Abstract
There is evidence that extracellular adenosine can attenuate cardiac hypertrophy, but the mechanism by which this occurs is not clear. Here we investigated the role of adenosine receptors and adenosine metabolism in attenuation of cardiomyocyte hypertrophy. Phenylephrine (PE) caused hypertrophy of neonatal rat cardiomyocytes with increases of cell surface area, protein synthesis, and atrial natriuretic peptide (ANP) expression. These responses were attenuated by 5 μM 2-chloroadenosine (CADO; adenosine deaminase resistant adenosine analog) or 10 μM adenosine. While antagonism of adenosine receptors partially blocked the reduction of ANP expression produced by CADO, it did not restore cell size or protein synthesis. In support of a role for intracellular adenosine metabolism in regulating hypertrophy, the adenosine kinase (AK) inhibitors iodotubercidin and ABT-702 completely reversed the attenuation of cell size, protein synthesis, and expression of ANP by CADO or ADO. Examination of PE-induced phosphosignaling pathways revealed that CADO treatment did not reduce AKTSer473 phosphorylation but did attenuate sustained phosphorylation of RafSer338 (24–48 h), mTORSer2448 (24–48 h), p70S6kThr389 (2.5–48 h), and ERKThr202/Tyr204 (48 h). Inhibition of AK restored activation of these enzymes in the presence of CADO. Using dominant negative and constitutively active Raf adenoviruses, we found that Raf activation is necessary and sufficient for PE-induced mTORC1 signaling and cardiomyocyte hypertrophy. CADO treatment still blocked p70S6kThr389 phosphorylation and hypertrophy downstream of constitutively active Raf, however, despite a high level phosphorylation of ERKThr202/Tyr204 and AKTSer473. Reduction of Raf-induced p70S6kThr389 phosphorylation and hypertrophy by CADO was reversed by inhibiting AK. Together, these results identify AK as an important mediator of adenosine attenuation of cardiomyocyte hypertrophy, which acts, at least in part, through inhibition of Raf signaling to mTOR/p70S6k.
Keywords: p70S6 kinase, Raf, 2-chloroadenosine
adenosine is known to exert cardioprotective effects, including protection against ischemia/reperfusion injury (5, 30, 34), reduction of oxidative stress (31, 37), and attenuation of hypertrophy and heart failure during pressure overload (26, 27, 43). In support of a protective role for adenosine, we observed that deletion of cd73 (the ectonucleotidase that produces extracellular adenosine from AMP) exacerbated left ventricular hypertrophy and contractile dysfunction in mice exposed to chronic pressure overload produced by transverse aortic constriction (TAC) (27, 43). Mammalian target of rapamycin (mTOR)/p70S6k signaling is critical for cell enlargement (24, 38), so that treatment with the mTOR inhibitor rapamycin reduces hypertrophy and heart failure in mice exposed to pressure overload (15, 28). We found that activation of the mTOR/p70S6k signaling pathway in response to TAC was greater in hearts of CD73 knockout mice than in wild-type mice and that treatment of neonatal cardiomyocytes with 2-chloroadenosine (CADO) reduced phenylephrine (PE)-induced mTOR/p70S6K signaling and hypertrophy (43). However, the mechanism by which adenosine reduces mTOR/p70S6k signaling and hypertrophy is not known.
Extracellular adenosine can modulate cell signaling through G-protein-coupled adenosine receptors on the cell membrane, four of which have been identified (A1, A2A, A2B, and A3) (7, 12, 23, 39). Data from Liao et al. (26) suggest the A1 receptor plays a role in mediating the antihypertrophic effects of adenosine, as treatment with the selective adenosine A1 receptor agonist N6-cyclopentyladenosine acid (CPA) attenuated pressure overload left ventricular hypertrophy or PE-induced cardiomyocyte hypertrophy equal to that produced by the nonselective adenosine agonist CADO. On the other hand, Gan et al. (14) reported that each of the adenosine receptors has antihypertrophic effects in neonatal cardiomyocytes. Recently, however, we (27) observed that hypertrophy and heart failure were not significantly exacerbated in A1 receptor knockout mice compared with wild-type mice exposed to TAC (although A1 receptor knockout mice did exhibit increased sudden death early after TAC). Furthermore, knockout of the A3 receptor in mice did not exacerbate hypertrophy. Rather, when mice were subjected to TAC, A3 receptor knockout paradoxically decreased hypertrophy and heart failure compared with WT mice, suggesting that the A3 receptor may play a maladaptive role during pressure overload. Black et al. (3) reported that transgenic overexpression of the A3 receptor exacerbated pressure overload induced hypertrophy and heart failure, while Funakoshi et al. (13) reported that cardiac A1 receptor overexpression promoted ventricular dilation, expression of hypertrophy markers, and systolic dysfunction. While activation of A2B receptors inhibits cardiac fibroblast proliferation, collagen synthesis (10), and maladaptive tissue remodeling after infarct (42), a contribution of A2 receptors to the attenuation of cardiomyocyte hypertrophy in vivo has not yet been demonstrated. Thus the role of specific adenosine receptors in modulating cardiomyocyte hypertrophy is unclear.
Besides stimulating cell membrane receptors, adenosine can be transported into the cell via concentrative or equilibrative nucleoside transporters. There is evidence that up to 70% of myocardial interstitial adenosine is transported back into the cardiomyocyte (9) where it is estimated that 90% is converted to AMP by adenosine kinase (AK) (Ref. 21), with the remainder degraded to inosine by adenosine deaminase. However, the role of intracellular adenosine metabolism in cell signaling and cardiomyocyte hypertrophy has not been thoroughly examined.
Here we investigated the role of adenosine receptors and AK in modulation of cardiomyocyte hypertrophy and antihypertrophic signaling by adenosine. Our results indicate that AK is important in the attenuation of cardiomyocyte hypertrophy by adenosine and its stable analog CADO, while adenosine receptors play a less prominent role. In addition, we identify Raf signaling to mTOR/p70Sk (mTORC1) as an important component of cardiomyocyte hypertrophy that is disrupted by CADO through a mechanism dependent on AK.
EXPERIMENTAL PROCEDURES
Chemicals.
