Abstract
Wound healing is an appropriate response to inflammation and tissue injury in the gastrointestinal tract. If wound healing responses are excessive, perpetuated, or prolonged, they lead to fibrosis, distortion of tissue architecture, and loss of function. This introductory editorial and the minireviews or reviews in this themes series highlight the diversity in severity and location of fibrosis in response to gastrointestinal inflammation. The multiplicity of cellular and molecular mediators and new players, including stem cells or extracellular matrix-producing cells derived from nonmesenchymal cell types, is reviewed. Comparisons of inflammation-induced fibrosis across organ systems and the need for integrated and systems-based molecular approaches, new imaging modalities, well-characterized animal models, cell culture models, and improved diagnostic or predictive markers are reviewed. To date, intestinal fibrosis has received much less attention than inflammation in terms of defining mechanisms and underlying causes. This themes series aims to illustrate the importance of research in this area in gastrointestinal health and disease.
Keywords: stem cells, extracellular matrix, cellular and molecular mediators
inflammation is a universal response to all events disrupting tissue homeostasis, such as infection, injury, ischemia, stress, and malfunction. Although inflammation has traditionally been viewed as a pathophysiological phenomenon with negative consequences, its primary roles are actually positive, those of eliminating the offending cause and mediating host defense, thereby allowing tissue repair and restoring tissue homeostasis (33). Because inflammation can assume drastically different features depending on the trigger, location, and duration, its outcome can have numerous and diverse pathological consequences. This is because inflammatory and immune processes act simultaneously on many different cell types, leading to both shared and distinct disease outcomes (29). Prominent among the latter is fibrosis, a response characterized by an excessive deposition of collagen and other extracellular matrix (ECM) molecules formation of scar tissue to heal a wound. Paralleling the behavior of inflammation, fibrosis can also display several diverse features depending on the trigger, location, and duration. Fibrosis varies from minor and inconsequential scars to major and extensive fibrotic responses that severely compromise tissue and organ function (64).
All tissues and organs of the body are susceptible to fibrotic disorders (36). Some of these have received considerable attention from clinical and basic investigators alike, such as liver cirrhosis, idiopathic pulmonary fibrosis, and kidney fibrosis. Other organs affected by fibrosis have received far less attention even though significant organ dysfunction and morbidity is associated with the fibrotic response. Fibrosis of the gastrointestinal tract occurs in inflammatory bowel diseases (IBD) as a consequence of chronic inflammation and can have very serious clinical and pathophysiological consequences, including morbidity, hospitalizations, and associated costs. Yet relatively little attention has been devoted to this important problem. Therefore, this and the articles in this themes series on fibrosis in the gastrointestinal tract and liver are intended to specifically address this important topic, assess current knowledge of the intestinal response to inflammation, compare with other organs, review inflammation-driven fibrosis of gastrointestinal tract or liver, highlight research needs, propose new research pathways, and discuss diagnostic and therapeutic implications.
Components of the Intestinal Fibrogenic Response
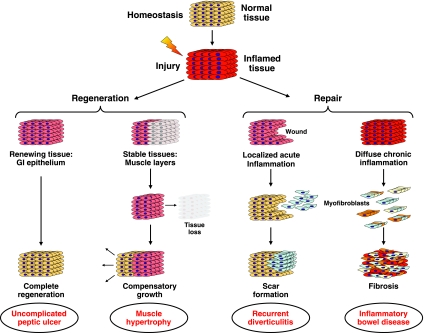
When normal tissue homeostasis is disrupted by an inflammatory insult, the affected cells set in motion a multiplicity of mechanisms whose ultimate goal is to fully restore normal tissue composition and function. This can be done through two basic mechanisms, regeneration or repair, each having distinct implications regarding success in reestablishing tissue homeostasis. As hinted in the introduction, reestablishment of tissue homeostasis will depend on the type, extent, severity, and duration of the inflammatory process, and also the type of tissue being compromised. In tissues with a high potential for renewal, such as the gastrointestinal epithelium, complete regeneration and restoration of normal tissue architecture are possible as long as inflammation is superficial and of limited duration. This is, for example, the case with uncomplicated gastroduodenal peptic ulcers (Fig. 1). Regeneration is probably also possible in stable tissues, such as the intestinal muscle layers that can respond to tissue injury and loss with compensatory growth, a phenomenon more often observed in parenchymal organs like the liver and kidney (Fig. 1). When regeneration and repair fail to achieve restitutio ad integrum, then the intestinal tissue will continue to try to repair itself. A localized but recurrent inflammatory wound can lead to formation of a discrete scar, as seen in a sigmoid colon subjected to repeated bouts of acute diverticulitis with inflammation followed by circumscribed fibrosis with or without stricture formation (Fig. 1). In situations in which inflammation is severe and persistent, as typically observed in IBD and particularly Crohn's disease (CD), the intestine continuously responds to chronic inflammatory aggression by relentlessly attempting to repair tissue. This can lead to scar tissue that permeates the whole tissue architecture, and ultimately to major functional impairment (Fig. 1).
