Abstract
With the dawn of the 21st Century, imaging has assumed a new role in disease-oriented science. Regarding pain, the emphasis clearly turned from structural to functional imaging with functional molecular imaging assuming the leading edge. This trend parallels the efforts of biologists working to understand the molecular messages of cell and cell systems’ of relevance to human disease processes. While originally imaging has been a stand-alone, documentary tool, today’s metabolic and molecular imaging technologies provide quantitative insight into inter-and intra-individual pathogenetic processes of relevance to human disease, complementing and expanding upon bench-type research. Imaging has become an indispensable tool in pain research.
Keywords: Functional imaging, metabolic imaging, molecular imaging, fMRI, PET, SPECT, complex disease
1. Introduction
This chapter focuses on the contemporary opportunities and emerging frontiers enabled by modern imaging technologies when it comes to the study of pain. Specifically, it is intended to provide a contemporary view of the role of current and future imaging opportunities with respect to advancing the research agenda in the field of pain. The goal is to link disease-oriented imaging science, with emphasis on pain, to the efforts of molecular biologists working to understand, by using experimental models, the behavior of cell and cell systems’ of relevance to human disease processes.
Contemporary imaging offers valuable understanding of complex human disease, providing much needed crosstalk between clinical phenomena and bench science where intra-and intercellular systems are isolated, identified and manipulated. While once purely documentary, a research field in isolation, today’s imaging methodologies provide quantitative insight into complex disease phenomena that assist in assessing the relevance and utility of fundamental model systems used in bench research with respect to their validity for clinical pain phenomena encountered in humans.
2. Advancing Understanding through Imaging
When it comes to the study of pain by means of imaging, the diversity of the pain phenotype needs to be recognized. Pain is experienced in the context of injury or disease. Its onset can be sudden or gradual and its presentation can be fleeting, continual, or recurrent and persistent. Although knowledge of the exact mechanistic underpinning is lacking, classifiers like “persistent” or “chronic” are reserved for those non-terminal pain conditions that impose recurrent or persistent activity limitations upon the patient for prolonged times, typically months to years (1). Besides temporal descriptors, pain conditions also range in severity and the extent of bodily involvement with the most devastating forms of suffering often demonstrating little explanatory, notably structural findings. When it comes to the most distressing presentations, lack of knowledge of the pathogenesis and the absence of validated biomarkers mean that the pain condition is in effect syndromic in nature with the diagnostic assignment relying on a combination of clinically observable features, often limited to a particular anatomical domain. Given the phenomenological diversity of human pains, it is important to recognize that not all pains will show the same brain activation pattern (2).
Epidemiological data of most pain conditions further suggest that women are more susceptible to experience pain at greater frequency and severity than men, particularly beginning with and during the reproductive ages (3, 4). With respect to scientific opportunities, animal models of pain often exhibit limitations when it comes to modeling the complex processes unique to human function, particularly those in effect in pain conditions for which therapeutic options fall short. Given this background, strong interests exist in elucidating the complex regulation and individual vulnerabilities underlying the human experience of pain and for which imaging studies hold great promise in advancing critical knowledge.
Beginning with the late Nineties, notably following the milestone paper published by the Bushnell group in Science in 1997 (5), imaging studies have greatly expanded the understanding of human brain function in pain. Recent imaging studies extended upon the earlier work and established the intriguing, functional neural underpinnings of pain, pleasure and reward (6). These manuscripts and related work point to a complex representation of the pain experience in the human brain. They highlight the concept that pain is not a simple sensory phenomenon but a complex experience, a stressor that threatens the homeostasis of the organism. As such, it has additional cognitive, emotional and motivational components. Functional neuro-imaging work has perhaps most clearly brought this concept forward, as various modalities of pain, whether experimental or clinical, are studied and their telencephalic correlates become clarified.
In contrast to animal experimentation, the psychophysical correlates provided by human subjects offer an unrivaled level of sensitivity and specificity in the assessment of pleasure, reward and pain. As a result, new knowledge of higher order brain processing of humans has widened the range of research questions pursued in bench-type research, particularly applicable to the study of pain.
In the first decade of the 21st Century, in-vivo functional imaging in humans has reached a level of sophistication that complements and expands upon the bench research in cell systems, brain-slices and animal models. In fact, today’s ability to image with metabolic or molecular measures the state of mind of the living human in response to a defined stimulus, even tackling mind-body interactions, opens the door to understanding complexly regulated disease phenomena at the level of systems’ biology that are unparalleled by the opportunities offered by animal models. Imaging the awake, behaving human, experiencing complex behavioral states, such as pain, capturing feelings with validated psychophysical tools has become an indispensable research endeavor for advancing critically needed understanding at the system’s level. This is exemplified by a series of recent imaging studies that elucidate the role of belief, anticipation, expectation, empathy, social loss and distraction with respect to pain (7–13).
In this respect, imaging techniques and methodologies in living humans are expected to drive in a meaningful way further advances in the understanding of the complex and higher order regulation in effect in human pain conditions, which are believed to result from the combined action of many genes, risk-conferring behaviors and environmental factors and with expectations and beliefs greatly impacting on the individual experience of pain (14). Many brain areas are activated in pain (15). As far as the human brain in pain is concerned, evidence from imaging studies points to the involvement of the prefrontal cortex, insula, orbito-frontal cortex, anterior cingulate, dorsal striatum, nucleus accumbens and ventral striatum, ventral pallidum, thalamus, hypothalamus, midbrain, amygdala, hippocampus, cerebellum and brainstem (5, 13, 16–24). As noted above, this body of work points to the complex representation and integration of pain information in the human brain. As a result, intra-individual variations in the pain experience are likely to arise at multiple levels, from initial perception to integrative and complex behavioral levels.
