Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that promotes cardiomyocyte survival and contributes to ischemic preconditioning. S1P lyase (SPL) is a stress-activated enzyme responsible for irreversible S1P catabolism. We hypothesized that SPL contributes to oxidative stress by depleting S1P pools available for cardioprotective signaling. Accordingly, we evaluated SPL inhibition as a strategy for reducing cardiac ischemia-reperfusion (I/R) injury. We measured SPL expression and enzyme activity in murine hearts. Basal SPL activity was low in wild-type cardiac tissue but was activated in response to 50 min of ischemia (n = 5, P < 0.01). Hearts of heterozygous SPL knockout mice exhibited reduced SPL activity, elevated S1P levels, smaller infarct size, and increased functional recovery after I/R compared with littermate controls (n = 5, P < 0.01). The small molecule tetrahydroxybutylimidazole (THI) is a Federal Drug Administration-approved food additive that inhibits SPL. When given overnight at 25 mg/l in drinking water, THI raised S1P levels and reduced SPL activity (n = 5, P < 0.01). THI reduced infarct size and enhanced hemodynamic recovery in response to 50 min of ischemia and to 40 min of reperfusion in ex vivo hearts (n = 7, P < .01). These data correlated with an increase in MAP kinase-interacting serine/threonine kinase 1, eukaryotic translation initiation factor 4E, and ribosomal protein S6 phosphorylation levels after I/R, suggesting that SPL inhibition enhances protein translation. Pretreatment with an S1P1 and S1P3 receptor antagonist partially reversed the effects of THI. These results reveal, for the first time, that SPL is an ischemia-induced enzyme that can be targeted as a novel strategy for preventing cardiac I/R injury.
Keywords: sphingosine-1-phosphate, sphingolipid, Sgpl1
sphingosine-1-phosphate (S1P) is a sphingolipid metabolite that promotes cell survival and inhibits apoptosis through activation of its cognate G protein-coupled receptors, resulting in the induction of multiple cell survival signaling pathways (4). S1P signaling contributes to cardiac development, angiogenesis, HDL-mediated effects, and regulation of vascular tone and permeability (7, 17, 21, 28). Recent studies (8–13, 15–18, 21, 29, 31, 32, 36) have implicated sphingolipid signaling in protection from ischemia-reperfusion (I/R) injury in the heart and other tissues as well as activation by pre- and postconditioning regimens. These studies collectively indicate that reduced S1P synthesis and signaling contribute to I/R injury, whereas increasing S1P signaling through specific S1P receptors may provide therapeutic benefits in the setting of I/R. Unfortunately, delivery of S1P is challenging due to binding by carrier proteins, ubiquitously expressed receptors, and rapid metabolism. S1P receptor agonists, such as FTY-720, enhance functional recovery after I/R (6). However, FTY-720's clinical utility may be limited by bradycardia stemming from a lack of receptor specificity. Thus, the development of alternative methods for modulating S1P signaling for therapeutic benefits is warranted.
S1P lyase (SPL) catalyzes the irreversible degradation of S1P (14). By reducing S1P pools available for autocrine and paracrine signaling, SPL promotes apoptosis under stress conditions, whereas SPL downregulation promotes cell survival. We hypothesized that modulation of SPL would affect the outcome of I/R injury in the heart. Accordingly, we measured SPL protein expression and activity in cardiac tissue at baseline and during ischemia. We further tested the contribution of SPL to cardiac injury after I/R using a genetic SPL knockout mouse model and a chemical SPL inhibitor. Our results suggest that SPL contributes to I/R injury and that SPL inhibition represents a promising strategy to mitigate cardiac I/R injury.
METHODS
Animals and drug treatment.
This investigation conformed with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). This study was approved by the Institutional Animal Care and Use Committees of the Veterans Affairs Medical Center and Children's Hospital Oakland Research Institute. Male wild-type (WT) C57Bl/6 mice weighing 19–25 g were from Charles River (Hollister, CA). SPL gene-trap knockout mice were a kind gift from Philippe Soriano (Mount Sinai School of Medicine, New York, NY) (22). These mice were maintained on a mixed C57BL6/129sv background by mating of heterozygotes, and WT littermates were used as controls. Genotyping was performed as previously described (3). All mice received standard rodent chow. WT C57Bl/6 mice received vehicle or 25 mg/l tetrahydroxybutylimidazole (THI; ∼200-fold more than would be ingested in a normal human diet per day) administered ad libitum in water containing 5.5 mmol/l glucose for 24 h before euthanasia (23). Glucose was added to the water to improve palatability of the THI solution; water intake was not different between vehicle- and THI-treated groups. SPL knockout mice received only vehicle.
I/R injury.
