Abstract
MicroRNAs are evolutionarily conserved, small (~20–25 nucleotides), single-stranded molecules that suppress the expression of protein-coding genes by translational repression, messenger RNA degradation, or both. More than 700 microRNAs have been identified in the human genome. Amazingly, a single miRNA can regulate the expression of hundreds of mRNAs/proteins within a cell. The small RNAs are fast emerging as master regulators of innate and adaptive immunity, and very likely to play a pivotal role in transplantation. The clinical application of RNA sequencing (“next – generation sequencing”) should facilitate transcriptome profiling at an unprecedented resolution. We provide an overview of microRNA biology and their hypothesized roles in transplantation.
Keywords: Transplantation, Biomarker, microRNA, Immune Monitoring, Diagnosis
INTRODUCTION
MicroRNAs (miRNAs) are a recently discovered class of non-coding RNAs thought to play a critical role in cell biology by regulating gene expression at the post-transcriptional level. miRNAs are evolutionarily conserved, small (~20–25 nucleotides), single-stranded molecules that suppress the expression of protein-coding genes by directing translational repression, mRNA degradation, or both (1). miRNAs mediate their regulatory action through imperfect binding to the 3’ untranslated region of target mRNAs carrying the complementary sites.
lin-4 and let-7 were the first two miRNAs to be discovered in caenorhabditis elegans and more than 700 different miRNAs have now been identified in the human genome (miRBase, Release 14.0, http://www.mirbase.org) (2–4). A single miRNA can regulate hundreds of functional targets within a single cell type (5, 6). Moreover, each mRNA can be targeted by multiple miRNAs which can interact with each other by synergism or competition. It is now considered that most protein-coding genes are regulated by miRNAs, and it has been demonstrated that the miRNAs play an important role in many diverse biological processes such as development (reviewed in (7), cell proliferation, differentiation, apoptosis, fat metabolism and oncogenesis (8). The discovery of RNA interference, a widely conserved mechanism for post-transcriptional gene regulation, holds considerable promise for improving not only our understanding of normal physiology but also several disease states. Indeed, dysregulated expression of miRNAs has already been associated with several cancers (9).
miRNAs can be encoded in independent transcription units, in polycistronic clusters or within the introns of protein-coding genes (10). The expression of miRNAs is initially controlled at the transcriptional level by transcription factors that regulate the production of miRNA-containing primary transcripts in specific cell types during development or in response to different environmental signals. Mature miRNAs are produced from long primary transcripts through a series of endonucleolytic maturation steps. In the canonical pathway of miRNA biogenesis, miRNA is transcribed mainly by RNA polymerase II, which produces a long primary transcript (Pri-miRNA) containing the mature miRNA sequence and a varying amount of flanking region. Pri-miRNA transcripts are then processed into corresponding precursor miRNA (pre-miRNA) stem-loops of ~60 nucleotides in length by the Drosha nuclease. This pre-miRNAs are then actively transported to the cytoplasm by Exportin-5 and are further processed into ~22-nucleotide duplexes by the cytoplasmic RNase III enzyme Dicer. The functional miRNA strand is then selectively loaded into the RNA-induced silencing complex (RISC).
The first miRNA, lin-4 was characterized using the northern blot method and a number of detection methods including microarray based analysis, quantitative PCR assays and next-generation sequencing have been developed for miRNA profiling (11). Northern blot analysis is a robust technique but its limitations include low throughput and limited sensitivity for detecting miRNAs. Higher throughputs platforms include massively parallel sequencing (‘next-generation sequencing’), multiplex bead-based flow cytometry, microarrays tailored for miRNA detection, and quantitative PCR-based approach using low density arrays. Next-generation sequencing (deep sequencing) technologies have opened the door to detecting and profiling known and novel miRNAs at unprecedented resolution (11, 12). This new massively parallel sequencing technology enables hundreds of thousands of short sequencing tags to be generated in one run. In addition, deep sequencing strategy offers exquisite sensitivity and the additional benefit of discovery of new small RNA species. However, these technologies present formidable computational challenges and remain expensive. Multiplex bead-based flow cytometry, microarrays and quantitative PCR-based low density arrays are highly efficient in detecting a defined set of known miRNA sequences in a rapid and simple manner using commercially available kits. However, they have incomplete coverage of known miRNAs and can only measure previously identified miRNAs and are therefore not equipped to discover and characterise miRNAs of unknown sequence. A useful approach might be to use deep sequencing as a discovery tool and apply quantitative PCR assays as the validation technology. Additionally, in situ hybridization methods for miRNA detection have been developed and can be used to determine the spatio-temporal expression patterns of candidate miRNAs. Finally, the identification of the function of candidate miRNAs involves functional analysis including up-regulation of the miRNAs to identify gain-of-function phenotypes and down-regulation or inhibition to identify loss-of-function phenotypes. The combination of up- and down-regulation can be used to identify genes that are regulated by specific miRNAs as well as to identify cellular processes that are affected by specific miRNAs.