PE, adenosine, CADO, CPA, CGS 21680, 5-(N-ethylcarboxamido)adenosine (NECA), 2-Cl-N 6-(3-iodobenzyl)adenosine-5′-N-methyluronamide (2-Cl-IB-MECA), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), ZM 241,385, MRS 1754, MRS 1191, and 8-sulfophenyltheophylline (8-SPT) were from Sigma (St. Louis, MO). Iodotubercidin (ITU) and ABT-702 were from Tocris (Ellisville, MO). CADO, adenosine, and PE were dissolved in H2O. AK inhibitors and other adenosine receptor agonists and antagonists were dissolved in DMSO at a 5-mM concentration.
Western blots.
Lysates were collected in 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 100 mM NaCl, 1 mM PMSF, 1× protease inhibitor cocktail (Boehringer-Mannheim), 1× phosphatase inhibitor cocktail (Boehringer-Mannheim), and 10% glycerol, in 10 mM Tris·HCl pH 7.4. Protein concentration was determined using the Bradford Bio-Rad protein assay (Hercules, CA). Samples were boiled in 1× SDS loading buffer for 3 min and separated on 10% polyacrylamide gels for Western blot analysis. Proteins were detected using antibodies against atrial natriuretic peptide (ANP; Penninsula Laboratories, San Carlos, CA), p-AktSer473, ERK, p-ERKThr202/Tyr204, p70S6K, p-p70S6KThr389, mTOR and p-mTORSer2448, p-RafSer338, p-AMPKThr172 (Cell Signaling Technology, Beverly, MA), Raf, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA) followed by visualization using horseradish-peroxidase-labeled secondary antibodies (Santa Cruz Biotechnology).
Neonatal rat cardiomyocyte isolation and culture.
This study was approved by the Institutional Animal Care and Use Committee of the University of Minnesota. Cardiomyocytes were isolated from 2-day-old Sprague-Dawley rats by enzymatic digestion (45) and separated from nonmuscle cells on a discontinuous Percoll gradient as previously described (43). A total of 2–4 million viable myocytes were isolated per ventricle with very little fibroblast contamination (<2%) and plated at 0.5 × 105 cells/cm2 in DMEM containing 10% FCS. Myocytes were incubated for 48 h to allow attachment and spreading, after which the medium was replaced with serum-free media for 24 before treatment. For RNAi treatment, wells were coated with 1% gelatin to promote cell attachment and spreading and cells were plated at 1 × 105 cells/cm2. After 24 h, cells were transfected overnight using Lipofectamine 2000 (Invitrogen) with 10 pm/well adenosine kinase small interfering or nontargeting control RNAi (Dharmacon, Lafayette, CO) in the continued presence of 10% FCS. The next day, the media were replaced with DMEM containing insulin/transferrin/selenium solution (Sigma) and incubated for an additional 24–48 h before replacement with growth factor free media (DMEM) for 24 h before treatment with PE or CADO.
Leucine incorporation by cardiomyocytes.
1 μCi of [3-H]leucine (Perkin-Elmer, Waltham, MA) was added per well in 24-well plates. After 48 h of treatment, cells were washed once in PBS, fixed 10 min in 10% TCA, washed again in TCA, and dissolved in 5% SDS, 0.1 M NaOH, and [3-H]incorporation was measured in a liquid scintillation counter.
Cell area determination and cellular ANP expression.
After treatment, cells were washed in PBS, fixed with 4% paraformaldehyde/PBS pH 7.4, washed three times in PBS, permeabilized with 0.1% Triton X-100 for 5 min, and stained using rhodamine-conjugated phalloidin (5 U/ml in PBS pH 7.4; Invitrogen) at room temperature for 20 min or mouse anti-cardiac myosin heavy chain (Abcam, Cambridge, MA) for 1 h followed by Alexa-fluor 555 anti-rabbit (Molecular Probes). Nuclei were stained using DAPI (300 nM; Invitrogen) for 5 min. Cell area was analyzed using Image J 1.34s (NIH). At least 100 individual cells were measured per experiment. Cells fixed as described above were also used for immunocytochemical analysis of ANP expression, using primary rabbit anti-ANP, followed by Alexa-fluor 488 anti-rabbit (Molecular Probes) as previously described (43).
Data and statistical analyses.
All values were expressed as means ± SE. Statistical significance was defined as P < 0.05. One-way ANOVA was used to test each variable for differences among the treatment groups with StatView (SAS Institute). If the ANOVA demonstrated a significant effect, post hoc pairwise comparisons were made with the Student's t-test.
RESULTS
Roles of adenosine receptors and AK in attenuation of cardiomyocyte hypertrophy.
To mimic adenosine and avoid possible interference from nonspecific effects of adenosine deaminase inhibitors (17, 29), cells were treated with CADO (adenosine deaminase resistant adenosine analog). In dose-response experiments (data not shown) and previous studies (27, 43), we determined that 5 μM CADO was effective in reducing the PE-induced increases in cardiomyocyte cell area, total protein accumulation, and [3H]leucine incorporation.
