Abstract
We previously demonstrated that endothelin (ET)-mediated coronary vasoconstriction wanes with increasing exercise intensity via a nitric oxide- and prostacyclin-dependent mechanism (Ref. 23). Therefore, we hypothesized that the waning of ET coronary vasoconstriction during exercise is the result of decreased production of ET and/or decreased ET receptor sensitivity. We investigated coronary ET receptor sensitivity using intravenous infusion of ET and coronary ET production using intravenous infusion of the ET precursor Big ET, at rest and during continuous treadmill exercise at 3 km/h in 16 chronically instrumented swine. In the systemic vasculature, Big ET and ET induced similar changes in hemodynamic parameters at rest and during continuous exercise at 3 km/h, indicating that exercise does not alter ET production or receptor sensitivity in the systemic vasculature. In the coronary vasculature, infusion of ET resulted in similar dose-dependent decreases in coronary blood flow and coronary venous oxygen tension and saturation at rest and during exercise. In contrast, administration of Big ET resulted in dose-dependent decreases in coronary blood flow, as well as coronary venous oxygen tension and saturation at rest. These effects of Big ET were significantly reduced during exercise. Altogether, our data indicate that continuous exercise at 3 km/h attenuates ET-mediated coronary vasoconstriction through reduced production of ET from Big ET rather than through reduced ET sensitivity of the coronary vasculature. The decreased ET production during exercise likely contributes to metabolic coronary vasodilation.
Keywords: big endothelin, vasoconstriction, coronary circulation
endothelin (et)-1 is one of the most potent vasoconstrictors known. It is mainly produced in endothelial cells through cleavage from its inactive precursor, Big ET, primarily by ET converting enzyme (ECE) and, to a lesser degree, by chymase, in concert with neutral endopeptidase (NEP) (31). Several studies have shown that ET receptor blockade results in systemic and coronary vasodilation (3, 21, 32), and hence that ET contributes to basal vascular tone in the systemic vasculature, as well as the coronary circulation. Our laboratory (21) has previously demonstrated that recruitment of coronary flow reserve during exercise is mediated, at least in part, through withdrawal of the vasoconstrictor effect of ET on the coronary vasculature. Moreover, we showed that nitric oxide (NO) and prostanoids (PGI2) act synergistically to inhibit this vasoconstrictor influence of ET during exercise (23). Indeed, NO and PGI2 have been shown to reduce the production and release of ET (11, 28), while ETA receptors can be nitrosylated by NO, thereby reducing their affinity for ET (34). Thus, next to their direct vasodilator effects, NO and PGI2 can induce vasodilation indirectly by limiting the production of, and/or vasoconstriction induced by, ET.
We hypothesized that the exercise-induced blunting of ET-mediated vasoconstriction in the coronary circulation results from a decrease in ET production, a decrease in ET receptor sensitivity, or a combination of these two effects. Therefore, the aim of the present study was to investigate each of these putative mechanisms by comparison of the in vivo coronary vasoconstrictor responses to Big ET and ET, in chronically instrumented swine at rest and during continuous treadmill exercise. Previous work has established that Big ET has little, if any, direct vasomotor effects, and that the vasoconstriction induced by Big ET is dependent on its conversion to ET (7, 8, 26, 27). Hence, the magnitude of constriction to Big ET-1 approximates the extent of ET production at the vascular wall (assuming no change in ET receptor sensitivity). Comparison of the vasoconstrictor effects of Big ET-1 and ET-1 in vivo, therefore, allows examination of the ET system, with the former reflecting ET production and the latter representing ET receptor sensitivity.
METHODS
Animals
Studies were performed in accordance with the American Physiological Society's “Guiding Principles in the Care and Use of Laboratory Animals” and with approval of the Animal Care Committee of the Erasmus University Medical Centre. A total of 21 Yorkshire × Landrace swine (2 to 3 mo old; 23 ± 1 kg at the time of surgery) entered the study.
Surgery
Swine were sedated (20 mg/kg ketamine and 1 mg/kg midazolam im), anesthetized (thiopental sodium 15 mg/kg iv), intubated, and ventilated with a mixture of O2 and N2 (1:2) to which 0.2–1.0% (vol/vol) isoflurane was added (6). Anesthesia was maintained with midazolam (2 mg/kg iv) and fentanyl (10 μg·kg−1·h−1 iv). Swine were instrumented under sterile conditions, as previously described (21, 22). Briefly, a thoracotomy was performed in the fourth intercostal space. Subsequently, a polyvinylchloride catheter was inserted into the aortic arch, for the measurement of blood pressure and blood sampling for the determination of the Po2, Pco2, pH, O2 saturation (So2), and hemoglobin concentration (ABL 820, Radiometer). A high-fidelity Konigsberg pressure transducer was inserted into the left ventricle (LV) via the apex for measurement of LV pressure and maximum rate of rise in LV pressure. Fluid-filled catheters were implanted in the LV, left atrium, and the pulmonary artery. Furthermore, a small angiocatheter was inserted into the anterior interventricular vein for coronary venous blood sampling (6). Transonic flow probes were placed around the ascending aorta and proximal left anterior descending coronary artery for measurement of cardiac output and coronary blood flow (CBF), respectively (3). Ultrasonic crystals (Sonometrics) were placed midmyocardially in the anterior wall of the LV to determine regional myocardium function. Electrical wires and catheters were tunneled subcutaneously to the back. The chest was closed, and the animals were allowed to recover.
Animals received analgesia (0.3 mg buprenorphine im) for 2 days and antibiotic prophylaxis (25 mg/kg amoxicillin and 5 mg/kg gentamycin iv) for 5 days. Studies were preformed approximately 2 wk after surgery.
Experimental Protocols
Four different protocols with infusion of ET or Big ET were performed (see below). The number of swine in each protocol, as well as the overlap between protocols, is shown in Table 1.
Table 1.
Schematic representation of the overlap of animals used in the various protocols
| ET |
Big ET |
||||
|---|---|---|---|---|---|
| Protocol | Rest | Exercise | Rest | Exercise | Total |
| ET | |||||
| Rest | 10H/8B | 6H | 5H | 6H | |
| Exercise | 3B | 9H/6B | 5H | 5H | |
| Big ET | |||||
| Rest | 4B | 5B | 9H/7B | 8H | |
| Exercise | 5B | 5B | 6B | 11H/9B | |
| Total | 16H/13B | ||||
ET, endothelin; Big ET, big endothelin; H, hemodynamic measurements; B, coronary venous blood samples.
ET.
REST (n = 10).
With swine lying quietly on the treadmill, resting hemodynamic measurements and blood samples were obtained. An intravenous infusion of ET (Sigma, E7764, dissolved in saline) was started at 10 pmol·kg−1·min−1; arterial and coronary venous blood samples and hemodynamic measurements were collected at the end of the 10-min infusion period. Subsequently, the dose of ET was increased to 20 pmol·kg−1·min−1 for 10 min, at the end of which hemodynamic measurements and blood samples were taken (22). In previous experiments, a higher dose of ET resulted in vomiting (22), which precluded its use in exercise experiments.
EXERCISE (n = 9).
