Abstract
Some clinical studies have suggested that lower IGF-I levels may be associated with an increased risk of ischemic heart disease. We generated atherosclerosis-prone apolipoprotein E-deficient (ApoE−/−) mice with 6T alleles (6T/ApoE−/− mice) with a 20% decline in circulating IGF-I and fed these mice and control ApoE−/− mice with normal chow or a Western diet for 12 wk to evaluate the effect of low serum IGF-I on atherosclerosis progression. We found that the 6T/ApoE−/− phenotype was characterized by an increased atherosclerotic burden, elevated plaque macrophages, and increased proinflammatory cytokine TNF-α levels compared with ApoE−/− controls. 6T/ApoE−/− mice had similar body weight, blood pressure, serum total cholesterol levels, total plaque and smooth muscle cell apoptosis rates, and circulating levels of endothelial progenitor cells as ApoE−/− mice. 6T/ApoE−/− mice fed with normal chow had reduced vascular endothelial nitric oxide synthase mRNA levels and a trend to increased aortic expression of chemokine (C-C motif) receptor (CCR)1, CCR2, and monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2. Western diet-fed 6T/ApoE−/− mice had a trend to increased expression of macrophage scavenger receptor-1/scavenger receptor-A, osteopontin, ATP-binding cassette (subfamily A, member 1), and angiotensin-converting enzyme and elevated circulating levels of the neutrophil chemoattractant chemokine (C-X-C motif) ligand 1 (KC). Our data establish a link between lower circulating IGF-I and increased atherosclerosis that has important clinical implications.
Keywords: vascular biology, growth factors, congenic mice, chemokines, chemokine receptors, apolipoprotein E
insulin-like growth factor (IGF)-I is a small 7,700-Da polypeptide that is synthesized in the liver and in multiple tissues and exerts both endocrine and autocrine/paracrine effects on growth, differentiation, and cell survival (4, 10). IGF-I is present at high concentrations in plasma, where it is associated with specific binding proteins (IGF-binding proteins). IGF-I is a critical regulator of developmental growth, and its circulating levels increase postnatally and into puberty but decline progressively with advancing age (8, 21). Some epidemiological studies have found that low IGF-I is a predictor of ischemic heart disease, but this finding has not been consistent, and the opposite association has been reported (1, 2, 7, 11).
IGF-I is expressed in the vasculature, stimulates the growth, differentiation, and survival of vascular smooth muscle cells (SMCs) (4, 10), and inhibits oxidized LDL (oxLDL)-induced apoptosis (9, 14). We (16) have previously reported that oxLDL downregulates IGF-I and IGF-I receptor expression in vascular SMCs and that expression of IGF-I/IGF-I receptor in areas of advanced human plaque staining positive for oxLDL is reduced, suggesting that decreased IGF-I activity could contribute to the atherosclerotic process. Furthermore, infusion of IGF-I into apolipoprotein E (ApoE)-deficient (ApoE−/−) mice on a Western diet (WD) reduced the atherosclerotic plaque burden (22). However, whether a genetically determined reduction in IGF-I levels has an effect on atherosclerosis development is unknown.
Bouxsein et al. (3) have generated a congenic strain of mice (C3H.6T mice, simplified as 6T mice) with a 20% reduction in circulating IGF-I levels compared with the parent C57BL/6J strain. To study the effect of low levels of circulating IGF-I on atherosclerosis development, we crossed 6T mice with ApoE−/− mice (these mice are also on the C57BL/6J background) to obtain mice homozygous for both 6T and ApoE-null alleles (6T/ApoE−/− mice) and used these 6T/ApoE−/− mice for experiments.
MATERIALS AND METHODS
Animals.
All animal experiments were performed according to protocols approved by the Institutional Committee for Use and Care of Laboratory Animals. Mice were housed individually and maintained on a 12:12-h light-dark cycle. C3H.6T mice, obtained from Dr. Clifford Rosen of the Jackson Laboratory (Bar Harbor, ME), were identified by PCR with the following three pairs of primers: D6 93, forward 5′-ATCCCAAGATTCTTGCCCTC-3′ and reverse 5′-TTAAAATCTCCACTTG CTAACCTG-3′; D6 124, forward 5′-TAGTGCCCTCTTCCAATTGG-3′ and reverse 5′-TTTCCTTCAACATAGATGTTTCTCA-3′; and D6 150, forward 5′-GGTAACAAAAAAAGGAAACCACC-3′ and reverse 5′-ATTATTGAGCATTTCCCCCC-3′. C3H.6T mice were bred with ApoE−/− mice (C57BL/6 background) for 10 generations to obtain homozygous C3H.6T+/+ and ApoE−/− double mutants (6T+/+/ApoE−/−, 6T/ApoE−/−). Eight-week-old 6T/ApoE−/− mice were fed with normal chow (NC) or WD (42% of total calories from fat, 0.15% cholesterol, Harlan Teklad) for 12 wk (n = 24 mice/group). Body weight and blood pressure were measured every week, and fasting blood was withdrawn before the beginning of the experiment and before death.
Atherosclerosis quantification, immunohistochemistry, and apoptosis detection.
Atherosclerosis was quantified by measuring the surface area of oil red O-positive lesions on en face preparations of whole aortas and by determining the plaque area on aortic valve cross-sections as previously described (22). Serial sections of aortic valves were stained using anti-Mac-3 antibody (BD Pharmingen) or anti-TNF-α antibody (Fitzgerald Industries) for the assessment of macrophage infiltration and TNF-α expression, respectively, as previously described (22). Cell apoptosis was quantified in paraffin-embedded aortic valve cross-sections with an Apoptosis TUNEL Detection kit-fluorescein (Roche) as per the manufacturer's instructions. To identify apoptotic SMCs in the atherosclerotic plaques, TUNEL-stained sections were costained with anti-α-smooth muscle actin (α-SMA) antibody (Chemicon) followed by an incubation with biotinylated secondary antibody and streptavidin-Alexa 594 complex (Invitrogen). Sections were mounted with 4′,6-diamidino-2-phenylindole-containing mounting media and imaged using fluorescence microscopy and Image-Pro plus version 6.0 software. Total cell apoptosis was defined as the TUNEL-positive cell number per 1,000 plaque cells, and SMC apoptosis was measured as the number of α-SMA/TUNEL double-positive cells per 1,000 α-SMA-positive cells.
