Abstract
In complex neural circuits of the brain, massive information is processed with neuronal communication through synaptic transmissions. It is thus fundamental to delineate information flows encoded by various kinds of transmissions. Here, we show that glutamate signals from two distinct sensory neurons bidirectionally affect the same postsynaptic interneuron, thereby producing the opposite behaviours. EAT-4/VGLUT (vesicular glutamate transporter)-dependent glutamate signals from AFD thermosensory neurons inhibit the postsynaptic AIY interneurons through activation of GLC-3/GluCl inhibitory glutamate receptor and behaviourally drive migration towards colder temperature. By contrast, EAT-4-dependent glutamate signals from AWC thermosensory neurons stimulate the AIY neurons to induce migration towards warmer temperature. Alteration of the strength of AFD and AWC signals led to significant changes of AIY activity, resulting in drastic modulation of behaviour. We thus provide an important insight on information processing, in which two glutamate transmissions encoding opposite information flows regulate neural activities to produce a large spectrum of behavioural outputs.
Keywords: C. elegans , glutamate, neural circuit, neurotransmission, thermotaxis
Introduction
Immense neural information is processed in complex neural circuits of the brain. In the course of processing, neurons communicate neural information through synaptic transmissions. To gain insight on information processing, it is fundamental to delineate how each information flow communicates in the circuits, thereby modulating behavioural outputs. Although studies in vertebrates have provided a wealth of data on the neural communication through synaptic transmissions, dissection of information flow conveyed between neurons has been hindered by difficulty of the identification of neural codes because of the complexity of the vertebrate nervous system (Di Maio, 2008).
The nematode Caenorhabditis elegans is well suited for the analysis of information processing in the circuits, because of its accessible genetics (Brenner, 1974), in vivo physiological techniques (Kerr et al, 2000), and the simple nervous system with entirely known synaptic connections and gap junctions (White et al, 1986). Of various behaviours analysed to date (de Bono and Maricq, 2005), thermotaxis is best known for its behavioural plasticity: the animals remember the ambient temperature in association with the past feeding condition, and migrate to and move isothermally around the previous experienced temperature on a temperature gradient (Hedgecock and Russell, 1975; Mori and Ohshima, 1995; Mohri et al, 2005). According to the neural circuit model for thermotaxis (Mori and Ohshima, 1995), temperature is sensed and remembered by AFD and AWC sensory neurons, thermal information from AFD and AWC is transmitted to AIY interneuron, and the subsequent information from AIY is transmitted to AIZ and RIA interneurons for further neural information processing (Figure 2D; Mori and Ohshima, 1995; Clark et al, 2006; Biron et al, 2008; Kuhara et al, 2008). Several molecular components related to temperature sensing signal transduction have been identified. In AFD neurons, three guanylyl cyclases, GCY-8, GCY-18, and GCY-23, appear to redundantly produce cGMP, and cGMP-dependent cation channel composed of TAX-2 and TAX-4 increase internal calcium concentration on reception of temperature change (Komatsu et al, 1996; Kimura et al, 2004; Inada et al, 2006). In AWC neurons, ODR-1 (guanylyl cyclase) produces cGMP through activation of ODR-3 (G-α), and TAX-4 (cGMP-dependent cation channel) increases internal calcium concentration on reception of temperature stimuli (Kuhara et al, 2008). However, it remains to be understood how neurons communicate with each other to modulate the neural activity that generates thermotactic behaviour.
EAT-4 is a C. elegans homologue of mammalian vesicular glutamate transporter (VGLUT) that concentrates glutamate into synaptic vesicles (Lee et al, 1999; Bellocchio et al, 2000; Takamori et al, 2000), and involved in chemotaxis, habituation of the tap withdrawal response, local search, and migration towards colder temperature (Rankin and Wicks, 2000; Hills et al, 2004; Chalasani et al, 2007; Clark et al, 2007). In this study, molecular, genetic, and calcium imaging analysis revealed that coordinated functions of EAT-4-dependent glutamate signals from AFD and AWC thermosensory neurons and from RIA interneurons are essential for thermotaxis. EAT-4-dependent glutamate signals from AFD inhibit AIY through activation of GLC-3/GluCl inhibitory glutamate receptor (Horoszok et al, 2001), whereas EAT-4-dependent glutamate signals from AWC stimulate AIY. Alteration of the strength of EAT-4-dependent AFD and AWC glutamate signals onto AIY leads to significant changes of AIY activity, which, through EAT-4-dependent glutamate signals from RIA, results in drastic modulation of thermotaxis. Our results provide an important insight on information processing, in which two glutamatergic synaptic transmissions encoding opposite information flows regulate neural activities to generate various behavioural outputs.
Results
VGLUT homologue EAT-4 is essential for thermotaxis behaviour
In our attempt to analyse thermotaxis-defective mutants through forward genetic approach, we isolated nj2 and nj6 mutants that exhibit abnormal migration in a radial temperature gradient (Supplementary Figure S1A and B). Both nj2 and nj6 mutations were mapped to the region including eat-4 gene and did not complement with eat-4(ky5) loss-of-function mutation, suggesting that nj2 and nj6 mutations are allelic to eat-4(ky5) mutation. Our sequencing of eat-4(nj2) and eat-4(nj6) genomes revealed that nj2 is associated with two missense mutations of the conserved amino acid (G16E) and the putative transmembrane domain (G355R), and that nj6 is a missense mutation of the conserved amino acid (G494R; Supplementary Figure S1C; Ni et al, 1994).
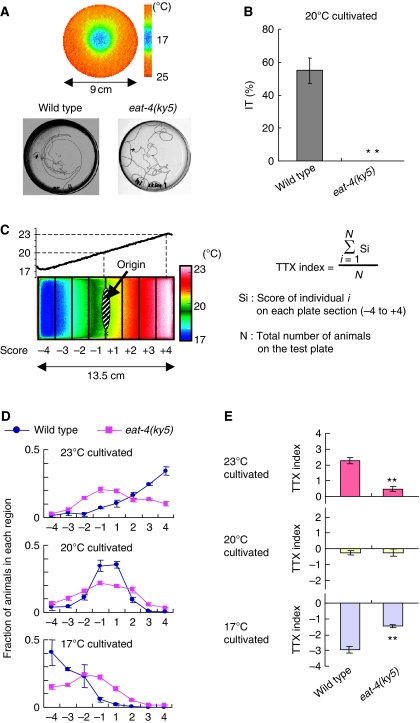
Two types of thermotaxis assays were performed for eat-4(ky5) mutants. The individual thermotaxis assay is suitable for scoring isothermal tracking (IT) behaviour (Figure 1A and B; Gomez et al, 2001). Although many wild-type animals (55±8%) exhibited IT behaviour in a radial thermal gradient from 17 to 25°C after cultivation at 20°C in well-fed conditions, no eat-4(ky5) mutants (0%) exhibited IT behaviour (Figure 1A and B). In addition, eat-4(ky5) mutants showed severe impairment in the population thermotaxis assay that is suitable for quantitatively assessing the migration ability towards the cultivation temperature (Figure 1C–E; Ito et al, 2006). After cultivation at 23, 20, or 17°C in well-fed condition, most of wild-type animals migrated up or down the linear temperature gradient (0.45°C/cm) until they reached the region nearly corresponding to the previous cultivation temperature (Figure 1C and D). However, eat-4(ky5) animals exhibited little tendency to migrate towards cultivation temperature and mostly dispersed in a wide area (Figure 1D). TTX indices (Figure 1C) of eat-4(ky5) mutants cultivated at both 23°C and 17°C (0.47±0.14 at 23°C and −1.46±0.08 at 17°C) differed significantly from those of wild-type animals (2.27±0.19 at 23°C and −2.96±0.22 at 17°C; Figure 1E). Although eat-4(ky5) mutants did not show locomotion defect, they dispersed less than wild-type animals in the absence of a temperature gradient (Supplementary Figure S2A and B; Ségalat et al, 1995), indicating the possibility that abnormal TTX indices of eat-4(ky5) mutants could result from other defects, such as local search defect. Nevertheless, eat-4(ky5) mutants dispersed more broadly from cultivation temperature than wild-type animals on a thermal gradient (Figure 1D). This tendency was also observed in the population thermotaxis assay of eat-4(ky5) mutants cultivated at 20°C after being placed at the higher and lower temperature positions in the gradient (Supplementary Figure S2C and D). These results suggest that eat-4(ky5) mutants indeed exhibit thermotaxis defect, implicating the behavioural regulation by EAT-4-dependent glutamatergic neurotransmission.
