Abstract
Transforming growth factor (TGF)-βs are dimeric polypeptides that have vital roles in regulating cell growth and differentiation. They signal by assembling a receptor heterotetramer composed of two TβRI:TβRII heterodimers. To investigate whether the two heterodimers bind and signal autonomously, one of the TGF-β protomers was substituted to block receptor binding. The substituted dimer, TGF-β3 WD, bound the TβRII extracellular domain and recruited the TβRI with affinities indistinguishable from TGF-β3, but with one-half the stoichiometry. TGF-β3 WD was further shown to retain one-quarter to one-half the signalling activity of TGF-β3 in three established assays for TGF-β function. Single-molecule fluorescence imaging with GFP-tagged receptors demonstrated a measurable increase in the proportion of TβRI and TβRII dimers upon treatment with TGF-β3, but not with TGF-β3 WD. These results provide evidence that the two TβRI:TβRII heterodimers bind and signal in an autonomous manner. They further underscore how the TGF-βs diverged from the bone morphogenetic proteins, the ancestral ligands of the TGF-β superfamily that signal through a RI:RII:RII heterotrimer.
Keywords: signal transduction, signalling, TβRI, TβRII, TGF-β
Introduction
Transforming growth factor-β (TGF-β) isoforms are secreted signal ligands that have vital roles in coordinating wound healing, modulating immune cell function, maintaining the extracellular matrix, and regulating epithelial and endothelial cell growth and differentiation (Massagué, 1998). The importance of the TGF-βs is underscored by their conservation among vertebrates and their demonstrated roles in a variety of human diseases, including tissue fibrosis (Blobe et al, 2000) and cancer (Derynck et al, 2001). TGF-βs are members of an extended signalling superfamily that arose in early metazoans (Kingsley, 1994). The superfamily has greatly diversified, with >30 known members in vertebrates, including the prototypical TGF-βs, the bone morphogenetic proteins (BMPs), the closely related growth and differentiation factors (GDFs), and the activins and inhibins (Massagué, 1998).
TGF-βs are disulphide-linked dimers of identical 112-residue protomers. The protomers include four disulphide bonds, three of which form a conserved structure known as a cystine knot (Sun and Davies, 1995). BMPs, GDFs, activins, and most other ligands of the TGF-β superfamily share a similar structure, though the cysteine that forms the inter-chain disulphide bond is lacking in three family members, GDF-3, GDF-9, and BMP-15 (McPherron and Lee, 1993; Dube et al, 1998). The ligands of the superfamily signal by binding and bringing together two single pass transmembrane receptor kinases, known as receptor types I and II (Derynck, 1994). This initiates a transphosphorylation cascade where the type II kinase phosphorylates and activates the type I (Wrana et al, 1994). The type I kinase phosphorylates Smad proteins (Shi and Massagué, 2003) and other effectors (Zhang, 2009), which regulate the transcription of target genes (Massagué and Wotton, 2000).
TGF-βs have been shown to assemble a receptor heterotetramer on the cell surface comprising two molecules of its type I receptor, TβRI, and two molecules of its type II receptor, TβRII, based on differential receptor tagging (Moustakas et al, 1993; Henis et al, 1994; Wells et al, 1999), two-dimensional gel electrophoresis (Yamashita et al, 1994), and genetic complementation (Weis-Garcia and Massagué, 1996). TβRI and TβRII have been further shown to form stable homodimers in the absence of TGF-β (Chen and Derynck, 1994; Gilboa et al, 1998; Rechtman et al, 2009), suggesting a two-step mechanism for assembly of a receptor heterotetramer.
The recently reported structures of TGF-β1 and -β3 bound to the TβRI and TβRII extracellular domains support the binding stoichiometry deduced on the basis of the cell-based experiments, with two molecules of each receptor symmetrically bound, TβRII at the ‘fingertips’, and TβRI directly adjacent on the underside of the ‘fingers’ (Groppe et al, 2008; Radaev et al, 2010) (Figure 1A). TβRI and TβRII directly contact one another in the complex and these receptor–receptor contacts are responsible for the pronounced stepwise manner with which TGF-βs bind TβRII and recruit TβRI (Groppe et al, 2008) first determined based on genetic complementation studies with receptor-deficient mink lung epithelial cells (Laiho et al, 1991). The additional constraint imposed by the receptor–receptor contact is thought to be further important for enhancing the specificity with which TβRI and TβRII bind TGF-βs and preventing activation of TGF-β responses by other ligands of the superfamily (Groppe et al, 2008; Massagué, 2008).
Figure 1.
Structure of the TGF-β receptor complex and effect of ligand monomerization. (A) Surface representation of the TGF-β3 homodimer bound to the TβRI and TβRII extracellular domains (PDB 2PJY). The two protomers of TGF-β3 are depicted in pink and dark blue. TβRII binds to the fingertips of each protomer and is depicted in green, whereas TβRI binds to the underside of the fingers and is depicted in yellow. The residues in the interface between TGF-β3 and TβRII are shown in the expanded view shown on the right. (B) Schematic representation of the TGF-β3 receptor complex where high affinity TβRI binding is dependent upon interactions with both TGF-β3 protomers and TβRII. (C) Schematic representation of the TGF-β3 monomer, which binds TβRII with the same affinity as wild-type TGF-β3, but which is impaired in its ability to bind and recruit TβRI. (D) Schematic representation of a TGF-β3 heterodimer comprising a wild-type protomer (blue) and a variant (pink) bearing substitutions that block TβRI and TβRII binding.
The binding of TGF-β by two well-separated TβRI:TβRII heterodimeric pairs suggests that the two heterodimers might bind and signal independently of one another. This is further suggested by the finding that low but measurable signalling was induced when TβRI and TβRII were artificially dimerized with small immunophilin domains (Stockwell and Schreiber, 1998) or when TGF-β responsive cells are treated with monomeric TGF-β1 or -β3 (Amatayakul-Chantler et al, 1994; Zúñiga et al, 2005). Monomeric TGF-β3, even though impaired 10–15-fold in its affinity for binding and recruiting TβRI (Groppe et al, 2008), retains significant reporter gene activity with a reduction in potency of just 10-fold relative to wild-type homodimer (Zúñiga et al, 2005). Other studies, such as one in which the TβRI and TβRII kinases were fused to the extracellular domain of the erythropoieten receptor (Luo and Lodish, 1996) or another in which the TβRI kinase domain was fused to the TβRII extracellular domain (Okadome et al, 1994), do not however support independent signalling. Monomeric TGF-β3 has been further shown to possess an intrinsic propensity to non-covalently dimerize, especially in the presence of TβRI and TβRII (Zúñiga et al, 2005), suggesting that the retention of activity by the monomers might reflect their propensity to non-covalently dimerize and assemble TβRI:TβRII heterotetramers, not assemble and signal through TβRI:TβRII heterodimers.
The objective of this study was to thoroughly investigate if TGF-βs signal through two independently functioning TβRI:TβRII heterodimers. This was accomplished by isolating a disulphide-linked TGF-β3 dimer composed of a wild-type protomer and a variant bearing substitutions of Arg25, Tyr90, Arg94, residues previously shown (De Crescenzo et al, 2006; Baardsnes et al, 2009) or implicated (Groppe et al, 2008) to be critical for binding of TβRI and TβRII. Using a series of complementary biochemical techniques, the substituted TGF-β3 dimer was shown to bind the TβRII extracellular domain and recruit the TβRI with affinities indistinguishable from the wild-type homodimer, but with one-half the stoichiometry. Using three established assays for TGF-β function, the substituted dimer was further shown to retain one-quarter to one-half the signalling activity of the wild-type homodimer. Collectively, these results show that the two TβRI:TβRII heterodimers bind and signal nearly independently of one another.
