Abstract
Cell death provokes a robust inflammatory response. We have previously shown that this response is dependent on IL-α. Here we investigate the cellular mechanism used by a host to sense cell death, produce IL-α and also the role of IL-β in this response. In almost all cases examined, the IL-1 that stimulated the death-induced inflammatory response came from the host rather than the cell that was dying. In these situations, host bone marrow-derived cells were the key source of the IL-α that was required for the inflammatory response. Conditional cellular depletion and reconstitution in CD11b promoter- driven diphtheria toxin receptor transgenic mice revealed that host macrophages played an essential role in the generation of the inflammatory response and were the source of the required IL-α. In addition, we found a role for IL-β in the death-induced inflammatory response and that this cytokine was generated by both bone marrow-derived and radioresistant host cells. The one exception to these findings was that when dendritic cells were injected into mice, they provided a portion of the IL-1 that stimulated inflammation, and this was observed whether the dendritic cells were live or necrotic. Together, these findings demonstrate that macrophages play a key role as the primary sentinels that are required to sense and report cell death in ways that initiate the inflammatory response. One key way they accomplish this important task is by producing IL-α that is needed to initiate the inflammatory response.
Introduction
When cells die in vivo, particularly by necrosis, they stimulate a robust inflammatory response (1) (2). Local arterioles dilate, venules leak protein-rich fluid and neutrophils, followed by monocytes, extravasate into the tissues. This reaction to cell death is seen in virtually all vascularized tissues and is so stereotypical that its progression is used forensically to date the time of injury (3).
The inflammatory response to cell injury is important because it has a number of consequences for the host (4) (5, 6). On the positive side, the response rapidly delivers the soluble and cellular innate defenses to the site of death, where they may neutralize or sequester the injurious agent, such as an infection. Additionally, the recruited cells can also clear cellular corpses and promote tissue repair. On the negative side the innate defenses are indiscriminate and once mobilized can injure friend and foe alike. As a result, there is often collateral damage to otherwise non-diseased cells resident at the site of inflammation. In situations of sterile cell death there is often little or nothing that the mobilized innate immune mechanisms can defend against and consequently the net effect of the process may be to do more harm than good. Because of this the inflammatory response to sterile cell death can exacerbate or even cause disease (7–12).
Despite the importance of the cell-death induced inflammatory response and its universality, its underlying mechanisms are poorly understood. In general terms it is believed that the immune system generates this response because cell death may be a sign of a dangerous process that is a threat to the host (4–6, 13). To detect cell injury it is thought that the innate immune system has evolved mechanisms to recognize and respond to molecules that are exposed on or released from dying cells (5, 14). It has been suggested that complement, natural antibody, or various different leukocytes may be the elements of the immune system that are responsible for recognizing cell injury and initiating this inflammatory response (5, 6, 14–23); however, how the immune system actually does this is unresolved. This is an important issue because it is a fundamental response and one that is a potential target for therapeutic intervention.
A recent insight into cell death-induced inflammation was that the cytokine IL-1 played a critical role in this response (24–26). Mice that lack the IL-1 receptor type I (IL-1R) and therefore the ability to respond to IL-1 failed to generate almost any acute neutrophilic inflammation to dying cells but could respond normally to a microbial stimulus (24). Since the source of IL-1 is cellular, this discovery suggested that some type of cellular element was playing a key role in triggering the inflammatory response to tissue injury. Many different cell types can produce IL-1 and it is not known which of these plays the key role in this sterile inflammatory response (27, 28). Therefore, we undertook the present studies to investigate the nature of this cell and its role in the cell death-induced inflammatory response.
Materials and Methods
Reagent and antibodies
Antibodies against Ly-6G (clone 1A8), CD11b (M1/70) were obtained from BD Bioscience. Antibodies against F4/80 (BM8), MHC-class II (M5/114.15.2), CD11c (N418) were obtained from eBioscience. Anti- 7/4 antibody was purchased from Serotec. 7-AAD was obtained form Molecular probe. Recombinant mouse MIP-2 was from Peprotech (Rocky Hill, NJ). Diphtheria toxin A was obtained form Calbiochem. Acetaminophen was purchased form Sigma.
Animal and cell lines
Wild type C57BL/6, wild type FVB/N, IL-1 receptor deficient mice (29), CD11b-DTR mice on a FVB background (30, 31) were purchased from Jackson Laboratories. CD11c-DTR on a C57BL/6 background was obtained from Dr. Dan R. Littman (New York University Medical School, New York, NY) (32). IL-1α, IL-1β and IL-1αβ double deficient mice of C57BL/6 background were described before (33). Irradiation bone marrow chimera was prepared as described (34). All animal protocols were approved by the UMass animal care and use committee. EL4 cells were maintained in RPMI-1640 with 10% FCS and antibiotics. J2-virus immortalized macrophage cell line (a gift from Dr. Doug Golenbock, University of Massachusetts Medical School, Worcester, MA) was maintained in DMEM with 10% FCS. All the cultured cell liens are tested negative for mycoplasma (Lonza).
