. Adrenomedullin inhibits angiotensin II-induced oxidative stress and gene expression in rat endothelial cells. Hypertens Res 28: 165–172, 2005 [DOI] [PubMed] [Google Scholar]
- 291. You J, Johnson TD, Marrelli SP, Bryan RM., Jr Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol Heart Circ Physiol 277: H893–H900, 1999 [DOI] [PubMed] [Google Scholar]
- 292. Zanetti M, Dato J'I, Katusic ZS, O'Brien T. Gene transfer of superoxide dismutase isoforms reverses endothelial dysfunction in diabetic rabbit aorta. Am J Physiol Heart Circ Physiol 280: H2516–H2523, 2001 [DOI] [PubMed] [Google Scholar]
- 293. Zemse SM, Hilgers RH, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am J Physiol Heart Circ Physiol 292: H3103–H3108, 2007 [DOI] [PubMed] [Google Scholar]
- 294. Zhang JY, Cao YX, Xu CB, Edvinsson L. Lipid-soluble smoke particles damage endothelial cells and reduce endothelium-dependent dilatation in rat and man. BMC Cardiovasc Disord 6: 3, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience 69: 1195–1204, 1995 [DOI] [PubMed] [Google Scholar]
- 296. Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience 171: 852–858, 2010 [DOI] [PubMed] [Google Scholar]
- 297. Zhang M, Song P, Xu J, Zou MH. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol 31: 125–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Zhang R, Bai YG, Lin LJ, Bao JX, Zhang YY, Tang H, Cheng JH, Jia GL, Ren XL, Ma J. Blockade of AT1 receptor partially restores vasoreactivity, NOS expression, and superoxide levels in cerebral and carotid arteries of hindlimb unweighting rats. J Appl Physiol 106: 251–258, 2009 [DOI] [PubMed] [Google Scholar]
- 299. Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res 92: 308–313, 2003 [DOI] [PubMed] [Google Scholar]
- 300. Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PLD, Stein PE, Pipkin FB, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature 468: 108–111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]