Abstract
The healing process is a key determinant for postinfarction left ventricular (LV) remodeling and the development of heart failure, which could be influenced by mechanical (pressure and/or volume) load. So far, limited information exists regarding an indepth characterization of the postinfarct healing process in the mechanically unloaded state. In the present work, we performed isogenic Lewis-to-Lewis rat abdominal heterotopic heart transplantation, which is characterized by hemodynamic unloading in the left ventricle, and simultaneously ligated the left anterior descending coronary artery (T-infarct group). Pathological evolution was dynamically compared with that of in situ infarcted Lewis hearts (I-infarct group) on days 3, 7, 14, and 35. There was a remarkable myocardial salvage in the unloaded heart, as shown by the improvement in infarct size (T-infarct group: 25.47% ± 4.31% vs. I-infarct group: 38.46% ± 4.82%, P < 0.01) and the smaller fraction of fibrosis in infarct segments (T-infarct group: 42.12% ± 8.40% vs. I-infarct group: 75.65% ± 10.51%, P < 0.01). In addition, there was a progressive disorganization of the two-dimensional collagen fiber alignment as well as retarded collagen fiber maturation in the T-infarct group. We also observed enhanced angiogenesis, lymphangiogenesis, and inflammatory cell retention in the infarct region during mechanical unloading. Moreover, capillary density and collagen deposition were significantly increased in the noninfarcted area of the unloaded heart compared with the same region in the in situ infarcted heart. In conclusion, ischemic insult in the mechanically unloaded heart elicits an altered inflammatory and healing response, which is characterized by myocardial salvage, delayed resolution of inflammation, and disorganization of the collagen orientation in the infarcted region. These findings could provide novel insights into the contribution of hemodynamic load in the postinfarction healing process. Further studies are warranted to elucidate its potential mechanism.
Keywords: myocardial infarction, ventricular remodeling, heart transplantation, inflammation
the healing process is a key determinant for postinfarction left ventricular (LV) remodeling and the development of heart failure, which could be influenced by mechanical (pressure and/or volume) load (39). The mammalian heart has a limited capability for self-regeneration, and persistent ischemia could result in myocardial death, granulated tissue formation and scar remodeling, and, ultimately, replacement fibrosis (14, 15). Early (hours to days) after myocardial infarction (MI), the activity of matrix metalloproteinases (MMPs) is significantly increased to remove tissue and cell debris. In this period, fibroblast proliferation as well as new extracellular matrix synthesis have not been fully initiated, which makes the infarcted region vulnerable to any distortion forces (39, 43). Therefore, it is conceivable that using pharmacological approaches to lower pressure and/or volume load (within the limit of coronary autoregulation) would have salutary effects on infarct healing (22).
The murine model of abdominal heterotopic heart transplantation (HTx) is widely used to evaluate immunological responses and drug efficacy against organ rejection. The transplanted heart (donor heart) is fully vascularized and beats, but its LV is not hemodynamically loaded (a detailed illustration is shown in Fig. 1). In addition, isogenic transplantation (i.e., Lewis to Lewis) could avoid alloimmunity and was used to investigate the mechanisms underlying LV assist device-induced cardiac remodeling. Moreover, the isograft coronary artery was also ligated in some bioengineering and stem cell studies (1, 6, 25–27, 46), which enables continuous manipulation without endangering the host.
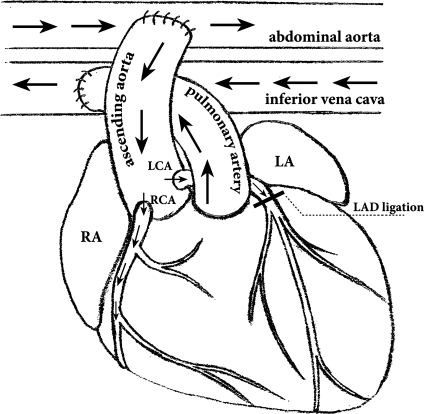
Fig. 1.
Schematic illustration of the abdominal heterotopic heart transplantation. The superior and inferior vena cava and pulmonary veins of the donor heart are ligated. The donor aorta is anastomosed to the host infrarenal aorta, and the donor pulmonary artery is anastomosed to the inferior vena cava. Arrows indicate the direction of blood flow. Blood from the host flows into the donor ascending aorta and then into the donor coronary arteries. Blood then returns to the donor right ventricle through the coronary sinus and finally drains into the host circulation via the donor pulmonary artery. In this setting, the donor heart is vascularized and beats, but the left ventricle (LV) is mechanically unloaded. In the transplanted group with myocardial infarction (MI), the donor left anterior descending coronary artery (LAD) was also ligated. RA, right atrium; LA, left atrium; LCA, left coronary artery; RCA, right coronary artery.
So far, to our knowledge, limited information exists regarding an indepth characterization of the postinfarction healing process in the mechanically unloaded state. Therefore, to address this issue, in the present work, we performed isogenic Lewis-to-Lewis rat abdominal heterotopic HTx and simultaneously ligated the coronary artery for permanent myocardial ischemia to dynamically compare related pathological changes with those of in situ infarcted isogenic hearts. Our work provides more insight into the contribution of LV mechanical loading to the postinfarction healing process.
MATERIALS AND METHODS
Study protocol.
Male Lewis inbred rats (n = 170, age: 12 wk) were obtained from Vital River Laboratory Animal Technology (Beijing, China) and received humane care in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). Coronary ligation of Lewis inbred rats has been reported to produce a uniform infarct size compared with other rat stains (32). The research protocol was approved by the institutional animal care and use committee. All rats were randomly assigned into the transplantation group (T group) or the in situ group (I group), designated for rats that underwent heterotopic HTx and rats with in situ heart operations, respectively. The T group was further divided into transplantation with MI (T-infarct group) and transplantation without MI (T-sham group). The I group was further divided into the in situ MI (I-infarct) group and in situ sham (I-sham) group. Surviving rats were killed on days 3, 7, 14, and 35 after the operations.
Animal models.
Animals for in situ MI were anesthetized with pentobarbital sodium (60 mg/kg ip), intubated under direct vision with polyethylene tubing, and placed on artificial ventilation (75 strokes/min, tidal volume: 8 ml/kg) using a volume-controlled rodent respirator (Inspira 55–7058, Harvard Apparatus, South Natick, MA). Ligation of the left anterior descending coronary artery (LAD) was performed as previously described (31). Briefly, after a left thoracotomy at the fourth intercostal space, the pericardium was opened, and the heart was exteriorized. The LAD was then permanently ligated by an intramural 5-0 silk suture inserted from below the tip of the left atrial appendage up to the pulmonary conus. Successful operation was confirmed by the appearance of deep S wave and subsequent ST segment elevation as well as ventricular arrhythmia in continuous ECG monitoring (MP150, Biopac Systems, Goleta, CA). The sham-operated groups received the same surgical procedure except that the LAD was not occluded.
