Abstract
Background
While community violence has been linked to psychological morbidity in urban youth, data on the physiological correlates of violence and associated posttraumatic stress symptoms are sparse. We examined the influence of child posttraumatic stress symptoms reported in relationship to community violence exposure on diurnal salivary cortisol response in a population based sample of 28 girls and 15 boys ages 7–13, 54% self-identified as white and 46% as Hispanic.
Methods
Mothers’ reported on the child’s exposure to community violence using the Survey of Children’s Exposure to Community Violence and completed the Checklist of Children’s Distress Symptoms (CCDS) which captures factors related to posttraumatic stress; children who were eight years of age or greater reported on their own community violence exposure. Saliva samples were obtained from the children four times a day (after awakening, lunch, dinner and bedtime) over three days. Mixed models were used to assess the influence of posttraumatic stress symptoms on cortisol expression, examined as diurnal slope and Area Under the Curve (AUC), calculated across the day, adjusting for socio-demographics.
Results
In adjusted analyses, higher scores on total traumatic stress symptoms (CCDS) were associated with both greater cortisol AUC and with a flatter cortisol waking to bedtime rhythm. The associations were primarily attributable to differences on the intrusion, arousal and avoidance CCDS subscales.
Conclusions
Posttraumatic stress symptomatology reported in response to community violence exposure was associated with diurnal cortisol disruption in these community-dwelling urban children.
Keywords: community violence, cortisol rhythm, posttraumatic stress symptoms
Introduction
Violence exposure among children growing up in the United States (US) is a leading pediatric public health problem (Thomson et al. 2002). Among youth living in urban communities in the US the prevalence of experiencing and witnessing serious and lethal violence is particularly high (Margolin and Gordis 2000). The majority of urban youth have witnessed violent events and, among older children, a third or more report being a direct victim of violence (Osofsky et al. 1993; Schubiner et al. 1993; Sheehan et al. 1997). In addition to being highly prevalent, exposure to violence is increasingly recognized as a major cause of childhood morbidity in urban US communities (Wright 2006). Research on children experiencing violence suggests that a number of domains related to cognitive, social and emotional functioning are adversely affected by exposure to such stressors. Children who are victims of violent acts or who witness violence have been found to have more externalizing and internalizing behavior problems, depressive symptoms, more aggression problems and are more likely to experience symptoms of posttraumatic stress disorder (PTSD) (Freeman et al. 1993; Martinez and Richters 1993; Campbell and Schwarz 1996; Mazza and Reynolds 1999; Schwab-Stone et al. 1999).
Chronic psychosocial stressors, including violence, may also result in physiologic alterations which may have even broader health effects (Wright 2006). Overlapping lines of evidence from both animal (Sanchez et al. 2001; Sanchez et al. 2005; Sanchez 2006) and human (Gunnar 1998; Gunnar and Donzella 2002) studies support the notion that physiologic stress pathways may be altered by chronic stress and early traumatic experiences. The health effects of early traumatic experiences, such as witnessing or being a victim of violent events, may be mediated through activation of the stress-response system resulting in dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and poor regulation of cortisol, the final product of the HPA system.
Typically, cortisol levels among adults as well as children rise in the morning, approximately thirty minutes after awakening, and decrease throughout the day (Gunnar and Donzella 2002). This pattern of cortisol activity has been observed in children as young as 2 months of age (Gunnar and Donzella 2002). A growing number of studies have demonstrated altered cortisol secretion among maltreated and neglected children (Gunnar et al. 2001; Tarullo and Gunnar 2006). For example, Cicchetti et al found higher morning and afternoon cortisol levels among maltreated children exhibiting internalizing symptoms (i.e., depression and anxiety disorders), compared to non-maltreated children experiencing symptoms (Cicchetti and Rogosch 2001). Maltreated children with posttraumatic stress symptoms have also been shown to have elevated cortisol levels throughout the day (Carrion et al. 2002). Elevated daytime salivary cortisol levels have also been found among 6- to 12-year-old children reared in Romanian orphanages several years after adoption (Gunnar, Morison et al. 2001). In contrast, other studies have found lower morning cortisol levels and a flattened daily cortisol rhythm among abused and maltreated children (Heim et al. 2000; Gunnar and Vazquez 2001). Data on the diurnal cortisol rhythm in pediatric samples outside of these selective populations and clinical samples is sparse.
