Abstract
Two major mechanisms have been postulated for the arrhythmogenic tendency observed in Brugada Syndrome (BrS): delays in conduction or increased heterogeneities in repolarization. We use a contact mapping system to directly investigate the interacting roles of these two mechanisms in arrhythmogenesis using a genetic murine model for BrS for the first time. Electrograms were obtained from a multielectrode recording array placed against the left ventricle and right ventricle (RV) of spontaneously beating Langendorff-perfused wild type (WT) and Scn5a+/− mouse hearts. Scn5a+/− hearts showed activation waves arriving at the epicardial surface consistent with slowed conduction, which was exacerbated in the presence of flecainide. Lines of conduction block across the RV resulting from premature ventricular beats led to the formation of reentrant circuits and polymorphic ventricular tachycardia. WT hearts showed an inverse relationship between activation times and activation recovery intervals measured at the epicardial surface, which resulted in synchronicity of repolarization times. In contrast, Scn5a+/− hearts, despite having smaller mean activation recovery intervals, demonstrated a greater heterogeneity compared with WT. Isochronal maps showed that their normal activation recovery interval gradients at the epicardial surface were disrupted, leading to heterogeneity in repolarization times. We thus directly demonstrate the initiation of arrhythmia in the RV of Scn5a+/− hearts. This occurs as a result of the combination of repolarization heterogeneities leading to lines of conduction block and unidirectional conduction, with conduction slowing allowing the formation of reentrant circuits. The repolarization heterogeneities may also be responsible for the changing pattern of block, leading to the polymorphic character of the resulting ventricular tachycardia.
Keywords: Brugada Syndrome, transgenic mouse, arrhythmia, conduction
brugada syndrome (BrS) is associated with a loss of Na+ channel function and increased risks of polymorphic ventricular tachycardia (VT). Right precordial ST elevation, right bundle branch block, and alterations in right ventricular (RV) epicardial action potential (AP) waveforms (13) localize the origin of arrhythmogenesis to the RV. Previous studies have implicated either slowed RV AP conduction (26) or alterations in AP repolarization (3, 13) in the arrhythmogenic mechanism.
However, evidence implicating conduction alterations mainly comes from clinical studies, which are limited by primarily using noninvasive techniques such as echocardiography, signal-averaged ECG, and body surface mapping (8, 26). A single ex vivo study of the heart from a BrS patient demonstrated local conduction delays in the RV outflow tract (RVOT) but in an absence of transmural differences in repolarization (5).
Furthermore, human evidence for the repolarization hypothesis is difficult to obtain due to the need for simultaneous endocardial and epicardial recordings. There are single catheter recordings that have shown differences in activation recovery interval (ARI) between RV endocardium and epicardium (18, 19). Most evidence for the repolarization hypothesis is, however, derived from studies in the canine arterial wedge preparation, in which BrS is modeled through pharmacological maneuver with potentially nonspecific effects (1, 12). So far, there is only one available clinical study that has showed both conduction and repolarization disturbances (14).
Experimental systems that contain genetic modifications directly replicating those known to exist in BrS may provide more specific models to clarify physiological abnormalities associated with the disease condition, while permitting invasive studies impractical in human subjects. BrS is a genetically heterogeneous condition. Nevertheless, up to 30% of patients have mutations in the SCN5A gene, which encodes the cardiac Nav1.5 α-subunit (9). Thus far it is the only gene that has been extensively studied in connection with BrS. Our group has produced a heterozygotic Scn5a+/− mouse, which shows a 50% reduction in the transmembrane Na+ current (20). While loss of function mutations in the SCN5A gene both clinically (30) and in our mouse model (20) may lead to a complex range of phenotypes, incorporating sick sinus syndrome and progressive conduction disorders, the Scn5a+/− mouse has been shown to closely reproduce many of the key human features of BrS. Thus it demonstrates ST elevation (16) and an enhanced ventricular arrhythmogenesis that is exacerbated by flecainide and relieved by quinidine (16, 17). Its monophasic AP recordings have shown delayed response latencies and increased transmural gradients of repolarization across the RV (17), as well as increased heterogeneity of refractory periods and steeper restitution curves within the RV epicardium (15). Furthermore, the Scn5a+/− mice have been shown to exhibit fibrosis specific to the RV, which worsens with age (28), in line with similar clinical findings (5).
The present studies now apply a multielectrode mapping system to perform a geometrical analysis of activation times (ATs), repolarization times (RTs), and ARIs at the epicardial surface and to calculate their dispersions, clarifying the interaction of depolarization and repolarization disorders in Scn5a+/− hearts. Furthermore, we generated isochronal maps that demonstrated the production of arrhythmogenic substrate through reentrant circuits. This information may contribute to future work investigating possible pharmacological treatments for a disease where the current mainstay of treatment is implantable cardioverter-defibrillator implantation.
While the majority of previous mapping studies have used epicardial pacing to give activation and repolarization data, we use spontaneously beating hearts to provide a more accurate representation of the electrical patterns occurring at the epicardium through physiological activation. While we can still only measure the outcomes of these electrical wavefronts in the form of their arrival at the epicardial surface, we believe that these provide a more representative insight into possible arrhythmogenic mechanisms occurring in vivo in Scn5a+/− hearts.
MATERIALS AND METHODS
Langendorff perfusion.
Heterozygote Scn5a+/− 129/sv mice aged 4 to 8 mo were used, with littermate WT controls. Experiments used a Langendorff-perfused preparation adapted for the murine heart (17). The heart was then carefully but rapidly excised and placed in ice-cold bicarbonate-buffered Krebs-Henseleit solution that contained the following (in mM): 119 NaCl, 25 NaHCO3, 4 KCl, 1.2 KH2PO4, 1 MgCl2, 1.8 CaCl2, 10 glucose, and 2 sodium pyruvate (pH 7.4). The solution was bubbled with a 95% O2-5% CO2 gas mixture (British Oxygen, Manchester, UK). Tissues surrounding the heart were carefully removed leaving a small (3–4 mm) section of aorta. The aorta was then cannulated under the buffer surface with a 21-gauge tailor-made cannula constructed from a blunted needle attached to small length of plastic tubing that had been prefilled with ice-cold buffer solution to prevent air bubbles from entering the system.