Abdominal heterotopic HTx was performed as previously described with modifications (see Fig. 1 for an illustration) (38). Briefly, donor rats were heparinized (1,000 units ip) and anesthetized with pentobarbital sodium (60 mg/kg ip). A midline incision was made from the sternum to the abdomen, and the chest wall was removed for heart exposure. Blunt dissection of the heart, aorta, vena cava, and pulmonary vessels was the performed. The pulmonary veins and inferior vena cava were ligated, and the graft was excised. Suture of the LAD was performed just after cardiac graft harvesting and trimming, in a similar fashion as described above, and the sham operation was done without ligation. The donor heart was then placed in precooled (4°C) University of Wisconsin solution (ViaSpan, Barr Pharmaceuticals, Woodcliff Lake, NJ) for cardioplegia and temporary preservation. Recipient rats were anesthetized with pentobarbital sodium (60 mg/kg ip), and a midline laparotomy was performed. The abdominal aorta and vena cava were carefully dissected and clamped with straight vascular clamps to prevent bleeding. A longitudinal aortotomy and venotomy ∼5 mm in length were performed. We used standard microvascular techniques with an 8-0 proline suture to perform end-to-side anastomoses of the aorta to the abdominal aorta and pulmonary artery to the inferior vena cava, respectively. The total cold ischemic time of the donor heart was ∼40 min. During the observation period, the survival of cardiac grafts was judged with daily manual palpation by a blinded investigator using a scoring system (19): 3 indicated a strong contraction and a soft graft with little turgor; 2 indicated a mild contraction and mildly hard turgor; 1 indicated a weak contraction and hard turgor; and 0 indicated no contraction. As we performed isogenic transplantation, any cardiac grafts with a score of <3 were mainly due to surgical failure. Thus, only cardiac grafts with a score of 3 were used for analysis.
Histological evaluation of the infarct healing process.
At the time of harvest, rats were anesthetized with pentobarbital sodium (60 mg/kg ip). ECG leads connected to a physiological data-acquisition system (MP150, Biopac Systems) were placed in paws and the abdominal region to detect the heart rate of in situ hearts and transplanted hearts, respectively. Rats were then perfused via the inferior vena cava with precooled PBS for 5 min. Transplanted and in situ hearts were quickly removed. The right ventricles were excised, and the remaining LV tissue was cut parallel to the atrioventricular groove in four slices in equal thickness. The third slice up from the apex was snap frozen in liquid nitrogen for protein extraction and biochemical assays. The second slice up from the apex, which has been reported to always contain the central part of the infarcted region (51), was prepared for paraffin embedding and sectioned at 5 μm for all histological analyses. Infarct size was determined using collagen-specific Sirius red F3BA (Sigma Aldrich, St. Louis, MO)-stained sections (see below). The equation was as follows (7): percent infarct of the LV = [epicardial infarct (in mm) + endocardial infarct (in mm)]/[LV epicardial circumference (in mm) + LV endocardial circumference (in mm)] × 100. To encompass the whole part of the infarcted free wall, a ×1 objective lens was used. The transmural thickness of the anterior free wall containing the scar was measured at four to five widely spaced locations and averaged (57). Due to the existence of viable myocardium in the infarcted LV free wall (especially in the T-infarct group), we applied another method to calculate infarct size, the fibrosis fraction in the infarcted segment (see Fig. 2 for an illustration).
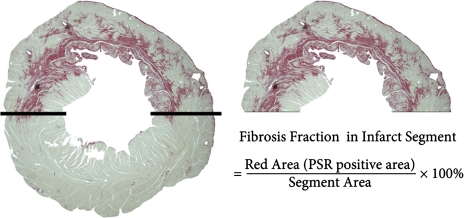
Fig. 2.
Measurement of the fraction of fibrosis in infarcted segments. The LV free wall (above the two solid lines that separate the infarcted area between the noninfarcted area) was defined as the infarcted segment. PSR, picrosirius red.
The mean cardiomyocyte cross-sectional area was determined in the LV viable septal region and stained with FITC-labeled wheat germ agglutinin (1:100 dilution, Invitrogen, Grand Island, NY). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA).
Histological analysis of collagen remodeling of the infarcted area was performed using picrosirius red-stained sections (31). First, tissue sections were rinsed in distilled water and incubated in saturated 0.1% picrosirius red for 90 min. Sections were then rinsed twice with 0.01 N HCl for 1 min, dehydrated with an ethanol gradient, and prepared for histological analysis. Images were visualized on a polarized microscope (E600POL, Nikon, Tokyo, Japan) equipped with a circular polarizer (Kenko, Tokyo, Japan) as previously described in detail (52) and digitized with a color charge-coupled device (Pro 150ES, Pixera, Los Gatos, CA). We used three quantitative parameters/methods, i.e., the collagen volume fraction (CVF), collagen maturation, and two-dimensional collagen fiber orientation to delineate postinfarction collagen remodeling. Quantification of collagen maturation was performed as previously described using a hue segmentation method (31), which is based on the notion that under polarized light, as the collagen fiber thickness increases, the color changes from green to yellow, orange, and red, which corresponds to the maturation of collagen fibers (40). The ratio of orange/red to yellow/green pixels is referred to as the scar maturation index, with a higher number representing increased maturation of scar tissue, as previously described (12). To quantify the two-dimensional collagen fiber orientation, we developed a novel analysis protocol using automatic angle recognition followed by circular statistics. Automatic angle recognition was achieved using Continuity software (version 6.3b, http://www.continuity.ucsd.edu/Continuity). We used the fiber angle calculation function in this software, which is based on an intensity gradient algorithm. Continuity automatically divides an image with a dimension of 640 × 480 pixels into small grids of 20 × 20 pixels in size and calculates the average angle of all fiber vectors inside the grid. Theoretically, 768 angular data are generated from each image. These data were further imported into NCSS 2007 (Number Cruncher Statistical Systems, Kaysville, UT) for circular data analysis (see Statistical analysis). The acquisition of images from picrosirius red-stained sections was done according to the following criteria: all images were obtained under the magnification of a ×10 objective lens and the sections were adjusted to allow the vertical central line of the image frame perpendicular to the tangent line of the epicardium.
Neovascularization and neutrophil and macrophage infiltration were evaluated using immunofluorescent staining. Slides were dewaxed, and antigen retrieval was performed. To define the small arteries in the infarcted region, tissue sections were stained with α-smooth muscle actin (α-SMA; A2547, 1:600, Sigma Aldrich). The endothelium was recognized by von Willebrand factor (vWF; sc-8068, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA). Neutrophils were stained using anti-myeloperoxidase (MPO) antibody (sc16128, 1:100, Santa Cruz Biotechnology). Macrophages were defined as ED-1 (MCA341R, 1:200, AbD Serotec, Oxford, UK)-positive cells. Tissue sections were then incubated with FITC-conjugated secondary antibody (goat anti-mouse IgG, Zymed Laboratories, San Francisco, CA). As negative controls, additional sections were exposed to the appropriate goat IgG isotype-matched antibody (Zymed) instead of incubation with primary antibodies under the same conditions to determine the specificity of antibody binding.
To define lymphatic endothelial cells, we used podopalnin, a transmembrane glycoprotein, as a lymphatic marker because of its relatively restricted expression in lymphatic endothelial cells (53). Slides were dewaxed, and antigen retrieval was performed. Sections were then incubated with antibody to podoplanin (sc-23565, 1:100; Santa Cruz Biotechnology). Horseradish peroxidase-labeled goat anti-mouse IgG and diaminobenzidine were used for immunohistochemical staining.
