Abstract
Myocardin is a serum response factor (SRF) coactivator exclusively expressed in cardiomyocytes and smooth muscle cells (SMCs). However, there is highly controversial evidence as to whether myocardin is essential for normal differentiation of these cell types, and there are no data showing whether cardiac or SMC subtypes exhibit differential myocardin requirements during development. Results of the present studies showed the virtual absence of myocardin−/− visceral SMCs or ventricular myocytes in chimeric myocardin knockout (KO) mice generated by injection of myocardin−/− embryonic stem cells (ESCs) into wild-type (WT; i.e., myocardin+/+ ESC) blastocysts. In contrast, myocardin−/− ESCs readily formed vascular SMC, albeit at a reduced frequency compared with WT ESCs. In addition, myocardin−/− ESCs competed equally with WT ESCs in forming atrial myocytes. The ultrastructural features of myocardin−/− vascular SMCs and cardiomyocytes were unchanged from their WT counterparts as determined using a unique X-ray microprobe transmission electron microscopic method developed by our laboratory. Myocardin−/− ESC-derived SMCs also showed normal contractile properties in an in vitro embryoid body SMC differentiation model, other than impaired thromboxane A2 responsiveness. Together, these results provide novel evidence that myocardin is essential for development of visceral SMCs and ventricular myocytes but is dispensable for development of atrial myocytes and vascular SMCs in the setting of chimeric KO mice. In addition, results suggest that as yet undefined defects in development and/or maturation of ventricular cardiomyocytes may have contributed to early embryonic lethality observed in conventional myocardin KO mice and that observed deficiencies in development of vascular SMC may have been secondary to these defects.
Keywords: embryonic stem cells, chimeric analysis, smooth muscle, heart, cardiac development, mouse
myocardin was initially identified by an in silico screen for cardiac cell-specific genes (40) and was shown to be a powerful serum response factor (SRF) coactivator in cardiac and smooth muscle cells (SMCs) (2, 7, 43, 45). However, whether myocardin is absolutely required for normal development of SMCs or whether its functions can be replaced by the myocardin-related transcription factors (MRTF) A and B and/or other genes in vivo is controversial. Li et al. (20) demonstrated that conventional myocardin knockout (KO) mice showed reduced numbers of vascular SMCs in the developing aorta, but this phenotype may be secondary to other developmental defects because KO embryos also showed gross developmental retardation. These mice also had no overt evidence of vascular leakage (e.g., hemorrhage) common to other models of defective SMC-pericyte maturation/differentiation and investment such as KLF2 (17) or PDGF receptor/ligand KO mice (10, 21). Moreover, myocardin KO mice died earlier in development [i.e., 10.5 days postcoitum (dpc)] than would be expected if lethality were due to defective SMC-pericyte investment and differentiation alone. Whereas conventional myocardin KO mice showed no overt defect in heart development, there was evidence that embryonic lethality at 10.5 dpc was secondary to pericardial effusion (20). In addition, these investigators showed that morpholino-induced suppression of myocardin in Xenopus embryos was associated with impaired heart development. Together, these observations raise the possibility that failed vascular SMC differentiation in conventional myocardin KO mice may have been secondary to as yet undefined defects in heart function, rather than reflecting a direct absolute requirement for myocardin in SMC differentiation.
Although the preceding results suggest that myocardin may be required for normal heart development, Huang et al. (12) showed that cardiomyocyte-selective KO of myocardin was not associated with any detectable changes in heart development, and KO mice were born at expected Mendelian ratios. However, mice subsequently developed lethal cardiomyopathy postnatally due to impaired cardiomyocyte structural organization, loss of contractile function, and programmed cell death. These results clearly indicate that myocardin is required for maturation and/or maintenance of differentiated function in cardiomyocytes. However, it is important to note that the conditional KO of myocardin in cardiomyocytes in these studies would have occurred after cardiomyocyte differentiation, and thus the studies do not address whether myocardin is actually required for initial development of atrial or ventricular myocytes.
Selective KO of myocardin in neural crest-derived cells has been shown to be associated with defective differentiation of SMC within the ductus arteriosis and perinatal lethality due to failed ductus closure (11). These observations are significant in that the ductus functions as a static shunt and requires no SMC contraction in embryos such that there would likely be little or no stimulus for compensatory activation of MRTFs until parturition, when it would be too late for compensation to be effective to permit survival. Together, these results suggest that myocardin is required for development of fully differentiated SMCs within the ductus arteriosis, although a limitation of these studies is that they did not directly assess SMC contractility. As such, it is possible that failed ductus closure could have been due to structural defects, rather than impaired SMC contraction secondary to defective differentiation.
Our understanding of the role of myocardin in SMC development was greatly confounded by studies by Pipes et al. (29) in collaboration with our laboratories, showing that myocardin−/− embryonic stem cells (ESCs) contribute to development of aortic SMC in the setting of chimeric KO mice produced by injection of lineage-tagged myocardin−/−/LacZ+ ESCs into wild-type (WT) host blastocysts. Significantly, these myocardin−/− aortic SMCs expressed a number of SMC differentiation marker genes, including smooth muscle α-actin (SMαA) and smooth muscle myosin heavy chain (SM-MHC), and were morphologically indistinguishable from SMCs derived from WT ESCs from the host blastocyst, at least as observed at the level of low-resolution light microscopy. However, there were several limitations in these studies, including the failure to test whether myocardin was required for SMC development in other blood vessels or other SMC-containing tissues, whether there were functional or ultrastructural alterations in the myocardin−/− aortic SMCs, and whether myocardin−/− ESCs were able to form SMC lineages at a frequency equivalent to that of WT ESCs. Consistent with the studies in the mouse chimeric injection model, results of our studies in embryoid bodies (EBs) (29), an in vitro development model that bypasses potential embryonic lethality due to failed morphogenesis and organogenesis, showed that myocardin was not required for development of SMC lineages. However, there was evidence of altered kinetics of activation of several SMC marker genes and a quantitative decrease in SM-MHC expression.
At present, there is uncertainty as to whether myocardin is absolutely required for development of cardiomyocyte or SMC lineages. It is also unknown whether myocardin dependence differs between different SMC subtypes, including SMCs derived from distinct embryological origins (25), and/or whether there are functional deficits or other abnormalities in myocardin−/− vascular SMCs that form in chimeric KO mice. The present studies address each of these questions and provide novel evidence showing that myocardin is essential for SMC development in the bladder, gut, and several other visceral SMC tissues in that there was a virtual absence of myocardin-null LacZ-tagged (i.e., myocardin−/−/LacZ+ ESCs) SMCs in these tissues in chimeric KO mice. We also confirm previous observations showing that myocardin−/− ESCs populate vascular SMC tissues (29) but extend previous studies by showing that myocardin−/− ESCs are present at a much reduced frequency in vascular SMC tissues, indicating they are at a selective disadvantage compared with the WT host cells. However, of interest, we found that the myocardin−/− aortic SMCs that did form had myofilaments and an overall ultrastructural appearance indistinguishable from that of adjacent WT (i.e., myocardin+/+) SMCs as determined using a unique transmission electron microscopic (TEM) lineage tracing method developed by our laboratory. Finally, of major significance, we provide completely unexpected results showing the virtual exclusion of myocardin−/− ESCs from ventricular, but not atrial, cardiomyocytes, indicating there is normally an absolute requirement for myocardin for development of these cells but that in the setting of conventional myocardin KO mice, this requirement is somehow circumvented, possibly through activation of compensatory genes such as MRTF A and B.
MATERIALS AND METHODS
Chimeric analyses.
Myocardin−/−/Gt(ROSA)26Sor-lacZ (LacZ+) and myocardin+/−/Gt(ROSA)26Sor-lacZ (LacZ+) chimeras were generated by both the University of Texas Southwestern Transgenic Core and the University of Virginia Gene Targeting and Transgenic Facility as described (29), with similar results from each facility over multiple blastocyst injections and n > 20 for both heterozygous and null populations. Animal experiments were done at 4–5 wk of age and were approved by the Animal Care and Use Committee Institutional Review Boards at the University of Virginia Health Sciences Center and University of Texas Southwestern.
Fixation and staining.
Smooth muscle tissues harvested from mice were washed twice in phosphate-buffered saline (PBS), fixed in ice cold 4% paraformaldehyde in PBS for 1 h or in 2% formaldehyde-0.2% glutaraldehyde in PBS at room temperature for 10 min (tissues were stained using 2 methods to ensure no difference in quality of staining was observed between the protocols used routinely by the Olson and Owens laboratories). Because of their small size, aortic strips for the histological assays of percent chimerism were fixed for 2 min at 37°C in 2% formaldehyde-0.2% glutaraldehyde in PBS to prevent loss of β-galactosidase signal, washed again in PBS, and stained for 48 h using standard X-gal staining solution: 5 mM ferrocyanide, 5 mM ferricyanide, 2 mM MgCl2, 0.1% NP-40, 0.1% sodium deoxycholate, and 1 mg/ml 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal; Sigma, St. Louis, MO) in dimethylformamide. For electron microscopy analysis, rings of thoracic aorta were washed with 10 ml of HEPES-buffered Krebs solution and then fixed in 2% glutaraldehyde plus 6% sucrose in 0.075 M Na-cacodylate buffer (pH 7.4; EM-fix) for ∼25 min. The aortic rings were then incubated overnight in EM-Bluo-gal stain solution: 0.1 M Na-cacodylate, 5 mM ferrocyanide, 5 mM ferricyanide, 2 mM MgCl2, 6% sucrose, and 1 mg/ml 5-bromo-3-indolyl β-d-galactopyranoside (Bluo-gal; Sigma) solubilized in dimethylformamide. The aortic rings were then postfixed in EM-fix for an additional 4–6 h and postfixed in osmium tetroxide, followed by uranyl acetate staining and embedding in Spurr's resin (4). Hearts analyzed at postnatal day 10 (P10) were fixed in 4% paraformaldehyde at 4°C for 1 h, washed in PBS twice, and stained overnight in standard X-gal staining solution in dimethylformamide. Embryos from 10.5 and 12.5 dpc were fixed for 30 or 60 min at 4°C in 2% formaldehyde-0.2% glutaraldehyde, washed in PBS twice, and stained overnight in standard X-gal staining solution. Embryos from 14.5 dpc were fixed for 60 min in 2% formaldehyde-0.2% glutaraldehyde at 4°C, decapitated and bisected below the ribcage to allow penetration of fix and stain into the thoracic cavity, fixed for an additional 30 min, and then washed and stained as described above. For electron microscopy, hearts were first perfused with 10 ml of HEPES-buffered Krebs solution supplemented with 3 mg/ml 2,3-butanedione monoxime (BDM; Sigma) and 2.5 units of heparin and then perfusion-fixed in EM-fix. Hearts were then carefully removed under a stereoscopic microscope and fixed for an additional 25 min. Next, the atria and ventricles were flushed with and incubated overnight in EM-Bluo-gal stain solution solubilized in dimethylformamide. Hearts were then postfixed in EM-fix for an additional 4–6 h before being postfixed in osmium tetroxide, stained with uranyl acetate, and embedded in Spurr's resin (8).