Preparation of necrotic cells
Bone marrow-derived dendritic cells (BMDCs) were obtained from cultures of bone marrow cells of C57BL/6 or IL-1 deficient mice with IL-4 (10 ng/mL, Invitrogen) and GM-CSF (5 ng/mL, Invitrogen) in Hybridoma culture medium for 7 days. A single cell suspension of liver was obtained by collagenase type IV treatment as previously described (35). To induce necrosis from thermal injury, BMDCs, liver or EL4 cells were washed 5 times with PBS and then resuspended in PBS at 10 million cells / 50 microL and then heat-shock at 45°C for 10 min followed by 37°C incubation for 5 hours; this resulted in necrosis (7-AAD/PI positive cells). To induce mechanical necrosis, brain, liver or heart from C57BL/6 or IL-1 deficient mice were weighed and added with 5 times of weight of PBS and subjected to mechanical injury by a motor-driven tissue tearer followed by nitrogen cavitation for 10 min at 500 psi. Similarly, mechanical necrosis was induced in EL4 and BMDCs by nitrogen cavitation for 10 min at 500 psi. After thermal injury there were no viable cells observed by microscopy with Trypan blue staining. After mechanical injury no viable cells were seen by microscopy and when analyzed by flow cytometry typically 99.9% of the cells were disrupted into fragments. The resulting necrotic cell suspensions were used for experiments without any clarification (i.e. containing all released cellular components and debris).
Neutrophil recruitment to peritoneal cavity
Mice were injected i.p. with 5 million necrotic BMDCs in 500 microL of PBS, 30 million of necrotic EL4 cells in 150 microL of PBS, 150 microL of liver homogenate, or 500 microL of heart or brain homogenate unless otherwise indicated. The total protein amounts in necrotic cell preparations that were injected were 4.1 mg (dendritic cells), 12.6 mg (EL4), 72.4 mg (brain), 25.4 mg (liver) or 77.0 mg (heart). The amount of the necrotic dendritic cells, brain, liver and heart preparations that was injected was one that was determined to induce a non-maximal response so as to be on a sensitive portion of the dose response curve (data not shown). After 13 –15 hour of injection, the peritoneum was lavaged with 6 mL of PBS with 2%FCS, 3mM of EDTA and 10U/mL of heparin. The absolute number of neutrophil (Ly-6G+ 7/4+) in 100 microL of lavage was counted using flow cytometer equipped with a high throughput sampler (BD bioscience).
Diphtheria toxin receptor transgenic mice
CD11c-DTR mice were injected i.p. with 100 ng of diphtheria toxin A (DTA) to eliminate CD11c+ cells in vivo. CD11b DTR mice received 500 ng of DTA i.p. or i.v. to deplete CD11b+ macrophages in vivo. Twenty four hours after DTA administration, CD11b-DTR mice were reconstituted with either 4 million naïve peritoneal cells, thioglycolate-induced peritoneal elicited cells (PECs), magnetic beads –purified F4/80+ PECs, an immortalized macrophage cell clone. Five hours after reconstitution, mice were challenged with either 150 microL of liver homogenate or 30 million of necrotic EL4 cells. F4/80+ macrophages were purified from thioglycolate -PECs of FVB/N mice using biotinylated anti F4/80 antibody and anti biotin antibody - magnetic beads (Mylteni).
Acetaminophen induced liver injury
Mice were fasted for 18 hours and injected i.p. with 300 mg/kg of acetaminophen. Liver was perfused with PBS and harvested after 18 hours of acetaminophen injection. One hundred mg of liver was homogenated in 1 mL of myeloperoxidase buffer (0.5% hexadecyl trimethyl ammonium bromide, 10 mM EDTA, 50 mM Na2HPO4, pH 5.4), sonicated and subjected to myeloperoxidase activity measurement as described (24).
Statistical analyses
Data are reported as means ± standard errors. Statistical analysis in each independent experiment was performed with an unpaired, two-tailed Student'st-test. One - way ANOVA and Dunnett's multiple comparison post- test were used to compare the means of multiple groups to the control group. In some cases Bonferroni's multiple comparison post- test was used to compare among multiple groups. P < 0.05 was considered statistically significant.
Results
The source of IL-1 in the cell death-induced inflammatory response: Release from dying cells or production by the host?
We have previously reported that IL-1α was essential for the acute neutrophilic inflammatory response stimulated by sterile cell death, however, the source of this cytokine was not known. It is possible that IL-1α comes from a pool of preformed cytokine released from dying cells, as recently suggested for bone marrow- derived dendritic cells (36). Alternatively, IL-1α could be produced by cells in the host that recognize and respond to dying cells. To evaluate the role of these different mechanisms we performed several experiments.
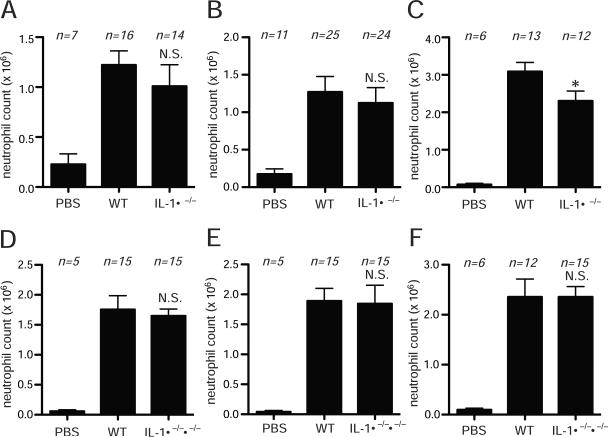
To examine the role of IL-1 from dying cells, we injected i.p. buffer or a variety of primary necrotic cells from wild type or IL-1α -deficient animals and quantified the resulting influx of neutrophils into the peritoneum. Injection of necrotic brain, and liver from IL-1α −/− mice (killed by mechanical injury) stimulated as much neutrophilic inflammation as did the same tissues from wild type animals (Fig 1A, B). Similarly, inflammation to necrotic heart from IL-1α −/− mice was only modestly less that to the same tissue from wild type animals (Fig. 1C) (and whether this small reduction in inflammatory activity is meaningful is uncertain because it was not observed with necrotic heart from IL-1αβ-double deficient mice, as is described next). Similarly there was no reduction in inflammation to liver cells from IL-1α−/− mice that were made necrotic by thermal injury (Supplementary Fig. 1). Since dying cells could also release IL-1β that might contribute to inflammation, we also examined tissues from IL-1αβ double-deficient animals. The proinflammatory activity of brain, liver and heart was equivalent to wild type tissues (Fig. 1D, E, F).