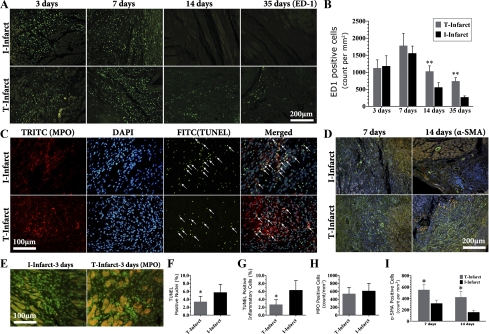
Immunostaining was further quantified with the following steps (31). vWF-positive vessels with an external diameter of <6 μm were calculated for capillary density as N/A, where N is the number of cross-sectioned vWF-positive capillary profiles and A (in mm2) is the area in which capillaries were counted. α-SMA-positive vessel-like structure with an external diameter between 6 and 50 μm were quantified with the above method. Quantification of histological staining was performed in 5–6 fields/section and digitized with magnification (×20 objective). MPO- and ED-1-positive cells and podoplanin-positive vessel-like structures were determined in a similar protocol. Specifically, lymphatic vessels were counted in only the inner and midlayer portions of the infarcted free wall, which contains the central region of infarcted tissue.
The TUNEL technique was used to analyze apoptosis in the infarcted region using an in situ cell death detection kit (Roche Diagnostics). First, we performed antigen retrieval and treatment, and the other steps followed the manufacturer's instructions. Nuclei were counterstained with DAPI. After TUNEL staining, slides were incubated with anti-MPO (sc-16128, 1:100, Santa Cruz) antibody to detect apoptotic inflammatory cells. Tetraethyl rhodamine isothiocyanate-conjugated secondary antibody was added for the visualization of MPO-positive cells. TUNEL-positive nuclei, TUNEL and MPO double-positive cells, and total nuclei were counted from the central infarcted regions. Data are expressed as percentages of TUNEL-positive nuclei or TUNEL and MPO double-positive cells to the total number of nuclei evaluated per section. Fluorescence was examined with a fluorescent microscope (80i, Nikon). All image analysis was performed in a blinded manner using Image Pro Plus (version 4.5, Media Cybernatics, Silver Spring, MA).
Gelatin zymography and Western blot analysis.
LV infarcted tissue from the third slice up from the apex was excised and homogenized individually in 5 ml of ice-cold extraction buffer [1:4 (wt/vol) containing 200 mmol/l NaCl, 1% Triton X-100, 0.1% NaN3, 20 mmol/l Tris·HCl, and 2 μg/ml aprotinin], and homogenates were then agitated in 4°C for 18 h. Samples were centrifuged at 4°C for 10 min (14,000 g), and the supernatant was aliquoted after protein concentration measurements by the bicinchoninic acid method. Gelatin zymography was carried out to detect the activity of MMP-2 (72 kDa) and MMP-9 (92 kDa). Samples (20 μg) were directly loaded on a 10% SDS polyacrylamide gel containing 0.5 mg/ml gelatin under nonreducing conditions. After electrophoresis, gels were washed in 2.5% Triton X-100 for 30 min twice and incubated for 24 h in buffer (50 mmol/l Tris·HCl, 5 mmol/l CaCl2, and 0.02% NaN3; pH 8) at 37°C. After incubation, gels were stained using 0.5% Coomassie blue R250, destained, and then scanned for image analysis.
To determine the protein abundance of transforming growth factor (TGF)-β1, MPO, VEGF-A, and VEGF-C, Western blot analysis was performed. Samples (40 μg) were loaded onto a 10% SDS polyacrylamide gel, separated under reducing conditions, and then transferred to a polyvinylidene fluoride membrane. Membranes were incubated with specific rabbit anti-TGF-β1 (sc-146, 1:400, Santa Cruz Biotechnology), anti-MPO (sc-16128, 1:200, Santa Cruz Biotechnology), anti-VEGF-A (ab46154, 1:250, Abcam, Cambridge, UK), and anti-VEGF-C (ab9546, 1:400, Abcam) antibodies as the primary antibodies and with horseradish peroxidase-conjugated goat anti-rabbit antibody as the secondary antibody at a dilution of 1:5,000–1:10,000 and detected with an enhanced chemiluminescent kit (Amersham Biosciences, Piscataway, NJ) following the manufacturer's instruction.
Statistical analysis.
Noncircular data are presented as means ± SD. For circular data analysis, only the images whose angular data passed a von Mises distribution test were used. Using descriptive circular analysis, three groups of variables were generated: 1) circular data dispersion tendency (circular variance, circular SD, and circular dispersion); 2) concentration tendency [mean resultant length and von Wiese concentration (κ)]; and 3) skewness and kurtosis of circular data. As concentration parameter κ is a characteristic parameter of von Mises distribution, we used κ to quantitatively represent the collagen fiber orientation in the infarcted area. Statistical comparisons of the difference were performed by an unpaired t-test or ANOVA followed by the least-significant-difference method for multiple comparisons. A Mann-Whitney U-test or Kruskal-Wallis H-test was used if the data failed tests for normality or equal variance. Two-tailed P values of <0.05 were considered statistically significant.
RESULTS
Enhanced myocardial salvage in the unloaded heart after permanent coronary ischemia.
A total of 110 male Lewis rats underwent HTx operation with or without MI (55 donors and 55 recipients), and 45 successful transplants were enrolled for analysis (Table 1). Another 60 Lewis rats underwent the in situ heart operation, and the 42 surviving rats were used for further assays (Table 1). Finally, approximately five to seven animals were included for each subgroup. As shown in Fig. 3 and Table 2, LVs in the T groups exhibited significant atrophy (decreased LV weight and progressive diminution of viable cardiomyocyte sizes). In contrast, cardiomyocytes of the I-infarct group were in a sustained process of compensatory hypertrophy. At the time of death (35 days postinfarction), heart rates were significantly decreased in the T groups compared with the I groups (Table 2). Interestingly, compared with the I-infarct group at 35 days, gross inspection of the heart sections of infarcted hearts showed a remarkable salvage of the ischemic myocardium compared with the T-infarct group at 35 days, as demonstrated by increased LV infarcted wall thickness (2.55 ± 0.49 vs. 1.46 ± 0.35 mm, P < 0.01, a ∼75% increase), decreased infarct size (25.47 ± 4.31% vs. 38.46 ± 4.82%, P < 0.01, a ∼34% reduction), and smaller fraction of fibrosis in infarct segments (42.12 ± 8.40% vs. 75.65 ± 10.51%, P < 0.01, a ∼44% reduction) (Figs. 2 and 3 and Table 2). The increased LV infarcted wall thickness was not fully the result of unloading-induced myocardial salvage because intraventricular pressure would undoubtedly influence the packing of collagen fibers and stretching of the viable myocardium.
Table 1.