Swine ran continuously for 20 min at 3 km/h without (control run) or with (ET run) infusion of ET-1 (10 and 20 pmol·kg−1·min−1). Blood samples and hemodynamic measurements were obtained at 10 and 20 min during each protocol. Animals were allowed to rest on the treadmill for 90 min between protocols, during which time hemodynamic values returned to baseline.
Big ET.
DOSE FINDING (n = 5).
We aimed to use doses of Big ET (Sigma, E8887, dissolved in saline) that would elicit similar increases in blood pressure as the doses of ET describe above. Studies in rats have shown that, both in vivo and ex vivo, approximately twice the dosage of Big ET is needed to reach constrictor responses similar to ET at a given dose (31, 33). Therefore, Big ET was infused intravenously at rest in dosages of 10, 20 and 40 pmol·kg−1·min−1. Pilot experiments revealed a further increase in blood pressure upon cessation of Big ET infusion, which stabilized after ∼5 min and likely reflects the time necessary for the conversion of the vaso-inactive Big ET into the vasoactive ET. Consequently, in subsequent experiments, we infused Big ET and measured its effects 10 min after the completion of each infusion.
REST (n = 9).
With swine lying quietly on the treadmill, resting hemodynamic measurements were obtained and blood samples were collected. Big ET was infused at 20 pmol·kg−1·min−1 for 10 min. Approximately 10 min after completion of infusion, blood samples were taken, and hemodynamic measurements were collected. Subsequently, Big ET was infused at 40 pmol·kg−1·min−1 for 10 min. Again, hemodynamic measurements and blood samples were taken ∼10 min after completion of the infusion. The total duration of the protocol was 40 min.
EXERCISE (n = 11).
Swine ran continuously on the treadmill at 3 km/h for 40 min without (control run) or with (Big ET run) infusion of Big ET (20 and 40 pmol·kg−1·min−1). Identical to the rest protocol of Big ET, the two periods of infusion were followed by a 10-min conversion period. Hence, blood samples and hemodynamic measurements were obtained at 20 and 40 min. Between protocols, animals were allowed to rest on the treadmill for 90 min, during which time hemodynamic values returned to baseline.
PHENYLEPHRINE (n = 5).
To investigate if the coronary vasoconstriction produced by ET or Big ET was the result of a direct vasoconstrictor effect of ET on the coronary vasculature rather than an autoregulatory response to an increase in blood pressure, we performed intravenous infusions of the α1-agonist phenylephrine (PE; Erasmus MC pharmacy, dissolved in saline; incremental dosages of 1–5 μg·kg−1·min−1, 10 min each) in resting swine. Since swine lack α1-receptors in their coronary microcirculation (30), infusion of PE results in systemic vasoconstriction and an increase in blood pressure without a direct effect on the coronary microvasculature.
Data Analysis
Digital recording and offline analysis of hemodynamic data have been described in detail elsewhere (6, 21). Systemic vascular conductance was calculated by dividing cardiac output by blood pressure. End-diastolic length (EDL) and end-systolic length (ESL) lengths were normalized to EDL resting baseline (both at rest and during exercise). Systolic segmental shortening was calculated as [(EDL − ESL)/EDL] × 100. Arterial and venous O2 content, as well as myocardial O2 consumption, were calculated as previously described (6, 21).
Blood pressure, systemic vascular conductance, CBF, coronary venous Po2 (cvPo2), and coronary venous So2 (cvSo2) as well as their changes from baseline values were used as indexes for vasoconstriction in the systemic and coronary circulations at rest and during exercise. In the resting protocols, baseline values were obtained before initiation of drug infusion, whereas, in the exercise protocols, baseline values were obtained from the corresponding samples taken during the control exercise run.
Statistical Analysis
Statistical analysis of hemodynamic and blood-gas data was performed using one-way (dose or exercise), or two-way (dose and exercise) ANOVA for repeated measures. When significant effects were detected (P < 0.05), post hoc testing for the effects of exercise and drug treatment was performed using Scheffé's test. Changes in the relation between myocardial oxygen consumption and cvPo2 were assessed using linear regression analysis with dose of ET or Big ET and animal as dummy variables. Data are presented as means ± SE.
RESULTS
Dose Finding
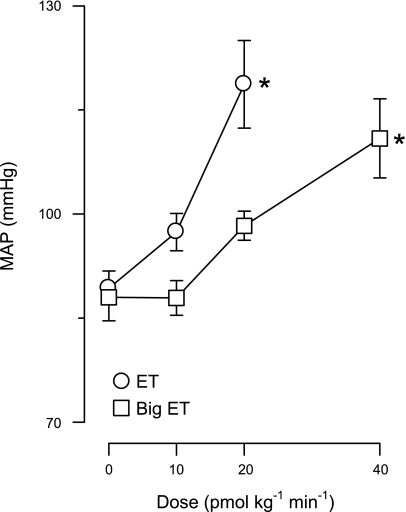
Increases in blood pressure at rest resulting from various doses of Big ET were matched to ET-induced increases at 10 and 20 pmol·kg−1·min−1. As shown in Fig. 1, infusion of Big ET at 10 pmol·kg−1·min−1 did not increase blood pressure; however, infusion of Big ET at 20 pmol·kg−1·min−1 resulted in an identical increase in blood pressure as ET at 10 pmol·kg−1·min−1. Infusion of Big ET at a rate of 40 pmol·kg−1·min−1 resulted in an increase in blood pressure comparable to 20 pmol·kg−1·min−1 ET. Therefore, in the remainder of the study, Big ET doses of 20 and 40 pmol·kg−1·min−1 were used for comparison with ET doses of 10 and 20 pmol·kg−1·min−1.
Fig. 1.
Dose-response study of exogenous big endothelin (Big ET) compared with ET. Shown is a dose-response curve for ET and Big ET at doses from 0 to 40 pmol·kg−1·min−1. MAP, mean arterial pressure. Values are means ± SE. *P ≤ 0.05, drug effect vs. baseline (no drug).
Effects of ET and Big ET on the Systemic Circulation
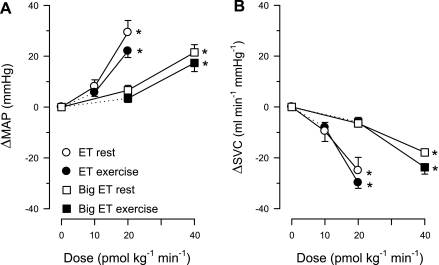
ET resulted in dose-dependent systemic vasoconstriction, as evidenced by a decrease in systemic vascular conductance (Fig. 2) and an increase in blood pressure (Table 2). The increase in blood pressure was accompanied by decreases in heart rate and cardiac output (Table 2), likely due to activation of the baroreflex. Continuous exercise at 3 km/h (∼60% of estimated maximal heart rate) resulted in systemic vasodilation, reflected by the increase in systemic vascular conductance (not shown), whereas blood pressure remained essentially constant (Table 3). These exercise-induced changes in systemic hemodynamics stabilized within 3 min (not shown) and were then maintained during the entire 40 min of the longest control exercise protocol (Table 3). The responses of the systemic vasculature to infusion of either ET or Big ET were similar at rest and during exercise. Moreover, infusion of Big ET at doses of 20 and 40 pmol·kg−1·min−1 resulted in an identical systemic vasoconstriction compared with ET at 10 and 20 pmol·kg−1·min−1, both at rest and during exercise (Fig. 2). Together, these data show that both ET and Big ET exert a vasoconstrictor effect on the systemic vasculature, and that this effect is not influenced by exercise.