In this overview, we focus on the miRNAs considered to influence both the development and function of cells participating in innate immunity and adaptive immunity, and summarize emerging data regarding miRNA expression patterns predictive of human allograft status.
MICRORNAS AND THE IMMUNE RESPONSE
MicroRNAs with demonstrated participation in hematopoiesis and immune regulation are summarized in Table 1, and illustrated in Figure 1. miRNAs have unique expression patterns in cells of the innate and adaptive immune responses and play pivotal role in their development, maturation, and function (reviewed in (13–18). Though the impact of miRNAs in models of alloimmunity has not been studied in detail, the exponential growth of the body of knowledge about miRNAs as immune regulators is likely to stimulate such studies in the near future.
Table 1.
| MicroRNA | Target Gene(s) | Function and Process Regulated |
|---|---|---|
| let-7e | TLR4 | Regulation of innate immune response |
| miR-9 | NFKB1 | Regulation of response to inflammation |
| miR-16 | TNF-α | Binding to ARE motifs of 3’ UTR ; induces the degradation of TNF-α |
| miR-17-5p | RUNX1 | Inhibition of proliferation, differentiation and maturation of monocytes |
| miR-17~92 (cluster) | BIM, PTEN | Regulation of the pro- to pre- transition during development of B and T cells ; promotes B cell and T cells survival ; |
| miR-20a | AML-1 | Inhibition of monocytes proliferation, differentiation and maturation |
| miR-21 |
PTEN, PDCD4, IL12A |
Upregulated in macrophage in response to inflammation ; induced by TLRs ; negatively regulates macrophage activation |
| miR-32 | PFV1, ORF2 | Inhibition of PFV1 replication by targeting viral genome |
| miR-34 |
JAG1, WNT1, FOXP1 |
Regulates myeloid-derived DC differentiation |
| miR-106a | AML-1 | Inhibition of proliferation, differentiation and maturation of monocytes |
| miR-122 | - | Required for HCV replication |
| miR-121/miR-122 | KIT | Regulate proliferation of stem cells and progenitor cells |
| miR-125b | TNF- α | Decreases during response to inflammation thus allowing TNF- α production |
| miR-126 | HOXA9, PLK2 | Promotes expansion of progenitor cells |
| miR-132 | - | Regulates immune response to bacterial infections; involved in the CREB signaling |
| miR-142 | AC9 | Is repressed by FOXP3, leading to increased production of cAMP and suppressor functions of Treg cells |
| miR-146a |
TRAF6, IRAK1, IRAK2 |
Increased expression in macrophages and epithelial cells in response to TLR-2, -4 et –5 activation or exposition to TNF- α or IL-10 ; regulates the immune response to bacterial infections |
| miR-150 | Myb | Regulates mature B cells production; regulates the pro- to pre-B transition; regulates T cell activation |
| miR-155 |
PU.1, MAF, SHIP1, AID, SOCS1, BACH1, CEBPB, CSFR, TAB2, JARID2 |
Regulates immune response to bacterial/viral infections ; induced by TLR signaling ; regulates TNF- α ; required for normal lymphocyte functions, response of the germinal centre, class switching, plasmacyte generation; Th1/Th2 polarization, Treg cell thymic development, granulocyte and monocyte proliferation |
| mir-181a |
DUSP5, DUSP6, SHP2, PTPN22, BCL-2, CD69 |
Regulates the development of B and T cells ; modulates the sensitivity of T lymphocytes to antigenes by regulating expression levels of various phosphatases from the TCR signaling |
| MiR-181b | AID | Involved in B cell class switching |
| miR-196b | HOX family | functional role in modulating hematopoietic stem cell homeostasis and lineage commitment |
| miR-223 | MEF2C | Granulopoiesis regulation |
| miR-326 | ETS1 | Promotes TH17 cells development |
| miR-424 | NFIA, SPI1 | Involved in monocyte differentiation and maturation |
Figure 1. A Schema of microRNA Participation in hematopoiesis and immune cell development and function.
DC, dendritic cell; GMP, granulocyte-macrophage progenitor; MEP, megakaryocyte-erythrocyte progenitor; RBC, red blood cell; DN, double-negative T cell; DP, double positive T cell; SP, single-positive T cell; TH-1, T helper type 1 cell; TH-2, T helper type 2 cell; TH-17, interleukin 17-producing helper T cell; Treg, regulatory T cell; Pro-B, pro–B cell; B-1, B-1 type B cell; B-2, B-2 type ‘conventional’ B cell; NK, natural killer; NKT, natural killer T; Pre-B, pre–B cell; pDC, plasmacytoid dendritic cell. (Figure was produced using Servier Medical Art).