Ex vivo I/R experiments were performed essentially as previously described (8). WT and heterozygous SPL-null mice (males, weight: 22–25 g) were heparinized (500 U/kg ip) and anaesthetized with pentobarbital sodium (60 mg/kg ip). Hearts were rapidly excised, washed in ice-cold arresting solution (120 mmol/l NaCl and 30 mmol/l KCl), and cannulated via the aorta on a 20-gauge stainless steel blunt needle. Hearts were perfused at 70 mmHg on a modified Langendorff apparatus using Krebs-Henseleit solution containing (in mmol/L) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 24 NaHCO3, 5.5 glucose, and 5.0 sodium pyruvate bubbled with 95% O2-5% CO2 at 37°C.5 Platinum electrodes connected to a stimulus generator (Grass Instruments, West Warwick, RI) were used to pace hearts at 360 beats/min. An isovolumic balloon filled with degassed distilled water was inserted into the left ventricle (LV) to record hemodynamics as previously described (9–11). The protocol consisted of a 20-min equilibration period followed by 50 min of global ischemia and 40 min of reperfusion. In some experiments, the index ischemia time was reduced to 40 min. For ischemic preconditioning (IPC), hearts were equilibrated for 16 min and then subjected to two short cycles of I/R, each consisting of 2 min of global ischemia and 2 min of reperfusion followed immediately by prolonged I/R as described above. Some hearts received the S1P1 and S1P3 receptor antagonist VPC-23019 (Avanti Polar Lipids, Alabaster, AL) administered at 1 μmol/l for 20 min during the 20-min equilibration period.
For the measurement of infarct size after I/R ± IPC, hearts were infused with 15 ml of 1% triphenyltetrazolium chloride (TTC; Sigma) in PBS at a rate of 1.5 ml/min as previously described (9–11). Hearts were then removed from the cannula, weighed, and fixed overnight in 10% formalin. Hearts were removed from formalin and stored at −20°C until being sectioned for the analysis of LV infarct size as previously described by our laboratory (9–11). The infarct size of each section was expressed as a fraction of the area at risk, which was defined as the total area of the LV in this global ischemia model.
Antibodies and reagents.
Antibodies were raised against the murine SPL COOH-terminal peptide VTQGNQMNGSPKPR. Anti-ribosomal S6 protein-phosphoprotein (S6), anti-phospho-eukaryotic translation initiation factor 4E (eIF4E), anti-phospho-MAPK-interacting serine/threonine kinase 1/2 (Mnk1/2), and horseradish peroxidase-linked anti-rabbit IgG were from Cell Signaling (Danvers, MA). Anti-actin and TTC were from Sigma-Aldrich. Rabbit polyclonal anti-β-galactosidase was from Abcam. THI was from Albany Molecular Research. ω-(7-Nitro-2–1,3-benzoxadiazol-4-yl)-d-erythro-S1P (NBD-S1P) and VPC-23019 were from Avanti Polar Lipids.
Western blot analysis and immunohistochemistry.
Measurements of SPL, phospho-eIF4E, phospho-S6, and phospho-Mnk1/2 were performed using standard SDS-PAGE and Western blot analysis as previously described by our laboratory (20). Immunoreactive bands were detected by enhanced chemiluminescence (Amersham Bioscience, Piscataway, NJ) and quantified by the densitometric analysis of digitized autoradiograms with NIH Image 1.61 software.
For immunohistochemistry, β-galactosidase antibody was diluted to 1:500 and incubated for 1 h with fixed cardiac tissue from SPL reporter mice or littermate control mice lacking the reporter. Incubation with secondary antibody (anti-rabbit IgG, diluted 1:1,000) followed for 1 h. Paraffin-fixed sections (4 μm thick) were stained with Mayer's hematoxylin-eosin or immunostained. Dectection of the secondary antibody was performed with the Vectastain ABC Elite Kit (Vector Laboratories). Images of slides were captured using ×20 and ×10 objectives on a Carl Zeiss (Thornwood, NY) AxioScope microscope with an AxioCam camera and processed using Adobe Photoshop (Adobe Systems, San Jose, CA) software.
SPL activity.
SPL activity was determined using a fluorescent assay as previously described by our laboratory (2). Briefly, 20 μM of NBD-S1P in SPL reaction buffer (0.6 mM EDTA, 0.4 mM pyridoxal 5′-phosphate, 3 mM DTT, 70 mM sucrose, 36 mM potassium phosphate buffer, and 36 mM NaF) were mixed with 0.08% of Triton X-100 and briefly sonicated. The reaction was carried out by adding 25–50 μg protein at 37°C for 30 min. At the end of the reaction, lipids were extracted into the organic phase by two-phase separation. The lower organic phase was dried, resuspended in methanol, and injected onto a 4.6 × 75-mm Luna C18 HPLC column (Phenomenex, Torrance, CA). The mobile phase consisted of solvent A (water) and solvent B [methanol-5 mM acetic acid in water-1 M tetra-n-butylammonium dihydrogen phosphate at 95:4:1 (vol/vol/vol)]. The peaks corresponding to fluorescent NBD-containing compounds were integrated for quantification.
S1P quantification.
For S1P measurements, mouse heart tissue, equivalent to 25% of a whole heart, was homogenized in 0.5 ml methanol using a glass homogenizer and a tip sonicator. After homogenization, 1.0 ml chloroform-methanol (1:1) was added, and the sample was incubated over night at 48°C. The sample was dried down and resuspended in 3 ml chloroform-methanol (2:1), and the extract was made basic by adding 50 μl of 1 M KOH in methanol. A two-phase separation was obtained by adding 0.5 ml water. The aqueous phase was recovered and made acidic by adding 0.1 ml concentrated acetic acid. Another two-phase separation was obtained by adding 1 ml chloroform-methanol (2:1), and the organic phase was recovered. S1P was extracted from 10 μl mouse plasma by adding 0.4 ml methanol followed by vortexing and incubation for 30 min at 30°C. The sample was spun in a tabletop centrifuge at 14,000 g, and the supernatant was recovered. [17C]S1P was used as an internal standard. Lipids were separated on a C18 column (2.1 × 50 mm, Kinetex, Phenomenex) at a flow rate of 0.25 ml/min. The gradient used was from 45% to 99% methanol containing 1% acetic acid and 5 mM ammonium acetate. Data were acquired in positive mode on a Micromass Quattro LCZ (Waters) mass spectrometer. Lipids were identified based on their specific precursor and product ion pair and quantitated using multiple reaction monitoring as previously described (27).
