Abstract
Obesity is a global epidemic with more than 1 billion overweight adults and at least 300 million obese patients worldwide. Diabetes is characterized by a defect in insulin secretion or a decrease in sensitivity to insulin, which results in elevated fasting blood glucose. Both obesity and elevated fasting glucose are risk factors for nonalcoholic fatty liver disease, a disease spectrum that includes hepatic steatosis (nonalcoholic fatty liver), nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Increased adiposity and insulin resistance contribute to the progression from NASH to fibrosis through the development of a profibrotic mileau in the liver, including increased hepatocellular death, increased reactive oxygen species generation, and an altered adipokine/cytokine balance. This review will summarize recent advances in our understanding of the pathological interactions among excessive fat accumulation, insulin resistance, and hepatic fibrogenesis and discuss specific molecular pathways that may be of interest in the development of therapeutic interventions to prevent and/or reverse hepatic fibrosis.
Keywords: nonalcoholic steatohepatitis, nonalcoholic fatty liver disease
“overweight” and “obesity” are defined as abnormal or excessive fat accumulation that presents a risk to health. A crude population measure of obesity is the body mass index (BMI), with a BMI of 30 or more considered obese and BMI equal to or more than 25 considered overweight. Obesity has become a global epidemic, with more than 1 billion overweight adults and at least 300 million obese patients worldwide (47a). Diabetes is characterized by a defect in insulin secretion or a decrease in sensitivity to insulin, which results in elevated fasting blood glucose. Both obesity and elevated fasting glucose are risk factors for nonalcoholic fatty liver disease (NAFLD), a disease spectrum that includes hepatic steatosis (nonalcoholic fatty liver, NAFL), nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Prospective studies have suggested the link between obesity and the development of type 2 diabetes and liver fibrosis (47). This review will summarize recent advances in our understanding of the pathological interactions among excessive fat accumulation, insulin resistance, and hepatic fibrogenesis and identify certain molecules or pathways that could be exploited for therapeutic interventions.
Mechanisms of Interaction
Obesity and NAFL.
Excess calorie intake over energy expenditure results in energy storage in the form of body fat. Weight gain of 10% by overfeeding of fast food and sedentary lifestyle in 18 young healthy subjects has been shown to increase liver fat by 2.5-fold in 4 wk (19). Fat is stored as triglycerides in the liver. Evidence from stable isotope studies indicates that serum free fatty acid from lipolysis of visceral adipose tissue is the main source of hepatic triglycerides in NAFLD. In addition, de novo hepatic lipogenesis is also significantly increased in NAFLD subjects (10). The hepatic gene expression of sterol regulatory element-binding protein 1c, a key transcriptional activator of lipogenic genes, as well as of acetyl-CoA carboxylases and fatty acid synthase is increased in subjects with NAFLD (14).
NAFL and insulin resistance.
The underlying mechanisms that contribute to obesity-induced insulin resistance are not entirely understood. Recent studies suggest that obesity may lead to hepatic insulin resistance through the activation of the proinflammatory M1 macrophages in adipose tissues and the release of proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α (40). Proinflammatory cytokines may decrease the cellular response to insulin and result in insulin resistance. IL-6 blocks the insulin signaling pathway at least in part through the induction of suppressor of cytokine signaling-3 (29). In addition, inflammatory stress may upregulate the production of IL-6 in adipocytes though the activation of the c-Jun NH2-terminal kinase (JNK1) (38). Several observational studies in humans have also shown that hepatic lipid accumulation (steatosis) is involved in the pathogenesis of insulin resistance (44).
NAFL and NASH.
NAFL progresses to NASH in as many as 40% of patients over time (25, 48). Multiple, interconnected pathways contribute to the progression from steatosis to steatohepatitis. An excess supply of nonesterified free fatty acids (NEFA) to the liver increases mitochondrial and peroxisomal β-oxidation and promotes microsomal induction of CYP4A1 and CYP2E1; this leads to increased reactive oxygen species (ROS) production (22). Elevated ROS then promotes organelle toxicity through increased lipid peroxidation and inflammation. Indeed, hepatic mitochondrial dysfunction is found in subjects with steatosis (39). Furthermore, structural abnormalities in the mitochondria, including megamitochondria and paracrystalline inclusion bodies (39), and a marked decrease in the activity of mitochondrial respiratory chain complexes are observed in patients with NASH (35). In addition to these deleterious effects on mitochondria, NEFA also induce apoptosis of hepatocytes through upregulation of Fas, activation of JNK, and destabilization of lysosomes (23).