The aorta was then attached to the cannula needle with a metal clip and transferred to the perfusion apparatus, where perfusion was commenced with the aforementioned bicarbonate-buffered Krebs-Henseleit solution. The perfusate was maintained at a flow rate of 2–2.5 ml/min using a peristaltic pump (Watson-Marlow Bredel Pumps model 505S; Falmouth, Cornwall, UK). It was passed through 200-μm and 5-μm pore-size filters (Millipore, Watford, UK) and warmed and maintained at 37°C via a water jacket and circulator (Techne model C-85A; Techne, Cambridge, UK). An equilibration time of 15 min was used before experiments were commenced.
In experiments with drugs, 10 μM flecainide and 5 μM quinidine (Sigma-Aldrich, Poole, UK) were perfused for 15 min before data acquisition. Concentrations were within the same range as clinical therapeutic levels used in BrS (flecainide: 0.2–0.9 mg/l or 4.8–21.7 μM; quinidine: 1.0–3.0 mg/l or 3.5–11 μM) (21). All procedures conformed to the UK Animals (Scientific Procedures) Act 1986. Experiments were performed under personal and project licenses (PIL 80/1243 and PPL 80/1974) approved by local ethical committees and issued by the UK Home Office.
Monophasic action potential recording.
Epicardial monophasic action potentials (MAPs) were recorded using a MAP electrode as previously described (17). MAP waveforms were analyzed using Spike2 software (Cambridge Electronic Design) and the action potential duration (APD) to 70% repolarization measured.
Multielectrode array.
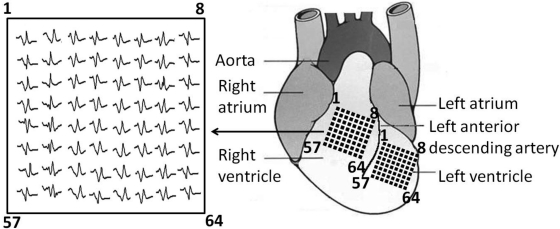
The custom-built multielectrode array consisted of 64 separate electrodes (Teflon-coated silver wires; 0.125-mm diameter; Science Products) arranged in an 8 × 8 configuration (Fig. 1). The interelectrode distance was 0.55 mm, and thus the total dimensions of the entire array were 3.85 × 3.85 mm. The array was connected through shielded wires to two 32-channel amplifiers (SCXI-1102C, 10-kHz bandwidth; National Instruments, Newbury, UK). The sampling frequency of the recording system was 2 kHz. The array was placed against either the left ventricular (LV) or RV surface with channel 1 near the base of the heart and channel 64 near the apex. The position of the array was determined in a consistent manner using the anatomical landmarks of the left anterior descending artery, the aorta, and the atria. Unipolar electrogram recordings were made from hearts beating spontaneously. A reference electrode was placed on the opposite ventricle, remote from the recording sites. An ECG was simultaneously recorded by placing ECG leads on either side of the heart.
Fig. 1.
Diagram showing the multielectrode array placed on the surface of the left ventricle (LV) or right ventricle (RV), with channel 1 near the base of the heart and channel 64 near the apex. Position of the array was determined using the anatomical landmarks of the left anterior descending artery, the aorta, and the atria. Inset: typical set of electrogram traces.
As repolarization is dependent on cycle length, to be able to compare RTs between hearts only recordings with hearts beating spontaneously with a cycle length of between 130 and 150 ms were used in our subsequent analyses. In hearts where cannulation was performed accurately with no ischemic damage, the majority had a spontaneous cycle length falling within these limits. There was no statistically significant difference in cycle lengths between any of the experimental groups compared (e.g., WT: 141 ± 2 ms; Scn5a+/−: 144 ± 3 ms; P = 0.35; n = 12). Furthermore, in recordings in individual hearts where cycle length fluctuated between these values, there was seen to be no significant effect on the ARI, with the mean difference between ARIs measured at given points in individual hearts at shortest cycle lengths and those at longest cycles lengths only 0.7 ± 0.06 ms, which is significantly smaller than the spatial ARI heterogeneity recorded even in the WT LV (mean ARI difference = 4.4 ± 0.6 ms; P < 0.001; n = 12). Lastly, the qualitative pattern of repolarization was similar within groups at any cycle length within the range studied.
Electrograms were biphasic, consisting of an initial negative waveform followed by a positive waveform. The AT, determined as the point of initial maximal negative slope dV/dtmin of the initial negative deflection, was measured using Spike2 software. Isochronal propagation maps of ATs, measured relative to the earliest AT in the array, were derived using Origin (MicroCal). The ARI was defined as the interval between AT and RT, and isochronal maps were drawn of ARIs, measured relative to the shortest ARI in the array. The RT was measured at the dV/dtmin of the second negative deflection, and isochronal maps of RTs, measured relative to the earliest RT in the array, were drawn. Use of the ARI as measured from unipolar elecrograms has been previously validated in humans using the dV/dtmax of the T wave (22). However, we acknowledge that the unipolar electrograms generated from mice have a somewhat different waveform to those seen in humans, which reflects the lack of plateau in the AP waveform in mice. This precluded accurate measurement of the RT in this manner. Instead, we empirically established a point on the murine electrogram that corresponded to a set repolarization point on the AP and that could be reproducibly measured. We thus validated our use of the ARI by comparing it to APD measurements made using the MAP catheter (Fig. 2, A and B; see results for further details).
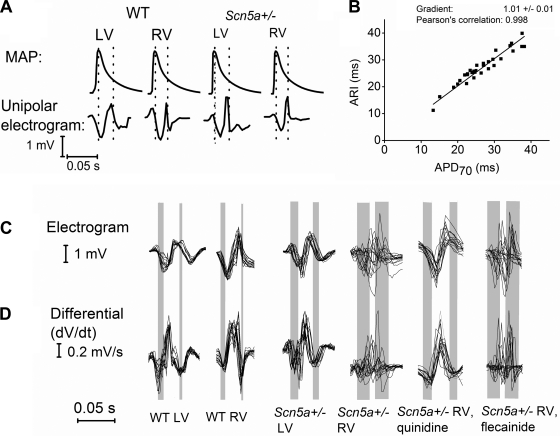
Fig. 2.