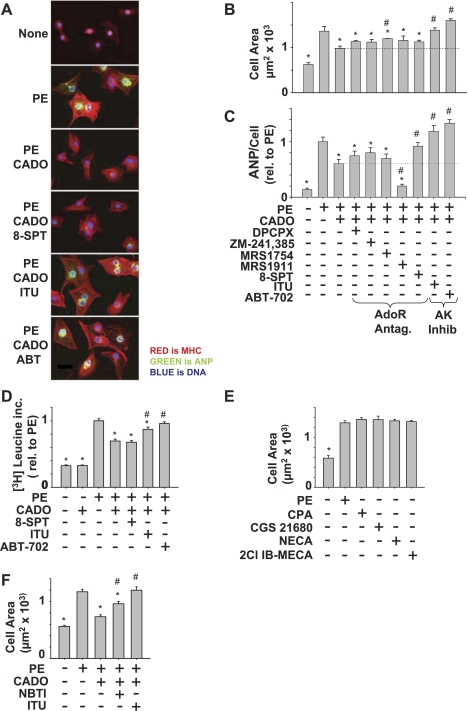
Cells can respond to extracellular adenosine through four distinct adenosine receptors. Cells can also take up extracellular adenosine, where most of it is metabolized by AK (21). To examine the influence of adenosine receptors vs. AK in attenuation of hypertrophy, we pretreated cardiomyocytes with 5 μM of selective adenosine receptor antagonists (A1: DPCPX; A2A: ZM 241,385; A2B: MRS 1754; or A3: MRS 1191), 5 μM of a nonselective adenosine receptor antagonist (8-SPT), or 0.2 μM of AK inhibitors (ITU and ABT-702) before treatment with CADO and stimulation of hypertrophy with 50 μM PE. As shown in (Fig. 1, A–D), CADO significantly reduced PE-induced increases in cell area (Fig. 1, A and B), ANP expression (Fig. 1C), and protein synthesis (Fig. 1D). Although each of the selective adenosine receptor antagonists, as well as the nonselective adenosine receptor antagonist 8-SPT, showed a trend toward reversing the attenuation of cell size produced by CADO (Fig. 1B), only the A2B receptor effect was significant, and even this was relatively modest. Likewise, selective antagonism of adenosine A1 or A2 receptors showed a trend toward reversing CADO attenuation of ANP expression (Fig. 1C). A3 receptor antagonism, however, potentiated reduction in ANP expression by CADO, suggesting A3 receptor activity may oppose antihypertrophic effects of A1 and A2 receptors. Nonselective antagonism of adenosine receptors using 8-SPT did modestly but significantly increase ANP expression in CADO-treated cells (Fig. 1C), but this was not accompanied by an increase in [3H]leucine incorporation (Fig. 1D), suggesting adenosine receptors were not mediating attenuation of cardiomyocyte protein synthesis by CADO. In agreement with a minimal role for adenosine receptors in attenuation of hypertrophy, selective adenosine receptor agonists did not reduce cell area in PE-treated cardiomyocytes (Fig. 1E). Remarkably, both AK inhibitors completely blocked the attenuation of cell enlargement (Fig. 1, A and B) and dramatically increased ANP expression (Fig. 1C) and protein synthesis (Fig. 1D) in CADO-treated cells. Importantly, CADO treatment alone did not reduce basal protein synthesis (Fig. 1D) or cause evidence of increased cell death as would be expected if this dose of CADO were cytotoxic. Additionally, in the absence of CADO, AK inhibition did not increase protein synthesis or cell area under basal conditions or in PE-treated cells (data not shown), indicating that AK inhibition was reversing effects of CADO, rather than independently enhancing cell growth.
Fig. 1.
Adenosine kinase (AK) inhibitors block effects of 2-chloroadenosine (CADO) on cell area (A and B), atrial natriuretic peptide (ANP) expression (A and C), and protein synthesis (D). Cells were pretreated for 10 min with 5 μM of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; A1 antagonist), ZM 241,385 (A2A antagonist), MRS 1754 (A2B antagonist) MRS 1191 (A3 antagonist), 8-sulfophenyltheophylline (8-SPT; nonselective adenosine receptor antagonist), 0.2 μM iodotubercidin (ITU; AK inhibitor), or 0.2 μM ABT-702 (AK inhibitor), followed by CADO (5 μM) for 10 min and then with 50 μM phenylephrine (PE) for 48 h. Treatment with 1 μM adenosine receptor agonists CPA (A1 agonist), CGS 21680 (A2A agonist), 5-(N-ethylcarboxamido)adenosine (NECA; nonselective agonist), or 2-Cl-N 6-(3-iodobenzyl)adenosine-5′-N-methyluronamide (2Cl-IB-MECA; A3 agonist) did not attenuate PE-induced increase in cell area (E). Inhibition of equilibrative nucleoside transporter with S-(4-nitrobenzyl)-6-thioinosine (NBTI; 1 μM ) increased cell area in CADO-treated cells (F). Cell area (B) is the average of 4–8 independent experiments, measuring at least 100 cells per condition. ANP expression (C) is the average ANP expression in at least 100 cells per condition normalized to PE to compare between experiments. [3H]leucine incorporation (D) is the average of 3 experiments, each run in triplicate, and normalized to the PE sample to compare between experiments. For cell area and ANP measurement, cells were fixed and immunostained for α-cardiac myosin heavy chain, ANP, and DNA (DAPI). *P < 0.05, compared with PE. #P < 0.05, compared with PE/CADO. Scale bar = 20 μm.
Because inhibition of AK restored hypertrophy in CADO-treated cells, a role for uptake and intracellular metabolism of CADO is implied. In support of this, inhibition of CADO uptake using the equilibrative nucleoside inhibitor S-(4-nitrobenzyl)-6-thioinosine (NBTI) diminished the antihypertrophic effects of CADO by ∼50% (Fig. 1F). In the same experiment, inhibition of AK completely blocked CADO attenuation of hypertrophy. One explanation for this finding is that NBTI inhibition of equilibrative nucleoside transport did not completely block CADO uptake, since rat cardiomyocytes also express concentrative nucleoside transporters that are insensitive to NBTI (35). Together these data suggest cellular uptake and metabolism by AK activity play an important role in mediating the antihypertrophic effects of CADO.
Adenosine reduction of cardiomyocyte hypertrophy is dependent on AK.
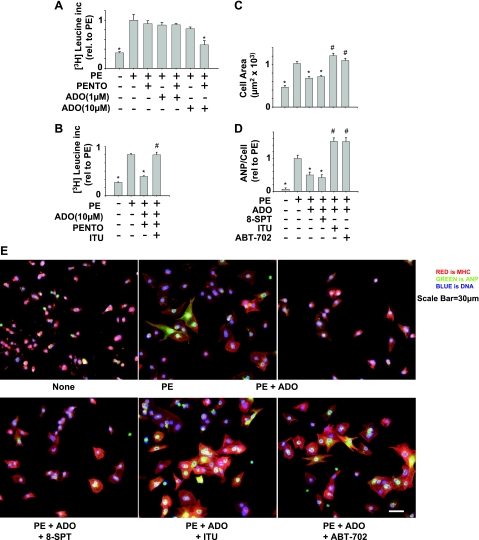
To verify that AK attenuates the antihypertrophic effect of adenosine, and not just its stable analog CADO, we repeated these experiments using 10 μM adenosine. For the protein synthesis experiment, we included pentostatin (an adenosine deaminase inhibitor) to preserve adenosine levels over the extended time course. The results using adenosine were the same as those using CADO. Inhibition of AK, but not inhibition of adenosine receptors with 8-SPT, completely reversed adenosine attenuation of protein synthesis (Fig. 2, A and B), cell area (Fig. 2, C and E) and ANP expression (Fig. 2, D and E). Together, these results indicate that AK was instrumental in the attenuation of cardiomyocyte hypertrophy produced by adenosine or its stable analog CADO, while adenosine receptors played a much less prominent role.
Fig. 2.