Numbers of animal in each group eligible for analysis
| 3 Days | 7 Days | 14 Days | 35 Days | Total | |
|---|---|---|---|---|---|
| T-infarct group | 6 | 7 | 5 | 5 | 23(29) |
| T-sham group | 6 | 5 | 6 | 5 | 22(26) |
| I-infarct group | 5 | 5 | 6 | 6 | 22(40) |
| I-sham group | 5 | 5 | 5 | 5 | 20(20) |
Numbers in parentheses indicate total operations performed in each group. Animals were divided into the following groups: transplantation with myocardial infarction (T-infarct), transplantation without myocardial infarction (T-sham group), in situ with myocardial infarction (I-infarct group), and in situ without myocardial infarction (I-sham group). Animals in the transplantation groups eligible for analysis excluded those alive at the end of the specified period of followup but with a manual palpation score of <3 (see materials and methods for more detail).
Fig. 3.
General pathological changes of in situ and transplanted hearts with and without MI. Animals were divided into the following groups: in situ without MI (I-sham group), in situ with MI (I-infarct group), transplantation without MI (T-sham group), and transplantion with MI (T-infarct group). A: representative images of wheat germ agglutinin (WGA)-stained (FITC labeled) noninfarcted areas (top) and gross inspection of PSR-stained heart transverse sections (bottom). B: statistical comparisons of cardiomyocyte cross-sectional areas derived from WGA staining. *P < 0.05 by intragroup comparisons with data of 3 days; §P < 0.05 by intergroup comparisons (i.e., I-infarct vs. T-infarct groups and I-sham vs. T-sham groups) between data from the same time point.
Table 2.
General characteristics of surviving rats eligible for analysis at 35 days
| No. of Animals/Group | Body Weight, g | LV Weight, g | Heart Rate, beats/min | Infarct Size (Length Based), % | Fraction of Fibrosis in the Infarcted Segment | LV Free Wall Thickness, mm | |
|---|---|---|---|---|---|---|---|
| T-infarct group | 5 | 297.0 ± 15.7 | 0.74 ± 0.16 (donor heart) | 222.2 ± 34.2 (donor heart) | 25.47 ± 4.31 (donor heart) | 42.12 ± 8.40 (donor heart) | 2.55 ± 0.49 (donor heart) |
| T-sham group | 5 | 313.8 ± 14.2 | 0.76 ± 0.14 (donor heart) | 227 ± 21.9 (donor heart) | N/A | N/A | 2.71 ± 0.29 (donor heart) |
| I-infarct group | 6 | 315.0 ± 14.1 | 1.16 ± 0.15c,d | 368.0 ± 22.0c,d | 38.46 ± 4.82c | 75.65 ± 10.51c | 1.46 ± 0.35c,d |
| I-sham group | 5 | 323.6 ± 26.3 | 1.24 ± 0.16c,d | 350.4 ± 22.2c,d | N/A | N/A | 3.36 ± 0.24a,b,e |
Values are means ± SD.
LV, left ventricular; N/A, not applicable.
P < 0.05 vs. the T-infarct group;
P = 0.06 vs. the T-Sham group;
P < 0.01 vs. the T-infarct group;
P < 0.01 vs. the T-sham group;
P < 0.01 vs. the I-infarct group.
Altered collagen remodeling in the infarcted region of the unloaded heart.
In the infarcted area, from day 3 to day 35, there was a continuously increasing trend for CVF and maturation in in situ hearts, as shown by an increased CVF and increased scar maturation index (as the result of increased collagen cross-linking; see Fig. 4, A, C and D). In the T-infarct groups, due to the existence of viable myocardium in the infarct segment, we only measured indexes of collagen remodeling in the scarred area. In these scattered regions, the collagen-positive area and collagen maturation index were all significantly decreased (Fig. 4, B–D). We also observed a progressive disorganization of the two-dimensional orientation of newly formed collagen fibers in the T-infarct groups from day 3 to day 35 after MI compared with the I-infarct groups (Fig. 4, A and B). To quantitatively delineate these dynamics, we used Continuity software for automatic angular recognition (collagen fiber orientation) and performed circular statistics. Figure 4A shows the dynamics of the two-dimensional organization of the infarcted region from the I-infarct group. As collagen deposition increases, fiber orientation undergoes a process from well-organized to partially disorganized structure and then again to well-organized structure (as shown in Fig. 4F, the dynamic change of concentration parameter κ). However, the newly synthesized collagen fibers of the T-infarct group exhibited a continuous disorganization from day 3 until day 35 after MI (Fig. 4, B and G).
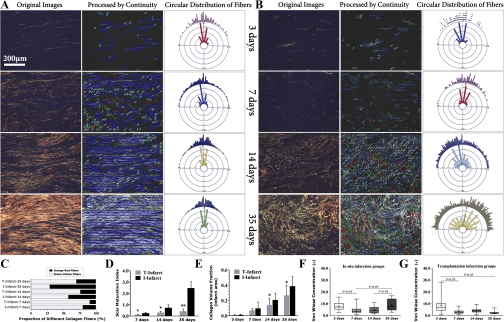
Fig. 4.
Dynamics of two-dimensional collagen fiber remodeling in infarcted regions. A and B: collagen deposition in the I-infarct group (A) and T-infarct group (B). Each image from left to right shows PSR-stained sections viewed under circularly polarized light (original images), the corresponding images processed by Continuity software (short colored lines indicate the automatically recognized fiber orientation in a grid with 20 × 20 pixels in dimension), and the circular distribution of collagen fibers derived from the corresponding images that calculated by Continuity and NCSS software, respectively. C and D: histological comparisons of the proportion of collagen fibers with different birefringence (scar maturation index). E: comparisons of the collagen volume fraction in fibrotic scars of the infarct segment. F and G: comparisons of von Wiese concentration (κ) derived from circular statistics of collagen fibers. In the box and whisker plots, the boxes extend from the 25th percentile to the 75th percentile, with a line at the median. The whiskers extend above and below the box to show the 5th–95th percentiles of values, and “+” within the box-whisker plot indicates the mean. *P < 0.05 vs. the I-infarct group at the same time point; **P < 0.01 vs. the I-infarct group at the same time point.
Altered neovascularization and lymphangiogenesis in the infarct region of the unloaded heart.
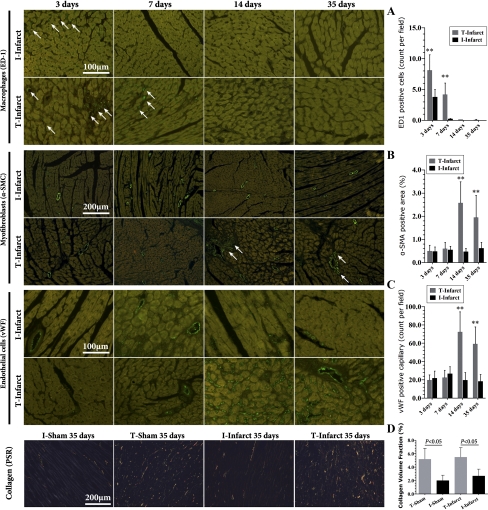
The density of newly formed small arteries and capillary vessels peaked at 7 days postinfarction in the I-infarct group and then gradually decreased (Fig. 5). This decreasing trend was significantly hampered from 14 days after ischemia in the T-infarct group. Even at 35 days after MI, a period when granulated tissue should be replaced with a matured scar during the pathological evolution of in situ rat hearts, capillary vessels could still be visible in the ischemic region in LV mechanically unloaded hearts (Fig. 5, A and C). In addition, compared with the I-infarct group, small arteries from the ischemic region of the T-infarct group lost their preferred orientation (Fig. 5, A and D).