A: monophasic action potentials (MAPs) recorded from the base of either LV or RV using a contact MAP catheter and unipolar electrograms recorded using multielectrode mapping. Vertical dotted lines on the MAP tracings denote activation recovery interval (ARI) calculated from the unipolar electrograms. B: graph comparing paired ARI measurements using multielectrode array mapping with action potential duration 70 (APD70) measurements using a MAP electrode at specific locations on the ventricle in individual hearts. C: typical electrograms from the LV and RV of wild-type (WT) and Scn5a+/− hearts and for RV Scn5a+/− traces after addition of quinidine and flecainide. From each 2 × 2 square of the 8 × 8 electrode array grid, a representative trace is shown so that 16 traces are overlaid in each case to provide an indication of the electrical responses over the recording area as a whole. D: differential of each trace, to demonstrate the maximal negative slope dV/dt, from which activation times (ATs) and repolarization times (RTs) were calculated. Vertical bars represent the range of times from first to last activation, and from first to last repolarization. Thus the left edge of the first bar represents the time of the earliest first peak in the dV/dt trace and the right edge of the first bar is the latest first peak in the dV/dt trace. Similarly, the left edge of the second bar is the earliest repolarization peak in the dV/dt trace and the right edge of the second bar is the last repolarization peak in the dV/dt trace. In this way, the bars represent the spatial dispersion of depolarization and repolarization. In each individual trace, the time between activation and repolarization was taken to be the ARI. Thus the interval between the 2 bars gives an indication of the time between activation and recovery of the array as a whole.
The difference between first and last AT obtained in each set of 64 electrograms, defined as the “activation time difference” (ATD); the difference between first and last RT, defined as the “repolarization time difference” (RTD); and the difference between shortest and longest ARI, defined as the “ARI difference” (ARID), were also measured.
Statistical procedures.
A set of 12 WT and 12 Scn5a+/− mice were used, with half exposed to flecainide and half to quinidine. Differences in incidences of VT were analyzed using Fisher Exact tests. All electrophysiological measurements, including ATs, ARIs, RTs, ATDs, ARIs, and RTDs, were expressed as means ± SE values. Their significant differences were analyzed using ANOVA applied to all groups with post hoc Tukey's honestly significant different tests then applied to paired groups. Correlations between ATs and ARIs were analyzed using Pearson's correlation. A P value of <0.05 was taken as a criterion for significance.
RESULTS
Figure 1 shows a diagram representing the 64-channel multielectrode array placed on the surface of the LV or RV. A unipolar electrogram was recorded from each channel with hearts beating spontaneously.
ARIs correlate well with APD70 measurements made by MAP electrode.
Initial experiments compared ARIs, calculated from unipolar electrograms and measured by the multielectrode array, with APDs measured by contact MAP electrode. Both sets of measurements were made in individual hearts (2 WT and 2 Scn5a+/−) to allow direct comparison of results. The MAP catheter was positioned either near the apex of the heart, corresponding to position 64 on the array, or near the base of the heart, corresponding to position 1 on the array. Both sets of measurements were made both before and after drug treatment. Although the two sets of measurements could not be made simultaneously as the electrodes needed to be on the same position on the heart, they were made with as little time elapsing between them as possible.
Figure 2A depicts representative pairs of MAPs and unipolar electrograms taken from the base of either LV or RV (corresponding to position 1 in the array) in individual hearts, while Fig. 2B plots all pairs of ARI measurements against APD70 measurements. These were taken at both the base and apex of both the LV and RV of two WT and two Scn5a+/− hearts, of which one of each genotype was exposed to flecainide and one was exposed to quinidine. In this way, the relationship between ARIs and APD70s was tested across a wide range of experimental conditions. The graph demonstrates a linear relationship with a gradient of 1.01 ± 0.01, which is not significantly different from unity (P < 0.33; n = 32) and a Pearson's correlation of 0.998. This empirically demonstrates an excellent correlation between the two methods of measuring RT. It is likely that ARIs equate to APD70 measurements rather than APD90s, as measurements from MAP electrodes inherently record from a number of cells and thus tend to overestimate values due to a degree of local dispersion. The tight linear relationship validates the use of the multielectrode array to calculate repolarization dispersion in the measurements described below. Furthermore, the mean difference between paired sets of ARI and APD70 measurements was 1.7 ± 0.3 ms. This is significantly smaller than the spatial ARI heterogeneity recorded even in the WT LV (mean ARID = 4.4 ± 0.6 ms; P < 0.001) and suggests that any observed heterogeneity in ARI is not due to the measurement error but is a true finding.
Scn5a+/− hearts show a low incidence of spontaneous VT but this is exacerbated by flecainide.
The incidences of ventricular ectopics (VEs) and of VT, defined as occurrences lasting over 1 s, were compared between WT and Scn5a+/− hearts before and after flecainide or quinidine (Table 1). Four records, each lasting 1 min, were made from each ventricle of each heart, two before and two after drug treatment. Comparable incidences of VEs and VT were found when recording from the LV as from the RV, which is likely as any arrhythmia would spread rapidly to affect both ventricles. Therefore, the denominator reflects the total number of records made in hearts of a given genotype and under a given pharmacological condition.
Table 1.
Incidence of VEs and VT
| WT |
Scn5a+/− |
|||
|---|---|---|---|---|
| VE | VT | VE | VT | |
| Before drug | 0/48 (0%) | 0/48 (0%) | 4/48* (8%) | 2/48† (4%) |
| Flecainide (10 μM) | 1/24 (2%) | 0/24 (0%) | 12/24* (50%) | 5/24† (21%) |
| Quinidine (5 μM) | 1/24 (2%) | 0/24 (0%) | 2/24 (4%) | 0/24 (0%) |
Denominator is the total number of traces analyzed for each condition. Percentage incidence is given in parentheses.