Results
Design and isolation of TGF-β3 WD
The objective was to generate a form of TGF-β that bound TβRII and recruited TβRI with affinities comparable to TGF-β1 or -β3, but with one-half the stoichiometry. This necessitated that a dimeric form of TGF-β1 or -β3 be used as TβRI binds across the dimer interface and requires both protomers, as well as TβRII, to bind with high affinity (Zúñiga et al, 2005; Groppe et al, 2008; Radaev et al, 2010) (Figure 1B and C). This was accomplished by generating a heterodimer with one wild-type protomer and one protomer in which Arg25 and Arg94 were substituted with glutamate and Tyr90 was substituted with alanine (Figure 1D). The importance of Arg25 and Arg94 for high affinity TβRII binding was first suggested based on the fact that these, along with Val92, are the only residues in the interface with TβRII that are substituted in TGF-β2 (Hart et al, 2002), the isoform that binds TβRII weakly (Cheifetz et al, 1990). This was later confirmed by TGF-β3–β2 and TGF-β2–β3 chimeras in which swaps of these residues between isoforms, Arg25 and Arg94 in TGF-β3 and Lys25 and Lys94 in TGF-β2, decreased or increased affinity several hundred fold to that of the other isoform (De Crescenzo et al, 2006; Baardsnes et al, 2009). The hydrogen-bonding arrangement of these arginines with the sidechain carboxylates of Asp32 and Glu119 on TβRII (Figure 1A, right), together with the dramatic decrease in affinity when conservatively replaced, led to the idea that substitution with glutamate would abolish TβRII binding altogether.
The blockade of TβRII binding would be expected to greatly impair the binding of TβRI due to the loss of receptor–receptor contacts essential for binding and recruiting TβRI (Groppe et al, 2008). This alone would probably be sufficient based on the weak apparent affinity of the TβRI extracellular domain for TGF-β1, -β2, and -β3 (Kd∼30–100 μM) (Groppe et al, 2008; Radaev et al, 2010), though to further diminish binding, Tyr90 was substituted. This residue is centrally located within the TβRI interface and was replaced with a less bulky alanine sidechain, with the goal to reduce TβRI binding based on its substantial contact with TβRI (Groppe et al, 2008; Radaev et al, 2010).
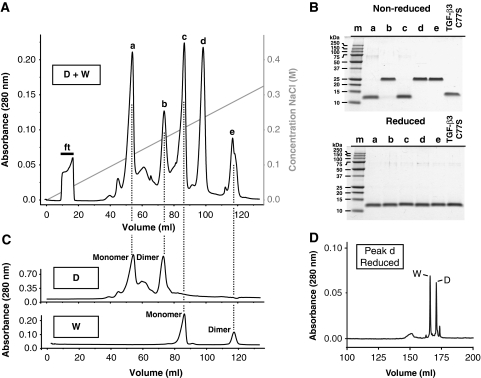
The heterodimer was prepared by first producing wild type and R25E, Y90A, R94E triply substituted human TGF-β3 monomers (designated, WT or W and dead or D, respectively) in bacteria. These were reconstituted from inclusion bodies, purified to near homogeneity in 8 M urea, and then diluted, in a 1:1 molar ratio, into refolding buffer (Cerletti, 2000). The folding mixture, which contained the desired heterodimer, TGF-β3 WD, as well as wild-type and substituted homodimers, TGF-β3 WW and TGF-β3 DD, respectively, was then fractionated using high-resolution cation-exchange chromatography at pH 4.0 (Figure 2A).
Figure 2.
Isolation of the TGF-β3 heterodimer. (A) HPLC cation-exchange elution profile of an oxidative folding reaction including equal amounts of TGF-β3 WT and TGF-β3 R25E, Y90A, R94E monomers. Five major peaks are eluted (a, b, c, d, and e) as a function of increasing concentration of NaCl. Flow through following loading is indicated by ‘ft’. (B) SDS–PAGE analysis of the peaks eluted in panel (A) and TGF-β3 C77S under both non-reducing (top) and reducing conditions (bottom). Peaks b, d, and e are shown to be reductant-sensitive dimers, whereas a and c are monomers. (C) HPLC cation-exchange elution profiles, exactly as in panel (A), except for refolding reactions of TGF-β3 WT (‘W’, bottom) or TGF-β3 R25E, Y90A, R94E monomer (‘D’, top) alone. (D) C18 reverse phase chromatogram of reduced peak d gives two major peaks, one of which has an intact ESI mass that matches the predicted mass for the WT monomer (‘W’, 12722.5 Da) and one that matches the predicted mass for the R25E Y90A R94E monomer (‘D’, 12576.2 Da).
This separation yielded five major species, and as anticipated, three of these, b, d, and e, corresponded to reductant-sensitive 25 kDa dimers (Figure 2B). The other two, a and c, corresponded to 12.5 kDa monomers (Figure 2B). The three dimers, as well as the two monomers were predicted to be positively charged under the experimental conditions, though reductions in the positive charge were expected for each arginine to glutamate substitution. Thus, peaks e, d, and b were predicted to correspond to the TGF-β3 WW, WD, and DD dimers, respectively, while peaks c and a, the TGF-β3 W and D monomers. To confirm this, TGF-β3 W and TGF-β3 D monomers were folded and fractionated under identical conditions. This yielded the anticipated chromatograms, with peaks e and c corresponding to dimeric and monomeric forms of wild-type TGF-β3, peaks b and a to the dimeric and monomeric forms of dead TGF-β3, and peak d, the purported wild-type–dead heterodimer, TGF-β3 WD, with no matching counterpart. To confirm the identity of the TGF-β3 WD, the protein was reduced and applied to a reverse phase C18 column. This led to two peaks, the ESI-MS determined masses of which were within 1.0 Da of the predicted masses of the W and D monomers, 12722.5 and 12576.2 Da, respectively.
TGF-β3 C77S, a variant of TGF-β3 in which the cysteine residue that forms the inter-chain disulphide has been substituted, was also produced. This variant is covalently monomeric (Figure 2B) and as shown previously binds TβRII with nearly the same affinity as the wild-type dimer, but is impaired in its ability to bind and recruit TβRI (Ilangovan et al, 2004; Zúñiga et al, 2005; Groppe et al, 2008).
Qualitative analysis of receptor binding by native gel electrophoresis
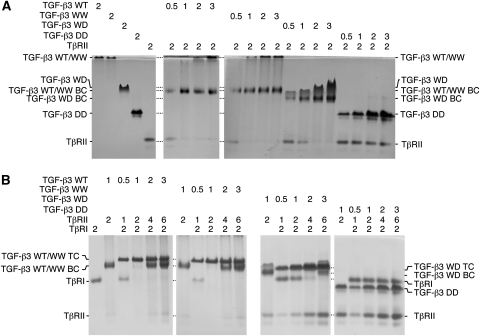
The relative affinities and stoichiometries of receptor binding by the isolated ligands were assessed by analysing the complexes formed with the purified TβRI and TβRII extracellular domains using native gel electrophoresis. The initial experiments focused on TβRII binding and were performed by titrating a fixed amount of TβRII extracellular domain, or TβRII-ED, with increasing molar amounts of the isolated TGF-β3 WW, WD, and DD dimers and a TGF-β3 WT dimer control (there in principle should be no difference between TGF-β3 WW and WT, though these are designated differently to emphasize that TGF-β3 WW was isolated from the folding reaction in which both wild-type and dead monomers were added, whereas TGF-β3 WT was isolated from a folding reaction in which only the wild-type monomer was added). The results showed that TGF-β3 WT, WW, and WD dimers formed detectable complexes with TβRII-ED, whereas TGF-β3 DD did not (Figure 3A). The fact that TGF-β3 DD failed to yield a detectable complex was consistent with expectations regarding its reduced affinity for TβRII. Though less convincing, the results also support the anticipated stoichiometry, with the intensity of the complex bands maximizing in intensity at a 2:1 TβRII-ED:TGF-β dimer ratio for TGF-β3 WT and WW, and a 2:2 ratio (same as 1:1) for TGF-β3 WD.
Figure 3.
Qualitative native gel analyses of receptor-binding properties. (A) TGF-β:TβRII binary complexes were formed by adding 0.5–3.0 molar equivalents of ligand dimers (WT, WW, WD, and DD) to 2.0 molar equivalent of TβRII, then electrophoresed through a native 12% polyacrylamide gel at room temperature and stained with Commassie brilliant blue. Positions of ligands alone and TGF-β:TβRII binary complexes (BC) are labelled. TGF-β3 WD runs with a pronounced smile, and while the underlying basis for this is not known, it may be related to alternate conformational states that it adopts (see also Figure 6D and accompanying explanation). (B) TGF-β:TβRI:TβRII ternary complexes were formed by adding 0.5–3.0 molar equivalents of ligands (WT, WW, WD, and DD), together with 2.0 molar equivalents of TβRII (relative to ligands), to 2.0 molar equivalents of TβRI and analysed as above. Positions of TβRI and TβRII alone and the corresponding TGF-β:TβRII binary (BC) and TGF-β:TβRII:TβRII ternary complexes (TC) are labelled.