In sum, enticed by technical advances and stimulated by the first demonstration in living humans of pain affect being encoded in the anterior cingulated but not somatosensory cortex (5), imaging tools became increasingly employed by clinician-scientists(25–27). With the turn of the century, not only have imaging technologies become less expensive, the number of institutions that have implemented respective services and the number of investigators taking advantage of the increased availability of such facilities has steadily increased over the past decade. As a consequence, publications utilizing imaging tools have sharply increased in numbers. The use of imaging in research also shifted from the purely descriptive documentation of event-related changes to adopting a more mechanistic, hypothesis-driven framework that has shaped much of the discovery process in the sciences at-large in past decades (28).
3. Each Tool Has Its Place
To gain insight into the unresolved questions linked to human pain conditions, and with pain being both a sensory and significant emotional experience, it is not surprising that the main focus has been on visualizing the brain – first its structure and more recently its function - much more than on depicting peripheral phenomena observable in the region reported in pain. Images of brain structures based on magnetic resonance imaging (MRI) and brain function, visualized by means functional magnetic resonance imaging (fMRI), positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have emerged as the most notable imaging applications for gaining insight into the central neurobiology, the latter two applications being analogous to in vitro autoradiography and radioimmunoassays used in bench research. Today’s in-vivo imaging techniques have matured to the point that enables the valid and reliable measurement of neuronal activity, neurochemistry and pharmacology in the living human brain, complementing and validating results obtained in bench-type research.
According to MEDLINE, taking into account the literature up to the 3rd week of November, 2008, the number of hits involving “pain” and “imaging” reached 267 for years 1950–1980, 507 for years 1981–1990, 3,575 for years 1991–2000, and 7,001 for the remaining years of 2001–2008. Much of the growth is attributable to the expansive use of structural imaging modalities, notably computer-aided tomography (CT) and MRI that increasingly became available in the past three decades of the last Century. While the trend looks great at first sight, a closer look identifies that the field of pain is not a leader when it comes to frontiers in imaging in the molecular age. The field of cancer with a literature body of about three times that of pain shows 1,295 publication that fit the descriptors of “molecular imaging” between 2001–2008; the field of pain only has 37 in the corresponding time window of which three overlap with cancer, although the number of publications fitting the generic terms “molecular” and “pain” has risen from 24 for years 1950–1980 to 60 for years 1981–1990, 560 for years 1991–2000, and 1,180 for the remaining period.
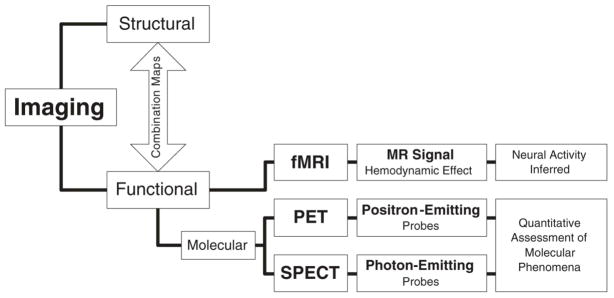
Overall, the overwhelming majority of the work is employing structural imaging methodologies. These have provided particular insight as to the changes in brain structure that take place in the context of chronic disease (29, 30). Newer technologies like functional imaging and even molecular functional imaging, the tools needed to complement laboratory research, make up the smallest portion of all imaging studies combined. It is increasingly understood that progress focusing on radiographically detectable change, captured by structural imaging modalities, represents the often late consequence of molecular processes, which – in turn -should be viewed as the clinically and therapeutically meaningful phenomenon to be captured (Figure 1).
Figure 1.
Imaging literature classified by the nature of the imaged signal and explanatory yield. Molecular imaging uses molecular probes for the purpose of image generation.
By no means should the various imaging tools, for both structural and functional imaging classes, be understood as interchangeable, particularly when it comes to their utility in the assessment of pain-related brain function. Differences exist in terms of the degree of the mechanistic specificity of the acquired data based upon the choice of the biological probes used for signal generation, and aspects of the inherent spatial and temporal limitations of the various imaging applications. Indication for one or the other imaging tool is determined by the scientific question under investigation and should not be made on the basis of the convenient availability of a particular imaging modality. For example, some pain effects are phasic in nature while others exert a tonic regulatory influence, e.g., long-lasting effects on neurotransmitter release, signal transduction and/or neurotransmitter interactions, necessitating imaging technologies with appropriately matched temporal resolution to answer the question posed.
In the case of lower spatial resolution, structural and functional imaging modalities are often combined by means of image co-registration techniques and processed using instrumentation-specific mathematical models to enhance the validity of assigning changes in biological brain function of lower spatial resolution to identifiable anatomical brain structures obtained using high-resolution MRI or CAT imaging. In fact, the newer classes of high-end imaging platforms incorporate multimodality imaging, e.g. PET-MRI or PET-CAT, permitting the convenient acquisition and fusion of multimodality images in the same recording session and without having to change the subject’s head support with respect to the image focus.
Without any question, the evolutionary trend in medical imaging involves the migration from capturing structure to the visualization of specific molecular functions that are superimposed on detailed anatomical maps. Targeting and mapping the role of specific genes and their expressed and functional proteins in living humans, known or presumed to be of relevance to human disease, including the pain conditions, replacing non-specific information with functional maps relevant to the mechanistic laboratory knowledge base constitutes the future of biomedical imaging (31). To compute inter-individual average response maps from individual data sets, validated spatial image normalization into internationally accepted standard stereotactic space using parametric mapping techniques (e.g., SPM, FSL) is required for which (linear and nonlinear) transformation algorithms are applied to match each individual image to a standard template (www.fil.ion.ucl.ac.uk/spm).
Functional Magnetic Resonance Imaging (fMRI)
Because of its noninvasiveness and widespread availability, fMRI constitutes the most used functional imaging modality to produce maps of human brain function. Its principle is founded on the hemodynamic response, the regional increase in blood flow that parallels neural activity, occurring with a delay of about 1–5 s after the onset of such activity. Regional changes in blood volume, blood flow and the relative concentration of oxygenated (diamagnetic) and deoxygenated (paramagnetic) hemoglobin are the consequences.
