Abstract
A simple, fast, and reproducible sample preparation procedure was developed for relative quantification of metabolites in adherent mammalian cells using the clonal β-cell line INS-1 as a model sample. The method was developed by evaluating the effect of different sample preparation procedures on high performance liquid chromatography- mass spectrometry quantification of 27 metabolites involved in glycolysis and the tricarboxylic acid cycle on a directed basis as well as for all detectable chromatographic features on an undirected basis. We demonstrate that a rapid water rinse step prior to quenching of metabolism reduces components that suppress electrospray ionization thereby increasing signal for 26 of 27 targeted metabolites and increasing total number of detected features from 237 to 452 with no detectable change of metabolite content. A novel quenching technique is employed which involves addition of liquid nitrogen directly to the culture dish and allows for samples to be stored at −80 °C for at least 7 d before extraction. Separation of quenching and extraction steps provides the benefit of increased experimental convenience and sample stability while maintaining metabolite content similar to techniques that employ simultaneous quenching and extraction with cold organic solvent. The extraction solvent 9:1 methanol: chloroform was found to provide superior performance over acetonitrile, ethanol, and methanol with respect to metabolite recovery and extract stability. Maximal recovery was achieved using a single rapid (~1 min) extraction step. The utility of this rapid preparation method (~5 min) was demonstrated through precise metabolite measurements (11% average relative standard deviation without internal standards) associated with step changes in glucose concentration that evoke insulin secretion in the clonal β-cell line INS-1.
Keywords: metabolomics, liquid chromatography-mass spectrometry, adherent mammalian cell sample preparation, INS-1, β-cells, HILIC, solvent, extraction time
INTRODUCTION
Metabolomic analysis of cells and tissues has emerged as an important technique for studying cellular biochemistry. With the advent of powerful high performance liquid chromatography - mass spectrometry (HPLC-MS) and gas chromatography - mass spectrometry (GC-MS) methods, large numbers of metabolites can be quantified to provide detailed insight into the metabolic status of cells. Despite the power of these methods, sample preparation is critical to achieving meaningful results. In these studies, we sought to develop a novel sample preparation procedure for adherent mammalian cells that would be simpler and faster than conventional methods. We chose INS-1 832/13 cells1,2 as a model cell line. This clone has proven to be a useful model for the study of insulin secretion with many properties in common with primary β-cells including physiological response to glucose. Accurate measurement of metabolites and their changes during glucose-stimulated insulin secretion is expected to add to our understanding of insulin secretion in normal and pathological states.
Many studies on sample preparation for metabolomic analysis of yeast and bacteria have been reported (see recent review3). Because of substantial differences in eukaryotic and prokaryotic cells, the findings from these investigations may not be fully applicable to adherent mammalian cells. While minimal media used to culture prokaryotic cells and mammalian culture media both contain interfering anions such as Cl−, SO4−, and PO4−, mammalian culture media also contains up to millimolar concentrations of amino acids, Good's buffers, organic acids, and complex biological mixtures such as fetal bovine serum. Unless these components are removed, they can cause substantial electrospray ionization suppression and contaminate the intracellular metabolite pool. These issues are compounded with adherent mammalian cells since they cannot be concentrated by centrifugation or filtration prior to quenching without trypsination or physical removal, which has been demonstrated to significantly alter the metabolite profile4.
Limited work has focused on development of sample preparation techniques for metabolomic analysis of adherent mammalian cells. One study employed rigorous experimental design to maximize nucleotide recovery, energy charge, fructose 1,6 bisphosphate (FBP) content, and minimize residual protein/DNA5. While valuable, the method may not be applicable to global metabolomic studies as it employs a biphasic extraction solvent that may remove non-polar metabolites from the assayed extract and employs elevated temperatures, which may degrade thermally labile metabolites such as nicotinamide adenine dinucleotide (NADH), nicotinamide adenine dinucleotide phosphate (NADPH)6, and succinyl-CoA (sCoA)7.
Although rigorous studies of sample preparation for adherent mammalian cells are rare, many metabolomic studies of such cells have been described (see Table 1). Extraction approaches vary widely in volume of solvent (e.g., 1 to 26 mL/10 cm plate of cells), number of repeated extractions (1 to 3), and duration of incubation per cycle of extraction (as long as 60 min). A variety of rinsing, quenching, heating, and sample concentration procedures are also used. Without comparative studies, it is difficult to know how performance, e.g., sample stability and metabolite recovery, is impacted by different procedures or if a method could be improved, e.g., by simplification or better removal of interferences.
Table 1.