Figure 1.
Requirement of IL-1 released from dying cells for neutrophil recruitment. (A, B, C) Necrotic brain homogenate (A), liver homogenate (B), or heart homogenate (C) from C57BL/6 (WT) or IL-1α−/− mice were injected i.p. into C57BL/6 mice. Total neutrophil number in the peritoneal cavity was measured 14 hours post injection. (D, E, F) Total neutrophil number in the peritoneal cavity of C57BL/6 mice in response to i.p. injected necrotic brain homogenate (D), liver homogenate (E) or heart homogenate (F) derived from C57BL/6 (WT) or IL-1α−/−β−/− mice. All data are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). PBS groups, WT mice received i.p. PBS. *P<0.05, N.S., not significant, versus WT group.
The above results implied that the IL-1 driving the sterile inflammatory response was coming from cells in the host. To directly test this point, we injected necrotic EL4 cells i.p. into wild type or IL-1-deficient mice and again quantified the resulting influx of neutrophils into the peritoneum. The dead EL4 cells stimulate strong neutrophilic inflammation in wild type mice (Fig. 2A) as we have previously reported (24). In contrast, the neutrophil response to the injection of the dead cells into IL-1-deficient mice was markedly reduced. The neutrophilic inflammatory response was completely inhibited in mice lacking both IL-1α and IL-1β or the IL-1R (Fig. 2A). These responses were also substantially reduced, although not to background, in mice lacking just IL-1β or IL-1β (Fig. 2A). Similar results were obtained regardless of whether the EL4 cells were killed by mechanical or thermal injury (Supplementary Figure 2).
Figure 2.
Host derived IL-1 is required for neutrophil recruitment to dead cells. (A) Total neutrophil number of peritoneal cavity 14 hours after i.p. injection of heat - shocked necrotic EL4 cells in C57BL/6 WT, IL-1α−/−, IL-1β−/−, IL-1α−/−β−/− or IL-1R−/−. One - way ANOVA and Bonferroni's multiple comparison tests were used to compare all of the groups. There were no significant differences among groups of IL-1α−/−, IL-1β−/−, IL-1α−/−β−/− or IL-1R−/− (not indicated in the figure). (B) WT C57BL/6 derived liver homogenate was injected i.p. to C57BL/6 or IL-1αβ−/− mice. The total neutrophil number of peritoneal cavity 14 hours after i.p. injection are shown. All of them are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). PBS groups, WT mice received i.p. PBS. ***P<0.001, versus WT group.
Similarly, a substantial component of the neutrophilic inflammatory response to a necrotic primary tissue (liver) was also dependent on IL-1 production from the host (Fig. 2B). These results are consistent with our findings that IL-1-deficient cells stimulate robust neutrophilic inflammation and indicate that for many dying cells much if not all of the IL-1 driving the sterile inflammatory response is coming from cells of the host.
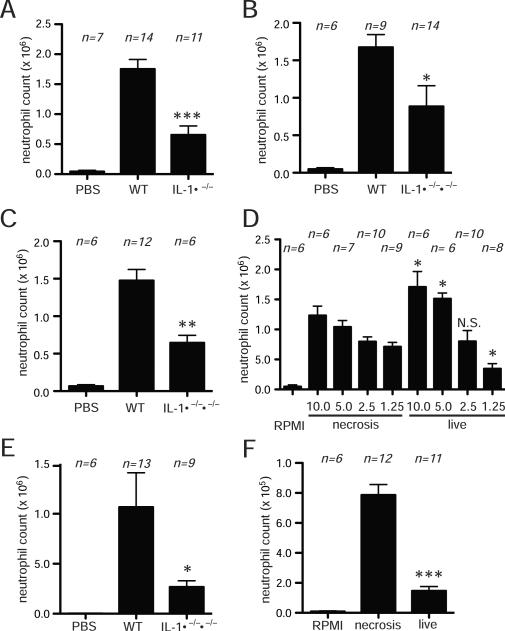
The one exception we observed was with dendritic cells. Similar to Eigenbrod et al. (36), we found that injection of necrotic bone marrow-derived dendritic cells from IL-1a−/− mice stimulated less inflammation than the ones from wild type mice, (Fig. 3A). Similar results were observed whether the bone marrow-derived dendritic cells were killed by thermal (Fig. 3A) or mechanical injury (Supplementary Fig. 3). We also found that the inflammatory response to IL-1ab −/− dendritic cells was reduced to a similar degree (Fig. 3B). In both of these situations, the inflammatory responses to the IL-1-deficient dendritic cells were still well above the buffer-injected background. This implied that the host was also producing some of the required IL-1. Indeed we found that the inflammatory response to necrotic dendritic cells was partially reduced (~50%) in IL-1-deficient hosts (Fig. 3C).
Figure 3.
Both host derived- and dead cell derived- IL-1 is contributing to neutrophil recruitment to necrotic bone marrow derived dendritic cells (BMDCs). (A, B) The total neutrophil numbers in peritoneal cavity of WT C57BL/6 mice after 14 hours i.p. injection of heat-shocked necrotic BMDCs from WT and IL-1α−/− (A), or IL-1α−/−β−/−(B) mice. (C) The total neutrophil numbers in peritoneal cavity of WT C57BL/6 or IL-1α−/−β−/− mice after 14 hours i.p. injection of heat-shocked necrotic BMDCs from WT mice. (D) The total neutrophil numbers in peritoneal cavity of WT C57BL/6 mice after 4 hours i.p. injection of indicated number (million) of live or heat shocked necrotic BMDCs from WT mice. (E) The total neutrophil numbers in peritoneal cavity of WT C57BL/6 mice after 4 hours i.p. injection of 5 million of live BMDCs from WT or IL-1α−/−β−/− mice. (F) The total neutrophil numbers in peritoneal cavity of WT C57BL/6 mice after 4 hours i.p. injection of live or heat-shocked necrotic EL4 cells. The small amount of inflammation seen after injection of live EL4 is presumably due a small number of dead cells in the injected suspension. All of data displays are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group) ***p<0.001, **p<0.01, *p<0.05, N.S., not significant versus WT group (A, B, C, E) or same amount of necrosis group (D, F).