Fig. 1.
Hypothesized models of the link between different types of inflammation and regenerative, wound healing, or fibrotic responses. Left: regeneration and restoration of tissue architecture occur after superficial inflammation of limited duration such as uncomplicated gastroduodenal peptic ulcers or acute injury or loss of stable tissue such as intestinal muscle layers. Right: fibrosis occurs when regeneration and repair fail to restore normal tissue architecture. Discrete circumscribed scars form after localized but recurrent inflammation as occurs with repeated bouts of acute diverticulitis. Extensive scar tissue and fibrosis can permeate the entire tissue when the intestine is subject to chronic inflammation, as in inflammatory bowel disease, and tissue is constantly attempting wound repair. GI, gastrointestinal.
Intestinal Inflammation Drives Intestinal Fibrosis
Regeneration and repair in intestinal inflammation represent biological goals that have practical clinical implications. In fact, in IBD, attaining endoscopic and histological mucosal healing is currently considered a key parameter in assessing response to therapy (30) and is viewed a positive step toward long-term resolution of disease. Evidence supporting the practical importance of returning intestinal tissue to normality is provided by long-term follow-up of CD and ulcerative colitis (UC) after treatment with anti-inflammatory drugs: those who achieve mucosal healing have a significantly lower rate of surgery compared with those who do not (15). Therefore, how the intestine responds to inflammation is critical not only in regard to treatment, but also to the long-term outcome of the primary disease process. Patients with CD provide useful examples of differing responses to inflammation. Although all patients implicitly have inflammation at the beginning of the disease, patients typically present with continued inflammation, fistula (a connection or passageway between organs or vessels that do not connect), or a stricture (a narrowing of the basal lumen). The longer the follow-up of CD patients, the fewer of them will remain free of inflammation, fistulae, and strictures, implying that the underlying inflammation may result in different clinical outcomes but is essentially a life-long process (6). These clinical observations are central to the biological argument that, in conditions like CD and probably other chronic intestinal inflammatory conditions, inflammation is accompanied by a response where fibrosis is an inevitable or very common component. The location of inflammation is probably a major determining factor of where fibrosis will develop and whether it is clinically symptomatic. In CD, inflammation is typically transmural and so is the ensuing fibrostenotic response, whereas, in UC, inflammation is limited to the mucosal layer and a fibrotic response is virtually limited to the mucosal layer (24). In CD, transmural inflammation and fibrosis typically affect the narrow-caliber small bowel such that thickening of the bowel wall due to fibrosis more frequently results in symptomatic stenosis or stricture.
Intestinal Fibrosis Follows Diverse Types of Inflammation
Accepting the premise that intestinal fibrosis is the consequence of the response to components of inflammation, then the question becomes which cellular and soluble components incite and perpetuate the local fibrogenic process. Chronic inflammatory infiltrates, like those observed in IBD, are eminently nonspecific and rich in neutrophils, T and B cells, macrophages, and other numerically less prominent cells. Many of these immune cell types interact with and activate local mesenchymal cells and stimulate them to mediate tissue remodeling and repair, through either direct cell-to-cell contact or the secretion of soluble factors (3, 7, 17). This response of mesenchymal cells is not limited to nonspecific immune responses. Even when the inflammatory infiltrate is triggered by a specific type of immune response, like the T helper (Th)-2 response that accompanies allergic disorders, it is possible to have a florid profibrotic process, as has been recently documented in patients with eosinophilic esophagitis (1). This, of course, supports the notion that gastrointestinal fibrosis is indeed a broadly based reaction to many different types of inflammatory injury.