Figure 1.
Thermotaxis of eat-4(ky5) mutants. (A) Individual thermotaxis assay of wild-type and eat-4(ky5) mutant animals. Adult animals cultivated at 20°C were individually placed on a radial thermal gradient with 17°C at the centre and 25°C at the periphery (9 cm diameter), and were allowed to move freely for 60 min (see Supplementary data for details). Most wild-type animals leave striking isothermal tracks on the assay plate, whereas eat-4(ky5) mutants move randomly. (B) Fraction of wild-type and eat-4(ky5) mutant animals that moved isothermally in the individual thermotaxis assay; n=60 animals for both strains. Error bar indicates s.e.m. Double asterisk indicates P<0.01 in ANOVA for a comparison with wild-type animals. (C) Procedures for population thermotaxis assay using a linear temperature gradient (Ito et al, 2006). About 50–200 animals cultivated at 17, 20, or 23°C were placed on the agar surface of 20°C (origin) and allowed to move freely for 60 min. The steepness of the temperature gradient was stably kept at 0.45°C/cm during the assay. The thermotactic behaviour was quantified as TTX index (see Supplementary data for details). (D) Distributions of wild-type and eat-4(ky5) mutant animals cultivated at 17, 20, or 23°C in the population thermotaxis assay. (E) TTX indices of wild-type and eat-4(ky5) mutant animals. Red bars, yellow bars, and blue bars represent TTX indices of animals cultivated at 23, 20, and 17°C, respectively; n=3 or more assays. Error bar indicates s.e.m. Double asterisk indicates P<0.01 in post hoc Tukey–Kramer tests for a comparison with wild-type animals.
EAT-4/VGLUT is expressed in subsets of neurons, including constituents of the thermotaxis neural circuit
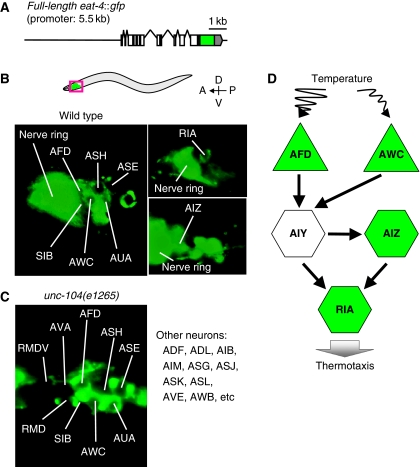
Previously, eat-4∷lacZ and eat-4∷gfp fusion genes, both containing the 2.4 kb fragment upstream of eat-4 as a promoter, did not appear to be expressed in critical neurons for thermotaxis (Lee et al, 1999). The eat-4 gene driven by this 2.4 kb promoter did not restore the normal thermotaxis in eat-4 mutants (Supplementary Figure S3A), suggesting that the 2.4 kb promoter does not drive sufficient expression of eat-4 gene for thermotaxis. Given that another gene resides 5.5 kb upstream of eat-4 gene, we constructed the fusion gene containing the eat-4 genomic fragment and the 5.5 kb upstream fragment (Figure 2A). This full-length eat-4∷gfp fusion gene rescued the abnormal thermotaxis of eat-4 mutants (Supplementary Figure S3A).
Figure 2.
Expression pattern of EAT-4. (A) The full-length eat-4∷gfp fusion gene with 5.5 kb of promoter region. (B,C) The expression of full-length eat-4∷gfp in the head of wild-type animals (B) and of unc-104(e1265) mutants (C). Anterior is to the left and dorsal is on the top. (B) Fluorescence was observed in nerve ring and cell body of many neurons including AFD, AWC (left panel), RIA (top-right panel), and AIZ neurons (bottom-right panel) in wild type. (C) Fluorescence was not observed in the nerve ring but in the cell body of many neurons, including AFD and AWC in unc-104(e1265) mutants. (D) The proposed thermotaxis neural circuit (Mori and Ohshima, 1995; Kuhara et al, 2008). Temperature is sensed by AFD and AWC sensory neurons, thermal information from AFD and AWC is conveyed to AIY interneuron, and the subsequent neural information from AIY is further conveyed to AIZ and RIA interneuron. Neurons expressing the full-length eat-4∷gfp are coloured green.
To identify cells expressing EAT-4, we observed the expression pattern of full-length eat-4∷gfp in wild-type animals (Figure 2B), and also in unc-104(e1265) mutants defective in UNC-104/KIF1A kinesin-like motor protein, to prevent the EAT-4∷GFP-caused strong fluorescence of the nerve ring (Figure 2C; Hall and Hedgecock, 1991; Otsuka et al, 1991). On the basis of cell-body positions and morphologies (Sulston and Horvitz, 1977; Sulston et al, 1983), we observed consistent expression of EAT-4∷GFP in many head neurons including AFD, AWC, AIZ, and RIA, which are constituents of the thermotaxis neural circuit (Figure 2B–D; Mori and Ohshima, 1995; Kuhara et al, 2008).
EAT-4/VGLUT-dependent glutamatergic neurotransmissions from AFD, AWC, and RIA neurons induce multiple behavioural outputs
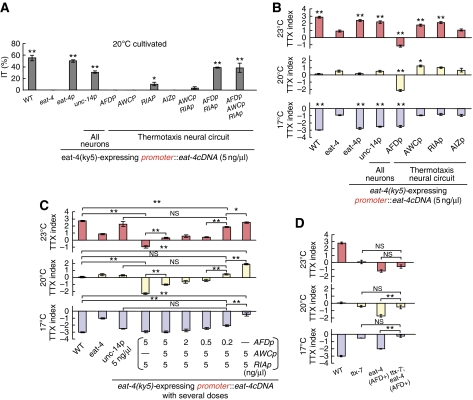
Consistent with the important neural function of EAT-4, expression of eat-4 cDNA from its own promoter (5.5 kb) and in all neurons strongly rescued the IT behaviour defect of eat-4(ky5) mutants (50±2% and 31±2%, respectively, Figure 3A) and restored normal migrations to cultivation temperature (P>0.05 compared with wild-type animals at every cultivation temperature; Figure 3B). To identify the neurons in which EAT-4 functions for thermotaxis, we introduced eat-4 cDNA under the control of various cell-specific promoters into eat-4(ky5) mutants. Expression of EAT-4 in AFD, AWC, or AIZ did not rescue IT behaviour defect of eat-4(ky5) mutants (0%), whereas expression of EAT-4 in RIA weakly did (10±2%; Figure 3A). Remarkably, simultaneous expression of EAT-4 in AFD and RIA rescued the defect (39±1%) as strongly as the transgenic animals expressing EAT-4 from the eat-4 promoter (P>0.05; Figure 3A). The rescue efficiency of the animals expressing EAT-4 in AFD and RIA was not increased by additional expression of EAT-4 in other neurons (Figure 3A). These results suggest that EAT-4-dependent glutamate transmission from RIA to downstream motor neurons is critical for conveying thermal information from AFD.
Figure 3.
Cell-specific rescue experiments for thermotaxis defects of eat-4(ky5) mutants. (A) Rescue experiments for defective isothermal tracking (IT behaviour) of eat-4(ky5) mutants by introducing cell-specific promoters∷eat-4 cDNA at 5 ng/μl; n=58 or more animals. Error bar indicates s.e.m. Single and double asterisk indicate P<0.05 and 0.01, respectively, in ANOVA for a comparison with eat-4(ky5) mutants. (B, C) Rescue experiments for defective migration to cultivation temperature of eat-4(ky5) mutants by introducing individual cell-specific promoters∷eat-4 cDNA at 5 ng/μl (B) or by introducing AFDp∷eat-4 cDNA, AWCp∷eat-4 cDNA, and RIAp∷eat-4 cDNA simultaneously at several doses (C); n=3 or more assays. Error bars indicate s.e.m. Single asterisk, double asterisk, and NS indicate P<0.05, P<0.01, and P>0.05 respectively, in post hoc Tukey–Kramer tests for a comparison with eat-4(ky5) mutants (B) and for a comparison of each genotype (C). (D) The population thermotaxis assays of eat-4 transgenic mutants carrying genetically abnormal function in RIA. eat-4 (AFD+) represents the transgenic eat-4(ky5) mutants expressing EAT-4 in AFD; n=⩾3 assays. Error bars indicate s.e.m. Double asterisk and NS indicate P<0.01 and P>0.05, respectively, in post hoc Tukey–Kramer tests for a comparison of each genotype.