Only a few studies have begun to examine the impact of community violence exposure on physiologic markers of stress response in children (Wilson et al. 2002; Murali and Chen 2005; Clark et al. 2006; Kliewer 2006). Murali and Chen examined the effects of violence on laboratory assessments of cardiovascular and neuroendocrine measures, both at baseline and in response to an interpersonal laboratory stressor (a debate task or a puzzle task) in 115 urban high school students ages 16 to 19 years. Increased frequency of lifetime violence exposure was associated with higher basal diastolic blood pressure, heart rate, and cortisol level. These relationships held even when they considered only remote exposure to violence (i.e., occurring more than a year ago). Higher levels of violence exposure also predicted decreased cardiovascular reactivity to the acute laboratory stressor based on the physiologic indicators (i.e., blood pressure, heart rate, heart rate variability); there was no difference in the change in cortisol in relation to the acute laboratory stressor comparing those with high violence vs. low violence exposure (Murali and Chen 2005). Kliewer examined the association between salivary cortisol levels and exposure to violence in 101 African American youth (Kliewer 2006). Witnessed violence was associated with both lower baseline cortisol (measured in the laboratory) and with increases in cortisol in response to a mild stressor (viewing and discussing a video depicting community violence). These authors additionally assessed morning levels of cortisol in these adolescents taken in the home setting. Girls with higher levels of witnessed violence had atypical awakening response patterns (i.e., declining cortisol from awakening to 1 hour after awakening without a rise). These data link community violence exposure with disruption of stress reactivity pathways, including the HPA axis albeit neither considered diurnal variation in cortisol expression throughout the day in a non-laboratory setting.
Moreover, existing evidence on stress hormones in PTSD is based in clinical samples and samples of other selective populations (Breslau 2006). Although a few studies have begun to examine the relationships among PTSD symptomatology and cortisol expression in community adult samples, (Young and Breslau 2004; Young et al. 2004) none have assessed these associations in non-clinical urban samples of children.
We expand the literature in the current study which aims to examine the relationships among posttraumatic stress symptoms reported in relationship to community violence experiences, and basal diurnal salivary cortisol response among a community-based cohort of urban school-aged children.
Methods and Materials
Study Population
The study is nested in a larger community-based cohort of mothers and their children aimed at examining the influence of environmental factors, including chronic stress, on respiratory outcomes as previously described in detail (Hanrahan et al. 1992). In brief, pregnant women receiving prenatal care at an urban community health center in Boston, MA, were enrolled between March 1986 and October 1992 prior to the 20th week of gestation. Women who did not speak either English or Spanish, who did not plan to have pediatric follow-up at the clinic, and whose age was less than 18 years at the time of delivery were excluded. One thousand women were eligible and enrolled, of whom 848 continued participation and delivered a live-born infant. In November 1996 new study initiatives were implemented at which time approximately 500 women continued active follow-up in the parent study. Of them, 412 gave voluntary written consent and completed a survey on their children’s violence exposure. Those who did not participate in the violence assessment were more likely to be White- Non Hispanics and current smokers (Hispanics, 45% non-responders vs. 52% responders; current smokers, 42% non-responders vs. 28% responders). The subsample presented in these analyses was randomly selected from the larger community-based study for the purpose of conducting a pilot study of a home-based collection protocol for salivary cortisol. Participants did not differ from non-participants based on demographics, violence exposure or distress symptoms. Fifty-five children were asked to participate and 43 completed the protocol. In the longitudinal study, detailed data had been ascertained through standardized questionnaires administered at baseline, clinic follow-up visits and medical record review as previously described (Hanrahan, Tager et al. 1992). The study protocol was approved by the human studies committees at both the Brigham & Women’s Hospital and the Beth Israel Deaconess Medical Center.
Exposure to Violence
Violence exposure was ascertained when the children were between four and ten years of age, using a modified version of the Survey of Children’s Exposure to Community Violence (ETV) (Richters and Saltzman 1990). The ETV is a multi-item survey structured to gather data on direct victimization and witnessing violence as well as factors known to influence the impact of violence (e.g. familiarity with the perpetrator or victim, frequency of events and the setting of the exposure. i.e. whether the events occurred at home) (Selner-O'Hagan et al. 1998). The survey measured lifetime exposure to specific violent events including hearing gunshots, and witnessing and/or experiencing shoving, hitting or punching, knife attacks, shootings, and witnessing verbal abuse of their primary caregiver. Mothers were asked to report on their child’s lifetime exposure to violence. In addition children who were eight years of age or greater (N=22) reported on their own community violence exposure. Acceptable internal consistency, test-retest reliability, and validity have previously been described for this scale (Selner-O'Hagan, Kindlon et al. 1998; Thomson, Roberts et al. 2002).