Statistical analysis.
Statistical significance was determined by a Student's two-tailed t-test for comparison of the means of two samples. Significance was set at P < 0.05.
RESULTS
SPL expression and activity in the mammalian heart.
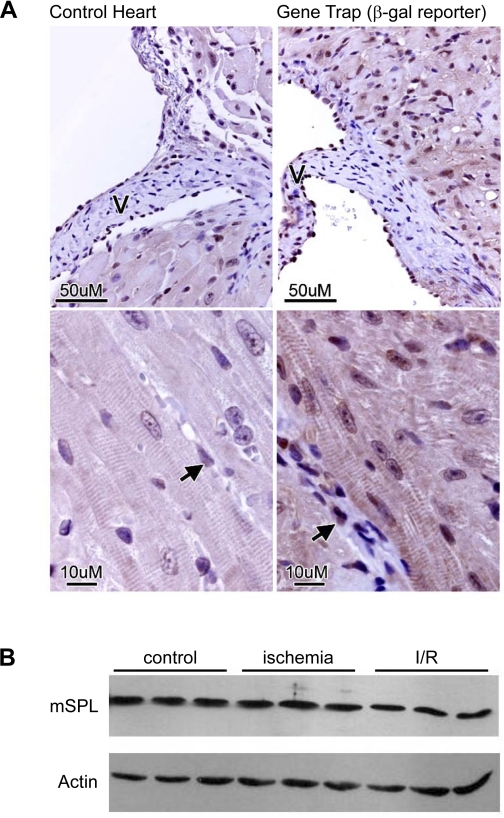
The SPL gene Sgpl1 is transcriptionally active in murine heart tissues (37). Therefore, we compared immunohistochemical detection of a β-galactosidase reporter in hearts of SPL gene-trap knockout mice and WT littermate controls. Our results revealed specific signals in the endothelium, endocardium lining the valve, and cardiomyocytes (Fig. 1A). In contrast, fibroblasts comprising the valve stroma and scattered throughout the myocardium appeared negative. To confirm SPL expression in WT cardiomyocytes, immunoblot analysis of isolated adult murine cardiomyocytes was performed. As shown in the three control lanes in Fig. 1B, SPL protein was readily expressed in isolated heart cells. Consistent with this finding, SPL activity of 2–5 pmol·mg−1·min−1 was detected in adult cardiac tissues.
Fig. 1.
Sphingosine-1-phosphate (S1P) lyase (SPL) expression and activity in the murine heart. A: immunohistochemical detection of a β-galactosidase (β-gal) reporter in hearts of SPL gene-trap reporter mice and reporterless littermate controls. β-Gal was detected in the endothelium (arrows), endocardium lining the valve (V), and cardiomyocytes. B: SPL protein expression in wild-type (WT) heart tissue. See text for details. mSPL, murine SPL.
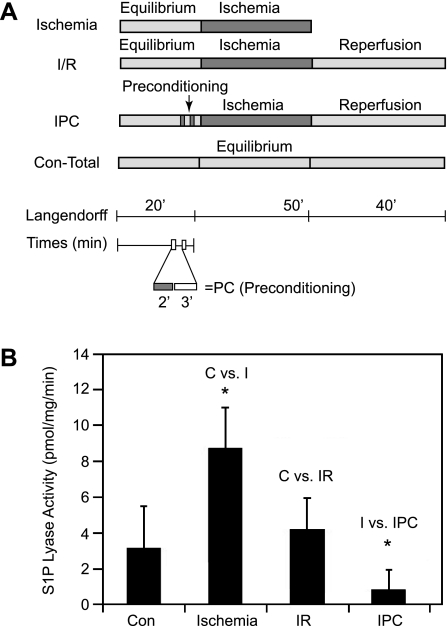
SPL activity is induced during ischemia.
To determine whether cardiac SPL activity is affected by ischemia, the Langendorff model was used to induce ischemia and I/R in ex vivo WT murine hearts using the treatment scheme shown in Fig. 2A. We have previously shown that IPC enhances the biosynthesis of S1P by increasing sphingosine kinase activity (11). Considering these previous findings, we also examined whether IPC influences the activity of the major enzyme responsible for S1P degradation, namely, SPL. As shown in Fig. 2B, SPL activity in ischemic tissues increased threefold over baseline. SPL activity returned to near baseline levels in I/R-treated tissues during reperfusion. SPL activity was lower in IPC plus ischemia-treated tissues than baseline, although the results did not reach statistical significance. In contrast to the dynamic response of SPL activity, SPL protein expression remained constant under these conditions (Fig. 1B). Based on these results, we conclude that SPL is activated by ischemia and may be inhibited by IPC.