Southern analysis and densitometry.
A probe complementary to the myocardin locus was generated 3′ to the targeting insert described in the KO strategy (20) using the following PCR primers (MWG Biotech, High Point, NC): forward, CTGGCATCTAAACACCTACACAAG, and reverse, TTAAACTAGAGCCCCATTCATCATT. The fragment was amplified from mouse genomic DNA and cloned using a TOPO-TA kit (Invitrogen, Carlsbad, CA), amplified, and subsequently digested out of the purified plasmid with EcoRI.
Mice were killed by halothane inhalation, and dissected tissues were placed in DNA digestion buffer (100 mM NaCl, 50 mM Tris, pH 8.0, 25 mM EDTA, pH 8.0, 0.5% SDS, an 0.5 mg/ml proteinase K). The remainder of the animal was homogenized in a blender in 200 ml of PBS plus 25 mM EDTA, pelleted at 3,000 g, and then digested in 5 times the volume of DNA digestion buffer. Organs were incubated for 2–3 h at 55°C with rotation, and DNA was purified by phenol-chloroform extraction and precipitation in isopropanol. Ten micrograms of each DNA (∼2.5 μg for aorta, because this was the maximum amount of DNA that could be purified from a single aorta) were digested with EcoRI, electrophoresed in a 0.8% agarose-Tris-acetate-EDTA (TAE) gel, depurinated in 0.25 N HCl for 15 min, washed in 1.5 M NaCl-0.5 N NaOH for 30 min, and then transferred to Hybond-N+ nitrocellulose membrane (Amersham, Piscataway NJ) using 0.4 N NaOH overnight. Probe was radiolabeled with α-[32P]dCTP using the RediPrime II random-prime labeling kit (Amersham), hybridized to the membrane, and exposed to film for 1–5 days. A standard curve using DNA purified from myocardin−/− and myocardin+/− ESCs was used to determine that exposures were in the linear range of the film (r2 of fit >0.9). We used Fluorchem AlphaEaseFC software (Alpha Innotech, San Leandro, CA) to perform densitometry on the films.
Histological staining.
Tissues for histological analysis were postfixed in 2% formaldehyde-0.2% glutaraldehyde overnight and submitted to the University of Virginia Research Histology Core, where they were paraffin-embedded, sectioned, and stained with eosin or nuclear fast red (Vector Labs, Burlingame, CA).
Percent chimerism histological assays.
Three rings of thoracic aorta from the same region of the distal end of the vessel of 4- to 6-wk-old mice were cut, fixed, and stained for X-gal as described above, postfixed, and sectioned longitudinally. The sections were stained with nuclear fast red according to the manufacturer's protocol. The entire area of three strips was imaged at ×100 magnification. Sections of liver were processed similarly and imaged. A student, blinded to the samples, counted nuclei positive and negative for X-gal in five images chosen at random from each strip, as well as five images from each liver as a control measure of chimerism in each animal.
Electron microscopy and electron probe X-ray microanalysis.
Myocardin−/−/LacZ+ cells were definitively identified in aortic sections at the electron microscopic level by spectroscopic detection of the bromine emission peak arising from the reaction product of the Bluo-gal reagent. This reagent gives rise to a fine electron-dense precipitate localized at cell membranes (44). Electron probe X-ray microanalysis (EPMA) is based on the generation of X-ray spectra by atomic core shell excitation of elements in a given cellular microvolume, along with the simultaneous measurement of the mass of this microvolume by detection of continuum (background) X-rays. The ratio between the characteristic X-ray count of a certain element and the continuum X-ray count is thus proportional to the concentration of that element in the microvolume being analyzed (33). The accuracy of this technique in measuring subcellular element concentrations has been well documented (37). EPMA was performed with a Phillips CM12 electron microscope operated at 120 keV in transmission mode and fitted with a LaB6 filament. The microscope was equipped with an ultrathin window energy-dispersive X-ray detector and an XP3 pulse processor (Oxford Link) interfaced to a 4-pi spectral engine. X-ray spectra were collected and displayed at 2.5 eV per channel. The electron beam was focused on electron-dense deposits found in some of the cells as well as in regions adjacent to the deposits in the same cell, and spectra were collected. Bromine peaks (1.48 and 1.52 keV, Lα and Lβ edges, respectively) arising from the deposits were used to identify the myocardin−/−/LacZ+ cells. These cells were photographed for morphological analysis and compared with cells lacking deposits.
EB culture and formation of EB-derived purified myocardin+/+ and myocardin−/− SMC lines.
We cultured EBs and purified SMCs from myocardin−/− and myocardin+/− ESCs (29) as described previously (34) with the exception that the SMαA-puromycin plasmid carried a constitutive zeocin resistance gene instead of neomycin to establish the stable ESC lines, since the KO cells were already resistant to neomycin from the KO targeting construct. A detailed protocol is available on request. Stable ESC clones with the SMαA-puromycin construct were maintained in zeocin selection medium while they were expanded in an undifferentiated state. The cells were confirmed to be undifferentiated by Sox-2 staining with an Abcam (ab15830; Cambridge, MA) rabbit polyclonal antibody per the manufacturer's protocol (data not shown). EB-derived purified SMC lines (henceforth referred to as “EB-derived SMCs”) were maintained in puromycin to prevent overgrowth of possible non-SMαA-positive cell contaminants after selection.
Contractile measurements in chimeric mouse thoracic aortas and reconstituted muscle fibers generated from myocardin+/+ and myocardin−/− EB-derived purified SMCs.
Thoracic aortas were carefully removed from the mouse (under a dissection stereomicroscope) and cleaned of periadventitial fat and loose connective tissue in physiological HEPES-buffered Krebs solution. Rings (600–800 μm wide) cut from the distal end of the thoracic aorta were cut again such that a strip was formed. The ends of the strips were attached with silk monofilaments to wire hooks connected a length-adjusting device and a force transducer (AE 801; SensoNor, Horten, Norway; http://sni.nextframe.net/index.html) on a “bubble plate” (36). Once mounted to the force transducer, the strip was stretched to 1.1× its resting length and allowed to equilibrate in HEPES-buffered Krebs solution for 30 min before agonist stimulation. Strips were challenged with depolarizing solution (154 mM K+) and the stable thromboxane A2 mimetic U-46619. All experiments were performed at 37°C.
EB-derived SMCs purified from three independent myocardin+/+ and myocardin−/− ESC clones were cultured and aggregated into reconstituted muscle fibers according to the methods previously described by Sinha et al. (34). After 7 days in culture (5% CO2, 37°C) with EB medium, longitudinal strips (0.75–0.80 mm wide, ∼1.5 mm long) were cut with a razor knife from the reconstituted muscle fibers, transferred to HEPES-buffered Krebs solution, and mounted to a bubble-plate force transducer as previously described (36). All experiments were performed at 37°C.
Western blotting.
Western blotting for smooth muscle differentiation markers was performed as previously described (34) using mouse monoclonal SMαA (clone 1A4; Sigma) and rabbit polyclonal SM-MHC (BT-562; Biomedical Technologies, Stoughton, MA). GAPDH (clone 6C5; Chemicon International, Temecula, CA) chemifluorescence was detected and densitometry performed using a Fluorchem 8800 digital imaging system and AlphaEaseFC software (Alpha Innotech). Rho GDI (a specific GDP dissociation inhibitor), Gα12, Gα13, and Gαq/11 protein rabbit polyclonal antibodies, as well as anti-RhoA antibodies (clone 26C4), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Thromboxane A2 and the endothelin A and B receptors were detected using rabbit polyclonal antibodies from Cayman Chemical (Ann Arbor, MI) and Abcam (Cambridge, MA), respectively. The rabbit polyclonal anti-calponin antibody was a generous gift from Dr. M. P. Walsh (University of Calgary). Rho-dependent kinase ROCK I/β and ROCK II/α mouse monoclonal antibodies were purchased from BD Biosciences (San Jose, CA). The COOH terminus-derived anti-cGMP-dependent protein kinase I antibody (which detects both α- and β-isoforms) was purchased from Assay Designs (Ann Arbor, MI). Primary antibodies were labeled with goat anti-mouse Alexa 680 (Invitrogen)-or goat anti-rabbit IRDye800 (Rockland Immunochemicals, Gilbertsville, PA)-conjugated secondary antibodies and were detected and quantified using an Odyssey Imaging System (Licor, Lincoln, NE).
Real-time RT-PCR.
Reverse transcription and real-time RT-PCR were carried out as previously described (32). Primers for real-time RT-PCR will be provided on request.
Flow cytometry.
Flow cytometry for SMαA in EBs was performed as previously described (32), with the exception that a SMαA-Cy3-conjugated 1A4 antibody was used (Sigma), and fluorescence-minus-one controls were used to set the gate for positive cells rather than IgG controls.
Statistics.
For significance testing in nuclear counting, densitometry, and real-time RT-PCR assays, we used the Student's t-test (Microsoft Excel).
RESULTS
Myocardin−/−/LacZ+ ESC-derived SMCs were virtually absent within visceral and bladder SMC tissues in chimeric KO mice.