Fig. 5.
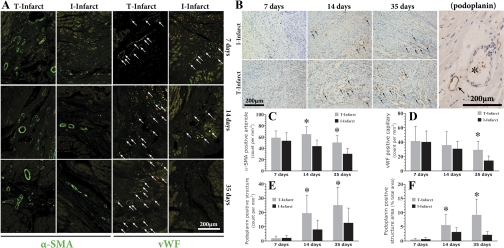
Dynamics of arteriogenesis, angiogenesis, and lymphangiogenesis in the infarcted area. A: representative images of immunofluorescent staining of LV specimens for the detection of arteriogenesis and angiogenesis in the infarcted area (FITC labeled). B: representative images of podoplanin immunohistochemical staining. Note that in the right image with higher magnification, the blood vessel (*) is negative for podoplanin staining and the adjacent small circle structure is positive for podoplanin (shown by an arrow), indicating lymphatic endothelial cells. C and D: related statistical comparisons derived from A. E and F: related statistical comparisons derived from B. α-SMA, α-smooth muscle actin; vWF, von Willebrand factor. *P < 0.05 vs. the I-infarct group at the same time point.
The pathological dynamics of lymphatic vessels are shown in Fig. 5B. Early after MI (7 days), there were almost no detectable lymphatic vessels in the infarcted region in both T and I groups. The number of lymphatic vessels underwent a significant increase thereafter, and the magnitude was more remarkable in the T-infarct group. Furthermore, this proliferative response was accompanied by a persistent increase in lymphatic vessel size in the T-infarct group (Fig. 5, E and F), an indication of lymphedema. For the I-infarct group, as time went on, lymphatic size was slightly decreased (Fig. 5F).
Delayed resolution of inflammation in the infarcted area during LV unloading.
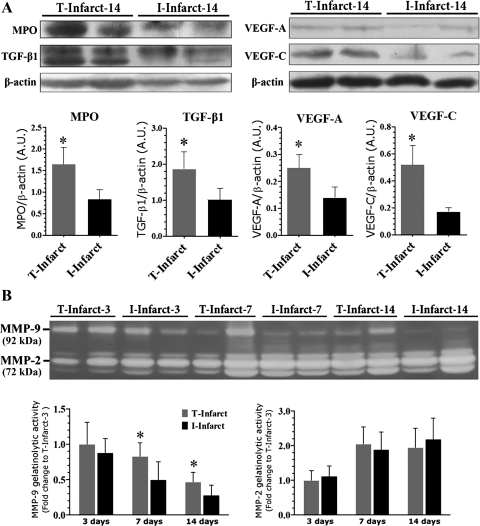
We next evaluated histological and molecular markers for inflammatory cell infiltration in the infarcts. There was no difference in the number of ED-1-positive macrophage infiltration in the early stage (3 and 7 days) between T-infarct and I-infarct groups. The macrophage number then began to decrease in the I-infarct group and statistically differed from the T-infarct group (Fig. 6, A and B). We next examined the difference of apoptosis in the infarcts. As shown in Fig. 6, C and F, 14 days after MI, TUNEL-positive cells as well as TUNEL and MPO double-positive cells were significantly decreased in the T-infarct group compared with the I-infarct group. As MPO is predominantly found in neutrophils, monocytes, and macrophages (36) and functions as a survival signal for neutrophils, contributing to the prolongation of inflammation (11), the decrease in MPO and TUNEL double-positive cells indicates a lower degree of inflammatory cell apoptosis (Fig. 6, C and G). Moreover, MPO protein levels in the infarcted region were significantly upregulated in the T-infarct group 14 days after MI (Fig. 7A), supporting increased infiltration of inflammatory cells. To determine whether or not this difference was due to a different degree of the inflammatory response at baseline in these two models, we quantified MPO-positive cells in infarcted regions on day 3 after the operations. The results showed that MPO-positive cell infiltration was similar in T-infarct and I-infarct groups (Fig. 6, E and H). We next evaluated the phenotypical change of fibroblasts in the infarcted area. As shown in Fig. 6, D and I, the number of nonvascular α-SMA-positive cells (myofibroblasts) was significantly increased in the T-infarct group compared with the I-infarct group. Of note, during this time period, TGF-β1, VEGF-A, and VEGF-C levels in the ischemic region in the T-infarct group were also significantly increased compared with the I-infarct group (Fig. 7). Furthermore, the increased inflammatory response in the T-infarct group was further confirmed by increased MMP-9 activity in T-infarct ischemic regions at 7 days after MI, whereas MMP-2 activity remained relatively stable compared with the I-infarct group (Fig. 7B).
Fig. 6.
Dynamics of the inflammatory response in the infarcted region. A: representative images of immunofluorescent staining of heart tissue specimens for the detection of ED-1-positive cells (macrophages) in the infarcted area (the secondary antibody was conjugated with FITC). B: statistical comparisons. C: infarcted region at 14 days after MI, showing representative images of double immunofluorescent staining for the detection of myeloperoxidase (MPO; TRITC labeled) and TUNEL (FITC labeled). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Arrows indicate TUNEL and MPO double-positive cells. D: dynamics of myofibroblast infiltration in infarct scars from infarcted segment (α-SMA immunofluorescent staining, FITC labeled; nuclei were stained with DAPI). E: MPO immunofluorescent staining (FITC labeled) in the infarcted area at 3 days after MI. F and G: related comparisons from C. H: related comparisons from E. I: related comparisons from D. *P < 0.05 vs. the I-infarct group at the same time point; **P < 0.01 vs. the I-infarct group at the same time point.
Fig. 7.
Western blot analysis of infarcted tissue at 14 days after MI (A) and dynamics of gelatinase [matrix metalloproteinase (MMP)-2 and MMP-9] activity of infarcted tissue revealed by substrate gelatin zymography (B). TGF-β1, transforming growth factor-β1. *P < 0.05 vs. the I-infarct group.
Increased angiogenesis and collagen deposition in the noninfarcted area during LV unloading.
We finally evaluated inflammatory, angiogenic, and fibrotic responses in noninfarcted regions. As shown in Fig. 8A, early after MI (3 days), macrophage (ED-1) infiltration could be observed in both I groups, but with a lesser extent in the I-infarct group, and could still be detected in the T-infarct group 7 days after MI. Macrophages were no longer observed 14 days postinfarction in both groups. We did not found any evidence of macrophage infiltration in the I-sham group at any time point (data not shown). The T-sham group presented a similar pattern of macrophage infiltration compared with their infarction counterparts (data not shown). These data indicate that reperfusion injury resulting from the transplantation procedure may contribute to early (3–7 days postoperation) inflammatory responses in T groups in noninfarcted related artery-supplied regions, but LV unloading itself is not associated with persistent inflammatory cell infiltration in these regions.