We implemented Rasch modeling techniques to summarize responses to the community ETV questionnaire into a continuous score as previously described (Suglia et al. 2007). The Rasch model produces two scores, one based on the parent’s responses to the violence survey, the other on the child’s responses to the survey. If a child is missing his or her self-report the model predicts the rasch score based on the existing correlation between the parent and child responses in the remainder of the cohort, so that all children have two scores. The final violence score is the average of the two rasch scores (Suglia et al. 2008; Horton et al. 2008). This is a desirable approach here as children are unlikely to be able to recall events occurring during very early childhood at the same time that parents may be more likely to underreport events that occur when children are older and violence occurs outside of the supervision of parents (Howard et al. 1999; Kuo et al. 2000).
Psychological Distress Symptoms
At the time of the violence assessment, mothers also reported on child posttraumatic stress symptoms over the past 6 months related to their exposure to community violence using the Checklist of Children’s Distress Symptoms (CCDS) (Richters and Martinez 1993). The CCDS which is based on diagnostic criteria described in the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (American Psychiatric Association 1987) was originally developed to assess distress in youth related to community violence exposure. The 28-item scale assesses children’s posttraumatic stress symptoms anchored to the experiences of community violence. Examples of symptoms assessed on this scale include difficulty with attention or sleep, intrusive thoughts, flashbacks, worries, and reminders of things that have happened in the past. Responses are based on a 5-point scale ranging from 1, “never” to 5, “most of the time”. The scale includes subscales on intrusive thoughts, emotional numbness, avoidant behavior, arousal and hopelessness or despondency about the future (Li et al. 1998; Howard et al. 2002). Many of these symptoms and behaviors including hypervigilance and emotional numbing as well as avoidant behavior and intrusive thoughts are defined components of PTSD while the despondency subscale is more closely related to depression and hopelessness (Wilson and Keane 1997). An overall total CCDS score was considered in the analyses as well as the separate subscale scores. Higher scores correspond to more adverse psychological symptoms.
Cortisol Measures
During longitudinal follow-up, salivary cortisol was collected from the children when they were between 7 and 13 years of age. The protocol consisted of a home-based assessment of salivary cortisol collected by the passive drool technique on 3 days (Kirschbaum and Hellhammer 1994; Strazdins et al. 2005). Parents and their children were given verbal instructions by trained research staff when saliva tubes were supplied as well as brief daily diaries repeating these instructions for recording adherence to the collection protocol. Subjects were instructed not to eat, brush their teeth, or drink liquids for at least 15 minutes before taking a sample. They provided 4 samples each day of collection: 30 minutes after awakening (“when your eyes open and you are ready to get up”), before lunch, before dinner, and at bedtime (“right before getting into bed”) similar to collection protocols in prior research (Carrion, Weems et al. 2002). They were also instructed to record the time that they woke up (in the log book) and the date and the exact time that each sample was taken (on the tube label and log book). After each collection, the tubes were placed in a home freezer. Samples were picked up in person by research staff on the fourth day and kept frozen in a cooler until they arrived at the Reproductive Ecology Laboratory at Harvard University where the cortisol assays were conducted. Salivary cortisol samples were assayed using radioimmunoassay (RIA) following procedures detailed by Ellison (Ellison 1988). Data were analyzed using a computerized RIA program, which calculated cortisol concentrations (nmol/l) from the counts per minute. The inter- and intra-assay coefficients of variation were 10% and 7.37% respectively. The sensitivity limit (the lowest value of cortisol distinguishable from 0 with 95% confidence) averaged 8 × 10−4 pmol/l for all assays.
Forty-three children completed the saliva protocol and provided samples. To limit variation between collection times we restricted our analyses to samples that were taken during the following time windows: 30 minutes after awakening to 1.5 hours (time 1), between 3 and 6.5 hours after waking (time 2), 7.5 to 11.5 hours after waking (time 3) and more than 11.5 hours after waking (time 4). These time windows were selected a priori as previously done in other studies (Cohen et al. 2006). The variation in sampling times within each sampling interval appeared random in that they were not related to the level of community violence exposure or CCDS symptoms (Pearson correlation). The children provided 516 saliva samples, 88 samples were excluded from analyses because they did not meet the sampling time windows, leaving 428 samples for analyses.
Statistical Analyses
Guided by previously described methodologies (Polk et al. 2005), we examined the relationships among exposure to violence, child posttraumatic stress symptoms and cortisol levels at each time point of collection as well as the area under the curve (AUC) and the diurnal slope over the waking day. Cortisol values were log transformed prior to analyses due to their skewed distribution. Diurnal slope is the slope of the log cortisol measurements over the course of the day. Slopes were estimated using the best linear unbiased predictor from a hierarchical mixed model that included random effects for subject and day within subject (Cohen et al. 2006). These estimates are "best" in the sense that they are unbiased and less variable than other linear estimators such as ordinary least squares estimates. The AUC was calculated as the area under a linear interpolation of the log cortisol measures over time for each day of measurement for each child.