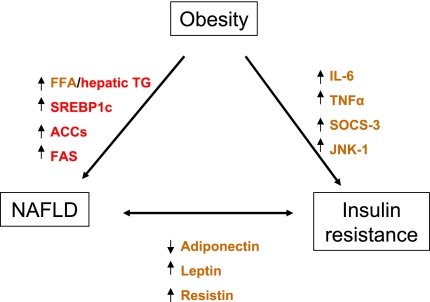
Adipokines and inflammatory cytokines from adipocytes may mediate pathological interactions between adipose tissue and liver. Because adipokines possess both pro- and anti-inflammatory functions, an imbalance between particular adipokines and cytokines may promote liver injury in steatohepatitis. In patients with NAFLD, plasma levels of adiponectin thought to derive from adipocytes are decreased and inversely related to hepatic insulin resistance and hepatic inflammation (16). High TNF-α and low adiponectin plasma levels have been suggested as independent predictors of NASH in patients with NAFLD (16). On the other hand, serum leptin and resistin are positively correlated with insulin resistance, hepatic steatosis, and liver injury (7, 32). These studies suggest a pathogenic link between obesity-induced insulin resistance and NAFLD (Fig. 1).
Fig. 1.
Proposed mechanisms of interaction among obesity, insulin resistance, and nonalcoholic fatty liver disease (NAFLD). The mediators are color-coded based on the source of origin (red, liver; brown, adipose tissue). FFA, free fatty acid; TG, triglyceride; SREBP1c, sterol regulatory element-binding protein 1c; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; SOCS-3, suppressor of cytokine signaling-3; JNK, c-Jun NH2-terminal kinase.
NASH and fibrosis.
Hepatic stellate cells (HSC) are liver-specific pericytes localized to an area between hepatocytes and sinusoidal endothelium called the space of Disse (11). In a healthy liver, quiescent HSCs store 80% of the body's vitamin A as retinyl esters in their cytoplasm (11). In an acutely injured liver, HSCs lose their vitamin A stores, transdifferentiate into contractile myofibroblasts, remodel extracellular matrix (ECM), and contribute to hepatic wound healing. In contrast, in a chronically injured liver, HSCs promote the development of fibrosis through excessive ECM production and reduced ECM metabolism (11).
Although 30–40% of people with simple steatosis progress to NASH, 74% of NASH patients progress to more severe liver injury, including fibrosis and cirrhosis (25). Increased adiposity and insulin resistance in obese subjects contribute to the progression of NASH to fibrosis. Increased presence of dead and dying hepatocytes, increased ROS, and altered adipokine/cytokine production are thought to promote a profibrotic milieu in the liver. We will briefly describe each of these contributing factors in the following sections of this review.
A major difference between NAFL and NASH is the increased number of apoptotic and/or necrotic hepatocytes detected in the liver (17). These dead and/or dying cells provide HSCs with critical signals to promote their activation; these signals can act both directly and/or indirectly on HSCs. For example, Kupffer cells, activated in part by hepatocyte cell death, produce transforming growth factor-β, ROS, and lipid peroxidation products, each of which is a potent stimulus for HSC activation (17).
Production of ROS promotes HSC activation and progression to fibrosis. Indeed, in a hyperinsulinemic and insulin-resistant state, ROS production is increased, in part, through increased CYP2E1 induction and activity, even in the absence of alcohol consumption (25). This is because of the loss of the repressive effect of insulin on CYP2E1 expression (25). Increased oxidant stress then can directly increase collagen production by activated HSC, particularly in the antioxidant-depleted hepatic microenvironment that occurs in advanced NASH (17, 25). Increased CYP2E1-mediated oxidant stress can further impair hepatic insulin signaling, exacerbating the insulin resistance in the liver, as well as liver injury/pathology. Findings in nrf-1-deficient mice provide experimental evidence for a role for oxidant stress in progression from NASH to fibrosis. nrf-1 is a transcription factor that modulates the expression of oxidative stress responsive genes; in its absence, mice develop a hepatic pathology similar to NASH. Specifically, hepatocyte-specific nrf-1-deficient mice exhibit repression of metallothionein 1 and metallothionein 2, two cysteine-rich proteins that protect the liver against free radicals, excessive zinc and cadmium, and toxic agents, including ethanol (30). Indeed, the full spectrum of liver disease, including steatosis, apoptosis, necrosis, inflammation, fibrosis, and cancer, is readily observed in liver-specific nrf-1−/− mice (25).