Biochemical assays.
IGF-I levels were measured with mouse/rat-specific IGF-I ELISA kits (Diagnostic Systems Laboratories, Webster, TX). Total cholesterol levels were measured using a commercially available kit (Cholesterol/Cholesteryl Ester Quantitation kit, BioVision, Mountain View, CA). Mouse cytokine levels and other proteins of interest were measured from sera samples using Milliplex Mouse Cytokine/Chemokine 32 or Mouse Cardiovascular Panel 1 ELISA panels (Millipore). The ELISA was performed as per the manufacturer's guidelines and read on a BioPlex system (Bio-Rad).
Endothelial progenitor cell measurements.
White blood cells in EDTA-treated whole blood were immunostained with R-phycoerythrin (PE)-conjugated anti-mouse Flk-1 monoclonal antibody and FITC-conjugated anti-mouse Sca-1 monoclonal antibody (BD Biosciences) and were immediately analyzed by a flow cytometer. Sca-1-positive/Flk-1-positive cells were considered to be endothelial progenitor cells (EPCs) and were counted to evaluate circulating EPC numbers.
Quantitative real-time RT-PCR.
Aortas were homogenized on ice in TriPure isolation reagent (Roche), and total RNA was isolated as per the manufacturer's protocol using Phase Lock Gels (Eppendorf, Hamburg, Germany) to assist in phase separation. RNA was then purified using a RNeasy mini kit (Qiagen, Valencia, CA). cDNA synthesis was performed using a RT2 first-strand kit (Qiagen/SABiosciences). Gene expression profiling was performed in a 96-well plate with an Atherosclerosis RT2 Profiler PCR Array system (Qiagen SABiosciences) to examine the aortic expression of 86 atherosclerosis-related genes of interest. Real-time PCR was performed using a 40-cycle 2-step PCR protocol in an iCycler IQ real-time detection system (Bio-Rad).
Cell culture.
Human THP-1 mononuclear cells were purchased from the American Type Culture Collection (ATCC) and were cultured in RPMI-1640 media (ATCC) as per the manufacturer's recommendations. PMA (100 ng/ml, Sigma-Aldrich) was added to THP-1 monocytes for 48 h to promote cell differentiation into macrophages. PMA-treated cells were considered to be macrophages based on a typical macrophage-like phenotype and immunopositivity for CD36 and CD16 markers. For experiments with IGF-I, THP-1 monocytes or macrophages were treated with 50 ng/ml human recombinant IGF-I (Tersica) for 16 h, and gene expression analysis was performed by quantative real-time RT-PCR with the Human Atherosclerosis RT2 Profiler PCR Array system (Qiagen SABiosciences) for macrophages or using monocyte chemoattractant protein-1 (MCP-1)/chemokine (C-C motif) ligand (CCL)2, chemokine (C-C motif) receptor (CCR)1, and CCR2 sequence-specific primer pairs (SABiosciences) for monocytes.
Tissue IGF-I determination.
The dissected aorta and liver were immediately snap frozen in liquid nitrogen and kept at −80°C until analysis. Tissue samples were homogenized in ice-cold 1 M acetic acid containing protease inhibitors (Halt Protease Inhibitor Cocktail, Pierce, Rockford, IL) using a Bullet Blender with stainless steel beads (Next Advance, Averill Park, NY) at 4°C. Tissue IGF-I was extracted by rotating homogenates at 4°C for 2 h followed by centrifugation at 10,000 g for 10 min. Supernatants were neutralized by sodium hydroxide and subjected to IGF-I determination using mouse/rat IGF-I ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis.
A two-tailed unpaired Student's t-test was used for data analysis, and differences were considered significant at P < 0.05. All numerical data are expressed as means ± SE.
For statistical analysis of the RT array, the cycle threshold (Ct) value of each gene of interest was normalized to five housekeeping genes, which were included in this commercially available kit. P values were calculated based on a Student's t-test of the replicate 2−ΔCt values for each gene in the control group (ApoE−/− mice) and low circulating IGF-I mice (6T/ApoE−/− mice) fed with the same chow (either NC or WD) using the Superarray Analysis software provided by manufacturer. Taking into account testing of multiple genes, a Bonferroni adjustment for multiple comparisons was used (18). Differences were considered significant when the observed P values were <0.05/k, where k is the number of tested genes. A similar correction was used for the analysis of cytokine arrays.
RESULTS
6T/ApoE-deficient mice have reduced circulating IGF-I levels and no changes in body weight or blood pressure compared with ApoE-deficient mice.