Adenosine attenuation of hypertrophy is blocked by inhibition of AK. Cells were treated with PE (50 μM) and/or adenosine [ADO (1 or 10 μM), pentostatin (PENTO; 1 μM), or ITU (0.2 μM)], and [3-H]leucine incorporation was measured over 48 h in triplicate (A and B). For cell area (C and E) and ANP expression (D and E), adenosine deaminase inhibitors were not included, but media, adenosine, and antagonists were replaced at 24 h. At least 100 cells were measured per condition to obtain average cell area and ANP expression. Scale bar = 30 μM. *P < 0.05, compared with PE treated. #P < 0.05, compared with PE/CADO treated.
AK regulation of cell signaling.
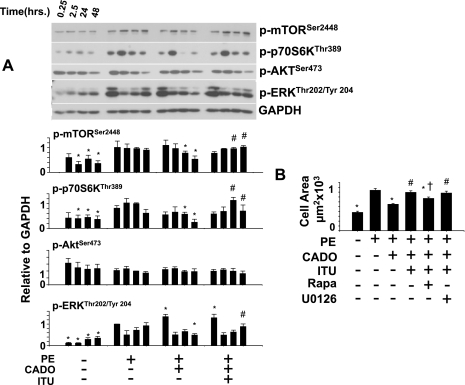
The Raf/MEK/ERK, Akt, and mTOR/p70S6k signaling pathways have each been implicated in promoting hypertrophy. Because sustained exposure to PE is required to drive cardiomyocyte hypertrophy (2), we monitored activation of these pathways over 48 h of PE stimulation and examined how these are regulated by CADO and AK. Examination of cell signaling pathways (Fig. 3A) revealed that PE induced a biphasic phosphorylation of ERKThr202/Tyr204 and a sustained phosphorylation of mTORSer2448 and p70S6kThr389 but had little effect on Aktser473 phosphorylation. Treatment with CADO significantly potentiated early ERK activation (15 min) by PE but reduced the second peak of ERK activation (48 h). CADO treatment also prevented the sustained activation of mTOR and p70S6k but had little effect on AKT activation. Consistent with a role for AK in mediating the antihypertrophic effects of CADO, ITU restored both sustained activation of p70S6k and the second peak of ERK activation (Fig. 3A).
Fig. 3.
CADO (5 μM) attenuates sustained phosphorylation of mTORSer2448 and p70S6KThr389 and late phosphorylation of ERKThr202/Tyr204 but does not alter AKTSer473 in PE (50 μM)-treated cells. Iodotubercidin (ITU; 200 nM) restores phosphorylation of p70S6kThr389 and ERKThr202/Tyr204 (A). Cell area increase between 24 and 48 h in PE/CADO-treated cells in response to AK inhibition (ITU; 200 nM) is blocked by inhibition of mammalian target of rapamycin (mTOR; rapamycin; 100 nM) but not inhibition of MEK/ERK signaling (U0126; 10 μM; B). Graphs for cell signaling represent phosphorylation state of indicated proteins relative to GAPDH and normalized to 15-min time point for ERKThr202/Tyr204 or 24-h time point for mTORSer2448, p70S6kThr389 or AktSer473 to compare between experiments. *P < 0.05, compared with PE at same time point. #P < 0.05, compared with PE/CADO at same time point. †Significant difference compared with PE/CADO/ITU (B). For cell signaling in A, bars represent average of at least 3 experiments for each time point. For cell area, at least 100 cells were measured per condition.
Because inhibition of AK restored both sustained activation of p70S6k (24–48 h) and the second peak of ERK activation (48 h), which had been blocked by CADO, we examined which of these pathways was important for the increased cell area observed in cells treated with CADO + AK inhibitors. We added either the MEK inhibitor U0126 (10 μM) or the mTOR inhibitor rapamycin (100 nM) for the last 24–48 h (during which the effect of AK inhibition was the most prominent) and observed that only rapamycin significantly inhibited continued cell expansion (Fig. 3B). This suggests restoration of cell growth in CADO-treated cells by AK inhibition requires sustained mTOR/p70S6k signaling, while the increase in MEK/ERK plays a less significant role. Together these results demonstrate that CADO reduces sustained activation of mTOR/p70S6k signaling independent of AKT, that sustained mTOR/p70S6k activity is critical for PE-induced hypertrophy, and that CADO inhibition of mTOR/p70S6k and hypertrophy is reversed by blocking AK.
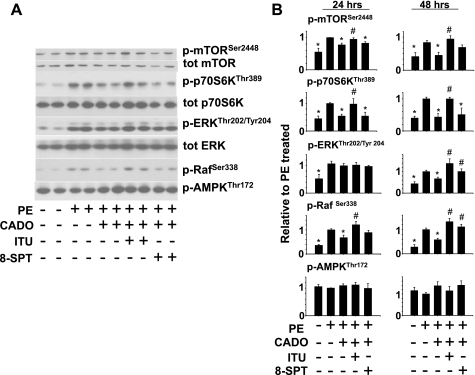
CADO/AK effects on Raf and AMPK.
The restoration of ERK and p70S6k signaling in CADO-treated cells by AK inhibition suggests that AK activity may be targeting a pathway that is common to both sustained mTOR/p70S6k signaling and the late peak in ERK. Raf is a component of the canonical ras/Raf/MEK/ERK signaling pathway but can also activate mTOR/p70S6k independent of its effects on ERK (25, 33). Therefore, we examined activity of Raf at 24 and 48 h of PE stimulation, when p70S6k activation was most dramatically upregulated by AK inhibition. Interestingly, phosphorylation of RafSer338, which prevents inhibition of its activity through an N-terminal autoinhibitory domain (41), was elevated in response to PE treatment (Fig. 4, A and B). CADO treatment reduced sustained phosphorylation of RafSer338 (24–48 h) while AK inhibition restored RafSer338 phosphorylation in CADO-treated cells, suggesting that AK may mediate the CADO inhibition of mTOR/p70S6k in part through effects on Raf. Interestingly, however, inhibition of adenosine receptors with 8-SPT also increased phosphorylation of RafSer338 (24 and 48 h), ERKThr202/Tyr204 (48 h), and mTORSer2448 (48 h) in CADO-treated cells (Fig. 4) but did not significantly increase p70S6kThr389 phosphorylation. Importantly, the reduction in p70S6kThr389 phosphorylation by CADO treatment observed from 24 to 48 h was completely restored by ITU. While the increased phosphorylation of RafSer338 at 48 h by 8-SPT treatment may account for the increased ANP expression observed (Fig. 1C), the sustained inhibition of p70S6k by CADO, which was reversed by ITU but not 8-SPT, suggests an adenosine receptor-independent mechanism by which CADO attenuates p70S6k activity and hypertrophy that is mediated by AK. Analysis of total p70S6k and total ERK levels did not reveal significant differences between CADO-treated cells and cells treated with CADO + ITU (Fig. 4 and data not shown), suggesting regulation of these pathways was through altered phosphorylation. Because CADO inhibition of mTOR itself was rather modest and CADO reduced p70S6kThr389 phosphorylation but not AktSer473 phosphorylation (Fig. 3), CADO appears to be selectively targeting the mTOR signaling complex known as mTORC1.