Changes in the expression of adipokines and cytokines are integral mediators of HSC activation and fibrogenesis (11, 42). Indeed, fibrosis is a common end point to chronic inflammation in an insulin-resistant state. Increased expression of leptin, TNF-α, IL-6, and monocyte chemoattractant protein (MCP)-1 are all associated with the insulin resistance and obesity (43). Reports suggest that leptin directly activates HSC (27) and also indirectly activates HSC through stimulatory effects on Kupffer cells (45). In addition to its effects on HSC activation, exposure of HSC to leptin in vitro reduces FasL-mediated apoptosis (36). These data suggest that increased leptin in NASH patients may promote survival of activated HSC and thereby contribute to fibrogenesis. Increased TNF-α expression by adipose tissue depots, as well as by hepatocytes and Kupffer cells, the resident macrophages in the liver, perpetuate insulin resistance, hyperinsulinemia, and hyperglycemia, as well as promote HSC activation and fibrogenesis. Finally, HSC produce and respond to MCP-1, a chemokine with potent activation and chemoattractant effects on HSCs and immune cells (11). Increased expression of MCP-1 increases hepatic inflammation and cell death and can therefore perpetuate HSC activation signals and progression from NASH to fibrosis (Fig. 2).
Fig. 2.
Potential pharmaceutical targets of interest in the progression of nonalcoholic steatohepatitis (NASH) to fibrosis in the setting of obesity and insulin resistance. ROS, reactive oxygen species; TGF-β, transforming growth factor-β; MCP-1, monocyte chemoattractant protein-1; CTGF, connective tissue growth factor.
In contrast to increased production of proinflammatory mediators, insulin resistance and obesity are often associated with reductions in the potent adipose-derived anti-inflammatory mediator, adiponectin. Reduced adiponectin facilitates or exacerbates increased production of inflammatory mediators, as well as HSC activation and fibrosis. Indeed, fibrosis is more severe in adiponectin knockout mice maintained on a high-fat diet compared with wild-type controls, while adiponectin administration attenuates CCl4-induced fibrosis in mice (42). In vitro, adiponectin potently suppresses platelet-derived growth factor-BB-induced HSC proliferation and migration (18). Finally, NASH patients who are diabetic, insulin resistant, and/or obese often exhibit reduced plasma adiponectin levels (42). However, recent studies demonstrate that increased adiponectin is associated with advancing fibrosis in patients with chronic hepatitis B (15). These data suggest that the regulation of adiponectin expression and its impact on liver during chronic injury and disease is likely to be more complex than originally proposed.
Additional factors that promote progression of NASH to fibrosis include increased sympathetic neurotransmitters, as well as angiotensin II, connective tissue growth factor (CTGF), and endocannabinoids. In vitro, norepinephrine promotes activation of NF-κB, proinflammatory chemokine expression, and contraction of isolated human HSC as well as collagen gene expression and proliferation of mouse HSC (5). The renin-angiotensin system is activated in the diseased liver (5), and there is evidence to suggest that blockade of angiotensin II can attenuate fibrosis in animal models (31). CTGF, a potent HSC activating cytokine, is overexpressed in patients with NASH, and increased expression of CTGF is positively associated with increased severity of hepatic fibrosis in humans (33). Consistent with these findings, Zucker rats exhibit increased hepatic CTGF mRNA and protein, and CTGF is induced in HSC incubated with high glucose or insulin (33). Endocannabinoids are increased, and endocannabinoid signaling enhanced, in livers from obese and insulin-resistant patients (26). Indeed, there is considerable evidence to suggest that not only does the endocannabinoid system contribute to insulin resistance and liver steatosis but it also, via the CB1 receptor, directly promotes progression to liver fibrosis in mice; by contrast, CB2 receptors exhibit antifibrotic function in the liver (26).
Synergy between obesity and insulin resistance in fibrosis progression.