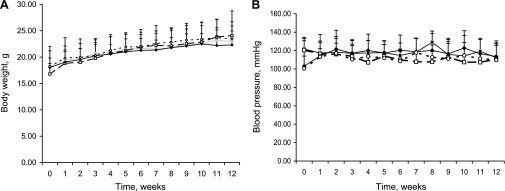
To study the effect of low circulating IGF-I levels on atherosclerosis, we bred 6T congenic mice on the ApoE−/− background to generate mice homozygous for 6T alleles and ApoE−/− alleles (6T/ApoE−/− mice). Serum IGF-I levels in 8-wk-old 6T/ApoE−/− mice were 208.8 ± 58.3 ng/ml compared with 221.4 ± 58.3 ng/ml in 6T mice and 354.5 ± 76.3 ng/ml in ApoE−/− mice [6T/ApoE−/− vs. ApoE−/− mice, P < 0.0001; 6T vs. ApoE−/− mice, P < 0.001; and 6T/ApoE−/− vs. 6T mice, P = not significant (NS)]. We found no differences in aortic or liver IGF-I mRNA and protein levels between 6T/ApoE−/− and ApoE−/− mice (Supplemental Material, Supplemental Fig. S1).1 After mice were fed with either NC or WD for 12 wk, there were no significant differences in either mean body weight or mean systolic blood pressures between 6T/ApoE−/− and ApoE−/− mice (Fig. 1).
Fig. 1.
Body weight (A) and blood pressure (B) of apolipoprotein E (ApoE)-deficient (ApoE−/−) mice with 6T alleles (6T/ApoE−/− mice; filled symbols) and control ApoE−/− mice (open symbols) fed with normal chow (NC; circles) or Western diet (WD; squares). Eight-week-old 6T/ApoE−/− and ApoE−/− mice were fed with NC or WD for 12 wk (n = 13–20 mice/group).
6T/ApoE−/− mice developed a greater plaque burden than ApoE−/− mice after 12 wk on NC or WD.
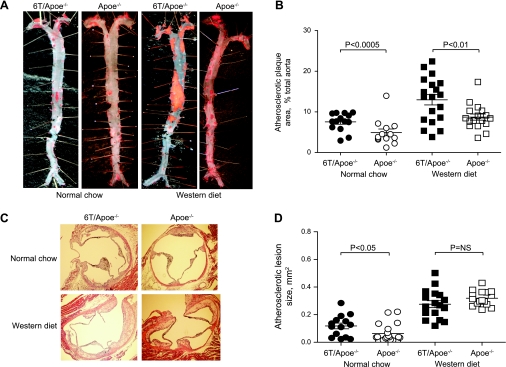
After 12 wk on NC or WD, we determined the plaque burden in mouse aortae by en face analysis and by measuring the lesion area in aortic root cross-sections. The total atherosclerotic plaque area by en face analysis was 108 ± 14% higher and 62 ± 10% higher in 6T/ApoE−/− mice fed NC or WD, respectively (6T/ApoE−/− vs. ApoE−/− mice, P < 0.0005 and P < 0.01, respectively; Fig. 1B). Atherosclerotic lesion area quantification of aortic root cross-sections indicated that NC-fed 6T/ApoE−/− mice had an 88 ± 17% increase in lesion area compared with ApoE−/− mice (P < 0.05; Fig. 2D). In WD-fed animals, there were no significant differences in lesion size between 6T/ApoE−/− and ApoE−/− mice (Fig. 2D).
Fig. 2.
6T/ApoE−/− mice have an increased atherosclerotic plaque burden compared with ApoE−/− mice. A: representative photographs of en face preparations of aortas stained with oil red. B: quantitative data. C: representative aortic valve cross-sections stained with hematoxylin-eosin. D: quantitative data.
IGF-I suppresses apoptosis of cultured SMCs (14) and reduced the expression of IGF-I colocalized with apoptotic SMCs in advanced human atherosclerotic plaques (16). Thus, we quantified total cell apoptosis and SMC apoptosis in atherosclerotic plaques from 6T/ApoE−/− and ApoE−/− mice. 6T/ApoE−/− mice fed with either NC or WD had total cell apoptosis rates and SMC apoptosis rates that were similar to ApoE−/− mice (Fig. 3, A and B). Assessment of SMC content in atherosclerotic plaques as the plaque area positive for the SMC marker α-SMA indicated no changes in plaque SMCs between 6T/ApoE−/− and ApoE−/− mice (Fig. 3C).
Fig. 3.
Atherosclerotic plaque apoptotic rates and smooth muscle cell (SMC) content. Apoptotic SMCs and total apoptotic cells were quantified in atherosclerotic plaques from 6T/ApoE−/− and ApoE−/− mice after aortic root cross-sections were stained by TUNEL assay and anti-α-smooth muscle actin (α-SMA) antibody plus 4′,6-diamidino-2-phenylindole (DAPI; A) or by staining with TUNEL assay plus DAPI (B). Data shown are the number of apoptotic SMCs per 1,000 plaque SMCs (A) or apoptotic cells per 1,000 total plaque cells (B). C: plaque SMC content was quantified using sections stained with anti-α-SMA antibody and DAPI using Image Pro software. NS, not significant.
We (22) have previously shown that IGF-I infusion into ApoE−/− mice increased circulating levels of EPCs. We evaluated EPC levels (number of Flk-1/Sca-1 double-positive white blood cells) and found that 6T/ApoE−/− mice had similar EPC numbers as ApoE−/− mice fed with either NC (6T/ApoE−/− mice: 0.29 ± 0.09% and ApoE−/− mice: 0.50 ± 0.17%, n = 7, P = NS) or WD (6T/ApoE−/− mice: 0.20 ± 0.03% and ApoE−/− mice: 0.26 ± 0.07%, n = 7, P = NS). Serum total cholesterol levels were not different between 6T/ApoE−/− and ApoE−/− mice fed with WD (6T/ApoE−/− mice: 1,147 ± 107 mg/dl vs. ApoE−/− mice: 1,011.6 ± 72.3 mg/dl, n = 9, P = NS).
6T/ApoE−/− mice have increased plaque macrophage infiltration and TNF-α expression.