Summary of sample preparation procedures reported for metabolomic analysis of cultured adherent mammalian cells.
| Cell Type | Analytical Technique |
Rinse | Quench | Extraction | Dry | Ref |
|---|---|---|---|---|---|---|
| Human rhabdomyosarcoma | NMR | trypsination/ 3X 0 °C PBS | LN2 (pelleted cells) | 10% ice cold TCA in H2O | yes | 21 |
| Human breast cancer | NMR | 2X ice cold PBS | MeOH | MeOH/ CHCl3/ H2O biphase | yes | 4 |
| Human fibroblasts | LC/MS | No | −75 °C 80% MeOH | −75 °C 80% MeOH | yes | 15 |
| Generic | LC/MS | No | −75 °C 80% MeOH | 4 °C 80% MeOH | optional | 10 |
| Hepatic | GC/MS and LC/MS | No | 150 °C Air | boiling H2O | yes | 10 |
| INS-1 | GC/MS | 1× MES, 1× H2O | −75 °C MeOH | 70 °C heating followed by CHCl3/ H2O biphase | yes | 22 |
| Canine kidney | LC/UV and conductivity | 1× PBS | 4 °C MeOH / CHCl3 | 2× buffered MeOH / CHCl3/ H2O biphase and 90 °C heating | yes | 5 |
| CHO | enzyme and GC/MS | 60% MeOH | −40 °C 60% MeOH | 100% MeOH | yes | 23 |
| HeLa | LC/MS | 2× NH4OAc | 80% MeOH | 80% MeOH | no | 24 |
To better understand how different sample preparation techniques affect the metabolome we measured the quantitative effect of novel and commonly used sample preparation procedures on recovery and stability of 27 known metabolites associated with glycolysis and the tricarboxylic acid (TCA) cycle as well as ~700 unidentified features using an undirected approach. Our initial method incorporated a water rinse to improve sensitivity and remove contaminants, a single rapid extraction with cold organic solvent to minimize sample preparation time, and a novel liquid nitrogen (LN2) quench step to rapidly halt metabolism and allow for storage of unextracted samples. We then evaluated the impact of additional and/or alternate sample preparation steps on metabolite recovery and stability.
Based on this study, we developed a procedure that removes interfering media components from the cell surface, quenches metabolism, extracts a wide range of metabolites, and generates stable extracts with sufficient concentration to detect many metabolites by anion exchange / hydrophilic interaction liquid chromatography - electrospray ionization- mass spectrometry (AEX/HILIC-ESI-MS). This method requires as little as 5 min, is convenient, and provides good sensitivity for a variety of metabolites.
MATERIALS AND METHODS
Materials
All chemicals were purchased form Sigma-Aldrich (St. Louis, MO) unless otherwise noted. HPLC grade acetonitrile was purchased from Burdick & Jackson (Muskegon, MI). RPMI media, fetal bovine serum, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and penicillin-streptomycin were purchased from Invitrogen Corp. (Carlsbad, CA). Cells lifters and 10 cm polystyrene non-pyrogenic culture dishes were purchased from Corning (Lowell, MA).
Cell Culture
Culture media was prepared with RPMI-1640 (+L-glutamine) supplemented with 10% fetal bovine syrum (FBS), 1 mM pyruvate, 10 mM HEPES, 50 µM 2-beta mercaptoethanol, and 1 unit penicillin-streptomycin. KRHB (Krebs-Ringer-HEPES buffer) was prepared containing 20 mM HEPES, 118 mM NaCl, 5.4 mM KCl, 2.4 mM CaCl, 1.2 mM MgSO4, and 1.2 mM KH2PO4 and adjusted to pH 7.4 with HCl. INS-1 cells were grown to confluence (~4 × 107 cells) in 10 cm polystyrene dishes at 37 °C and 5% CO2. RPMI media was replaced with KRHB containing 10 mM glucose 30 min prior to quench to generate metabolite profiles comparable to those expected from stimulation experiments. (Stimulus-secretion coupling studies of β-cells are generally carried out in KRHB instead of culture media to measure the effect of specific stimulants without other potential fuels and unknown compounds from the media impacting results).
HPLC-MS
Chromatographic separations were performed with an Agilent Technologies (Santa Clara, CA) 1200 HPLC system equipped with a Phenomenex (Torrance, CA) Luna NH2 2.0 × 150 mm, 3 µm HPLC column equipped with a 2.0 × 4 mm guard column using the following conditions: mobile phase A was 100% acetonitrile (ACN); mobile phase B was 100% 5 mM ammonium acetate pH 9.9 with ammonium hydroxide; gradient program was (time, %B, flow rate) 0 min, 20%, 200 µL/min, 20 min, 100%, 200 µL/min, 20.1 min, 100%, 300 µL/min; column temperature was 25 °C; injection volume was 80 µL, and autosampler temperature was 4 °C. These chromatographic conditions are similar to those reported previously8–9 and provide for a weak AEX/HILIC separation afforded by the polar and partially deprotonated propylamine stationary phase. A representative INS-1 extract chromatogram with additional method details is provided in Figure S-1. Detection was performed on an Agilent Technologies LC/MSD TOF using a dual ESI source in negative-ion mode. Instrument parameters were as follows: gas temp: 350 °C, drying gas: 10 L/min, nebulizer: 20 psig, VCap 3500 V, fragmentor: 150 V, skimmer: 65 V, acquisition rate: 1 spectra/s, mass range: 50–1200 m/z, data storage: centroid with 20 count threshold, reference spray: on.