MICRORNAS AND INNATE IMMUNITY
miRNAs involved in the regulation of granulocytes, monocytes, macrophages, dendritic cells, NK and NKT cells have been identified. miR-21 and miR-196b are involved in the regulation of neutrophil development; miR-223 is considered a master regulator of neutrophil function and is induced by the myeloid transcription factors PU.1 and CCAAT/enhancer-binding-protein-β (C/EBPβ), and negatively regulates both the proliferation and activation of neutrophils.
The miR-17~92 cluster has been implicated in monocyte differentiation and maturation. Several studies have evaluated the response of macrophages to inflammation/infection and identified TLR - induced miRNAs including miR-146, miR-9, miR-147, miR-21, and miR-155. It has been suggested that many of the miRNAs induced by TLRs negatively regulate the activation of inflammatory pathways in myeloid cells. For example, miR-146 inhibits pro-inflammatory signaling molecules (e.g. IL-1R-associated kinase 1 [IRAK1], IRAK2, and TNFR-associated factor 6 [TRAF6]), miR-9 represses NF-kB subunit 1 (NFKB1), and miR-155 inhibits the expression of pro-inflammatory proteins (Table 1).
MICRORNA AND ADAPTIVE IMMUNITY
miRNAs have also been identified as key regulators of B- and T-cell differentiation, regulatory T (Treg) cell function and antigen signaling. The first evidences of the involvement of miRNAs in T cells homeostasis was provided by T-cell specific deletion of Dicer, which revealed a non-redundant requirement for miRNAs for T cell maturation and function. The miR-17~92 cluster has also been identified as a key regulator of T cell proliferation. This cluster impairs the expression of mRNAs encoding pro-apoptotic proteins (Table 1), and thereby increases T cell survival during development. Indeed, mice expressing high level of miR-17~92 display lymphoproliferative diseases and auto-immunity. miR-181a, which is increased during early T cell development, enhances TCR signaling strength by targeting phosphatases (Table 1).
miRNAs have also been implicated in T helper cells polarization. miR-155 promotes T helper 2 (TH-2)-cell differentiation, while miR-326 promotes TH-17 cell development. The development of mice models of targeted deletion of Dicer or Drosha in Treg cells have revealed the critical role of miRNAs in the stable functioning of Treg cells, the deficient mice developing fatal auto-immune diseases. Using this model, it was shown also that miR-155 is critical for Treg cell homeostasis and survival.
miRNAs are also critically implicated in B-cell maturation and function. Whereas miRNA-181 over-expression induces a specific B-cell development program, a targeted deletion of Dicer in B-cells results in a complete block in B-cell development. Ablation of Dicer in B cell progenitors resulted in a block at the pre- to pro-B-cell transition, an effect that linked to the dysregulation of the pro-apoptotic protein BIM, a target of the miR-17~92 cluster. Constitutive expression of miR-150 induced a block at an early stage of B-cell development and miR-150-deficient mice have increased levels of antibody secretion both at baseline and following T-cell-dependent antigenic stimulation. miR-155 is well-characterized as a critical miRNA for B cell homeostasis and miRNA-155 deficient mice have defective antibody class switching and differentiation into plasma cells, resulting in impaired humoral response.
MICRORNA BIOGENESIS AND INFLAMMATION
Inflammation has been reported to regulate miRNA biogenesis (10, 18, 19), and TLR ligands, antigens, or cytokines can modulate miRNA expression level through regulation of specific transcription factors. Phorbol myristic acid (PMA) induces miR-21 in HL-60 cell line in an AP1 dependent manner (20), the TLR4 ligand lipopolysaccharide (LPS) induces many miRNAs in the human monocyte cell line THP1, in particular miR-155, miR-146 and miR-132, and interleukin 1β0323 induces miR-146a (21). We have shown that the polyclonal mitogen PHA induces miR-155 in normal human peripheral blood cells (22). Interestingly, miR-155 can also be induced by the TLR3 ligand poly-inositol-cytidine (poly [I: C]), and also by interferon-β0323 and TNF-α. Cytokines such as interferon-β has been shown to inhibit Dicer expression, decreasing the processing of pre-miRNAs, whereas interferon-γ induces processing (23).