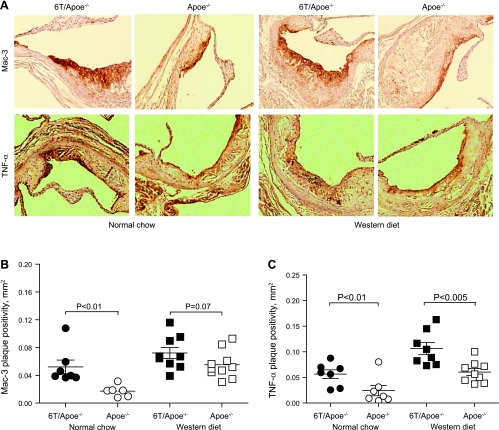
Macrophage accumulation plays a critical role in atherosclerotic plaque development. We analyzed the distribution of macrophages in plaques by staining aortic root cross-sections with anti-Mac-3 antibodies. NC-fed 6T/ApoE−/− mice had a marked increase in Mac-3-positive plaque area compared with ApoE−/− mice (3.0 ± 0.2-fold increase, P < 0.01; Fig. 4, A and B). There was a similar strong trend toward increased Mac-3-positive plaque area in WD-fed 6T/ApoE−/− (1.31-fold increase, P = 0.07; Fig. 4B). TNF-α is a cytokine that is expressed by multiple cells, including macrophages, and is abundant in atherosclerotic plaques. Immunostaining revealed that TNF-α-positive plaque area was greater in 6T/ApoE−/− mice compared with ApoE−/− mice fed with either NC or WD (115 ± 14% and 76 ± 11% increase, respectively, P < 0.01 and P < 0.005; Fig. 4C).
Fig. 4.
Low serum IGF-I increases plaque macrophage levels (A and B) and plaque TNF-α expression (A and C). Macrophage plaque levels or TNF-α expression were quantified by immunostaining of aortic root cross-sections with anti-Mac-3 or anti-TNF-α antibody, respectively, and immunopositive plaque areas were measured using Image Pro software. A: representative sections. B and C: quantitative data.
Atherosclerosis-related gene and protein profiling.
To obtain initial insights into mechanisms underlying the increased plaque burden in 6T/ApoE−/− mice, we analyzed the expression of 86 atherosclerosis-related genes of interest in atherosclerotic aortas from 6T/ApoE−/− and ApoE−/− mice using commercially available RT arrays (Table 1). In addition, we determined the plasma levels of 25 atherosclerosis-related cytokines/chemokines and other proteins of interest with a Milliplex ELISA (Table 2). Gene or protein expression levels of several proatherogenic molecules were upregulated in 6T/ApoE−/− mice compared with ApoE−/− controls, including macrophage scavenger receptor (MSR)-1/scavenger receptor (SR)-A (2.7 ± 0.1-fold increase, P = 0.04), secreted phosphoprotein 1 (osteopontin, 4.8 ± 0.2-fold increase, P = 0.01), CCR1 (2.2 ± 0.1-fold increase, P = 0.04), and chemokine chemokine (C-X-C motif) ligand 1 [CXCL1; a potent neutrophil chemoattractant (5), 3.0 ± 0.3-fold increase, P = 0.015]. We also found a trend toward increased MCP-1 aortic mRNA and circulating protein levels (2.9-fold and 2.6-fold increase, P = 0.20 and P = 0.15, respectively) and MCP-1 receptor (CCR2) aortic mRNA levels (2.0-fold increase, P = 0.08) in 6T/ApoE−/− mice. 6T/ApoE−/− mice also had increased aortic mRNA levels of angiotensin-converting enzyme (ACE; 2.2 ± 0.1-fold increase, P = 0.01) and ATP-binding cassette, subfamily A, member 1 (Abca1, 2.1 ± 0.1-fold increase, P = 0.01). Aortic mRNA levels of endothelial nitric oxide (NO) synthase (eNOS) were reduced in 6T/ApoE−/− mice compared with control mice (6T/ApoE−/− mice: 0.3 ± 0.1 and ApoE−/− mice: 1.0 ± 0.3, relative expression, n = 6, P < 0.05). Circulating levels of eotaxin (CCL11), a potent basophil chemoattractant, were slightly reduced in 6T/ApoE−/− mice (1.6 ± 0.4-fold decrease, P = 0.046). It is of note that none of these changes in gene or protein expression levels can be considered statistically significant since statistical correction for multiple comparisons using Bonferroni's adjustment established the threshold of significance at P < 0.05/k, namely, P < 0.00058 for the gene array and P < 0.0019 for the protein arrays, respectively.
Table 1.