Nominal Preparation, Quenching, Extraction, and Assay Procedure
Unless otherwise stated, metabolism was quenched and metabolites extracted from INS-1 cells using the following procedure (see also illustration in Figure S-2). Cells were rapidly rinsed by gently dispensing ~10 mL of 37 °C deionized water to the cell surface. The plate was rocked briefly (~2 s), aspirated, and quenched by directly adding ~15 mL of LN2 to the dish. Approximately 5 s passed between addition of water and quenching by addition of LN2. The plates were briefly stored on dry ice, transferred to a −80 °C freezer, and extracted and assayed within 7 d.
For extraction, plates were immediately transferred to a 4 °C cold room and 1.5 mL of ice cold 90% 9:1 MeOH: CHCl3 (MC) was immediately added to each plate and cells scraped/suspended with a cell lifter. The extraction solvent also contained 13C6-fructose-6-phosphate (F6P) (10 µM), 13C1-phosphoenolpyruvate (PEP) (10 µM), 13C6-citrate (CIT) (10 µM), 13C4-succinate (SUC) (10 µM), 13C10 15N5-adenosine monophosphate (AMP) (2 µM), 13C10-adenosine triphosphate (ATP) (20 µM), and 13C10-guanosine triphosphate (GTP) (10 µM) as internal standards. Extracts were transferred to 1.5 mL microcentrifuge tubes and pelleted at 4 °C for 3 min at 16,100 g. Supernatants were transferred to autosampler vials and assayed. Using this rapid procedure, a single sample can be quenched, extracted, pelleted, and ready for injection in ~5 min. All experiments were performed in triplicate.
Solvent Screen
INS-1 cells were extracted with 0.7 mL of 100% ethanol (EtOH), ACN, MeOH, and 9:1 MC yielding extracts containing ~70% organic (based on an estimated 300 µL residual water/plate). Samples were assayed within 10 min of extraction solvent and reinjected after 8 h of storage at 4 °C.
Impact of Water Rinsing
To assess suppression, cells were rinsed with either 10 mL 37 °C Milli-Q water or 10 mL 37 °C KRHB prior to quenching. To assess rinsing impact on the metabolic profile, control cells were rinsed twice with 10 mL KRHB and experimental cells were rinsed once with 10 mL 37 °C Milli-Q water (either rapidly or with 30 s incubation) and re-rinsed with an additional 10 mL KRHB to achieve an identical final residue matrix.
Evaluation of Quenching Methods
Following aspiration of the water rinse solution, metabolism was quenched with ~15 mL LN2, 1.5 mL of −75 °C 90% 9:1 MC, or 1.5 mL ice cold 90% 9:1 MC. Scraping and pelleting was conducted immediately following quench per the nominal method.
Comparison of Metabolite Changes with Glucose Stimulation Using Different Quenching and Preparation Methods
INS-1 cells were grown in 6 cm plates and preincubated in 3 mL KRHB containing 0.5 mM glucose for 30 min. Cells were then quenched (control) or stimulated with 95 µL of 1 M glucose (final concentration 10 mM), incubated 20 min, then quenched. Quenching and extraction were performed using our method and two established methods for adherent mammalian cells. The first, 80% MeOH, involves no rinse, −80 °C 8:2 MeOH:H2O quench, 3 extraction cycles, and drying/reconstitution of the sample10. The second, B-MTC, involves PBS rinse, 4 °C 2:1 MeOH:CHCl3 quench, 2 biphasic extraction cycles with 1:1 MeOH:2.8 mM tricine and CHCl3, heating at 90 °C for 4 minutes, and drying/reconstitution of the sample5. All volumes were scaled accordingly to 6 cm plate size. Dried samples were reconstituted in 1:1 MeOH:H2O for compatibility with the HILIC method.
Residual Water in INS-1 Cell Plates
Plates of INS-1 cells quenched and stored at −80 °C were thawed, scraped, and the resulting suspension transferred to individual microcentrifuge tubes containing a granule of trichloroacetic acid. The samples were sonicated in an ice bath for 10 min, centrifuged to pellet debris, and aqueous volume measured. The average volume was 290 µL plus an estimated 10 µL of residual water remaining in the plate yielding ~300 µL residual water per plate.