The subsequent experiments focused on TβRI recruitment and were performed by titrating a fixed amount of TβRI extracellular domain, or TβRI-ED, with increasing amounts of TβRII-ED:TGF-β dimer complex. The TβRII:TGF-β complex was always added in a 2:1 molar ratio, regardless of whether the TβRII-ED was needed or not, to ensure that binding of TβRI-ED was not limited by TβRII-ED. The ligands that bound TβRII, TGF-β3 WT, WW, and WD, also bound and recruited TβRI-ED. TGF-β3 DD, which did not detectably bind TβRII, also failed to bind and recruit TβRI (Figure 3B). The stoichiometries in this case are more convincing, with the TβRI-ED:TβRII-ED:TGF-β complex appearing neither undertitrated (i.e. excess TβRI present) nor overtitrated (i.e. excess TβRII:TGF-β complex present) at a 2:1 TβRI-ED:TGF-β dimer ratio for TGF-β3 WT and WW and a 2:2 (same as a 1:1) ratio for TGF-β3 WD. These results also support the binding stoichiometry with TβRII as excess TβRII is present when TGF-β3 WD, TβRII-ED, and TβRI-ED are combined in a ratio of 2:4:2 (same as 1:2:1), but not when TGF-β3 WT (or WW), TβRII-ED, and TβRI-ED are combined in this same ratio. These results, though qualitative, indicate that TGF-β3 WD binds and assembles a 1:1:1 TGF-β3:TβRII:TβRI complex, not a 1:2:2 as does TGF-β3 WT or TGF-β3 WW.
Quantitative analysis of receptor-binding affinities using SPR
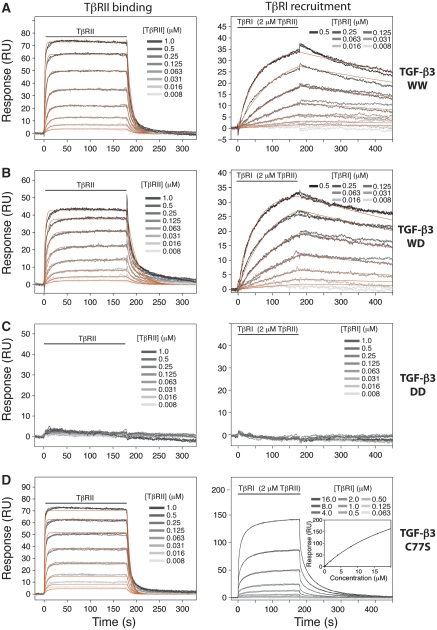
TGF-β3 WW, WD, and DD were quantitatively characterized in terms of their ability to bind TβRII-ED and recruit TβRI-ED using SPR. To accomplish this, the ligands were biotinylated in the presence of excess of TβRI-ED and TβRII-ED, separated away from the bound receptors by HPLC, and captured at moderate-to-low density, 100–150 resonance units (RU), on a streptavidin surface. Two sets of measurements were made, one in which increasing concentrations of TβRII-ED was injected and another in which the running buffer was supplemented with a near-saturating concentration of TβRII and increasing concentrations of TβRI-ED were injected. The former provided information about TβRII binding, while the latter, TβRI recruitment (Groppe et al, 2008; Radaev et al, 2010).
The series of sensorgrams obtained from these two sets of measurements are presented in Figure 4 (left and right panels, respectively). Through visual inspection, the results are consistent with expectations: TGF-β3 WW and WD robustly bind TβRII and recruit TβRI, while TGF-β3 DD is neither capable of binding TβRII nor recruiting TβRI. The low surface density, together with the uniformity of the immobilized ligands, allowed the sensorgrams to be globally fit to a simple kinetic model (orange lines), yielding the association (ka) and disassociation (kd) rate constants as well as the dissociation constant (Kd) (Table I). These data show that TGF-β3 WW and WD are indeed indistinguishable in their ability to bind TβRII and recruit TβRI, with Kds of 0.18±0.02 and 0.16±0.01 μM, respectively for binding TβRII, and Kds of 0.031±0.002 and 0.027±0.001 μM, respectively, for TβRI recruitment. These values are further shown to be equivalent to those of TGF-β3 WT (Supplementary Figure S1; Table I). TGF-β3 DD did not yield any detectable response, indicating it either binds TβRII and recruits TβRI very weakly or is non-native. The reason for the systematic deviation in the kinetic fits during the dissociation phase for TβRII binding to TGF-β3 WT, WW, and WD is not known, but does not alter our conclusions as near identical Kd values were obtained by fitting the equilibrium response, Req, as a function of receptor concentration to a standard binding isotherm (not shown).
Figure 4.
Quantitative SPR measurements of receptor-binding properties with biotinylated ligands. (A) Sensorgrams obtained as TβRII alone was injected (left) or TβRI was injected in the presence of 2.0 μM TβRII (right) over a TGF-β3 WW surface. The traces shown (grey) correspond to triplicate measurements of a two-fold serial dilution of the receptor over the concentration range shown. The orange curves correspond to global fits of each data set to a 1:1 binding model. The horizontal line indicates the time during which the analyte was injected. (B–D) Same SPR experiments as in panel (A), except over TGF-β3 WD, TGF-β3 DD, and TGF-β3 C77S (W) surfaces, respectively. The sensorgrams for TβRI recruitment by TGF-β3 C77S in the presence of 2.0 μM TβRII was analysed by fitting the equilibrium response as a function of concentration to a standard binding curve (panel D, right panel, inset).
Table 1. Binding constants for TGF-β3 and variants to the signalling receptors.
| Surface | Analyte | Dissassociation constant (μM) |
||||
|---|---|---|---|---|---|---|
| Buffer suppl. | kon (M−1 s−1) | koff (s−1) | Kd (μM) | Rmax (RU) | ||
| TGF-β3 WT | TβRII | None | 7.4 (±0.4) × 105 | 0.10 (±0.01) | 0.14±0.01 | 99±9 |
| TGF-β3 WW | TβRII | None | 5.5 (±0.1) × 105 | 0.10 (±0.00) | 0.18±0.02 | 86±2 |
| TGF-β3 WD | TβRII | None | 3.3 (±0.1) × 105 | 0.052 (±0.002) | 0.16±0.01 | 50±1 |
| TGF-β3 DD | TβRII | None | NDa | NDa | NDa | NDa |
| TGF-β3 C77S | TβRII | None | 1.1 (±0.1) × 106 | 0.15 (±0.01) | 0.14±0.01 | 80±3 |
| TGF-β3 WT | TβRI | 2 μM TβRII | 3.5 (±0.1) × 104 | 1.2 (±0.1) × 10−3 | 0.034±0.002 | 59±2 |
| TGF-β3 WW | TβRI | 2 μM TβRII | 3.5 (±0.1) × 104 | 1.1 (±0.1) × 10−3 | 0.031±0.002 | 37±2 |
| TGF-β3 WD | TβRI | 2 μM TβRII | 4.2 (±0.1) × 104 | 1.1 (±0.1) × 10−3 | 0.027±0.001 | 35±1 |
| TGF-β3 DD | TβRI | 2 μM TβRII | NDa | NDa | NDa | NDa |
| TGF-β3 C77S | TβRI | 2 μM TβRII | NDb | NDb | 30±2 | 400±20 |
| aNot determined due to weak binding. | ||||||
| bNot determined due to large systematic deviations in the fits to a simple (1:1) kinetic model. | ||||||
TGF-β3 C77S was reexamined in terms of its ability to bind TβRII-ED and recruit TβRI-ED (Figure 4D). The sensorgrams, together with the fitted parameters, confirmed that TGF-β3 C77S bound TβRII with nearly the same affinity as TGF-β3 WT, WW, and WD (Table I). TGF-β3 C77S, in contrast, was significantly impaired in its ability to bind and recruit TβRI. The Kd in this case could not be obtained by kinetic analysis using a simple (1:1) model due to large systematic deviations in both the association and disassociation phases. This is likely because the TβRI-binding site was partially modified during the biotinylation reaction. To derive the Kd, the data were therefore analysed by fitting the equilibrium response, Req, as a function of receptor concentration to a standard binding isotherm (not shown). This yielded a Kd nearly 100-fold greater than TGF-β3 WT, WW, and WD (Table I), consistent with the reduced affinity previously reported (Groppe et al, 2008). These data show that the TGF-β3 WD dimer, unlike the TGF-β3 C77S monomer, has not altered its affinity for the signalling receptors.