The finding that necrotic IL-1+/+ dendritic cells were much more inflammatory than IL-1−/− ones implied that the dendritic cells must have been making IL-1 before they were killed. Indeed we found that injection of live wild type dendritic cells elicited inflammation to a similar degree to necrotic ones and that this was reduced if the live dendritic cells lacked the ability to produce IL-1 (Fig 3D, E). This contrasts with other cell types where necrotic cells are strongly proinflammatory but live ones are not (figure 3F).
Does the cell death-induced IL-1 generated in the host come from parenchymal and/or bone marrow-derived cells?
We next examined the nature of the cells in the host that are producing the IL-1 needed for the neutrophilic inflammatory response to cell death. Many different cell types can potentially make this cytokine (27). To begin to elucidate which cell lineages were the source of IL-1 in this inflammatory response, we generated chimeric mice whose bone marrow and/or radioresistant parenchyma cells lacked functional IL-1 genes and challenged them i.p. with necrotic EL4 cells.
Neutrophilic inflammation to dead cells was significantly reduced in radiation chimeras whose bone marrow lacked either functional IL-1α or IL-1β genes (Fig. 4A). The neutrophil recruitment was slightly further attenuated in mice whose bone marrow lacked the ability to make both IL-1α and IL-1β (Fig. 4A). Although the inhibition of the inflammatory responses was quite marked, it was not as complete as was observed in mice lacking these cytokines in all cells; this suggested that IL-1 from radioresistant cells might also be contributing to responses, albeit in a minor way.
Figure 4.
Bone marrow-derived cells are the major producer of IL-1 in inflammation to dead cells. (A) Total neutrophil numbers in peritoneal cavity were determined in bone marrow chimeric mice 14 hours after i.p. challenge of heat-shocked necrotic EL4 cells. Chimeric mice were generated using C57BL/6 (WT) mice as hosts and the indicated IL-1−/− mice as donors (WT→WT, IL-1α−/−→WT, IL-1β−/−→WT, IL-1αβ−/−→WT), or using IL-1−/− mice as hosts and IL-1−/− mice as donors (IL-1α−/−→IL-1α−/−, IL-1β−/−→IL-1β−/−, IL-1α−/−β−/−→IL-1α−/−β−/−). (B) Total neutrophil numbers in the peritoneal cavity were determined in bone marrow chimeric mice 14 hours after i.p. challenge of heat-shocked necrotic EL4 cells. Chimeric mice were generated by using IL-1−/− as hosts and C57BL/6 as donors (WT→ IL-1α−/−, WT→IL-1β−/−). (A, B) All of data displays are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group In panel A, One – way ANOVA and Bonferroni's multiple comparison test for all of the groups showed a significant difference for WT→WT to all the other groups (P<0.001), but there was no significant difference among the groups of cells fromIL-1−/− donors (not shown in the figure). In panel B, Bonferroni's multiple comparison test for all of the groups showed a significant difference for WT→ IL-1α−/− to WT→ IL-1β−/−, IL-1α−/− → IL-1α−/− or IL-1β−/− → IL-1β−/− (P<0.01, not shown in the figure). ***p<0.001, **p<0.01, N.S., not significant versus EL4 stimulated WT→WT group.
To directly evaluate the contribution of IL-1 from parenchymal elements, we injected necrotic EL4 cells into mice whose parenchymal, but not bone marrow-derived cells, lacked IL-1 genes (Fig. 4B). Neutrophilic inflammation was not reduced in mice whose parenchymal elements lacked a functional IL-1α gene. In contrast, in chimeras whose parenchyma lacked functional IL-1β, there was a significantly reduced response. Collectively these results indicate that bone marrow-derived cells are a major source of IL-1α (and much of the IL-1β) driving the sterile inflammatory response to dying cells. These findings raise the question of what the precise identity is of the key bone marrow-derived cell(s) needed for the cell death-induced inflammatory response. This is an important question because it addresses the issue of what is the sentinel system that senses and responds to cell death. We hypothesized that this cell would be a macrophage or dendritic cell as these elements are normally resident in tissues and interact with dead cells.
Role of Dendritic cells in cell death-induced inflammation
To investigate the role of dendritic cells in the death-induced inflammatory response, we utilized a transgenic mouse that expressed the human diphtheria toxin receptor (DTR) under the control of the CD11c promoter (32). Normally, murine cells are resistant to diphtheria toxin because they lack a receptor to bind and internalize the toxin. However, in this model most dendritic cell subsets and the CD11c+ subset of CD8+ T cells are deleted by diphtheria toxin A (DTA) (32). We treated the CD11c-DTR or wild type mice with DTA and then challenged them with necrotic EL4 cells or liver homogenate i.p.. The DTA treatment by itself did not induce inflammation; this is presumably because the number of cells that are killed are too few to stimulate this response although it is also possible that DTA-killing is not proinflammatory and/or that the dead cells are too rapidly cleared.