Particularly striking in the analysis of the myocardin−/−/LacZ+ chimeric mice was the failure of the myocardin−/− ESCs to contribute to visceral SMCs while contributing consistently to vascular SMCs and cell types other than ventricular myocytes (see below). In >30 bladders from myocardin−/−/LacZ+ animals from multiple independent blastocyst injections at both the University of Virginia and University of Texas Southwestern facilities, a similar pattern was observed. That is, myocardin+/−/LacZ+ cells were observed in the SMC layer of bladders from heterozygous control chimeric mice (Fig. 1A) but were consistently absent from bladder SMCs of the myocardin−/−/LacZ+ chimeras (Fig. 1B). When evaluated in cross-sectional histological sections, the heterozygous KO myocardin+/−/LacZ+ ESCs contributed to bladder SMCs (Fig. 1C), whereas myocardin−/−/LacZ+ ESCs only contributed to the bladder epithelium and interstitial cells, not SMCs (Fig. 1D). In light of this observation, we carefully examined other SMC tissues to see whether the contribution by myocardin−/−/LacZ+ cells was altered. A similar pattern was observed in the stomach, with myocardin+/−/LacZ+ controls showing staining of the SMC layer of the gut (Fig. 1, E and I), whereas myocardin−/−/LacZ+ cells did not (Fig. 1F). Interestingly, at the transition between skeletal muscle and smooth muscle in the esophagus, the myocardin−/−/LacZ+ cell contribution rapidly fell off (Fig. 1F). Indeed, examination of cells at the transition region showed myocardin−/−/LacZ+ cells contributing to striated skeletal muscle but not to nonstriated SMCs (Fig. 1F, inset) compared with myocardin+/−/LacZ+ cells, which contributed to both muscle types (Fig. 1J). It should also be noted that myocardin−/−/LacZ+ cells consistently gave rise to vascular SMCs within blood vessels within visceral SMC tissues.
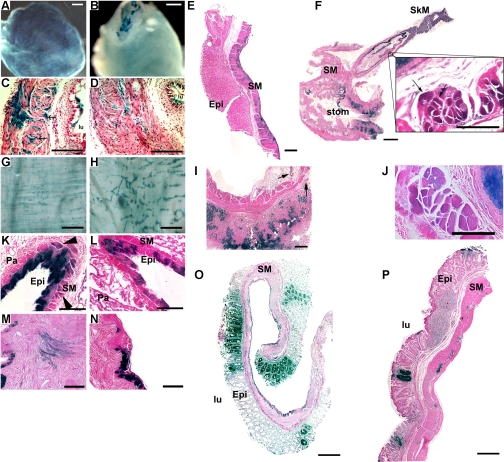
Fig. 1.
Myocardin−/− embryonic stem cells (ESCs) fail to differentiate into a variety of visceral smooth muscle cell (SMC) types. A variety of visceral smooth muscle tissues from myocardin+/−/LacZ+ control and myocardin−/−/LacZ+ chimeric mice were dissected and stained with X-gal to identify cells derived from ESCs, and their gross and cross-sectional histology was evaluated. A: representative bladder from a myocardin+/−/LacZ+ control ESC chimera in which at least one-half of the bladder SMCs are LacZ+. Scale bar, 500 μm. B: in contrast, myocardin−/−/LacZ+ ESCs are not observed to differentiate into bladder SMCs despite significant contributions to bladder epithelial cells (top) and interstitial cells (faint blue staining). Scale bar, 500 μm. C: histological section of a bladder, stained with nuclear fast red, obtained from a myocardin+/−/LacZ+ control ESC chimera, displaying multiple blue SMC fibers (arrows). Scale bar, 100 μm. D: histological section of a bladder from a myocardin−/−/LacZ+ chimera in which blue SMCs are not observed, despite labeling of all other bladder cell types. Scale bar, 100 μm. lu, Lumen. E: representative stomach from a myocardin+/−/LacZ+ control mouse, stained with eosin, showing staining throughout the SMC layers. Epi, epithelium; SM, smooth muscle. Scale bar, 200 μm. F: histological section through the stomach and esophagus of a myocardin−/−/LacZ+ chimera, stained with eosin, showing the absence of LacZ+ cells in the SMC layers (stom, stomach). Interestingly, a transition is clearly visible in the esophagus as the muscular layer transitions from voluntary skeletal muscle (SkM) with striations to involuntary SM. Scale bar, 1 mm. At the transition between the 2 layers (inset; scale bar, 100 μm), the LacZ+ cells show striations whereas the LacZ− cells do not, indicating myocardin−/− cells adequately contribute to esophageal striated muscle but fail to contribute to SMC layers. G: whole mount preparation of the SMC layer of the large intestine of a myocardin+/−/LacZ+ chimera that has been dissected away from the epithelial lining, showing LacZ+ SMC fibers in the circular and longitudinal SMC layers. Scale bar, 500 μm. H: whole mount preparation of the SMC layer of the large intestine from a myocardin−/−/LacZ+ chimera, dissected away from the epithelial lining, showing LacZ+ cells contributing to the parasympathetic ganglia but not to SMC fibers in the muscular layers. Scale bar, 500 μm. I: cross section from the stomach of a myocardin+/−/LacZ+ chimera (corresponding to stomach in E) showing LacZ+ cells in the epithelial layer and parasympathetic ganglia (arrows) but not in SMC layers. Scale bar, 200 μm. J: section similar to that in I, from the esophagus of a myocardin+/−/LacZ+ chimera, showing staining in the epithelial layer and SMC layer corresponding to a similar region shown in the inset in F. Scale bar, 100 μm. K: cross section of the lungs of a myocardin+/−/LacZ+ chimera showing LacZ+ cells in the SMC surrounding the bronchi (arrowheads). Pa, parenchyma. Scale bar, 100 μm. L: section similar to that in K, from the lungs of a myocardin−/−/LacZ+ chimera, showing no contribution of −/− cells to the airway SMCs of the lung. Scale bar, 100 μm. M: cross section of the uterus of a myocardin+/−/LacZ+ chimera showing LacZ+ cells contributing to the myometrium. Scale bar, 100 μm. N: cross section of the uterus from a myocardin−/−/LacZ+ chimera showing LacZ-stained cells within the epithelium but no LacZ+ cells in the myometrium. Scale bar, 100 μm. O: cross section from the large intestine of a myocardin+/−/LacZ+ chimera showing LacZ+ cells in the epithelial and SMC layers as well as parasympathetic ganglia. Scale bar, 200 μm. P: section similar to that in O, from the large intestine of a myocardin−/−/LacZ+ chimera, showing staining in the epithelial layer and parasympathetic ganglia but not the muscular layer. Scale bar, 200 μm.
The pattern of myocardin−/− cells failing to contribute to visceral SMC extended throughout the gut. For example, there was little, if any, contribution of myocardin−/−/LacZ+ cells to the SMC layers (Fig. 1, H and P) of the large intestine, whereas myocardin−/−/LacZ+ cells did contribute to the parasympathetic ganglia. In contrast, SMCs from myocardin+/−/LacZ+ ESCs were readily observed in SMC layers of the large intestine (Fig. 1, G and O). Similar to observations of reduced contribution of myocardin−/−/LacZ+ ESCs to visceral SMC, we also observed a marked decrease in these cells investing into SMCs with the lung bronchi (Fig. 1, K vs. L) and uterus (Fig. 1, M vs. N).
Myocardin−/− cells exhibited a marked decrease in investment into cardiac ventricles but contributed normally to coronary blood vessels and atrial myocytes.
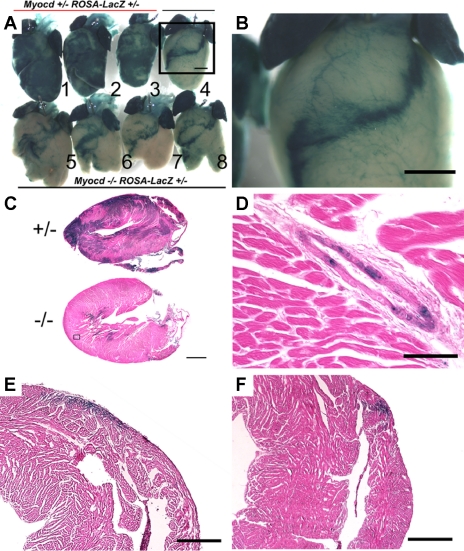
Hearts of myocardin−/−/LacZ+ chimeric mice showed an unexpected profound decrease in the contribution of myocardin−/−/LacZ+ ESCs into the ventricles of the heart at P10 (Fig. 2A). Indeed, myocardin−/−/LacZ+ cells that persisted in the adult ventricle were confined to a superficial band beginning at the intraventricular septum and wrapping around the right ventricle (Fig. 2B). The same pattern was seen in 100% of 30 chimeric mice examined at 10 days, 28 days, and 6 mo of age and across multiple independent blastocyst injections. Longitudinal histological sections through the ventricle showed occasional myocardin−/−/LacZ+ cells within the myocardium, indicating that at least some myocardin−/−/LacZ+ cells were capable of differentiation into ventricular myocytes (Fig. 2C). In contrast, myocardin+/−/LacZ+ ESCs showed homogenous distribution throughout the heart (Fig. 2, A, hearts 1–3, and C, top image). Investment of myocardin−/−/LacZ+ ESCs within the coronary vasculature appeared to be unaffected, indicating that myocardin is not required for differentiation of coronary SMCs that are derived from the proepicardial organ (25) (Fig. 2, B and D). These latter results extend observations from our previously published studies showing that cell autonomous myocardin is not required for differentiation of vascular SMCs derived from mesodermal sources (29).
Fig. 2.