Quantitative analysis of receptor-binding stoichiometries using SPR
To determine the stoichiometry with which TβRI and TβRII bind, the endoglin-like domain of betaglycan, or BGe, was studied. BGe binds all three TGF-β isoforms with high affinity (Mendoza et al, 2009) and its binding site does not overlap with TβRII (Verona et al, 2008). The rationale was that the maximal SPR response attained with BGe should reflect the amount of immobilized binding-competent TGF-β and allow one to infer stoichiometry based on the normalized maximal SPR response for binding of TβRI and TβRII.
The measurements were made using surfaces in which TGF-β3 WW, WD, and DD were immobilized using standard carbodiimide-based amine coupling. The rationale for this was to ensure that all three ligands were uniformly modified, which may not have been so with the biotinylated ligands described earlier since these were prepared in the presence of excess TβRI and TβRII and may have been differentially modified. The sensorgrams obtained upon injection of increasing concentrations of BGe over these surfaces are provided as Supplementary data (Supplementary Figure S2, panels A–C). To derive the dissociation constant, Kd, and maximal response, Rmax, the data were analysed by fitting the equilibrium response, Req, as a function of concentration to a simple binding model. The derived parameters show that the Kds are similar, with all three ligands binding in the low micromolar range (Table II). The same surface was used to assess TβRII binding and TβRI recruitment by injecting increasing concentrations of TβRII-ED alone or TβRI-ED in the presence of a near-saturating concentration of TβRII-ED. The sensorgrams show that TGF-β3 WW and WD exhibit robust concentration-dependent responses, but TGF-β3 DD does not (Supplementary Figure S2, panels D–F and G–I, respectively). The fact that TGF-β3 DD failed to bind TβRII and recruit TβRI, but bound BGe in a manner essentially indistinguishable from TGF-β3 WW and WD, showed that its inability to bind TβRI and TβRII is a consequence of the R25E Y90A R94E substitutions, not conformational changes or misfolding.
Table 2. Binding constants for TGF-β3 and variants to the betaglycan endoglin-like domain.
| Surface | Analyte | Dissassociation constant (μM) |
|||
|---|---|---|---|---|---|
| Buffer suppl. | Kd (μM) | Rmax (RU) | Norm Rmax | ||
| TGF-β3 WW | BGe | None | 3.5±0.4 | 700±30 | 1.00±0.05 |
| TGF-β3 WD | BGe | None | 2.0±0.2 | 1000±100 | 1.00±0.04 |
| TGF-β3 DD | BGe | None | 5.6±0.2 | 1600±100 | 1.00±0.02 |
| TGF-β3 WW | TβRII | None | 1.2±0.1 | 330±10 | 0.47±0.02 |
| TGF-β3 WD | TβRII | None | 0.60±0.10 | 190±10 | 0.19±0.02 |
| TGF-β3 DD | TβRII | None | NDa | NDa | NDa |
| TGF-β3 WW | TβRI | 4 μM TβRII | 0.27±0.05 | 270±50 | 0.39±0.02 |
| TGF-β3 WD | TβRI | 4 μM TβRII | 0.30±0.05 | 150±10 | 0.15±0.01 |
| TGF-β3 DD | TβRI | 4 μM TβRII | NDa | NDa | NDa |
| aNot determined due to weak binding. | |||||
The amplitudes of the responses at the highest concentration of injected receptor over the TGF-β3 WW surface are each lower than BGe, which is expected, even for a 2:1 receptor:ligand stoichiometry, as BGe is 38 kDa in size whereas TβRII-ED is 14 kDa and TβRI-ED is 11 kDa. The responses at the highest receptor concentration over the TGF-β3 WD surface are decreased even further relative to BGe, presumably due to the reduced stoichiometry. To quantify this effect, the equilibrium response as a function of concentration for TβRII binding and TβRI recruitment were normalized by the corresponding maximal response for BGe and then fitted to a standard binding equation as before. The fits for TβRII binding and TβRI recruitment yielded normalized Rmax values of 0.47±0.02 and 0.39±0.02 for TGF-β3 WW and 0.19±0.02 and 0.15±0.01 for TGF-β3 WD, respectively (Figure 5; Table II). The normalized Rmax values for TβRII binding and TβRI recruitment differ by a factor 2.47±0.37 and 2.05±0.21, respectively, providing the first quantitative demonstration of the reduced stoichiometry with which TGF-β3 WD binds TβRII and recruits TβRI.
Figure 5.
SPR equilibrium analysis to determine receptor-binding stoichiometries. (A) Normalized equilibrium response curves for binding of BGe (red) and TβRII (orange) to TGF-β3 WW, or TβRI to TGF-β3 WW in the presence of 4 μM TβRII (blue). The normalized equilibrium responses shown were obtained by dividing the observed equilibrium responses from the raw sensorgrams (Supplementary data; Figure 2, panels A–C) by the fitted maximal response value (Rmax) for BGe. The latter was obtained by fitting the equilibrium response for BGe (the region of the sensorgram near the end of the injection period) as a function of concentration to a standard binding equation. (B, C) Normalized equilibrium response as in panel (A), except for binding of the three receptors to TGF-β3 WD (panel B) or TGF-β3 DD (panel C).
Isolation of ligand–receptor complexes and direct determination of stoichiometries
To directly demonstrate the reduced stoichiometry, an excess of TβRI-ED and TβRII-ED were added to TGF-β3 WW and WD and the complexes were isolated using size exclusion chromotography. The elution profiles, and corresponding SDS gel, show that the TGF-β3 WW complex elutes before the TGF-β3 WD complex and both elute before the uncomplexed receptors (Figure 6A and B). The isolated complexes were analysed using native gel electrophoresis to ascertain that they were fully saturated with TβRI and TβRII. This was accomplished by challenging the isolated complexes with additional TβRII-ED, TβRI-ED, or both TβRII-ED and TβRI-ED (Figure 6C). This resulted in no apparent changes, indicating that the ligands were bound by their full complement of receptors.
Figure 6.
Stoichiometry of TGF-β receptor complexes determined by HPLC analysis. (A) Isolation of complexes of TGF-β3 WW (solid line) and TGF-β3 WD (dashed line) with the TβRI and TβRII extracellular domains using size exclusion chromotography. Small molecules that elute at the total volume are indicated by ‘SM’. (B) Peaks from panel (A) were analysed by SDS–PAGE gel under non-reducing conditions; peaks a and b correspond to the complexes with TβRI and TβRII, whereas a’ and b’ correspond to excess unbound receptors. (C) Native gel analysis of the isolated TGF-β3 WW and WD receptor complexes (left and right panels, respectively) either alone (lane 6) or with 2.0 molar equivalents of added TβRII (lane 7), TβRI (lane 8), or TβRI and TβRII (lane 9). (D) HPLC cation-exchange analysis of the isolated TGF-β3 WW and WD receptor complexes (solid and dashed lines, respectively) under denaturing conditions (8 M urea, pH 4.0). Identity of the eluted peaks is indicated.
To analyse the stoichiometry, the isolated complexes were separated using high-resolution ion-exchange chromotography in the presence of 8 M urea. The UV absorption profiles, recorded at 280 nm, included three components as anticipated (Figure 6D). The split TβRII peak is a consequence of deamidation of Asn19 and has no effect on TβRII's ability to bind TGF-β (Hinck et al, 2000). The splitting of the TGF-β3 WD peak is unexpected, but is not due to contamination of TGF-β3 WD with either TGF-β3 WW or TGF-β3 DD as reanalysis of the TGF-β3 WD peak from Figure 6D in the absence of urea yields a single peak well resolved from either TGF-β3 WW or DD (Supplementary Figure S3, panel A). The splitting may instead arise from alternate slowly converting conformations under the conditions used to dissociate the complex, as reanalysis of material from the leading edge of the split peak in the presence of 8 M urea yields an identical split peak (Supplementary Figure S3, panel B).
To quantitate stoichiometries, the areas under the peaks were measured and compared with those for 2:2:1 and 1:1:1 TβRI:TβRII:TGF-β3 dimer complex calculated from the corresponding molar extinction coefficients at 280 nm. The results show that the relative integrated HPLC peak areas uncorrected for differences in extinction coefficients for the TβRI:TβRII:TGF-β3 WW complex, 0.099:0.45:1.00, closely match those expected for a 2:2:1 TβRI:TβRII:TGF-β complex, 0.085:0.41:1.00, whereas those for TGF-β3 WD complex, 0.043:0.16:1.00, match those expected for a 1:1:1 TβRI:TβRII:TGF-β complex, 0.043:0.20:1.00 (Table III). These results unambiguously demonstrate that the TGF-β3 WD heterodimer binds TβRII-ED and recruits TβRI-ED with an affinity indistinguishable from the TGF-β3 WT homodimer, but with one-half the stochiometry.