WT, wild type; VE, ventricular ectopic; VT, ventricular tachycardia.
Significantly different pairs.
There were no VEs in the WT mice before drug and only very low incidences after treatment with either flecainide or quinidine. The incidence of VEs was 8% in the Scn5a+/− hearts, and this rose significantly to 50% in the presence of flecainide, while falling to 2% in the presence of quinidine. There were no incidences of VT in the spontaneously beating WT hearts before drug, and neither quinidine nor flecainide treatment provoked VT. In the Scn5a+/− hearts, 2 out of 48 records (4%) displayed polymorphic VT before drug treatment. No traces displayed VT with quinidine, but the incidence of polymorphic VT rose significantly to 5 of 24 (21%) with flecainide treatment (P = 0.037, Fisher's exact test). The average cycle length of the VT was 50 ms, and all episodes were polymorphic.
Scn5a+/− hearts show evidence of slowed conduction and lines of block.
Figure 2C shows typical electrograms from the epicardial surface of the LV and RV of WT and Scn5a+/− hearts and for RV Scn5a+/− traces after addition of quinidine and flecainide. Figure 2D shows the differential of each trace, to demonstrate the maximal negative slope dV/dt, from which ATs and RTs were calculated. The vertical bars represent the range of times from first to last activation and from first to last repolarization.
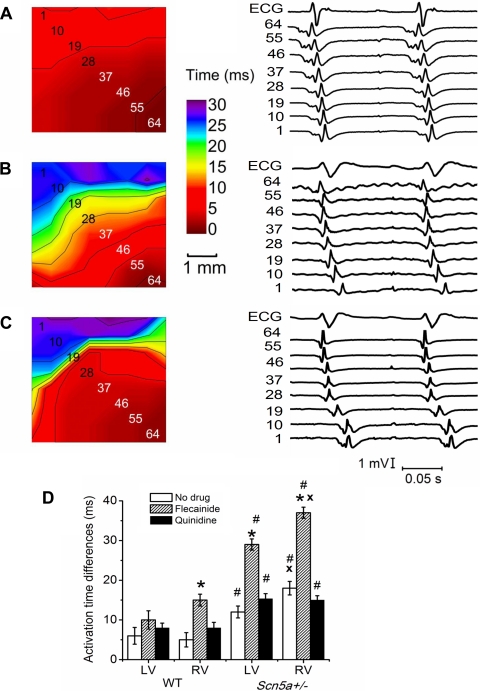
Figure 3, A–C, shows typical ECGs, electrograms, and isochronal AT maps for electrical activity arriving at the epicardial surface for the RV of WT (Fig. 3A) and Scn5a+/− (Fig. 3, B and C) hearts in sinus rhythm of ∼7 Hz in WT hearts and 6 Hz in Scn5a+/− hearts. In the majority of recordings, both in LV and RV of WT and Scn5a+/− hearts, there was a single planar wavefront arriving at the epicardium with the intraepicardial vector travelling from the apex to the base of the heart (Fig. 3, A and B). To provide controlled conditions under which electrophysiological parameters could be independently measured, analysis of activation and RTs was only carried out in those recordings demonstrating such a wavefront in the intraepicardial plane travelling from apex to base. Arrival times of the waves seen in Scn5a+/− hearts were spread over a larger time scale, particularly in the RV (Fig. 3B). There were also instances of heterogeneities in arrival times in the RV of Scn5a+/− hearts, seen by a region of isochronal line crowding (Fig. 3C), a pattern that was not observed in the LV.
Fig. 3.
A–C: typical isochronal maps for the RV of (A) WT and (B and C) Scn5a+/− hearts. Electrogram numbers correspond to the channel numbers of the array, as shown in the maps. A and B: single planar propagating wavefronts, but with considerably delayed arrival of activation times in B. C: heterogeneities in activation times, with isochronal line crowding. Electrograms are shown for each map along the diagonal axis of the multielectrode array shown by the numbers. D: graph of AT differences. Significant differences: *: effect of drug (i.e., results with flecainide or quinidine compared with those without drug); #: effect of genotype (i.e., Scn5a+/− compared with WT); x: effect of cardiac ventricle (i.e., RV compared with LV).
In WT hearts, LV and RV ATDs were similar, but in Scn5a+/− hearts, the ATDs were significantly larger in RV than LV (Fig. 3D). Both LV and RV ATDs were larger in Scn5a+/− hearts than the corresponding WT hearts. While quinidine did not significantly alter ATDs in either the LV or RV of either WT or Scn5a+/− hearts, they were significantly increased by flecainide in the RV (but not the LV) of WT hearts, and in both LV and RV of Scn5a+/− hearts, the effect being particularly strong in the RV. These effects can also be seen in Fig. 2, C and D, with loss of AT synchronicity in Scn5a+/− hearts that was worsened by flecainide but improved by quinidine, as demonstrated by the variation in width of the first vertical bar in each trace.
VT originates in the RV and arises from lines of conduction block leading to the induction of reentrant circuits.
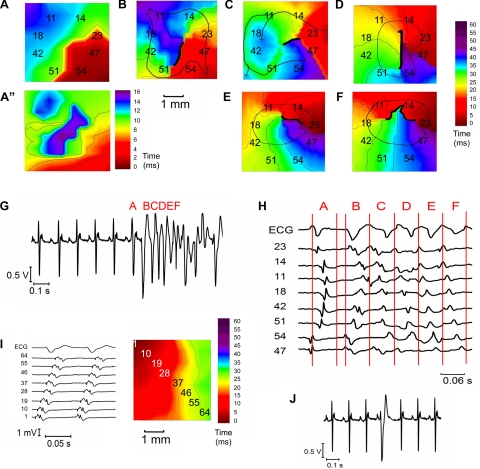
Figure 4, A–F, shows typical isochronal maps, ECG, and electrograms of activation arriving at the epicardial surface of the RV of an Scn5a+/− heart after flecainide treatment, illustrating a typical premature ventricular beat leading to the initiation of polymorphic VT. Figure 3, A–F, is sequential in time, as shown in the ECG in Fig. 3G. In the last sinus beat, crowded isochronal lines indicate an area of delayed arrival of activation at the epicardial surface (Fig. 4A). The corresponding repolarization map shows increased repolarization heterogeneities in this area (Fig. 4A”). A premature ventricular beat superimposed on this leads to a line of block with impulse propagation flowing around it in the intraepicardial plane and evidence of fractionated electrograms (Fig. 4B). A second VE results in the formation of a circuit running anticlockwise (Fig. 4C). The circuit continues into the next beat, initiating VT (Fig. 4D). In each beat the line of block changes, creating a nonstationary vortex, which results in a polymorphic character of the arrhythmia (Fig. 4, E and F).