Multiple Cellular Sources of the Excessive ECM in Intestinal Fibrosis
A wealth of evidence has demonstrated that “activated” hepatic stellate cells are the sources of ECM in fibrotic liver. In the gastrointestinal tract, the situation is complicated because there are multiple types of mesenchymal cells, and under normal conditions these cells elaborate ECM, which is important for normal tissue architecture. In uninjured intestine, subepithelial myofibroblasts, and subepithelial, submucosal and serosal fibroblasts are major sources of ECM. Healthy gastrointestinal smooth muscle layers show little accumulation of ECM. In IBD, fibroblasts, myofibroblasts, and smooth muscle have all been implicated as cellular mediators of fibrosis. This themes series highlights evidence that additional cell types are involved. For example, stellate cells are not exclusively found in liver but are found in the pancreas and human intestinal mucosa, where stellate cells have also been isolated and characterized in normal and inflamed gut mucosa (27). Intestinal stellate cells display the same basic morphological, phenotypic, and functional characteristics of hepatic stellate cells and also produce collagen in response to traditional stimuli such as transforming growth factor (TGF)-β. Some cells of nonmesenchymal origin undergo a process of transdifferentiation into mesenchymal cells to become efficient ECM-producing cells. The best example are epithelial cells that, under the pressure of TGF-β and other factors, undergo epithelial-to-mesenchymal transition (21, 22). Another example is mucosal endothelial cells that transform into mesenchymal cells under the pressure of IL-1β (F. Rieder and colleagues, work in progress). Other types of cells that may contribute to intestinal fibrosis are pericytes, circulating fibrocytes (CD45-positive leukocytes capable of producing collagen), and bone marrow-derived stem cells (39), which will be reviewed in detail in this series.
Multiple Soluble Factors Contribute to Intestinal Fibrosis
Another aspect of the intestinal profibrogenic response is to consider the various triggers that activate immune and nonimmune cells to become ECM-producing cells. These triggers include a very vast array of products. Several cytokines and chemokines can activate intestinal mesenchymal cells, and traditionally TGF-β has been considered the most potent profibrogenic molecule (46). TGF-β profibrogenic pathways are fairly well defined, but depending on the stage of the fibrotic response this molecule is not necessarily the dominant fibrogenic molecule. In fact, a large number of additional soluble mediators have been reported to activate fibroblasts and enhance ECM production, including insulin-like growth factor I, epithelial growth factor, basic fibroblast growth factor, platelet-derived growth factor, IL-1, IL-4, IL-6, IL-13, and IL-21; other soluble mediators have an opposite effect, like IL-10, IL-12, and interferon-γ (61). Although TGF-β is probably the most effective in stimulating ECM production by mature mesenchymal cells, other molecules may instead set the stage for this response at earlier stages of fibrogenesis. For instance, connective tissue growth factor (CTGF) directs fibroblast differentiation in mesenchymal stem/stromal cells and conditions the response of these cells to subsequent stimuli (26). Thus it is likely that multiple molecules are involved in intestinal fibrogenesis and most are yet to be investigated and their relative contribution defined. A complementary new aspect to be considered in regard to these molecules is that some may be encoded by genetic variants that render them more effective in exerting the profibrogenic activity. Such is the case, for example, for CTGF. Variants of its gene are associated with a more severe degree of hepatic fibrosis in subjects infected with schistosomes (8). This observation makes it possible that patients with particularly severe intestinal fibrosis and strictures, as typically seen in some CD patients, may also have variants of profibrogenic genes, an area that certainly deserve attention and further investigation.
Gut Microbiota Contributes to Intestinal Fibrosis
An increasingly appreciated component of the body's overall homeostasis is the role of the microbiome in health and disease, a concept that must now be taken into consideration in regard to fibrosis in general and intestinal fibrosis in particular. The human organism is composed of ∼1012 cells expressing 104 genes. Normal commensal microbes are composed of an estimated 1014 bacteria (in addition to archea, fungi, and viruses) and express 106 genes (12). Together, the integration of the mammalian and microbial genomes and metabolic products (metabolomes) creates a superorganism whose health is greatly dependent on the composition and function of the microbial as well as the human component. Within this ground-breaking concept it is extremely important to remember that the bulk of the microbial load is found in the gastrointestinal tract (37), making the microbiota a dominant force in intestinal health and disease. It is already established that bacterial products can induce a profibrogenic response in mesenchymal cells in vivo (16). This response is of particular importance in the intestine, and products of the abundant and diversified gut microbiota can activate mesenchymal cells through Toll-like and NOD-like receptors (TLR and NLR, respectively). Evidence of the contribution of the gut microbiota to intestinal fibrogenesis can be found in vivo in various animal models (34, 42, 59) as well as in vitro models. One example is the enhancement of the fibrogenic action of human intestinal fibroblasts in response to TLR5 activation by the ligand flagellin (F. Rieder and colleagues, work in progress). Interestingly, not all bacterial products are profibrogenic, and some of them, like zwitterionic polysaccharides, actually protect against intestinal fibrosis in a model of surgical fibrosis (48). Thus whether intestinal fibrosis develops or not may depend not only on the type of local inflammatory process but also on the specific composition of the luminal or mucosa-adherent microbiota in the affected segment, a notion with potential therapeutic implications.