MICRORNA, INFLAMMATION AND EPITHELIAL CHANGES
During acute rejection, the resident graft parenchymal cells undergo phenotypic as well as functional changes. For example, during acute rejection of the renal allograft, the tubular epithelial cells acquire an activated phenotype and secrete cytokines, chemokines, and display various cell surface molecules. The molecular mechanisms for the phenotypic changes and the gain or loss of function are largely unknown. Existing data are consistent with the hypothesis that miRNAs target key components of signaling pathways in the epithelial cells and alter their cell surface phenotype and functional attributes (24). IL1-β stimulation of human lung alveolar epithelial cells has been shown to result in a rapid time and concentration-dependent increase in miR-146a (25). Transfection of these cells with miR-146 precursor prior to IL1-β exposure decreases the release of chemokines CXCL8 (IL-8) and CCL5 (RANTES) indicating that miR-146 is likely to be involved in a negative feed back loop serving to down regulate inflammation during the innate immune response (25).
miRNAs have also been shown to regulate the expression of cell surface proteins implicated in immunoregulation. B7-H1, a member of the B7 costimulatory molecule family, is critical in regulating cell-mediated immune responses including epithelial-T cell interactions (26), and has been found to be upregulated in renal tubular epithelial cells during acute rejection of the renal allograft (27). miR-513 has been shown to regulate B7-H1 translation and to be involved in IFNγ-induced B7-H1 expression in cholangiocytes (26). In a model of human biliary epithelial cells, transfection of miR-513 precursor decreased IFNγ-induced B7-H1 expression, demonstrating that miR-513 down-regulation is key to the IFNγ-induced B7-H1 induction. Moreover, transfection of biliary epithelial cells with the miR-513 precursor inhibited B7-H1-associated apoptotic cell death in co-cultured human T cells, demonstrating the functional significance of miR-513 in biliary epithelial cell-T cell interactions during an immune response (24, 26).
Our analysis of miRNA expression profiles of renal allograft biopsies revealed that a number of tubular epithelial cell miRNAs are under-expressed during acute cellular rejection (22). We have also found that pro-inflammatory cytokines down-regulate miR-30a in primary cultures of human renal epithelial cells (22). It would be of interest to determine whether the inflammation associated alterations in miRNA expression levels in resident graft parenchymal cells are significant contributors to the tissue injury.
MICRORNA AND FIBROSIS
Emerging data suggest that miRNAs participate in the regulation of fibrosis process (28). Inflammation related to a sustained alloimmune injury is a well identified factor for allograft fibrosis. The key cellular mediator of fibrosis is the myofibroblast, which when activated serves as the primary collagen-producing cell. In kidney tissue, activated myofibroblasts are thought to be derived from multiple sources including the activation of local fibroblasts and the epithelial cells in a process called epithelial-mesenchymal transition (EMT). EMT is a phenomenon whereby a number of facilitating factors induce tubular cells to lose their epithelial phenotype, express mesenchymal markers, acquire migratory and invasiveness potential, and secrete ECM.
Several recent studies have identified the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and miR-149) and miR-205 as key regulators of EMT. These miRNAs were shown to mediate EMT in response to TGFβ1 in Madin–Darby canine kidney (MDCK) cells. The miR-200 family participates in a signaling network with the E-cadherin transcription repressors ZEB1/δEF1 and ZEB2/SIP1. Down-regulation of these miRNAs relieves their cooperative repression of the mesenchymal transcription factors ZEB1 and SIP1 that, in turn, are free to inhibit E-cadherin expression promoting epithelial dedifferentiation (29, 30). Interestingly, we found that several members of the miR-200 family (i.e. miR-200a, miR-141 et miR-429) were under-expressed in biopsy samples of human renal allograft with acute T cell mediated rejection as compared to normal renal allografts (22).
miRNAs may also be involved in the fibrogenic process of organs in addition to kidneys. Altered expression of miRNAs have been reported in myocardial fibrosis (31) whereas miRNA-133a has been found to protect against myocardial fibrosis (32). In the liver, the participation of miRNAs in the activation of hepatic stellate cells, a major driver for liver fibrosis, has been advanced (33).
Additional miRNAs that could play a role in fibrogenesis have also been identified with the use of animal model of diabetic nephropathy. Kato et al demonstrated that miR-192 expression is enhanced by TGFβ1 in mesangial cells in vitro and in vivo in the glomeruli from diabetic mice (34). miR-192 was shown to target S1P1 (E-box repressor Smad-1 interacting protein), a regulatory protein that binds to promoter regions of Col1a2. Consequently, the repression of S1P1 by miR-192 increases collagen synthesis in response to TGFβ1. The same group recently reported the critical participation of miRNAs in the regulation of Akt by TGFβ1 (35). Indeed, it has been shown that TGFβ1 activates Akt in mesangial cells by inducing miR-216a and miR-217, the two miRNAs that target PTEN (phosphatase and tensin homologue), an inhibitor of Akt activation. These two miRNAs are localized in the second intron from a non-coding gene whose promoter is activated by TGFβ1 and miR-192 (34). Together, these studies highlighted a new mechanism of Akt activation due to miRNA-induced PTEN repression. Wang et al showed that miR-377 expression is increased in several models of diabetic nephropathy and in mesangial cells exposed to high levels glucose or TGFβ1 (36). The stable expression of miR-377 in mesangial cells increased fibronectin expression.