Table 3. Relative HPLC peak areas at 280 nm for TβRI:TβRII:TGF-β dimer complexes.
| Complex | Relative integrated HPLC peak areasa |
||
|---|---|---|---|
| TβRI | TβRII | TGF-β dimer | |
| Theoretical, 2:2:1 | 0.085 | 0.41 | 1.00 |
| Theoretical, 1:1:1 | 0.043 | 0.20 | 1.00 |
| HPLC, TGF-β3 WW | 0.099 | 0.45 | 1.00 |
| HPLC, TGF-β3 WD | 0.043 | 0.16 | 1.00 |
| aIntegrated HPLC peak areas are uncorrected for differences in molar extinction coefficients. | |||
Biological activities of the heterodimer
The biological activity of the TGF-β3 WD heterodimer was compared with TGF-β3 WT and TGF-β3 WW to determine the effect of its altered stoichiometry on signalling. The first assay involved measuring the induction of phospho-Smad3, a direct downstream target of TβRI. MCF10A human breast epithelial cells were treated with 40 and 80 pM TGF-βs for 30 min and the cell lysates were analysed by western blotting with a phospho-Smad3 antibody (Figure 7A, upper panel). The results show that the activity of TGF-β3 WD was partially compromised compared with TGF-β3 WT and TGF-β3 WW, whereas the other two variants tested, TGF-β3 DD and TGF-β3 C77S, were fully compromised. Time-dependent Smad3 phosphorylation assays were performed to compare the rate and amplitude of signalling (Figure 7B). The appearance and disappearance of phospho-Smad3 upon stimulation with TGF-β3 WD, WW, and WT had similar overall kinetics, but the amplitude at all time points for TGF-β3 WD was reduced by roughly a factor or four compared with TGF-β3 WW and TGF-β3 WT (Figure 7B, lower panel).
Figure 7.
Cell-based assays of TGF-β function. (A) Induction of Smad3 phosphorylation in human breast epithelial cells as a function of increasing concentrations of added TGF-βs. Control for equal loading (actin) is aligned below. (B) Induction of Smad3 phosphorylation as in panel (A), but as a function of treatment time as shown. Relative amounts of pSmad3 are plotted below. Values shown are normalized relative to actin and are expressed as a percentage of the maximum detected with TGF-β3 WT at 60 min. (C) Luciferase-reporter gene activity in mink lung epithelial cells transiently transfected with a fixed amount of CAGA12-Luc and β-galactosidase reporters as a function of increasing concentrations of added TGF-βs. Luciferase are expressed as a percentage of the maximum value attained by the wild-type protein. Lines shown correspond to the best fit to an equation describing the EC50. (D) Growth inhibitory activity examined by measuring the incorporation of 5-[125I]-iodo-2′-deoxyuridine into total DNA in cultured mink lung epithelial cells as a function of increasing concentrations of added TGF-βs. Values shown are expressed as percentage relative to untreated cells. Lines shown correspond to the best fit to an equation describing the IC50.
The ligands were further tested in a luciferase-reporter gene assay by transfecting cultured mink lung epithelial (Mv1Lu) cells with a CAGA12 luciferase reporter and by treating them with the TGF-βs over a range of concentrations for 18 h. The dose–response curves, presented in Figure 7C, show that TGF-β3 WD is essentially indistinguishable from TGF-β3 WW and TGF-β3 WT, with EC50 values in the range of 10–16 pM (Table IV). TGF-β3 C77S is ∼11-fold less potent, with an EC50 of ∼140 pM, and TGF-β3 DD is significantly less potent, with no detectable activity at 200 pM, the highest concentration tested (Table IV).
Table 4. Biological activity of TGF-β ligands.
| Ligand | Reporter gene assay EC50 (pM) | Growth inhibition assay IC50 (pM) |
|---|---|---|
| TGF-β3 WT | 16±2 | 0.4±0.1 |
| TGF-β3 WW | 13±1 | 0.6±0.1 |
| TGF-β3 WD | 10±1 | 0.8±0.2 |
| TGF-β3 DD | NDa | 1300±200 |
| TGF-β3 C77S | 140±10 | 17±2 |
| aNot determined due to the absence of a detectable response. | ||
The ligands were also characterized in terms of their ability to growth inhibit mink lung epithelial cells by treating them with the TGF-βs over a range of concentrations for 24 h. The dose–response curves, presented in Figure 7D, show that TGF-β3 WD exhibits comparable potency to TGF-β3 WW and TGF-β3 WT, especially at lower concentrations, though at higher concentrations, TGF-β3 WD appeared approximately two-fold less potent, with an IC50 close to 0.8±0.2 pM versus 0.4±0.1 and 0.6±0.1 pM for TGF-β3 WT and WW, respectively (Table IV). TGF-β3 C77S, in contrast, weakly inhibited growth, with an IC50 close to 17±2 pM, and TGF-β3 DD exhibited marginal inhibition, with an IC50 of ∼1300 pM (Table IV).
These results show that TGF-β3 WD heterodimer possesses one-quarter to one-half the biological activity of the wild-type homodimer, TGF-β3 C77S is significantly compromised, and TGF-β3 DD is severely compromised. These results, together with the studies presented above, show that the diminishment of receptor binding, either partially as in TGF-β3 C77S or fully as in TGF-β3 DD, attenuates biological activity, but eliminating the binding of one of the two TβRI:TβRII pairs reduces the activity by no more than a factor of four.
Receptor complex assembly on the cell surface
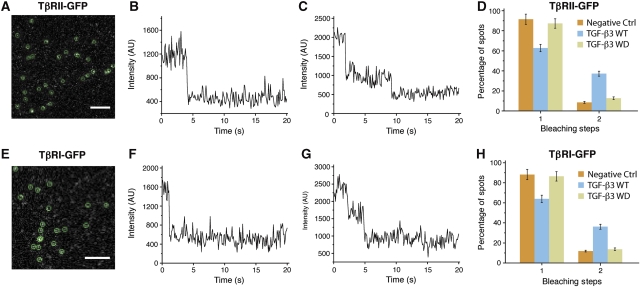
TGF-β3 WD may not bind the cell surface receptors in the same overall manner as the purified receptor extracellular domains due to interactions between the transmembrane or cytoplasmic domains that promote assembly of a TβRI:TβRII heterotetramer. To investigate this possibility, single-molecule TIRF-based fluorescence imaging was used. This method measures the proportion of receptors that are monomeric or dimeric based on an analysis of the bleaching statistics, that is the fraction of molecules that photobleach in a single step versus those that bleach in two (Iino et al, 2001). This technique revealed that TGF-β treatment leads to a significant increase in the proportion of dimeric receptors on the cell surface, with both TβRI and TβRII being about 90% monomeric and 10% dimeric in the absence of TGF-β and about 65% monomeric and 35% dimeric in its presence (Zhang et al, 2009, 2010).
The same procedure was employed to determine whether TGF-β3 WD led to any significant dimerization of TβRI or TβRII on the cell surface. This involved transiently transfecting cultured HeLa cells with either C-terminally GFP-tagged TβRI or TβRII, expressing these for a limited time to ensure expression at endogenous levels, treatment with TGF-β3 WT or WD, and analysis of the fixed cells using single-molecule TIRF-based imaging. Typical TIRF images and bleaching patterns for cells transfected with TβRII-GFP and TβRI-GFP are shown in Figure 8 (panels A–C and E–G, respectively). These, as well as the corresponding bleaching statistics, are similar to those reported earlier, with TGF-β3 treatment increasing the proportion of TβRI and TβRII dimers from 11.8±1.3 to 36.1±2.6% and 8.5±0.9 to 37.2±2.5%, respectively (Figure 8D and H). This readily measurable increase in dimers was not however apparent upon treatment with TGF-β3 WD, with the proportion of TβRI and TβRII dimers essentially within the error limits of the control, 13.6±1.2 and 12.7±1.3%, respectively. These results show that the TGF-β3 WD heterodimer is incapable of assembling a TβRI:TβRII heterotetramer on the cell surface.
Figure 8.