Fig. 2.
Systemic effects of Big ET and ET infusions at rest and during exercise. A: effects of ET (10 and 20 pmol·kg−1·min−1) and Big ET (20 and 40 pmol·kg−1·min−1) on MAP are not different at rest and/or during exercise. B: effects of ET and Big ET on systemic vascular conductance (SVC) are not different at rest and/or during exercise. Δ, Change. Values are means ± SE. *P ≤ 0.05, drug effect vs. control.
Table 2.
Effects of Big ET and ET in swine at rest
| Control | Dose 1 | Dose 2 | |
|---|---|---|---|
| Systemic hemodynamics | |||
| Heart rate, beats/min | |||
| ET | 126 ± 6 | 103 ± 2* | 90 ± 3*† |
| Big ET | 113 ± 5 | 102 ± 8 | 83 ± 7*† |
| Cardiac output, l/min | |||
| ET | 5.2 ± 0.5 | 4.7 ± 0.4 | 3.8 ± 0.3*† |
| Big ET | 4.6 ± 0.2 | 4.2 ± 0.4 | 3.7 ± 0.3*† |
| Aortic blood pressure, mmHg | |||
| ET | 89 ± 2 | 97 ± 3* | 119 ± 6*† |
| Big ET | 90 ± 3 | 96 ± 3* | 111 ± 5*† |
| Left ventricular systolic pressure, mmHg | |||
| ET | 110 ± 4 | 118 ± 4* | 137 ± 10*† |
| Big ET | 110 ± 5 | 120 ± 4* | 134 ± 7*† |
| Left ventricular dP/dtmax, mmHg/s | |||
| ET | 2,650 ± 150 | 2,290 ± 120* | 2,100 ± 120*† |
| Big ET | 2,700 ± 180 | 2,550 ± 140 | 2,220 ± 120*† |
| Left atrial pressure, mmHg | |||
| ET | 3 ± 1 | 7 ± 1* | 9 ± 2*† |
| Big ET | 0 ± 1 | 4 ± 1* | 6 ± 1* |
| Rate pressure product, 102·beats·min−1·mmHg | |||
| ET | 139 ± 9 | 122 ± 6* | 123 ± 10* |
| Big ET | 123 ± 9 | 122 ± 10 | 114 ± 10 |
| Myocardial oxygen balance | |||
| Coronary blood flow, ml/min | |||
| ET | 41 ± 3 | 39 ± 3 | 34 ± 2*† |
| Big ET | 46 ± 6 | 43 ± 6 | 36 ± 5*† |
| Arterial Po2, mmHg | |||
| ET | 101 ± 2 | 96 ± 2* | 99 ± 2 |
| Big ET | 98 ± 3 | 97 ± 3 | 96 ± 3 |
| Arterial So2, % | |||
| ET | 97 ± 1 | 96 ± 1* | 96 ± 1* |
| Big ET | 96 ± 1 | 95 ± 1 | 95 ± 1 |
| Coronary venous Po2, mmHg | |||
| ET | 25.1 ± 1.1 | 22.1 ± 1.2* | 18.9 ± 1.5*† |
| Big ET | 23.1 ± 0.4 | 19.9 ± 0.6* | 18.5 ± 0.6*† |
| Coronary venous So2, % | |||
| ET | 19.5 ± 1.8 | 14.4 ± 1.7* | 10.5 ± 2.1*† |
| Big ET | 15.3 ± 0.9 | 10.7 ± 1.1* | 8.4 ± 0.5*† |
| Coronary venous Pco2, mmHg | |||
| ET | 52 ± 2 | 54 ± 1 | 56 ± 2 |
| Big ET | 51 ± 2 | 58 ± 2* | 59 ± 2* |
| Myocardial oxygen consumption, μmol/min | |||
| ET | 171 ± 16 | 159 ± 14 | 147 ± 15* |
| Big ET | 198 ± 18 | 191 ± 20 | 182 ± 17 |
| Regional myocardial function | |||
| End-diastolic length, mm | |||
| ET | 10 ± 0 | 10.1 ± 0.2 | 10.3 ± 0.2 |
| Big ET | 10 ± 0 | 10.2 ± 0.2 | 10.4 ± 0.3 |
| End-systolic length, mm | |||
| ET | 8.4 ± 0.2 | 8.5 ± 0.3 | 8.9 ± 0.4 |
| Big ET | 8.5 ± 0.2 | 8.5 ± 0.2 | 9.0 ± 0.3 |
| Systolic shortening, % | |||
| ET | 16.4 ± 2.4 | 15.8 ± 2.2 | 13.3 ± 2.7 |
| Big ET | 15.5 ± 1.8 | 16.2 ± 2.7 | 14.0 ± 2.3 |
Values are means ± SE. Big ET dose 1 = 20 pmol·kg−1·min−1; dose 2 = 40 pmol·kg−1·min−1. ET dose 1 = 10 pmol·kg−1·min−1; dose 2 = 20 pmol·kg−1·min−1. dP/dtmax, maximum rate of rise in pressure; Po2, oxygen tension; So2, oxygen saturation; Pco2, carbon dioxide tension.
P < 0.05 vs. control.
P < 0.05 vs. dose 1.
Table 3.