Due to the increase in blood flow and volume without a corresponding increase in oxygen extraction from blood, deoxyhemoglobin concentration is lowered. The hemodynamic response is relatively short-lived, peaking after about 4 s before returning back to baseline again. Being an indirect measure of neuronal activity, other factors - regional or otherwise - have a bearing on the MR signal. With the MR signal being a measure of the hemodynamic response, it needs to be understood that activation involving excitatory or inhibitory neuronal networks is indistinguishable.
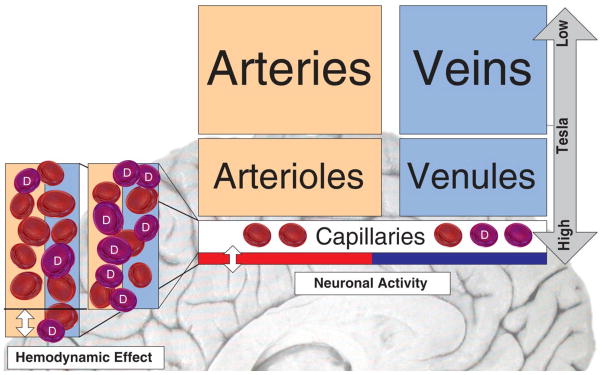
It is important to understand that the magnetic resonance (MR) signal captures the combined effect of arteries, veins, arterioles, venules and capillaries with scanners producing magnetic fields of lesser strength being more influenced by events occurring in larger vessels. Scanners with greater field strength (>4 tesla) bias the signal towards smaller vessels and consequently more to the source of neuronal activity. Blood oxygen-level-dependent (BOLD) fMRI, the most widely used assessment technique in research is based upon measurable differences of the magnetic resonance signal dependent upon the level of oxygenation and due to differences in the magnetic properties of oxyhemoglobin and deoxyhemoglobin. T1-weighted pulse-sequences are better suited to detect changes in blood flow while T2-weighted images are more suited to detect changes in the local concentration of paramagnetic deoxyhemoglobin (Figure 2).
Figure 2.
Schematic illustration of the BOLD effect used for MR image generation based on the change in the ratio of oxyhemoglobin to deoxyhemoglobin (D). MR signals generated by scanners with high tesla value (> 4 tesla) represent higher information content from relevant smaller vessels that are in the vicinity of active neurons.
Functional Molecular Imaging Using PET/SPECT
The rapid progress and new understanding gained by bench research using molecular probes, including the need to rapidly transfer findings into humans, calls for comparable tools to be applied in the intact human, allowing in-vivo localization and quantification of specific molecules and their function in the human brain or other body regions of interest. Probes capable of monitoring the biological activity of enzymes, transporters, receptors and other relevant target proteins will increasingly shape the future of medicine. Such methodologies require – optical imaging techniques excluded - attaching radioactive atoms to relevant molecules and observing the emitted radioactivity by a network of spatially arranged sensors around the subject, often referred to as “gamma camera”. This sensor array defines the limitations of PET/SPECT in terms of spatial and temporal resolutions by its geometrical layout and sensor properties.
Suitable radioactive atoms for PET - positron emitters - include 15oxygen, 11carbon or 18fluorine, having a half-life of 2 min, 20 min or 120 min, respectively. Radiotracers that are labeled with these atoms employed decay by positron emission. For SPECT, γ-ray emitters, such as 99technetium, 123iodine and 133xenon with a half-life of 6 h, 13 h and 5 d are used. Unlike radioligands suitable for PET, these compounds emit single or multiple uncorrelated γ-rays. Appropriate radioligands must show high selectivity for their target combined with low nonspecific binding to brain tissue devoid of the target. They also must exhibit rapid permeation of the compound through the blood-brain barrier and be metabolized in a way not to interfere with the ongoing measurement process.
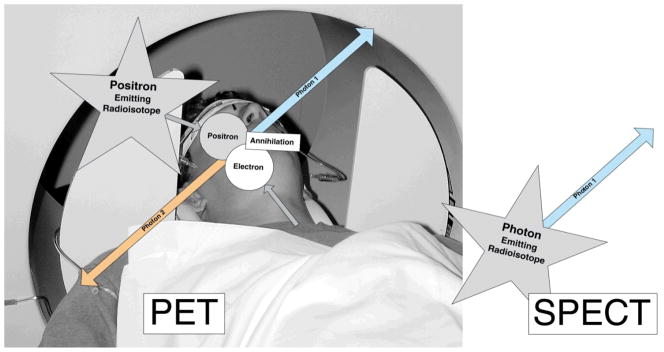
Compounds suitable for PET emit a positron that when colliding with an electron in its close vicinity results in the annihilation of the two particles and the generation of two photons, travelling in opposing directions, each with an energy of 511 keV. The phenomenon of two annihilation photons simultaneously traveling in opposing directions, hitting opposite γ-sensors within a very short time window (typically 5–7 ns) is taken advantage of to eliminate false-positive sensor hits and thereby improving the signal-to-noise ratio in PET data acquisitions. The acquisition of coincidence events over time forms the basis for the sinograms that – in turn – are used for PET 3-D image reconstruction.
After decay, isotopes used for SPECT imaging produce a single γ-ray (in case of some compounds, multiple, unrelated γ-rays) of varying, however lower energy than PET compounds, depending upon the type of radionuclide used. Although SPECT radionuclides are more readily available due their longer half-lives, radiopharmaceuticals with single emitting radionuclides are more difficult to prepare, limiting the current use of SPECT. On the other hand, the potential to radioactively label, biologically meaningful compounds with 11carbon and 18fluorine is substantial (Figure 3).
Figure 3.
Principles underlying PET and SPECT image generations. PET uses positron emitting isotopes while SPECT imaging employs single photon-emitting radiotracers. For PET, images are generated on the basis of the two simultaneously emitted annihilation photons, traveling in opposing directions. For SPECT, radioactivity is captured through a collimator to form an image on the detector.