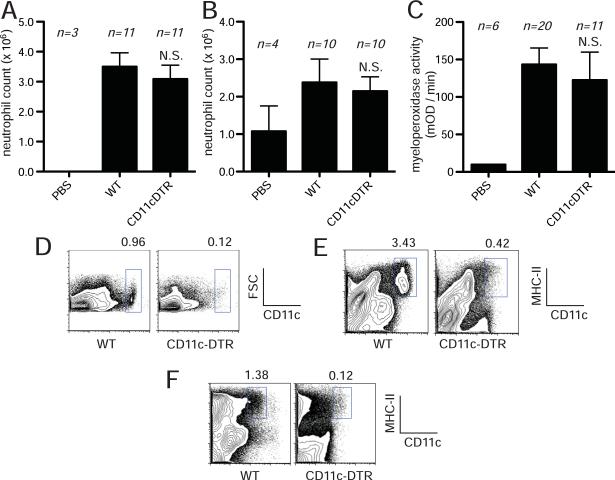
We found that the acute neutrophilic inflammatory response to injected necrotic cells was not reduced in the DTA-treated CD11c-DTR-transgenic mice compared to wild type animals (Fig. 5A, B). Similarly, the neutrophil recruitment to acetaminophen-induced liver injury was not reduced in CD11c-DTR transgenic mice (Fig. 5C). Give these results we sought to verify that DTA was eliminating dendritic cells in the transgenic mice. We found that CD11c+ cells were efficiently depleted in the treated mice as was previously reported (Fig. 5D, E, F) (32) (37). Therefore, we found no evidence that dendritic cells are required for the death-induced inflammatory response in our model and this is consistent with the findings with the CD11b-DTR mice described below, suggesting that dendritic cells are not major participants in driving responses in our models.
Figure 5.
CD11c+ dendritic cells are not required for recruiting neutrophils to cell death. (A, B) C57BL/6 or CD11c-DTR mice were treated with i.v. injection of 100 ng of diphtheria toxin A and then challenged with i.p. injection of heat-shocked necrotic EL4 cells (A) or liver homogenate (B). Total number of neutrophil was determined 14 hours after injection of necrotic cells. (C) Myeloperoxidase activity in the liver of mice challenged with i.p. injection of 300 mg/kg of Acetaminophen in C57BL/6 or CD11c-DTR mice treated with i.v. injection of 100 ng of diphtheria toxin A. (D, E, F) Flow cytometry histogram of CD11c+ cells in the spleen (D), liver (E), or peritoneal cavity (F) of C57BL/6 or CD11c-DTR mice treated with diphtheria toxin A. All data displays (A–C) are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). N.S., not significant versus WT groups. PBS groups, WT mice received i.p. PBS. FSC, forward scatter.
Role of CD11b+ cells in cell death-induced inflammation
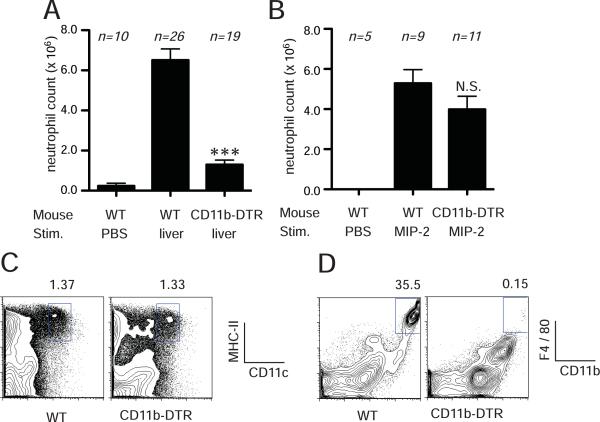
In one set of experiments we examined the effect on the sterile inflammatory response of conditionally depleting CD11b+ cells by injecting DTA into a transgenic mouse that expresses the diphtheria toxin receptor under the control of the CD11b promoter (30). In the CD11b-DTR transgenic mice, CD11b+hi cells selectively express the DTR and are eliminated upon treatment with DTA. Transgenic or nontransgenic mice were injected with or without DTA and then challenged i.p. with buffer or necrotic liver cells. Toxin treatment markedly inhibited the neutrophilic inflammatory response to dead cells in transgenic mice but not wild type ones (Fig. 6A). Similar results were obtained injecting necrotic EL4 cells (described further below). The toxin treatment by itself (with no dead cell challenge) did not induce inflammation presumably because the CD11b+ cells that are killed are needed for the inflammatory response, the number of cells that die are too few and/or their mechanism of death does not stimulate inflammation (see below).
Figure 6.
CD11b+ cells are required for neutrophil recruitment to dead cells. (a) FVB/N or CD11b-DTR mice were treated i.v. injection of 500 ng of diphtheria toxin A and then challenged with i.p. injection of liver homogenate. Total neutrophil number in the peritoneal cavity was determinced after 14 hours of stimulation. (B) Neutrophil number in response to i.p. injection of MIP-2 in FVB/N or CD11bDTR mice. (C, D) Flow cytometry histograms of CD11c+ cells or F4/80+ cells in the peritoneal cavity of FVB/N or CD11b-DTR mice treated with diphtheria toxin A. All data displays (A, B) are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). ***p<0.001, N.S., not significant versus WT stimulated with liver homogenate (A) or MIP-2 (B). PBS groups, WT mice received i.p. PBS.
Although neutrophils express CD11b, previous studies have shown that these cells are not depleted by diphtheria toxin treatment in the CD11b-DTR transgenic mice, presumably because their levels of CD11b expression (and the DTR) are too low. Nevertheless, to critically test whether neutrophils were present and functional in our experiments, we challenged the CD11b-depleted transgenic mice i.p. with the recombinant chemokine MIP-2 and quantified the influx of neutrophils into the peritoneum. Diphtheria toxin treatment did not significantly reduce the MIP-stimulated neutrophil response (Fig. 6B). These results argue that diphtheria toxin depletion of CD11b positive cells is not inhibiting the inflammatory response to dead cells by direct effects on neutrophils or other common components needed for the response such as vascular endothelium. Data further supporting this conclusion will be presented in sections below.