Fig. 2.
SPL activity and expression in response to ischemia/reperfusion (I/R) injury in WT hearts. A: I/R protocols. IPC, ischemic preconditioning; Con-Total, 110-min equilibrium normoxic control. B: SPL enzyme activity. n = 5 hearts per treatment. Con and C, control; I, ischemia. *P < 0.05.
SPL loss-of-function mutant mice are resistant to I/R-induced cardiac injury.
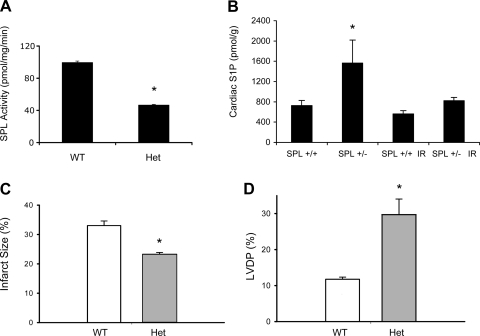
To ascertain whether SPL activation contributes to cardiac I/R injury, we used a genetic model of SPL deficiency. Because mice homozygous for a targeted deletion of the murine SPL gene Sgpl1 do not survive beyond ∼1 mo after birth, we compared mice heterozygous for the Sgpl1-null allele to age- and sex-matched WT littermate controls. Furthermore, to evaluate the importance of SPL in regulating cardiac S1P, we quantified S1P levels in WT and SPL-deficient mouse hearts at baseline and after I/R. As shown in Fig. 3A, tissue SPL activity in heterozygous SPL-null mice is approximately half that of littermate controls. Hearts were isolated from heterozygotes and WT littermate control mice, mounted on a Langendorff apparatus, equilibrated, and subjected to I/R. Hemodynamic function was followed by the continuous monitoring of LV developed pressure (LVDP), and infarct size was measured at the end of the experiment, as described in methods. As shown in Fig. 3B, SPL heterozygotes showed a twofold increase in cardiac S1P levels compared with WT littermates under baseline conditions. After I/R, S1P levels decreased in both heterozygote and WT hearts, consistent with SPL activation. Importantly, as shown in Fig. 3, C and D, mice constitutively deficient in SPL demonstrated significantly reduced infarct size and increased hemodynamic recovery from I/R injury compared with controls.
Fig. 3.
Mouse models with reduced SPL expression are less susceptible to I/R injury. A and B: tissue (intestine) SPL activity (A) and cardiac S1P levels (B) in mice heterozygous (Het) for a gene-trap targeted disruption of Sgpl1 and littermate controls. C and D: infarct size (C) and left ventricular (LV) developed pressure (LVDP; D) after I/R. Four WT and 5 heterozygous mouse hearts were used. *P < 0.05.
Brief oral THI pretreatment affords cardioprotection from I/R-induced injury.
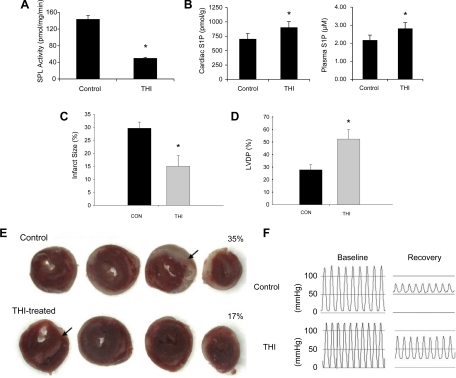
To address whether the cardioprotective effects afforded by SPL reduction observed in a genetic model have translational relevance, it became important to assess the potential cardioprotective effects of pharmacological SPL inhibition. Recently, THI, an imidazole compound found in Federal Drug Administration (FDA)-approved caramel food coloring no. 3, was shown to inhibit SPL when administered orally to mice (23). The only other small-molecule SPL inhibitor that could be used safely in vivo is the vitamin B6 analog deoxypyridoxine, which has nonspecific activity against all vitamin B6-dependent enzymes. Therefore, we used THI to achieve pharmacological SPL inhibition and tested the effect of this strategy on ischemic heart injury in WT hearts. To that end, THI was placed in the drinking water for 24 h before euthanasia. Initially, we tested several different regimens of THI administration, including 24-h treatment with 25 mg/l, 24-h treatment with 50 mg/l, and 3 days of treatment at both 25 and 50 mg/l concentrations. Surprisingly, maximal cardioprotection was obtained using the lower dose of THI for 24 h, whereas increasing the concentration or the length of exposure resulted in diminished treatment efficacy (data not shown).
As shown in Fig. 4, A and B, THI at 25 mg/l given for 24 h reduced tissue SPL activity and raised cardiac and plasma S1P levels. THI treatment reduced the size of infarction from an average of 29.6 ± 2.4% of the risk area in control hearts to 15.1 ± 4.0% in hearts from mice treated with THI (Fig. 4, C and E). Functional recovery was also improved by THI treatment. As shown in Fig. 4D, LVDP at the end of the experiment averaged 27.8 ± 4.1 mmHg in control hearts versus 52.3 ± 7.5 mmHg in THI-treated hearts.
Fig. 4.