Myocardin−/− ESCs readily form atrial ventricular myocytes and coronary smooth muscle cells but not ventricular myocytes. Hearts from chimeric animals at 10 days of age were dissected out and stained with X-gal. A: myocardin−/−/LacZ+ (Myocd−/− ROSA-LacZ+/−) ESCs failed to contribute to the ventricles (hearts 4–8) compared with myocardin+/−/LacZ+ (Myocd+/− ROSA-LacZ+/−) cells (hearts 1–3). Scale bar, 1 mm. The myocardin−/−/LacZ+ cells typically formed a superficial band of myocytes extending from the aortic root in the left ventricle, across the ventricular septum, and continuing in a band across the right ventricle. B: higher magnification of heart 4 (box in A) shows myocardin−/−/LacZ+ cells contributed to coronary vasculature (see also enlarged image in D) and atria. C: longitudinal sections show abundant myocardin+/−/LacZ+ cells contributing to the ventricles (top) compared with myocardin−/−/LacZ+ cells (bottom). D: high magnification of a coronary artery from a myocardin−/− chimera showing abundant myocardin−/−/LacZ+ cells in a coronary artery. Scale bar, 100 μm. E and F: saggital sections of the right ventricle show that myocardin−/−/LacZ+ cells contributed to a superficial strip across the anterior right ventricle in an inferior (E) and superior section (F) across the heart. Scale bar, 500 μm. Results were consistent over 30 myocardin−/−/LacZ+ animals at ages from 10 days to 6 mo.
Myocardin−/−/LacZ+ ESCs were underrepresented in visceral SMC tissues based on quantitative analysis of genomic DNA by Southern analyses.
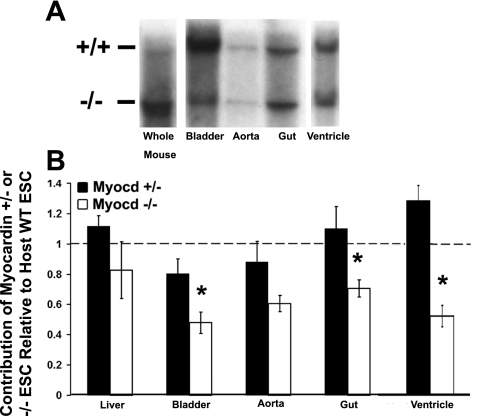
Although unlikely, it is possible that myocardin−/−/LacZ+ ESCs contributed to formation of SMC tissues in the gastrointestinal tract, but somehow the LacZ transgene may have been silenced, thus abrogating our ability to detect myocardin−/−/LacZ+ SMCs. To rule out this possibility, we performed quantitative Southern blot analyses to detect the relative number of cells present in various tissues of mixed chimeric mice that were derived from WT ESCs (having +/+ myocardin alleles) vs. either the myocardin+/− or myocardin−/− ESCs. We analyzed tissues from five myocardin+/−/LacZ+ and four myocardin−/−/LacZ+ mice and performed densitometry of the bands. If myocardin−/− and myocardin+/− ESCs contribute equally to all tissues, one would expect the ratio of −/− and +/− alleles relative to +/+ cells derived from host blastocyst ESCs to be equivalent between a whole animal DNA lysate (designated “whole mouse” in Fig. 3) and individual organ DNA lysate within the same animal. Comparison of the ratios of −/− to +/+ and +/− to +/+ alleles from each tissue to the ratios in a whole mouse lysate indicates whether there is an increase or decrease in the relative contribution of the myocardin+/− or myocardin−/− ESCs relative to +/+ host blastocyst within any given tissue. Visceral and vascular smooth muscle lysates were compared with whole mouse lysate. Results of the Southern analysis showed that bladder and gut tissues had a reduced contribution of myocardin−/− ESCs compared with WT host blastocyst ESCs when normalized to overall chimerism using whole mouse lysates. In contrast, results of analysis of liver homogenates showed an equivalent contribution of myocardin−/−/LacZ+ ESCs compared with WT host ESCs, indicating no detectable effect of loss of myocardin for development of hepatocytes or other liver cell types (Fig. 3B). These results are in full agreement with histological results showing a marked reduction in myocardin−/−/LacZ+ ESCs to visceral SMCs tissues, as shown in Fig. 1. Southern results from the aorta showed a trend toward a decreased contribution of myocardin−/−/LacZ+ ESCs, but this did not achieve statistical significance, perhaps due to the fact these samples contain a substantial proportion of non-SMCs, including adventitial fibroblasts, neurons, and other non-SMCs that are not myocardin dependent.
Fig. 3.
Quantitative Southern analyses show reduced investment of myocardin−/− cells in visceral SMC tissues, bladder, and cardiac ventricles. Bladder, aorta, small intestine/gut, and ventricle were dissected from 28-day-old myocardin+/−/LacZ+ control and myocardin−/−/LacZ+ animals, lysed, DNA purified and electrophoresed, and transferred to a nylon membrane for Southern analysis. A: representative Southern blots. If cells contributed equally to all tissues, the ratio between the +/+ lane, representing cells derived from ESCs from the host blastocysts, which have myocardin wild-type (WT) alleles, and the −/− lane, representing cells derived from ESCs containing knockout alleles, would be equivalent in all tissues (i.e., a ratio of 1). The “whole mouse” lysate lane represents homogenized tissue from an entire mouse (less dissected organs and tissues), and the ratio between the bands in this lane represents the overall contribution of chimeric cells in the individual animal assayed (i.e., %chimerism). Analysis of tissues from myocardin−/−/LacZ+ chimeras showed a decreased ratio of cells derived from ESCs with myocardin−/− alleles in visceral SMC tissues and ventricles compared with that observed in whole mouse lysates, suggesting myocardin is required for appropriate contribution of ESC-derived cells to these tissues. B: results of densitometric analysis of multiple independent Southern blots. Myocardin−/−/LacZ+ chimeras showed a statistically significant decrease in the contribution of myocardin−/− ESCs in the bladder, gut, and ventricles compared with WT ESCs from the host blastocysts (n = 4 myocardin+/−/LacZ+ and 5 myocardin−/−/LacZ+). Values are means ± SD. *P < 0.05. Heterozygous myocardin+/− ESCs exhibited no significant change in their contribution to any of these tissues, indicating that loss of 1 myocardin allele had no effect.
Myocardin−/−/LacZ+ ESC-derived SMCs were underrepresented in vascular SMC tissues based on quantitative analyses of the proportion of LacZ-positive cells.
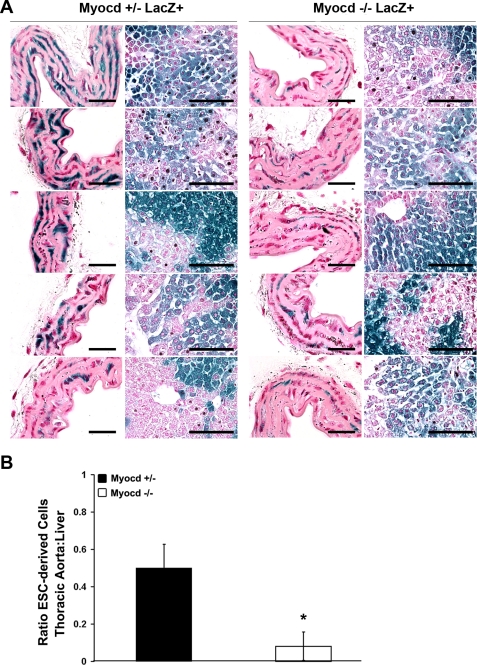
Southern blots are limited in sensitivity due to the tissues being composed of multiple cell types other than SMCs, thus lowering the signal-to-noise ratio. For example, aortic results are limited by the fact that the aortic samples also include DNA derived from endothelial cells, adventitial fibroblasts, and other non-SMC that would presumably not show any dependence on myocardin, since it is not known to be expressed in these cells at any point in their development. We therefore designed a more sensitive assay to detect quantitative differences in the contribution of myocardin−/−/LacZ+ ESCs to vascular SMC tissues. We isolated corresponding thoracic aorta segments from five myocardin+/−/LacZ+ and five myocardin−/−/LacZ+ chimeric animals, fixed and stained them with X-gal, and then sectioned them en face to allow for easier identification of individual SMCs within the media of blood vessels. Nuclear fast red was used to identify nuclei, and an evaluator blinded to the samples counted LacZ-positive (LacZ+) and -negative (LacZ−) nuclei in thoracic aorta and liver controls to determine whether there was a difference in contribution to vascular SMCs (Fig. 4A). Assuming that the contribution to the liver by either myocardin+/−/LacZ+ or myocardin−/−/LacZ+ ESCs is not different (Fig. 3), we normalized the number of LacZ+ cells in the sections of the thoracic aorta to that in the liver. These analyses revealed a highly significant, quantitative decrease (P < 0.01) in the fraction of myocardin−/−/LacZ+ SMCs within the aortas of chimeric KO mice. Indeed, the myocardin−/−/LacZ+ cells comprised fewer than 20% of the cells in the aortic media compared with ∼50% of the cells in the liver. Myocardin+/−/LacZ+ cells were present in blood vessels at the same frequency as in liver, indicating that loss of one myocardin allele had no effect on formation of aortic SMCs.
Fig. 4.
Histological analysis shows reduced investment of myocardin−/− ESCs into vascular SMC lineages. Tissues were fixed and stained for LacZ. Aortic strips were prepared en face and sectioned, and LacZ+ and LacZ− nuclei were counted to determine the percentage of cells that were ESC derived in each tissue. A: cross sections of aortas prepared en face and examined with nuclear fast red (NFR) staining (left) at high magnification show fewer LacZ+ cells in myocardin−/−/LacZ+ aortas compared with liver tissue (right) relative to myocardin+/−/LacZ+ controls. Aorta: scale bar, 25 μm; liver: scale bar, 100 μm. B: quantification of the ratio of LacZ+ nuclei in the thoracic aorta relative to the liver shows a markedly decreased contribution of myocardin−/−/LacZ+ ESCs to vascular SMCs compared with the contribution of myocardin+/−/LacZ+ ESCs. Values are means ± SD. *P < 0.05.
Myocardin−/−/LacZ+ ESC-derived cardiomyocytes were underrepresented in cardiac ventricles as determined by quantitative Southern analysis.