When employing radiotracers, compounds that lend themselves to be radiolabeled and used in tracer amounts at occupancy levels typically below 1% of the respective receptor sites, factors specific to the radioactive compound used for image generation, such as its metabolism and protein binding properties require corrective adjustments for accurate assessment of the process of interest. The total radioactivity in a region of interest represents the sum of the radioactivity of the specifically bound compound and the presence of (1) non-specifically-bound and (2) any free compound in the region of interest. The latter two fractions are inferred from the radioactivity observed in a brain region known to be devoid of specific binding sites, or calculated using tracer kinetic analyses and the decay and metabolite corrected radiotracer concentrations in plasma. Reference region quantification models, as opposed to plasma input kinetic analyses, are currently favored if properly validated. Reasons for that include experimental and analytical convenience (without need for challenging arterial lines, and the collection and analysis of blood “on the fly” for the quantification of the parent compound and its radiolabeled metabolites in plasma), as well as the lesser statistical uncertainty in the quantification of the molecular process of interest (e.g., receptor binding sites, enzymatic activity).
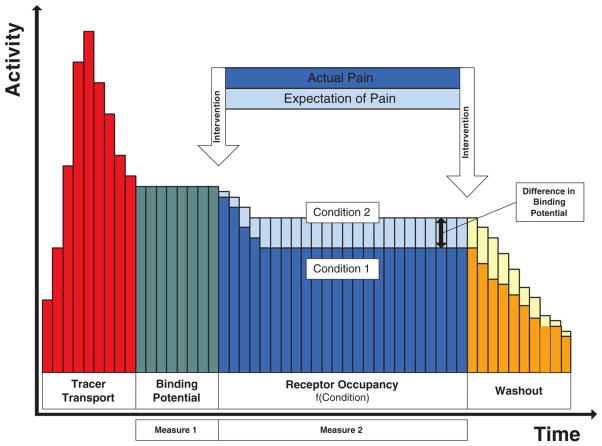
PET or SPECT image data typically consist of time activity curves from which images are generated for a defined period of time following the administration of the radiotracer. As an example, Figure 4 schematically illustrates the PET time activity curve using the radiotracer [11C]carfentanil that binds to μ-opioid receptor for conditions of pain and the expectation of pain without actual pain to illustrate the methodological capability for functional assessments of the μ-opioid system at baseline and when activated in pain and the expectation of pain (24). Carfentanil, a potent μ-opioid agonist is preferred over 11C-labeled morphine, heroin or codeine because of metabolic complexities and high levels of unspecific binding encountered with these other compounds.
Figure 4.
Schematic time activity curve reflecting changes in regional radioactivity emitted from [11C]carfentanil bound to the μ-opioid receptor when corrected for radioactivity due to unspecifically bound and free compound. Due to the greater availability of endogenous opioids in pain than in the saline control condition with the subject expecting pain but no pain is felt, less radioligand binding occurs because of the increased competition for available binding sites. Sequences are counterbalanced to control for order effects.
Suitable radioligands in combination with tracer kinetic models, representing the time-dependent local activity of the radiotracer in compartments, such as blood and brain tissue – free, unspecifically bound and specifically bound – modeling equilibrium during timed data acquisition (e.g., using traditional compartment analyses or reference regions, such as in the Logan plots) turn a time sequence of PET images into a quantitative biological assay (32–35). Functional molecular imaging has matured to the point to permit unprecedented insight into the regulatory effect of systems visualized by highly selective molecular probes.
4. Cracking Complex Systems’ Biology
With the turn of the Century, patient-oriented research has become more exciting than ever and imaging data, obtained by metabolic and molecular imaging studies started to play a major role in advancing the understanding of pain. Emerging biotechnologies, including the access, affordability and experimental yield of various functional imaging modalities offer unprecedented insight into the regulation of human disease and the mechanistic action of therapeutic interventions. Novel molecular methodologies support the discovery of explanations why certain treatments do not work and/or particular devices cause complications in individual patients. The specificity by which suitable biological probes, labeled with radioactive transmitters, are capable to dissect molecular processes in the intact human will increasingly influence laboratory investigations (36). For example, existing positron probes offer insight into the glucose metabolism by 2-[18F]fluoro-2-deoxy-D-glucose (FDG), oxygen utilization by 15O2, the function of neurotransmitter systems, such as the μ-opiate system by [11C]carfentanil, the dopamine D2/D3 system by [11C]raclopride and [18F]fallypride, the serotonin transporter with [11C]DASB, the serotonin receptor 5-HT1A by [11C]WAY-100635, benzodiazepine receptors by [11C]flumazenil, or even enzyme functions, such as the monoamine oxidase (MAO) by [11C]deprenyl, just to mention a few. However, development in this area has progressed slowly. Establishing accurate pharmacokinetics of the labeled molecule are not always simple, making only a few of the potentially suitable agents actually useful for molecular quantification in humans. In addition, the lack of access to existing molecular libraries (largely present in the drug industry) that includes ligands not necessarily destined to become therapeutic targets, limits the total number of compounds that could become available for molecular imaging.