Effects of ET and Big ET in swine during exercise
| Control 1 | Dose 1 | Control 2 | Dose 2 | |
|---|---|---|---|---|
| Systemic hemodynamics | ||||
| Heart rate, beats/min | ||||
| ET | 180 ± 5 | 165 ± 3* | 178 ± 5 | 153 ± 4*† |
| Big ET | 184 ± 8 | 169 ± 8* | 183 ± 10 | 159 ± 10* |
| Aortic blood pressure, mmHg | ||||
| ET | 82 ± 2 | 88 ± 2* | 81 ± 3 | 103 ± 3*† |
| Big ET | 84 ± 2 | 87 ± 2 | 84 ± 3 | 101 ± 4*† |
| Cardiac output, l/min | ||||
| ET | 7.7 ± 0.3 | 7.5 ± 0.3 | 7.7 ± 0.3 | 6.9 ± 0.3*† |
| Big ET | 8.1 ± 0.3 | 7.9 ± 0.3 | 8.4 ± 0.4 | 7.6 ± 0.4* |
| Left ventricular systolic pressure, mmHg | ||||
| ET | 106 ± 4 | 111 ± 3* | 105 ± 3 | 122 ± 4*† |
| Big ET | 110 ± 3 | 112 ± 3 | 113 ± 5 | 124 ± 5*† |
| Left ventricular dP/dtmax, mmHg/s | ||||
| ET | 3,460 ± 150 | 3,260 ± 140 | 3,390 ± 150 | 3,110 ± 100 |
| Big ET | 3,290 ± 310 | 3,280 ± 260 | 3,410 ± 400 | 3,250 ± 320 |
| Left atrial pressure, mmHg | ||||
| ET | 5 ± 1 | 8 ± 1* | 6 ± 1 | 9 ± 1* |
| Big ET | 5 ± 1 | 6 ± 1 | 4 ± 1 | 9 ± 1*† |
| Rate pressure product, 102·beats·min−1·mmHg | ||||
| ET | 190 ± 8 | 183 ± 6 | 187 ± 7 | 186 ± 6 |
| Big ET | 208 ± 14 | 195 ± 14 | 213 ± 18 | 203 ± 18 |
| Myocardial oxygen balance | ||||
| Coronary blood flow, ml/min | ||||
| ET | 68 ± 5 | 66 ± 5 | 68 ± 5 | 62 ± 7* |
| Big ET | 70 ± 6 | 68 ± 5 | 72 ± 8 | 68 ± 7 |
| Arterial Po2, mmHg | ||||
| ET | 99 ± 2 | 96 ± 3 | 94 ± 4 | 89 ± 4 |
| Big ET | 100 ± 2 | 101 ± 3 | 97 ± 3† | 99 ± 3 |
| Arterial So2, % | ||||
| ET | 97 ± 1 | 96 ± 1* | 96 ± 1 | 93 ± 1 |
| Big ET | 97 ± 1 | 97 ± 1 | 96 ± 1 | 96 ± 1 |
| Coronary venous Po2, mmHg | ||||
| ET | 22.2 ± 0.3 | 20.3 ± 0.9* | 20.7 ± 0.5 | 14.9 ± 1.1*† |
| Big ET | 21.1 ± 0.5 | 20.7 ± 0.6 | 20.2 ± 0.8 | 17.2 ± 0.8*† |
| Coronary venous So2, % | ||||
| ET | 14.9 ± 1.5 | 11.8 ± 2.0* | 12.7 ± 1.4† | 5.8 ± 1.0*† |
| Big ET | 12.6 ± 0.7 | 11.5 ± 0.6 | 12.2 ± 1.2 | 8.1 ± 0.7*† |
| Coronary venous Pco2, mmHg | ||||
| ET | 52 ± 2 | 53 ± 1 | 51 ± 2 | 52 ± 2 |
| Big ET | 50 ± 1 | 50 ± 1 | 46 ± 1† | 48 ± 1 |
| Myocardial oxygen consumption, μmol/ min | ||||
| ET | 338 ± 29 | 337 ± 30 | 340 ± 30 | 317 ± 31 |
| Big ET | 355 ± 25 | 337 ± 24 | 362 ± 33 | 357 ± 33 |
| Regional myocardial function | ||||
| End-diastolic length, mm | ||||
| ET | 10.2 ± 0.1 | 10.2 ± 0.1 | 10.2 ± 0.1 | 10.3 ± 0.1 |
| Big ET | 10.7 ± 0.4 | 10.5 ± 0.4 | 10.6 ± 0.4 | 10.6 ± 0.4 |
| End-systolic length, mm | ||||
| ET | 8.5 ± 0.2 | 8.6 ± 0.2 | 8.5 ± 0.2 | 8.7 ± 0.1*† |
| Big ET | 8.5 ± 0.3 | 8.3 ± 0.3 | 8.5 ± 0.2 | 8.5 ± 0.3 |
| Systolic shortening, % | ||||
| ET | 16.1 ± 3.1 | 16.4 ± 2.4 | 16.5 ± 2.6 | 15.1 ± 2.2 |
| Big ET | 20.2 ± 1.9 | 20.7 ± 1.8 | 20.0 ± 1.7 | 19.8 ± 1.5 |
Values are means ± SE. Big ET dose 1 = 20 pmol·kg−1·min−1; dose 2 = 40 pmol·kg−1·min−1. ET dose 1 = 10 pmol·kg−1·min−1; dose 2 = 20 pmol·kg−1·min−1.
P < 0.05 vs. corresponding control.
P < 0.05, dose 1 vs. dose 2 or control 1 vs. control 2.
Effects of Big ET and ET on the Coronary Circulation
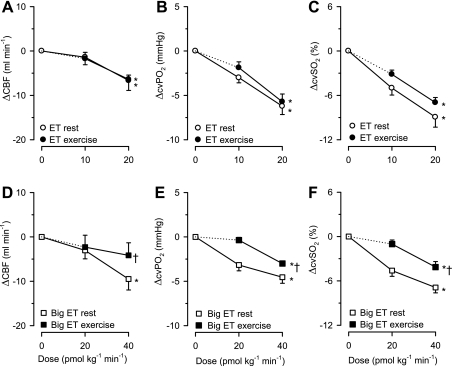
Infusion of ET resulted in a dose-dependent decrease in CBF (Table 2, Fig. 3). The decreased CBF resulted in a decrease in myocardial oxygen delivery that exceeded the (small) decrease in myocardial oxygen consumption. As a result, myocardial oxygen extraction increased, resulting in a decrease in cvPo2 and cvSo2 (Fig. 3). Altogether, these data indicate a direct vasoconstrictor effect of ET on the coronary vasculature at rest. To compare ET-induced coronary vasoconstriction at rest and during exercise, changes in CBF, cvPo2, and cvSo2 due to exercise need to be taken into account. The CBF, cvPo2, and cvSo2 during exercise in the presence of ET were, therefore, compared with their respective values in a control exercise trial (Table 3). Progressive infusions of ET induced similar decreases in CBF, cvPo2, and cvSo2 at rest and during exercise (Fig. 3), indicating that ET receptor sensitivity was unaltered during exercise. The shift in the relation between myocardial oxygen consumption and cvPo2 is commonly used to assess changes in coronary resistance vessel tone. Figure 4 shows that ET caused a parallel shift in the relation between myocardial oxygen consumption and cvPo2, supporting the interpretation that the vasoconstrictor effect of ET is not influenced by exercise. At the highest dose of ET, myocardial contractility tended to be reduced, as evidenced by a tendency toward a reduction in systolic shortening, which occurs red both at rest and during exercise (P < 0.1, Tables 2 and 3). Altogether these findings indicate that ET receptor sensitivity in the coronary circulation is not altered by exercise.
Fig. 3.
Coronary vasoconstrictor effects of ET (A–C) and Big ET (D–F) infusions at rest and during exercise. A and D: coronary blood flow (CBF). B and E: coronary venous oxygen tension (cvPo2). C and F: coronary venous oxygen saturation (cvSo2). A–C: effects of ET (10 and 20 pmol·kg−1·min−1) are similar at rest and during exercise. D–F: effects of Big ET (20 and 40 pmol·kg−1·min−1) are reduced during exercise. Values are means ± SE. *P ≤ 0.05, drug effect vs. control; †P ≤ 0.05, effect of Big ET or ET altered during exercise.
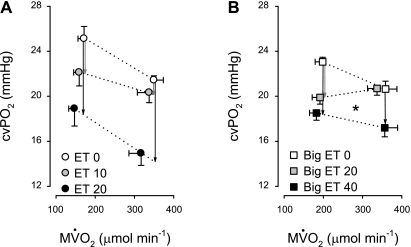
Fig. 4.