In the population thermotaxis assay, expression of EAT-4 in AIZ did not induce any changes in thermotaxis of eat-4(ky5) mutants as in individual thermotaxis assay (Figure 3B). Interestingly, expression of EAT-4 in AFD, AWC, or RIA induced different migration pattern from that of eat-4(ky5) mutants (Figure 3B), namely, the transgenic animals expressing EAT-4 in AFD migrated towards colder temperature than eat-4(ky5) mutants, the animals expressing EAT-4 in AWC migrated towards warmer temperature after being cultivated at 20 and 23°C, and the animals expressing EAT-4 in RIA migrated towards warmer temperature after being cultivated at 23°C (Figure 3B). Thus, EAT-4-dependent glutamate transmissions from AFD, AWC, or RIA to their postsynaptic neurons are involved in regulation of thermotaxis and each glutamate transmission induces different behavioural outputs.
Because expression of EAT-4 in RIA seems essential for thermotaxis (Figure 3A), it was difficult to detect the effect of EAT-4 only on AFD or AWC. To reconcile this problem, we introduced several doses of AFDp∷eat-4 cDNA and AWCp∷eat-4 cDNA with constant 5 ng/μl dose of RIAp∷eat-4 cDNA into eat-4(ky5) mutants, and performed the population thermotaxis assay (Figure 3C). Simultaneous expression of EAT-4 in AFD and RIA, both in 5 ng/μl doses, enhanced migration towards colder temperature than wild-type animals after cultivation at 20 and 23°C (cryophilic movement; Figure 3C). Although tracks of these transgenic animals in the individual thermotaxis assay did not indicate cryophilic phenotype (Supplementary Figure S3B), cryophilic phenotype would not be detectable on the assay plate, in which the steepness of thermal gradient differs in different areas. Simultaneous expression of EAT-4 in AFD, AWC, and RIA with introduction of 5 ng–5 ng–5 ng/μl, 2 ng–5 ng–5 ng/μl, and 0.5 ng–5 ng–5 ng/μl of AFDp∷eat-4 cDNA, AWCp∷eat-4 cDNA, and RIAp∷eat-4 cDNA, respectively, into eat-4(ky5) mutants still drove the tendency to migrate towards cold temperature, although the cryophilic response of these transgenic animals weakened as compared with the animals expressing EAT-4 in AFD and RIA (Figure 3C). By contrast, simultaneous expression of EAT-4 in AWC and RIA with 5–5 ng/μl of AWCp∷eat-4 cDNA and RIAp∷eat-4 cDNA, respectively, enhanced migration towards warmer temperature after cultivation at 17 and 20°C (thermophilic movement; Figure 3C). These results suggest that glutamate transmission from AFD induces cryophilic movement and that glutamate transmission from AWC induces thermophilic movement. Considering that EAT-4 in AWC was not necessary for IT behaviour (Figure 3A) and simultaneous expression of EAT-4 in AFD, AWC, and RIA induced migration towards cold temperature until the introduction dose of AFDp∷eat-4 cDNA was reduced up to less than one-tenth of AWCp∷eat-4 cDNA (Figure 3C), AFD-mediated glutamate transmission is likely to be more influential than AWC-mediated glutamate transmission for thermotaxis behaviour. Noticeably, simultaneous expression of EAT-4 in AFD, AWC, and RIA with 0.2 ng–5 ng–5 ng/μl of AFDp∷eat-4 cDNA, AWCp∷eat-4 cDNA, and RIAp∷eat-4 cDNA, respectively, almost restored the normal migration to cultivation temperature and showed similar behaviour to that of eat-4(ky5) mutants expressing EAT-4 in all neurons (Figure 3C). These results further support that EAT-4-dependent glutamate transmission from AFD, AWC, and RIA regulates thermotaxis behaviour (Figure 3B).
RIA neurons have multiple neurotransmitter outputs
As already described in Figure 3A, EAT-4-dependent glutamate transmission from RIA is crucial for thermotaxis. Nevertheless, eat-4 transgenic mutants expressing EAT-4 only in AFD but not in RIA (eat-4 (AFD+)) could migrate to colder temperature, as opposed to eat-4(ky5) mutants that dispersed in broader area in the population thermotaxis assay (Figures 1D and 3B). In addition, the phenotype of eat-4 (AFD+) did not change with additional expression of EAT-4 in RIA (Figure 3C). These results raise two possibilities: signals from AFD flow either through RIA-independent pathway or through RIA-dependent pathway coupled with both EAT-4-dependent and -independent synaptic transmissions. To distinguish these possibilities, we examined the effect of genetically imparied RIA on eat-4 (AFD+) (Figure 3D). Correct localization of synapses in RIA requires TTX-7/IMPase, and ttx-7(nj50) mutants showed abnormal thermotactic phenotype, similar to that of wild-type animals in which RIA was ablated (Tanizawa et al, 2006). Thermotactic defect of ttx-7(nj50) mutants completely masked the defect of eat-4 (AFD+) (Figure 3D), suggesting the essential role of RIA-dependent pathway involving both EAT-4-dependent and -independent transmissions.
Glutamatergic neurotransmissions from AFD and AWC neurons oppositely modulate activity of postsynaptic neuron AIY
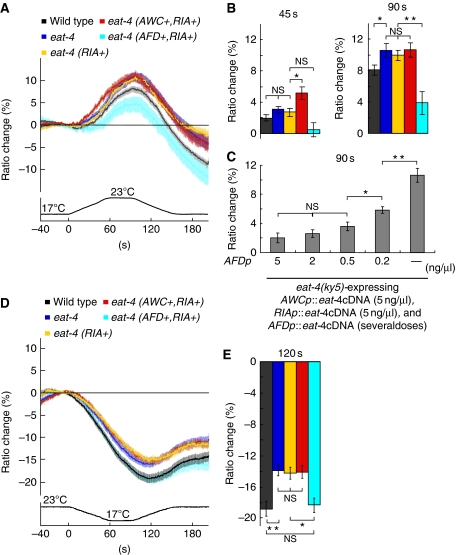
To analyse molecular physiology of the EAT-4-dependent glutamate transmission for thermotaxis, we conducted calcium imaging of AIY, which is postsynaptic to both AFD and AWC (Figure 2D). We monitored temperature stimulus-evoked calcium concentration changes of AIY using cameleon, a genetically encoded calcium indicator (Miyawaki et al, 1997; Nagai et al, 2004). We verified that expression of cameleon (yc3.60) in AIY itself did not affect thermotaxis (Supplementary Figure S3C). Calcium imaging was carried out in animals cultivated at 20°C (Figure 4). Consistent with our previous results, the calcium concentration in AIY of wild-type animals increased with warming and decreased with cooling (Figure 4A and D; Kuhara et al, 2008). The fluorescence resonance energy transfer (FRET) ratios did not return to baseline, which might be caused by faster degradation of YFP fluorescence than CFP fluorescence in yc3.60 (Figure 4A and D; Supplementary Figure S4A and B). On warming, FRET ratios in AIY of eat-4(ky5) mutants increased more than that of wild-type animals (11±1% for eat-4(ky5) mutants and 8±0.6% for wild-type animals at 90 s after starting temperature change; Figure 4A and B). Thus, AIY of eat-4(ky5) mutants appear to be hyper-responsive to warming, suggesting that EAT-4-dependent glutamate transmission is involved in the thermal response of AIY.
Figure 4.