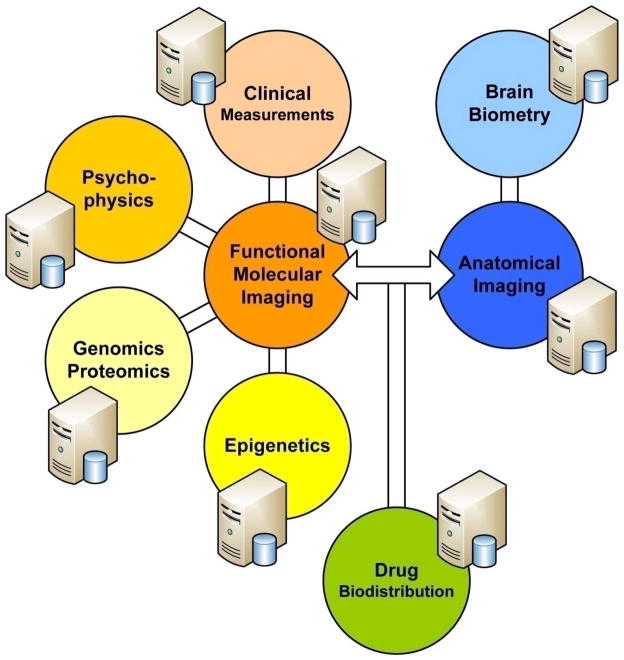
Structural and functional imaging in humans, paired with psychophysics, brain biometry, bioinformatics and clinical, environmental and putative risk data enable clinician-scientists to pursue hypotheses for which animal model systems exhibit limitations in characterizing the psychophysical phenotype. With the dawn of the genomic age, molecular tools became available that combined with state-of-the art imaging studies permit the modeling of complexly regulated processes in living humans down to the level of molecular function. Driven by the idea to link variations in brain biometry and function to behavioral differences of humans, this type of research is believed to generate knowledge of vulnerabilities to illnesses, including insight into the mechanisms by which genetic liability is conferred (Figure 5). Traditional, single-laboratory based science is increasingly complemented by team science that addresses the organized problem complexities of interdisciplinary and multidisciplinary nature with imaging data playing a major role in modeling complexity. This is particularly true for those complex systems in which mind-body interactions influence outcomes.
Figure 5.
Tackling biological complexity, linking phenotype to genotype using functional (molecular) brain imaging to explain complexly regulated phenomena, such as pain in humans.
The prevailing thinking states that clinical pain conditions are believed to develop from the combined action of many genes, risk-conferring behaviors and environmental factors, not understood from the function of each of these factors alone (37). Because traits, such as the sensitivity to a particular type of pain as well as the suppression of pain, are subject to considerable variation from subject to subject, the overall system response results from the interaction of a multitude of factors impacting upon the signal strength in pro-and anti-nociceptive signaling systems. Influences on these complexly regulated pro-and anti-nociceptive processes are difficult to resolve due to a multitude of inputs, which include endocrine signaling, genetic factors, neuroplastic changes linked to the persistence of pain and even the lasting effects of prior treatments.
Besides untangling the regulatory complexity and difficulties that arise from defining the clinical phenotype, distinguishing between pro-and anti-nociceptive processes constitutes a challenge for conventional, non-molecular imaging insofar that any regional activation could be due to either pro-or anti-nociceptive signaling. Given the need to further distinguish between excitatory and inhibitory neuronal activity in both pro-and anti-nociceptive systems, signal specificity greater than what is obtained from image generation based upon changes in hemodynamics is needed to resolve scientific questions with certainty, calling for highly selective radioligands. For this purpose, specific biological probes, capable of isolating receptor systems of interest are required to unequivocally link changes in brain activity to one or the other process.
It should be understood that any kind of imaging study cannot occur in isolation. While on one hand, animal models and cell systems establish the scientific framework for hypothesis testing, results of imaging studies may call for novel lines of bench research. In particular, radioligands with high target specificity allow imaging of neurobiological and/or neuro-pharmacological processes in the functioning human brain, providing an exciting opportunity for the cross-validation of basic and clinical research findings.
Brain Receptor Imaging
Knowledge of the function of the human brain is fundamental to understanding the response to all types of injury and disease, not just neuropsychiatric conditions. Due to the pivotal role of receptors in neurotransmission and neuromodulation, in-vivo studies of receptor systems are gaining appeal (38). Using highly selective radioligands, the regional distribution, density and activity of labeled receptor systems can be visualized (a) at rest and (b) in an experimental state that presumably involves the activation or deactivation of neurotransmission in the system under study. As stated by the law of mass action, free ligand binds to its receptor dependent on the concentration and affinity of both the ligand and the receptor. As noted above, receptor binding is quantified using suitable tracer kinetic models; receptor tracers for PET/SPECT imaging have been developed for dopamine, serotonin, cholinergic, γ-aminobutyric acid (GABA), adenosine and the opioid systems. There is also growing understanding of the selective affinity of various ligands to receptor subtypes, including possible regional differences that may exist in terms of receptor affinity.
Regarding pain, quantifying neurotransmission at the neuroreceptor site using functional molecular assays permits the dissection of neural activity by assigning it to either a pro-nociceptive or anti-nociceptive regulatory phenomenon as the regional activation of a specific neurotransmission system can be correlated with psychophysical and/or clinical variables. As receptor-specific data of the neurotransmitter system under study are entered into statistical analyses(ANOVAs, correlations) -data unconfounded by unspecific activity in adjacent neural networks-such functional molecular imaging assays are expected to surge in popularity in neurobiological and neuro-pharmacological applications, notably by moving such imaging approaches into the early phases of drug development. This opens the door for drug activity – in a very early stage of testing -to be assessed down the level of receptor function in a dynamic assay in the phylogentically most relevant model system, the human, considering the powerful effects of emotions on the modulation of pain, or the modulation of emotions by neuro-pharmacological drugs on the experience of pain that remain a challenge to be untangled in animal models.
Competitive ligand-receptor assays are employed to visualize the degree to which radioligands are displaced by exogenously administered and endogenous ligands. The latter case, and in the instance of displaceable radiotracers, enables the study of the effect of physical or pharmacological challenges on neurotransmitter release in a particular neuroreceptor system and in turn, to modulate or to relate any changes to the clinical endophenotype. Such assays permit the study of the modulatory effect of a particular genetic makeup on neurotransmission(e.g., anti-nociceptive processes, presumably influencing a subject’s ability (a) to suppress signaling in pro-nociceptive channels, or (b) to activate to a greater degree than average those circuits that influence pain suppression) (Figure 6).
Figure 6.
The complex pain experience is shaped by pro-nociceptive and anti-nociceptive processes, which are influenced by a host of factors, including gender, present state of mood and pain history. To resolve questions whether neural activity is attributed to pro-nociceptive or anti-nociceptive signaling, specific molecular probes are required for image generation as unspecific neural activity, inferred by the hemodynamic effect for example, is not able to make the distinction with any degree of certainty.
