Abstract
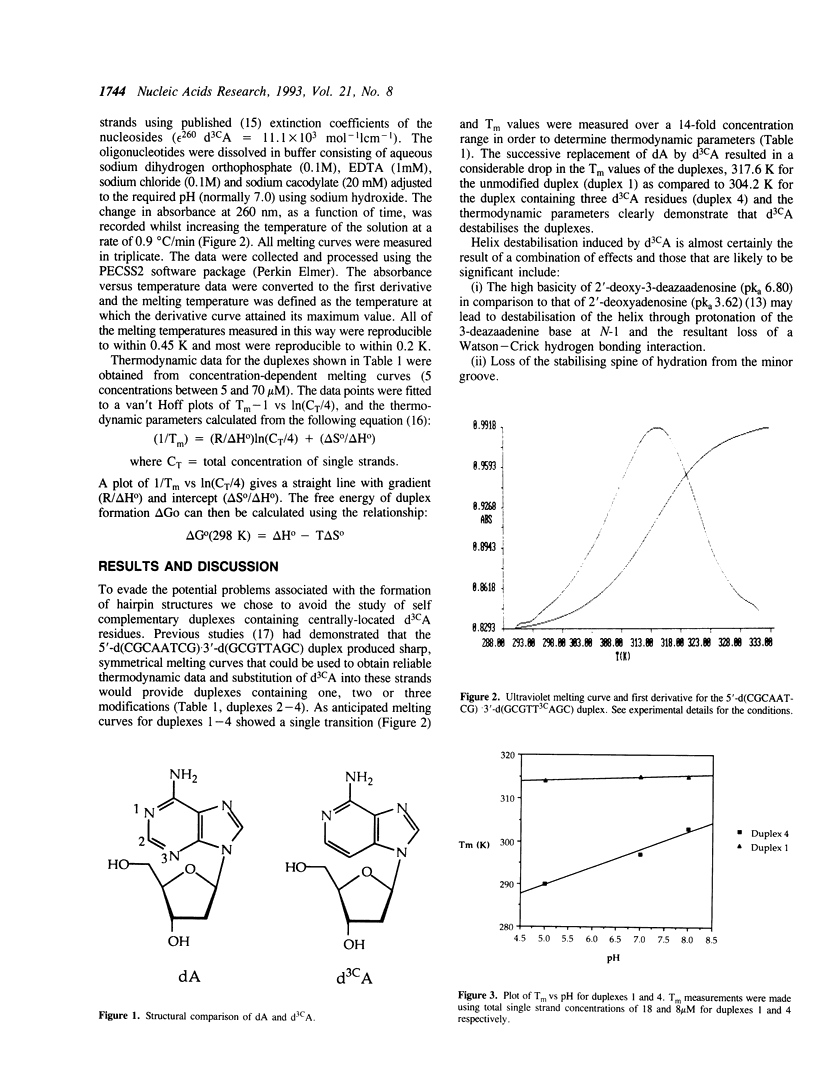
We have used ultraviolet melting techniques to study the effect on stability of incorporating the nucleoside analogue 2'-deoxy-3-deazaadenosine (d3cA) into the duplex 5'-d(CGCAATCG)-3'-d(GCGTTAGC). Our results demonstrate that the successive replacement of dA by d3CA increasingly destabilises the duplex. The destabilising effect of this analogue is considerably enhanced as the pH is lowered and the results are consistent with protonation of 3-deazaadenine (probably at N-1) contributing to duplex destablisation. Surprisingly, the incorporation of d3CA does not significantly affect the binding of distamycin-A.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuprina V. P. Anomalous structure and properties of poly (dA).poly(dT). Computer simulation of the polynucleotide structure with the spine of hydration in the minor groove. Nucleic Acids Res. 1987 Jan 12;15(1):293–311. doi: 10.1093/nar/15.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosstick R., Li X., Tuli D. K., Williams D. M., Connolly B. A., Newman P. C. Molecular recognition in the minor groove of the DNA helix. Studies on the synthesis of oligonucleotides and polynucleotides containing 3-deaza-2'-deoxyadenosine. Interaction of the oligonucleotides with the restriction endonuclease EcoRV. Nucleic Acids Res. 1990 Aug 25;18(16):4771–4778. [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Ebel S., Lane A. N., Brown T. Very stable mismatch duplexes: structural and thermodynamic studies on tandem G.A mismatches in DNA. Biochemistry. 1992 Dec 8;31(48):12083–12086. doi: 10.1021/bi00163a017. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Gaines P. C., Karlinsey J. E., Vinayak R., Simon M. I. Sequence-specific interaction of the Salmonella Hin recombinase in both major and minor grooves of DNA. EMBO J. 1992 Jul;11(7):2695–2705. doi: 10.1002/j.1460-2075.1992.tb05335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Fukui T., Uesugi S. Polynucleotides. XXI. Synthesis and properties of poly 1-deazaadenylic acid and poly 3-deazaadenylic acid. J Biochem. 1974 Jul;76(1):107–115. doi: 10.1093/oxfordjournals.jbchem.a130534. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987 Sep;26(9):1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- Newman P. C., Nwosu V. U., Williams D. M., Cosstick R., Seela F., Connolly B. A. Incorporation of a complete set of deoxyadenosine and thymidine analogues suitable for the study of protein nucleic acid interactions into oligodeoxynucleotides. Application to the EcoRV restriction endonuclease and modification methylase. Biochemistry. 1990 Oct 23;29(42):9891–9901. doi: 10.1021/bi00494a020. [DOI] [PubMed] [Google Scholar]
- Newman P. C., Williams D. M., Cosstick R., Seela F., Connolly B. A. Interaction of the EcoRV restriction endonuclease with the deoxyadenosine and thymidine bases in its recognition hexamer d(GATATC). Biochemistry. 1990 Oct 23;29(42):9902–9910. doi: 10.1021/bi00494a021. [DOI] [PubMed] [Google Scholar]
- Ono A., Ueda T. Minor-groove-modified oligonucleotides: synthesis of decadeoxynucleotides containing hypoxanthine, N2-methylguanine and 3-deazaadenine, and their interactions with restriction endonucleases Bgl II, Sau, 3AI, and Mbo I (Nucleosides and Nucleotides Part 75). Nucleic Acids Res. 1987 Apr 10;15(7):3059–3072. doi: 10.1093/nar/15.7.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton J. G., Wemmer D. E. Structural modeling of the distamycin A-d(CGCGAATTCGCG)2 complex using 2D NMR and molecular mechanics. Biochemistry. 1988 Oct 18;27(21):8088–8096. doi: 10.1021/bi00421a018. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Schultz P. G., Dervan P. B. Distamycin and penta-N-methylpyrrolecarboxamide binding sites on native DNA. A comparison of methidiumpropyl-EDTA-Fe(II) footprinting and DNA affinity cleaving. J Biomol Struct Dyn. 1984 Mar;1(5):1133–1147. doi: 10.1080/07391102.1984.10507508. [DOI] [PubMed] [Google Scholar]
- Seela F., Grein T. 7-Deaza-2'-deoxyadenosine and 3-deaza-2'-deoxyadenosine replacing dA within d(A6)-tracts: differential bending at 3'- and 5'-junctions of d(A6).d(T6) and B-DNA. Nucleic Acids Res. 1992 Jul 11;20(13):2297–2306. doi: 10.1093/nar/20.9.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]