In vivo calcium imaging of AIY according to temperature change in eat-4(ky5) mutants and the eat-4(ky5) transgenic animals. (A) The intracellular calcium concentration change was measured as the YFP/CFP fluorescence change (ratio change) of cameleon (yc3.6). eat-4 (AWC+, RIA+), eat-4 (AFD+, RIA+), and eat-4 (RIA+) represent the transgenic eat-4(ky5) mutants expressing EAT-4 in AWC and RIA but not in AFD, in AFD and RIA but not in AWC, and in RIA but not in AFD and AWC, respectively (top). Response curves represent the average of ratio change according to temperature change. Temperature change along time is shown at the bottom of the response curve. 0 s is the time starting temperature change from 17 to 23°C (bottom). (B) The average of ratio changes to temperature stimuli at 45 s (left) and at 90 s (right), regarding the results shown in Figure 4A. The colour of bars in the graphs corresponds to the colour of response curves described in Figure 4A. (C) The average of ratio changes of eat-4(ky5) expressing EAT-4 in AFD, AWC, and RIA simultaneously by introducing several doses of AFDp∷eat-4 cDNA with 5 ng/μl of AWCp∷eat-4 cDNA and RIAp∷eat-4 cDNA at 90 s. (D) Ratio change of yc3.6 in AIY in response to temperature change from 23 to 17°C. (E) The average of ratio changes to temperature stimuli at 120 s for the results shown in D; (A–E) n=18 or more animals. Error bar indicates s.e.m. Single asterisk, double asterisk, and NS indicate P<0.05, P<0.01, and P>0.05, respectively, in Steel–Dwass tests for a comparison of each genotype.
Previous reports showed that activation of AIY mediates movement towards warmer temperature (Mori and Ohshima, 1995; Hobert et al, 1997; Kuhara et al, 2008), implicating the correlation between the activity of AIY and behavioural output. We then hypothesized that glutamate transmissions from AFD and AWC have opposite effects on the activity of AIY neurons, consistent with the opposite effect of AFD and AWC on behavioural outputs. To test this hypothesis, we monitored the activity of AIY on warming in transgenic eat-4(ky5) animals expressing EAT-4 in either AFD or AWC (Figure 4A and B) by calcium imaging. As EAT-4 in RIA is essential for thermotaxis (Figure 3A), we also expressed EAT-4 in RIA of transgenic animals (Figure 4A and B). The FRET ratio changes in AIY of eat-4(ky5) mutants expressing EAT-4 in RIA (eat-4 (RIA+)) were larger than those of wild-type animals and similar to those of eat-4(ky5) mutants (eat-4; 10±0.6% at 90 s; Figure 4A and B), suggesting that expression of EAT-4 in RIA has almost no effect on the AIY activity. The larger increment in the FRET ratio of eat-4 (RIA+) on warming was consistent with our behavioural results showing that eat-4 (RIA+) exhibited the tendency to migrate towards warmer temperature after cultivation at 23°C, whereas the similar larger increment in the FRET ratio of eat-4(ky5) mutants was not coincided with their behavioural output (Figure 3B). Thus, EAT-4 in RIA is likely to be important for coupling AIY activity and behavioural output. Additional expression of EAT-4 in AFD of eat-4(ky5) mutants (eat-4 (AFD+, RIA+)) induced significantly smaller change of FRET ratios in AIY (3.9±1.5% at 90 s) as compared with eat-4(ky5) mutants and wild-type animals (Figure 4A and B). Notably, smaller changes in FRET ratio observed in eat-4 (AFD+, RIA+) were similarly observed in eat-4(ky5) mutants expressing EAT-4 only in AFD (eat-4 (AFD+)), which argues against any feedback pathway to AIY through RIA (Supplementary Figure S4C and D). Expression of EAT-4 in AWC and RIA (eat-4 (AWC+, RIA+)) caused not only the larger change, such as eat-4(ky5) mutants (eat-4; 10.6±1% at 90 s), but also faster change of FRET ratios in AIY (5.1±0.7% at 45 s; Figure 4A and B). Our results on calcium imaging of AIY in animals cultivated at 20°C, therefore, closely correlated with behavioural data shown in Figure 3C. We also conducted calcium imaging of AIY in wild-type animals and eat-4(ky5) transgenic animals cultivated at both 17°C and 23°C, and found that correlation between calcium imaging of AIY and thermotaxis behaviour was held in every cultivation temperature (Supplementary Figure S5). The data on calcium imaging suggest that glutamate transmissions from AFD or from AWC inhibit or stimulate AIY, respectively.
To verify the effect of glutamate signals on the response of AIY, we also conducted calcium imaging of AIY on cooling (Figure 4D and E). On cooling, FRET ratios in AIY of eat-4(ky5) mutants did not decrease as much as those of wild-type animals (−14±0.7% for eat-4(ky5) mutants and −19±1.0% for wild-type animals at 120 s; Figure 4D and E). Consistent with the results on warming (Figure 4A and B), this result likely represents hyperactive state of AIY in eat-4(ky5) mutants (Figure 4D and E). The FRET ratios in AIY of eat-4 (RIA+) and eat-4 (AWC+, RIA+) showed a similar change to those of eat-4(ky5) mutants (−14±0.8% for eat-4 (RIA+) and for eat-4 (AWC+, RIA+) at 120 s; Figure 4D and E), implicating no effects of glutamate signals from AWC and RIA on the response of AIY to cooling. By contrast, the FRET ratios in AIY of eat-4 (AFD+, RIA+) decreased to the similar level to those of wild-type animals (−18±0.9% for eat-4 (AFD+, RIA+) at 120 s; Figure 4D and E), suggesting that inhibition through glutamate transmission from AFD regulates the response of AIY to cooling. Altogether, these results demonstrate that glutamate transmission from AFD inhibits AIY activity in response to both warming and cooling, and glutamate transmission from AWC stimulates AIY activity in response to only warming, thereby implying that AFD-mediated glutamate transmission more effectively contributes to thermotaxis behaviour than AWC-mediated glutamate transmission.
To elucidate whether various behavioural outputs shown in Figure 3C are caused by modulation of AIY activity through EAT-4-dependent glutamate transmission from AFD and AWC, we investigated dose dependency of AIY activity in transgenic eat-4(ky5) mutants expressing relative different doses of EAT-4 in AFD, AWC, and RIA with calcium imaging on warming (Figure 4C). Intriguingly, FRET ratios showed similar small changes when introducing 5, 2, or 0.5 ng of AFDp∷eat-4 cDNA to the eat-4 mutants, whereas FRET ratios dramatically increased when introducing 0.2 or 0 ng of AFDp∷eat-4 cDNA (Figure 4C), which is consistent with the results of cell-specific rescue experiments (Figure 3C). These results also demonstrated that modulation of the AIY activity by opposite glutamate signals from AFD and AWC induces drastic changes in behavioural output.
GluCl homologue GLC-3 inhibits the activity of AIY interneurons in thermotaxis
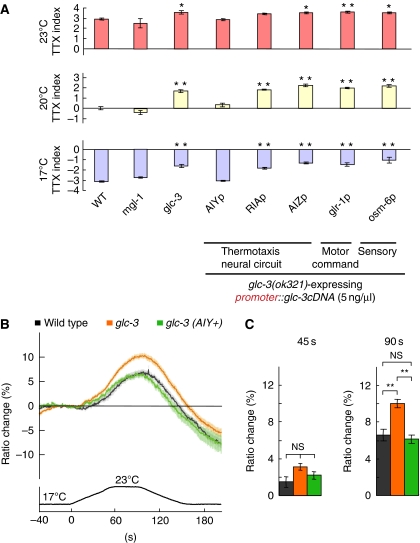
Our analysis of EAT-4/VGLUT suggested that glutamate receptors receive EAT-4-dependent glutamate signals in AIY. Four classes of glutamate receptors are predicted in C. elegans; AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)-type glutamate-gated cation channels encoded by eight glr genes, NMDA (N-methyl-D-aspartic acid)-type glutamate-gated cation channels encoded by two nmr genes, glutamate-gated chloride channels (GluCl) encoded by four glc genes and two avr genes, and metabotropic G-protein-coupled glutamate receptors (mGluR) encoded by three mgl genes (Brockie and Maricq, 2003; Yates et al, 2003; Dillon et al, 2006). Of those, MGL-1/mGluR and GLC-3/GluCl were found to be expressed in AIY (Horoszok et al, 2001; Wenick and Hobert, 2004), implicating that either MGL-1 or GLC-3 is required for thermotaxis as a glutamate receptor in AIY. Although mgl-1(tm1811) mutants migrated to their cultivation temperature normally, glc-3(ok321) mutants showed migration to warmer temperature than the cultivation temperature (Figure 5A). This thermotaxis defect was fully rescued by expressing GLC-3 in AIY but not in any other neurons (Figure 5A), suggesting that GLC-3/GluCl acts as a glutamate receptor in AIY for thermotaxis.