To determine whether the myocardin−/−/LacZ+ cells persisted in the cardiac ventricles but were not detectable through X-gal staining, we performed Southern blot analysis with a probe directed toward the 3′ region of the myocardin gene locus (Fig. 3B). The signal from +/+ and −/− alleles in the whole mouse was compared with the signal from the liver and ventricles. Assuming that the contribution to the liver by either myocardin+/−/LacZ+ or myocardin−/−/LacZ+ cells is not different (Fig. 3), we normalized the number of LacZ+ cells in the ventricle to that in the liver. Myocardin−/−/LacZ+ cells had a ventricle/whole mouse signal significantly less than the ratio for myocardin+/−/LacZ+ controls, indicating that the contribution of myocardin−/−/LacZ+ cells to the ventricles was significantly less than the contribution to other tissues. The ratios for ventricular samples are also likely to underestimate the deficiency in myocardin−/−/LacZ+ ESCs forming ventricular myocytes, since these samples also contain coronary SMCs, interstitial fibroblasts, and endocardial and epicardial cells that appeared to contain abundant myocardin−/−/LacZ+ cells (see Fig. 2, B and D).
Myocardin−/−/LacZ+ ESCs formed aortic SMC within chimeric KO mice and had normal myofilaments and overall ultrastructural appearance based on examination by electron microscopy and electron probe microanalysis.
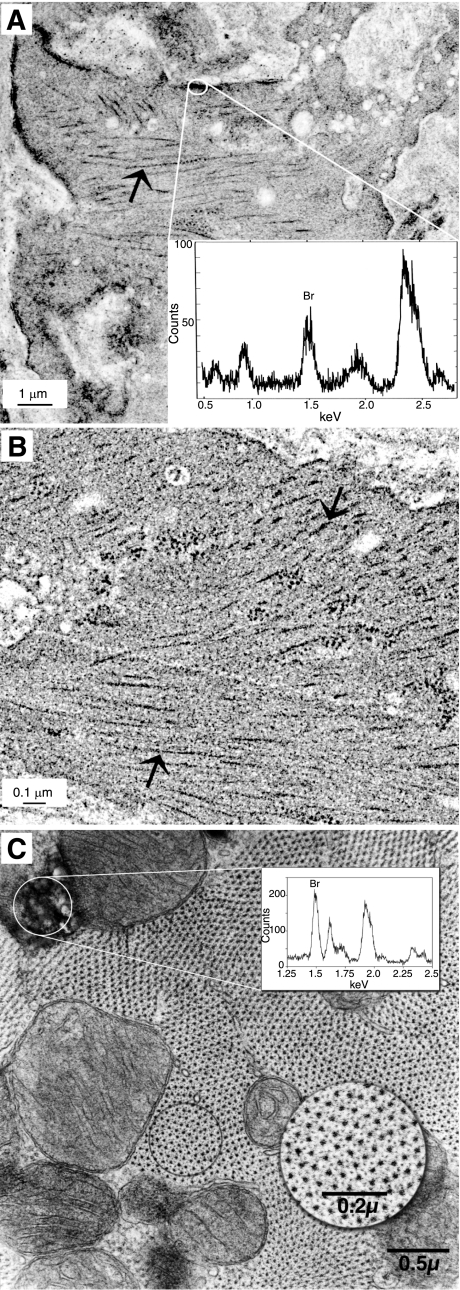
To determine whether the myocardin−/−/LacZ+ cells that populate the aorta are fully differentiated SMCs, we developed a novel method for identifying and assessing the ultrastructural appearance of myocardin−/−/LacZ+ cells within the aorta. The unequivocal identification was accomplished by staining aortas with Bluo-gal (Fig. 5A), which forms dense precipitates of nonsoluble Bluo-gal crystals in the presence of galactosidase activity that can be readily identifiable by a combination of TEM appearance and detection of bromine in the Bluo-gal crystals through electron probe microanalysis (Fig. 5, graph insets in A and C). The myocardin−/−/LacZ+ SMCs within the aorta had ultrastructural characteristics consistent with a functional contractile apparatus, including arrays of myosin filaments that appeared indistinguishable from those present in adjacent WT ESC-derived aortic SMCs (large arrows in Fig. 5, A vs. B). We should note that although our methods have a high degree of fidelity such that we are confident that a LacZ+ cell is indeed derived from a myocardin−/− ESC, due to inherent methodological limitations associated with balancing the need for adequate TEM fixation while retaining the ability to detect LacZ enzymatic activity, we believe there are likely many false negatives with this method. As such, there may be myocardin−/− SMCs that have not been identified as such. Thus our results show that at least some myocardin−/− SMCs have ultrastructural characteristics indistinguishable from adjacent myocardin+/+ SMCs, but we cannot conclude whether this is true of all myocardin−/− SMCs.
Fig. 5.
Ultrastructural appearance of myocardin−/−/LacZ+ adult vascular SMCs and ventricular cardiomyocytes is unchanged from adjacent myocardin+/+ host cells. Thoracic strips were prepared from the aorta of myocardin−/−/LacZ+ animals, fixed briefly, stained with Bluo-gal for LacZ+, and embedded for analysis by electron microscopy (EM). LacZ+ cells were identified by electron probe microanalysis (EPMA) of the Bluo-gal precipitate to ascertain whether a given cell was derived from myocardin−/−/LacZ+ ESCs vs. WT ESCs from the recipient blastocyst. No differences in the ultrastructural appearance were observed between WT cells and the −/− cells identified by EPMA. A: electron micrograph of a LacZ+ myocardin−/− SMC in the thoracic aorta of a myocardin−/−/LacZ+ chimera showed a normal ultrastructural phenotype, including normally appearing myofilaments (arrow). The boxed region was subjected to EPMA analysis. EPMA (inset) of Bluo-gal precipitates showed a strong bromine peak, indicating cells are positive for LacZ and confirming their myocardin−/−/LacZ+ ESC origin. B: electron micrograph of an adjacent WT SMC derived from a host ESC is shown for comparison. The arrows denote arrays of myosin filaments that were indistinguishable between SMC derived from the myocardin−/− ESC shown in A. Results are consistent with at least some myocardin−/−/LacZ+ ESC-derived aortic SMCs having a normal functional contractile apparatus. C: hearts from 28-day-old myocardin−/−/LacZ+ were perfusion fixed with EM-fix, stained with Bluo-gal rather than X-gal to enhance detection by EM, postfixed, and then embedded for EM analysis. The electron micrograph shows a myocardin−/−/LacZ+ ventricular myocyte in an adult heart that is positive for Bluo-gal crystals. EPMA (graph inset) of the Bluo-gal precipitates shows a strong bromine peak, confirming the presence of Bluo-gal crystals within the cell and myocardin−/−/LacZ+ genotype. Myocardin−/−/LacZ+ cardiac ventricular myocytes show a normal ultrastructural phenotype with regularly arrayed myosin filaments surrounded by actin filaments (circular inset).
Myocardin−/−/LacZ+ ESCs formed cells in the adult heart that were phenotypically normal based on ultrastructural analyses.
The preceding analyses clearly showed that myocardin−/−/LacZ+ ESCs exhibited reduced investment into ventricular myocytes. However, a small subfraction of myocardin−/−/LacZ+ ESCs were present within ventricles (Fig. 5C). Bluo-gal staining was used to identify myocardin−/−/LacZ+ ventricular myocytes in sections of hearts from myocardin−/−/LacZ+ chimeras. Although myocardin−/−/LacZ+ cells were grossly underrepresented within ventricles, those that did invest had an ultrastructural appearance indistinguishable from those derived from WT host ESCs, including a regular lattice of myosin filaments surrounded by actin filaments (Fig. 5C, circular inset). These results indicate that cell autonomous myocardin gene expression is not an absolute requirement for formation of ventricular myocytes. However, the extraordinarily low frequency of myocardin−/− ESCs that formed ventricular myocytes clearly indicates there is a critical myocardin-dependent step in the early development of these cells that coincides with their location along the axis between the ventricles as shown in Fig. 2A.
Myocardin−/−/LacZ+ SMCs exhibited normal contractile properties.
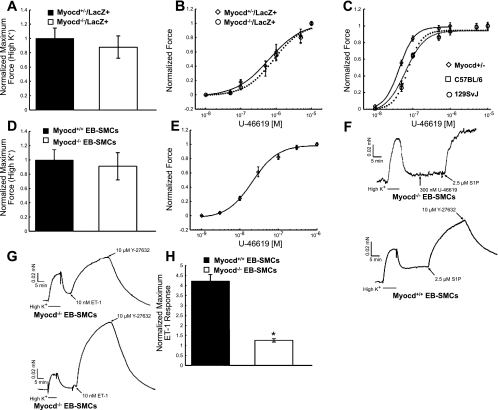
To determine whether SMCs derived from myocardin−/−/LacZ+ ESCs were capable of normal contraction in response to agonists, we measured the contractility of aortic strips. Strips (0.8 wide × 1.5 mm long) cut from both myocardin−/−/LacZ+ (n = 15 total strips, 3 strips from 5 mice) and myocardin+/−/LacZ+ chimeric mouse aortas (n = 15 total strips, 3 strips from 5 mice) contracted similarly following stimulation with 154 mM K+ and relaxed back to baseline on switching to HEPES-buffered Krebs solution (data not shown). There were no significant differences in maximal 154 mM K+-induced force between myocardin+/−/LacZ+ and myocardin−/−/LacZ+ chimeric mouse aortas (Fig. 6A).
Fig. 6.