Fig. 4.
Inhibition of AK with ITU (0.2 μM) restores phosphorylation of Raf Ser338, mTOR2448, and p70S6kThr389 in CADO-treated cells at 24 and 48 h but does not significantly affect AMPKThr172 phosphorylation. A and B: graphs represent averages of phosphorylation level from 3–10 experiments, relative to PE-treated samples. *P < 0.05, compared with PE. #P < 0.05, compared with PE/CADO.
AMPK can also reduce mTOR/p70S6k signaling (4, 16, 18, 22). Because AK produces AMP, which activates AMPK (6), and because AK can also phosphorylate CADO(20), we examined phosphorylation of AMPKThr172 in response to CADO treatment and in the presence of AK inhibitor ITU. CADO treatment did not significantly increase AMPK phosphorylation in PE-treated cells nor did ITU treatment significantly reduce AMPK phosphorylation (Fig. 4). At the same time, ITU did block the inhibitory effect of CADO on p70S6kThr389, suggesting AK-dependent inhibition of mTORC1 by CADO is independent of changes in AMPK activation.
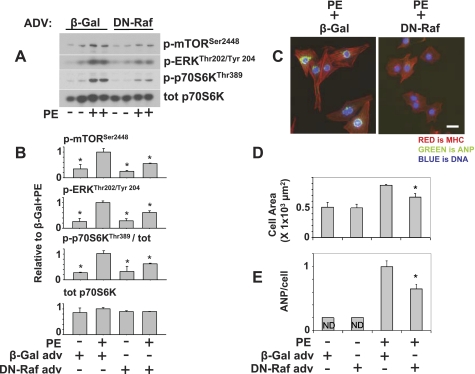
Full induction of mTOR/p70S6k pathway by PE requires Raf.
Because Raf can activate mTOR/p70S6k independent of its effects on ERK (25, 33), we examined the role of Raf in PE stimulation of mTOR/p70S6k and hypertrophy. Infection of cardiomyocytes with dominant negative Raf (kinase dead; DN Raf) adenovirus (19) reduced the PE-induced increase of mTOR, p70S6k, and ERK activation, as well as cell area and ANP expression (Fig. 5, A-E). This demonstrates that Raf activity is required for PE-induced hypertrophy, as previously observed (44) and that Raf is an important link between PE stimulation and the mTOR/p70S6K pathway in cardiomyocytes.
Fig. 5.
PE activates mTOR/p70S6k through a Raf-dependent mechanism. Dominant negative Raf (DN-Raf) blocks PE induction of mTOR/p70S6k and ERK (A and C), as well as PE-induced increase in cell area (B and D) and ANP expression per cell (B and E). For immunofluoresence (B), cells were stained for ANP, α-cardiac myosin heavy chain, and DNA (DAPI). There was very little ANP stain in absence of PE and no difference between β-Gal and DN-Raf. For p70S6kThr389, graph represents average from 2 independent experiments (n = 3) relative to PE + β-Gal. For cell area, graph represents averages from 2 independent experiments, in which >100 cells were measured per condition. For ANP expression, graph represents average ANP expression measured in ≥100 cells per condition *P < 0.05, compared with PE treated. ND is not determined. Scale bar = 20 μm.
AK mediates CADO inhibition of Raf signaling to mTOR/p70S6K.
Results in Figs. 4 and 5 suggest that CADO reduces sustained activation of Raf and p70S6k through an AK-dependent mechanism and that Raf activity is necessary for PE-induced mTOR/p70S6k signaling. To determine if restoring Raf activation can overcome the antihypertrophic effects of CADO, we infected cardiomyocytes with a constitutively active Raf mutant (CA-Raf; N terminus deleted; Ref. 19) for 24 h before treatment with CADO. CA-Raf increased cell area, and this was associated with increased activation of mTOR, p70S6k, and ERK (Fig. 6). While CADO was unable to reduce AKT or ERK activation in the presence of CA-Raf, CADO strongly inhibited mTOR and p70S6K activation (Fig. 6, A and B). Interestingly, CADO treatment still reduced cell area (Fig. 6, C and D) despite high levels of ERK and AKT activation. As in PE-stimulated cells, the inhibitory effects of CADO on the Raf-induced mTORC1 pathway and cell size were reversed by the AK inhibitor ITU. The inhibition of p70S6K by CADO, and the inability of CADO to suppress ERK or AKT signaling downstream of Raf, suggest CADO did not reduce activity of CA-Raf but was interfering with a downstream effector of Raf that is necessary specifically for activation of mTORC1 (mTOR/p70S6K) but not mTORC2 (mTOR/AKT) or MEK/ERK signaling.
Fig. 6.
Active Raf induces mTOR/p70S6kThr389, ERKThr202/Tyr204, and AKTSer473 phosphorylation (A and B) and hypertrophy (C and D), but CADO inhibits p70S6k and hypertrophy through an AK-dependent mechanism. Cells were infected with adenovirus expressing constitutively active Raf or β-Gal for 24 h, followed by CADO or CADO + ITU for an additional 24 h. Cells were fixed and stained for α-cardiac myosin heavy chain, ANP, and DNA (DAPI). *P < 0.05, compared with CA-Raf. #P < 0.05, compared with Raf/CADO. Graphs of p-ERKThr202/Tyr204 and p-p70S6kThr389 represent the average of 2 experiments each performed in duplicate (n = 4). For images, scale bar = 20 μm.