MICRORNA AS BIOMARKERS OF ALLOGRAFT STATUS
miRNAs are stable, present in high abundance, and can be examined even in formalin-fixed tissue. They are measurable in bodily fluids such as plasma, serum and urine and all these attributes facilitate measurement of miRNAs in biologic specimens of interest and investigation of miRNA expression patterns as biomarkers of allograft status.
In our study, we utilized a two-step sequential approach to discover and validate miRNA signatures predictive of human renal allograft status. First, we ascertained global miRNA expression patterns of allograft biopsies with the use of microfluidic cards containing TaqMan® primers and probes for 365 mature human miRNAs (Training/Discovery set). Unsupervised hierarchical clustering, principal component analysis (PCA), and supervised analysis were used to determine whether miRNA levels distinguished acute rejection biopsies from allograft biopsies with normal biopsy findings. In the second step, we used the stem-loop reverse transcription (RT) TaqMan quantitative miRNA assay to measure intragraft levels of miRNAs in an independent set of allograft biopsies to test whether the miRNA levels predict allograft status (Validation set).
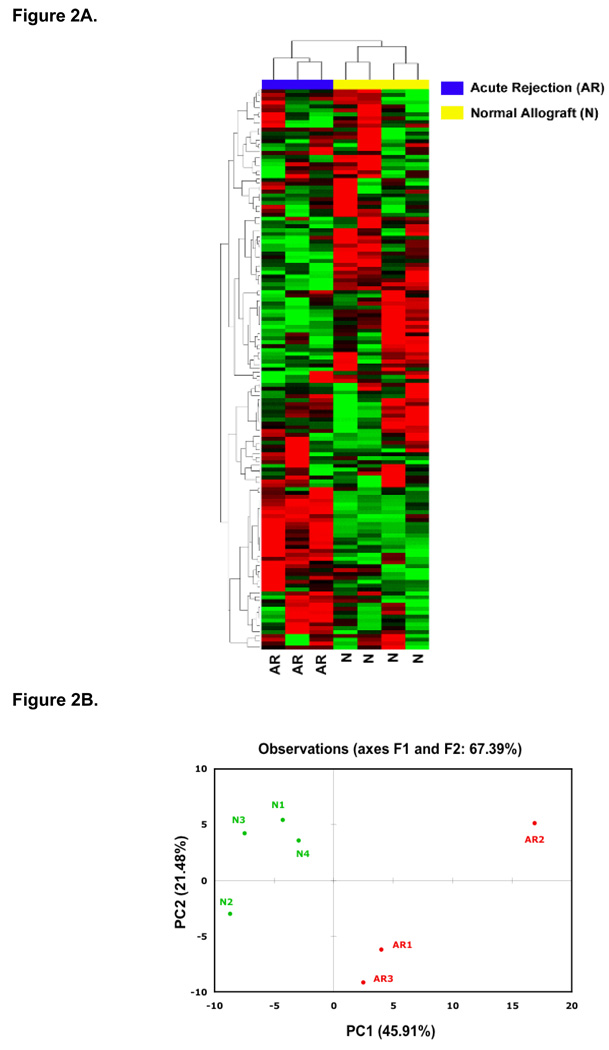
Figure 2A illustrates that unsupervised hierarchical clustering of miRNA expression patterns of human renal allografts correctly classifies the normal allograft biopsies and the biopsies showing acute rejection changes. The clear separation of acute rejection biopsies from normal allograft biopsies was further confirmed by displaying the relationships among miRNA expression patterns using principal component analysis (Fig. 2B). Supervised analysis showed that a subset of 17 miRNAs was differentially expressed at a P-value < 0.01; among the 17, ten miRNAs (let-7c, miR-10a, miR-10b, miR-125a, miR-200a, miR-30a-3p, miR-30b, miR30c, miR30e-3p, miR-32) were under-expressed in acute rejection biopsies compared to normal allograft biopsies, and 7 miRNAs (miR-142-5p, miR-142-3p, miR-155, miR-223, miR-146b, miR-146a, miR-342) were over-expressed.
Figure 2. Unsupervised hierarchical clustering and principal component analysis of miRNA expression profiles differentiate acute rejection biopsies from normal allograft biopsies of human renal allografts.