Obesity is not only a risk factor for hepatic fibrosis through the progression of NAFLD but also has a synergistic effect on a superimposed or secondary hepatic injury. Prospective cohort studies from the United Kingdom indicate that the combination of obesity and alcohol consumption of 150 g or more each week in women is associated with a marked increased risk of cirrhosis compared with obese women who drank <70 g of alcohol per week (24). Furthermore, excess body weight and alcohol appear to have a synergistic rather than additive effect on the progression of liver disease (13). Indeed, obesity is an independent risk factor for alcohol-induced liver damage that appears to exacerbate each stage in the disease progression (8); this appears to be related to excessive alcohol consumption, since moderate alcohol consumption in obese patients is associated with reduced fibrotic progression (50). Although there are likely shared mechanisms responsible for the hepatic pathology associated with NASH and alcohol-induced liver injury, the presence of ethanol and ethanol metabolism is one important difference between the two conditions. Ethanol metabolism and subsequent generation of toxic metabolites, such as acetaldehyde, have potent effects on HSC activation (4). Another mechanism by which alcohol can accelerate fibrosis in obese individuals is through ethanol-induced increases in CYP2E1, which are greater than the levels of CYP2E1 induced by obesity alone (41). Increased CYP2E1-mediated production of ROS and lipid peroxidation products in obese humans who are also heavy alcohol consumers could therefore worsen obesity-induced liver injury and accelerate progression to fibrosis.
Obesity and alcohol abuse are both associated with increased proinflammatory gene expression (41). Therefore, in obese individuals who concurrently abuse alcohol, increases in molecules that directly or indirectly cause liver injury appear to accelerate the progression from NASH to fibrosis.
Pharmacological Targets of Interest
The cornerstone of intervention for chronic liver disease and fibrosis is removal of the primary etiologic agent responsible for initiating and perpetuating liver injury. In obese patients, weight loss remains the only effective therapy. In fact, weight loss of 5–10% of body weight decreases liver fat by 40–80% in nondiabetic subjects and type 2 diabetic patients (21). Histological improvement in liver is observed after bariatric surgery. Patients who lose weight after bariatric surgery also show attenuated inflammation and fibrosis at two years postsurgery (9, 12). Advances in our understanding of the molecular mechanisms that contribute to NAFLD, NASH, and fibrosis in obese and/or diabetic subjects has led to active investigation of several promising targets of intervention as outlined in Fig. 2. A select number of these potential therapeuctic options will be described in the following section.
Insulin sensitizers, peroxisome proliferator-activated receptors, and antioxidants.
Insulin sensitizers peroxisome proliferator-activated receptor agonists and antioxidants are rational approaches to the treatment of NASH based on proposed mechanisms of pathogenesis. However, the largest randomized controlled trial to date comparing oral pioglitazone at 30 mg daily, oral vitamin E at 800 international units (IU) daily, or placebo for 96 weeks showed no evidence for global histological improvement in patients receiving pioglitazone vs. placebo, although there were improvements in some histological features, such as hepatic steatosis and lobular inflammation. In addition, patients receiving pioglitazone gained more weight than those receiving vitamin E or placebo. Treatment with vitamin E was associated with significant improvement in the global recommended daily allowance for adults at 22.5 IU. Two meta-analyses of large randomized trials have revealed increased mortality in individuals receiving high-dose vitamin E supplementation (1, 28). The implications of these analyses for the potential adverse effects of high-dose vitamin E as a treatment for patients with obesity and NAFLD are unclear. Whether pioglitazone or vitamin E may be beneficial to a subgroup of patients is yet to be determined.
Angiogenesis inhibitors.
Hepatic angiogenesis is a required multistep mechanism in response to tissue injury and/or hypoxia to restore tissue integrity and oxygen homeostasis. Angiogenesis and the disruption of normal liver vasculature are linked to the progression of cirrhosis in chronic liver diseases. Early studies using angiogenesis inhibitors demonstrated promising results in fibrosis prevention (46, 49). However, a recent study showed that integrin αvβ3 may aggravate experimental liver fibrosis despite suppression of angiogenesis in rat models (34). Given that angiogenesis is an integral part of wound healing response, normalization of excessive, pathological angiogenesis may be a potential therapeutic approach to attenuate fibrosis.