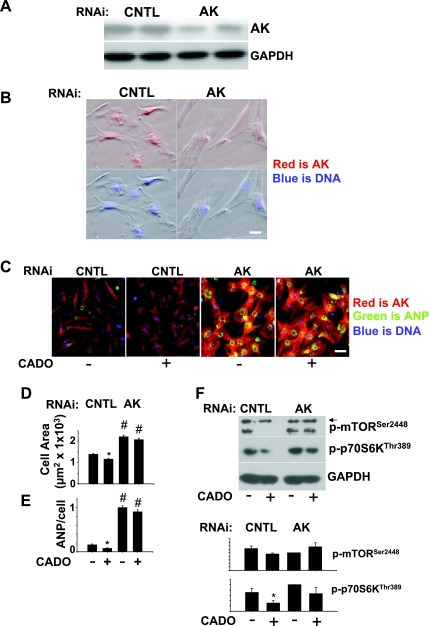
AK knockdown induces spontaneous hypertrophy.
To confirm our results using AK inhibitors, we used RNAi to reduce AK expression. For liposome-mediated transfection of RNAi, we cultured cardiomyocytes on gelatin-coated plates, which improved survival and transfection efficiency during liposome-mediated RNAi transfection. Additionally, cells were cultured for a longer time period (72 h after transfection) to allow for RNAi depletion of AK before treatment with CADO. By Western blot, AK was reduced by ∼65% after 72 h of RNAi treatment (Fig. 7A). Using immunocytochemistry to examine AK subcellular localization, we observed that AK was localized predominantly in the nucleus in control RNAi-treated cells while AK RNAi reduced this staining (Fig. 7B). Surprisingly, RNAi reduction of AK induced a spontaneous increase in hypertrophy of cardiomyocytes, characterized by a nearly twofold increase in cell surface area and strong ANP expression (Fig. 7, B-E). Additionally, cardiomyocytes treated with AK RNAi demonstrated enhanced staining for cardiac myosin heavy chain (Fig. 7C) and increased cytoskeletal organization, suggesting that AK knockdown was promoting maturation of the incompletely differentiated neonatal cardiomyocytes, as is commonly seen in response to hypertrophic stimulation of neonatal cardiomyocytes (1). We did not observe a change in AK localization or expression level in response to PE or CADO treatment (data not shown). In AK RNAi-treated cells, there was a trend towards higher levels of p70S6kThr389 phosphorylation under basal conditions (Fig. 7F) and greater resistance to inhibition by CADO, again supporting a role for AK in regulating cell size through the mTORC1 pathway. In these transfected cells, however, we found that PE caused adjacent cells to pull together and form condensed islands of cells, and this occurred to a greater extent in the already hypertrophied AK knockdown cells, making it difficult to compare the effects of CADO on cell area or compare side-by-side effects of CADO on the PE response in control and AK RNAi-treated cells. Taken together, these results suggest a novel role for AK in attenuation of cardiomyocyte hypertrophy.
Fig. 7.
RNAi knockdown of AK (72 h) results in reduction in AK protein by western blot compared with nontargeting control RNAi-treated cells (A). Immunofluorescent staining of AK is localized in the nucleus in cardiomyocytes, and this staining is lost after 96 h of RNAi treatment (B). RNAi knockdown of AK promotes spontaneous hypertrophy and ANP expression (B, C, D, and E), as well as reduced sensitivity of p70S6k to inhibition by CADO (F). For cell area and ANP expression, cardiomyocytes were transfected with RNAi for 72 h and then treated an additional 48 h with CADO (5 μM). Cells were then fixed and stained for α-cardiac myosin heavy chain, ANP, and DNA (Hoescht). For analysis of p70S6k and mTOR phosphorylation, cell lysates were collected after 24 h of treatment (96 h after RNAi). Graphs represent average of 3 experiments relative to AK RNAi. *P < 0.05, comparing untreated and CADO-treated cells. #P < 0.05, comparing control RNAi-treated cells and AK RNAi-treated cells. Scale bar = 20 μM for B and 40 μM for C.
DISCUSSION
Adenosine and cardiomyocyte hypertrophy.
We initially undertook this work to determine the signaling mechanism(s) by which the A1 receptor attenuated cardiomyocyte hypertrophy. While we were able to detect A1-dependent responses to CADO or CPA (unpublished observations), we were unable to demonstrate a significant inhibition of cardiomyocyte hypertrophy by selective A1 agonists or a robust reversal of CADO attenuation of hypertrophy by adenosine receptor antagonists. Additionally, we did not observe an increase in hypertrophy in A1 knockout mice exposed to aortic banding. It is not clear why our results using adenosine agonists contrast with other reports finding antihypertrophic effects mediated by adenosine receptors (14, 26, 32). We did observe a significant increase in RafSer338 phosphorylation and ERKThr202/Tyr204 phosphorylation (48 h time point) as well as a significant increase in ANP expression in response to nonselective adenosine receptor antagonism (8-SPT treatment). We also observed a trend toward increased p70S6k activation in response to A1 receptor antagonism (data not shown) and a slight but significant reversal of CADO attenuation of cell surface area by A2B receptor antagonism. It is possible that these effects are amplified in other experimental conditions. In agreement with the protective effect of A3 receptor deletion against hypertrophy and heart failure that we previously observed in vivo (27), antagonism of the A3 receptor potentiated attenuation of ANP expression by CADO. Together these data suggest A1 and A2 receptors may contribute modestly to adenosine attenuation of hypertrophy, while the A3 receptor opposes these effects.
In support of intracellular metabolism of adenosine playing a role in regulating cardiomyocyte hypertrophy, the AK inhibitors ITU and ABT-702 reversed the inhibitory effects of CADO or Ado on PE-induced cardiomyocyte hypertrophy and ANP expression. The likelihood that this outcome is due to specific effects on AK activity is strengthened by the fact that two different AK inhibitors (ITU and ABT-702, nucleoside-based and nonnucleoside-based inhibitors of AK, respectively) showed the same effect. While our data do not exclude a role for adenosine receptors in attenuation of cardiomyocyte hypertrophy under different experimental conditions in other studies, our results identify for the first time a prominent role for AK in mediating the antihypertrophic effect of adenosine.
RNAi depletion of AK induces spontaneous hypertrophy.
In support of our findings using chemical inhibitors of AK, we observed that RNAi knockdown of AK (but not control RNAi) induced spontaneous hypertrophy. The spontaneous hypertrophic response in cells depleted of AK was surprising, because chemical inhibition of AK activity did not induce the same response. It is possible that different culture conditions (the use of gelatin-coated plates, higher density cells, and longer period of culture) used for transfection or cell stress from the lipid transfection reagent resulted in increased endogenous adenosine production, so that growth of control RNAi-treated cells was attenuated, while AK-depleted cells were resistant to effects of endogenous adenosine. These results support a role for AK in attenuating cell growth, and suggest that under certain conditions, AK activity may limit cell growth in the absence of exogenous adenosine.