Gene expression profiles in aortas from 6T/ApoE−/− mice compared with ApoE−/− mice
| NC |
WD |
|||||
|---|---|---|---|---|---|---|
| Gene Name | Gene Abbreviation | GenBank Accession Number | Relative Expression | P value | Relative Expression | P value |
| Chemokine (C-C motif) receptor 1 | Ccr1 | NM_009912 | 2.16 | 0.04 | −1.07 | 0.52 |
| Chemokine (C-C motif) receptor 2 | Ccr2 | NM_009915 | 1.99 | 0.08 | −1.65 | 0.38 |
| Chemokine (C-C motif) ligand 2 | Ccl2 | NM_011333 | 2.85 | 0.20 | 1.13 | 0.66 |
| Platelet-derived growth factor B polypeptide | Pdgfb | NM_011057 | 1.71 | 0.11 | 1.49 | 0.29 |
| Connective tissue growth factor | Ctgf | NM_010217 | −2.04 | 0.09 | −1.58 | 0.16 |
| ATP-binding cassette, subfamily A, member 1 | Abca1 | NM_013454 | 1.83 | 0.23 | 2.14 | 0.01 |
| Angiotensin I-converting enzyme 1 | Ace | NM_009598 | 1.22 | 0.87 | 2.22 | 0.01 |
| Fibrinogen, α-chain | Fga | NM_010196 | −2.01 | 0.91 | 2.38 | 0.19 |
| Fibrinogen, β-chain | Fgb | NM_181849 | −2.01 | 0.91 | 2.83 | 0.18 |
| Interleukin-4 | Il4 | NM_021283 | −2.01 | 0.91 | 2.38 | 0.19 |
| Interleukin-5 | Il5 | NM_010558 | −1.68 | 0.94 | 2.22 | 0.19 |
| Integrin, α5 | Itga5 | NM_010577 | −1.08 | 0.53 | 1.57 | 0.15 |
| Kruppel-like factor-2 | Klf2 | NM_008452 | −2.01 | 0.91 | 2.38 | 0.19 |
| Laminin, α1 | Lama1 | NM_008480 | −2.01 | 0.91 | 2.64 | 0.15 |
| Lipoprotein lipase | Lpl | NM_008509 | −1.14 | 0.72 | 1.83 | 0.09 |
| Matrix metallopeptidase-1a | Mmp1a | NM_032006 | −2.01 | 0.91 | 2.22 | 0.20 |
| Macrophage scavenger receptor-1 | Msr1 | NM_031195 | 1.51 | 0.57 | 2.69 | 0.04 |
| Nuclear receptor, subfamily 1, group H, member 3 | Nr1 h3 | NM_013839 | −1.33 | 0.87 | 1.65 | 0.11 |
| Serine (or cysteine) peptidase inhibitor, member 1 | Serpine1 | NM_008871 | 1.31 | 0.51 | 1.77 | 0.0495 |
| Secreted phosphoprotein-1 | Spp1 | NM_009263 | 1.44 | 0.43 | 4.76 | 0.01 |
| Tenascin C | Tnc | NM_011607 | 1.26 | 0.69 | 1.80 | 0.14 |
| B cell leukemia/lymphoma 2 | BclII | NM_009741 | −1.14 | 0.55 | −1.74 | 0.11 |
| Heparin-binding EGF-like growth factor | Hbegf | NM_010415 | −1.55 | 0.25 | −2.18 | 0.16 |
| Lysophospholipase-1 | Lypla1 | NM_008866 | 1.24 | 0.57 | −2.14 | 0.13 |
| Collagen, type III, α1 | Col3a1 | NM_009930 | 1.13 | 0.64 | 1.60 | 0.29 |
| Peroxisome proliferator activated receptor-γ | Pparg | NM_011146 | 1.06 | 0.65 | −4.14 | 0.50 |
| Tumor necrosis factor-α | Tnf | NM_013693 | −2.01 | 0.91 | 1.34 | 0.72 |
ApoE, apolipoprotein E; ApoE−/− mice, ApoE-deficient mice; 6T/ApoE−/− mice, ApoE−/− mice with 6T alleles; NC, normal chow; WD, Western diet.
Table 2.
Cytokine/chemokine levels in 6T/ApoE−/− mice compared with ApoE−/− mice
| NC |
WD |
|||||
|---|---|---|---|---|---|---|
| Protein Name | 6T/ApoE−/− mice | ApoE−/− mice | P value | 6T/ApoE−/− mice | ApoE−/− mice | P value |
| Total plasminogen activator inhibitor-1 | 3,094 ± 1,597 | 2,853 ± 1,077 | 0.70 | 6,348 ± 2,196 | 5,532 ± 656 | 0.44 |
| Vascular cell adhesion molecule | 1,092,000 ± 186,700 | 1,184,000 ± 179,800 | 0.29 | 1,015,000 ± 455,000 | 1,096,000 ± 559,800 | 0.77 |
| Intercellular cell adhesion molecule | 35,990 ± 10,620 | 36,680 ± 5,407 | 0.86 | 50,600 ± 19,790 | 56,490 ± 18,240 | 0.58 |
| Matrix metalloproteinase-9 | ND | ND | 14,590 ± 4,160 | 14,880 ± 9,070 | 0.94 | |
| Soluble E-selectin | 55,420 ± 50,440 | 46,380 ± 19,930 | 0.63 | 70,050 ± 16,530 | 48,920 ± 24,390 | 0.08 |
| Eotaxin [chemokine (C-C motif) ligand 11] | 336.1 ± 264.6 | 535.6 ± 122.4 | 0.046 | 408.2 ± 86.4 | 508.7 ± 94.99 | 0.06 |
| Granulocyte colony-stimulating factor | 317.4 ± 194.4 | 218.2 ± 84.9 | 0.16 | 519.4 ± 216.8 | 580.5 ± 278.1 | 0.65 |
| Interleukin-1a | 9.3 ± 0.8 | 34.9 ± 36.4 | 0.29 | 31.8 ± 25.2 | 26.5 ± 4.8 | 0.62 |
| Interleukin-6 | 17.5 ± 7.8 | 19.5 ± 8.5 | 0.78 | 88.0 ± 135.7 | 44.5 ± 50.3 | 0.62 |
| Interleukin-7 | 198.8 ± 221.3 | 21.3 ± 15.5 | 0.11 | 41.8 ± 28.6 | 198.1 ± 319.8 | 0.29 |
| Interleukin-12 p40 | 25.08 ± 9.01 | 47.94 ± 55.78 | 0.41 | ND | ND | |
| Interleukin-12 p70 | 215.7 ± 329.3 | 60.0 ± 68.5 | 0.42 | ND | ND | |
| Interleukin-15 | 2245 ± 4259 | 302 ± 190 | 0.22 | 431 ± 634 | 281 ± 168 | 0.62 |
| Interleukin-13 | 262 ± 127 | 468 ± 429 | 0.28 | 348 ± 227 | 339 ± 94 | 0.94 |
| Interferon-γ-induced protein, 10 kDa | 98.4 ± 106.1 | 72.3 ± 21.3 | 0.46 | 133.2 ± 65.3 | 152.6 ± 137.7 | 0.73 |
| Chemokine (C-X-C motif) ligand 1 (KC) | 21.07 ± 15.05 | 15.39 ± 8.80 | 0.47 | 74.41 ± 32.57 | 24.9 ± 18.41 | 0.01 |