(A) MicroRNA (miRNA) expression patterns of 7 human kidney allograft biopsies (3 showing histological features of acute rejection [AR] and 4 with normal allograft biopsy results [N]) were examined using microfluidic cards containing TaqMan® probes and primer pairs for 365 human mature miRNAs. A total of 174±7 miRNAs were expressed at a significant level (i.e. CT < 35) in all samples. The biopsies were grouped by unsupervised hierarchical clustering on the basis of similarity in expression patterns. The degree of relatedness of the expression patterns in biopsy samples is represented by the dendrogram at the top of the panel. Branch lengths represent the degree of similarity between individual samples (top) or miRNA (left). Two major clusters (top) accurately divided AR biopsies from normal allograft biopsies. Each column corresponds to the expression profile of a renal allograft biopsy, and each row corresponds to a miRNA. The color in each cell reflects the level of expression of the corresponding miRNA in the corresponding sample, relative to its mean level of expression in the entire set of biopsy samples. The increasing intensities of red mean that a specific miRNA has a higher expression in the given sample and the increasing intensities of green mean that this miRNA has a lower expression. The scale (shown at bottom right) reflects miRNA abundance ratio in a given sample relative to the mean level for all samples. (B) Principal Component Analysis of seven kidney allograft biopsies based on the expression of 174 small RNAs significantly expressed (i.e. CT < 35) in all the samples. PCA is a bilinear decomposition method designed to reduce the dimensionality of multivariable systems and used for over viewing clusters within multivariate data. It transforms a number of correlated variables into a smaller number of uncorrelated variables called principal components (PC). The first PC accounts for as much of the variability in the data as possible, and each succeeding component accounts for as much of the remaining variability as possible. PCA showed evident clustering and confirmed the separation of AR samples from normal allograft biopsies. Samples were accurately grouped by PC1, which explained 45.91% of the overall miRNA expression variability, whereas PC2 explained 21.48% of variability and did not classified the samples according to their diagnosis (Reprinted with Permission from [20]).
We used the stem-loop RT TaqMan quantitative miRNA assay to measure intragraft levels of miRNA panel members in an independent set of 26 allograft biopsy specimens (9 acute rejection biopsies and 17 normal allograft biopsies) to test whether the miRNA panel predicted allograft status (Validation set). We selected 6 of 17 miRNAs for the validation study and our selection was based on their expression in immune cells and/or renal tubular epithelial cells. As observed in the training set, we found that miR-142-5p (P<0.0001), miR-155 (P<0.0001), and miR-223 (P<0.0001) were over-expressed in acute rejection biopsies in the validation set, and miR-10b (P=0.01), miR-30a-3p (P=0.04) and let-7C (P=0.08) were under-expressed. Analysis involving the receiver-operating-characteristic curve identified that acute rejection can be predicted with a high degree of accuracy with the use of intragraft levels of miR-142-5p (100 % sensitivity and 95% specificity, P<0.0001, Table 2) or miR-155 (100% sensitivity and 95% specificity, P<0.0001, Table 2). Intragraft levels of miR-223, -10b, -30a-3p and let-7c were also diagnostic of acute rejection but with a lesser level of accuracy (Table 2)
Table 2.
Diagnostic Accuracy of Intragraft miRNA Levels (22).
| Cutoff Point | % Sensitivity | % Specificity | AUC (95% CI) | P-Value | |
|---|---|---|---|---|---|
| miRNAs* | |||||
| miR-142-5p | 0.11 | 100 | 95 | 0.99 (0.96–1.02) | <0.0001 |
| miR-155 | 0.06 | 100 | 90 | 0.98 (0.94–1.01) | <0.0001 |
| miR-223 | 0.44 | 92 | 90 | 0.96 (0.90–1.02) | <0.0001 |
| miR-10b | 1.33 | 100 | 62 | 0.83 (0.69–0.97) | 0.002 |
| miR-30a-3p | 0.57 | 67 | 76 | 0.79 (0.63–0.95) | 0.007 |
| let-7c | 0.64 | 92 | 61 | 0.73 (0.55–0.92) | 0.03 |
| RNU44 | - | - | - | 0.50 (0.29–0.72) | 0.97 |
Receiver-operating-characteristic (ROC) curve analysis was used to determine the cutoff points that yielded the highest combined sensitivity and specificity. The ratio of miRNA copies to RNU44 copies were used to perform the ROC curve analysis. Analysis involving RNU44 small nucleolar RNA was performed using copies per one microgram total RNA. CI: Confidence intervals. (Adapted from [20]).