Fig. 4.
A–F: typical isochronal propagation maps in the RV of an Scn5a+/− heart after flecainide treatment, showing ventricular tachycardia (VT) initiation. G: ECG trace showing a ventricular ectopic (VE) initiating a run of polymorphic VT. H: part of the same ECG trace, together with 8 electrogram traces, at the initiation of VT. Electrogram numbers correspond to the channel numbers of the array, as shown in the maps. The portion of electrogram trace delineated by each letter is synchronous with the corresponding propagation map. Thick black lines on the propagation maps denote lines of block. Thin arrows denote lines of propagation. In A, this demonstrates a crowded isochronal lines in the last sinus beat, indicating an area of conduction slowing. A”: repolarization map of the last sinus beat shows increased repolarization heterogeneity in the same area. A premature ventricular beat superimposed on this leads to a line of block with impulse propagation flowing around it (B). C: a second VE results in the formation of a reentrant circuit. D: circuit continues into the next beat to initiate VT. However, the line of block changes, creating a nonstationary vortex, which results in a polymorphic arrhythmia (E and F). I: propagation map, ECG, and electrogram traces of the VT propagating as a wave front across the LV from its onset in the RV. J: ECG trace of a VE occurring after the T wave in an Scn5a+/− heart.
Lines of functional conduction block from premature ventricular beats, leading to the initiation of reentrant circuits, were seen in all seven of the cases in which VT occurred. In contrast in the LV, propagation of VT occurs as a wavefront across the LV in the intraepicardial plane from its onset in the RV (Fig. 4I). Premature ventricular beats did not appear to originate from any one particular region of the ventricle; however, the location where they were able to transform into reentrant circuits of VT coincided with localized areas of repolarization heterogeneity in all cases. Traces in which VEs occurred were also associated with a significantly higher mean RT difference in preceding sinus beats over the recording array of 17.2 ± 2.3 ms, compared with 11.3 ± 1.2 ms in traces where no VEs occurred (P < 0.03; n = 12). In five of seven of the VT cases, there were two or more successive premature ventricular beats. Out of the 19 total episodes of premature ventricular beats that occurred, the ones that were synchronized with the T wave led to the initiation of VT (Fig. 4G), while those occurring after the T wave did not (Fig. 4J).
ARIs are shorter, but more heterogeneous, in Scn5a+/− hearts.
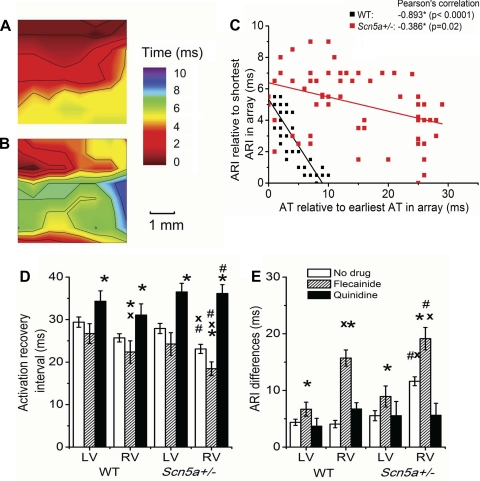
The ARI is a measure of the RT course, as indicated by the initial studies comparing unipolar electrogram recordings with MAP recordings (see Fig. 2, A and B). Figure 2, C and D, shows variation in the ARIs within recordings and among different experimental conditions of ventricle, genotype, and drug treatment. Figure 5, A and B, demonstrates maps of ARIs, measured relative to the shortest ARI in the array in the RV of WT and Scn5a+/− hearts from the same experimental runs as Fig. 3, A and B. Figure 5C, again using the data from the experimental runs depicted in Fig. 3, A and B, and Fig. 5, A and B, plots each ARI measurement in the array relative to the shortest ARI in the array, against the corresponding AT relative to the earliest AT in the array, for each of 64 points on the 8 × 8 electrode grid.
Fig. 5.
A and B: maps of ARIs in the RV of WT (A) and Scn5a+/− (B) hearts from the same runs as Fig. 3, A and B, measured relative to the shortest ARI in the array. C: graph of paired ARI and AT measurements, relative to the shortest ARI or earliest AT in the array, at each of 64 points on the 8 × 8 electrode grid for the experimental runs depicted in Fig. 3, A and B, and Fig. 5, A and B. Graphs of ARIs (D) and ARI differences (E). Significant differences: *: effect of drug; #: effect of genotype; x: effect of cardiac ventricle.
ARIs were of similar length in the LV and RV of WT hearts but were longer in the LV than the RV of Scn5a+/− hearts (Fig. 5D). They were shorter in Scn5a+/− hearts than WT for the RV but not LV. Flecainide decreased the ARI in both WT and Scn5a+/− hearts, while quinidine increased it. The ARIDs, measured from the differences between shortest and longest ARIs, were equal between LV and RV in WT hearts but greater in the RV than LV for Scn5a+/− hearts (Fig. 5E). They were greater in Scn5a+/− hearts than WT in the RV but not the LV. Addition of quinidine did not alter ARID under any condition, but addition of flecainide consistently increased it in LV and RV for both WT and Scn5a+/− hearts. In doing so, the result was a greater ARID in the RV of the Scn5a+/− compared with the WT. This could be further analyzed in the isochronal maps (Fig. 5, A and B) that show that RTs were spread over a wider time course in the Scn5a+/− hearts. The ARI map in the WT hearts (Fig. 5A) follows a very similar pattern to that of the activation maps, with the longest ARIs in the regions of earliest activation and the shortest ARIs in the region of latest activation. In contrast, in the Scn5a+/− map (Fig. 5B), ARI is disorganized and does not follow a clear gradient.