SMCs derived in vitro from myocardin−/− ESCs using a novel ESC-embryoid body (EB) SMC differentiation model system previously developed in our laboratories (34) and in aortas of myocardin−/− chimeric mice exhibit normal contractile properties and agonist sensitivity. A: comparison of normalized maximal K+ contraction between thoracic aortic strips from myocardin+/−/LacZ+ (n = 14) and myocardin−/−/LacZ+ chimeric mice (n = 15). B: U-46619 dose-response curves in strips cut from the thoracic aorta of myocardin+/−/LacZ+ (EC50 = 625 ± 150 nM, n = 14 strips from 5 animals) and myocardin−/− ROSA26+/− (EC50 = 835 ± 146 nM, n = 15 strips from 5 animals) chimeric mice. C: dose-response curves to the stable thromboxane A2 analog U-46619 in strips cut from the thoracic aorta of C57BL/6 (EC50 = 73.9 ± 4.78 nM, n = 4) and 129SvJ strain controls (EC50 = 64.1 ± 11.9 nM, n = 4) as well as myocardin+/−/LacZ+ animals (EC50 = 44.4 ± 1.29 nM, n = 4). D: comparison of normalized maximal K+ contraction between myocardin+/+ (n = 14) and myocardin−/− SMCs (n = 11) produced in vitro in our ESC-EB SMC differentiation model system (34) (cells are henceforth referred to as “EB-derived SMC”). E: dose-response cure to U-46619 in myocardin+/+ EB-derived SMC fibers (EC50 = 23.9 ± 3.2 nM, n = 3). F: representative force trace from myocardin−/− (top) and myocardin+/+ EB-derived SMCs (bottom). The traces demonstrate rapid induction and maintenance of contractile force in the presence of depolarizing (154 mM K+) solution, which rapidly relaxes to near baseline once the bathing solution is switched back to Krebs. The myocardin−/− EB-derived SMC failed to respond to 300 nM U-46619 but did respond with force comparable to depolarizing solution when stimulated with spingosine-1-phosphate (S1P). The vertical bars represent times when the Krebs buffer was changed. G: representative force traces from myocardin−/− (top) and myocardin+/+ EB-derived SMCs (bottom) stimulated with 10 nM ET-1. The traces demonstrate that myocardin is not necessary for the development of ET-1 contractile responses. H: myocardin−/− EB-derived SMCs (n = 3) generated significantly less 10 nM ET-1-induced maximal force than myocardin+/+ EB-derived SMCs (n = 4). ET-1 maximal forces were normalized to maximal K+ contraction. Value are means ± SD. *P < 0.05.
To further explore the contractile capabilities of myocardin−/−/LacZ+ SMCs, we measured dose-response curves to the stable thromboxane A2 mimetic U-46619. Thromboxane A2 signaling is known to result in a large contractile force with a relatively small increase in intracellular calcium (1, 12). No differences in either maximal force development or thromboxane sensitivity were evident between thoracic aortic strips cut from myocardin+/−/LacZ+ and myocardin−/−/LacZ+ chimeric mice (Fig. 6B).
Because of the reduced fraction of myocardin−/−/LacZ+ cells within aortic strips based on histological analysis (Fig. 4B), the preceding studies lack sensitivity for detecting changes in contractility within the subset of myocardin−/− SMCs within this tissue. To address this limitation, we generated pure populations of myocardin−/− SMCs in vitro using a unique EB SMC differentiation model previously described in our laboratories (34). In brief, either myocardin+/+ or myocardin−/− ESCs were aggregated into EBs and induced to differentiate, and SMCs were purified from the heterogeneous cell population. These EB-derived SMCs were then embedded into three-dimensional collagen gels on which they further differentiate and align along the axis of stress applied to the gel. In this manner, we were able to study contractile properties and agonist sensitivities in strips composed of pure populations of SMCs derived from either myocardin+/+ or myocardin−/− ESCs. All strips cut from both myocardin+/+ (n = 14) and myocardin−/− reconstituted muscle fibers (n = 11) demonstrated a rapid phasic contraction when stimulated with depolarizing solution (154 mM K+), indicating that not only is the SM contractile apparatus fully functional in myocardin−/− SMCs, but cells also have all the necessary signaling pathways and functional attachments to the extracellular matrix necessary to generate force. All strips tested were able to maintain (5–8 min in duration) 154 mM K+-induced tonic force until extracellular K+ was removed by switching the bathing solution to HEPES-buffered Krebs solution, at which time the strips relaxed to near baseline, indicating that a sufficient complement of Na+/K+/Ca2+ ATP-(in)dependent exchangers/pumps are present and functional (Fig. 6, F and G). Of major significance, there were no significant differences in maximal 154 mM K+-induced force capacity between myocardin+/+ (n = 14) and myocardin−/− EB-derived SMC reconstituted muscle fibers (n = 11) (Fig. 6D). As such, these studies provide compelling evidence that fully functional SMCs can develop in the absence of myocardin, at least within the in vitro experimental model system tested in the present study.
To determine whether there may be more subtle differences in contractility, we measured responses to various contractile agonists in EB-derived SMC reconstituted muscle fibers derived from myocardin+/+ or myocardin−/− ESCs. Myocardin+/+ EB-derived SMC fibers exhibited an EC50 of 23.9 ± 3.2 nM to the thromboxane agonist U-46619 (Fig. 6E). In contrast, to our surprise, we observed a complete lack of a contractile response to 300 nM U-46619 in myocardin−/− EB-derived SMC fibers (Fig. 6F), suggesting a defect in thromboxane A2 receptor (TXA2R)-mediated calcium-independent (calcium sensitized) force generation. In contrast, myocardin+/+ and myocardin−/− EB-derived SMC fibers contracted similarly (qualitatively) to 2.5 μM spingosine-1-phosphate (S1P) and 10 nM endothelin-1 (ET-1) (Fig. 6, F and G, respectively), indicating that the absence of myocardin does not abrogate the development of either the S1P or ET-1 contractile signaling cascades within the EB-derived SMC fiber model system. On further analysis of the ET-1 responses, we found that myocardin−/− SMCs also generated significantly less maximal force (Fig. 6H) relative to 154 mM K+-induced force (1.25 ± 0.08, n = 3, P < 0.05) compared with myocardin+/+ EB-derived SMCs (4.22 ± 0.28, n = 4).
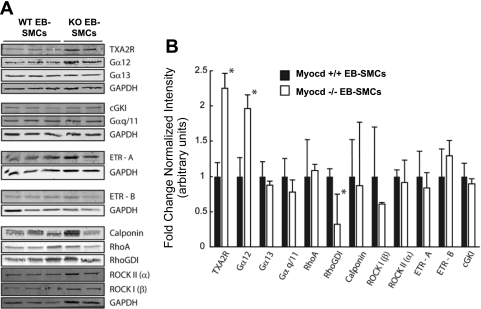
Reduced thromboxane-induced contraction in myocardin−/− EB-derived SMC fibers was not due to loss of expression of the thromboxane receptor.
Western blot analyses of TXA2R were performed to determine whether the failure of myocardin−/− EB-derived SMCs to contract in response to thromboxane was the result of defective receptor expression. Surprisingly, TXA2R expression was increased more than twofold in myocardin−/− compared with myocardin+/+ EB-derived SMCs (Fig. 7). In contrast, protein expression levels of both the A and B isoforms of the endothelin receptor showed no significant differences. It has been demonstrated that TXA2R activation in platelets disproportionately signals through Gα13, with Gα12 activation occurring as a secondary event following the binding of ligand (13). Therefore, we performed Western blots for both Gα13 and Gα12 to determine whether the absence of thromboxane A2 response observed in the myocardin−/− EB-derived SMCs was due to a defect in TXA2R activation of the heterotrimeric G proteins responsible for calcium-sensitized force (35). Interestingly, we observed no significant difference in the protein expression of Gα13 but a significant twofold increase (1.96 ± 0.18, P < 0.05, n = 3 myocardin+/+ and 2 myocardin−/− independent clones) in Gα12 protein expression in myocardin−/− compared with myocardin+/+ EB-derived SMCs. It is possible that the observed defect in thromboxane A2 signaling was due to either the TXA2R becoming desensitized to ligand (30) or the loss of coupling between the Gα13 subunit to its βγ-subunits, thus inhibiting RhoA activation (26). Because cGMP can phosphorylate and inhibit RhoA activity, we next examined the protein expression levels of cGMP-dependent protein kinase I (cGKI). There were no significant differences in cGKI expression levels, although we cannot rule out differences in basal cGKI activities within myocardin−/− and myocardin+/+ EB-derived SMCs. We also observed a significant reduction (0.32 ± 0.39-fold change, P < 0.05, n = 3 myocardin+/+ and 2 myocardin−/− independent clones) in Rho GDI protein expression but no significant differences in the protein expression levels of RhoA, calponin, ROCK I, and ROCK II between myocardin−/− and myocardin+/+ EB-derived SMCs. Thus the lack of response to U-46619 in the myocardin−/− SMCs was not due to an absence of these downstream signaling molecules.
Fig. 7.
Myocardin−/− ESC-EB-derived SMCs show a paradoxical upregulation of thromboxane A2 receptor (TXA2R) and Gα12 but decreased expression of Rho GDP dissociation inhibitor (GDI). EB-derived SMCs derived from myocardin+/+ and myocardin−/− ESCs were grown to confluence, and protein was harvested and analyzed by quantitative Western blotting. A: Western blots for 3 WT lines and 2 −/− knockout (KO) lines were compared with determine signaling pathways altered by loss of the myocardin gene. cGKI, cGMP-dependent protein kinase I; ETR, endothelin receptor; ROCK, Rho-dependent kinase. B: densitometric results show statistically significant downregulation of Rho GDI signaling molecules, possibly providing an explanation for the observed defect in signaling. Receptors for other agonists and signaling molecules were equivalent between WT and myocardin−/− EB-derived SMCs. Values are means ± SD. *P < 0.05.
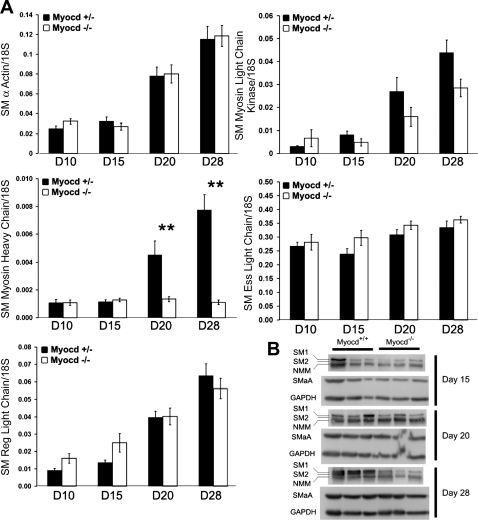
Myocardin−/− EB-derived SMCs showed reduced expression of the SM2 isoform of SM-MHC.