Fig. 8.
Dynamics of inflammatory, fibrotic, and angiogenic responses in noninfarcted regions. A: representative images and related comparisons of macrophage infiltration (ED-1 staining, FITC labeled) in the noninfarcted area. B: myofibroblast immunofluorescent staining (α-SMA, FITC labeled) and comparisons. C: endothelial cell immunofluorescent staining (vWF, FITC labeled) and comparisons. D: representative images of collagen deposition (PSR staining) in the noninfarcted region 35 days after operations. **P < 0.01 vs. the I-infarct group at the same time point.
In noninfarcted regions of the I-infarct group, α-SMA-positive myofibroblasts were mainly located in the artery wall (Fig. 8B; a similar pattern was observed in the I-sham group; data not shown). However, in the T-infarct group, as shown in Fig. 8B, there was a significantly increasing trend for nonvessel myofibroblast infiltration from 14 days postinfarction, which was more obvious in the perivascular region. Similarly, vWF-positive capillary density did not change significantly across time in the I-infarct groups (Fig. 8C; as did the I-sham group; data not shown), whereas capillary density was significantly increased from 14 days postinfarction in the T-infarct group (as did the T-sham group; data not shown). Finally, we found that collagen deposition was significantly increased in T groups 35 days after transplantation compared with their infarction counterparts (no differences between T groups, Fig. 8D). These lines of evidence suggest that LV mechanical unloading could lead to enhanced angiogenic and fibrotic responses, but these changes are not likely the result of reperfusion injury of cardiac transplantation because there is no sustained inflammatory cell infiltration in noninfarcted related artery-supplied regions.
DISCUSSION
The principal findings from this work, compared with in situ infarcted “working heart” model, are fourfold: 1) the postinfarction healing process during LV mechanical unloading is characterized by improved infarct size and considerable myocardial salvage in the ischemic region; 2) in the infarct scar, collagen remodeling is featured by disorganization of collagen alignment and delayed maturation; 2) the healing process is accompanied by aberrantly enhanced neovascularization, lymphangiogenesis, and lymphedema and a subsequently delayed resolution of inflammation; and 4) LV unloading per se induces enhanced angiogenic and fibrotic responses in the noninfarcted area.
LV mechanical unloading, heart rate reduction, and myocardial salvage.
The first work concerning the impact of LV unloading on postinfarction healing was done by Suzuki and colleagues (45) and showed a >50% reduction in infarct size in a mouse isogenic heterotopic HTx model. They attributed this finding to enhanced cardiac self-regeneration during LV unloading as demonstrated by the increased existence of c-Kit and Sca-1-positive cells in infarcted regions. To our knowledge, this bulk mass of surviving myocardium has never been reported in the published literature. Whether or not the existence of massive myocardium in the infarcted region is due to myocardial regeneration warrants future elegantly designed studies to confirm. In our opinion, this phenomenon is not likely the result of myocardial regeneration but a consequence of myocardial salvage.
An important contributor for myocardial salvage in this work, compared with the in situ infarcted heart, is decreased donor heart rate, which could be ascribed to a decrease of innervation in the transplanted heart and an increased ratio of sympathetic over parasympathetic tone in in vivo mice (16, 33). Moreover, reduced venous return in heterotopic HTx also contributes to the decreased heart rate (referred as the “Bainbridge reflex”) by affecting stretch-induced modulation of sinoatrial node automaticity (33).
Heart rate reduction plays important roles in different phases during infarction healing. Early after MI, heart rate reduction- and mechanical unloading-induced downregulation of O2 consumption could limit or even reuse myocardial necrosis/apoptosis in adjacent regions to the infarct center. In addition, as coronary collateral flow is a purely diastolic phenomenon, prolongation of diastole by heart rate reduction could augment collateral circulation flow and facilitate the opening of recruitable (immature) collaterals. This process could be further accentuate when the LV is unloaded by reducing wall stress transmission-induced compression to intramyocardial collateral vessels (5). In the granulation phase of infarct healing, our results are in agreement with previous work showing that heart rate reduction could enhance postinfarction angiogenesis by upregulating proangiogenic factors (28, 30, 56). Moreover, pharmacologically induced chronic heart rate reduction could give rise to a dramatic improvement of infarct size and myocardial survival in rats (55). Taken together, from the very beginning until the chronic phase after MI, LV mechanical unloading and heart rate reduction could be the key mechanisms underlying the massive myocardial salvage observed in this work.
Impact of LV mechanical unloading on collagen remodeling and organization.
In the infarcted area, it has been suggested that the local pattern of stretching in the LV cavity determines the collagen alignment in healing myocardial infarct scars (13). Therefore, it is conceivable that in the hemodynamically unloaded state, the collagen fibers are wavy and loosely packed. Specifically, collagen orientation in the T-infarct group lost their preferred orientation progressively, which, if occurs in situ, should be in parallel with the preexisting matrix scaffold and perpendicular to the direction of wall stress, theoretically. In addition, the majority of the infarcted area during unloading is composed of thinner collagen fibers (green-yellow fibers) compared with in situ infarcted hearts, indicating a delayed collagen cross-linking process (delayed scar maturation). Although the effect of mechanical and physical forces on collagen cross-linking is poorly understood, our results support those of a previously published report (34) showing that mechanical load (strains) determine the degree of collagen cross-linking in the infarcted region.
In the T-sham group and in noninfarcted areas of the T-infarct group, we observed a pronounced upregulation of angiogenic and fibrogenic responses. The HTx procedure could inevitably result in reperfusion injury to the donor heart, which was confirmed by macrophage infiltration early after unloading (3 and 7 days postoperation). However, the lack of a temporal coincidence between inflammatory cell infiltration and angiogenesis and fibrosis suggests that these two processes are independent. Thus, increased interstitial fibrosis in these regions was predominantly influenced by mechanical unloading. With regard to the impact of mechanical unloading on cardiac extracellular matrix deposition, the results from human LV assist device and murine isogenic HTx studies on cardiac fibrosis are still conflicting (8, 9, 23, 24, 35, 42). Results from our work and a recent report (8) using biopsies of LV assist device-implanted patients showed that LV unloading significantly increased the interstitial collagen deposition and was accompanied by increased microvascular density, supporting the hypothesis that unloading per se could enhance the myocardial fibrogenic response. In addition, the observation that phenotypically transformed fibroblasts (myofibroblasts) are mainly located adjacent to the vessel wall in the T groups bolsters the interpretation that cardiac fibrosis is associated with the infiltration of fibroblasts originated from endothelial cells via the endothelial-mesenchymal transition (54) and that this process is augmented during LV unloading.
Delayed resolution of inflammation in the infarcted region during LV unloading.
The third finding of this work, that infarct healing during unloading is characterized by a delayed resolution of inflammation, may be related to morphological and functional changes of cardiac lymphatics. First, the role of epicardial and intracardiac lymphatic cells should be address. It is well established that the cardiac lymphatic system provides an exit route for extravasated inflammatory cells, whereas the epicardial lymphatic connection to adjacent to the lymph node is completely destroyed during HTx. Early in this situation, drainage of lymph and inflammatory cells are dependent on the epicardial transudation and resorption of the lymph that drains blindly from the disrupted myocardial lymphatic system into the peritoneal cavity (17, 44). Evidence has shown that even 1 day posttransplantation, traffic of antigen-presenting cells from cardiac transplants to the spleen can occur (29). However, with regard to the chronic phase, to our knowledge, there are no available data concerning the time course of reestablishment of the epicardial lymphatic system to regional lymph nodes after HTx.