External Environment Contributes to Intestinal Fibrosis
Whereas the microbiota is part of the internal microenvironment of the gut, this organ is also subject to the influence of the external macroenvironment. The concept that all elements present in the environment can potentially influence disease development is established, and several examples illustrate how environmental factors can lead to lung, liver, and kidney fibrosis (55, 57, 62). The same is likely to be true for intestinal fibrosis. Several environmental contaminants influence the immune response. A recent example is the skewing of the regulatory T cell-Th17 cell balance by activation of the aryl hydrocarbon receptor, whose ligand is dioxin, a known environmental toxin and also a by-product of the food component tryptophan, and numerous other substances (52). The number and diversity of environmental factors makes it impossible to study each one of them and determine its profibrogenic potential. Some unexpected factors, however, are emerging and underscore the highly complex nature of how their fibrogenic activity is exerted. Recent reports have shown that obesity is associated with changes in the gut microbiota, with a decrease in bacteroidetes and increase in firmicutes (28, 58). Obesity induces a state of low-grade inflammation (53), and various immune abnormalities are present in the fat tissue microenvironment, including loss of Th2 and regulatory T cells, and increase in Th1 and CD8+ effector T cells (31). Although still incompletely understood, these changes predispose to organ inflammation and fibrosis, as firmly established in nonalcoholic fatty liver disease (9). A similar response may also occur in the intestine, where the combination of bacteria and a high-fat diet results in activation of specific cell types, including leukocytes, endocrine cells, and vascular cells (10). Although not formally proven yet, it is theoretically possible that intestinal mesenchymal cells can also be affected by obesity, as indicated by a recent report. In this intriguing study obesity was induced in ewes by feeding them a high-fat diet; when their offspring were examined, intestinal inflammation and fibrosis were detected and were sustained up to 2 years after delivery (65). Thus it is possible that environmental factors not only condition intestinal fibrosis through direct individual exposure but also through prenatal conditioning. Given the enormity and diversity of the external environment, new tools and approaches are needed to evaluate the impact of what has been recently named the “exposome” on intestinal disease in general and intestinal fibrosis in particular (38).
Novel Inducers and Regulators of Intestinal Fibrosis
In addition to factors known to be relevant to intestinal fibrosis, new inducing and modulatory factors are emerging that must undergo intense scrutiny and investigation. It is now evident that activation of immune and nonimmune cells can be accomplished not only by bacterial products (pathogen-associated molecular patterns) but also products derived from injured cells, the so-called damage-associated molecular patterns (DAMPs) (47). These include a wide range of products, such as DNA, RNA, ATP, HMGB1, uric acid, fragments of ECM molecules, IL-1α, IL-18, and IL-33, among others in a rapidly expanding list. Each of these DAMPs can cause “sterile inflammation,” a term that describes triggering of an inflammatory response in the absence of any culpable microbial agent (44, 45). There is limited information on whether and how DAMPs may promote a fibrogenic response, but the cytokine IL-1α, which belongs to the “alarmin” groups of DAMPs, has been reported to induce a fibrogenic phenotype in fibroblasts of systemic sclerosis patients (23). Supporting these findings are ongoing studies showing that necrotic epithelial cell-derived IL-1α can induce activation of human intestinal fibroblasts (M. Scarpa et al., unpublished observations). It is possible that this response also results in enhanced ECM production, which would establish a link between a typical DAMP and intestinal fibrosis.