Initially, Pearson correlation coefficients were performed to assess the relationships among community violence exposure, and posttraumatic stress symptoms assessed on the CCDS. To model the relationship between cortisol levels and posttraumatic stress symptoms we employed mixed models allowing for repeated diurnal slope and AUC measures (one per each day per each child). Additionally we used mixed models regression to estimate the effect of violence exposure and posttraumatic stress symptoms on log cortisol at each time point of measurement. All analyses were adjusted for race/ethnicity, socioeconomic status (maternal education), age and gender. While tobacco smoke exposure has been shown to alter cortisol secretion we did not adjust for tobacco exposure due to the high correlation with race/ethnicity in these data (i.e., of the 20 Hispanic women in our sample only 1 smoked during pregnancy, 35% of the White women smoked during pregnancy). All statistical analyses were done using SAS version 9.0 (SAS Institute, Cary, NC).
Results
Table 1 summarizes the demographic characteristics of the population and descriptive statistics for all other covariates. Of the 43 children, 65% were female, 54% white, 46% Hispanic and 42% had mothers with less than a high school education. Means and standard deviations for the violence exposure scale, cortisol levels aggregated across the three days for each time interval of collection as well as summary variables, and the CCDS subscale scores are also depicted.
Table 1.
Population Characteristics and Descriptive Statistics for Covariates
| Demographics | N | % |
|---|---|---|
| Child’s age | ||
| (Mean, SD and Range) | 10.3 ± 1.8 (7–13) | |
| Child’s Gender | ||
| Male | 15 | 34.9 |
| Female | 28 | 65.1 |
| Race/Ethnicity | ||
| White | 23 | 53.5 |
| Hispanic/Black/Other* | 20 | 46.5 |
| Mother’s Education Level | ||
| Some College | 7 | 16.3 |
| High School Graduate / Technical School | 18 | 41.9 |
| Less than High School/ No Graduation | 18 | 41.9 |
| Mean | SD | |
| Cortisol Measures 1 (time after awakening) | ||
| Time 1 (5min to 1.5hr) | 5.23 | 4.13 |
| Time 2 (3–6.5hr) | 2.06 | 2.76 |
| Time 3 (7.5–11.5hr) | 1.88 | 2.94 |
| Time 4 (11.5hr + ) | 1.04 | 1.78 |
| Diurnal Slope | −0.13 | 0.03 |
| AUC | 87.6 | 16.4 |
| Checklist of Child Distress Symptoms (CCDS) | ||
| CCDS overall score | 1.73 | 0.70 |
| Hopelessness | 1.42 | 0.68 |
| Numbness | 1.3 | 0.50 |
| Arousal | 2.1 | 0.87 |
| Intrusion | 1.45 | 0.80 |
17 Children identified as Hispanic, 1 Black and 2 as other race/ethnicity
Units for cortisol in nmol/l; for conversion to mg/dl divide by 27.6
The community violence scale was modestly correlated with the overall posttraumatic stress symptoms score (Pearson correlation r=0.26 p=.09) as well as the subscales derived from the CCDS measure (r ranged from 0.07 to 0.31), similar to what has been found in other urban populations (Howard, Feigelman et al. 2002). The community violence scale was most strongly correlated with intrusive symptoms (r=0.31, p=0.04) on the posttraumatic stress scale which also corroborates previous findings by Howard and colleagues (Howard, Feigelman et al. 2002). The community violence scale was not significantly associated with the cortisol indicators.
We did find consistent and robust relationships between the overall posttraumatic stress symptom scale and subscale scores and cortisol disruption. In unadjusted analyses (data not shown) the posttraumatic stress symptom overall score from the CCDS was positively associated with cortisol AUC and diurnal slope as well as the cortisol measures taken at time 3 (before lunch) and time 4 (before dinner) (all p < 0.05).