| Chemokine (C-X-C motif) ligand 5 | 1708 ± 1250 | 2453 ± 1585 | 0.27 | 982 ± 1388 | 1159 ± 1129 | 0.80 |
| Monocyte chemoattractant protein-1 | 22.48 ± 20.03 | 32.94 ± 45.97 | 0.72 | 19.70 ± 12.60 | 7.62 ± 3.79 | 0.15 |
| Mitogen-inducible gene | 150.2 ± 88.7 | 112.2 ± 81.4 | 0.41 | 221.4 ± 101.6 | 156.7 ± 100.1 | 0.29 |
| Interleukin-4 | 4.42 ± 10.69 | 2.72 ± 3.37 | 0.64 | 0.92 ± 0.22 | 1.35 ± 1.19 | 0.41 |
| Interleukin-5 | 49.4 ± 76.9 | 6.2 ± 9.4 | 0.12 | 26.3 ± 35.1 | 8.1 ± 6.5 | 0.24 |
| Macrophage inflammatory protein-1a | ND | ND | 227.4 ± 345.5 | 166.7 ± 223.3 | 0.77 | |
| RANTES [chemokine (C-C motif) ligand 5] | 5.4 ± 3.9 | 12.1 ± 10.8 | 0.10 | 28.1 ± 63.9 | 15.2 ± 7.7 | 0.63 |
| Tumor necrosis factor-α | 24.0 ± 39.2 | 11.2 ± 10.8 | 0.50 | 6.1 ± 9.3 | 5.2 ± 2.4 | 0.87 |
| Vascular endothelial growth factor | 1.75 ± 0.74 | 1.95 ± 1.11 | 0.66 | 7.04 ± 14.03 | 1.42 ± 1.06 | 0.35 |
Values are means ± SE (in pg/ml). RANTES, regulated upon activation, normal T cell expressed, and secreted; ND, not detectable.
IGF-I downregulates chemokine/chemokine receptors in cultured cells.
Since we found that mice with low circulating IGF-I levels have increased aortic expression of several chemokines and chemokine receptors, we measured the potential effect of IGF-I on human THP-1 monocytes and THP-1 macrophages using RT arrays or RT-PCR. RT arrays indicated that IGF-I decreased the expression of the proapoptotic molecule Bax, ICAM-1, MSR-1/SR-A, matrix metalloproteinase (MMP)-1, and α5-integrin in THP-1 macrophages (changes were not statistically significant considering the threshold for significance with Bonferroni adjustment was 0.05/25 = 0.002; Table 3). Quantitative RT-PCR indicated that IGF-I induced a trend toward downregulation of MCP-1/CCL2 chemokine mRNA levels in macrophages (48% decrease, P = 0.07); however, gene expression levels of the chemokine receptors CCR1 and CCR2 were not altered by IGF-I (Fig. 5). In contrast, we found that IGF-I induced marked and significant downregulation of MCP-1/CCL2, CCR1 and CCR2 in THP-1 monocytes (Fig. 5). These data suggest that IGF-I-dependent regulation of mononuclear cell chemokine/chemokine receptors could contribute to the increased atherosclerotic burden in mice with low circulating IGF-I levels.
Table 3.
Gene expression profiles in IGF-I-treated THP-1 macrophages
| Relative Expression |
||||
|---|---|---|---|---|
| Gene Name | Abbreviation | GenBank Accession Number | IGF-I vs. control | P value |
| Bcl2-associated X protein | BAX | NM_007527 | 0.5791 | 0.031558 |
| Chemokine (C-C motif) ligand 2 | Ccl2 | NM_011333 | 0.5219 | 0.071173 |
| Chemokine (C-C motif) receptor 1 | Ccr1 | NM_009912 | 1.0082 | 0.92297 |
| Chemokine (C-C motif) receptor 2 | Ccr2 | NM_009915 | 2.0876 | 0.376459 |
| CD44 molecule (Indian blood group) | CD44 | NM_000610 | 0.4869 | 0.20049 |
| Colony-stimulating factor 1 (macrophage) | CSF1 | NM_000757 | 0.2012 | 0.098647 |
| Fibronectin 1 | FN1 | NM_010233 | 0.1663 | 0.114014 |
| Heparin-binding EGF-like growth factor | HBEGF | NM_001945 | 0.3565 | 0.058545 |
| Intercellular adhesion molecule 1 | ICAM1 | NM_000201 | 0.2233 | 0.034123 |
| Interferon (α, β, and ω) receptor 2 | IFNAR2 | NM_000874 | 0.7007 | 0.102391 |
| Interleukin 1 receptor, type I | IL1R1 | NM_000877 | 0.3157 | 0.055 |
| Interleukin 1 receptor, type II | IL1R2 | NM_010555 | 0.2846 | 0.206441 |
| Integrin, α5 (fibronectin receptor-α) | ITGA5 | NM_010577 | 0.2797 | 0.014981 |
| Integrin, β2 | ITGB2 | NM_008404 | 0.4313 | 0.152814 |
| Matrix metallopeptidase-1 (interstitial collagenase) | MMP1 | NM_002421 | 0.1579 | 0.01098 |
| Matrix metallopeptidase-3 (stromelysin-1, progelatinase) | MMP3 | NM_002422 | 0.1978 | 0.160551 |
| Macrophage scavenger receptor-1 | MSR1 | NM_031195 | 0.191 | 0.067798 |
| Nuclear factor of κ-light polypeptide gene enhancer in B cells-1 | NFKB1 | NM_003998 | 0.4313 | 0.1815 |
| Platelet-derived growth factor B polypeptide | PDGFB | NM_011057 | 0.369 | 0.159103 |
| Prostaglandin-endoperoxide synthase-1 (prostaglandin G/H synthase and cyclooxygenase) | PTGS1 | NM_000962 | 0.4313 | 0.223673 |
| Serine (or cysteine) peptidase inhibitor, member 1 | SERPINE1 | NM_008871 | 0.5892 | 0.207759 |
| Secreted phosphoprotein-1 | SPP1 | NM_009263 | 0.3213 | 0.067696 |
| Transforming growth factor, β1 | TGFB1 | NM_000660 | 0.5041 | 0.195858 |
| Transforming growth factor, β2 | TGFB2 | NM_003238 | 0.4389 | 0.0537 |
| Tumor necrosis factor-α | TNF | NM_013693 | 0.5403 | 0.20983 |
Fig. 5.