Rarely is reference given to the experimental complexity involved in imaging work, in particular the molecular imaging techniques. A host of problems, linked to equipment, instrumentation, research personnel and subject selection can easily affect the successful completion of a functional imaging study. Patient movement is a common difficulty in fMRI studies that is difficult to correct for. In addition, molecular imaging adds elements related to cyclotron operation, such as the acquisition of enough “radio-label” and factors related to the synthesis of the radiotracer, e.g., failure to synthesize the compound of sufficient purity, and to achieve radio-labeling with low quantity of “cold” (unlabelled) compound, to avoid physiological effects. Unlike fMRI studies, PET studies often require the acquisition of blood samples, particularly if traditional kinetic analyses using plasma radiotracer concentrations are contemplated. Needless to say, these types of imaging studies depend on a committed team where each member understands his/her role and everyone recognizes that their contribution counts.
Imaging μ-Opioidergic Neurotransmission
The study of the opioidergic neurotransmission represents a good example to illustrate the scientific yield of target-specific neuro-receptor imaging for the study of the complex modulation of tissue-damaging signals at various levels of the pain system (39). Regarding opioidergic neurotransmission, four opioid receptor classes, OP1 (δ1–2), OP2 (κ1–3), OP3, also referred to as μ-opioid receptors(μ1, μ2 and μ3), and OP4, known as the nociceptin receptor, represent potentially insightful radioligand binding sites for functional molecular studies of opioidergic neurotransmission. All opioid receptors consist of a seven trans-membrane domain with a highly conserved amino acid sequence and belong to the family of G-protein-coupled receptors (40–45).
Of particular significance to pain regulation, μ-opioid receptors are widely distributed throughout the central and peripheral nervous system(46–49); μ-opioid receptors happen to be the binding site of endogenous opioid peptides, including endorphin, dynorphin and enkephalin and, at normal therapeutic dosages, the principal receptor involved in morphine and in general, any exogenously administered opiate induced analgesia (49). At higher dosages, and in the case of morphine and some of its derivatives, however, receptor selectivity is lost and opioid receptors other than the μ-opioid receptor are activated as well (50).
To assess the anti-nociceptive effects mediated by the μ-opioid system, we currently use [11C]carfentanil in our laboratory for PET imaging. Regarding the selectivity of exogenous μ-opioid receptor agonists, fentanyl, followed by morphine and meperidine exhibit the highest receptor affinity (51–53). Carfentanil, a fentanyl derivative, is a potently selective μ-opioid receptors agonist, which when radio-labeled with [11C] meets the requirements to act as a suitable radiotracer for the study of central μ-opioid receptor binding (54–57). Low occupancy of the receptors by the radioligand – typically only about 1% of receptors are labeled – is required to neglect, on a theoretical basis, any significant system’s effect due to the presence of the radiotracer. Figure 7 illustrates the [11C]carfentanil binding in humans at baseline, in the absence of any challenges, with binding regions and densities matching locations identified in post-mortem studies (58, 59). Other commonly used compounds, such as [11C]dyprenorphine and [11C]cyclofoxy, display non-selective receptor pharmacology.
Figure 7.
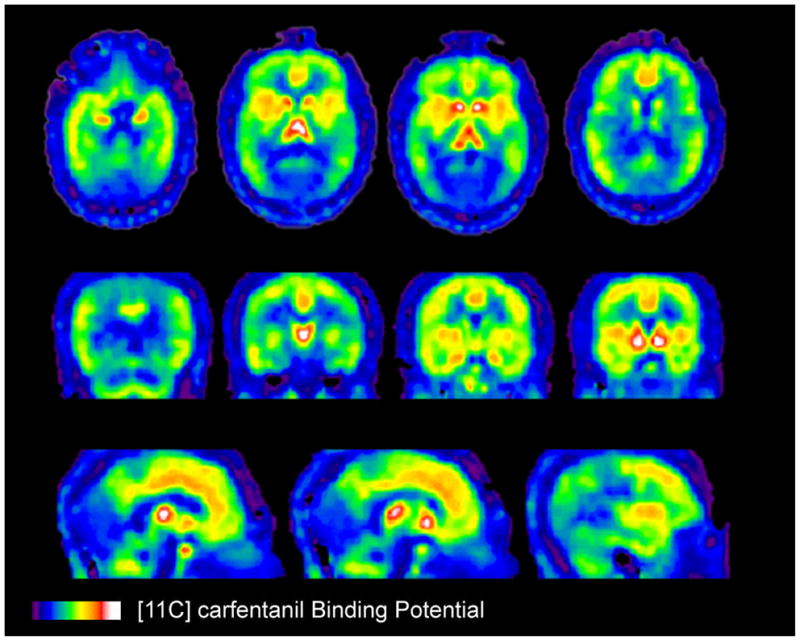
In-vivo [11C]carfentanil baseline binding in the human brain. Top, middle and lower rows correspond to horizontal, frontal and sagittal brain cuts. Binding values are represented by the pseudocolor scale in the lower part of the figure.
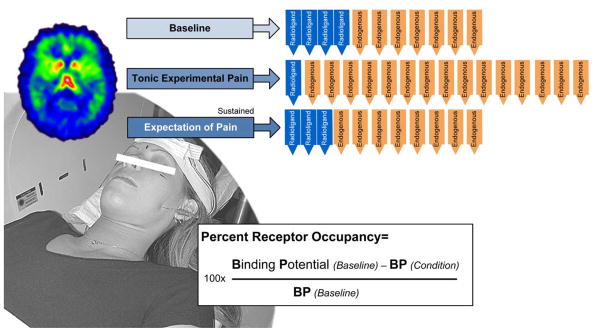
[11C]carfentanil is also a compound that has demonstrated its capacity to be displaceable during endogenous opioid release in response to sustained or repetitive pain (24, 60). The reduction in the measured receptor signal during a noxious challenge is interpreted as reflecting processes associated with the release of the endogenous neurotransmitter. These may include (a) competition between the endogenous neurotransmitter and the radiolabeled tracer; (b) changes in the conformational state of the receptors in the context of high concentrations of endogenous ligand in the synaptic cleft (a change from high to low affinity states, the latter being less capable of binding the agonist radioligand); and (c) receptor internalization and recycling (61). Activation of the endogenous neurotransmitter system results in the reduction of the externally acquired binding measure, often termed binding potential (Figure 8).