Effects of ET and Big ET infusions on the myocardial oxygen balance. A: incremental dosages of ET (0, 10, and 20 pmol·kg−1·min−1) resulted in a parallel shifts of the relation between myocardial oxygen consumption (MV̇o2) and cvPo2. P value for rotation: 0.9. Vertical arrows indicate the change in cvPo2 in response to ET at rest (left) and during exercise (right) at constant MV̇o2. B: Big ET (20 pmol·kg−1·min−1) resulted in a significant change in slope of the relation between MV̇o2 and cvPo2, indicating a decreased conversion of Big ET to ET during exercise. In the presence of 40 pmol·kg−1·min−1 Big ET, the rotation was not significant. However, when both doses were combined, a significant rotation was observed (P = 0.049). Vertical arrows indicate the change in cvPo2 in response to Big ET at rest (left) and during exercise (right). Dotted lines were used because the measurements at rest (low MV̇o2) and during exercise (high MV̇o2) were performed in different groups of animals. *P ≤ 0.05, rotation of the relation between MV̇o2 and cvPo2 compared with control conditions.
Infusion of Big ET also resulted in a dose-dependent decrease in CBF at rest (Table 2). Since myocardial oxygen consumption was not influenced by infusion of Big ET, the resultant decrease in myocardial oxygen delivery necessitated an increase in myocardial oxygen extraction, and hence cvPo2 and cvSo2 progressively decreased (Fig. 3). These data show that Big ET induces vasoconstriction of the coronary vasculature at rest. Moreover, especially at the highest dose, Big ET tended to reduce systolic shortening (P = 0.1, Table 2). The effects of Big ET on the coronary vasculature, as well as systolic shortening at rest, were similar to the effects induced by ET. During continuous exercise at 3 km/h, the vasoconstrictor effect of Big ET on the coronary vasculature was significantly reduced compared with resting conditions, as evidenced by blunted decreases in CBF, cvPo2, and cvSo2 (Fig. 3). Furthermore, Big ET produced a significant increase in slope (P = 0.049 vs. control) of the relation between myocardial oxygen consumption and cvPo2 (Fig. 4). Additionally, there was no effect of Big ET infusion on systolic shortening during exercise (Table 3).
Altogether, our data suggest that, during exercise, the conversion of Big ET to ET is decreased in the coronary vasculature compared with resting conditions. In contrast, the sensitivity of the coronary vasculature to ET seems unperturbed.
Effects of PE on the Systemic and Coronary Vasculature at Rest
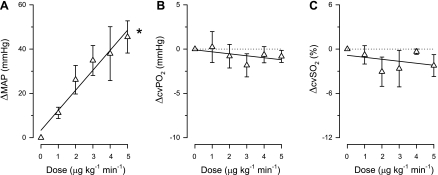
PE was infused intravenously in incremental dosages up to 5 μg·kg−1·min−1, and its infusion resulted in systemic vasoconstriction, as evidenced by dose-dependent increases in blood pressure that were accompanied by decreases in heart rate (Table 4). PE infusion at a dose of 2 μg·kg−1·min−1 resulted in a similar blood pressure increase and heart rate decrease compared with the highest doses of Big ET and ET. Infusion of PE at a dose of 5 μg·kg−1·min−1 resulted in an even more pronounced increase in blood pressure and further decreases in heart rate and systemic vascular conductance. CBF also decreased upon infusion of PE, whereas cvPo2 and cvSo2 remained essentially unchanged (Fig. 5). The discrepant response between CBF and cvPo2 and cvSo2 indicates that the decrease in CBF is due to a decrease in myocardial oxygen consumption rather than a direct coronary vasoconstrictor effect of PE.
Table 4.
Effects of phenylephrine on the systemic and coronary circulations
| Control | 2 μg·kg−1·min−1 | 5 μg·kg−1·min−1 | |
|---|---|---|---|
| Systemic hemodynamics | |||
| Heart rate, beats/min | 139 ± 8 | 98 ± 6* | 88 ± 6* |
| Aortic blood pressure, mmHg | 93 ± 2 | 114 ± 5* | 135 ± 7* |
| Rate pressure product, 102·beats·min−1·mmHg | 156 ± 10 | 134 ± 5 | 140 ± 8 |
| Myocardial oxygen balance | |||
| Coronary blood flow, ml/min | 62 ± 8 | 56 ± 10* | 56 ± 7* |
| Coronary venous Po2, mmHg | 23.0 ± 1.7 | 22.6 ± 1.8 | 22.7 ± 1.2 |
| Coronary venous So2, % | 19.6 ± 2.2 | 17.1 ± 2.0 | 17.7 ± 1.5 |
| Coronary venous Pco2, mmHg | 59 ± 2 | 60 ± 1 | 59 ± 3 |
| Myocardial oxygen consumption, μmol/min | 261 ± 38 | 227 ± 40 | 237 ± 29 |
Values are means ± SE of 5 swine.
P < 0.05 vs. corresponding control.
Fig. 5.
Systemic and coronary effects of phenylephrine (PE) infusion at rest. A: MAP; B: cvPo2; C: cvSo2. A: PE (1–5 μg·kg−1·min−1) causes dose-dependent systemic vasoconstriction. B and C: PE does not cause coronary vasoconstriction. Values are means ± SE. *P ≤ 0.05, drug effect vs. control.
DISCUSSION
The main findings of the present study were that: 1) the systemic vasoconstrictor effects of both ET and Big ET in resting swine were similar to those produced during exercise; 2) the coronary vasoconstrictor effects of ET were also maintained during exercise; however, 3) the coronary vasoconstrictor effect of Big ET during exercise was attenuated compared with resting conditions. Together, these findings indicate that, during exercise, in the coronary vasculature, ET receptor sensitivity is maintained, while the conversion of Big ET to ET is blunted. The implications of these findings will be discussed below.
Exercise-induced Modulation of the ET System in the Systemic Circulation
Twofold higher dosage of Big ET compared with ET were required to obtain a similar vasoconstrictor response in both the systemic and the coronary vasculature, indicating that the conversion of Big ET to ET occurs at a similar rate in the systemic and coronary vasculature. The finding that the dose ratio of Big ET to ET is approximately two is consistent with data in anesthetized rodents (31, 33), but contrasts results in humans that demonstrate a comparable potency of Big ET vs. ET (25). These differences are difficult to explain, but may be related to species differences in ECE activity or dosage of Big ET that was 4- to 10-fold lower in the human studies.
In the present study, we found that the systemic vasoconstriction induced by ET infusion was similar at rest and during continuous exercise, suggesting that ET receptor sensitivity in the systemic vasculature does not change during exercise. Moreover, the systemic vasoconstrictor effects of Big ET were also similar at rest and during exercise, indicating that the conversion of Big ET to ET is maintained during exercise. These data appear to be at odds with our laboratory's previous study, in which we observed that contribution of endogenous ET to systemic vascular tone decreased during exercise (21). In that study, however, the withdrawal of ET-mediated vasoconstriction only occurred at the highest exercise intensities (4 and 5 km/h), whereas swine ran at 3 km/h in the present study, since it was not possible to maintain voluntary exercise at a higher intensity for 40 min. It is, therefore, possible that the exercise intensity used in the present study was not sufficient to demonstrate a reduced production of ET in the systemic vasculature.