One of the cell types that expresses CD11b is a subset of dendritic cells. We therefore investigated whether these cells were depleted by diphtheria toxin in the CD11b-DTR transgenic mice. We found no reduction in dendritic cells, including the CD11c+CD11b+ subset, in the peritoneal cavity of transgenic mice treated with diphtheria toxin (Fig. 6C). In contrast, DTA caused a marked depletion of macrophages (CD11b+ F4/80+) from the peritoneal cavity (Fig. 6D).
Identification of the CD11b+ cell required for the cell death-induced inflammatory response
To verify that the inhibition of sterile inflammation in the DTA-treated CD11b-DTR mice was due to the loss of a specific cell type we attempted to reconstitute responses in these treated animals by adoptive transfer of wild type (DTA-insensitive) cells (Fig. 7). In initial experiments we injected resident peritoneal cells from wild type mice into the DTA-treated CD11b-DTR mice. The adoptive transfer of these live cells did not by itself cause any inflammation. However, these transferred cells were able to reconstitute the ability of the DTA-treated transgenic animals to respond to necrotic cells (Fig. 7A). This result indicates that the inhibition of the sterile inflammatory response in the treated transgenic mice is not due to pleiotropic effects of the DTA treatment.
Figure 7.
Macrophages restore inflammatory response to cell death in CD11b-DTR mice. FVB/N or CD11b-DTR mice were injected i.v. with 500 ng of diphtheria toxin A and then reconstituted with naïve peritoneal cells (PCs) (A), thioglycolate peritoneal elicited cells (PECs) (B), F4/80+ thioglycolate PECs (C) or immortalized macrophage cell line (D). Neutrophil number was determined 14 hour after i.p. injection of liver homogenate (A, B) or heat-shocked necrotic EL4 cells (C, D). All data displays are combined results of 3 or more experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). ***P<0.001, versus CD11b-DTR Tg mice without reconstitution and stimulated with liver homogenate (A, B) or necrotic EL4 (C, D). MP = macrophage
We next used this adoptive transfer approach to determine the identity of the CD11b+ cell required for the cell death-induced inflammatory response. We found that adoptive transfer of thioglycolate-induced peritoneal exudate cells, which are primarily macrophages, also reconstituted responses (Fig. 7B). Similar results were obtained by transferring F4/80+ macrophages purified from the peritoneal exudates (Fig. 7C). Neither of these cell populations caused inflammation by themselves (without dead cell challenge).
The findings above strongly suggested that the CD11b+ cell that is required for the sterile inflammatory response is a macrophage. However, it is difficult to completely exclude the possibility that the reconstituting activity is coming from some contaminating cell type in the macrophage populations. To critically test this point we sought to reconstitute the DTA-treated transgenic mice with a macrophage population that could have no possible contaminates and for this we used a macrophage clone immortalized by J2 virus (38) (Fig. 7D). One potential caveat of this approach is that the transgenic mice are on the FVB background while the macrophage clone is of C57BL6 origin and therefore genetic differences could in theory stimulate an allogeneic response. However, such responses should take much longer than the 15 hour time course of our assay and most importantly we found that transfer of the immortalized macrophages by themselves (with no dead cell challenge) caused no inflammation. Just as we found with purified primary macrophages, the macrophage clone was sufficient to reconstitute the inflammatory response to necrotic cells. These results unambiguously indentify macrophages as a key cell participating in the sterile inflammatory response to cell death.
Macrophages are required for the production of IL-1α
In a final set of experiments we sought to determine whether macrophages were the key host cells that were required to produce the IL-1 that drives the sterile inflammatory response. To address this issue we used DTA to deplete the CD11b+ cells from the CD11b-DTR mice and then compared the ability of wild type versus IL-1-deficient peritoneal macrophages to reconstitute the sterile inflammatory response to dead cells. This experimental design again involved the transfer of macrophages from C57BL6 mice (the background of the mutant mice) into the FVB transgenics. However, like the results we observed above, the transferred cells induced no inflammation by themselves and the wild type macrophages reconstituted responses to necrotic cells (Fig. 8). In contrast to wild type macrophages, IL-1α/β double deficient macrophages failed to reconstitute responses. Similar results were obtained using macrophages that lacked IL-1α. Surprisingly, IL-1β-deficient macrophages did reconstitute responses.
Figure 8.
IL-1α in macrophages is required for reconstituting neutrophil response to cell death in CD11b-DTR mice. FVB/N or CD11b-DTR mice were injected i.v. with 500 ng of diphtheria toxin A and then reconstituted (recon.) with thioglycolate PEC from wild type C57BL/6, IL-1α−/−, IL-1β−/− or IL-1α−/−β−/− mice. Neutrophil number was determined 14 hour after i.p. injection (stim.) of heat-shocked necrotic EL4 cells or PBS. Data displays are combined results of 3 experiments and represented as means ± SEM (n = total number of mice from the multiple experiments for each group). One - way ANOVA and Dunnett's multiple comparison tests were used to compare the necrotic EL4 stimulated and WT PEC reconstituted CD11b-DTR group with others. **p<0.01, *p<0.05, N.S., not significant.
Discussion
Although it has long been known that cell death in vivo stimulates a robust acute inflammatory response, the underlying cellular mechanisms that sense cell death and drive the inflammatory response have been poorly understood. This report describes a number of new insights into this process.