Figure 8.
Percent receptor occupancy (Bmax/Kd) is used for the quantification of scans. Note the schematically captured different states of activation of the endogenous system as a function of the experimental condition, changing the availability of receptor sites for the competitive radioligand according to the law of mass action.
Neuro-imaging studies in the human have shown the activation of μ-opioid neurotransmission during sustained pain in brain regions, most notably the insular cortex, both anterior and posterior, the anterior cingulate and prefrontal cortex, as well as the ventral basal ganglia (24, 62). These are regions respectively implicated in the representation of interoceptive states, emotional and cognitive integration, as well as motivation and reward processing. From the perspective of the regulation of the pain experience, present data point to a distributed network of regions regulated by the μ-opioid neurotransmitter system, with important implications for the understanding of the complexity of the pain experience and its relief. For example, it has been shown that during the administration of a placebo, expectancies of pain relief can activate endogenous opioid neurotransmission in a number of these regions (i.e., anterior cingulate, prefrontal cortex, insula, thalamus, ventral basal ganglia, amygdala, periaqueductal gray), with the magnitude of activation relating to the subjectively reported level of pain relief (13, 63).
Given the complex regulatory impact of the μ-opioid receptor system, it should be understood that (1) the number of available receptor sites, and (2) the strength, and (3) the rate by which a subject activates his/her endogenous opioid system will have a bearing on the individual response to a pain-stressor. These parameters are quantified by recording the intra-individual binding potential (BP), the proportional receptor occupancy (a) before the initiation of the pain-stressor, and (b) during pain-stress.
Using this type of functional neuro-receptor imaging, the effect size of the changes in the in vivo μ-opioid receptor availability from the condition of (a) non-painful control to (b) sustained pain of moderate intensity amounts to a reduction in the binding measure of as much as 20%, varying from subject-to-subject and with brain region(typically ranging from 8–20% for regions within which significant changes occur). Both sensory and affective components are modulated with the individual pain report being negatively correlated with the degree to which the μ-opioid system is activated on an individual basis(24). In this respect, genomic individuality - based upon the genetic variations that distinguish one person from another -is being explored to understand the individual response behavior to pain. The large effect size also makes the exploration of complex genetic effects (e.g., gene-gene interactions) feasible without extremely large sample sizes (19, 20).
5. Understanding Me
It is increasingly recognized that not all individuals are equally susceptible to encounter severe or lasting pain. In fact, individuality among humans (or animals) can no longer be dismissed as it appears to explain much of the variance in the response to an insult, disease or any type of stressor in general. Just focusing on gender, women in their reproductive years represent the majority of those seeking care for a host of clinical pain conditions. Women also report greater pain severity on average (3).
In addition, molecular individuality appears to drive the subject-specific sensory, motor, affective and autonomic complaints, including those linked to stress-related illness and/or pain (24). Besides gender and age, different genotypes are increasingly studied with respect to their contribution in (a) the amplification of symptoms, (b) the susceptibility to a particular clinical course of the disease, and (c) the observation of a unique treatment response. The estimated >5 million single nucleotide polymorphisms (SNPs) of the 6 billion bits of the human genetic code that exhibit a minor allele frequency of >10 percent, have become the target of investigation for select gene products that confer risk. The majority of SNPs, about 15 million show minor allele frequencies of less than 10 percent of humans (http://www.hapmap.org).
To unravel the effect of common SNPs and their expressed and functional gene products, imaging, notably functional molecular imaging, will play a major role in examining differences in brain function as a consequence of SNP variants that either predispose to or protect from disease. As an example, in a PET radioligand study, a common functional polymorphism, homozygotes for the met158met allele of the catechol-O-methyltransferase (COMT), shows significantly lower μ-opioid regional system activation in sustained pain-stress of 10 min and more when compared to subjects characterized as met158val heterozygotes and val158val homozygotes (20).
The fact that gender, age and the genetic/molecular individuality influence the behavioral pain phenotype also impacts upon the design of imaging studies in terms of the assembly of matched control groups, particularly when study samples are small and differences in ancestry between experimental groups could confound results. Because molecular imaging studies are extremely resource-intensive and therefore study samples tend to be small, controlling for ancestry (more than just ethnicity) between contrasts, such as low and high responders, becomes necessary (19).