Figure 5.
Cell-autonomous function of GLC-3 in thermotaxis. (A) The population thermotaxis assays of mgl-1 and glc-3 mutants, and cell-specific rescue experiments for glc-3 mutants; n=3 or more assays. Error bar indicates s.e.m. Single and double asterisk indicate P<0.05 and 0.01, respectively, in post hoc Tukey–Kramer tests for a comparison with wild-type animals. (B, C) In vivo calcium imaging of AIY in glc-3(ok321) mutants and the glc-3(ok321) transgenic animals. (B) Ratio change of yc3.6 in AIY according to temperature change. glc-3 (AIY+) represents the transgenic glc-3(ok321) mutants expressing GCL-3 in AIY. (C) The average of ratio changes to temperature stimuli at 45 s (left) and at 90 s (right) regarding the results shown in B; (B, C) n=24 or more animals. Error bar indicates s.e.m. Double asterisk and NS indicates P<0.01 and P>0.05, respectively, in Steel–Dwass tests for a comparison with wild-type animals.
Given that the activation of AIY mediates movement towards warmer temperature (Mori and Ohshima, 1995; Hobert et al, 1997; Kuhara et al, 2008) and GluCls mediate inhibitory neurotransmission (Dent et al, 1997), GLC-3/GluCl presumably regulates thermotaxis through inhibition of the AIY activity. Consistent with this possibility, FRET ratios in AIY of glc-3(ok321) mutants changed more than those of wild-type animals (10±0.5% for glc-3(ok321) mutants and 6.6±0.6% for wild-type animals at 90 s; Figure 5B and C), implicating the hyper-responsiveness of AIY in glc-3(ok321) mutants. The increased change of FRET ratios was rescued by expressing GLC-3 in AIY (6.2±0.4% at 90 s; Figure 5B and C), suggesting that GLC-3 cell autonomously inhibits the activity of AIY.
GLC-3/GluCl receives glutamatergic signals from AFD thermosensory neurons
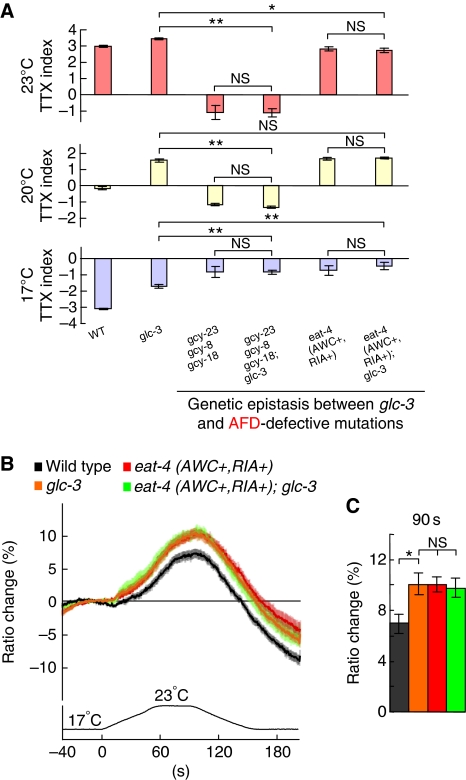
We addressed whether GLC-3/GluCl in AIY receives EAT-4-dependent glutamate signals from AFD or AWC. Thermosensory signal transduction in AFD is thought to be mediated by the change in intracellular concentration of cGMP through GCY-23, GCY-8, and GCY-18 guanylyl cyclases (Inada et al, 2006), and gcy-23(nj37) gcy-8(oy44) gcy-18(nj38) triple mutants showed abnormal thermotactic phenotype similar to that of AFD-ablated wild-type animals (Inada et al, 2006; Kuhara et al, 2008). gcy-23 gcy-8 gcy-18; glc-3 quadruple mutants showed thermotactic abnormality quite similar to that of gcy-23 gcy-8 gcy-18 triple mutants, suggesting that the abolishment of thermosensory signalling in AFD entirely suppressed the glc-3(ok321) mutation (Figure 6A). In addition, thermotactic defect of the eat-4 mutants expressing EAT-4 in AWC and RIA, but not in AFD (eat-4 (AWC+, RIA+)), completely masked the defect of glc-3 mutants (Figure 6A). These results suggest that GLC-3 glutamate receptors in AIY receive EAT-4-mediated glutamate signals from AFD.
Figure 6.
Effect of AFD defect on glc-3(ok321) mutants. (A) The population thermotaxis assays of AFD-defective mutants and glc-3 mutants in the background of abnormal neural signalling in AFD; n=3 or more assays. Error bar indicates s.e.m. Single asterisk, double asterisk, and NS indicate P<0.05, P<0.01, and P>0.05, respectively, in post hoc Tukey–Kramer tests for a comparison of each genotype. (B, C) In vivo calcium imaging of AIY in eat-4 (AWC+, RIA+); glc-3 and in each single mutant. (B) Ratio change of yc3.6 in AIY according to temperature change. (C) The average of ratio changes to temperature stimuli at 90 s regarding the results shown in Figure 6B; (B, C) n=22 or more animals. Error bar indicates s.e.m. Single asterisk and NS indicate P<0.05 and P>0.05, respectively, in Steel–Dwass tests for a comparison of each genotype.
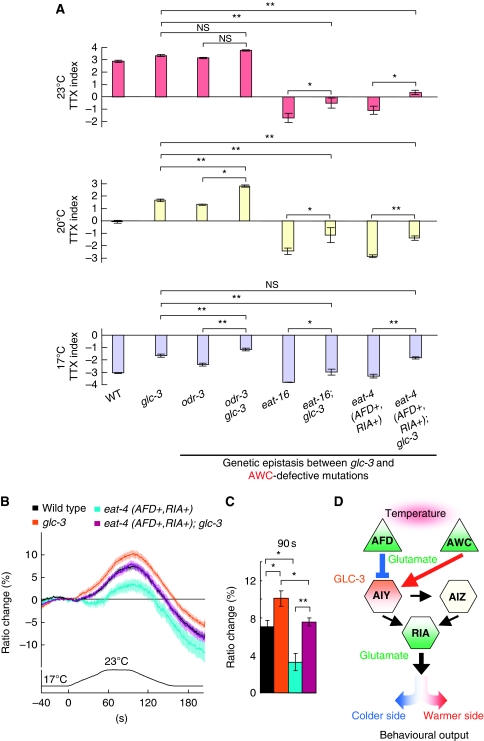
Recently, ODR-3 G-α-dependent heterotrimeric G-protein-coupled signalling was found to mediate thermosensory signal transduction in AWC, and EAT-16 RGS (regulator of G-protein signalling) suppressed the G-protein-coupled signalling in AWC (Kuhara et al, 2008). odr-3(n1605) and eat-16(nj8) mutants migrated towards warmer and colder temperatures than the cultivation temperature, respectively (Figure 7A; Kuhara et al, 2008). glc-3 odr-3 double mutants migrated to much warmer temperature than both glc-3 and odr-3 single mutants after cultivation at 17 and 20°C, implicating the additive effect of odr-3 and glc-3 mutation (Figure 7A). Similarly, thermotactic defect of eat-16(nj8) mutants and the eat-4(ky5) mutants expressing EAT-4 in AFD and RIA (eat-4 (AFD+, RIA+)) influenced additively on the defect of glc-3 mutants (Figure 7A), implicating GLC-3 independent glutamate transmission from AWC. Our genetic analyses suggest that GLC-3/GluCl is required for reception of EAT-4-dependent glutamate signals from AFD.
Figure 7.