Adenosine regulation of cardiomyocyte hypertrophic signaling.
We previously observed that activation of mTOR/p70S6k, ERK (unpublished observations), and AKT signaling pathways were increased in CD73 knockout mice (adenosine deficient) in response to pressure overload compared with wild-type mice (43). Here, using an isolated cardiomyocyte hypertrophy model, we investigated the mechanism(s) by which adenosine regulates these pathways. Interestingly, CADO treatment potentiated early ERK activation by PE (15 min) but reduced sustained ERK activation (48 h). While potentiation of early ERK activation by CADO was dependent on the A1 receptor (unpublished observations) and was not affected by AK inhibition, the second peak of ERK activation was fully restored by AK inhibition (Fig. 3). Although recent evidence suggests that ERK activity is not essential for hypertrophy (36), the increased activation of ERK is indicative of the activity of upstream components of the ras/Raf/MEK/ERK signaling cascade. Indeed, we found that phosphorylation of RafSer338 at 24 and 48 h was attenuated by CADO treatment but restored by inhibition of AK. Raf is necessary and sufficient for cardiac hypertrophy, so that AK-dependent reduction of RAF activation by CADO may have contributed to attenuation of hypertrophy. However, the finding that inhibition of adenosine receptors using 8-SPT also increased activation of Raf and ERK at the later time point (48 h) but did not restore hypertrophy suggests increasing Raf signaling alone is not sufficient to restore protein synthesis and cell enlargement in CADO-treated cells.
Interestingly, AKT phosphorylation was not inhibited by CADO, but at the same time, sustained p70S6K activation was attenuated by CADO. mTOR/p70S6k signaling is critical for protein synthesis and cell enlargement (24, 38). The specific reduction of p70S6kThr389 phosphorylation by CADO, while AKTSer473 phosphorylation was preserved, suggests CADO treatment was specifically inhibiting the mTOR signaling complex known as mTORC1 (which phosphorylates p70S6KThr389) but not inhibiting mTORC2 (which phosphorylates AKTSer473). In examining the signaling pathway(s) linking α-adrenergic stimulation (PE) to mTORC1, we found that dominant negative Raf blocked the PE-induced increase in p70S6kThr389 phosphorylation. Furthermore, constitutively active Raf promoted p70S6kThr389 phosphorylation and hypertrophy in the absence of additional stimuli. This suggests an important role for Raf in cardiomyocyte mTORC1 signaling. However, in cells expressing constitutive Raf activity, CADO still reduced p70S6kThr389 phosphorylation and hypertrophy without inhibiting ERK or AKT. The reduction in Raf-dependent p70S6kThe389 phosphorylation by CADO was completely reversed by inhibition of AK. These data suggest that CADO or adenosine can limit cell growth downstream of Raf activation through an AK-dependent mechanism that targets mTORC1, while preserving protective antiapoptotic signaling pathways that are dependent on ERK and AKT (8).
How does AK inhibit hypertrophy?
While the inhibition of hypertrophy, as well as the targeting of mTORC1 by CADO, appears to be dependent upon AK, the mechanism by which AK mediates these effects is not clear. Because AMPK can inhibit mTORC1 signaling (4) and AK has been shown to play a role in activation of AMPK by extracellular adenosine (6), we examined the possibility that CADO was inhibiting mTORC1 through AMPK activation. We did not observe a significant increase in AMPK activation in PE-treated cells treated with CADO nor was AMPK activation blocked by inhibition of AK. However, from the present data we cannot rule out the possibility that a compartmentalized AK-dependent activation of a specific population of AMPK proteins occurs that is not apparent when examining total phospho-AMPKThr172 levels but that contributes to reduced activation of mTOR/p70S6k signaling through tuberin/hamartin or some other pathway.
Because the rescue of p70S6k activation in CADO-treated cells by AK inhibition appears greatest at later time points (24–48 h), it is also possible that AK regulation of p70S6k is indirect. We have previously observed alterations in cardiomyocyte microtubule dynamics in response to adenosine or CADO (11), which may over time alter formation of membrane signaling complexes, thereby indirectly regulating signaling between Raf and mTORC1. We are currently investigating the role of AK in adenosine-dependent cytoskeletal changes and the potential effects this has on sustained activity of hypertrophic signaling pathways.
Another possible mechanism by which AK might mediate the antihypertrophic effects of adenosine is through regulation of methylation reactions. We found that AK is localized in the nucleus in neonatal cardiomyocytes. It is possible that nuclear localization of AK might relate to its role in transmethylation reactions. High levels of intracellular adenosine may inhibit S-adenosylmethionine-dependent trans-methylation reaction by increasing levels of S-adenosylhomocysteine (40). A reduced ability to methylate DNA or histones may alter gene expression, which might also account for indirect effects on mTORC1. However, we did not observe a difference in methylated histone levels in response to CADO or inhibition of AK (unpublished observations), suggesting an overall change in the ability to perform methylation reactions was not likely the cause of attenuation of hypertrophy by CADO.
Together, our findings suggest that AK plays a critical role in the attenuation of cardiomyocyte hypertrophy by adenosine or its stable analog CADO, while adenosine receptors play a less prominent role. A potential role for AK in mediating the protective effects of adenosine in the stressed heart must now be considered.