Adenosine receptor antagonists.
Hypoxia results in hepatic adenosine accumulation and A2A receptor activation, which, in turn, leads to increased phosphatidylinositol 3-kinase and protein kinase B/Akt phosphorylation in animal models (2). A2A receptor activation enhances HSC activation, collagen production, and profibrotic collagen production. A2A receptor-deficient mice are protected from CCl4- and thioacetamide-induced fibrosis (3). Therefore, A2A receptor antagonist may be a potential treatment for liver fibrosis.
Inhibition of HSC activation and promotion of ECM metabolism.
HSC activation and subsequent ECM deposition are hallmarks of hepatic fibrogenesis. A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAMTS2) is an enzyme responsible for processing procollagen proteins into collagen fibrils, which is an important determinant of hepatic fibrosis reversibility. In ADAMTS2 knockout mice, the extent of liver injury after CCl4 was similar to that of wild-type mice. However, collagen fibril diameter was reduced and collagen bundles less dense in the ADAMTS2-deficient mice; this was associated with slightly faster fibrosis reversal after cessation of CCl4 exposure (20). Sustained production of tissue inhibitors of metalloproteinases (TIMPs) during liver injury could inhibit the activity of interstitial collagenases, leading to reduced degradation of the ECM. In addition, TIMP-1 has antiapoptotic effects on HSC. Therefore, inhibition of TIMPs is a potential therapy for liver fibrosis. In fact, use of matrix metalloproteinase-9 mutant proteins as TIMP-1 scavengers in mice reduces fibrosis accumulation by enhancing matrix resorption (37). Given the central role of activated HSC in fibrogenesis, the inhibition of HSC activation, clearance of activated HSC, or reversal of activated HSC into a quiescent phenotype is a rational approach for antifibrotic therapies.
Conclusion
Available evidence from animal models and population-based studies suggest a causal relationship between excess fat accumulation, insulin resistance, and progression to fibrosis. Although the mechanisms of obesity-induced insulin resistance and its association with liver fibrosis are incompletely understood, recent studies indicate an intricate cross talk between adipose tissue and liver through adipokines and inflammatory cytokines. The adipokines and inflammatory cytokines released from adipose tissue may promote hepatic insulin resistance and contribute to the progression of NAFLD. Furthermore, the presence of hepatic steatosis may also aggravate the progression of liver fibrosis in the setting of secondary or superimposed liver injury such as in alcohol abusers. Activation of the JNK-1 pathway is central in the pathogenesis of obesity-induced insulin resistance. Excess hepatic triglycerides and oxidative stress may lead to tissue injury and activation of repair mechanisms. These repair processes involve recruitment of immune cells, angiogenesis, activation of HSC, and subsequent ECM deposition. Weight loss remains the cornerstone of current therapy. Recent studies have identified several potential targets for pharmacological intervention in the fibrogenesis pathway, including the JNK-1 signaling pathway, antiangiogenic agents, cytokines and their receptors, and the adenosine receptor pathway. The modulation of HSC activation and ECM remodeling is an area of active investigation and may also lead to novel therapeutic interventions.
GRANTS
This work was supported by National Institutes of Health grants awarded to L. E. Nagy (RO1AA-11975; RO1AA-11876) and M. T. Pritchard (AA-017918), DOD Grant 10248754 to L. E. Nagy, and an American Liver Foundation Postdoctoral Fellowship Award to D. J. Chiang.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank David Schumick from the Cleveland Clinic Center for Medical Art and Photography for assistance with Fig. 2.