Data Processing and Statistics
Compounds were identified based on retention time and m/z match to injections of authentic standards. Quantification was performed using Agilent Technologies MassHunter Quantitative software. Peak areas were measured from extracted ion chromatograms of [M-H]− metabolite ions with ± 70 ppm detection windows centered on the theoretical mass. [M-2H]2− ions were used for acetyl-CoA (aCoA) and sCoA to improve sensitivity. Peak areas from internal standards were measured using an identical procedure; however, values were only used to verify instrument stability and not used in endogenous metabolite quantification. Undirected data processing was performed using Agilent Technologies MassHunter Qualitative software for peak picking and MassProfiler Professional for data alignment, statistical analysis, and visualization.
Data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined using a non-corrected two-tailed Student's t test, unpaired assuming equal variance. A p-value of < 0.05 was considered significant.
RESULTS AND DISUCSSION
Solvent Screen
Initial experiments evaluated EtOH, ACN, MeOH, and 9:1 MC (all 70:30 organic:H2O) for their ability to extract and stabilize metabolites. All samples were injected within 10 min of extraction solvent addition to minimize metabolite peak area variability due to potential extract instability. The following compounds were measured in the extracts: Glucose-6-phosphate + fructose-6-phospate (G6P+F6P), FBP, 2-phosphoglycerate + 3-phosphoglycerate (2PG+3PG), PEP, aCoA, citrate + isocitrate (CIT+ICIT), alphaketogluterate (AKG), sCoA, SUC, fumarate (FUM), malate (MAL), AMP, adenosine diphosphate (ADP), ATP, guanosine mono and diphosphate (GMP and GDP), GTP, flavin adenine dinucleotide (FAD), NAD+, NADH, NADP+, NADPH, Asp, and Glu. The peak area for each metabolite relative to 9:1 MC is shown in Figure 1a. Metabolite recoveries with MeOH and 9:1 MC were similar and the highest for nearly all analytes. In contrast to most metabolites, NADP+ showed substantially higher recovery with ACN. These results are consistent with most metabolite extraction studies which find methanolic solutions to be suitable extraction solvents.
Figure 1.
Recovery and stability (4 °C, 8 h) for metabolites extracted from INS-1 cells using various solvents at 70:30 solvent: water ratio in the final extract. Samples injected within 10 min of extraction solvent addition. (a) Metabolite peak area ratio in specified solvent versus 9:1 MC. (b) Log 2 of metabolite peak area ratio after 8 h of 4 °C storage versus initial injection. ACN (acetonitrile), EtOH (ethanol), MeOH (methanol), 9:1 MC (9:1 MeOH: CHCl3). Error bars represent 1 SEM, n = 3.
Extract Stability
Metabolomic studies often require the analysis of many samples prepared simultaneously to reduce variability and improve work flow efficiency. Preparing multiple samples at once can lead to storage times of several hours prior to injection because LC-MS analysis may require 10–60 min per sample; therefore, metabolite stability is a critical parameter. Metabolite instability can be caused by inherent chemical liability, enzymatic action3, 8, and degradation of macromolecules to release “metabolites”11. In this regard, metabolite concentrations may either increase or decrease depending on the dominant effects.
To evaluate the impact of organic solvent on stability, samples were reanalyzed after 8 h at 4 °C (mimicking typical autosampler storage) and metabolite peak areas were compared to initial values obtained following immediate injection (Figure 1b). Stability of peak areas for metabolites such as G6P+F6P, FBP, CIT+ICIT, SUC, FUM, MAL, ATP, GTP, NAD, Asp, and Glu were impacted minimally by organic solvent (areas between 96 to 120% of initial values), whereas 2PG+3PG, PEP, AKG, AMP, ADP, GMP, GDP, FAD, NADH, NADP, and NADPH showed substantial variability in peak area (20 to 220% of initial).
Further study of the nucleotides revealed that much of the instability was due to enzymatic activity (data not shown), in agreement with other reports12–14. This finding highlights a disadvantage of cold aqueous-organic mixtures as extraction solvents; they fail to achieve complete enzyme deactivation. Reports have shown that brief heating ameliorates this effect3, 5. An advantage of the 75% 9:1 MC mix and cold sample storage used here is low enzymatic activity without risking loss of labile metabolites by heating.
Overall, 9:1 MC produced the most stable extracts of the solvents studied; therefore, further work was performed with this solvent. The ratio of 9:1 MC to water was investigated and 75% 9:1 MC was found to provide the best balance between polar metabolite recovery and soluble protein removal (see Supporting Information). We also found that additional extraction cycles and longer extraction incubation time did not improve recovery of a broad range analytes compared to a single cycle ~1 min extraction with 9:1 MC (see Figure S-4). Stability of INS-1 extracts containing 75% 9:1 MC in the final extract (% water adjusted for residual water content of the plate) was comparable to that shown in Figure 1b (see Figure S-5). Metabolite extracts are stable when stored at −80 °C for 7 d with less than 25% decrease in peak area over that period (see Figure S-6).