After conversion of Big ET to ET, secretion occurs predominantly on the abluminal side of the vessel. Therefore, local concentrations can vary and the vasoconstriction in response to Big ET infusion may also vary between vascular beds. Withdrawal of the vasoconstrictor influence of endogenous or exogenous ET during exercise in active skeletal muscle can, therefore, be offset by an enhanced effect of ET in nonexercising muscle or visceral tissues. Indeed, it was recently observed in humans that the vasoconstrictor effect of exogenously administered ET was lower in the exercising leg compared with the resting leg (36). Moreover, studies by Maeda et al. (13, 14, 17), in humans exercising with one leg, showed that the concentration of ET increased in the venous blood of the nonexercising leg, whereas it remained unchanged in the exercising leg. We, therefore, cannot exclude the possibility that the effects of Big ET and/or ET during exercise decreased in the vasculature of exercising muscle, whereas they may have increased in vascular beds of inactive muscle and/or visceral organs.
Exercise-Induced Modulation of the ET System in the Coronary Circulation
Under resting conditions, the coronary vasculature is maintained in a state of constriction, so-called basal tone, by a balance of vasodilator and vasoconstrictor factors released from the autonomic nervous system, the cardiac myocytes, as well as from within the vasculature itself. ET is an important factor in the maintenance of basal tone. Thus, at rest, there is continuous production of ET, that is in equilibrium with its clearance, resulting in a modest vasoconstrictor effect, as illustrated by the modest vasodilator effect of ET receptor blockade with tezosentan (21). Our data show that an increase in ET concentration through infusion of ET or Big ET results in vasoconstriction, most likely through a temporary disbalance between “production” and clearance. Studies in literature show that, not only the magnitude, but also the duration of this constriction, depends on the concentration of ET. Thus a bolus injection of a low dose of ET (4 pmol) induces a partially transient decrease in CBF in isolated rat hearts, whereas a high dose of ET (400 pmol) resulted in a sustained decrease in CBF (24). This is also true for prolonged infusions in vivo (12): ET infusions at 5 ng·kg−1·min−1 iv for 1 h in conscious dogs result in a 10-fold increase in ET plasma levels, but only in a small decrease in CBF that is almost instantaneously reversed upon cessation of the infusion. Higher dosages of 10 and 20 ng·kg−1·min−1, which resulted in a 40- and 80-fold increase in ET plasma levels, not only result in more severe vasoconstriction, but also in progressively slower reversal of the constriction (12), suggesting that clearance receptors may be overwhelmed by the high circulating plasma concentrations of ET.
During exercise, the balance between vasodilators and vasoconstrictors is shifted in favor of vasodilation to maintain myocardial perfusion, and hence myocardial oxygen delivery, commensurate with the increased oxygen demand of the myocardium. Our laboratory has previously shown that the coronary vasoconstrictor effect of the endogenous ET system wanes within minutes during exercise (21), thereby contributing to the exercise-induced vasodilation. Thus it appears that the balance between production and clearance of endogenous ET can be adjusted rapidly, most likely because the concentrations of endogenous ET present in the myocardial interstitium, as well as in the circulation, are low compared with the concentrations present in response to exogenous infusion of ET. To provide evidence that this waning of the ET-induced vasoconstriction during increased metabolic demand is not simply the result of masking the vasoconstrictor influence of ET by an increasing number of vasodilator systems, we previously simulated exercise in vitro, by electrical stimulation of cardiac myocytes and adding their supernatant to isolated coronary arterioles, thereby uncoupling myocardial oxygen supply (i.e., the vasculature) and demand (i.e., the myocytes), while simultaneously preventing the influence of volatile and neurohumoral vasoactive factors (20). Supernatant of unstimulated myocytes resulted in ET-mediated vasoconstriction that decreased with increasing stimulation rates, providing further evidence that withdrawal of ET-mediated vasoconstriction contributes to metabolic coronary vasodilation. Our laboratory subsequently showed in vivo that this effect of blunting the ET vasoconstrictor influence was mediated by NO and prostacyclin (PGI2) (23). NO has been shown to reduce the affinity of the ETA receptor (34, 35), and, since both NO (1, 11) and prostacyclin (29) can limit ET production, we hypothesized that the waning of the ET vasoconstriction with increasing exercise intensity was due to a reduction in ETA receptor sensitivity and/or ET production.
In the present study, we showed that the vasoconstriction induced by ET infusion is similar at rest and during exercise, whereas the vasoconstriction induced by infusion of Big ET was reduced during exercise. Hence, the reduced vasoconstrictor influence of ET during exercise is unlikely to be caused by decrease in receptor sensitivity. Yet the baroreceptor reflex response to the increase in blood pressure may have caused a decrease in sympathetic activity and an increase in parasympathetic activity that could potentially influence coronary resistance vessel tone and may differentially affect the responses to (Big) ET at rest and during exercise. Our laboratory has previously shown that, in swine, blocking muscarinic receptors with atropine results in coronary vasodilation, but that this vasodilation is mediated through an increase in sympathetic activity, as it is abolished after prior β-blockade (4, 5). Thus an increase in parasympathetic activity could influence coronary vascular tone by decreasing β-adrenergic activity, possibly resulting in vasoconstriction. Since β-adrenergic activity is already minimal under resting conditions, it is unlikely that activation of the parasympathetic nervous system contributed to the coronary vasoconstriction in response to either ET or Big ET at rest. It is, however, possible that, when sympathetic activity is high during exercise, a reduction in β-adrenergic coronary vasodilation contributed to the vasoconstriction. This would then result in an overestimation of the vasoconstriction induced by ET and Big ET during exercise, and an underestimation of the difference between rest and exercise. Following this line of reasoning, it is possible that a reduction in β-adrenergic vasodilation during exercise following ET and Big ET masked an exercise-induced decrease in coronary sensitivity to ET. As the magnitude of the increase in blood pressure and the decrease in heart rate were similar for ET and Big ET, the reduced vasoconstrictor response to Big ET compared with ET is unlikely to be caused by baroreceptor reflex-mediated activation of the parasympathetic or inhibition of the sympathetic nervous system, but rather results from a decreased production and/or release of ET.
Due to its abluminal secretion (2), ET plasma concentrations do not adequately reflect its concentrations in the interstitium, and production/release of ET cannot simply be measured by its arteriovenous concentration difference. To our knowledge, only a few studies have investigated the production of ET in the heart during exercise, although production of ET from Big ET in the heart has been demonstrated at rest (25). Furthermore, Maeda and coworkers (15, 16) demonstrated that prolonged exercise (∼45 min) increased mRNA for preproendothelin, as well as ET protein levels in the rat heart. However, since these measurements were made using the entire LV of the rat, it is unclear whether these results are indicative of elevated ET production in cardiac myocytes, vascular cells, or both, during exercise. Moreover, in these experiments, rats were anesthetized immediately following exercise, and their hearts were removed. It is, therefore, possible that the increase in ET is due to the termination of exercise rather than to exercise itself. Recent studies have shown that ET production can be reduced by increases in endothelial cGMP (11), whereas oxidative stress enhances ET production through stabilization of preproendothelin mRNA (18). As NO alleviates oxidative stress and enhances cGMP production, this may provide a pathway by which NO can reduce ET production. In accordance with this suggestion, our laboratory previously demonstrated that endothelial NO synthase inhibition results in an increase in ET-mediated vasoconstriction in the coronary vasculature during exercise (23).