Analysis of bleaching steps of single TβRII and TβRI spots imaged with fixed HeLa cells. (A) A typical image with diffraction-limited fluorescent spots of TβRII-GFP on the cell membrane of transfected HeLa cells. The spots enclosed with the green circles (3 × 3 pixels) were chosen for single-molecule bleaching analysis. Scale bar: 4 μm. (B, C) Two representative time courses of TβRII-GFP emission after background correction show one-step (B) and two-step bleaching (C). (D) Frequency of one- and two-step bleaching events for TβRII-GFP in the absence (yellow) or presence of TGF-β3 WT (blue) or TGF-β3 WD (grey) with fixed HeLa cells. (E–H) As in panels (A–D) but for TβRI-GFP. The data presented were obtained from three independent experiments.
Discussion
The objective of this study was to thoroughly investigate if TGF-βs signal through two independently functioning TβRI:TβRII heterodimers. This was accomplished by investigating a heterodimeric form of TGF-β3 bearing substitutions in one of its protomers to block TβRII binding and TβRI recruitment. The heterodimer was shown using a series of complementary biochemical techniques to bind the TβRII extracellular domain and recruit the TβRI with affinities indistinguishable from the wild-type homodimer but with one-half the stoichiometry. TGF-β3 C77S bound TβRII-ED indistinguishably from the wild-type homodimer, but was impaired nearly 100-fold in its ability to bind and recruit TβRI-ED. TGF-β3 DD, though native, as shown by its ability to bind the betaglycan endoglin-like domain, was reduced at least 200-fold in its ability to bind TβRII-ED and recruit TβRI-ED.
The functional data showed that TGF-β3 WD, which bound the receptor extracellular domains with affinities indistinguishable from wild-type homodimer, but with one-half the stoichiometry, had four-fold lower activity compared with TGF-β3 in the Smad phosphorylation assay, a two-fold lower IC50 in the growth inhibition assay, and an indistinguishable EC50 in the reporter gene assay. TGF-β3 C77S, which was significantly impaired in its ability to bind and recruit TβRI-ED, had a nine-fold higher EC50 in the reporter gene assay and a 43-fold higher IC50 in the growth inhibition assay. TGF-β3 DD, which did not detectably bind TβRII-ED or recruit TβRI-ED, had no detectable activity in the reporter gene assay and an IC50 three to four orders of magnitude higher than TGF-β3 in the growth inhibition assay.
The fact that TGF-β3 WD exhibits a small, but detectable decrease in activity compared with wild-type dimer in the Smad phosphorylation assay and growth inhibition assay, but not the reporter gene assay is likely due to lower intrinsic sensitivity of this assay compared with the others. This is illustrated by the data of Amatayakul-Chantler et al (1994) who showed that monomeric TGF-β1 (TGF-β1 C77S) was reduced in its potency eight-fold compared with dimeric (WT) TGF-β1 in a reporter gene assay, but >100-fold in a growth inhibition assay. Thus, it is not surprising that TGF-β3 WD, which is reduced in its growth inhibitory activity by no more than two-fold, exhibits no detectable difference in its reporter gene activity.
The four-fold reduction in Smad phosphorylation activity for the TGF-β3 WD heterodimer shows that the two TβRI:TβRII pairs bind TGF-β and function in a nearly autonomous manner. The diminishment in activity of the heterodimer compared with the wild-type homodimer by an additional factor of two beyond that anticipated for independent binding and signalling may be a consequence of increased apparent affinity of the wild-type homodimer for the cell surface receptors. This could occur by membrane-localization effects, where the apparent affinity of the wild-type homodimer for cell surface TβRII is increased after it binds TβRII through one of its two sites and becomes localized on the membrane surface. The other possible explanation is that the two TβRI:TβRII pairs functionally interact, but this seems unlikely given the limited magnitude of the effect.
The conclusion that the two TβRI:TβRII pairs bind and function in a near-autonomous manner presumes that TGF-β3 WD binds the cell surface receptors in the same manner as the purified receptor extracellular domains. This seems likely because the TIRF-based single-molecule fluorescence data obtained with C-terminally GFP-tagged TβRI and TβRII showed that treatment with TGF-β3 WD leads to a negligible increase in the proportion of dimeric TβRI and TβRII on the cell surface, whereas treatment with TGF-β3 leads to more than a three-fold increase (Figure 8).
The current results indicate that receptor transactivation occurs exclusively within TβRI:TβRII heterodimers, not between. This is probably determined by the arrangement of the receptors as the last structurally ordered residue on the C-terminus of TβRI and TβRII are separated by just 46 Å within a TβRI:TβRII heterodimer, but 80 Å between heterodimers (Figure 9A). The current results stand in contrast to results obtained in earlier studies in which the receptors were artificially dimerized. Two such studies employed TβRI and TβRII variants bearing dimerization domains, one employing the extracellular domain of the erythropoietin receptor (Luo and Lodish, 1996) and the other small immunophilin domains inserted between the kinase and transmembrane domains (Stockwell and Schreiber, 1998). The erythropoietin-dimerized receptors led to TβRII-TβRI transactivation, but not activation of downstream signalling, while the immunophilin-dimerized receptors led to the activation of downstream signalling, though at a significantly reduced level compared with wild-type homodimer. A third study employed TGF-β as the dimerizer, but utilized a chimeric receptor comprising the TβRI kinase domain and the TβRII extracellular domain (Okadome et al, 1994). This construct, designated TβRII-I, yielded no detectable signalling when transfected into a TβRI-deficient cell line.
Figure 9.
Positioning of the TGF-β3 and BMP receptor complexes on a membrane surface. (A) Surface representation of the TGF-β3 homodimer bound to the TβRI and TβRII extracellular domains (PDB 2PJY). The two protomers of TGF-β3 are depicted in magenta and blue, TβRII in green, and TβRI in yellow. C-terminal residues of the receptor extracellular domains are shaded grey. Distances between the C-terminal residues of the receptor extracellular domains are shown on the projection onto the cell surface. Circled numbers correspond to the number of amino acids between the last structurally ordered residue in receptor extracellular domain and the first predicted residue of the transmembrane domain. (B) Surface representation of the BMP-2 homodimer bound to the BMPRIa and ActRII extracellular domains (PDB 2GOO) shown in a similar manner to the TGF-β complex in panel (A). The two protomers of BMP-2 are depicted in orange and brown, ActRII in magenta, and BMPRIa in cyan.
The discrepancy between these results and the previous emphasizes that the precise positioning of the receptors is important, with wild-type-like signalling when TβRI and TβRII are arranged natively, but diminished or no detectable signalling when arranged non-natively. The spacing between the last structurally ordered residue on the C-terminus of the two bound TβRIIs in the TGF-β receptor complex is 104 Å (Figure 9A) and that between those of the two bound Epo receptors in the Epo receptor complex is 30 Å (Syed et al, 1998). The l04-Å spacing, which would be expected for signalling with the combination of TβRII and TβRII-I, is likely too large compared with the 46-Å spacing when TβRI and TβRII are arranged within a TβRI:TβRII heterodimer. The 30-Å spacing, which would be expected for the chimeric Epo-TβRI and Epo-TβRII receptors, enables efficient transactivation, but perhaps brings the TβRI and TβRII kinases too closely together so that Smad phosphorylation is inhibited. These observations suggest that the separation between the kinases is a critical factor for efficient signalling, though it should be emphasized that this conclusion is tentative given that orientation effects might also be important and that both TβRI and TβRII include 10 to 14 structurally disordered residues bridging the last structurally ordered residue of their extracellular domain and the first predicted residue of the transmembrane domain (Figure 9A). Thus, further studies are required to ascertain whether receptor activity is determined by distance alone, or whether orientation or other effects might also have a role.
The isolated TβRI and TβRII kinase domains have previously been shown to weakly interact using yeast two hybrid and other methods (Ventura et al, 1996). Thus, it seems likely that the close proximity between the TβRI and TβRII extracellular domains, as in TGF-β3 or TGF-β3 WD complexes, promotes this otherwise intrinsically weak interaction, and positions the kinases for optimal transactivation and signalling. The current results therefore emphasize that binding of TβRI and TβRII adjacent to one another and with direct contact in the TGF-β receptor complex has roles beyond TβRI recruitment and enhancing specificity (Groppe et al, 2008), but also promoting transphosphorylation that leads to downstream signalling.