Water Rinsing
After aspiration, residual media or buffer remains on the cell and dish surface which is subsequently dissolved into extraction solvent yielding potential for contamination of the intracellular metabolite pool and lowered analytical performance, e.g. due to ionization suppression. A solution to this problem is to rinse residual media/buffer from the cell surface with water prior to quenching; however, legitimate concerns have been raised that such rinsing of mammalian cells may alter intracellular metabolites15. We therefore evaluated the impact of rinsing cells with water to eliminate media and buffer residue prior to quenching.
Peak areas for metabolites of glycolysis, the TCA cycle, and related cofactors were compared for cells incubated in KRHB and then rinsed with either KRHB or water prior to quenching. (Non-water rinsed cells were rinsed with KRHB to act as a control by removing extracellular metabolites excreted by cells during incubation). Substantial increases in metabolite peak areas, from 1.5 to 22-fold, were observed for 26 of the measured metabolites in water rinsed samples compared to KRHB rinsed samples (Figure 2a). LC-MS chromatograms (Figure 2b) illustrate clear enhancement of metabolite signals with water rinse, especially in the 17–25 min range where TCA and glycolysis metabolites elute. A region of broad peaks observed at ~12 min, attributed to HEPES in the KRHB, is decreased by rinsing showing removal of media components. Removing this background simplifies the chromatogram and reveals more metabolites. Overall, water rinsing increased the number of high quality detectable features (i.e., features present in 3 of 3 samples with relative standard deviation (RSD) < 30%) from 237 to 452. The increased signals are attributed at least in part to removal of salts that affect ESI.
Figure 2.
Enhancement of metabolite peak area with water rinsing. (a) Fold change enhancement of INS-1 metabolite peak area with water rinse versus KRB rinse. INS-1 cells rinsed with water or KRHB prior to quench and re-rinsed with KRHB to yield an identical residue matrix. (b) 3D MS-chromatograms of KRHB and water rinsed INS-1 cells. Broad peaks from 10 to 15 min are attributed to residual HEPES buffer. Substantial signal enhancement is observed for water rinse sample in 15–20 min region where TCA and glycolysis components elute. Error bars represent 1 SEM, n = 3.
Although the increase in signal and detectable features with water rinsing is attractive, it is necessary to determine if the procedure alters the metabolome. To assess this possibility, INS-1 cells were rinsed with water for 2 or 30 s and compared to cells rinsed only with KRHB. To account for differences in MS sensitivity due to ion suppression, all INS-1 cells were rapidly re-rinsed with KRHB for ~2 s prior to aspiration and quenching. Using this procedure, all cells had the same final matrix, but some had been pretreated with water prior to quenching. For the glycolysis and TCA compounds, no significant differences were found for cells rinsed briefly with water compared to those rinsed with KRHB (see Figure 3a) suggesting little alteration of these metabolic pathways or leakage during a short water rinse. In contrast, with a 30 s exposure to pure water significant differences (p < 0.05) in peak area are observed for G6P+F6P, PEP, aCoA, SUC, ADP, and GDP (Figure 3a) demonstrating that alteration of metabolite content does occur with water exposure, but only after longer times than necessary to rinse away media.
Figure 3.
Effect of water rinse on metabolite peak areas from INS-1 cells on a directed and undirected basis. INS-1 cells were rinsed with KRHB, water (rapid ~2 s), or water (incubate 30 s) prior to a second rapid rinse with KRHB to yield an equivalent matrix in all samples prior to quenching. (a) Peak areas ratios for specified metabolites in extracts of cells rinsed with water versus KRHB prior to quench. Error bars represent 1 SEM, n = 3. Asterisk indicates significant difference in peak area with p < 0.05. (b) Log-log plot of peak areas for all features detected in extracts of INS-1 cells treated with rapid water or KRHB rinse prior to quenching. Features plotted are detected in all replicates and peak areas have RSD < 25% within each group.
To further expand on these observations, we compared peak areas for all features detected following a 2 s water rinse and a KRHB rinse (Figure 3b). Peak areas ranged from 84% to 123% of control with a mean of 104 ± 8% and no significant features were identified following p-value correction for multiple testing. Thus, little change in peak area was observed for nearly all detected features with rapid water rinse over a broad range of hydrophobicity. We conclude that the short water rinse improves signal without substantially altering the detectable metabolome.