REFERENCES
- 1. Bjelakovic GND, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. J Am Med Assoc 297: 842–857, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Carini R, Grazia De Cesaris M, Splendore R, Baldanzi G, Nitti MP, Alchera E, Filigheddu N, Domenicotti C, Pronzato MA, Graziani A, Albano E. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology 127: 914–923, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol 148: 1144–1155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis 29: 211–221, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Day CP. From fat to inflammation. Gastroenterology 130: 207–210, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Diehl AM. Lessons from animal models of NASH. Hepatol Res 33: 138–144, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Diehl AM. Obesity and alcoholic liver disease. Alcohol 34: 81–87, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Dixon JB, Bhathal PS, O'Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg 16: 1278–1286, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88: 125–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furuya CK, Jr, de Oliveira CP, de Mello ES, Faintuch J, Raskovski A, Matsuda M, Vezozzo DC, Halpern A, Garrido AB, Jr, Alves VA, Carrilho FJ. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol 22: 510–514, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. Br Med J 340: c1240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi R, Enjoji M. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res 38: 1122–1129, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Hui CK, Zhang HY, Lee NP, Chan W, Yueng YH, Leung KW, Lu L, Leung N, Lo CM, Fan ST, Luk JM, Xu A, Lam KS, Kwong YL, Lau GK. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol 47: 191–202, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology Baltimore 40: 46–54, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 28: 370–379, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 125: 1796–1807, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kechagias S, Ernersson A, Dahlqvist O, Lundberg P, Lindstrom T, Nystrom FH. Fast-food-based hyper-alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 57: 649–654, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kesteloot F, Desmouliere A, Leclercq I, Thiry M, Arrese JE, Prockop DJ, Lapiere CM, Nusgens BV, Colige A. ADAM metallopeptidase with thrombospondin type 1 motif 2 inactivation reduces the extent and stability of carbon tetrachloride-induced hepatic fibrosis in mice. Hepatology Baltimore 46: 1620–1631, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest 105: 1067–1075, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology Baltimore 47: 1495–1503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu B, Balkwill A, Reeves G, Beral V. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. Br Med J 340: c912, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med 87: 679–695, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Mallat A, Lotersztajn S. Endocannabinoids and their role in fatty liver disease. Dig Dis 28: 261–266 [DOI] [PubMed] [Google Scholar]
- 27. Marra F, Bertolani C. Adipokines in liver diseases. Hepatology Baltimore 50: 957–969, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 142: 37–46, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract 4: 619–626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem 283: 33554–33562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM, Brenner DA. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology Baltimore 50: 929–938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pagano C, Soardo G, Pilon C, Milocco C, Basan L, Milan G, Donnini D, Faggian D, Mussap M, Plebani M, Avellini C, Federspil G, Sechi LA, Vettor R. Increased serum resistin in nonalcoholic fatty liver disease is related to liver disease severity and not to insulin resistance. J Clin Endocrinol Metab 91: 1081–1086, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C, Bedossa P. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology Baltimore 34: 738–744, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Patsenker E, Popov Y, Stickel F, Schneider V, Ledermann M, Sagesser H, Niedobitek G, Goodman SL, Schuppan D. Pharmacological inhibition of integrin alphavbeta3 aggravates experimental liver fibrosis and suppresses hepatic angiogenesis. Hepatology Baltimore 50: 1501–1511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology Baltimore 38: 999–1007, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Qamar A, Sheikh SZ, Masud A, Jhandier MN, Inayat IB, Hakim W, Mehal WZ. In vitro and in vivo protection of stellate cells from apoptosis by leptin. Dig Dis Sci 51: 1697–1705, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roderfeld M, Weiskirchen R, Wagner S, Berres ML, Henkel C, Grotzinger J, Gressner AM, Matern S, Roeb E. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J 20: 444–454, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322: 1539–1543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118: 2992–3002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Syn WK, Teaberry V, Choi SS, Diehl AM. Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis. Semin Liver Dis 29: 200–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 131: 934–945, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6: 772–783, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Jarvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis 42: 320–330 [DOI] [PubMed] [Google Scholar]
- 45. Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 137: 713–723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang YQ, Ikeda K, Ikebe T, Hirakawa K, Sowa M, Nakatani K, Kawada N, Kaneda K. Inhibition of hepatic stellate cell proliferation and activation by the semisynthetic analogue of fumagillin TNP-470 in rats. Hepatology Baltimore 32: 980–989, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373: 1083–1096, 2009. 19299006 [Google Scholar]
- 47a. World Health Organization Preventing chronic diseases: a vital investment. In: WHO Fact Sheet No 311, 2005 [Google Scholar]
- 48. Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci USA 102: 4120–4125, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Yamazaki M, Tsujinoue H, Imazu H, Masaki T, Fukui H. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut 52: 1347–1354, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zein CO, Unalp A RC, Liu YC, McCullough AJ. Smoking and severity of hepatic fibrosis in honalcoholic fatty liver disease. J Hepatol In press [DOI] [PMC free article] [PubMed] [Google Scholar]