The finding that TGF-βs signal through TβRI:TβRII heterodimers is of interest in light of recent single-molecule fluorescence imaging studies that show that both TβRI and TβRII are predominantly monomeric in the absence of ligand (Zhang et al, 2009, 2010). The discrepancy between these findings and the previous findings that showed that the receptors are dimeric (Chen and Derynck, 1994; Gilboa et al, 1998; Rechtman et al, 2009) is likely a consequence of differences in expression levels, as the single-molecule studies showed that TβRI and TβRII were predominantly monomeric when expressed at endogenous levels, but dimeric when overexpressed. The findings from the single-molecule study, together with the current findings, therefore show that the TGF-βs have adapted to bind and assemble TβRI:TβRII heterodimers, not TβRI:TβRII heterotetramers.
The data presented, even though they show that the two TβRI:TβRII pairs bind and function in an autonomous manner, does not imply that one of the two TβRI:TβRII pairs is dispensible. Thus, as explained above, one important function of the two pairs may be to increase potency by enhancing the apparent affinity for binding TGF-β via membrane-localization effects. The four-fold increase in pSmad levels with the wild-type homodimer versus the heterodimer might be vital in vivo where the local concentration of active dimer can lead to very different biological outcomes (McKarns et al, 2003).
The fact that TGF-βs activate the Smad pathway through two near-autonomously functioning TβRI:TβRII pairs stands in contrast to the BMPs, which are unable to activate the Smad pathway when one of the type II receptor-binding sites is blocked (Knaus and Sebald, 2001; Isaacs et al, 2010). This suggests that BMPs have a minimal requirement for a type I:type II:type II heterotrimer. The type I and type II receptor extracellular domains do not contact one another in the BMP receptor complex (Figure 9B) and thus the requirement for a heterotrimer in the BMP system must be a consequence of direct or indirect interactions between the transmembrane or kinase domains of the receptors. One possible role for the type I:type II:type II heterotrimer is to promote efficient receptor transactivation and signalling. Another is to enhance potency via multivalent binding, perhaps overcoming the low intrinsic affinity that many BMPs have for their type II receptors (Nickel et al, 2009).
The requirement for a heterotrimer in the BMP system, but not the TGF-β, may be related to differences in the manner by which these two subfamilies of ligands bind their receptors. The TGF-βs bind the type I and type II receptors as two well-separated heterodimeric pairs, whereas the BMPs bind their type I and type II receptors without any direct contact, but in much closer spatial proximity to one another (Figure 9). Thus, in analogy to the TGF-βs, where direct contact between the extracellular domains promotes recruitment of the low affinity receptor and signalling, so too may the close proximity between the transmembrane and/or kinase domains of the receptors in the BMP system promote functions critical for ligand binding and signalling. Though speculative, it might be this function is related to enhancement of ligand binding by dimerization of the type II receptor, rather than signalling, as the type II receptors are somewhat closer together in the BMP system (84 Å) compared with the TGF-β (104 Å) and the two type I—type II receptor distances are not that different from that in the TGF-β system, with one ‘short’ distance compatible for transactivation, 35 Å, and one ‘long’ distance that is not, 72 Å.
Materials and methods
Protein preparation
Human TGF-β3 was expressed, refolded, and purified as previously described (Cerletti, 2000) with modifications (Supplementary data). TGF-β3 WW, WD, and DD were prepared by expressing and purifying the wild-type (W) and R25E, Y90A, R94E (D) monomers separately and by combining them in a 1:1 ratio for refolding. The three dimers that formed, WW, WD, and DD, were separated from one another, as well as non-dimerized W and D monomers, using cation-exchange chromatography as described (Supplementary data). Other proteins were produced as described (Supplementary data).
Native gel assay
Native gel assays to assess the binding and stoichiometry of TGF-β3 WT, WW, WD, and DD to the TβRI and TβRII extracellular domains, TβRI-ED and TβRII-ED, respectively, were performed as previously described (Zúñiga et al, 2005)
SPR-binding assays
Binding studies were performed with BIAcore 3000 instrument (GE Healthcare) and were analysed using the software package Scrubber2 (Biologic Software). For kinetic experiments, TGF-βs were biotinylated and captured on streptavidin chip surfaces (GE Healthcare). TGF-β3 WT, WW, WD, and C77S were biotinylated in the presence of excess TβRI and TβRII, whereas TGF-β3 DD was biotinylated in the presence of excess soluble betaglycan, using sulfo-NHS-LC-LC-Biotin (Pierce) at pH 7.5. Singly biotinylated TGF-βs were separated from receptors and doubly and multiply biotinylated forms by Source S cation-exchange column (GE Healthcare) in the presence of 30% isopropanol at pH 4.0. Surface densities of captured TGF-βs were kept at 50–300 RU to minimize rebinding artifacts. For equilibrium experiments, TGF-β3 DD, WD, and WW were covalently attached to flow cells using an amine coupling kit (GE Healthcare). Binding assays were performed by injecting two-fold serial dilutions of the receptors in triplicate in HBS-EP buffer (GE Healthcare) at a flow rate of either 10 μl per min (equilibrium experiments) or 50 μl per min (kinetic experiments). Surfaces were regenerated by a brief injection of 4 M guanidine hydrochloride (10 s contact time at a flow rate of 100 μl per min). Instrument noise was removed by double referencing. Equilibrium analyses were performed by averaging the equilibrium binding response near the end of the injection. Kinetic analyses were performed by global fitting with a simple 1:1 model. Standard errors were obtained from the variation among the derived parameters.
Stoichiometry by HPLC
TGF-β3 WW and WD ternary complex with TβRI and TβRII were formed and purified as before (Groppe et al, 2008). Compositional analysis of the isolated complexes was determined by separating the components using high-resolution ion-exchange chromatography (Mono S HR 5/5 column, GE Healthcare) in the presence of 8 M urea and 50 mM sodium formate at pH 4.0 (0–400 mM NaCl in 50 mM sodium formate over 80 column volumes). Chromatograms were recorded at 280 nm and peaks areas of individual components were determined using the program Peakfit (Systat Software Inc., San Jose, CA).
Smad3 phosphorylation assays
MCF10A human breast epithelial cells were treated with 40 and 80 pM TGF-β ligands for 30 min. Western blot was carried out as previously described (Zúñiga et al, 2005) using a rabbit polyclonal anti-phospho-Smad3 antibody (Upstate Biotechnology) or an anti-actin antibody (Ambion). Time-dependent Smad3 phosphorylation was performed in the same manner, but with different treatment times.
Growth inhibition assay
The effect of TGF-β isoforms on the proliferation of cultured mink lung epithelial cells was tested as described (Cheifetz et al, 1990; Zúñiga et al, 2005).
Luciferase-reporter gene assays
The induction of a TGF-β CAGA12 luciferase-reporter construct was performed as previously described (Dennler et al, 1998) with modifications (Supplementary data).
Single-molecule fluorescence
HeLa cells were cultured and transfected with TβRII-GFP or TβRI-GFP plasmid and expressed at endogenous levels or lower as previously described (Zhang et al, 2009, 2010). For the ligand stimulation experiments, the transfected cells that were ready for fluorescence imaging, were added with 200 pM TGF-β3 (WT) or TGF-β3 (WD) in DMEM for 15 min at 4°C. Single-molecule fluorescence imaging was performed and analysed as previously reported (Zhang et al, 2010). The experiments were performed three times and 200–300 spots were selected and analysed every time.
Supplementary Material
Acknowledgments
We acknowledge Christian Zwieb and Thomas Thompson for critically reading the manuscript. This project was supported by the NIH (GM58670 and RR13879 to AH; CA75253 to LS; CA54174 to the Macromolecular Interactions and Mass Spectrometry Shared Resources of the UTHSCSA CTRC), the Robert A Welch Foundation (AQ1431 to APH), the Consejo National de Ciencia y Tecnología (49828 to FL-C), and the Chinese 973 project (2007CB935601 to XF).
Footnotes
The authors declare that they have no conflict of interest.