Evaluation of LN2, −75 °C, and 0 °C Quenching Methods
Most sample preparation procedures use cold organic solvents to simultaneously quench metabolism and initiate metabolite extraction. While these procedures are effective, we investigated applying LN2 directly to plates to quench metabolism and adding extraction solvent at a later time. This approach of separating quench and extraction steps was designed to allow the analyst to focus on time sensitive biological manipulations (e.g., changing cell media at fixed times) leaving solvent measuring and subsequent extraction steps to be performed later. This approach could prove especially useful when conducting complicated extraction protocols requiring extended incubation times and multiple extraction cycles. An additional benefit of postponing extraction is the flexibility to adjust internal standard concentrations in the extraction solvent based on a preliminary sample analysis without the need to repeat the full set of biological experiments as required by some protocols16. Finally, we also observed improved long term (7 d) stability of samples (mean recovery = 98 ± 6% compared to 90 ± 8 %) when stored as frozen plates and extracted shortly before analysis compared to stored as extracts at −80 °C (see Supporting Information).
To evaluate the performance of the technique, we compared metabolite profiles of INS-1 cells quenched with LN2 to those quenched and extracted simultaneously with −75 °C and 0 °C 75% 9 :1 MC (Figure 4). Peak areas for a majority of metabolites were similar; although, significant differences were observed in metabolite levels for 5 of 27 and 11 of 27 metabolites in −80 °C and 0 °C samples, respectively, relative to LN2 quench. We conclude that direct LN2 quenching provides benefits in work flow and stability (at least for the extraction procedures used here) while offering similar metabolite profiles to −75 °C and 0 °C quench methods.
Figure 4.
Comparison of metabolite peak areas with different quenching techniques. INS-1 cells quenched with LN2, −75 °C solvent, and 0 °C solvent, extracted, and assayed. Metabolite peak areas for each technique plotted versus peak areas from LN2 technique. Error bars represent 1 SEM, n = 3. Asterisk indicates significant difference in peak area with p < 0.05.
Comparison of Glucose Stimulation Results with Different Quenching/Preparation Methods
To evaluate the performance of the 9:1 MC method in quantifying changes in metabolite concentration associated with glucose stimulated insulin secretion, we subjected INS-1 cells to step changes in glucose concentration and prepared samples using this method and two established methods, 80% MeOH10 and B-MTC5 (Figure 5). The final volume of all samples was identical allowing for a direct comparison of results between methods.
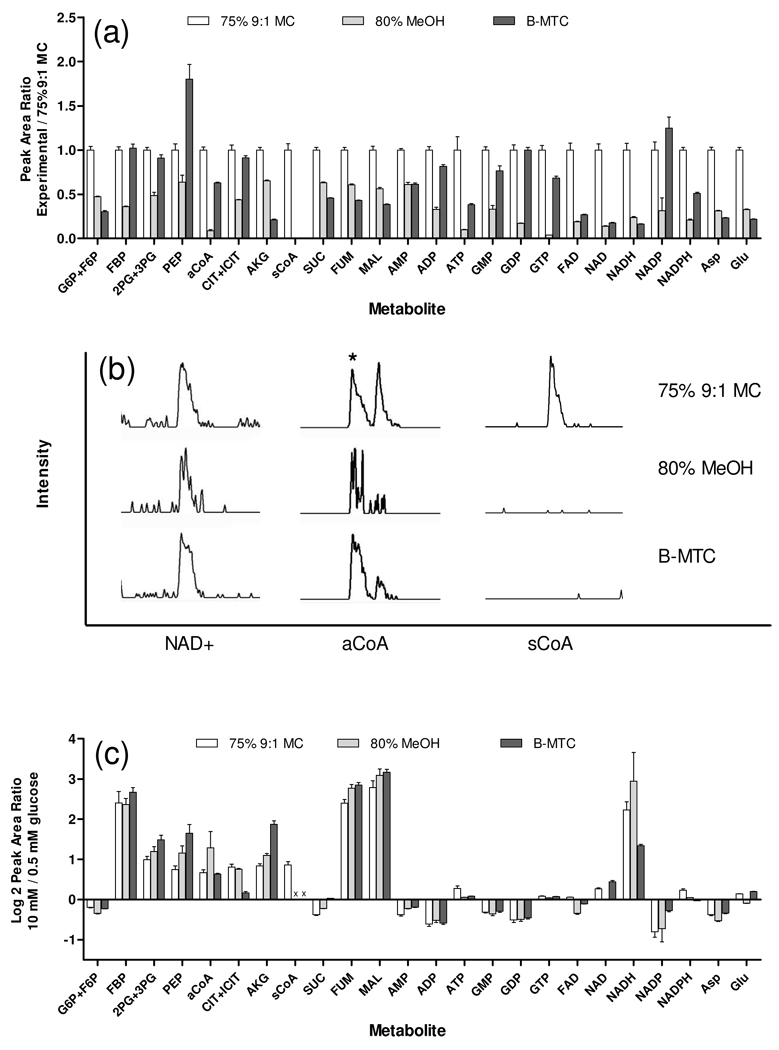
Figure 5.