In vitro analysis of the role of myocardin in SMC development was also pursued using an EB model of ESC-SMC differentiation previously described by our laboratory (34). Quantitative PCR analysis of the SMC differentiation markers and components of the contractile apparatus, including SMαA, SM-MHC, SM regulatory light chain, SM myosin light chain kinase, and SM essential light chain, showed no changes between myocardin+/+ and myocardin−/− EB-derived SMCs at 10, 15, 20, and 28 days of in vitro development, with the sole exception of the SM-MHC gene (Fig. 8A, n = 4 experiments in triplicate). Results of Western blot analyses showed similar changes in protein expression. For example, consistent with the mRNA findings, SMαA expression was unchanged between the two cell types, whereas the SM-1 isoform of SM-MHC was markedly downregulated and the SM-2 splice variant was completely absent in myocardin−/− cells even at 28 days of in vitro differentiation (Fig. 8B). This was not a result of selective expansion of myocardin+/+ EB-derived SMCs, because flow cytometric analysis showed an equal proportion of SMαA-positive cells in both myocardin+/+ and myocardin−/− SMC populations at all time points examined (data not shown).
Fig. 8.
Myocardin−/− ESCs show defective activation of smooth muscle myosin heavy chain (SM-MHC) expression within EBs. Myocardin+/+ and myocardin−/− ESCs were aggregated into EBs, and RNA and protein were harvested and analyzed at early and late time points of EB development to assess the ability of myocardin−/− cells to form mature SMCs in vitro. A: real-time RT-PCR analysis of smooth muscle contractile protein genes showed equivalent expression between myocardin+/+ and myocardin−/− cells. However, there was a specific failure of SM-MHC to upregulate in differentiating myocardin−/− cells during late EB development. D, day. Values are means ± SD (n = 4 experiments, each performed in triplicate). **P < 0.01. B: Western blotting showed global deficits in SM-MHC protein expression in myocardin−/− cells and a distinct absence of the mature SM2 SM-MHC splice variant in myocardin−/− EBs at 28 days of differentiation. Values are means ± SD (each band represents an independent EB experiment for n = 3 for each cell type at each time point). Consistent with the mRNA findings, smooth muscle α-actin (SMaA) protein expression was unchanged between WT and myocardin−/− EBs, whereas SM-MHC was downregulated. Ess, essential; Reg, regulatory; NMM, nonmuscle myosin.
DISCUSSION
Results of the present studies provide compelling evidence that myocardin requirements differ dramatically between different cardiomyocyte and SMC subtypes. Indeed, our observation that myocardin−/− ESCs virtually completely failed to contribute to formation of ventricular myocytes within the setting of chimeric KO mice was completely surprising in light of previous studies in conventional myocardin KO mice that showed no overt heart defects. Similarly, results of the present studies also unexpectedly showed that cell autonomous myocardin was required for development of multiple smooth muscle lineages in that we observed a virtual absence of myocardin−/− ESC-derived SMCs within the bladder, small intestine, uterus, and stomach in chimeric KO mice despite these cells contributing equivalent to myocardin+/+ host ESCs in all non-SMC lineages, other than ventricular cardiomyocytes. In contrast, we frequently observed myocardin−/− ESCs within vascular smooth muscle lineages (in multiple blood vessels in all tissues examined) albeit at a reduced frequency compared with myocardin +/+ host ESCs. We also present multiple lines of evidence that myocardin−/− SMCs develop normally based on their exhibiting 1) normal morphology as shown by TEM, 2) normal myofilament structures, and 3) full contractile capabilities, including maximal force development, responsiveness to most contractile agonists, and K+ depolarization-induced contraction. The few notable deficits detected included failed contractile responsiveness to thromboxane A2 and diminished expression of the late SMC differentiation marker gene, the SM2 variant of SM-MHC, when myocardin−/− ESCs were differentiated in vitro into SMCs in an EB model system. Our current understanding of the genetic and developmental differences between vascular and visceral smooth muscle is limited. However, of interest, we observed in a variety of blood vessels myocardin−/− SMCs that have different embryological origins, including SMCs from pulmonary arteries and cardiac outflow tract blood vessels that are derived from neural crest cells, coronary artery SMCs that are derived from proepicardial cells, and aorta/peripheral artery SMCs that are derived from local mesoderm as is the case with visceral SMCs (25). As such, it is unlikely that differential dependence on myocardin for development is a function of the embryological origins of SMCs.
One possibility is that differential myocardin dependence for SMC development is a function of unique environmental cues that exist in blood vessels vs. visceral and other SMC tissues. Although the precise nature of these differences is not clear, it is likely that at least part of the mechanism relates to whether the myocardin-related genes MRTF A and B are activated and/or whether they can fully compensate for myocardin within that SMC subtype. Indeed, although there are numerous overlapping functions of myocardin and the MRTFs (1, 41), there are also notable exceptions, including differential control of nuclear localization and Rho dependence (6, 23). Of interest, Huang et al. (11) showed that selective KO of myocardin in neural crest-derived tissues, generated by crossing floxed myocardin mice with either Wnt1 cre or Pax3 cre mice, showed 100% perinatal lethality and presented evidence for incomplete differentiation of SMC within the ductus arteriosus and failed ductus closure at birth. These results are informative in that the ductus serves as a shunt that allows blood flow to bypass the fetal lungs, but at birth, contraction of the ductus SMCs is required for its closure and perinatal survival. As such, these results suggest that myocardin is normally required for development of SMCs within the ductus arteriosis and that MRTF A/B (or other genes) fails to substitute, presumably because there is no selection pressure to initiate compensatory activation of these genes until it is too late. Consistent with the hypothesis that myocardin and the MRTFs have many overlapping but also distinct functions are results of studies by Li et al. (19) showing that selective knockout of MRTF B in neural crest cells resulted in embryonic lethality between embryonic day 17.5 and P1 due to cardiac outflow tract defects. These results indicate that in cardiac neural crest cells, MRTF A and myocardin were incapable of compensating for loss of MRTF B.
The results of the present studies extend our knowledge in this field by showing for the first time that myocardin is essential for development of multiple SMC lineages, thus implying that MRTF A and B have limited ability to compensate for loss of myocardin in these SMC subtypes. In contrast, we have shown that myocardin−/− ESCs readily form vascular SMC lineages, although at a somewhat reduced frequency compared with WT ESCs. Moreover, myocardin−/− vascular SMC have a normal morphology and myofilament structure in vivo, suggesting that MRTFs A and B compensate almost fully in vascular SMC lineages. We also have shown that myocardin−/− SMCs formed in vitro within EBs exhibit nearly completely normal contractile properties. An important unresolved question is why myocardin−/− SMCs developed in vitro in the EB model showed reduced expression of the SM2 variant of SM-MHC. Since SM2 SM-MHC is a marker of mature SMC (18, 27, 31), it is possible that myocardin is dispensable for initial SMC differentiation but is required for full maturation. This implies that myocardin, a transcription factor and potent SRF coactivator, is somehow selectively altering alternative splicing of the SM-MHC gene, since myocardin−/− SMCs expressed normal levels of the SM1 variant of SM MHC. Although at least some myocardin−/−/LacZ+ cells ultimately form mature SMCs as determined by the presence of myosin filaments, the majority of myocardin−/−/LacZ+ cells fail to activate the transcription of the contractile proteins required for normal SMC development within the proper temporal window and therefore fail to respond appropriately to extracellular signals and feedback cues. Consistent with this idea, our previous studies have linked contraction in SMCs with myocardin/SRF signaling, suggesting that development of the contractile phenotype may be critical for feedback on SMC gene expression and myocardin-mediated hypertrophy (39). MRTFs have been shown to respond to actin dynamics (38), as does the myocardin/MRTF-regulated actin and focal adhesion-associated protein palladin (14, 15). We previously reported that KO of palladin was associated with reduced expression of SMαA and SM-MHC as well as altered SMC contractility and migration. Thus feedback cues such as altered actin dynamics, migration, and palladin expression in the myocardin−/− SMCs during development may put them at a disadvantage compared with their WT normal neighboring cells.
The precise mechanisms whereby myocardin KO results in reduced numbers of myocardin−/− SMCs is unknown, as is why visceral SMCs exhibit greater dependency than other SMC subtypes. However, it is worth noting that chimeric KO lineage tracing approaches, as employed in the present studies, have remarkable sensitivity in detecting even subtle “cell autonomous” functions of genes that may give WT cells a slight advantage over KO cells at critical points in development. Specifically, the failure of appropriate morphogenic/local environmental cues to activate myocardin in a presumptive SMC at a critical juncture in development may result in this cell “being left behind.” Adjacent WT cells may progress in their development, eventually altering the local environment so that it is no longer conducive to formation of additional SMCs from multipotential embryonic cells. In contrast, within conventional or even conditional cell-selective KO models, there is a much greater possibility for activation of compensatory genes, including the MRTFs, since all cells have the same selective disadvantage.
In summary, whether a given multipotential myocardin−/− embryonic cell differentiates into a SMC or ventricular cardiomyocyte will be a function of complex factors, including 1) the morphogenic and environmental cues that induce differentiation into those cell types and how long these signals persist relative to the rate of activation of compensatory genes; 2) the rate at which WT embryonic cells in proximity to the myocardin−/− cells respond to the morphogenic and environmental cues by differentiating, which will eventually result in dissolution of the stimuli that originally induced differentiation of these cell lineages; and 3) the extent to which genes such as MRTF A and B can compensate for loss of myocardin and the kinetics of these compensatory processes. Clearly, further studies are needed to resolve these difficult questions, as well the interesting possibility that myocardin-positive cells may secrete factors that could promote differentiation of adjacent myocardin−/− cells through indirect non-cell autonomous mechanisms.