Effect of AWC defect on glc-3(ok321) mutants. (A) The population thermotaxis assays of AWC-defective mutants and glc-3 mutants in the background of abnormal neural signalling in AWC; n=3 or more assays. Error bar indicates s.e.m. Single asterisk, double asterisk, and NS indicate P<0.05, P<0.01, and P>0.05, respectively, in post hoc Tukey–Kramer tests for a comparison of each genotype. (B, C) In vivo calcium imaging of AIY in eat-4 (AFD+, RIA+); glc-3 and in each single mutant. (B) Ratio change of yc3.6 in AIY according to temperature change. (C) The average of ratio changes to temperature stimuli at 90 s regarding the results shown in B; (B, C) n=22 or more animals. Error bar indicates s.e.m. Single and double asterisk indicate P<0.05 and 0.01, respectively, in Steel–Dwass tests for a comparison of each genotype. (D) A model for glutamatergic neurotransmission in the thermotaxis neural circuit. AFD, AWC, and RIA release glutamate in EAT-4-dependent manner (green). GLC-3 acts in AIY (orange). EAT-4-dependent glutamate signals from AFD inhibit the activity of AIY through activation of GLC-3 inhibitory glutamate receptors, and induce eventual migration to colder side (blue). By contrast, EAT-4-dependent glutamate signals from AWC stimulate the activity of AIY, and induce eventual migration to warmer side (red).
Using calcium imaging, we clarified whether EAT-4-dependent glutamate transmission from AFD is mediated by GLC-3 in AIY (Figures 6B, C and 7B, C). FRET ratios in AIY of the double mutants (eat-4 (AWC+, RIA+); glc-3) constructed from the eat-4 mutants expressing EAT-4 in AWC and RIA (eat-4 (AWC+, RIA+)) and from glc-3 mutants showed a change larger than those of wild-type animals (9.8±0.8% for double mutants and 7±0.7% for wild-type animals at 90 s) and similar to those of both single mutants (10±0.6% for transgenic eat-4 mutants and 10±0.8% for glc-3 mutants at 90 s; Figure 6B and C), thereby demonstrating the same hyper-responsiveness of double mutants as both single mutants. By contrast, FRET ratios in AIY of the double mutants (eat-4 (AFD+, RIA+); glc-3) constructed from the eat-4 mutants expressing EAT-4 in AFD and RIA (eat-4 (AFD+, RIA+)) and from glc-3 mutants showed the change in between those in both single mutants (7.6±0.4% for double mutants, 3.3±0.9% for transgenic eat-4 mutants, and 10±0.8% for glc-3 mutants at 90 s), but similar to those of wild-type animals (Figure 7B and C). These physiological results are consistent with the results obtained by genetic analysis, further supporting the molecular model that GLC-3 inhibits AIY on receiving EAT-4-dependent glutamate signals from AFD.
Discussion
EAT-4/VGLUT-dependent glutamatergic neurotransmission in the thermotaxis neural circuit
In the current model for temperature sensing, thermal stimuli are transduced in AFD through three functionally redundant guanylyl cyclases and cGMP-gated channels (Komatsu et al, 1996; Inada et al, 2006) and in AWC through heterotrimeric G-protein signalling and cGMP-gated channels (Kuhara et al, 2008). In this study, our results suggest a model for signalling pathways downstream of temperature sensing (Figure 7D). After temperature sensing in AFD and AWC sensory neurons, thermosensory information is transmitted to postsynaptic interneuron AIY through EAT-4/VGLUT-dependent glutamatergic neurotransmission. EAT-4-dependent glutamate signals from AFD inhibit AIY through activation of inhibitory glutamate receptor GLC-3/GluCl, thereby inducing migration towards colder temperature. By contrast, EAT-4-dependent glutamate signals from AWC stimulate AIY to induce migration towards warmer temperature. Hence, we demonstrated that the bidirectional regulation of AIY through EAT-4-dependent glutamate transmissions from AFD and AWC is essential for terminal behavioural phenotypes.
Previous reports showed that AFD and AWC differ in contribution for thermotaxis; AFD-ablated animals exhibited severe defect (Mori and Ohshima, 1995; Kuhara et al, 2008), whereas AWC-ablated animals exhibited slight defect in thermotaxis (Biron et al, 2008; Kuhara et al, 2008). These results suggested that thermotaxis is regulated by the orchestrated information in major thermosensory neuron AFD and supportive thermosensory neuron AWC. Consistent with these previous results, our behavioural and calcium imaging results demonstrated that contributions of inhibitory glutamate signals from AFD and excitatory glutamate signals from AWC are not equivalent. For IT behaviour, EAT-4-dependent glutamate transmission from AFD was indispensable, while that from AWC was not necessary (Figure 3A). In addition, transgenic eat-4 mutants expressing EAT-4 in AFD, AWC, and RIA showed strong tendency to migrate towards colder temperature, except for the case in which the introduction dose of AFDp∷eat-4 cDNA to the eat-4 mutants was reduced to less than one-tenth of AWCp∷eat-4 cDNA (Figure 3C). Further, glutamate transmission from AFD inhibits AIY activity in response to both warming and cooling and that from AWC stimulates AIY activity in response to only warming (Figure 4), suggesting that the contribution of inhibitory glutamate signals from AFD is larger than that of excitatory glutamate signals from AWC. Our study also showed that well-balanced glutamate signals from AFD and AWC could drive nearly normal responsiveness of AIY to temperature changes and eventual normal thermotaxis (Figures 3C and 4C). Taken together, glutamate signals from AFD and AWC are implied to be the critical factor for mediating the orchestration of major information in AFD and supportive information in AWC. In addition, our study demonstrated that GLC-3 receives EAT-4-dependent glutamate signals from AFD, although glutamate receptors that receive glutamate signals from AWC remained to be identified (Figures 5,6,7). The failure of finding excitatory glutamate receptors may possibly be caused by the difference in contribution between AFD and AWC for thermotaxis, thus reflecting the biased detection ability of thermotaxis defect towards AFD over AWC.
The RIA interneuron, known as one of the most pivotal interneurons in C. elegans, integrates signals processed in the thermotaxis neural circuit and emits outputs to downstream neurons (Figure 2C; Mori and Ohshima, 1995; Tanizawa et al, 2006). Given those previous reports, our work revealed that multiple transmissions including EAT-4-dependent glutamate from RIA are involved in communicating processed information in the circuit to downstream neurons, thereby generating ultimate thermotactic outputs (Figures 3 and 7D). Although there are no solid evidences as to which neurons downstream of RIA are main component neurons in the circuit, RIA is heavily connected with numerous presynapses to SMD or RMD head motor neurons that regulate turning behaviour (White et al, 1986; Gray et al, 2005). Considering that temperature regulates turn frequency and run duration (Zariwala et al, 2003), it is quite likely that SMD and RMD control turn frequency, depending on thermal information transmitted by RIA. We expect that inspection of glutamate transmission from RIA to SMD or to RMD, including identification of glutamate receptors functioning in SMD or RMD for thermotaxis, reveal the information processing that consequently generates a variety of thermotactic behavioural outputs.
Information processing in the simple circuit composed of AFD, AWC, and AIY neurons
Mori and Ohshima (1995) proposed the neural circuit for thermotaxis, in which AFD thermosensory neurons transmit excitatory signal to AIY interneurons. Consistent with this proposal, Clark et al (2006) showed using calcium imaging that AIY of animals lacking AFD did not respond to temperature changes. Given these results and our results on EAT-4, one can propose that the neural signals transmitted from AFD to AIY are of at least two kinds, EAT-4-dependent inhibitory signals and EAT-4-independent excitatory signals. In this study, we further showed that the thermotactic abnormality of gcy-23 gcy-8 gcy-18 triple mutants with defective AFD function is considerably different from that of the eat-4 mutants expressing EAT-4 in AWC and RIA, but not in AFD (eat-4 (AWC+, RIA+); Figure 6A), and that AIY of eat-4 mutants are hyper-responsive to temperature change than wild-type animals (Figure 4A and B), thereby undoubtedly implicating EAT-4-independent excitatory signals from AFD to AIY. Recent electrophysiological study showing that AFD responds to both cooling and warming (Ramot et al, 2008) is consistent with these results.