Comparison of metabolite peak areas, sensitivity, and peak area changes, in INS-1 with glucose stimulation using proposed and established quenching and extraction methods. 75% 9:1 MC is water rinse, LN2 quench, and 1 cycle of 9:1 MC extraction. 80% MeOH is no rinse, −80 °C 8:2 MeOH:H2O quench, 3 extraction cycles, dried with N2, and reconstituted in 1:1 MeOH:H2O. B-MCT is PBS rinse, 4 °C 2:1 MC quench, 2 extraction cycles of 1:1 MeOH:3.8 mM tricine and CHCl3, heat 4 min at 90 °C, dry with N2, and reconstitute in 1:1 MeOH:H2O. INS-1 cells incubated in 0.5 mM glucose 30 min versus cells incubated in 0.5 mM glucose 30 min and stimulated to 10 mM glucose for 20 min. (a) Metabolite peak areas ratios versus 9:1 MC for 10 mM glucose samples. Error bars represent 1 SEM, n = 3. (b) Comparison of NAD+, aCoA, and sCoA chromatograms in 0.5 mM glucose samples. Each individual chromatogram scaled to maximum peak height. Asterisk indicates aCoA peak. (c) Log 2 of the peak area ratio for metabolites in 10 mM glucose versus 0.5 mM glucose.× indicates not detected. Error bars represent 1 SEM, n = 3.
We found that the 9:1 MC method tended to give higher peak areas for the glycolytic and TCA compounds (Figure 5a). This result was accompanied by higher signal to noise ratios, which was significant for compounds that produced small peak areas such as aCoA, but was less important for more abundant species like MAL (see Figure 5b). The 9:1 MC method also allowed detection of sCoA, unlike the other methods. These results suggest overall better sensitivity obtainable by the 9:1 MC method. Because of differences in rinsing, quenching, extraction, heating, and drying steps with each preparation method, it is not possible to directly pinpoint the reasons for this effect, but based on our study we believe that the rinsing step is a key factor in the improved sensitivity as evident in substantially larger peaks for suppressive components such as HEPES in the 80% MeOH chromatograms and phosphate ion in the B-MTC sample.
The improved detection NADH, NADPH, and sCoA may also be attributable to avoiding a heating step. In addition to the improved peak areas, the 9:1 MC method had the convenience of shorter sample preparation times. The total preparation time for six samples was ~15 min using the 9:1 MC method compared to ~5 h using 80% MeOH and B-MTC methods, with a large portion of preparation time for the 80% MeOH and B-MTC methods devoted to drying samples under N2 stream. Although the 9:1 MC method had advantages of sensitivity, detection of heat sensitive compounds, and decreased time requirements; we did find that B-MTC method had better reproducibility with average RSD for all glycolytic and TCA components of 11%, 12%, and 7% for 9:1 MC, 80% MeOH, and B-MTC, respectively.
All three methods produced comparable relative changes in metabolites with glucose stimulation (Figure 5c). Further, these changes tend to agree well with expectation and previous results. Most detected components in the glycolysis and TCA pathways increased substantially following glucose addition which is consistent with increased glycolysis and anaplerosis in these cells17. Using the 9:1 MC method, increases in most components of glycolysis and TCA pathways were moderate (1.6 to 2.0-fold) with the exception of FBP, FUM, and MAL which increased 5.3, 5.3 and 6.9-fold, respectively. The large increases in FUM and MAL likely arise from the high levels of pyruvate carboxylase found in β-cells and the INS-1 clone used in these studies18. These results agree with findings from a previous study which reported increases in CIT+ICIT, MAL, AKG, aCoA, and sCoA of 1.5, 2.0, 1.8, 1.5, and 1.9-fold, respectively, upon 30 min glucose stimulation19. The previous study also reported little change in Asp and Glu levels which is consistent with results we obtained all three extraction methods. In a similar study, CIT and MAL were observed to increase 2 and 5 fold, respectively, after a 30 min stimulation17. We found that NADH increased 4.7-fold which is consistent with an anticipated increase in catabolic reduction charge due to increased glycolysis and TCA cycle flux. Mono and diphosphonucleotides decreased from −1.2 to −1.5 fold while a slight increase of 1.2 fold was observed for ATP and GTP was little changed. This observed increase in the ATP/ADP ratio is consistent with previous observations for GSIS in islet β-cells20.
CONCLUSIONS
We have developed a sample preparation procedure for global metabolic analysis of adherent mammalian cells that uses water rinsing, LN2 quenching, and rapid single step extraction. Individual samples can be quenched, prepared, and injected within ~5 min using only 1.5 mL of solvent for a 10 cm plate to minimize dilution. Sensitivity is enhanced by use of water rinsing, which if performed rapidly, does not alter the metabolome. The choice of 75% 9:1 MC extraction solvent yields extracts that are stable for at least 8 h at 4 °C and 7 d at −80 °C. The method produces relative changes in the metabolome that are similar to previous methods that require longer times, but with an overall increase in peak area for better sensitivity. All three methods appear to produce valid metabolite profiles for glucose-stimulation experiments.