GRANTS
This study was supported by U.S. Public Health Service and National Heart, Lung, and Blood Institute Grants HL-21872 and HL-71790 and Research Grants 0330136N and 0160275Z from the American Heart Association. J. Fassett is a recipient of a Scientist Development Award from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aoki H, Sadoshima J, Izumo S. Myosin light chain kinase mediates sarcomere organization during cardiac hypertrophy in vitro. Nat Med 6: 183– 188, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Barron AJ, Finn SG, Fuller SJ. Chronic activation of extracellular-signal-regulated protein kinases by phenylephrine is required to elicit a hypertrophic response in cardiac myocytes. Biochem J 371: 71– 79, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Black RG, Jr, Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, Bolli R, Auchampach JA. Gene dosage-dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res 91: 165– 172, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem 279: 32771– 32779, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol 103: 203– 215, 2008 [DOI] [PubMed] [Google Scholar]
- 6. da Silva CG, Jarzyna R, Specht A, Kaczmarek E. Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res 98: e39– 47, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Jong JW, de Jonge R, Keijzer E, Bradamante S. The role of adenosine in preconditioning. Pharmacol Ther 87: 141– 149, 2000 [DOI] [PubMed] [Google Scholar]
- 8. de Jonge N, Goumans MJ, Lips D, Hassink R, Vlug EJ, van der Meel R, Emmerson CD, Nijman J, de Windt L, Doevendans PA. Controlling cardiomyocyte survival. Novartis Found Symp 274: 41– 51; discussion 51–47, 152–155, 272–156, 2006 [PubMed] [Google Scholar]
- 9. Deussen A, Stappert M, Schafer S, Kelm M. Quantification of extracellular and intracellular adenosine production: understanding the transmembranous concentration gradient. Circulation 99: 2041– 2047, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96: 2656– 2666, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Fassett JT, Xu X, Hu X, Zhu G, French J, Chen Y, Bache RJ. Adenosine regulation of microtubule dynamics in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 297: H523– H532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol 362: 364– 374, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Funakoshi H, Chan TO, Good JC, Libonati JR, Piuhola J, Chen X, MacDonnell SM, Lee LL, Herrmann DE, Zhang J, Martini J, Palmer TM, Sanbe A, Robbins J, Houser SR, Koch WJ, Feldman AM. Regulated overexpression of the A1-adenosine receptor in mice results in adverse but reversible changes in cardiac morphology and function. Circulation 114: 2240– 2250, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gan XT, Rajapurohitam V, Haist JV, Chidiac P, Cook MA, Karmazyn M. Inhibition of phenylephrine-induced cardiomyocyte hypertrophy by activation of multiple adenosine receptor subtypes. J Pharmacol Exp Ther 312: 27– 34, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens 24: 1663– 1670, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214– 226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haynes J, Jr, Killilea DW, Peterson PD, Thompson WJ. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic-3′,5′-guanosine monophosphate-stimulated phosphodiesterase to reverse hypoxic pulmonary vasoconstriction in the perfused rat lung. J Pharmacol Exp Ther 276: 752– 757, 1996 [PubMed] [Google Scholar]
- 18. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577– 590, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Klesse LJ, Parada LF. p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J Neurosci 18: 10420– 10428, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koshiba M, Kosaka H, Nakazawa T, Hayashi N, Saura R, Kitamura N, Kumagai S. 2-Chloroadenosine but not adenosine induces apoptosis in rheumatoid fibroblasts independently of cell surface adenosine receptor signalling. Br J Pharmacol 135: 1477– 1486, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kroll K, Decking UK, Dreikorn K, Schrader J. Rapid turnover of the AMP-adenosine metabolic cycle in the guinea pig heart. Circ Res 73: 846– 856, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Lantier L, Mounier R, Leclerc J, Pende M, Foretz M, Viollet B. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J 24: 3555– 3561 [DOI] [PubMed] [Google Scholar]
- 23. Lasley RD, Smart EJ. Cardiac myocyte adenosine receptors and caveolae. Trends Cardiovasc Med 11: 259– 263, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharmacol Toxicol 47: 443– 467, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lenormand P, McMahon M, Pouyssegur J. Oncogenic Raf-1 activates p70 S6 kinase via a mitogen-activated protein kinase-independent pathway. J Biol Chem 271: 15762– 15768, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Liao Y, Takashima S, Asano Y, Asakura M, Ogai A, Shintani Y, Minamino T, Asanuma H, Sanada S, Kim J, Ogita H, Tomoike H, Hori M, Kitakaze M. Activation of adenosine A1 receptor attenuates cardiac hypertrophy and prevents heart failure in murine left ventricular pressure-overload model. Circ Res 93: 759– 766, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Lu Z, Fassett J, Xu X, Hu X, Zhu G, French J, Zhang P, Schnermann J, Bache RJ, Chen Y. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation 118: 1713– 1721, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 109: 3050– 3055, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mery PF, Pavoine C, Pecker F, Fischmeister R. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP-stimulated phosphodiesterase in isolated cardiac myocytes. Mol Pharmacol 48: 121– 130, 1995 [PubMed] [Google Scholar]
- 30. Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res 52: 25– 39, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Narayan P, Mentzer RM, Jr, Lasley RD. Adenosine A1 receptor activation reduces reactive oxygen species and attenuates stunning in ventricular myocytes. J Mol Cell Cardiol 33: 121– 129, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Pang T, Gan XT, Freeman DJ, Cook MA, Karmazyn M. Compensatory upregulation of the adenosine system following phenylephrine-induced hypertrophy in cultured rat ventricular myocytes. Am J Physiol Heart Circ Physiol 298: H545– H553, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Pearson G, Bumeister R, Henry DO, Cobb MH, White MA. Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J Biol Chem 275: 37303– 37306, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther 114: 208– 221, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Podgorska M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T. Prevalence of unidirectional Na+-dependent adenosine transport and altered potential for adenosine generation in diabetic cardiac myocytes. Basic Res Cardiol 101: 214– 222, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Purcell NH, Wilkins BJ, York A, Saba-El-Leil M.K., Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA 104: 14074– 14079, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reichelt ME, Shanu A, Willems L, Witting PK, Ellis NA, Blackburn MR, Headrick JP. Endogenous adenosine selectively modulates oxidant stress via the A1 receptor in ischemic hearts. Antioxid Redox Signal 11: 2641– 2650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31: 342– 348, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15: 813– 827, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Tinton S, Buc-Calderon P. Homocysteine enhances the inhibitory effect of extracellular adenosine on the synthesis of proteins in isolated rat hepatocytes. Biochem J 310: 893– 896, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem 278: 11221– 11226, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Wakeno M, Minamino T, Seguchi O, Okazaki H, Tsukamoto O, Okada K, Hirata A, Fujita M, Asanuma H, Kim J, Komamura K, Takashima S, Mochizuki N, Kitakaze M. Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation 114: 1923– 1932, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Xu X, Fassett J, Hu X, Zhu G, Lu Z, Li Y, Schnermann J, Bache RJ, Chen Y. Ecto-5′-nucleotidase deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction. Hypertension 51: 1557– 1564, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yue TL, Gu JL, Wang C, Reith AD, Lee JC, Mirabile RC, Kreutz R, Wang Y, Maleeff B, Parsons AA, Ohlstein EH. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem 275: 37895– 37901, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Zhang W, Anger T, Su J, Hao J, Xu X, Zhu M, Gach A, Cui L, Liao R, Mende U. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J Biol Chem 281: 5811– 5820, 2006 [DOI] [PubMed] [Google Scholar]