Intracardiac lymphatic functions are closely associated with the extracellular matrix (48). Lymphatic capillaries have discontinuous junctions, which are connected to the surrounding collagen fibers by anchoring filaments. These structural bases render the opening of the lymphatic vessel lumen in the face of interstitial edema and collapse under conditions of well-organized packing collagen fibers. Therefore, a disorganized collagen network may serve as maintaining factor for lymphatic lumen opening. Another possible factor influencing lymphatic function in the present work is the upregulation of VEGF levels. In addition to their well-known effect on angiogenesis and lymphangiogenesis, these factors are potent vasodilator and vascular permeablilizing agents, contributing to the leakage of fluid and protein from the vasculature to the interstitium (2). These effects could further increase interstitial pressure and result in lymphatic lumen opening and lymphedema. Furthermore, it has been suggested that the cyclic change of LV pressure plays an important role in lymphatic drainage (50), as shown by the fact that epicardial lymph pressure (an indicator of interstitial volume) is dependent on ventricular pressure (18, 49). Therefore, LV mechanical unloading may have a direct negative impact on lymphatic drainage.
Lymphatic function is important for the clearance of inflammatory cells. It is generally accepted that the removal of neutrophils in the inflamed site is dependent on apoptosis, with the cellular debris and neutrophils further engulfed by infiltrated macrophages. Macrophages then exit the inflamed region via lymphatic vessels. Thus, it is reasonable to deduce that impaired lymphatic drainage could result in the prolonged retention of inflammatory cells and, consequently, the delayed resolution of inflammation, which is supported by the evidence of decreased inflammatory cell apoptosis, upregulation of MPO expression, increased macrophage infiltration, and MMP-9 activity as well as the active remodeling of the infarct scar (upregulation of TGF-β1 and massive infiltration of myofibroblasts) in T-infarct group.
Study limitations.
First, it is well known that sympathetic sprouting is important for infarct healing and scar formation, especially in angiogenesis and lymphangiogenesis (3, 4, 20), whereas the murine HTx preparation is devoid of innervation and lacks communication with cervical ganglions and the central nervous system. Therefore, its potential impact on the inflammatory response and on cardiac neural stem cell function (10) during LV unloading cannot be clarified in the present work. This is a critical limitation of our work. Second, in the T groups, the surgical procedure inevitably brings reperfusion injury to the heart. Although the cold ischemic time during transplantation is generally accepted and we performed related pathological analysis showing that there no temporal coincidence between macrophage infiltration and the enhanced fibrotic response, we still cannot totally rule out its potential impact. Third, the histological difference of infarct segments between in situ (almost uniformly occupied by scar tissue) and transplanted hearts (scattered distribution of scar tissue) warrants an elegantly designed research protocol and methods to quantify infarct size and molecular events in scar tissue. To address the first concern, traditionally used area- and length-based methods are both not suitable (47). Thus, we used the fraction of fibrosis in infarcted segments as a supplemental method. For the second issue, laser microdissection-based analysis is required, which could be a direction of our future work.
Implications.
The striking differences between the in situ and LV unloaded postinfarction healing processes make it unreasonable to use murine cardiac isografts to assess the efficacy of drugs and/or interventions targeting the postinfarction healing process. Care is needed in the interpretation of previous studies using this model to investigate the efficacy and mechanisms of stem cell therapy (1, 6) because the delayed resolution of inflammation provides a hostile microenvironment for stem cell survival in the damaged myocardium (41) and renders cell fusion (21, 37).
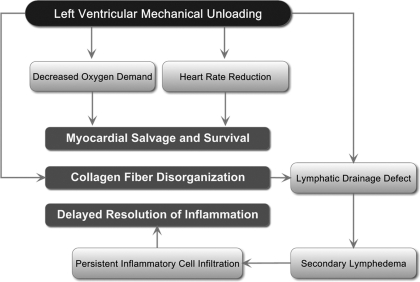
In summary, our work demonstrated an altered postinfarction healing process in the LV mechanically unloaded rat heart, which was characterized by considerable myocardial salvage, disorganization of collagen fiber alignment, delayed maturation in scar tissue, delayed resolution of inflammation, and, finally, enhanced angiogenic and fibrotic responses in the noninfarcted area (Fig. 9). Our work could provide novel insights into the role of hemodynamic load in the postinfarction healing process. Further studies are warranted to elucidate its potential mechanism.
Fig. 9.
Proposed mechanisms and summary of postinfarction healing responses in the mechanically unloaded LV.
GRANTS
This work was supported by National Natural Science Foundation of China Grants 30740010, 30871072, and 81070121, Tianjin Municipal Science and Technology Committee Grants 09ZCZDSF04200 and 09JCZDJC19500, and an intramural research program from Medical College of Chinese People's Armed Police Forces (WKH2009Z01). The authors gratefully acknowledge that we were licensed for the use of Continuity 6.3b software from National Biomedical Computation Resources (National Institutes of Health Grant P41-RR-08605), Cardiac Mechanics Research Group, University of California (San Diego, CA).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ausoni S, Zaglia T, Dedja A, Di Lisi R, Seveso M, Ancona E, Thiene G, Cozzi E, Schiaffino S. Host-derived circulating cells do not significantly contribute to cardiac regeneration in heterotopic rat heart transplants. Cardiovasc Res 68: 394–404, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 87: 262–271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damon DH. Sympathetic innervation promotes vascular smooth muscle differentiation. Am J Physiol Heart Circ Physiol 288: H2785–H2791, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296: H293–H302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Marchi SF, Oswald P, Windecker S, Meier B, Seiler C. Reciprocal relationship between left ventricular filling pressure and the recruitable human coronary collateral circulation. Eur Heart J 26: 558–566, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Dedja A, Zaglia T, Dall'Olmo L, Chioato T, Thiene G, Fabris L, Ancona E, Schiaffino S, Ausoni S, Cozzi E. Hybrid cardiomyocytes derived by cell fusion in heterotopic cardiac xenografts. FASEB J 20: 2534–2536, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Delyani JA, Robinson EL, Rudolph AE. Effect of a selective aldosterone receptor antagonist in myocardial infarction. Am J Physiol Heart Circ Physiol 281: H647–H654, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, Whitehead KJ, Salama ME, Selzman CH, Stehlik J, Clayson SE, Bristow MR, Renlund DG, Li DY. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol 56: 382–391, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drakos SG, Terrovitis JV, Anastasiou-Nana MI, Nanas JN. Reverse remodeling during long-term mechanical unloading of the left ventricle. J Mol Cell Cardiol 43: 231–242, 2007 [DOI] [PubMed] [Google Scholar]
- 10.El-Helou V, Beguin PC, Assimakopoulos J, Clement R, Gosselin H, Brugada R, Aumont A, Biernaskie J, Villeneuve L, Leung TK, Fernandes KJ, Calderone A. The rat heart contains a neural stem cell population; role in sympathetic sprouting and angiogenesis. J Mol Cell Cardiol 45: 694–702, 2008 [DOI] [PubMed] [Google Scholar]