Another new aspect is that of novel ways in which profibrogenic responses can be modulated. One such way is through the action of microRNAs (miRNAs), recently identified as key regulators of gene expression that function by repressing specific target genes at the posttranscriptional level (5, 35). miRNAs have been recently shown to regulate fibrosis and represent a brand new way of looking at regulation of fibrotic diseases (19). An additional way of regulating fibrosis is through epigenetic modifications, defined as the adaptation of chromosomal regions allowing the modulation of gene expression and perpetuation of local activation or repression states (43). Recent studies in the kidney link epigenetic histone methylation in mesangial cells to expression of genes associated with fibrosis (54). The importance of epigenetics in inflammation-driven intestinal fibrosis is substantial, because changes in gene function may become stable and potentially heritable. For example, if epigenetic changes induce a profibrotic fibroblast lineage in response to inflammation, such phenotype may persist in subsequent daughter cells. This will create a subpopulation of mesenchymal cells with the capacity to produce high levels of collagen and other ECM products, which would further promote and perpetuate intestinal fibrosis. Ongoing experiments have detected the presence of high levels of histone H4 lysine acetylation (H4K16Ac) on the collagen gene promoter in human intestinal fibroblasts from fibrotic CD tissue. Since histone acetylation is associated with gene activation, this preliminary observation lends support to the concept of epigenetic regulation of intestinal fibrogenesis (M. Scarpa, S. Kessler, C. Fiocchi, and E. Stylianou, unpublished observations).
Integrating Knowledge: a Sensible Approach to the Pathogenesis of Intestinal Fibrosis
From the above discussion and a growing body of literature, it is evident that the fibrogenic process is a highly complicated and dynamic one and involves a multitude of triggers, cells, molecules, and pathways in all tissues or organs affected. In gastrointestinal fibrosis, a further complexity is added by the presence and diversity of the nearby luminal microbiota. Another key point is the practical observation that, despite the generally accepted argument that intestinal fibrosis is considered an inevitable consequence of chronic intestinal inflammation, notable exceptions exist. Helicobacter pylori-induced gastritis is without a doubt a prototypical example of chronic inflammation, but even minor gastric fibrosis is rarely seen in this condition (4). Another example is celiac disease (gluten-sensitive enteropathy), also a typically chronic inflammatory disease of the upper small bowel, which is not accompanied by fibrosis, even though several of its pathogenic components are shared with IBD (50). Thus, although intestinal fibrosis is most often associated with and induced by inflammation, this is not a universal response. Therefore, events and mechanisms must exist that prevent fibrosis from developing even when all the ideal inflammatory conditions appear to be in place. This apparent paradox underscores our far-from-complete understanding of the intestinal fibrogenic process and its intrinsic variability. At least one reason for this variability is that the factors responsible for chronic gut inflammation interact with and modulate each other, and these same interactions probably occur during initiation, progression, or perpetuation of intestinal fibrosis. Thus, if complexity is the hallmark of intestinal fibrosis, as is the case for many biological processes (14), a logical approach is to embrace it and extend studies of individual components and contributions to their interactions with other fibrogenic mediators. What is needed to better understand the pathogenesis of intestinal fibrosis is the collection of a large bolus of molecular information and bioinformatic analyses to define networks linked to fibrosis. Such networks can be used to develop diagnostic and predictive models that will give integrated information on the mechanisms and clinical outcomes of intestinal fibrosis, similar to what is being attempted in cancer and other common human diseases (49).
Clinical and Therapeutic Considerations
The obvious lack of an understanding of the pathogenic events underlying intestinal fibrosis is translated to our current inability to prevent, detect, and treat this condition. Prevention of intestinal fibrosis is out of reach, at least for the time being, until we gain a far more thorough understanding of its pathogenesis. Still, detection of fibrosis and its quantification is making some progress on two fronts. The first is through new imaging modalities, the subject of another review in this series that will discuss this topic in some detail. The second is the development of laboratory markers of intestinal fibrosis. Progress in this area would allow identification of patients at risk and theoretically offer the option of an early aggressive therapy. Presently available tools for prediction of fibrostenosing CD include genetic variants, clinical phenotypes and serological markers. Among multiple genetic variants, those of NOD2 predispose to fibrostenosis of the terminal ileum and a higher risk for need of resection (13), and the combination of NOD2 variants with other risk variants, like those of the ATG16L1, IBD5, DLG5, and IL-23R genes, further increases the risk of having a stricturing (and fistulizing) clinical evolution and a higher risk of surgery (60). Clinical phenotypes of higher risk for intestinal fibrosis include ileal involvement, active smoking, long duration of disease, need for corticosteroid therapy, and family history of stricturing disease, but all these factors are nonspecific and are also associated with fistulizing disease (18). Finally, a number of serological markers are available that have some capacity of discriminating patients at greater risk of complicated CD and risk of surgery (11, 41), but they predict complications rather than specifically predicting fibrosis. In addition, measurement of several ECM molecules and growth factors has been proposed for detection or prediction of fibrotic CD, but their value and specificity are not yet established.