We are aware of a one other study in which miRNA expression patterns of human renal allograft were examined (37). Sui et al. studied renal allograft biopsies from 3 patients with acute rejection and 3 normal kidney cortex specimens (non-tumour renal tissue from patients with renal tumour) with the use of a microarray platform (miChip) containing 455 human miRNAs and reported that the expression pattern of 20 miRNAs (12 down-regulated and 8 upregulated) distinguished acute rejection biopsies from normal renal cortex. The miRNAs found in the Sui et al. study (37) to be informative were not predictive of allograft status in our investigation (20). The reasons for the lack of concordance between the two studies may include the fact that the comparison was made between acute rejection biopsies and normal renal cortex by Sui et al whereas acute rejection biopsies were compared with normal allograft biopsies in our study. Also, the three rejection specimens were combined and the three normal samples were also combined prior to miRNA expression profiling in the Sui et al study making it in essence a single comparison of acute rejection vs. normal renal cortex.
We have initiated miRNA profiling of renal allograft biopsies classified as IF/TA and our preliminary findings are consistent with the hypothesis that intragraft miRNA expression profiles are predictive of IF/TA.
PERSPECTIVES AND CONCLUSION
Increased understanding of the role of miRNAs in the alloimmune response and identification of miRNAs of pathogenic significance in transplantation immunobiology may offer several benefits. First, miRNAs have the potential of being excellent biomarkers of allograft status. In this regard, the revolutionary technology of deep sequencing should provide a comprehensive view of gene expression patterns and help identify and quantify rare transcripts even without prior knowledge of the gene, and facilitate detection of alternative splicing and sequence variations in the transcripts.
A second perspective is the potential for therapeutic applications. miRNA function is based on the RNA interference phenomenon. RNA interference is an RNA-dependent gene silencing process that is initiated by short double-stranded RNA molecules in a cell's cytoplasm. This double-stranded RNA can be exogenous (coming from infection by a virus with an RNA genome, or laboratory manipulations [small interfering RNA, siRNA], or therapeutic strategy) or endogenous (originating in the cell’s miRNAs). RNA interference is also one of the most promising new approaches for disease therapy (38), and clinical trials are ongoing using siRNA-targeting strategies, either delivered in situ (i.e. vascular endothelial growth factor-targeted siRNA for the treatment of macular diseases), or systemically administered via targeted nanoparticles (39). In transplantation, gene silencing strategies have been investigated to date in an attempt to reduce ischemia-reperfusion injury, by incorporating siRNA into organ storage solution (40) or by delivering systemically siRNAs in animal models of ischemia-reperfusion injury (41). Given the critical role of miRNAs in immune cells homeostasis, there is a distinct possibility that RNA interference strategies could also be developed to modulate the transplant immune repertory. Because manipulation of one miRNA may have impact on multiple mRNAs and because one mRNA may be regulated by multiple miRNAs, it is important to guard against off-target effects. Also, miRNAs, instead of causing translational repression and/or mRNA degradation, may relieve translational repression as well as promote transcription (42).
In the near future, increased understanding of the role of miRNAs in intracellular signaling, the expression of proteins involved in alloimmune responses, modulation of cytokines and chemokines, adhesion and co-stimulatory molecules, and graft response to injury should help better define the role of miRNAs in transplant immunobiology, and provide an exciting framework for developing new biomarkers as well as new therapeutic interventions in transplantation.
ACKNOWLEDGEMENT
DA is funded by the Fondation Centaure that supports a French Transplantation Research Network. MS is supported in part by awards (R37AI 51652 and RO1AI72790) from the National Institutes of Health, USA.
Abbreviations
- AC9
adenylate cyclase 9
- AID
activation-induced cytidine deaminase
- BACH1
BTB-, CNC- and bZIP-domain-containing transciption factor
- BCL2
B-cell lymphoma 2
- BIM
BCL-2-interacting mediator of cell death
- CEBPB
CCAAT/enhancer binding protein-β
- CSFR
colony-stimulating factor receptor
- DUSP
dual-specificity protein phosphatase
- ETS1
v-ets erythroblastosis virus E26 oncogene homolog 1
- FOXP1
forkhead box P1
- HOX
homeobox
- IL12A
interleukin-12A
- IRAK
IL-1R-associated kinase
- JAG1
Jagged1
- JARID2
jumonji, AT rich interactive domain 2
- MEF2C
myeloid ELF1-like factor 2C
- mRNA
messenger RNA
- miRNA
microRNA
- MYB
transcriptional activator Myb
- NFIA
nuclear factor I/A
- NFKB1
nuclear factor-kB subunit 1
- ORF2
open reading frame 2
- PDCD4
programmed cell death 4
- PFV1
primate foamy virus type 1
- PLK2
polo-like kinase 2
- PTEN
phosphatase and tensin homologue
- PTPN22
protein tyrosine phosphatase, non-receptor type 22
- PU.1
transcription factor PU.1
- RUNX1
runt-related transcription factor 1
- SHIP1
SH2-domain-containing inositol-5-phosphatase 1
- SHP2
SH2-domain-containing protein tyrosine phosphatase 2
- SOCS1
suppressor of cytokine signaling 1
- TAB2
TAK1-binding protein 2
- TLR4
Toll-like receptor 4
- TRAF6
TNFR-associated factor 6
- WNT1
wingless-type MMTV integration site family, member 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: DA, TM and MS have no conflicts of interest related to this study.