Figure 5C demonstrates that there is an inverse correlation between ARID and ATD. However, this is much stronger in the WT case than in the case of the Scn5a+/− hearts (Pearson's correlation: WT: −0.893, significant to P < 0.0001; Scn5a+/−: −0.386, significant to P = 0.02). The inverse correlation was present in recordings from all hearts studied, despite some variations in the direction of the propagating wavefront over the epicardium between recordings and between hearts. The mean Pearson's correlation of a set of 12 such graphs of ARID and ATD, from two recordings of each heart studied, was −0.777 ± 0.029 for the WT hearts and −0.449 ± 0.034 for the Scn5a+/− hearts, which represents a significant difference (P < 0.0001).
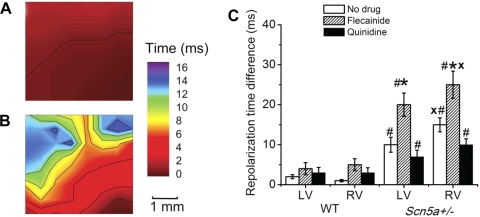
Heterogeneity in RTs is greatly increased in Scn5a+/− hearts. Figure 2, C and D, shows variation in RT within recordings and among different experimental conditions of ventricle, genotype, and drug treatment.
Figure 6, A and B, demonstrates maps of RTs at the epicardial surface of the RV of (Fig. 6A) WT and (Fig. 6B) Scn5a+/− hearts, measured relative to the earliest RT in the array. This shows greater synchronicity of RTs in the WT than Scn5a+/− hearts. RTDs were of similar length in the LV and RV of WT hearts, and although slightly increased by flecainide, this change was not significant (Fig. 6C). RTDs were significantly longer in the RV than LV of Scn5a+/− hearts. They were also longer in Scn5a+/− hearts than WT, both in the LV and RV. Flecainide increased the RTD in the LV and RV of Scn5a+/− hearts, while quinidine decreased it in both.
Fig. 6.
A and B: maps of RTs in the RV of (A) WT and (B) Scn5a+/− hearts from the same runs as Fig. 3, A and B. C: graph of RT differences. Significant differences: *: effect of drug; #: effect of genotype; x: effect of cardiac ventricle.
DISCUSSION
These experiments apply multielectrode mapping to integrate the two main proposed mechanisms for the arrhythmogenesis in BrS of slowed conduction and repolarization heterogeneity. We demonstrate localized areas of heterogeneity in ATs arriving at the epicardial surface specifically in the RV for the first time. We furthermore demonstrate a breakdown in the normal relationship between depolarization times and RTs, leading to increased repolarization heterogeneity. Both these factors were exacerbated by flecainide but alleviated by quinidine. Flecainide has an established clinical use in diagnostically unmasking arrhythmogenic phenotypes in BrS patients (23), while quinidine has been shown to have therapeutic effects in BrS (2).
In contrast to most other mapping studies, we have used recordings in which hearts are beating spontaneously, as opposed to being paced from a fixed point on the epicardium. To allow comparisons of RTs, which are cycle length dependent, we used only recordings with a cycle length falling within tight boundaries of between 130 and 150 ms. Using spontaneously beating hearts means that the activation pattern represents both electrical activity travelling through the intraepicardial plane and from the endocardium to the epicardium, while we were measuring only the former of these. This precluded accurate measurement of conduction velocities through the three-dimensional tissue as demonstrated by Bayly et al. (4); however, as the focus of our study was to examine patterns of activation and repolarization arriving at the epicardial surface and thus to provide insight into the mechanism of arrhythmogenesis occurring in vivo, it was crucial to preserve physiological activation sequences in our experiments.
However, it follows that the differences in the activation and repolarization patterns arriving at the epicardial surface, as well as resulting from abnormalities in conduction within the intraepicardial plane, may also reflect delays involving the transmural plane or indeed in the specialized conduction system. While we were only able to record from the epicardium, the finding of a higher incidence of VEs in the Scn5a+/− hearts than WT and their role in leading to polymorphic VT suggests that the Purkinje system could well play a significant role in the initiation of the electrophysiological abnormalities. The Purkinje system has previously been shown to play an important role in the initiation of polymorphic VT after myocardial infarction (25), and furthermore flecainide, which exacerbated the conduction block and repolarization heterogeneities in our recordings, is known to have a significant effect on the Purkinje system. However, previous studies (38) in the Scn5a+/− mice have shown that conduction velocity in the bundle branches was not affected and fibrosis and connexin 40 expression in the bundle branches was also unchanged.
Our experiments demonstrated that VEs were associated with regions of increased repolarization heterogeneity. Previous experiments in canine isolated RV preparation modeling BrS showed that VT could be initiated from a premature beat occurring in a region of increased epicardial repolarization heterogeneity (18). Other canine tissue studies (4, 5, 11, 19) have demonstrated increased extrasystolic activity resulting from repolarization heterogeneities in the presence of flecainide, pinacidil, or simulated ischemia in a mechanism termed “phase 2 reentry.” The authors suggest that drug-induced dispersion of repolarization gives rise to electrotonic currents that cause reentrant excitation via a circus movement mechanism. Computer modeling studies of BrS have also demonstrated that increased repolarization heterogeneities can generate this phenomenon, which can be observed as a premature beat that in turn can lead to functional block and VT (25).
Although Scn5a+/− hearts showed a relatively low incidence of VT before the introduction of flecainide, these experiments were conducted with spontaneously beating hearts, and the software limited data acquisition time frames to 1 min. Higher rates of arrhythmogenesis have been demonstrated under conditions of paced electrical stimulation and when longer recording time frames are used (17). Furthermore, BrS patients also have a low spontaneous incidence of VTs (9). Thus the low incidence of VT in these experiments before drug does not compromise the validity of this mouse model as a surrogate of clinical BrS.