- 11.El Kebir D, Jozsef L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ Res 103: 352–359, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Flanders KC, Major CD, Arabshahi A, Aburime EE, Okada MH, Fujii M, Blalock TD, Schultz GS, Sowers A, Anzano MA, Mitchell JB, Russo A, Roberts AB. Interference with transforming growth factor-β/Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol 163: 2247–2257, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fomovsky GM, Holmes JW. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am J Physiol Heart Circ Physiol 298: H221–H228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 13: 1877–1893, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res 81: 474–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol 279: H733–H740, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Geissler HJ, Dashkevich A, Fischer UM, Fries JW, Kuhn-Regnier F, Addicks K, Mehlhorn U, Bloch W. First year changes of myocardial lymphatic endothelial markers in heart transplant recipients. Eur J Cardiothorac Surg 29: 767–771, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Vergroesen I, Spaan JA. Stopped-flow epicardial lymph pressure is affected by left ventricular pressure in anesthetized goats. Am J Physiol Heart Circ Physiol 264: H1624–H1628, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T, Visovatti SH, Hyman MC, Hayasaki T, Pinsky DJ. Heterotopic vascularized murine cardiac transplantation to study graft arteriopathy. Nat Protoc 2: 471–480, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hosaka K, Rayner SE, von der Weid PY, Zhao J, Imtiaz MS, van Helden DF. Calcitonin gene-related peptide activates different signaling pathways in mesenteric lymphatics of guinea pigs. Am J Physiol Heart Circ Physiol 290: H813–H822, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Johansson CB, Youssef S, Koleckar K, Holbrook C, Doyonnas R, Corbel SY, Steinman L, Rossi FM, Blau HM. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol 10: 575–583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jugdutt BI. Pleiotropic effects of cardiac drugs on healing post-MI. The good, bad, and ugly. Heart Fail Rev 13: 439–452, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Klotz S, Foronjy RF, Dickstein ML, Gu A, Garrelds IM, Danser AH, Oz MC, D'Armiento J, Burkhoff D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation 112: 364–374, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Klotz S, Jan Danser AH, Burkhoff D. Impact of left ventricular assist device (LVAD) support on the cardiac reverse remodeling process. Prog Biophys Mol Biol 97: 479–496, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 114: I167–I173, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kutschka I, Chen IY, Kofidis T, von Degenfeld G, Sheikh AY, Hendry SL, Hoyt G, Pearl J, Blau HM, Gambhir SS, Robbins RC. In vivo optical bioluminescence imaging of collagen-supported cardiac cell grafts. J Heart Lung Transplant 26: 273–280, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kutschka I, Kofidis T, Chen IY, vonDegenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation 114: I174–I180, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Lamping KG, Zheng W, Xing D, Christensen LP, Martins J, Tomanek RJ. Bradycardia stimulates vascular growth during gradual coronary occlusion. Arterioscler Thromb Vasc Biol 25: 2122–2127, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med 171: 307–314, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei L, Zhou R, Zheng W, Christensen LP, Weiss RM, Tomanek RJ. Bradycardia induces angiogenesis, increases coronary reserve, and preserves function of the postinfarcted heart. Circulation 110: 796–802, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Li XH, Zhou X, Zeng S, Ye F, Yun JL, Huang TG, Li H, Li YM. Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron Artery Dis 19: 363–370, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Liu YH, Yang XP, Nass O, Sabbah HN, Peterson E, Carretero OA. Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. Am J Physiol Heart Circ Physiol 272: H722–H727, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev 88: 919–982, 2008 [DOI] [PubMed] [Google Scholar]
- 34.McCormick RJ, Musch TI, Bergman BC, Thomas DP. Regional differences in LV collagen accumulation and mature cross-linking after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 266: H354–H359, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Muranaka H, Marui A, Tsukashita M, Wang J, Nakano J, Ikeda T, Sakata R. Prolonged mechanical unloading preserves myocardial contractility but impairs relaxation in rat heart of dilated cardiomyopathy accompanied by myocardial stiffness and apoptosis. J Thorac Cardiovasc Surg 140: 916–922, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol 25: 1102–1111, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Nygren JM, Liuba K, Breitbach M, Stott S, Thoren L, Roell W, Geisen C, Sasse P, Kirik D, Bjorklund A, Nerlov C, Fleischmann BK, Jovinge S, Jacobsen SE. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol 10: 584–592, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg 57: 225–229, 1969 [PubMed] [Google Scholar]
- 39.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990 [DOI] [PubMed] [Google Scholar]
- 40.Rich L, Whittaker P. Collagen and picrosirius red staining : a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci 22: 1–12, 2005 [Google Scholar]
- 41.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature 451: 937–942, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Soppa GK, Barton PJ, Terracciano CM, Yacoub MH. Left ventricular assist device-induced molecular changes in the failing myocardium. Curr Opin Cardiol 23: 206–218, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Stewart RH, Rohn DA, Allen SJ, Laine GA. Basic determinants of epicardial transudation. Am J Physiol Heart Circ Physiol 273: H1408–H1414, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki R, Li TS, Mikamo A, Takahashi M, Ohshima M, Kubo M, Ito H, Hamano K. The reduction of hemodynamic loading assists self-regeneration of the injured heart by increasing cell proliferation, inhibiting cell apoptosis, and inducing stem-cell recruitment. J Thorac Cardiovasc Surg 133: 1051–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 112: I166–172, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Takagawa J, Zhang Y, Wong ML, Sievers RE, Kapasi NK, Wang Y, Yeghiazarians Y, Lee RJ, Grossman W, Springer ML. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102: 2104–2111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140: 460–476, 2010 [DOI] [PubMed] [Google Scholar]
- 49.VanTeeffelen JW, Merkus D, Bos LJ, Vergroesen I, Spaan JA. Impairment of contraction increases sensitivity of epicardial lymph pressure for left ventricular pressure. Am J Physiol Heart Circ Physiol 274: H187–H192, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev 86: 1263–1308, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation 84: 2123–2134, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 89: 397–410, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Wilting J, Becker J, Buttler K, Weich HA. Lymphatics and inflammation. Curr Med Chem 16: 4581–4592, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Zhang RL, Christensen LP, Tomanek RJ. Chronic heart rate reduction facilitates cardiomyocyte survival after myocardial infarction. Anat Rec (Hoboken) 293: 839–848, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Zheng W, Brown MD, Brock TA, Bjercke RJ, Tomanek RJ. Bradycardia-induced coronary angiogenesis is dependent on vascular endothelial growth factor. Circ Res 85: 192–198, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Zhou X, Li YM, Ji WJ, Jiang TM, Sun XN, Zhu Y, Shi R. Phenytoin can accelerate the healing process after experimental myocardial infarction? Int J Cardiol 107: 21–29, 2006 [DOI] [PubMed] [Google Scholar]