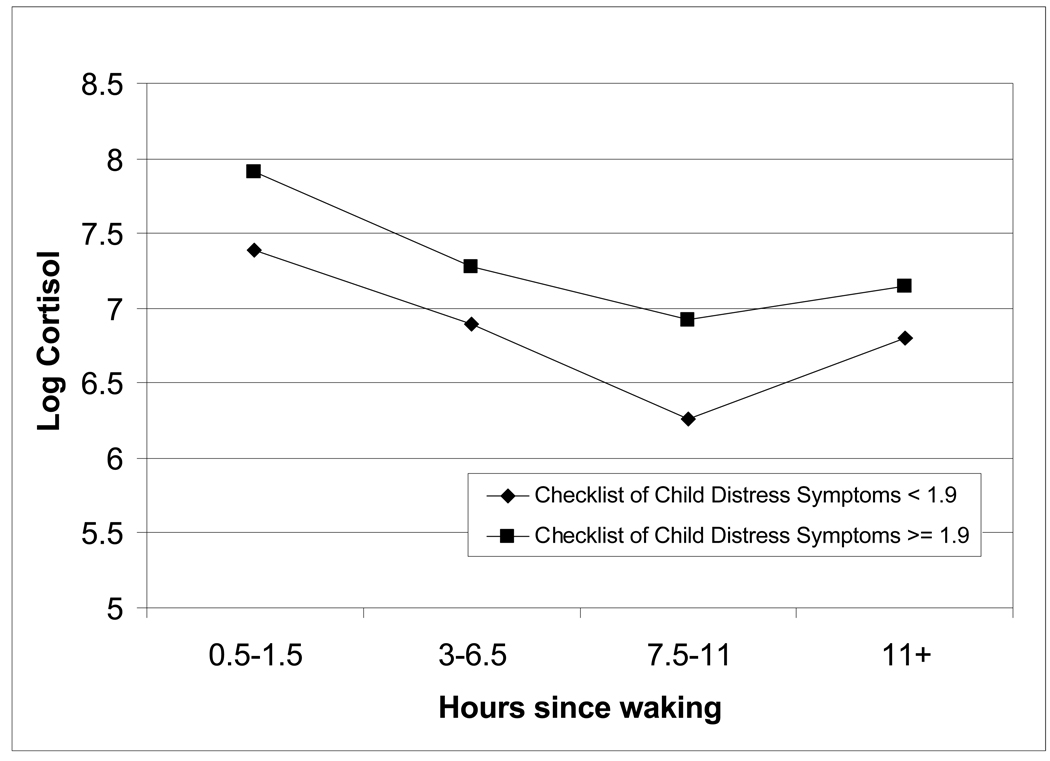
We assessed the relationship between the overall score and subscales on the CCDS (as a continuous measure) and cortisol outcomes, adjusting for socio-demographic factors (Table 2). A number of the posttraumatic stress symptom subscales as well as the overall score strongly predicted cortisol measures in these separate regression models. Diurnal slope was positively associated with the arousal, avoidance and intrusion subscales and with the overall posttraumatic stress score indicating that higher CCDS scores associate with cortisol levels that do not decrease as quickly over the course of the day. In addition the AUC was positively associated with the arousal, avoidance and intrusion subscale and with the overall CCDS score indicating that those with higher posttraumatic stress symptom scores also had higher average cortisol levels over the course of the day. In analyses of the time-specific cortisol measures, cortisol levels were positively associated with the arousal, avoidance, intrusion and numbness subscales and with the overall posttraumatic stress scores among measures obtained before dinner (time 3) and before bedtime (time 4). The adjusted association between, posttraumatic stress symptoms overall score and cortisol is also shown in Figure 1. Children with an overall posttraumatic symptom score greater than 1.9 (highest quartile of CCDS score), had higher salivary cortisol levels throughout the day.
Table 2.
Child distress symptoms (per one unit change) on cortisol response (nMol/L) by time of collection, AUC and Diurnal slope1
| Time 1 (waking- 1.5hr) |
Time 2 (3–6.5hr) |
Time 3 (7.5– 11.5hr) |
Time 4 (11.5+ hrs) |
AUC |
Diurnal Slope |
|
|---|---|---|---|---|---|---|
| Estimate | Estimate | Estimate | Estimate | Estimate | Estimate | |
| CCDS 2 | ||||||
| Overall Score | 0.1640 | 0.2720† | 0.4556* | 0.3883* | 5.2632* | 0.0102* |
| Hopelessness | −0.0516 | 0.2379 | 0.2116 | 0.2880†* | 1.9227 | 0.0080 |
| Numbness | −0.0163 | 0.4206† | 0.2589 | 0.4720* | 4.5320 | 0.0099 |
| Arousal | 0.1956 | 0.1988 | 0.3657* | 0.2860* | 4.7409* | 0.0071† |
| Avoidance | 0.0538 | 0.2102† | 0.3735* | 0.2502* | 3.6407* | 0.0079* |
| Intrusion | 0.1316 | 0.2008 | 0.4867* | 0.3916* | 5.2831* | 0.0114* |
Each line represents a separate regression model. All models are adjusted for age, gender, race/ethnicity, and maternal education
p-value <0.10;
p-value < 0.05,
Checklist of Child Distress Symptoms
Figure 1.