Although we have identified conditions that reproducibly extract and stabilize components from glycolysis, the TCA cycle, and nucleotide metabolites from INS-1 cells, it would be premature to conclude that this procedure would be fully applicable to all mammalian cell lines as presented. It is anticipated that most procedural aspects should translate effectively; nevertheless, we suggest that any experiment aimed at characterizing a metabolome should be preceded by careful characterization of the sample preparation procedure.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Charles Evans for insightful discussion, Jinghua Xu for training with cell culture, Erin Shellman and Katherine Lorenz for assistance with statistical analysis, and Xiangquan Li for samples of the INS-1 832/13 cell line. This work was supported by NIH Grant DK046960 to R.T.K., DK079084 to C.F.B. and the Michigan Nutrition Obesity Research Center (DK089503).
Footnotes
Supporting Information Available. Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 2.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 3.Canelas AB, Pierick A, Ras C, Seifar RM, van Dam JC, van Gulik WM, Heijnen JJ. Anal. Chem. 2009;81:7379–7389. doi: 10.1021/ac900999t. [DOI] [PubMed] [Google Scholar]
- 4.Teng Q, Huang W, Collette T, Ekman D, Tan C. Metabolomics. 2009;5:199–208. [Google Scholar]
- 5.Ritter JB, Genzel Y, Reichl U. Anal. Biochem. 2008;373:349–369. doi: 10.1016/j.ab.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Wu L, Knight J. Clin. Chem. (Washington, DC, U. S.) 1986;32:314–319. [PubMed] [Google Scholar]
- 7.Gao L, Chiou W, Tang H, Cheng X, Camp HS, Burns DJ. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2007;853:303–313. doi: 10.1016/j.jchromb.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Bajad SU, Lu W, Kimball EH, Yuan J, Peterson C, Rabinowitz JD. J. Chromatogr., A. 2006;1125:76–88. doi: 10.1016/j.chroma.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Uehara T, Yokoi A, Aoshima K, Tanaka S, Kadowaki T, Tanaka M, Oda Y. Anal. Chem. 2009;81:3836–3842. doi: 10.1021/ac9002062. [DOI] [PubMed] [Google Scholar]
- 10.Bennett BD, Yuan J, Kimball EH, Rabinowitz JD. Nat. Protoc. 2008;3:1299–1311. doi: 10.1038/nprot.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinowitz JD, Kimball E. Anal. Chem. 2007;79:6167. doi: 10.1021/ac070470c. [DOI] [PubMed] [Google Scholar]
- 12.Kimball E, Rabinowitz JD. Anal. Biochem. 2006;358:273–280. doi: 10.1016/j.ab.2006.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canelas AB, Pierick A, Ras C, Seifar RM, van Dam JC, van Gulik WM, Heijnen JJ. Anal. Chem. 2009;81:7379–7389. doi: 10.1021/ac900999t. [DOI] [PubMed] [Google Scholar]
- 14.Ritter JB, Genzel Y, Reichl U. Anal. Biochem. 2008;373:349–369. doi: 10.1016/j.ab.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD. PLoS Pathog. 2006;2:1165–1175. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett BD, Yuan J, Kimball EH, Rabinowitz JD. Nat. Protocols. 2008;3:1299–1311. doi: 10.1038/nprot.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M. J. Biol. Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 18.Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alarcon C, Rhodes CJ, Newgard CB. J. Biol. Chem. 2006;281:35624–35632. doi: 10.1074/jbc.M602606200. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald MJ. J. Biol. Chem. 2007;282:6043–6052. doi: 10.1074/jbc.M606652200. [DOI] [PubMed] [Google Scholar]
- 20.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane A, Fan T. Metabolomics. 2007;3:79–86. [Google Scholar]
- 22.Fernandez C, Fransson U, Hallgard E, Spegel P, Holm C, Krogh M, Warell K, James P, Mulder H. J. Proteom. Res. 2007;7:400–411. doi: 10.1021/pr070547d. [DOI] [PubMed] [Google Scholar]
- 23.Sellick CA, Hansen R, Maqsood AR, Dunn WB, Stephens GM, Goodacre R, Dickson AJ. Anal. Chem. 2008;81:174–183. doi: 10.1021/ac8016899. [DOI] [PubMed] [Google Scholar]
- 24.Myint KT, Uehara T, Aoshima K, Oda Y. Anal. Chem. 2009;81:7766–7772. doi: 10.1021/ac901269h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.