Since our laboratory previously found that withdrawal of the vasoconstrictor effect of endogenous ET occurred within 10 min of exercise (21), it is unlikely that this is due to a change in transcription of preproendothelin mRNA. We, therefore, investigated the ET pathway downstream of preproendothelin mRNA, using infusion of Big ET. In a preliminary study, we found that the vasoconstrictor effect of Big ET progressed after cessation of the infusion and stabilized after ∼5 min. This delay was most likely due to the necessary conversion of Big ET into the vasoactive ET and is in accordance with the 3-min half time of uptake of Big ET into endothelial cells (10). The measurements of the vascular effects of Big ET were, therefore, performed 10 min after cessation of its infusion. This required a longer exercise protocol for the Big ET infusions (40 min) compared with the ET infusions (20 min). Exercise resulted in significant decreases in cvPo2 and cvSo2 that were slightly larger after 20 min of exercise compared with 10 min of exercise, but stable thereafter. To correct for these small changes in cvPo2 and cvSo2 during control exercise, we calculated the effects of Big ET and ET during exercise by subtracting the values obtained during the control run from the values during Big ET or ET infusion at the corresponding time points. CBF and other cardiovascular parameters measured were very stable over the entire 40 min of control exercise, making it unlikely that the differences between the responses to ET and Big ET were induced by the differences in duration of exercise. Moreover, the repeated-measures design of the experiments enabled the use of each animal as its own control, thereby increasing statistical power for the analyses.
The present study shows that the loss of ET-mediated vasoconstriction during exercise is due to decreased conversion of Big ET to ET in the coronary vasculature. These data are in accordance with data from a previous study that showed that cardiac myocytes can modulate the production of ET (by a phosphoramidon-sensitive enzyme) in the coronary arterioles (19). There are two pathways by which Big ET can be converted into ET, both of which are sensitive to phosphoramidon. First, ECE can directly cleave Big ET at position 21, thereby resulting in formation of mature ET (9, 37). Second, chymase can cleave Big ET at position 31, resulting in ET1–31, that can be subsequently cleaved by NEP to produce ET (31). Future studies are necessary to determine whether the decreased conversion of Big ET to ET during exercise results from inhibition of ECE, chymase, and/or NEP.
Coronary Vasoconstriction: Direct Effect or Autoregulatory Response?
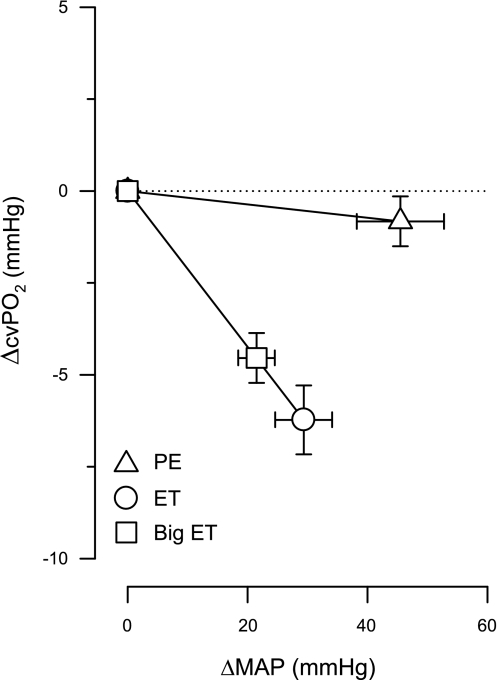
Administration of Big ET and ET resulted in significant increases in blood pressure that were accompanied by significant, probably baroreceptor reflex-mediated, decreases in heart rate. The increase in blood pressure (i.e., LV afterload) and the decrease in heart rate have counterbalancing effects on myocardial oxygen consumption. Thus only the highest dose of ET at rest resulted in a significant decrease in myocardial oxygen consumption. Nevertheless, significant vasoconstriction occurred as evidenced by the decreases in CBF and cvPo2 and cvSo2. It could be argued that this vasoconstriction is the result of an autoregulatory response to the increase in blood pressure or a metabolic response to the decrease in myocardial oxygen consumption, rather than a direct coronary vasoconstrictor effect caused by ET. To test this hypothesis, we performed experiments with infusion of the α1-agonist PE. Swine express α1-adrenoceptors in the systemic, but not the coronary, resistance vessels (30). Administration of PE causes systemic vasoconstriction, thereby increasing blood pressure, without directly affecting the coronary microcirculation. Thus administration of PE can be used to produce autoregulatory coronary vasoconstriction across a range of blood pressures. Administration of PE resulted in a small decrease in myocardial oxygen consumption, resulting in a decrease in CBF. However, myocardial oxygen delivery and myocardial oxygen demand decreased to the same extent, so that cvPo2 and cvSo2 remained essentially unchanged. In contrast, direct vasoconstriction in the coronary resistance vessels produced by infusions of Big ET and ET resulted in decreases not only in CBF but most importantly also in cvPo2 and cvSo2 (Fig. 6). These results underline the importance of studying myocardial oxygen balance, i.e., measuring cvPo2 and cvSo2, to assess vasomotor tone responses of coronary resistance vessels to drug interventions at rest and during exercise. Thus cvPo2 and cvSo2 reflect changes in resistance vessel tone independent of autoregulatory responses.
Fig. 6.
Effects of PE, ET, and Big ET infusions on coronary vasomotor tone and MAP. Comparison of infusions of PE (5 μg·kg−1·min−1), ET (20 pmol·kg−1·min−1), and Big ET (40 pmol·kg−1·min−1) at rest shows that coronary vasoconstriction induced by ET or Big ET is not due to autoregulation. Values are means ± SE.
Conclusion
During exercise, the production of ET from its precursor Big ET is reduced in the coronary vasculature, while ET receptor sensitivity appears to be unaffected. The reduced production of ET during exercise contributes to reduction of coronary vascular tone, thereby facilitating recruitment of flow reserve during exercise-induced metabolic coronary vasodilation.