Our results also suggest that AWC transmit excitatory signals to AIY through EAT-4-dependent glutamatergic neurotransmission (Figure 4), although we previously reported that hyper-responsiveness of AWC to temperature change induced lower responsiveness of AIY (Kuhara et al, 2008). It is probable that AWC transmits EAT-4-independent inhibitory signals in addition to EAT-4-dependent excitatory glutamate signals to AIY, which is reminiscent of the regulation of behavioural response to odourants, in which AWC releases both glutamate and neuropeptide NLP-1 to postsynaptic AIA interneurons (Chalasani et al, 2010).
AWC senses odourants as well as temperature (Bargmann et al, 1993; Kuhara et al, 2008). How does C. elegans distinguish these qualitatively different signals within a sensory neuron? Similar to thermal signal, olfactory signal is transduced through G-protein signalling and cGMP-gated channels in AWC, and is further transmitted to AIY through EAT-4-dependent glutamatergic neurotransmission (Coburn and Bargmann, 1996; Roayaie et al, 1998; L’Etoile and Bargmann, 2000; Chalasani et al, 2007). Intriguingly, olfactory signal from AWC inhibits AIY on reception of glutamate signal by GLC-3/GluCl (Chalasani et al, 2007), whereas, as shown in this study, thermal signal from AWC stimulates AIY through putative glutamate receptors other than GLC-3/GluCl. Hence, our results demonstrated that segregation of different sensory signals, such as olfactory and thermosensory signals, is mediated through not only distinct signal transductions in a sensory neuron but also discrete neurotransmissions in downstream neural circuits.
We demonstrated one of the simplest neural circuits consisting of two different sensory neurons and a single postsynaptic interneuron, in which AFD and AWC sensory neurons use the same EAT-4/VGLUT-dependent glutamatergic neurotransmission to inhibit or stimulate the postsynaptic neuron AIY. The balance between inhibition and stimulation of the AIY activity significantly affected thermotactic behaviour. Strong EAT-4-dependent glutamatergic transmission from AFD enhanced the tendency for animals to migrate towards colder temperature than the cultivation temperature, whereas weak EAT-4-dependent transmission from AFD relative to AWC weakened the tendency to migrate to colder temperature and rather strengthened the tendency to migrate towards warmer temperature than the cultivation temperature. Thus, two synaptic transmissions encoding opposite information flows regulate neural activities to generate various behavioural outputs. Studies on synaptic transmission in the simple neural circuit consisting of AFD, AWC, and AIY should shed light onto information processing underlying vertebrate intertwined neural networks.
Materials and methods
Strains and genetics
The techniques used for culturing and handling C. elegans were essentially as described by Brenner (1974). We used the following strains: wild-type C. elegans variety Bristol strain (N2), IK604 eat-4(ky5) III, IK600 eat-4(nj2) III, IK602 eat-4(nj6) III, IK708 glc-3(ok321) V, IK732 mgl-1(tm1811) X, CB1265 unc-104(e1265) II, IK589 ttx-7(nj50) I, IK597 gcy-23(nj37) gcy-8(oy44) gcy-18(nj38) IV, MT3644 odr-3(n1605) V, IK839 eat-16(nj8) I, IK813 gcy-23(nj37) gcy-8(oy44) gcy-18(nj38) IV; glc-3(ok321) V, IK815 glc-3(ok321) odr-3(n1605) V, IK840 eat-16(nj8) I; glc-3(ok321) V, IK818 eat-4(ky5); Ex[gcy-8p∷eat-4 cDNA, glr-3p∷eat-4 cDNA, ges-1p∷NLS-GFP] designated as eat-4 (AFD+,RIA+), IK819 eat-4(ky5); Ex[odr-3p∷eat-4 cDNA, glr-3p∷eat-4 cDNA, ges-1p∷NLS-GFP] designated as eat-4 (AWC+,RIA+), IK820 eat-4 (AFD+, RIA+) III; glc-3(ok321) V, IK822 eat-4 (AWC+, RIA+) III; glc-3(ok321) V, and many transgenic strains derived from them. eat-4(nj2) and eat-4(nj6) were isolated in genetic screens, as described by Okochi et al (2005) and mapped by multi-factor crosses and complementation tests with eat-4(ky5). The isolated eat-4 mutants were outcrossed to wild-type animals more than six times before the analyses.
Behavioural assays
The individual thermotaxis assay was performed as described by Mori and Ohshima (1995) and Mohri et al (2005). The population thermotaxis assay was performed as previously reported (Ito et al, 2006). Details of each assay are described in Supplementary data.
Molecular biology
The eat-4 genomic fragment, including 2.4 or 5.5 kb of the promoter region and 2 kb of the downstream region, was amplified by PCR from N2 genome. The full-length eat-4∷gfp translational fusion gene fragment, which was amplified by PCR from N2 genome and pPD95.75, contains a 5.5-kb fragment upstream of eat-4 gene, eat-4 gene (4.5 kb) including all exons and introns, gfp (0.9 kb), and 2-kb fragment downstream of eat-4 gene.
For cell-specific rescue experiments, cell-specific promoter∷eat-4 cDNA construct and cell-specific promoter∷glc-3 cDNA construct were generated. eat-4 cDNA was PCR amplified by using tagged primers from yk32h2 EST clone that contains eat-4 cDNA lacking initial 7 bp, and was cloned into pPD49.26 to generate pNR8. glc-3 cDNA was PCR amplified from yk1649c11 EST clone that contains full-length glc-3 cDNA, and was cloned into pPD49.26 to generate pNR56. The resultant plasmids were confirmed to contain the intact cDNA by sequencing. All cell-specific promoter∷eat-4 cDNA plasmids and cell-specific promoter∷glc-3 cDNA plasmids were generated by inserting cell-specific promoter fragments into pNR8 or pNR56. All cell-specific promoter constructs were previously generated by PCR to contain only non-coding region. Before conducting the rescue experiments, expression patterns of cell-specific promoter∷gfp constructs were verified by examining GFP fluorescence. Cell-specific promoters are 0.7 kb of gcy-8p for AFD, 2.3 kb of odr-3p for AWC, which also induces expression slightly in AWB, 0.8 kb of ttx-3p for AIY, 1.9 kb of glr-3p for RIA, 1.4 kb of lin-11p for AIZ, which also induces expression in ADF, AVJ, AVH, AVG, ADL, and RIC, 2.1 kb of osm-6p for sensory neurons, 5.3 kb of glr-1p for motor and command neurons, and 1.4 kb of unc-14p for all neurons.
For calcium imaging of AIY, ttx-3p∷yc3.60 construct was generated. Yellow cameleon 3.60 was amplified from YC3.60/pcDNA3 (Nagai et al, 2004) and cloned into pPD49.26 to generate pNR46. ttx-3p fragment was inserted into pNR46 to generate ttx-3p∷yc3.60 (pNR86). The resultant plasmid was confirmed to contain the intact ttx-3p∷yc3.60 by sequencing.
Transgenic animals
Germline transformation was performed by co-injecting experimental DNA (0.2–100 ng/μl) and an injection marker pKDK66 ges-1p∷NLS-GFP (50 ng/μl) or pNAS88 ges-1p∷NLS TagRFP (50 ng/μl) into the gonad (Mello et al, 1991). Multiple independent transgenic lines were established for each experimental DNA. For comparison of phenotypes on different genetic backgrounds, transgenic arrays were transferred by intercrossing.
In vivo calcium imaging and data analysis
In vivo calcium imaging was performed essentially according to previous reports with some modifications (Kimura et al, 2004; Kuhara et al, 2008). After being cultivated at 20°C, well-fed animals expressing ttx-3p∷yc3.60 were glued onto a 1.5% agar pad on glass, immersed in M9 buffer, and covered by cover glass. The agar pad and M9 buffer were kept at the initial imaging temperature. Sample preparation was completed within 2 min. The sample was then placed onto a peltier-based thermocontroller (Tokai Hit, Japan) on the stage of an Olympus BX61WI at the initial imaging temperature for 2 min, and fluorescence was introduced into a Dual-View (Molecular Devices, USA) optics systems. Cyan fluorescent protein (CFP; F480) and yellow fluorescent protein (YFP; F535) images were simultaneously captured by an EM-CCD camera C9100-13 ImagEM (Hamamatsu Photonics). Images were taken with a 500 ms exposure time with 2 × 2 binning. The temperature on the agar pad was monitored by a thermometer system, DCM-20 (Tokai Hit and Hamamatsu Photonics). For each imaging experiment, fluorescence intensities of F535 and F480 were measured using MetaMorph imaging analysis system (Molecular Devices). As a computer regulates all the recorded images and the outcome of the analysis, any intention of a researcher should be excluded. Relative increases or decreases in the intracellular calcium concentration were measured as increases or decreases in the YFP/CFP fluorescence ratio of the cameleon protein (Ratio Change).