Tetrahydroxybutylimidazole (THI) pretreatment protects against I/R injury of hearts from WT mice. A and B: tissue (thymus) SPL activity (A) and cardiac and plasma S1P levels (B) in vehicle- and THI-treated mice. C: infarction size in control and THI-treated mice as percentage of the area at risk, which is the entire LV in this global ischemia model. Seven control mice and six THI-treated mice were used. D: LVDP at the end of reperfusion. E: typical infarcts in control and THI-treated hearts. Arrows point to representative infarct areas. F: representative examples of LVDP in control and THI-treated hearts. As shown, the developed pressure was substantially greater in the THI-treated heart at the end of reperfusion. See D for group details. *Statistical significance (P < 0.05).
S1P cell surface receptor antagonism reduces the effect of THI.
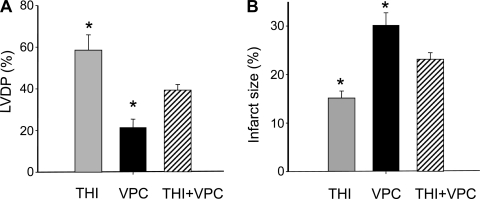
We next asked whether inhibition of cell surface S1P receptors would blunt the effects of THI. In isolated mouse hearts, we infused the S1P1 and S1P3 receptor antagonist VPC-23019 at a concentration of 1 μmol/l for 20 min during 20 min of equilibration. Hearts (n = 4 hearts/group) were then subjected to 50 min of ischemia and 50 min of reperfusion. Hearts were from mice treated with THI with or without VPC-23019 or controls treated with the S1P receptor antagonist alone. As shown in Fig. 5, A and B, VPC-23019 alone had no effect on infarct size and hemodynamic recovery compared with hearts from mice not treated with this agent (compare with Fig. 4, C and D). As expected, THI treatment by itself dramatically reduced infarct size and markedly improved hemodynamic recovery, also consistent with the data shown in Fig. 4, C and D. VPC-23019 pretreatment of hearts from mice that received THI showed an intermediate response: infarct size was significantly reduced but not to the level of THI alone. The same was true for LVDP, which was augmented but not to the level of the hearts treated with THI alone. These data establish receptor involvement in THI-mediated cardioprotection but do not exclude an intracellular role for S1P.
Fig. 5.
The S1P cell surface receptor antagonist VPC-23019 (VPC) reduces the effect of THI. Mouse hearts were subjected to I/R injury as described in methods (n = 4 hearts/group). A: compared with THI, the S1P antagonist VPC had no significant effect on LVDP at the end of reperfusion. Values, expressed as a percentage of baseline, were similar to those obtained in the absence of VPC (compare with Fig. 4D). In hearts harvested from THI-treated mice, the results were intermediate. B: Infarct size, expressed as a percentage of the risk area, which is the entire LV in this global ischemia model, was reduced by treatment with THI, and VPC alone had no effect on the extent of infarction (compare with Fig. 4C). However, in the presence of VPC, the results in THI-treated mice were intermediate. These data establish S1P receptor involvement but also suggest a possible intracellular role for S1P. Statistics were determined by one-way ANOVA with post hoc Student-Newman-Keuls testing. *P < 0.05 vs. all other conditions.
SPL inhibition potentiates the activation of key regulators of protein translation after I/R.
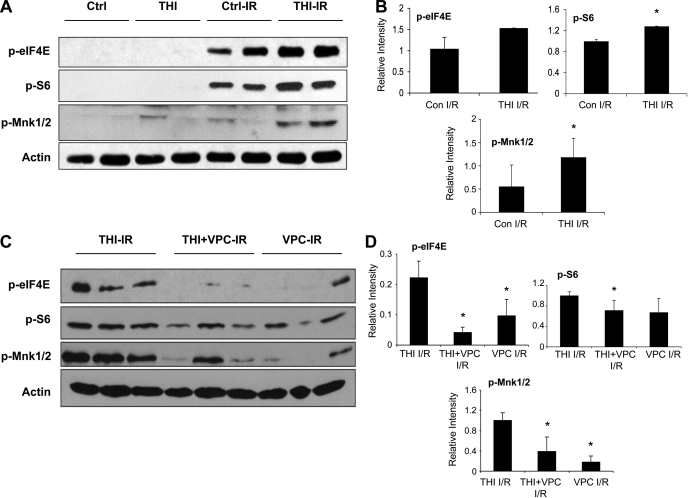
Cell survival and containment of tissue damage after I/R are facilitated by stress-induced signaling pathways that activate the protein translational machinery. To explore whether SPL inhibition influences this process, we measured the activation status of two key components of the translational machinery, ribosomal S6 protein and eIF4E, by monitoring their phosphorylation status in the hearts of WT THI-treated and vehicle-treated WT mice. As shown in Fig. 6A, phosphorylation of eIF4E and S6 were both elevated after I/R in control and THI-treated hearts. However, phosphorylation of both these proteins was significantly higher in THI-treated hearts. Similarly, hearts of THI-treated mice subjected to I/R exhibited elevated levels of phosphorylated Mnk1/2, the protein kinase responsible for eIF4E phosphorylation, compared with vehicle-treated I/R control hearts. Quantitative analysis of this immunoblot is shown in Fig. 6B. Quantitated differences in protein levels between THI-treated and vehicle-treated hearts reached statistical significance (P < 0.05) except for eIF4E (P = 0.05). Based on these results, we conclude that THI treatment activates a signaling pathway that involves Mnk/eIF4E and ribosomal S6 protein.