One of the major findings in the present report is that the initiation of an acute inflammatory response to sterile cell death requires a CD11bhigh leukocyte that is almost certainly a macrophage. Supporting this conclusion was the finding that conditionally depleting CD11b-positive cells in CD11b-DTR mice inhibited these responses (Fig. 5). The fact that in these same animals inflammation was intact to other stimuli (such as MIP) indicated that the essential CD11b+ cell was operating upstream in the pathway and that there was no negative effect of these depletions directly on neutrophils or other downstream components (e.g. vascular endothelium). This was also shown by the fact that after CD11b cell-depletion, the inflammatory response to dead cells could be reconstituted with CD11b+ leukocytes.
Several lines of evidence indicated that the key CD11b-positive leukocyte was a macrophage. In the CD11b-DTR model in vivo, macrophages are one of the principal CD11bhigh cells that are eliminated by DTA. Importantly, inflammatory responses in the peritoneum of CD11b-depleted animals were successfully reconstituted with resident and thioglycolate-induced peritoneal leukocytes, both cell populations in which the primary CD11b-expressing cells were macrophages (Fig. 7). The fact that purified primary macrophages and a pure cultured macrophage clone could also reconstitute responses in the depleted animals demonstrated that macrophages were critical upstream elements in this sterile inflammatory response.
Another important finding in our studies was that bone marrow-derived cells are the origin of most if not all of the IL-1α that is needed to drive the acute inflammatory response (Fig. 4). Moreover, while wild type macrophages reconstituted sterile inflammatory responses in mice depleted of CD11b cells, IL-1α-deficicient macrophages failed to do so (Fig. 8). This demonstrated that macrophages are almost certainly the major source of IL-1α needed for the cell death-induced inflammatory response in our models. In one circumstance, part of the initial source of IL-1α can come from the dead cell itself, as first suggested by Eigenbrod et al(36). However, among the dead cell types we examined, we only found this to be the case for necrotic dendritic cells. Presumably these cells have a higher content of IL-1α relative to other proinflammatory danger signals than do other cell types and indeed we found that injection of live dendritic cells stimulated IL-1 dependent inflammation. Perhaps agonal release of IL-1α could contribute to inflammation to other cell types in situations where the dying cell was previously stimulated to produce high levels of IL-α before or during the agonal stages of death (39, 40). However, even in situations where dead cells contribute IL-1 (as we saw with dendritic cells) there is a substantial component of inflammatory response that is being driven by IL-1 produced by the host.
Another new finding was that IL-1β was also required for the cell death-induced inflammatory response. This had not been seen in earlier antibody-blocking experiments (24) presumably because complete neutralization of this cytokine is notoriously difficulty (41) (27). Recently IL-1β's contributions along with NLRP3 inflammasomes to cell death-induced inflammation have been also reported (40, 42). We previously showed that Caspase-1-deficient mice did not show a reduction in neutrophil recruitment to necrotic EL4 cells. Although Caspase I plays a major role in many systems in cleaving pro-IL-1b to mature IL-1b, the differences we observe between IL-1β-deficient mice and Caspse-1 deficient mice suggest that proteases other than Caspase 1 can process pro IL-1β to active IL-1β in vivo, as previously suggested (43). We found that the dominant source of IL-1β in this response was from bone marrow-derived leukocytes, although there was also a contribution from radioresistant cells in the host (Fig. 4). We detected no contribution directly from dying cells, although again it is possible that this might be seen in situations where the dying cell has been induced to produce high levels of this cytokine.
Our experiments revealed some interesting and surprising findings that merit comment. One result is that there is a strong inhibition of responses in animals deficient in only IL-1α or IL-1β (Fig. 2A, Supplementary Fig. 2). This is surprising because both forms of this cytokine stimulate the same receptor, IL-1R1, and on this basis it would have been predicted that the loss of only one of the two forms of the cytokine would have led to more partial inhibitions. This raises the possibility that IL-1α and IL-1β may somehow stimulate in different ways and that both of these are needed for optimal responses. Another and perhaps more likely explanation is that these two cytokines can stimulate each others expression and as a result might create a positive feed back loop that is needed to amplify responses (27) (44). This may explain why some of the IL-1β driving the inflammatory response is coming from radioresistant cells, presumably non-bone marrow derived cells, in the host (i.e. these “host” cells are responding to the IL-1 produced by macrophages and in so doing helping to amplify the response).
Another interesting finding is that IL-1β−/− but not IL-1α−/− peritoneal macrophages reconstitute responses in CD11b-depleted mice (Fig. 8). This demonstrates that the IL-1α and IL-1β needed for the inflammatory responses can come from different cells and it is possible that IL-1α stimulates other cells to make IL-1β (33, 45). What the source of IL-1β is in this situation is not defined in our experiments but it may come from both bone marrow and host cells as suggested by our results with chimeras. It is also possible that under some conditions macrophages (e.g. different macrophage subsets or states of activation) would contribute both forms of IL-1.
Yet another interesting set of observations concerns the role of dendritic cells in the cell death-induced inflammatory response. Depleting CD11c-positive cells in the CD11c-DTR mouse did not inhibit inflammation to cell death in the peritoneum or liver suggesting that dendritic cells play little role in these responses (Fig. 5). It is possible that a role for these cells was missed because they were not sufficiently depleted; however we could find only few remaining dendritic cells and the CD11c-DTR experimental system eliminates dendritic cell function in other models. Moreover, in independent experiments we found that CD11b cell-depletions which eliminates macrophages without any detectable depletion of dendritic cells inhibited inflammatory responses; this again suggested that dendritic cells were not sufficient for responses (Fig. 6C). Therefore, the weight of evidence is that dendritic cells do not play a major role in this sterile inflammatory response, at least in the models we examined.