Acknowledgments
This work was supported by NIH RO1 DE15396 (CSS), NIH RO1 AT001415 (JKZ) and NIH RO1 DA016423 and RO1 DA022520 (JKZ)
References
- 1.Von Korff M, Dworkin SF, Le Resche L. Pain. 1990;40:279. doi: 10.1016/0304-3959(90)91125-3. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz J, Casey KL. European Journal of Pain: Ejp. 2005;9:163. doi: 10.1016/j.ejpain.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Unruh AM. Pain. 1996;65:123. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 4.Von Korff M, Le Resche L, Dworkin SF. Pain. 1993;55:251. doi: 10.1016/0304-3959(93)90154-H. [DOI] [PubMed] [Google Scholar]
- 5.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Science. 1997;277:968. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 6.Leknes S, Tracey I. Nature Reviews Neuroscience. 2008;9:314. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 7.Wager TD, et al. Science. 2004;303:1162. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 8.Singer T, et al. Science. 2004;303:1157. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 9.Panksepp J. Science. 2003;302:237. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- 10.Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain. 2000;85:19. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 11.Petrovic P, Kalso E, Petersson KM, Ingvar M. Science. 2002;295:1737. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 12.Ploghaus A, et al. Science. 1999;284:1979. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 13.Scott DJ, et al. Archives of General Psychiatry. 2008;65:220. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro SC, et al. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:1264. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Price DD. Molecular Interventions. 2:392–339. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz J, Minoshima S, Casey KL. Brain. 2003;126:5. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 17.Wager TD, et al. Science. 2004;303:1162. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 18.Rolls ET, et al. Cerebral Cortex. 2003;13:308. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, et al. Nature. 2008;452:997. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zubieta JK, et al. Science. 2003;299:1240. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 21.Zubieta JK, et al. Journal of Neuroscience. 2002;22:5100. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coghill RC, et al. Journal of Neuroscience. 1994;14:4095. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. European Journal of Pain: Ejp. 2005;9:463. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Zubieta JK, et al. Science. 2001;293:311. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 25.Casey KL. Proc Natl Acad Sci U S A. 1999;96:7668. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey KL. Progress in Brain Research. 2000;129:277. doi: 10.1016/S0079-6123(00)29020-1. [DOI] [PubMed] [Google Scholar]
- 27.Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Journal of Neurophysiology. 1997;78:450. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- 28.Frost JJ. Annals of the New York Academy of Sciences. 2008;1144:251. doi: 10.1196/annals.1418.027. [DOI] [PubMed] [Google Scholar]
- 29.Apkarian AV. Novartis Foundation Symposium. 261:239–245. [PubMed] [Google Scholar]
- 30.Apkarian AV, et al. Journal of Neuroscience. 2004;24:10410. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherry SR. Physics in Medicine & Biology. 2004;49:R13. doi: 10.1088/0031-9155/49/3/r01. [DOI] [PubMed] [Google Scholar]
- 32.Joshi A, Fessler JA, Koeppe RA. Journal of Cerebral Blood Flow & Metabolism. 2008;28:852. doi: 10.1038/sj.jcbfm.9600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naganawa M, et al. Neuroimage. 2008;40:26. doi: 10.1016/j.neuroimage.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 34.Naganawa M, et al. Neuroimage. 2005;26:885. doi: 10.1016/j.neuroimage.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Kimura Y, Naganawa M, Shidahara M, Ikoma Y, Watabe H. Annals of Nuclear Medicine. 2007;21:1. doi: 10.1007/BF03033993. [DOI] [PubMed] [Google Scholar]
- 36.Jones T. Journal of Psychopharmacology. 1999;13:324. doi: 10.1177/026988119901300402. [DOI] [PubMed] [Google Scholar]
- 37.Cookson W, Liang L, Abecasis G, Moffatt M, Lathrop M. Nature Reviews Genetics. 2009;10:184. doi: 10.1038/nrg2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heiss WD, Herholz K. Journal of Nuclear Medicine. 2006;47:302. [PubMed] [Google Scholar]
- 39.Henriksen G, Willoch F. Brain. 2008;131:5. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC. DNA & Cell Biology. 1992;11:1. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- 41.Kieffer BL, Black D, Hirth CG. Analytical Biochemistry. 1993;215:1. doi: 10.1006/abio.1993.1546. [DOI] [PubMed] [Google Scholar]
- 42.Kieffer BL. Trends in Pharmacological Sciences. 1999;20:19. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 43.Kieffer BL. Cellular & Molecular Neurobiology. 1995;15:615. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballet S, Pietsch M, Abell AD. Protein & Peptide Letters. 2008;15:668. doi: 10.2174/092986608785133672. [DOI] [PubMed] [Google Scholar]
- 45.Labuz D, Mousa SA, Schafer M, Stein C, Machelska H. Brain Research. 2007;1160:30. doi: 10.1016/j.brainres.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 46.Voorn P, Brady LS, Berendse HW, Richfield EK. Neuroscience. 1996;75:777. doi: 10.1016/0306-4522(96)00271-0. [DOI] [PubMed] [Google Scholar]
- 47.Nozaki-Taguchi N, Yaksh TL. Anesthesia & Analgesia. 94:968. doi: 10.1097/00000539-200204000-00036. [DOI] [PubMed] [Google Scholar]
- 48.Yaksh TL. Acta Anaesthesiologica Scandinavica. 1997;41 doi: 10.1111/j.1399-6576.1997.tb04623.x. [DOI] [PubMed] [Google Scholar]
- 49.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Trends in Neurosciences. 1988;11:308. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 50.Dykstra LA, Preston KL, Bigelow GE. Psychopharmacology. 1997;130:14. doi: 10.1007/s002130050208. [DOI] [PubMed] [Google Scholar]
- 51.Portoghese PS, el KR, Law PY, Loh HH, Le BB. Farmaco. 2001;56:191. doi: 10.1016/s0014-827x(01)01040-0. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs AM, Youngblood F. Journal of the American Podiatric Medical Association. 1992;82:520. doi: 10.7547/87507315-82-10-520. [DOI] [PubMed] [Google Scholar]
- 53.Simonds WF. Endocrine Reviews. 1988;9:200. doi: 10.1210/edrv-9-2-200. [DOI] [PubMed] [Google Scholar]
- 54.Sadzot B, Frost JJ. Anesthesia Progress. 1990;37:113. [PMC free article] [PubMed] [Google Scholar]
- 55.Frost JJ, et al. Journal of Cerebral Blood Flow & Metabolism. 1990;10:484. doi: 10.1038/jcbfm.1990.90. [DOI] [PubMed] [Google Scholar]
- 56.Frost JJ, et al. Journal of Cerebral Blood Flow & Metabolism. 1989;9:398. doi: 10.1038/jcbfm.1989.59. [DOI] [PubMed] [Google Scholar]
- 57.Frost JJ. NIDA Research Monograph. 1986;74:15. [PubMed] [Google Scholar]
- 58.Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Brain Research. 1990;530:312. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- 59.Gorelick DA, et al. Psychopharmacology. 2008;200:475. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wager TD, Scott DJ, Zubieta JK. Proc Natl Acad Sci U S A. 2007;104:11056. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narendran R, et al. Synapse. 2004;52:188. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- 62.Smith YR, et al. Journal of Neuroscience. 2006;26:5777. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zubieta JK, et al. Journal of Neuroscience. 2005;25:7754. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]