Fig. 6.
THI treatment potentiates the activation of protein translation after I/R in WT mouse hearts. A: phosphorylated eukaryotic translation initiation factor 4E (p-eIF4E), phosphorylated ribosomal S6 protein (p-S6), phosphorylated MAPK-interacting serine/threonine kinase 1/2 (p-Mnk), and actin levels in heart tissues from THI-treated and control (Ctrl) mice before and after I/R. B: ImageJ quantification of post-I/R signals normalized to actin. These blots are representative of 4 separate experiments. *P < 0.05 vs. control I/R. C: p-eIF4E, p-S6, p-Mnk, and actin levels in tissues from THI-, VPC-, and THI + VPC-treated hearts after I/R. D: ImageJ quantification of the results shown in C. *P < 0.05 vs. THI I/R.
Importantly, we found that the levels of eIF4E, Mnk1/2, and S6 phosphorylation observed in heart tissues exposed to the combination of THI pretreatment administered in vivo and VPC-23019 given ex vivo before I/R were markedly lower than those observed in heart tissues exposed to THI alone. In the case of eIF4E, phosphorylation was almost completely blocked, whereas Mnk1/2 and S6 phosphorylation levels were intermediate between those observed in hearts receiving either agent alone, as shown in Fig. 6, C and D. We observed some variability in our results, some of which may be accounted for by the use of different antibody lots between the two experiments. Despite these variations, our results clearly show that THI effects on the protein translational machinery are attenuated by VPC-23019. These results demonstrate that the activation status of the Mnk/eIF4E and S6 signaling pathway correlates with THI-mediated cardioprotection. Furthermore, both THI-mediated cardioprotection and its effects on the protein translation machinery appear to be at least partially dependent on S1P receptor signaling.
DISCUSSION
The principal and most remarkable finding of this study is that we show, for the first time, that short-term inhibition of SPL protects mouse hearts against I/R injury. Our study also reveals that SPL is expressed in the murine heart. Although cardiac SPL protein levels remained constant under different ex vivo conditions, SPL activity was rapidly induced in hearts subjected to I/R, presumably through a posttranslational mechanism. This possibility is consistent with the identification of SPL in a screen for nitrosylated proteins and with the presence of potential sites for protein kinase phosphorylation within mammalian SPL protein sequences and the recent identification of SPL as a target of phosphorylation by ataxia telangiectasia mutated/ ataxia telangiectasia and Rad3 related (ATM/ATR) (19, 24, 35).
In our study, a combination of genetic and pharmacological models of SPL inhibition was used to test the role of SPL in I/R, since no specific inhibitor of SPL is available to date. We observed cardioprotection in a genetically altered mouse model exhibiting reduced SPL expression and activity and increased S1P levels. This model was generated by gene-trap mutagenesis, resulting in a null allele (22). In the homozygous state, SPL mutants are runted and die early, whereas heterozygotes are reproductive and live a normal lifespan. In this mouse model, reduced SPL activity observed in the heterozygote does not confer a phenotype in the heart under resting conditions, but hearts from these mice exhibit diminished susceptibility to I/R injury.
Importantly, in WT C57/Bl6 mice, inhibition of SPL by placing the FDA-approved food additive THI (caramel coloring no. 3) in drinking water for 24 h increased S1P levels, reduced infarct size, and improved functional recovery after I/R. The results we obtained using both of these models strongly implicate SPL as the target of THI that is responsible for mediating cardioprotection. Some differences in the extent of responses between THI administration and SPL heterozygosity were noted. This may be accounted for by the fact that these two experiments were performed in mouse lines of different genetic backgrounds. However, the most likely explanation for this difference is that the heterozygous SPL-null mouse represents a chronic state of inhibition of the sphingolipid degradative pathway. In homozygotes, this has been shown to result in the accumulation of other sphingolipid intermediates upstream of S1P that may be damaging to the myocardium, such as ceramide or sphingosine (3). We suspect that targeting SPL using a small-molecule inhibitor for a brief time period avoids the potential untoward complications of the genetic model, thereby unmasking the full effect of S1P as a cardioprotectant.
The Langendorff system we used for these experiments is a constant-pressure system set at 70 mmHg (11). Since coronary perfusion pressure was constant, coronary flow reflects coronary vascular resistance. At baseline, coronary flow, as measured by the coronary sinus effluent over time, did not differ between the control and THI-treated groups (2.9 ± 0.04 vs. 2.92 ± 0.03 ml/min). After recovery from I/R injury, the coronary flow was higher in the THI-treated group (1.88 ± 0.07 vs. 1.52 ± 0.06 ml/min, n = 6, P < 0.01). The following factors may be involved: control hearts had a larger infarct size, which leads to a larger area of no flow and, hence, an overall reduction in coronary flow. Also, as shown in Fig. 4, I/R injury resulted in higher diastolic pressures and lower systolic pressures in control versus THI-treated hearts. The marked reduction in developed pressure would also be expected to contribute to a reduction in coronary flow. Similar results were found in the heterozygous SPL hearts subjected to I/R injury compared with WT controls.