We have shown that in WT hearts the normal pattern of impulse propagation in the intraepicardial plane is mirrored by a gradient of ARI from apex to base of heart, with the regions of the epicardium that are activated earliest having the longest ARI and the regions that are activated latest having the shortest ARI. This was true in all hearts studies, despite small variations in the direction of the propagating wavefront over the epicardium between recordings and between hearts. The ARIs were thus determined by the position of the cells in relation to the sinus activation pathway. This phenomenon has been widely demonstrated in tissues (7, 28), animal hearts (16, 42), and humans (8, 15, 42, 43), and it enables the RT to become spatially synchronized and reduce the likelihood of reentry phenomena. A likely mechanism facilitating the AT-ARI gradient is local electrotonic current flow via gap junctions from cells downstream to cells upstream (17, 35). However, in Scn5a+/− hearts, despite the mean values of ARI being shorter, the ordered spatial pattern of ARIs with respect to ATs was disrupted, which resulted in nonuniformities of RT across the epicardial ventricular surface. This is exacerbated in the presence of flecainide but somewhat reduced by quinidine. Disruption in the AT-ARI relationship has previously been documented in association with ventricular arrhythmias both clinically (8, 17) and in animal models (16).
We go on to demonstrate areas of repolarization heterogeneity in the RV epicardium in which lines of conduction block can form following a premature ventricular beat. Then, in the presence of delayed activation, this can result in the formation of reentrant circuits and initiation of polymorphic VT. The spatial repolarization heterogeneities may also cause the lines of block to be nonstationary and thus result in geometrically changing reentrant circuits and a VT of polymorphic nature (6).
This study adds to a recent series of articles by our group and others attempting to elucidate the mechanisms of arrhythmogenesis using a murine model of BrS. We have previously demonstrated both depolarization abnormalities, in the form of delayed conduction latencies, and repolarization abnormalities, in the form of heterogeneities in APDs (24). Further studies using multiple MAP recordings of APD restitution properties in both the LV and RV of Scn5a+/− hearts have shown steeper restitution slopes and higher incidences of discordant alternans localized to the RVOT (22). Thus spatial and temporal heterogeneities appear to occur both in the transmural and intraepicardial planes but are focused at the RVOT.
Previous studies have used multiarray mapping during epicardial ventricular pacing to measure both transverse and longitudinal conduction velocity, and calculate anisotropy ratios, to provide information on both passive and active activation heterogeneities (38). They showed that both longitudinal and transversal conduction velocities of the RV but not LV were significantly reduced in young Scn5a+/− mice, while in old Scn5a+/− mice further conduction slowing occurred, especially in the transverse plane in the RV. These studies also demonstrated increased fibrosis in the old Scn5a+/− mice, both in the RV and LV, which was associated with downregulation of Cx43 expression. Increased fibrosis, which has also been demonstrated in clinical cases of BrS (9), could exacerbate depolarization and repolarization gradients and therefore maximize the effect on electrophysiological heterogeneity. While these studies have provided useful data in establishing electrophysiological risk factors for the initiation and propagation of arrhythmias, in mapping the ventricular arrhythmias occurring in spontaneously beating hearts, our present studies are able to demonstrate the mechanism by which these abnormalities can coexist in the RV epicardium to lead to reentrant patterns of excitation and tachyarrhythmias.
One question that remains unanswered is why both the depolarization and repolarization changes seen in BrS are localized to the RV. It is possible that the LV may have an increased depolarization reserve compared with the RV, and under conditions where INa is functionally reduced, heterogeneity in Nav1.5 expression may become a significant determinant of conduction heterogeneities (29). Alternatively, the increased Ito current in the RV could influence the reduction of Na+ current to a different extent in the RV than the LV. The Ito blocking action of quinidine could thus underlie its effect in reducing arrhythmogenesis, in contrast to flecainide, the primary action of which is to block INa. More work is needed in this area to determine the underlying reason for the RV localization of effect.
The main limitations of our study come from the differences between mice and humans. The thickness of the RV wall is much reduced in the mouse, which might limit the degree of spatial repolarization possible. Due to the lack of a plateau phase in the AP, murine unipolar electrograms do have a different morphology from human ones. However, our recorded electrograms reproducibly show an initial negative waveform followed by a larger positive one and correlated well with multiple paired APD70 measurements using a MAP electrode. Furthermore, activation mapping using unipolar electrogram arrays both in the LV and RV has previously successfully shown electrophysiological heterogeneities in a range of transgenic mice (7, 24, 27). While an optical mapping system may have provided better spatial resolution, its use is hampered by concerns about motion artifacts and use of paralyzing agents. As we only had access to one electrode array, we were unable to map the LV and RV simultaneously. Furthermore, due to the small size of the mouse heart, technical limitations prevented us from recording endocardial data. Thus our study was principally concerned with measuring heterogeneities in the intraepicardial plane rather than transmural ones. However, this provided a contrast to previous clinical studies showing increased uniformity of depolarization in BrS patients but that only examined the endocardium and that have mapped the initiation of VT only during programmed stimulation protocols (14). Finally, our recordings were able to map the onset of spontaneous arrhythmogenesis and thus shed light on possible arrhythmogenic mechanisms occurring in vivo.
GRANTS
This work was supported by the British Heart Foundation, the Medical Research Council; the Wellcome Trust, and the Biotechnology and Biological Research Council, UK. C. A. Martin was supported by a Medical Research Council Clinical Research Fellowship and a Sackler Studentship of the University of Cambridge School of Clinical Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. W. Lammers and A. Wahab from the Dept. of Physiology, Faculty of Medicine and Health Sciences, UAE University, Al Ain, United Arab Emirates, who designed and constructed the multielectrode array used in this study (www.smoothmap.org).