Mean cortisol level (nmol/L) by severity of symptoms on the Checklist of Child Distress Symptoms and time of collection
Significant differences between CCDS groups were noted at time 1 and time 3 (p< 0.05).
Adjusted for age, gender, race/ethnicity, and maternal education
Given the variability in the timing of collection for the morning sample, it is possible that the peak value for the cortisol morning response is included for some subjects which may influence the diurnal slope (Cohen, Doyle et al. 2006). We thus ran a sensitivity analysis calculating the diurnal slope excluding the morning value and found that the effect estimates shown in Table 2 changed only slightly and remained statistically significant (data not shown).
Discussion
In this urban community-dwelling sample of school-aged children higher levels of posttraumatic stress symptoms reported in response to community violence exposure predicted elevated cortisol levels, especially in the afternoon and evening samples, and a blunted diurnal slope, even when adjusting for race, gender, age, and socioeconomic status. Stronger associations were found for the numbness, avoidance, intrusion and arousal subscales, defined components of PTSD, and not with the hopelessness subscale which is more closely related with depression (Wilson and Keane 1997; Li, Howard et al. 1998).
These findings are strikingly similar to diurnal salivary cortisol patterns reported by Carrion and colleagues in a pediatric clinical sample who met criteria for a diagnosis of PTSD in which a parallel home salivary cortisol collection protocol with similar timing of collection was used (Carrion, Weems et al. 2002). Subjects were also similarly aged (mean 10.7 years, range 7 to 14 years). Unlike our community sample, children in that study were recruited from local social service departments and mental health clinics. In the clinical sample, all children had been exposed to interpersonal trauma and met criteria for PTSD based on a score of 12 or greater on the PTSD Reaction Index (Nader et al. 1990). The clinical sample had significantly higher cortisol levels later in the day (samples collected in the afternoon and evening) relative to age- and gender-matched archival controls. Moreover, these investigators found no differences between children meeting criteria for PTSD and children with sub threshold symptoms in terms of elevated cortisol levels. These findings are also consistent with those of De Bellis and colleagues (De Bellis et al. 1999) who reported elevated levels of cortisol in 24-hour urine collections in children with a history of maltreatment when compared to controls. However, they conflict with a number of studies of adults and older adolescents with trauma histories and PTSD (Goenjian et al. 1996). This may reflect developmental differences given the ages across these studies as well as variation in cortisol collection procedures (De Bellis, Baum et al. 1999; Gunnar et al. 2001). While stress may elicit elevated cortisol levels among children (Gunnar, Morison et al. 2001; Carrion, Weems et al. 2002), under prolonged stress the stress-cortisol response may be further disrupted resulting in decreased cortisol expression in adulthood (Miller et al. 2007). It could be that, if symptoms persist these children would later show lower cortisol levels if followed longitudinally. Future studies with repeated assessment related to traumatic stress exposure and the subsequent development of psychopathology and disrupted stress responses (e.g., HPA axis) will be needed to better delineate the natural history of the psychobiological stress response (e.g., cortisol) following exposures such as community violence.
As in any epidemiologic study we acknowledge a number of limitations. As is typical with longitudinal studies, there was significant reduction in the sample available from the original cohort over time. The non-participation of subjects from the longitudinal study may be seen as a limitation albeit there were no differences based on socio-demographics, violence exposure or distress symptoms, comparing those who had cortisol assessed versus those who did not among the participants who remained in follow-up. Thus, this unlikely influenced our findings. In addition, although the relatively small sample size was large enough to reveal significant effects, a larger sample size could increase the precision of our estimates. Moreover, a larger sample size may allow for the more formal testing of a mediation model, i.e., community violence events leading to PTSD leading to cortisol disruption.
Notably we demonstrated associations between posttraumatic stress symptoms and cortisol measures later in the day (afternoon and evening) but not in the morning. It is possible these associations reflect an aggregate effect of daily challenges making the afternoon measures more sensitive to chronic stressors (Cohen, Doyle et al. 2006). Another explanation is that the association actually does occur across the day, but because the diurnal slope tend to be very steep from early morning to early afternoon, relatively small errors in the timing of samples (which is difficult to avoid in naturalistic studies) may result in large errors in cortisol measurement. Thus the explanation may lie more in the collection procedures. Indeed, while we asked mothers to provide the morning samples 30 minutes after the child awakened, only 75% of samples were collected within 25–35 minutes of awakening. Some of the morning samples were collected as close to 5 minutes after awakening while the rest were collected one hour after awakening with one additional sample collected one and a half hours after awakening. This wide sampling frame may include the peak morning cortisol response for some subjects which may influence the diurnal slope although a sensitivity analysis suggested this did not explain our results.