Based on our findings we propose that macrophages are the initial sensor of cell death and in response produce IL-1 and maybe other mediators that initiate the inflammatory response. Since these cells are resident in tissues they are strategically placed to carry out this function. Moreover, in this location they are the primary cells that seek out and eat dead cells and therefore are well positioned to be the initial sensor of death. Consistent with this model we had previously found that dead cells directly stimulate macrophages to produce IL-1 in vitro (24). However, we can not fully exclude the possibility that in vivo some other primary mechanism senses cell death and then stimulates macrophages to generate IL-1 as a secondary event.
Necrosis (oncosis) is defined as the cell death characterized by the morphologic changes of swollen cells and organelles, and loss of membrane integrity leading to the release of intracellular contents (2). Cell death due to membrane disruption by mechanical damage is classified as necrosis (46) and many different forms of cell injury can lead to this kind of cell death. Recent studies have revealed that the necrosis induced by deprivation of energy (e.g. ischemia) has highly organized processes (47, 48). Whether all forms of necrosis lead to the same biological outcomes is not known. Although the inflammatory response to both of the heat-shocked and mechanically disrupted cells are same regarding the dependence of host derived IL-1 (Figure 2, Supplementary figure 2) but not of dead cell-derived IL-1 (Figure 1, Supplementary figure 1), further studies are required to generalize these findings to other types of necrotic cell death.
In summary our results shed light on the key cellular elements and their mediators that help trigger the acute inflammatory response to sterile cell death. We believe this is important because it provides insight into a fundamental biological process. Moreover since this process contributes to disease pathogenesis it is a potential therapeutic target. The identification of the host cell that initiates the response and insights into how it then triggers inflammation should aid in the development and evaluation of therapeutics to selectively block this response.
Supplementary Material
Acknowledgement
The authors thank Fernando Ontiveros for critical reading of the manuscript, Zubin Patel, Sharlene Hubbard, Matthew Janko and Janice BelleIsle for technical assistance. HK contributed to the design, execution and analysis of all experiments and writing of the manuscript. DK performed the experiments. YI provided advice and IL-1-deficient mice. KLR contributed to the design and analysis of all experiments and writing of the manuscript.
This work was supported by grants from the NIH to KLR and core resources supported by the Diabetes Endocrinology Research Center grant DK32520 were also used.
Abbreviations
- IL-1R
IL-1 receptor type I
- DTR
diphtheria toxin receptor
- DTA
diphtheria toxin A
- FSC
forward scatter
- PCs
peritonea cells
- TG
thioglycollate
- PECs
peritoneal exudate cells
- MPs
macrophages
- BMDCs
bone marrow-derived dendritic cells
References
- 1.Majno G, La Gattuta M, Thompson TE. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- 2.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Antman EM, Braunwald E. ST-Elevation Myocardial Infarction: Pathology, Pathophysiology, and Clinical Features. In: Libby P, Bonow RO, Mann DL, Zipes DP, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 8th ed. Saunders; Philadelphia, PA: 2007. pp. 1207–1230. [Google Scholar]
- 4.Rock KL, Hearn A, Chen CJ, Shi Y. Natural endogenous adjuvants. Springer Semin Immunopathol. 2005;26:231–246. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- 5.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 8.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 9.Kishi M, Richard LF, Webster RO, Dahms TE. Role of neutrophils in xanthine/xanthine oxidase-induced oxidant injury in isolated rabbit lungs. J Appl Physiol. 1999;87:2319–2325. doi: 10.1152/jappl.1999.87.6.2319. [DOI] [PubMed] [Google Scholar]
- 10.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 11.Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 12.Kelly KJ, Williams C, Rb M, Sm S, Ta G-R, Jc B, Jv Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 14.Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit. Rev. Immunol. 2003;23:15–44. doi: 10.1615/critrevimmunol.v23.i12.20. [DOI] [PubMed] [Google Scholar]
- 15.Beg AA. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/s1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2006;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 17.Weiser MR, Williams JP, Moore K, L M, M H, Hb C, Mc Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–2348. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 19.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 20.Powell JD, Horton MR. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–218. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- 21.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 22.Inohara C, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 23.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 24.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 25.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009 doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 28.Hacham M, Argov S, White RM, Segal S, Apte RN. Different patterns of interleukin-1alpha and interleukin-1beta expression in organs of normal young and old mice. Eur Cytokine Netw. 2002;13:55–65. [PubMed] [Google Scholar]
- 29.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 30.Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill J, Hughes J, Lang RA. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 31.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock KL. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eigenbrod T, Park JH, Harder J, Iwakura Y, Nunez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1alpha released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plitas G, Burt BM, Stableford JA, Nguyen HM, Welles AP, DeMatteo RP. Dendritic cells are required for effective cross-presentation in the murine liver. Hepatology. 2008;47:1343–1351. doi: 10.1002/hep.22167. [DOI] [PubMed] [Google Scholar]
- 38.Blasi E, Mathieson BJ, Varesio L, Cleveland JL, Borchert PA, Rapp UR. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985;318:667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers HW, Sheehan KC, Brunt LM, Dower SK, Unanue ER, Schreiber RD. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc Natl Acad Sci U S A. 1992;89:1011–1015. doi: 10.1073/pnas.89.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Ambade A, Re F. Cutting edge: Necrosis activates the NLRP3 inflammasome. J Immunol. 2009;183:1528–1532. doi: 10.4049/jimmunol.0901080. [DOI] [PubMed] [Google Scholar]
- 43.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 44.Iwakura Y. Roles of IL-1 in the development of rheumatoid arthritis: consideration from mouse models. Cytokine Growth Factor Rev. 2002;13:341–355. doi: 10.1016/s1359-6101(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 45.Franchi L, Eigenbrod T, Nunez G. TNF-alpha Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. The Journal of Immunology. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.