IGF-I downregulates chemokines and chemokine receptors in mononuclear cells. Human THP-1 mononuclear cells were treated with IGF-I (left) or differentiated into macrophages with PMA and after that treated with IGF-I (right). Gene expression analysis was performed by quantative real-time RT-PCR. n = 5 mice/group. MCP-1/CCL2, monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2; CCR, chemokine (C-C motif) receptor.
DISCUSSION
The major finding of this study is that a mild reduction in circulating IGF-I levels promotes atherosclerosis in ApoE-deficient mice fed with NC or WD. These data are consistent with previous results from our laboratory (22) showing that an increase in circulating IGF-I levels has an antiatherogenic effect. A subject of great interest in the IGF-I field is the relative physiological importance of endocrine IGF-I (most circulating IGF-I is synthesized in the liver) and autocrine/paracrine IGF-I (most tissues, including blood vessels, synthesize IGF-I). We (19) have recently shown that an increase in smooth muscle-specific IGF-I does not change the atherosclerosis burden but increases features of plaque stability. Thus, the present report provides further evidence that specific changes in endocrine (circulating) IGF-I play a dominant role in alteration of the total atherosclerotic burden.
Here, we report that the 6T/ApoE−/− phenotype was associated with a trend toward altered expression of several atherosclerosis-related molecules in the vascular wall. Some of these molecules have been previously known to be regulated by IGF-I [e.g., eNOS and IL-6 (22, 25, 26)]; however, vascular expression levels of other molecules (namely, MSR-1/SR-A, CCR1, CCR2, and MCP-1/CCL2) have not previously been shown to be associated with changes in circulating IGF-I in atherosclerotic mice. Since PCR array and protein array data are, by necessity, mainly exploratory in nature and unlikely to yield statistically significant data because of the need to correct for multiple comparisons, we performed an independent analysis of IGF-I effects on cultured monocytes/macrophages and found that IGF-I caused statistically significant changes in MCP-1/CCL2, CCR1, and CCR2 expression.
There is evidence that eNOS has antiatherosclerotic effects (13), and IGF-I-induced eNOS upregulation and an increase in NO bioavailability are thought to be potential mechanisms mediating IGF-I's atheroprotective effect (22). Therefore, eNOS downregulation in 6T/ApoE−/− mice could contribute to the larger atherosclerotic burden seen in these animals.
Chemokine/chemokine receptor systems are a key regulator of macrophage chemotaxis toward sites of inflammation, such as atherosclerotic lesions (15). The aging-associated decline in circulating IGF-I levels correlates with increased chemokine expression in elderly subjects (6), and an increase in skeletal muscle-specific IGF-I levels reduces several proinflammatory chemokines (including MCP-1/CCL2) in mice (17). Here, we report that an increase in plaque macrophages levels in 6T/ApoE−/− mice fed with NC correlated with trends to elevated gene expression of CCR1 and the chemokine MCP-1/CCL2 and its cognitive receptor, CCR2. We also demonstrated that IGF-I downregulated MCP-1/CCL2 in cultured monocytes and macrophages and that IGF-I reduced the expression of CCR1 and CCR2 in monocytes. An increase in MCP-1 levels has been shown to increase the circulating CD11b-positive monocyte/macrophage subpopulation in mice (23), and CCR2 is a critical mediator of oxidized phospholipid-induced tissue macrophage accumulation in vivo (12). Therefore, activation of the MCP-1-CCR2 axis in 6T/ApoE−/− mice is another potential mechanism mediating the increased macrophage levels and greater atherosclerotic burden present in these mice.
It is of note that, when fed with WD, 6T/ApoE−/− mice did not have increased aortic expression of CCR1, CCR2, and CCL2 (although there was a trend toward higher circulating levels of CCL2) and that the effect of low IGF-I on plaque macrophage infiltration (1.3-fold increase, P = 0.07; Fig. 4B) was markedly less than when mice were fed ND (3-fold increase, P < 0.01; Fig. 4B). Additionally, when fed with WD, 6T/ApoE−/− mice had no significant increase in aortic valve lesion area (Fig. 2D), although the effect of low IGF-I on the total aortic plaque area was striking (Fig. 1B) and similar to that with NC. In contrast to the case of NC, 6T/ApoE−/− mice fed with WD had a trend to increased aortic gene expression of MSR-1, secreted phosphoprotein 1 (osteopontin), Abca1, and ACE and elevated circulating levels of the chemokine CXCL1 (KC). Taken together, our data suggest that molecular mechanisms mediating the effect of low IGF-I on the atherosclerotic burden in 6T/ApoE−/− mice may be, at least in part, different under normocholesterolemic and hypercholesterolemic conditions.