In summary, subclinical PTSD symptomatology reported in relation to community violence exposure significantly predicts cortisol disruption in this urban community-dwelling non-clinical pediatric sample. Moreover, the cortisol response among children with greater distress symptoms parallels diurnal cortisol patterns previously reported in a clinical sample of traumatized children with diagnosed PTSD in the same age range (Carrion, Weems et al. 2002). Further research to corroborate these preliminary findings is needed; if the posttraumatic stress symptoms are a result of the experiences these children encounter as exigencies of their urban environment (e.g., crime and violence), which is highly prevalent in these neighborhoods, a large number of children may be impacted. Psychological sequelae in this context may also result in physiologic alterations which may have even broader health effects (Wright 2006).
Acknowledgements
We would like to thank Andrew Curran and Grace Chan who were very helpful in the salivary cortisol collection. Data collection for this study was funded by K08 HL 04187 and a Deborah Monroe Noonan Foundation grant. During preparation of this manuscript Shakira Franco Suglia was supported by T32 MH073122; Rosalind J Wright was supported by R01 ES10932 and U01 HL072494.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association; 1987. [Google Scholar]
- Breslau N. Neurobiological research on sleep and stress hormones in epidemiological samples. Ann N Y Acad Sci. 2006;1071:221–230. doi: 10.1196/annals.1364.017. [DOI] [PubMed] [Google Scholar]
- Campbell C, Schwarz DF. Prevalence and impact of exposure to interpersonal violence among suburban and urban middle school students. Pediatrics. 1996;98(3 Pt 1):396–402. [PubMed] [Google Scholar]
- Carrion VG, Weems CF, et al. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry. 2002;51(7):575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol. 2001;13(4):783–804. [PubMed] [Google Scholar]
- Clark R, Benkert RA, et al. Violence exposure and optimism predict task-induced changes in blood pressure and pulse rate in a normotensive sample of inner-city black youth. Psychosom Med. 2006;68(1):73–79. doi: 10.1097/01.psy.0000195744.13608.11. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, et al. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68(1):41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- De Bellis M, Baum A, et al. Development traumatology: I. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Ellison P. Human salivary steroids: methodological considerations and applications in physical anthropology. Yearbook of Physical Anthropology. 1988;31:115–142. [Google Scholar]
- Freeman LN, Mokros H, et al. Violent events reported by normal urban school-aged children: characteristics and depression correlates. J Am Acad Child Adolesc Psychiatry. 1993;32(2):419–423. doi: 10.1097/00004583-199303000-00025. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, et al. Basal cortisol, dexamethasone suppression of cortisol, and MHPG in adolescents after the 1988 earthquake in Armenia. Am J Psychiatry. 1996;153(7):929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev Med. 1998;27(2):208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Bruce J, et al. Salivary cortisol response to stress in children. Adv Psychosom Med. 2001;22:52–60. doi: 10.1159/000059275. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, et al. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13(3):611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Tager IB, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145(5):1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, et al. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Horton NJ, Roberts K, et al. A maximum likelihood latent variable regression model for multiple informants. Stat Med. 2008 doi: 10.1002/sim.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DE, Cross SI, et al. Parent-youth concordance regarding violence exposure: relationship to youth psychosocial functioning. J Adolesc Health. 1999;25(6):396–406. doi: 10.1016/s1054-139x(99)00102-0. [DOI] [PubMed] [Google Scholar]
- Howard DE, Feigelman S, et al. The relationship among violence victimization, witnessing violence, and youth distress. J Adolesc Health. 2002;31(6):455–462. doi: 10.1016/s1054-139x(02)00404-4. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kliewer W. Violence exposure and cortisol responses in urban youth. International Journal of Behavioral Medicine. 2006;13(2):109–120. doi: 10.1207/s15327558ijbm1302_2. [DOI] [PubMed] [Google Scholar]
- Kuo M, Mohler B, et al. Assessing exposure to violence using multiple informants: application of hierarchical linear model. J Child Psychol Psychiatry. 2000;41(8):1049–1056. [PubMed] [Google Scholar]