As noted above, there is substantial evidence that the administration of S1P acutely is cardioprotective (8–12, 17, 21, 29, 31, 32, 36). Although the degree of S1P elevation in response to THI pretreatment was modest, it exceeded endogenous levels relative to baseline achieved by IPC. These levels are sufficient to mediate cardioprotection in rat models of I/R, as we have previously reported (32, 33). We observed that pretreatment of mice with a low concentration of THI for 24 h afforded greater protection than did a higher dose and/or longer treatment periods, such as those used by Schwab et al. (23) to induce lymphocyte depletion. In this connection, it has recently been reported that acute in vitro S1P degradation is primarily driven by lyase cleavage, a process that takes only 40–100 min (25). The translational relevance of these findings will require further testing using SPL inhibitory strategies in postischemic delivery regimens and/or through the use of inducible SPL knockout mouse models.
The shortened lifespan and congenital anomalies observed in homozygous SPL-null mice demonstrate that complete SPL inhibition is not well tolerated and that SPL is an essential protein (3, 22, 34). In contrast, mice exhibiting low levels of SPL expression and activity do not exhibit notable phenotypes, and pharmacological inhibition of SPL has proven useful for immunomodulation (1, 34). Thus, we interpret our data to indicate that brief and incomplete inhibition of SPL may serve to raise and maintain S1P during I/R injury at levels sufficient to capitalize on the protective effects of S1P signaling. Accordingly, the complications that may be associated with long-term total inhibition of sphingolipid degradation can be avoided.
We hypothesize that cardioprotection associated with SPL inhibition or reduced SPL expression can be attributed primarily to the accumulation of S1P and the resulting intracellular and/or receptor-mediated effects. Because THI increases S1P levels both in tissue and plasma, we sought to determine whether cell surface S1P receptors are involved in its beneficial effects. We found that the S1P1 and S1P3 receptor antagonist VPC-23019 significantly reduced the cardioprotective effects of THI feeding. However, there was still a residual reduction of infarct size and an increase in hemodynamic recovery. One possibility is that there is an additional effect of THI on intracellular or even on mitochondrial S1P. Recent evidence has identified a pool of S1P generated by sphingosine kinase-2 that regulates gene transcription (5). Of direct pertinence to the present study is that S1P produced by sphingosine kinase-2 in the mitochondria interacts with prohibitin 2 to regulate the assembly and function of a key member of the electron transport chain, complex IV, or cytochrome c oxidase (26). By raising intracellular S1P levels, THI might affect one or both of these processes. Whether this is the case awaits future investigation.
However, it is possible that other factors may be involved. For example, SPL catalyzes the formation of the reactive long-chain aldehyde hexadecenal. The latter could enhance oxidant stress produced by I/R, thereby potentiating tissue injury. Conversely, oxidant stress during ischemia may be equivalent or even additive to pharmacological postconditioning with S1P, which is cardioprotective (8, 32). Future studies will be required to clarify how modulation of SPL expression and activity influence the heart in the context of I/R and whether S1P and S1P receptors are necessary for mediating the cardioprotective effects of THI. SPL is expressed in cardiomyocytes, fibroblasts, and endothelial cells as well as in other cells and tissues extrinsic to the heart. Further studies using tissue-specific SPL knockout models may be helpful in identifying the specific tissue source of SPL that is required for modulating the cardiac response to I/R.
We observed no obvious differences in apoptotic or autophagic programmed cell death between THI- and vehicle-treated ischemic heart tissues, based on levels of the cleaved form of the caspase-3 substrate poly(ADP-ribose) polymerase as a marker of apoptosis and the processed form of the autophagy marker LC3 by immunoblot analysis (data not shown). These were not unexpected findings, since it would be unlikely for either intrinsic or extrinsic apoptotic pathways to be activated within the short time frame in which our experiments were conducted. Based on our results, we attribute the protection from myocardial tissue injury afforded by THI to the prevention of necrotic cell death.
The effects of THI pretreatment on I/R recovery and infarct size correlated with elevated levels of phosphorylated S6, Mnk1/2, and eIF4E. Furthermore, both THI-mediated cardioprotection and protein phosphorylation effects were partially reversed by inhibition of signaling through S1P1 and S1P3 receptors. Thus, the effects of THI on S1P receptor signaling appear to contribute to its effects on the protein translational machinery. All three of these proteins are downstream targets of stress-activated prosurvival signaling pathways that are influenced by S1P, including phosphatidylinositol 3-kinase, and MEK/ERK MAPK pathways (29, 30, 36). Our cumulative findings suggest that pretreatment with THI primes specific stress-activated signaling pathways, thereby preparing tissues to respond to ischemic injury and contributing to its cardioprotective effects. Preservation of protein translational activity may contribute to the ability of THI to mediate cardioprotection, but it is likely that other factors affected by the immediate response to stress signaling are also involved.
In summary, our findings demonstrate that short-term SPL inhibition may be a novel strategy to protect against acute cardiac I/R injury. Small-molecule inhibitors of SPL are currently in clinical trials as immunomodulatory agents. Based on our findings, we hypothesize that these agents could potentially mitigate I/R injury and other forms of cardiac insult or ischemic tissue damage. In conclusion, this is the first demonstration that short-term SPL inhibition using an FDA-approved food additive is cardioprotective.