It is generally accepted that congenic mouse strains are a useful tool to test the significance of a specific genetic region [quantitative trait loci (QTL)] in animal phenotypes (20). 6T congenic mice (the parental strain of 6T/ApoE−/− mice used in the present study) were generated by transferring a chromosome 6 QTL from C3H to the B6 genetic background (3). Since this QTL does not contain growth hormone-related genes (3), we assessed changes in atherosclerosis in 6T/ApoE−/− mice associated with alterations of circulating IGF-I that were likely at least partially independent of growth hormone. We (24) have recently shown that changes in growth hormone levels could blunt the ability of IGF-I to reduce atherosclerosis in ApoE−/− mice. However, it is possible that the transfer of this genomic region from the C3H background may have introduced into 6T/ApoE−/− mice as-yet-unidentified C3H genes that lead to increased atherogenesis. As such, the final phenotype of 6T/ApoE−/− congenic mice may reflect reduced serum IGF-I levels as well as other genetic contributions from the C3H background resulting in an increased sensitivity to atherosclerosis development. New congenic sublines with a smaller and more refined chromosome 6 QTL would help to distinguish between these two possibilities. Irrespective of the genetic mechanism, the phenotypic presentation of 6T/ApoE−/− mice suggests that the serum IGF-I regulatory system exerts a profound influence on atherosclerosis development.
In summary, we generated atherosclerosis-prone 6T/ApoE−/− mice with reduced circulating IGF-I levels and fed these mice and control ApoE−/− mice with NC or WD for 12 wk to evaluate the effect of low serum IGF-I on atherosclerosis progression. We found that the 6T/ApoE−/− phenotype was associated with an increased atherosclerotic burden, elevated plaque macrophages, and proinflammatory cytokine TNF-α levels compared with ApoE−/− controls. 6T/ApoE−/− mice fed with NC have a trend toward lower vascular levels of eNOS and upregulated aortic expression of chemokine receptors CCR1, CCR2, and MCP-1/CCL2 and that WD-fed 6T/ApoE−/− mice have a trend toward increased expression of MSR-1/SR-A, osteopontin, Abca1, and ACE and elevated circulating levels of the potent neutrophil chemoattractant CXCL1 (KC). Our data establish a link between a reduction in circulating IGF-I and increased atherosclerosis that has important clinical implications.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-070241, R01-HL-080682, and P20-RR-018766.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge Sarah Galvez and Catherine Kim for extensive assistance.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LO. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol 160: 25–31, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Boquist S, Ruotolo G, Skoglund-Andersson C, Tang R, Bjorkegren J, Bond MG, de Faire U, Brismar K, Hamsten A. Correlation of serum IGF-I and IGFBP-1 and -3 to cardiovascular risk indicators and early carotid atherosclerosis in healthy middle-aged men. Clin Endocrinol (Oxf) 68: 51–58, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bouxsein ML, Rosen CJ, Turner CH, Ackert CL, Shultz KL, Donahue LR, Churchill G, Adamo ML, Powell DR, Turner RT, Muller R, Beamer WG. Generation of a new congenic mouse strain to test the relationships among serum insulin-like growth factor I, bone mineral density, and skeletal morphology in vivo. J Bone Miner Res 17: 570–579, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24: 435–444, 2004 [DOI] [PubMed] [Google Scholar]
- 5.DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol 30: 547–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich N, Haring R, Nauck M, Ludemann J, Rosskopf D, Spilcke-Liss E, Felix SB, Dorr M, Brabant G, Volzke H, Wallaschofski H. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94: 1732–1739, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Hammerman MR. Insulin-like growth factors and aging. Endocrinol Metab Clin North Am 16: 995–1011, 1987 [PubMed] [Google Scholar]
- 9.Higashi Y, Peng T, Du J, Sukhanov S, Li Y, Itabe H, Parthasarathy S, Delafontaine P. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res 46: 1266–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol Metab 21: 245–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106: 939–944, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Kadl A, Galkina E, Leitinger N. Induction of CCR2-dependent macrophage accumulation by oxidized phospholipids in the air-pouch model of inflammation. Arthritis Rheum 60: 1362–1371, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS−/−Apoe−/− mice are ameliorated by enalapril treatment. J Clin Invest 105: 451–458, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P. Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol 23: 2178–2184, 2003 [DOI] [PubMed] [Google Scholar]
- 15.McNeill E, Channon KM, Greaves DR. Inflammatory cell recruitment in cardiovascular disease: murine models and potential clinical applications. Clin Sci (Lond) 118: 641–655, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol 33: 1777–1789, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, Wannenes F, Battistini L, Rosenthal N, Molinaro M, Musaro A. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J 21: 1393–1402, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Rosner B. Fundamentals of Biostatistics. Pacific Grove, CA: Duxbury, 2000, p. 511–532 [Google Scholar]
- 19.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe−/− mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol 30: 1916–1924, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver LM. Mouse Genetics: Concepts and Applications. New York: Oxford Univ. Press, 1995, p. xiii [Google Scholar]
- 21.Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci 54: B521–B538, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27: 2684–2690, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem 278: 46654–46660, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Titterington JS, Sukhanov S, Higashi Y, Vaughn C, Bowers C, Delafontaine P. Growth hormone-releasing peptide-2 suppresses vascular oxidative stress in ApoE−/− mice but does not reduce atherosclerosis. Endocrinology 150: 5478–5487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Nagase S, Koyama A. Stimulatory effect of IGF-I and VEGF on eNOS message, protein expression, eNOS phosphorylation and nitric oxide production in rat glomeruli, and the involvement of PI3-K signaling pathway. Nitric Oxide 10: 25–35, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Wickman A, Jonsdottir IH, Bergstrom G, Hedin L. GH and IGF-I regulate the expression of endothelial nitric oxide synthase (eNOS) in cardiovascular tissues of hypophysectomized female rats. Eur J Endocrinol 147: 523–533, 2002 [DOI] [PubMed] [Google Scholar]