- Li X, Howard D, et al. Distress symptoms among urban African American children and adolescents: a psychometric evaluation of the Checklist of Children's Distress Symptoms. Arch Pediatr Adolesc Med. 1998;152(6):569–577. doi: 10.1001/archpedi.152.6.569. [DOI] [PubMed] [Google Scholar]
- Margolin G, Gordis EB. The effects of family and community violence on children. Annu Rev Psychol. 2000;51:445–479. doi: 10.1146/annurev.psych.51.1.445. [DOI] [PubMed] [Google Scholar]
- Martinez P, Richters JE. The NIMH community violence project: II. Children's distress symptoms associated with violence exposure. Psychiatry. 1993;56(1):22–35. doi: 10.1080/00332747.1993.11024618. [DOI] [PubMed] [Google Scholar]
- Mazza JJ, Reynolds WM. Exposure to violence in young inner-city adolescents: relationships with suicidal ideation, depression, and PTSD symptomatology. J Abnorm Child Psychol. 1999;27(3):203–213. doi: 10.1023/a:1021900423004. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, et al. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Murali R, Chen E. Exposure to violence and cardiovascular and neuroendocrine measures in adolescents. Ann Behav Med. 2005;30(2):155–163. doi: 10.1207/s15324796abm3002_8. [DOI] [PubMed] [Google Scholar]
- Nade K, Pynoos R, et al. Children's PTSD reactions one year after a sniper attack at their school. Am J Psychiatry. 1990;147(11):1526–1530. doi: 10.1176/ajp.147.11.1526. [DOI] [PubMed] [Google Scholar]
- Osofsky JD, Wewers S, et al. Chronic community violence: what is happening to our children? Psychiatry. 1993;56(1):36–45. doi: 10.1080/00332747.1993.11024619. [DOI] [PubMed] [Google Scholar]
- Polk DE, Cohen S, et al. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30(3):261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Richters JE, Martinez P. The NIMH community violence project: I. Children as victims of and witnesses to violence. Psychiatry. 1993;56(1):7–21. doi: 10.1080/00332747.1993.11024617. [DOI] [PubMed] [Google Scholar]
- Richters JE, Saltzman W. Survey of Children's Exposure to Community Violence: Parent report. Rockville, MD: National Institute of Mental Health; 1990. [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, et al. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13(3):419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, et al. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Scott R, et al. Exposure to violence among inner-city youth. J Adolesc Health. 1993;14(3):214–219. doi: 10.1016/1054-139x(93)90008-d. [DOI] [PubMed] [Google Scholar]
- Schwab-Stone M, Chen C, et al. No safe haven. II: The effects of violence exposure on urban youth. J Am Acad Child Adolesc Psychiatry. 1999;38(4):359–367. doi: 10.1097/00004583-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Selner-O'Hagan MB, Kindlon DJ, et al. Assessing exposure to violence in urban youth. J Child Psychol Psychiatry. 1998;39(2):215–224. [PubMed] [Google Scholar]
- Sheehan K, DiCara JA, et al. Children's exposure to violence in an urban setting. Archives of Pediatrics and Adolescent Medicine. 1997;151(5):502–504. doi: 10.1001/archpedi.1997.02170420072012. [DOI] [PubMed] [Google Scholar]
- Strazdins L, Meyerkort S, et al. Impact of saliva collection methods on sIgA and cortisol assays and acceptability to participants. J Immunol Methods. 2005;307(1–2):167–171. doi: 10.1016/j.jim.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Ryan L, Laden F, Dockery DW, Wright RJ. Violence exposure, a chronic psychosocial stressor, and childhood lung function. Psychosom Med. 2008 Feb;70(2):160–169. doi: 10.1097/PSY.0b013e318160687c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Ryan L, Wright RJ. Creation of a community violence exposure scale: accounting for what, who, where, and how often. J Trauma Stress. 2008 Oct;21(5):479–486. doi: 10.1002/jts.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Thomson CC, Roberts K, et al. Caretaker-child concordance for child's exposure to violence in a preadolescent inner-city population. Arch Pediatr Adolesc Med. 2002;156(8):818–823. doi: 10.1001/archpedi.156.8.818. [DOI] [PubMed] [Google Scholar]
- Wilson DK, Kliewer W, et al. Violence exposure, catecholamine excretion, and blood pressure nondipping status in African American male versus female adolescents. Psychosom Med. 2002;64(6):906–915. doi: 10.1097/01.psy.0000024234.11538.d3. [DOI] [PubMed] [Google Scholar]
- Wilson J, Keane TM. Assessing Psychological Trauma and PTSD. New York, NY: The Guilford Press; 1997. [Google Scholar]
- Wright RJ. Health effects of socially toxic neighborhoods: the violence and urban asthma paradigm. Clin Chest Med. 2006;27(3):413–421. doi: 10.1016/j.ccm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biol Psychiatry. 2004;56(3):205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Young EA, Tolman R, et al. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol Psychiatry. 2004;55(6):621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]