GRANTS
This work was supported by National Institutes of Health Grants GM-66954 and CA-77528 (to J. D. Saba), HL-090606 (to J. S. Karliner), and K26-RR-0243037 (to A. D. Borowsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bagdanoff J, Donoviel M, Nouraldeen A, Tarver J, Fu Q, Carlsen M, Jessop T, Zhang H, Hazelwood J, Nguyen H, Baugh S, Gardyan M, Terranova K, Barbosa J, Yan J, Bednarz M, Layek S, Courtney L, Taylor J, Digeorge-Foushee A, Gopinathan S, Bruce D, Smith T, Moran L, O'Neill E, Kramer J, Lai Z, Kimball S, Liu Q, Sun W, Yu S, Swaffield J, Wilson A, Main A, Carson K, Oravecz T, Augeri D. Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J Med Chem 52: 3941– 3953, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Bandhuvula P, Fyrst H, Saba J. A rapid fluorescent assay for sphingosine-1-phosphate lyase enzyme activity. J Lipid Res 48: 2769– 2778, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bektas M, Allende ML, Lee BG, Chen W, Amar MJ, Remaley AT, Saba JD, Proia RL. Sphingosine 1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J Biol Chem 285: 10880– 10889, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fyrst H, Saba J. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol 6: 489– 497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325: 1254– 1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res 83: 285– 293, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res 82: 212– 220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin Z, Karliner J, Vessey D. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res 79: 134– 140, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Jin Z, Zhang J, Huang Y, Hoover H, Vessey D, Karliner J. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res 76: 41– 50, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Jin Z, Zhou H, Zhu P, Honbo N, Mochly-Rosen D, Messing R, Goetzl E, Karliner J, Gray M. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC-ε knockout mouse hearts. Am J Physiol Heart Circ Physiol 282: H1970– H1977, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation 110: 1980– 1989, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol 33: 1713– 1717, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Keul P, Sattler K, Levkau B. HDL and its sphingosine-1-phosphate content in cardioprotection. Heart Fail Rev 12: 301– 306, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kumar A, Saba JD. Lyase to live by: sphingosine phosphate lyase as a therapeutic target. Expert Opin Ther Targets 13: 1013– 1025, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levkau B, Hermann S, Theilmeier G, van der Giet M, Chun J, Schober O, Schäfers M. High-density lipoprotein stimulates myocardial perfusion in vivo. Circulation 110: 3355– 3359, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Means C, Xiao C, Li Z, Zhang T, Omens J, Ishii I, Chun J, Brown J. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H2944– H2951, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Means CK, Brown JH. Sphingosine 1-phosphate receptor signalling in the heart. Cardiovasc Res 82: 193– 200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nofer JR, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A, Ishii I, Kleuser B, Schäfers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest 113: 569– 581, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsen J, Vermeulen M, Santamaria A, Kumar C, Miller M, Jensen L, Gnad F, Cox J, Jensen S, Nigg E, Bruna S, Mann M. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3: ra3, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Oskouian B, Sooriyakumaran P, Borowsky A, Crans A, DIllard-Telm L, Tam Y, Bandhuvula P, Saba J. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is downregulated in colon cancer. Proc Natl Acad Sci USA 103: 17384– 17389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc Res 82: 201– 211, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Schmahl J, Raymond CS, Soriano P. PDGF signaling specificity is mediated through multiple immediate early genes. Nat Genet 39: 52– 60, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Schwab S, Pereira J, Matloubian M, Xu Y, Huang Y, Cyster J. Lymphocyte sequestration through S1P lyase inhibition an disruption of S1P gradients. Science 309: 1735– 1739, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul 50: 349– 362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siow D, Anderson C, Berdyshev E, Skobeleva A, Pitson S, Wattenberg B. Intracellular localization of sphingosine kinase 1 alters access to substrate pools but does not affect the degradative fate of sphingosine-1-phosphate. J Lipid Res 51: 2546– 2559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J 25: 600– 612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullards MC, Merrill AHJ. Analysis of sphingosine-1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography. Sci STKE 67: 1– 11, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta 1781: 483– 488, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Tao R, Hoover H, Honbo N, Kalinowski M, Alano C, Karliner J, Raffai R. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia/reoxygenation through lipoprotein-associated sphingosine-1-phosphate. Am J Physiol Heart Circ Physiol 298: H1022– H1028, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tao R, Hoover H, Zhang J, Honbo N, Alano C, Karliner J. Cardiomyocyte S1P1 receptor-mediated ERK signaling and desensitization. J Cardiovasc Pharmacol 53: 486– 494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tao R, Zhang J, Vessey D, Honbo N, Karliner J. A sphingosine kinase-1 mutation determines cell fate during hypoxia in adult mouse cardiomyocytes. Cardiovasc Res 74: 56– 63, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Vessey D, Li L, Honbo N, Karliner J. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol 297: H1429– H1435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit 12: BR318– BR324, 2006 [PubMed] [Google Scholar]
- 34. Vogel P, Donoviel M, Read R, Hansen G, Hazlewood J, Anderson S, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS ONE 4: e4112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal Biochem 354: 279– 289, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol 293: H3150– H3158, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Zhou J, Saba J. Identification of the first mammalian sphingosine phosphate lyase gene and its functional expression in yeast. Biochem Biophys Res Commun 242: 502– 507, 1998 [DOI] [PubMed] [Google Scholar]