REFERENCES
- 1.Shyu AB, Wilkinson MF, van Hoof A. Messenger RNA regulation: to translate or to degrade. Embo J. 2008;27(3):471. doi: 10.1038/sj.emboj.7601977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosshans H, Filipowicz W. Molecular biology: the expanding world of small RNAs. Nature. 2008;451(7177):414. doi: 10.1038/451414a. [DOI] [PubMed] [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 5.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 7.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9(3):219. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 8.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20(5):515. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 9.Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358(5):502. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38 Suppl:S2. doi: 10.1038/ng1794. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander MR, Chen W, Adamidi C, et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol. 2008;26(4):407. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 13.Gantier MP, Sadler AJ, Williams BR. Fine-tuning of the innate immune response by microRNAs. Immunol Cell Biol. 2007;85(6):458. doi: 10.1038/sj.icb.7100091. [DOI] [PubMed] [Google Scholar]
- 14.Lodish HF, Zhou B, Liu G, Chen CZ. Micromanagement of the immune system by microRNAs. Nat Rev Immunol. 2008;8(2):120. doi: 10.1038/nri2252. [DOI] [PubMed] [Google Scholar]
- 15.Pipkin ME, Monticelli S. Genomics and the immune system. Immunology. 2008;124(1):23. doi: 10.1111/j.1365-2567.2008.02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tili E, Michaille JJ, Calin GA. Expression and function of micro-RNAs in immune cells during normal or disease state. Int J Med Sci. 2008;5(2):73. doi: 10.7150/ijms.5.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136(1):26. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10(2):111. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 19.Sheedy FJ, O'Neill LA. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis. 2008;67 Suppl 3:iii50. doi: 10.1136/ard.2008.100289. [DOI] [PubMed] [Google Scholar]
- 20.Kasashima K, Nakamura Y, Kozu T. Altered expression profiles of microRNAs during TPA-induced differentiation of HL-60 cells. Biochem Biophys Res Commun. 2004;322(2):403. doi: 10.1016/j.bbrc.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 21.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anglicheau D, Sharma VK, Ding R, et al. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A. 2009;106(13):5330. doi: 10.1073/pnas.0813121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiesen JL, Tomasi TB. Dicer is regulated by cellular stresses and interferons. Mol Immunol. 2009;46(6):1222. doi: 10.1016/j.molimm.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Drescher KM, Chen XM. MicroRNAs and Epithelial Immunity. Int Rev Immunol. 2009;28(3–4):139. doi: 10.1080/08830180902943058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180(8):5689. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong AY, Zhou R, Hu G, et al. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol. 2009;182(3):1325. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Zhang J, Li J, et al. Expression of B7-H1 in inflammatory renal tubular epithelial cells. Nephron Exp Nephrol. 2006;102(3–4):e81. doi: 10.1159/000089686. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. Febs J. 277(9):2015. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 30.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diez J. Do microRNAs regulate myocardial fibrosis? Nat Clin Pract Cardiovasc Med. 2009;6(2):88. doi: 10.1038/ncpcardio1415. [DOI] [PubMed] [Google Scholar]
- 32.Matkovich SJ, Wang W, Tu Y, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106(1):166. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol. 2009;15(45):5633. doi: 10.3748/wjg.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104(9):3432. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato M, Putta S, Wang M, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11(7):881. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Wang Y, Minto AW, et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. Faseb J. 2008;22(12):4126. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sui W, Dai Y, Huang Y, Lan H, Yan Q, Huang H. Microarray analysis of MicroRNA expression in acute rejection after renal transplantation. Transpl Immunol. 2008;19(1):81. doi: 10.1016/j.trim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010 Mar 21; doi: 10.1038/nature08956. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X, Lian D, Wong A, et al. Novel small interfering RNA-containing solution protecting donor organs in heart transplantation. Circulation. 2009;120(12):1099. doi: 10.1161/CIRCULATIONAHA.108.787390. [DOI] [PubMed] [Google Scholar]
- 41.Zheng X, Feng B, Chen G, et al. Preventing renal ischemia-reperfusion injury using small interfering RNA by targeting complement 3 gene. Am J Transplant. 2006;6(9):2099. doi: 10.1111/j.1600-6143.2006.01427.x. [DOI] [PubMed] [Google Scholar]
- 42.Beitzinger M, Meister G. MicroRNAs: from decay to decoy. Cell. 2010;140(5):612. doi: 10.1016/j.cell.2010.02.020. [DOI] [PubMed] [Google Scholar]