GRANTS
This work was supported by grants from the Netherlands Heart Foundation (2000T042 to D. Merkus and V. J. de Beer) and the National Institutes of Health (HL-52490 and AR-048523 to M. H. Laughlin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest 85: 587–590, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chabrier PE. The role of endothelin in the vessel wall. Baillieres Clin Haematol 6: 577–591, 1993 [DOI] [PubMed] [Google Scholar]
- 3. de Beer VJ, Sorop O, Pijnappels DA, Dekkers DH, Boomsma F, Lamers JM, Duncker DJ, Merkus D. Integrative control of coronary resistance vessel tone by endothelin and angiotensin II is altered in swine with a recent myocardial infarction. Am J Physiol Heart Circ Physiol 294: H2069–H2077, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Duncker DJ, Haitsma DB, Liem DA, Verdouw PD, Merkus D. Exercise unmasks autonomic dysfunction in swine with a recent myocardial infarction. Cardiovasc Res 65: 889–896, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res 82: 1312–1322, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Duncker DJ, Stubenitsky R, Verdouw PD. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am J Physiol Heart Circ Physiol 275: H1663–H1672, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Haynes WG, Ferro CE, Webb DJ. Physiologic role of endothelin in maintenance of vascular tone in humans. J Cardiovasc Pharmacol 26, Suppl 3: S183–S185, 1995 [PubMed] [Google Scholar]
- 8. Hein TW, Ren Y, Yuan Z, Xu W, Somvanshi S, Nagaoka T, Yoshida A, Kuo L. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci 50: 3329–3336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hisaki K, Matsumura Y, Fujita K, Maekawa H, Takaoka M, Morimoto S. Differences in potency of big endothelin-1-induced pressor action in rat isolated perfused mesenteric artery, hindquarter and lung. Life Sci 54: 275–280, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Johnstrom P, Fryer TD, Richards HK, Maguire JJ, Clark JC, Pickard JD, Davenport AP. Positron emission tomography of [18F]-big endothelin-1 reveals renal excretion but tissue-specific conversion to [18F]-endothelin-1 in lung and liver. Br J Pharmacol 159: 812–819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol 286: L984–L991, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Leadley RJ, Jr, Zhu JL, Goetz KL. Effects of endothelin-1 and sarafotoxin S6b on regional hemodynamics in the conscious dog. Am J Physiol Regul Integr Comp Physiol 260: R1210–R1217, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Maeda S, Miyauchi T, Goto K, Matsuda M. Alteration of plasma endothelin-1 by exercise at intensities lower and higher than ventilatory threshold. J Appl Physiol 77: 1399–1402, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Maeda S, Miyauchi T, Goto K, Matsuda M. Differences in the change in the time course of plasma endothelin-1 and endothelin-3 levels after exercise in humans. The response to exercise of endothelin-3 is more rapid than that of endothelin-1. Life Sci 61: 419–425, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Maeda S, Miyauchi T, Sakai S, Kobayashi T, Goto K, Sugishita Y, Matsuda M. Endothelin-1 in the heart during exercise. J Cardiovasc Pharmacol 31, Suppl 1: S392–S394, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Maeda S, Miyauchi T, Sakai S, Kobayashi T, Iemitsu M, Goto K, Sugishita Y, Matsuda M. Prolonged exercise causes an increase in endothelin-1 production in the heart in rats. Am J Physiol Heart Circ Physiol 275: H2105–H2112, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Maeda S, Miyauchi T, Sakane M, Saito M, Maki S, Goto K, Matsuda M. Does endothelin-1 participate in the exercise-induced changes of blood flow distribution of muscles in humans? J Appl Physiol 82: 1107–1111, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Mawji IA, Robb GB, Tai SC, Marsden PA. Role of the 3′-untranslated region of human endothelin-1 in vascular endothelial cells. Contribution to transcript lability and the cellular heat shock response. J Biol Chem 279: 8655–8667, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Merkus D, Brzezinska AK, Zhang C, Saito S, Chilian WM. Cardiac myocytes control release of endothelin-1 in coronary vasculature. Am J Physiol Heart Circ Physiol 288: H2088–H2092, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Merkus D, Duncker DJ, Chilian WM. Metabolic regulation of coronary vascular tone: role of endothelin-1. Am J Physiol Heart Circ Physiol 283: H1915–H1921, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res 59: 745–754, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Merkus D, Houweling B, van den Meiracker AH, Boomsma F, Duncker DJ. Contribution of endothelin to coronary vasomotor tone is abolished after myocardial infarction. Am J Physiol Heart Circ Physiol 288: H871–H880, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Merkus D, Sorop O, Houweling B, Boomsma F, van den Meiracker AH, Duncker DJ. NO and prostanoids blunt endothelin-mediated coronary vasoconstrictor influence in exercising swine. Am J Physiol Heart Circ Physiol 291: H2075–H2081, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Neubauer S, Ertl G, Haas U, Pulzer F, Kochsiek K. Effects of endothelin-1 in isolated perfused rat heart. J Cardiovasc Pharmacol 16: 1–8, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Pernow J, Kaijser L, Lundberg JM, Ahlborg G. Comparable potent coronary constrictor effects of endothelin-1 and big endothelin-1 in humans. Circulation 94: 2077–2082, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Plumpton C, Haynes WG, Webb DJ, Davenport AP. Phosphoramidon inhibition of the in vivo conversion of big endothelin-1 to endothelin-1 in the human forearm. Br J Pharmacol 116: 1821–1828, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollock DM, Opgenorth TJ. ETA receptor-mediated responses to endothelin-1 and big endothelin-1 in the rat kidney. Br J Pharmacol 111: 729–732, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prins BA, Hu RM, Nazario B, Pedram A, Frank HJ, Weber MA, Levin ER. Prostaglandin E2 and prostacyclin inhibit the production and secretion of endothelin from cultured endothelial cells. J Biol Chem 269: 11938–11944, 1994 [PubMed] [Google Scholar]
- 29. Razandi M, Pedram A, Rubin T, Levin ER. PGE2 and PGI2 inhibit ET-1 secretion from endothelial cells by stimulating particulate guanylate cyclase. Am J Physiol Heart Circ Physiol 270: H1342–H1349, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Schulz R, Oudiz RJ, Guth BD, Heusch G. Minimal alpha 1- and alpha 2-adrenoceptor-mediated coronary vasoconstriction in the anaesthetized swine. Naunyn Schmiedebergs Arch Pharmacol 342: 422–428, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Simard E, Jin D, Takai S, Miyazaki M, Brochu I, D'Orleans-Juste P. Chymase-dependent conversion of Big endothelin-1 in the mouse in vivo. J Pharmacol Exp Ther 328: 540–548, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Takamura M, Parent R, Cernacek P, Lavallee M. Influence of dual ETA/ETB-receptor blockade on coronary responses to treadmill exercise in dogs. J Appl Physiol 89: 2041–2048, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Verbeuren TJ, Mennecier P, Merceron D, Simonet S, de Nanteuil G, Vincent M, Laubie M. Phosphoramidon inhibits the conversion of big ET-1 into ET-1 in the pithed rat and in isolated perfused rat kidneys. J Cardiovasc Pharmacol 22, Suppl 8: S81–S84, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Wiley KE, Davenport AP. Nitric oxide-mediated modulation of the endothelin-1 signalling pathway in the human cardiovascular system. Br J Pharmacol 132: 213–220, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wiley KE, Davenport AP. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery. Br J Pharmacol 133: 568–574, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wray DW, Nishiyama SK, Donato AJ, Sander M, Wagner PD, Richardson RS. Endothelin-1-mediated vasoconstriction at rest and during dynamic exercise in healthy humans. Am J Physiol Heart Circ Physiol 293: H2550–H2556, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]