Considering the restricted value and specificity of current diagnostic and predictive markers of intestinal fibrosis (in CD) and our narrow understanding of the related pathogenic events, it is not surprising that surgical rates for fibrotic complications of CD have remained essentially unchanged for the last three decades despite remarkable advances in the effectiveness of anti-inflammatory therapies (39, 40). From this important observation one has to infer that controlling intestinal inflammation alone is not sufficient to prevent or eliminate the associated fibrotic response. Thus it follows that fibrosis-specific therapies must be developed, and perhaps therapies specific for intestinal fibrosis, since the underlying pathophysiology may be different from other organs, particularly with regard to the presence of the luminal microbiota. We need to learn how to limit tissue damage, prevent activation of mesenchymal cells or other cell types, block excessive secretion and deposition of ECM components, or learn how to replace fibrogenic mesenchymal cells with new healthy cells. Another review in this series addresses the promise of targeting myofibroblasts for stem cell therapy. All these approaches are being attempted in the management of liver fibrosis (discussed in another chapter of this series) or fibrosis associated with other chronic inflammatory disorders (36, 51, 56) and should be applied to the intestine. Finally, we need to take into account not only how, but also when to intervene in the evolving profibrogenic process. Blockade of NF-κB activation in the model of chronic 2,4,6-trinitrobenzenesulfonic acid (TNBS) murine colitis decreases expression of inflammation-promoting genes, but not that of collagen genes (63); moreover, preliminary evidence from a murine model of infection-associated intestinal fibrosis indicates that early removal of the inflammatory stimulus reduces fibrosis, but once the fibrogenic response is set in motion, fibrosis continues to propagate even in the absence of the inflammatory stimulus (20). Therefore, timing of therapeutic intervention may be a critical variable determining the good or poor outcome of the inflammation-driven fibrotic process. This is a strong reason to focus our investigative efforts on its early pathogenic events.
Challenges in Investigating and Treating the Early Stages of Intestinal Fibrosis
Clinically, intestinal fibrosis is often identified at an advanced stage as a result of clinical symptoms. As mentioned, new and improved imaging modalities are needed to improve detection and early diagnosis. In addition, validated animal models of different gastrointestinal inflammatory disorders and associated fibrosis represent a major obstacle. Another chapter in this series will review animal models. To model fibrosis in CD, an animal model should feature inflammation and fibrosis in small bowel. Relatively few models of IBD develop small bowel inflammation, and fibrosis has been studied in only a handful of these models (2, 42). An additional issue for therapy testing and discovery is both ease and reproducibility of the animal model of inflammation and associated fibrosis, and ability to apply the model to genetically or microbially manipulated mice. TNBS and dextran sodium sulfate models offer promise (25, 32) but have limitations in terms of ease and reproducibility (TNBS) or common immune mechanisms with IBD (dextran sodium sulfate). Other needed models are reporter mice to visualize ECM activation, reporter models to visualize and isolate different mesenchymal cell subtypes and mice with mesenchyme subtype-specific expression of Cre-recombinase to permit gene disruption in the different mesenchymal cell subpopulations. Such models would open up avenues for defining cell-specific molecular, genetic, or epigenetic mechanisms at different stages of fibrosis, as well as evaluating cell-specific impact and outcomes of therapy.
Concluding Remarks
Fibrosis associated with gastrointestinal inflammation has been understudied relative to its important contributions to tissue dysfunction and disease. This themes series begins to highlight important cellular and molecular mechanisms, knowledge gaps, and technical or clinical challenges that must be overcome to improve our ability to prevent or treat the negative consequences of fibrosis triggered by inflammation. Our goal is to stimulate new directions and cross-talk among organ systems that will facilitate progress in understanding and controlling this natural process that represents an appropriate response to inflammation and tissue injury but that when excessive or uncontrolled leads to major and serious pathophysiological outcomes.