REFERENCES
- 1.Aiba T, Shimizu W, Hidaka I, Uemura K, Noda T, Zheng C, Kamiya A, Inagaki M, Sugimachi M, Sunagawa K. Cellular basis for trigger and maintenance of ventricular fibrillation in the Brugada syndrome model: high-resolution optical mapping study. J Am Coll Cardiol 47: 2074–2085, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alings M, Dekker L, Sadee A, Wilde A. Quinidine induced electrocardiographic normalization in two patients with Brugada syndrome. Pacing Clin Electrophysiol 24: 1420–1422, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 293: H2024–H2038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng 45: 563–571, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJ, Verkerk AO, de Groot JR, Bhuiyan Z, Bezzina CR, Veldkamp MW, Linnenbank AC, van der Wal AC, Tan HL, Brugada P, Wilde AA, de Bakker JM. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation 112: 2769–2777, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Costeas C, Peters NS, Waldecker B, Ciaccio EJ, Wit AL, Coromilas J. Mechanisms causing sustained ventricular tachycardia with multiple QRS morphologies: results of mapping studies in the infarcted canine heart. Circulation 96: 3721–3731, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet 17: 539–554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckardt L, Bruns HJ, Paul M, Kirchhof P, Schulze-Bahr E, Wichter T, Breithardt G, Borggrefe M, Haverkamp W. Body surface area of ST elevation and the presence of late potentials correlate to the inducibility of ventricular tachyarrhythmias in Brugada syndrome. J Cardiovasc Electrophysiol 13: 742–749, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Eckardt L, Probst V, Smits JP, Bahr ES, Wolpert C, Schimpf R, Wichter T, Boisseau P, Heinecke A, Breithardt G, Borggrefe M, LeMarec H, Bocker D, Wilde AA. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation 111: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gepstein L, Hayam G, Ben-Haim SA. Activation-repolarization coupling in the normal swine endocardium. Circulation 96: 4036–4043, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Hanson B, Sutton P, Elameri N, Gray M, Critchley H, Gill JS, Taggart P. Interaction of activation-repolarization coupling and restitution properties in humans. Circ Arrhythm Electrophysiol 2: 162–170, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Kimura M, Kobayashi T, Owada S, Ashikaga K, Higuma T, Sasaki S, Iwasa A, Motomura S, Okumura K. Mechanism of ST elevation and ventricular arrhythmias in an experimental Brugada syndrome model. Circulation 109: 125–131, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kurita T, Shimizu W, Inagaki M, Suyama K, Taguchi A, Satomi K, Aihara N, Kamakura S, Kobayashi J, Kosakai Y. The electrophysiologic mechanism of ST-segment elevation in Brugada syndrome. J Am Coll Cardiol 40: 330–334, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lambiase PD, Ahmed AK, Ciaccio EJ, Brugada R, Lizotte E, Chaubey S, Ben-Simon R, Chow AW, Lowe MD, McKenna WJ. High-density substrate mapping in Brugada syndrome: combined role of conduction and repolarization heterogeneities in arrhythmogenesis. Circulation 120: 106–117, 101–104, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Martin CA, Grace AA, Huang CL. Spatial and temporal heterogeneities are localized to the right ventricular outflow tract in a heterozygotic Scn5a mouse model. Am J Physiol Heart Circ Physiol 300: H605–H616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin CA, Zhang Y, Grace AA, Huang CL. In vivo studies of Scn5a+/− mice modeling Brugada syndrome demonstrate both conduction and repolarization abnormalities. J Electrocardiol 43: 433–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CA, Zhang Y, Grace AA, Huang CL. Increased right ventricular repolarization gradients promote arrhythmogenesis in a murine model of Brugada syndrome. J Cardiovasc Electrophysiol 21: 1153–1159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagase S, Kusano KF, Morita H, Fujimoto Y, Kakishita M, Nakamura K, Emori T, Matsubara H, Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: using the epicardial lead. J Am Coll Cardiol 39: 1992–1995, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Nagase S, Kusano KF, Morita H, Nishii N, Banba K, Watanabe A, Hiramatsu S, Nakamura K, Sakuragi S, Ohe T. Longer repolarization in the epicardium at the right ventricular outflow tract causes type 1 electrocardiogram in patients with Brugada syndrome. J Am Coll Cardiol 51: 1154–1161, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, Saumarez RC, Trezise AE, Huang CL, Vandenberg JI, Colledge WH, Grace AA. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci USA 99: 6210–6215, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, Priori SG, Tan HL, Hiraoka M, Brugada J, Wilde AA. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs org). Heart Rhythm 6: 1335–1341, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potse M, Vinet A, Opthof T, Coronel R. Validation of a simple model for the morphology of the T wave in unipolar electrograms. Am J Physiol Heart Circ Physiol 297: H792–H801, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Napolitano C, Schwartz PJ, Bloise R, Crotti L, Ronchetti E. The elusive link between LQT3 and Brugada syndrome: the role of flecainide challenge. Circulation 102: 945–947, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Schrickel JW, Brixius K, Herr C, Clemen CS, Sasse P, Reetz K, Grohe C, Meyer R, Tiemann K, Schroder R, Bloch W, Nickenig G, Fleischmann BK, Noegel AA, Schwinger RH, Lewalter T. Enhanced heterogeneity of myocardial conduction and severe cardiac electrical instability in annexin A7-deficient mice. Cardiovasc Res 76: 257–268, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Szumowski L, Sanders P, Walczak F, Hocini M, Jais P, Kepski R, Szufladowicz E, Urbanek P, Derejko P, Bodalski R, Haissaguerre M. Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction. J Am Coll Cardiol 44: 1700–1706, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Tukkie R, Sogaard P, Vleugels J, de Groot IK, Wilde AA, Tan HL. Delay in right ventricular activation contributes to Brugada syndrome. Circulation 109: 1272–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 27.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 109: 1048–1055, 2004 [DOI] [PubMed] [Google Scholar]
- 28.van Veen TA, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, Huang CL, Wilders R, Grace AA, Escande D, de Bakker JM, van Rijen HV. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation 112: 1927–1935, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Veeraraghavan R, Poelzing S. Mechanisms underlying increased right ventricular conduction sensitivity to flecainide challenge. Cardiovasc Res 77: 749–756, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Wilde AA, Coronel R. The complexity of genotype-phenotype relations associated with loss-of-function sodium channel mutations and the role of in silico studies. Am J Physiol Heart Circ Physiol 295: H8–H9, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 100: 1660–1666, 1999 [DOI] [PubMed] [Google Scholar]