Statistics
All error bars in the figures indicate standard error of mean (s.e.m.). Results of behavioural experiments were treated as parametric data. The statistical analysis for all behavioural experiments was performed by one-way analysis of variance for multiple comparisons. Comparisons between each result were followed by post hoc Tukey–Kramer test, except for data sets including results of ‘0%’. Results of calcium imaging were treated as non-parametric data. The statistical analysis for all calcium imaging was performed by Steel–Dwass tests. A single asterisk (*), double asterisk (**), and non-significant (NS) in the figures indicate P<0.05, P<0.01, and P>0.05, respectively. Detailed statistical results in each figure are described in Supplementary data.
Supplementary Material
Acknowledgments
We thank A Miyawaki for yc3.60 construct; A Fire for pPD plasmids; J McGree for ges-1 promoter; Y Tanizawa for glc-3 promoter; N Nishio for ges-1p∷NLS TagRFP and sharing unpublished results of thermotaxis assays; L Avery for eat-4(ky5) mutant; S Mitani at the National Bioresource Project (Japan) and Caenorhabditis Genetic Center for strains; H Inada for sharing unpublished results; M Okumura for technical advice; members of Mori laboratory for comments on this manuscript and stimulating discussion. NO was supported by the Japan Society for the Promotion of Science. AK was supported by Takeda Science Foundation and MEXT, Japan. IM is a Scholar of Institute for Advanced Research in Nagoya University. This work was supported by CREST, JST, and, in part, by Grant-in-Aid for Scientific Research on Priority Areas—Molecular Brain Science—from MEXT (17024023; to IM).
Footnotes
The authors declare that they have no conflict of interest.
References
- Bargmann C, Hartwieg E, Horvitz H (1993) Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527 [DOI] [PubMed] [Google Scholar]
- Bellocchio E, Reimer R, Fremeau RJ, Edwards R (2000) Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science 289: 957–960 [DOI] [PubMed] [Google Scholar]
- Biron D, Wasserman S, Thomas J, Samuel A, Sengupta P (2008) An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc Natl Acad Sci USA 105: 11002–11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie P, Maricq A (2003) Ionotropic glutamate receptors in Caenorhabditis elegans. Neurosignals 12: 108–125 [DOI] [PubMed] [Google Scholar]
- Chalasani S, Chronis N, Tsunozaki M, Gray J, Ramot D, Goodman M, Bargmann C (2007) Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450: 63–70 [DOI] [PubMed] [Google Scholar]
- Chalasani S, Kato S, Albrecht D, Nakagawa T, Abbott L, Bargmann C (2010) Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci 13: 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Biron D, Sengupta P, Samuel A (2006) The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci 26: 7444–7451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Gabel C, Lee T, Samuel A (2007) Short-term adaptation and temporal processing in the cryophilic response of Caenorhabditis elegans. J Neurophysiol 97: 1903–1910 [DOI] [PubMed] [Google Scholar]
- Coburn C, Bargmann C (1996) A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17: 695–706 [DOI] [PubMed] [Google Scholar]
- de Bono M, Maricq A (2005) Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28: 451–501 [DOI] [PubMed] [Google Scholar]
- Dent J, Davis M, Avery L (1997) avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J 16: 5867–5879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio V (2008) Regulation of information passing by synaptic transmission: a short review. Brain Res 1225: 26–38 [DOI] [PubMed] [Google Scholar]
- Dillon J, Hopper N, Holden-Dye L, O’Connor V (2006) Molecular characterization of the metabotropic glutamate receptor family in Caenorhabditis elegans. Biochem Soc Trans 34: 942–948 [DOI] [PubMed] [Google Scholar]
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann C, Nef P (2001) Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30: 241–248 [DOI] [PubMed] [Google Scholar]
- Gray J, Hill J, Bargmann C (2005) A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA 102: 3184–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Hedgecock E (1991) Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: 837–847 [DOI] [PubMed] [Google Scholar]
- Hedgecock E, Russell R (1975) Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 72: 4061–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie P, Maricq A (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G (1997) Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron 19: 345–357 [DOI] [PubMed] [Google Scholar]
- Horoszok L, Raymond V, Sattelle D, Wolstenholme A (2001) GLC-3: a novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br J Pharmacol 132: 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I (2006) Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 172: 2239–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Inada H, Mori I (2006) Quantitative analysis of thermotaxis in the nematode Caenorhabditis elegans. J Neurosci Methods 154: 45–52 [DOI] [PubMed] [Google Scholar]
- Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien R, Schafer W (2000) Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26: 583–594 [DOI] [PubMed] [Google Scholar]
- Kimura K, Miyawaki A, Matsumoto K, Mori I (2004) The C. elegans thermosensory neuron AFD responds to warming. Curr Biol 14: 1291–1295 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Mori I, Rhee J, Akaike N, Ohshima Y (1996) Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17: 707–718 [DOI] [PubMed] [Google Scholar]
- Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, Kimura K, Inada H, Matsumoto K, Mori I (2008) Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 320: 803–807 [DOI] [PubMed] [Google Scholar]
- L’Etoile N, Bargmann C (2000) Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron 25: 575–586 [DOI] [PubMed] [Google Scholar]
- Lee R, Sawin E, Chalfie M, Horvitz H, Avery L (1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci 19: 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Kramer J, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery J, Adams J, Ikura M, Tsien R (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887 [DOI] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Kimura K, Koike M, Mizuno T, Mori I (2005) Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Ohshima Y (1995) Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348 [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A (2004) Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B, Rosteck PJ, Nadi N, Paul S (1994) Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc Natl Acad Sci USA 91: 5607–5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi Y, Kimura K, Ohta A, Mori I (2005) Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J 24: 2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A, Jeyaprakash A, García-Añoveros J, Tang L, Fisk G, Hartshorne T, Franco R, Born T (1991) The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron 6: 113–122 [DOI] [PubMed] [Google Scholar]
- Ramot D, MacInnis B, Goodman M (2008) Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci 11: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin C, Wicks S (2000) Mutations of the Caenorhabditis elegans brain-specific inorganic phosphate transporter eat-4 affect habituation of the tap-withdrawal response without affecting the response itself. J Neurosci 20: 4337–4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie K, Crump J, Sagasti A, Bargmann C (1998) The G alpha protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20: 55–67 [DOI] [PubMed] [Google Scholar]
- Ségalat L, Elkes D, Kaplan J (1995) Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science 267: 1648–1651 [DOI] [PubMed] [Google Scholar]
- Sulston J, Horvitz H (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56: 110–156 [DOI] [PubMed] [Google Scholar]
- Sulston J, Schierenberg E, White J, Thomson J (1983) The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100: 64–119 [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee J, Rosenmund C, Jahn R (2000) Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 407: 189–194 [DOI] [PubMed] [Google Scholar]
- Tanizawa Y, Kuhara A, Inada H, Kodama E, Mizuno T, Mori I (2006) Inositol monophosphatase regulates localization of synaptic components and behavior in the mature nervous system of C. elegans. Genes Dev 20: 3296–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenick A, Hobert O (2004) Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell 6: 757–770 [DOI] [PubMed] [Google Scholar]
- White J, Southgate E, Thomson J, Brenner S (1986) The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Phil Trans R Soc London Series B Biol Sci 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Yates D, Portillo V, Wolstenholme A (2003) The avermectin receptors of Haemonchus contortus and Caenorhabditis elegans. Int J Parasitol 33: 1183–1193 [DOI] [PubMed] [Google Scholar]
- Zariwala H, Miller A, Faumont S, Lockery S (2003) Step response analysis of thermotaxis in Caenorhabditis elegans. J Neurosci 23: 4369–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.