Importantly, SMαA and SM-MHC expression were differentially impacted by the loss of myocardin in our EB SMC differentiation model system, indicating that differences are unlikely to be explained simply through alterations in myocardin/CArG/SRF signaling pathways critical for transcriptional control of both of these genes (28). Studies of the role of myocardin during embryonic development suggest that myocardin has both activator and repressor functions in the differentiation of multiple muscle types through interaction with the skeletal muscle transcription factor MyoD (24). Therefore, another possibility to explain reduced contribution of myocardin−/− ESCs to SMC lineages is that the observed phenotype is due, at least in part, to the absence of repressor functions of myocardin on MyoD, which, unopposed by myocardin in the −/− chimeras, may alter the fate of cells originally destined to form visceral SMC. Alternatively, repressors of SRF/CArG interaction such as KLF4 (22), ternary complex factors (42), HERP (5), or Msx1/2 (9) may inhibit SMC differentiation in myocardin−/− cells, since their activity is no longer opposed by myocardin. Finally, it is possible that myocardin−/− ESCs form SMCs equally effectively as WT ESCs but have increased rates of apoptosis and/or reduced proliferative capacity. However, the latter seems unlikely given the virtual absence of myocardin−/− SMCs within visceral SMC tissues and observations in cultured cell systems that loss of myocardin promotes, rather than retards, SMC proliferation (45).
An intriguing and completely unexpected observation in the present studies was that myocardin−/− SMCs within our EB model showed selective loss of thromboxane A2-induced contraction and a significant increase in expression of the TXA2R Gα12 and Rho GDI. Although the CArG/SRF/myocardin dependence of gene expression for the signaling proteins examined has not been tested using standard genetic methodologies to date, it is unlikely that the observed perturbations in protein expression are directly due to a lack of myocardin, because the proteins examined are expressed in multiple cell types throughout development. Because myocardin+/+ and myocardin−/− EB-derived SMCs demonstrated comparable responses to depolarizing solution, S1P, and ET-1 (albeit with decreased magnitude), defects in Ca2+ mobilization following receptor activation and/or depolarization seem unlikely, as well. Rather, the smooth muscle-specific expression of a guanine nucleotide exchange factor (GEF) specifically activated by the TXA2R could account for the decreased sensitivity to U-46619 in myocardin−/− SMCs without deleterious perturbations in the signaling cascades initiated by either ET-1 or S1P. Although CArG/SRF/myocardin-dependent expression of a GEF has not been found to date, Rho GEFs have been shown to be necessary components of TXA2R-coupled signaling in pulmonary artery smooth muscle cells (3) and others cell types (8, 16).
Of major interest, our results also showed that myocardin−/− ESCs were at a major disadvantage compared with host WT ESCs in contributing to development of ventricular but not atrial myocytes (Fig. 2). Intriguingly, the myocardin−/− ESCs formed a distinct band of superficial cells along the midline of the two ventricles, suggesting that the WT cells out compete the myocardin−/− ESCs during a critical stage of heart morphogenesis. The precise mechanisms responsible for these effects are unclear. However, of interest, the small number of myocardin−/− ventricular myocytes that do form appear to have a normal ultrastructural appearance compared with adjacent ventricular myocytes derived from WT ESCs. One interpretation of these observations is that myocardin is essential for proper migration of presumptive ventricular myocytes during early development but is dispensable for subsequent differentiation and maturation due to activation of compensatory gene networks. Further conditional cardiomyocyte-selective myocardin KO studies are required to test this possibility.
In summary, results of the present studies provide compelling evidence that cell autonomous myocardin plays a critical role in formation of visceral SMCs and ventricular cardiomyocytes in the developing mouse embryo. The precise mechanisms for defective investment of myocardin−/− ESCs into SMC tissues and into ventricular myocytes are unclear. However, it is interesting to hypothesize that myocardin−/− embryonic cells are at a selective disadvantage in responding to spatial and temporal cues necessary for programming these lineages during early development and that MRTFs are either not able to compensate and/or not appropriately induced within the microenvironments in which SMC and ventricular cardiomyocyte cell lineage programming occurs. Further studies are needed to address this hypothesis and to define the environmental cues that contribute to differences in myocardin dependency between different SMC and cardiomyocyte subtypes.
GRANTS
This study received funding support from National Institutes of Health Grants T32 GM007267 (fellowship support for M. H. Hoofnagle), P01 HL19242, P01 HL48807-15, R37 HL57353, R01 HL38854, and R01 HL 098538.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of Mary McCanna, Diane Raines, John Sanders, Melissa Bevard, Maggie Ober, Dominique Rose, Robert Hammer, Joseph Craft, and Kurt D. Marshall.
REFERENCES
- 1. Cen B, Selvaraj A, Burgess RC, Hitzler JK, Ma Z, Morris SW, Prywes R. Megakaryoblastic leukemia 1, a potent transcriptional coactivator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol Cell Biol 23: 6597–6608, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Derewenda U, Oleksy A, Stevenson AS, Korczynska J, Dauter Z, Somlyo AP, Otlewski J, Somlyo AV, Derewenda ZS. The crystal structure of RhoA in complex with the DH/PH fragment of PDZRhoGEF, an activator of the Ca2+ sensitization pathway in smooth muscle. Structure 12: 1955–1965, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Devine CE, Somlyo AV, Somlyo AP. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol 52: 690–718, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doi H, Iso T, Yamazaki M, Akiyama H, Kanai H, Sato H, Kawai-Kowase K, Tanaka T, Maeno T, Okamoto E, Arai M, Kedes L, Kurabayashi M. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler Thromb Vasc Biol 25: 2328–2334, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Du KL, Chen MM, Li J, Lepore JJ, Mericko P, Parmacek MS. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. J Biol Chem 279: 17578–17586, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Du KL, Ip HS, Li J, Chen MM, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 23: 2425–2437, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harenberg A, Girkontaite I, Giehl K, Fischer KD. The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. Eur J Immunol 35: 1977–1986, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Hayashi K, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced MSX1 and MSX2 inhibit myocardin-dependent smooth muscle gene transcription. Mol Cell Biol 26: 9456–9470, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGF-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS. Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest 118: 515–525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J, Min LM, Cheng L, Yuan LJ, Zhu X, Stout AL, Chen M, Li J, Parmacek MS. Myocardin is required for cardiomyocyte survival and maintenance of heart function. Proc Natl Acad Sci USA 106: 18734–18739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang JS, Ramamurthy SK, Lin X, Le Breton GC. Cell signalling through thromboxane A2 receptors. Cell Signal 16: 521–533, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Jin L, Gan Q, Zieba BJ, Goicoechea SM, Owens GK, Otey CA, Somlyo AV. The actin associated protein palladin is important for the early smooth muscle cell differentiation. PLoS One 5: e12823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin L, Yoshida T, Ho R, Owens GK, Somlyo AV. The actin-associated protein Palladin is required for development of normal contractile properties of smooth muscle cells derived from embryoid bodies. J Biol Chem 284: 2121–2130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly P, Stemmle LN, Madden JF, Fields TA, Daaka Y, Casey PJ. A role for the G12 family of heterotrimeric G proteins in prostate cancer invasion. J Biol Chem 281: 26483–26490, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev 11: 2996–3006, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuro-o M, Nagai R, Tsuchimochi H, Katoh H, Yazaki Y, Ohkubo A, Takaku F. Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J Biol Chem 264: 18272–18275, 1989 [PubMed] [Google Scholar]
- 19. Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci USA 102: 8916–8921, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA 100: 9366–9370, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 280: 9719–9727, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem 279: 42422–42430, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Long X, Creemers EE, Wang DZ, Olson EN, Miano JM. Myocardin is a bifunctional switch for smooth versus skeletal muscle differentiation. Proc Natl Acad Sci USA 104: 16570–16575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Manganello JM, Huang JS, Kozasa T, Voyno-Yasenetskaya TA, Le Breton GC. Protein kinase A-mediated phosphorylation of the Gα13 switch I region alters the Gαβγ13-G protein-coupled receptor complex and inhibits Rho activation. J Biol Chem 278: 124–130, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pipes GC, Sinha S, Qi X, Zhu C, Gallardo TD, Shelton J, Creemers E, Sutherland L, Richardson JA, Garry DJ, Wright WE, Owens GK, Olson EN. Stem cells and their derivatives can bypass the requirement of myocardin for smooth muscle gene expression. Dev Biol 288: 502–513, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Reid HM, Kinsella BT. The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for nitric oxide-mediated desensitization. Independent modulation of Tp alpha signaling by nitric oxide and prostacyclin. J Biol Chem 278: 51190–51202, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Rovner AS, Thompson MM, Murphy RA. Two different heavy chains are found in smooth muscle myosin. Am J Physiol Cell Physiol 250: C861–C870, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Shang Y, Yoshida T, Amendt BA, Martin JF, Owens GK. Pitx2 is functionally important in the early stages of vascular smooth muscle cell differentiation. J Cell Biol 181: 461–473, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shuman H, Somlyo AV, Somlyo AP. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy 1: 317–339, 1976 [DOI] [PubMed] [Google Scholar]
- 34. Sinha S, Wamhoff BR, Hoofnagle MH, Thomas J, Neppl RL, Deering T, Helmke BP, Bowles DK, Somlyo AV, Owens GK. Assessment of contractility of purified smooth muscle cells derived from embryonic stem cells. Stem Cells 24: 1678–1688, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Somlyo AV, Horiuti K, Trentham DR, Kitazawa T, Somlyo AP. Kinetics of Ca2+ release and contraction induced by photolysis of caged d-myo-inositol 1,4,5-trisphosphate in smooth muscle. The effects of heparin, procaine, and adenine nucleotides. J Biol Chem 267: 22316–22322, 1992 [PubMed] [Google Scholar]
- 37. Somlyo AV, Shuman H, Somlyo AP. Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis. Nature 268: 556–558, 1977 [DOI] [PubMed] [Google Scholar]
- 38. Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98: 159–169, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Wamhoff BR, Bowles DK, McDonald OG, Sinha S, Somlyo AP, Somlyo AV, Owens GK. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a Rho kinase/myocardin/SRF-dependent mechanism. Circ Res 95: 406–414, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci USA 99: 14855–14860, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428: 185–189, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA 100: 7129–7134, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weis J, Fine SM, David C, Savarirayan S, Sanes JR. Integration site-dependent expression of a transgene reveals specialized features of cells associated with neuromuscular junctions. J Cell Biol 113: 1385–1397, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang D, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 92: 856–864, 2003 [DOI] [PubMed] [Google Scholar]