References
- Amatayakul-Chantler S, Qian SW, Gakenheimer K, Bottinger EP, Roberts AB, Sporn MB (1994) [Ser77]transforming growth factor-beta 1. Selective biological activity and receptor binding in mink lung epithelial cells. J Biol Chem 269: 27687–27691 [PubMed] [Google Scholar]
- Baardsnes J, Hinck CS, Hinck AP, O’Connor-McCourt MD (2009) TbetaR-II discriminates the high- and low-affinity TGF-beta isoforms via two hydrogen-bonded ion pairs. Biochemistry 48: 2146–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF (2000) Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350–1358 [DOI] [PubMed] [Google Scholar]
- Cerletti N (2000) Process for the production of biologically active dimeric protein, US Patent 6057430
- Cheifetz S, Hernandez H, Laiho M, ten Dijke P, Iwata KK, Massagué J (1990) Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem 265: 20533–20538 [PubMed] [Google Scholar]
- Chen RH, Derynck R (1994) Homomeric interactions between type II transforming growth factor-beta receptors. J Biol Chem 269: 22868–22874 [PubMed] [Google Scholar]
- De Crescenzo G, Hinck CS, Shu Z, Zuniga J, Yang J, Tang Y, Baardsnes J, Mendoza V, Sun L, Lopez-Casillas F, O’Connor-McCourt M, Hinck AP (2006) Three key residues underlie the differential affinity of the TGFbeta isoforms for the TGFbeta type II receptor. J Mol Biol 355: 47–62 [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM (1998) Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17: 3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R (1994) TGF-beta-receptor-mediated signaling. Trends Biochem Sci 19: 548–553 [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29: 117–129 [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM (1998) The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 12: 1809–1817 [DOI] [PubMed] [Google Scholar]
- Gilboa L, Wells RG, Lodish HF, Henis YI (1998) Oligomeric structure of type I and type II transforming growth factor beta receptors: homodimers form in the ER and persist at the plasma membrane. J Cell Biol 140: 767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP (2008) Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell 29: 157–168 [DOI] [PubMed] [Google Scholar]
- Hart PJ, Deep S, Taylor AB, Shu Z, Hinck CS, Hinck AP (2002) Crystal structure of the human TbetaR2 ectodomain--TGF-beta3 complex. Nat Struct Biol 9: 203–208 [DOI] [PubMed] [Google Scholar]
- Henis YI, Moustakas A, Lin HY, Lodish HF (1994) The types II and III transforming growth factor-beta receptors form homo-oligomers. J Cell Biol 126: 139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck AP, Walker KP III, Martin NR, Deep S, Hinck CS, Freedberg DI (2000) Sequential resonance assignments of the extracellular ligand binding domain of the human TGF-beta type II receptor. J Biomol NMR 18: 369–370 [DOI] [PubMed] [Google Scholar]
- Iino R, Koyama I, Kusumi A (2001) Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys J 80: 2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangovan U, Deep S, Hinck CS, Hinck AP (2004) Sequential resonance assignments of the extracellular domain of the human TGFbeta type II receptor in complex with monomeric TGFbeta3. J Biomol NMR 29: 103–104 [DOI] [PubMed] [Google Scholar]
- Isaacs MJ, Kawakami Y, Allendorph GP, Yoon BH, Belmonte JC, Choe S (2010) Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol 24: 1469–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM (1994) The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8: 133–146 [DOI] [PubMed] [Google Scholar]
- Knaus P, Sebald W (2001) Cooperativity of binding epitopes and receptor chains in the BMP/TGFbeta superfamily. Biol Chem 382: 1189–1195 [DOI] [PubMed] [Google Scholar]
- Laiho M, Weis FM, Boyd FT, Ignotz RA, Massagué J (1991) Responsiveness to transforming growth factor-beta (TGF-beta) restored by genetic complementation between cells defective in TGF-beta receptors I and II. J Biol Chem 266: 9108–9112 [PubMed] [Google Scholar]
- Luo K, Lodish HF (1996) Signaling by chimeric erythropoietin-TGF-beta receptors: homodimerization of the cytoplasmic domain of the type I TGF-beta receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J 15: 4485–4496 [PMC free article] [PubMed] [Google Scholar]
- Massagué J (1998) TGF-beta signal transduction. Annu Rev Biochem 67: 753–791 [DOI] [PubMed] [Google Scholar]
- Massagué J (2008) A very private TGF-beta receptor embrace. Mol Cell 29: 149–150 [DOI] [PubMed] [Google Scholar]
- Massagué J, Wotton D (2000) Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19: 1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKarns SC, Letterio JJ, Kaminski NE (2003) Concentration-dependent bifunctional effect of TGF-beta 1 on immunoglobulin production: a role for Smad3 in IgA production in vitro. Int Immunopharmacol 3: 1761–1774 [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ (1993) GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem 268: 3444–3449 [PubMed] [Google Scholar]
- Mendoza V, Vilchis-Landeros MM, Mendoza-Hernandez G, Huang T, Villarreal MM, Hinck AP, Lopez-Casillas F, Montiel JL (2009) Betaglycan has two independent domains required for high affinity TGF-beta binding: proteolytic cleavage separates the domains and inactivates the neutralizing activity of the soluble receptor. Biochemistry 48: 11755–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Lin HY, Henis YI, Plamondon J, O’Connor-McCourt MD, Lodish HF (1993) The transforming growth factor beta receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem 268: 22215–22218 [PubMed] [Google Scholar]
- Nickel J, Sebald W, Groppe JC, Mueller TD (2009) Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev 20: 367–377 [DOI] [PubMed] [Google Scholar]
- Okadome T, Yamashita H, Franzen P, Moren A, Heldin CH, Miyazono K (1994) Distinct roles of the intracellular domains of transforming growth factor-beta type I and type II receptors in signal transduction. J Biol Chem 269: 30753–30756 [PubMed] [Google Scholar]
- Radaev S, Zou Z, Huang T, Lafer EM, Hinck AP, Sun PD (2010) Ternary complex of transforming growth factor-beta1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J Biol Chem 285: 14806–14814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtman MM, Nakaryakov A, Shapira KE, Ehrlich M, Henis YI (2009) Different domains regulate homomeric and heteromeric complex formation among type I and type II transforming growth factor-beta receptors. J Biol Chem 284: 7843–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massagué J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700 [DOI] [PubMed] [Google Scholar]
- Stockwell BR, Schreiber SL (1998) Probing the role of homomeric and heteromeric receptor interactions in TGF-beta signaling using small molecule dimerizers. Curr Biol 8: 761–770 [DOI] [PubMed] [Google Scholar]
- Sun PD, Davies DR (1995) The cystine-knot growth-factor superfamily. Annu Rev Biophys Biomol Struct 24: 269–291 [DOI] [PubMed] [Google Scholar]
- Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM (1998) Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 395: 511–516 [DOI] [PubMed] [Google Scholar]
- Ventura F, Liu F, Doody J, Massagué J (1996) Interaction of transforming growth factor-beta receptor I with farnesyl-protein transferase-alpha in yeast and mammalian cells. J Biol Chem 271: 13931–13934 [DOI] [PubMed] [Google Scholar]
- Verona EV, Tang Y, Millstead TK, Hinck AP, Agyin JK, Sun LZ (2008) Expression, purification and characterization of BG(E)RII: a novel pan-TGFbeta inhibitor. Protein Eng Des Sel 21: 463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis-Garcia F, Massagué J (1996) Complementation between kinase-defective and activation-defective TGF-beta receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J 15: 276–289 [PMC free article] [PubMed] [Google Scholar]
- Wells RG, Gilboa L, Sun Y, Liu X, Henis YI, Lodish HF (1999) Transforming growth factor-beta induces formation of a dithiothreitol-resistant type I/Type II receptor complex in live cells. J Biol Chem 274: 5716–5722 [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J (1994) Mechanism of activation of the TGF-beta receptor. Nature 370: 341–347 [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH (1994) Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-beta. J Biol Chem 269: 20172–20178 [PubMed] [Google Scholar]
- Zhang W, Jiang Y, Wang Q, Ma X, Xiao Z, Zuo W, Fang X, Chen YG (2009) Single-molecule imaging reveals transforming growth factor-beta-induced type II receptor dimerization. Proc Natl Acad Sci USA 106: 15679–15683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yuan J, Yang Y, Xu L, Wang Q, Zuo W, Fang X, Chen YG (2010) Monomeric type I and type III transforming growth factor-beta receptors and their dimerization revealed by single-molecule imaging. Cell Res 20: 1216–1223 [DOI] [PubMed] [Google Scholar]
- Zhang YE (2009) Non-Smad pathways in TGF-beta signaling. Cell Res 19: 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúñiga JE, Groppe JC, Cui Y, Hinck CS, Contreras-Shannon V, Pakhomova ON, Yang J, Tang Y, Mendoza V, López-Casillas F, Sun L, Hinck AP (2005) Assembly of TbetaRI:TbetaRII:TGFbeta ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J Mol Biol 354: 1052–1068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.