GRANTS
P. K. Lund's work on fibrosis is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK047769-11. C. Fiocchi's work is supported by NIDDK Grants DK50984-10 and DK069854-5.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1. Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol 119: 206–212, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Adler J, Swanson SD, Schmiedlin-Ren P, Higgins PD, Golembeski CP, Polydorides AD, McKenna BJ, Hussain HK, Verrot TM, Zimmermann EM. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology 2011. [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129: 969–984, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol 1: 63–96, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol 9: 839–845, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 8: 244–250, 2002 [DOI] [PubMed] [Google Scholar]
- 7. De La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C). J Biol Chem 274: 30747–30755, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Dessein A, Chevillard C, Arnaud V, Hou X, Hamdoun AA, Dessein H, He H, Abdelmaboud SA, Luo X, Li J, Varoquaux A, Mergani A, Abdelwahed M, Zhou J, Monis A, Pitta MG, Gasmelseed N, Cabantous S, Zhao Y, Prata A, Brandt C, Elwali NE, Argiro L, Li Y. Variants of CTGF are associated with hepatic fibrosis in Chinese, Sudanese, and Brazilians infected with schistosomes. J Exp Med 206: 2321–2328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diehl AM. Hepatic complications of obesity. Gastroenterol Clin North Am 34: 45–61, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One 5: e12191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubinsky MC, Kugathasan S, Mei L, Picornell Y, Nebel J, Wrobel I, Quiros A, Silber G, Wahbeh G, Katzir L, Vasiliauskas E, Bahar R, Otley A, Mack D, Evans J, Rosh J, Hemker MO, Leleiko N, Crandall W, Langton C, Landers C, Taylor KD, Targan SR, Rotter JI, Markowitz J, Hyams J. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol 6: 1105–1111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eberl G. A new vision of immunity: homeostasis of the superorganism. Mucosal Immunol 3: 450–460, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Economou M, Trikalinos TA, Loizou KT, Tsianos EV, Ioannidis JP. Differential effects of NOD2 variants on Crohn's disease risk and phenotype in diverse populations: a metaanalysis. Am J Gastroenterol 99: 2393–2404, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Embrace the complexity. Nat Immunol 10: 325, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 133: 412–422, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Grassl GA, Valdez Y, Bergstrom KS, Vallance BA, Finlay BB. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology 134: 768–780, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-κB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 282: G1035–G1044, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Henckaerts L, Van Steen K, Verstreken I, Cleynen I, Franke A, Schreiber S, Rutgeerts P, Vermeire S. Genetic risk profiling and prediction of disease course in Crohn's disease patients. Clin Gastroenterol Hepatol 7: 972–980.e2, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Jiang X, Tsitsiou E, Herrick SE, Lindsay MA. MicroRNAs and the regulation of fibrosis. FEBS J 277: 2015–2021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson LA, Luke A, Blanco LP, Sauder KL, Higgins PD. Late removal of inflammatory stimulus does not abrogate fibrosis development in vivo in a mouse model of inflammation and fibrosis. Gastroenterology 138, Suppl 1: S–758, 2010 [Google Scholar]
- 21. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112: 1776–1784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawaguchi Y, McCarthy SA, Watkins SC, Wright TM. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J Rheumatol 31: 1946–1954, 2004 [PubMed] [Google Scholar]
- 24. Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis 7: 16–26, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology 125: 1750–1761, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120: 3340–3349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leite AZ, de la Motte C, Strong SA, Fiocchi C. Isolation and functional characterization of human intestinal mucosal stellate cells (Abstract). Gastroenterology 122: A-111, 2002 [Google Scholar]
- 28. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev 65: S140–S146, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis 16: 338–346, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med 15: 846–847, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Lund PK, Zuniga CC. Intestinal fibrosis in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol 17: 318–323, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Medzhitov R. Origin and physiological roles of inflammation. Nature 454: 428–435, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Mourelle M, Salas A, Guarner F, Crespo E, Garcia-Lafuente A, Malagelada JR. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology 114: 519–526, 1998 [DOI] [PubMed] [Google Scholar]
- 35. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10: 111–122, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Paz Z, Shoenfeld Y. Antifibrosis: to reverse the irreversible. Clin Rev Allergy Immunol 38: 276–286, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Pennisi E. Body's hardworking microbes get some overdue respect. Science 330: 1619, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science 330: 460–461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 6: 228–235, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease: progress in basic and clinical science. Curr Opin Gastroenterol 24: 462–468, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Rieder F, Schleder S, Wolf A, Dirmeier A, Strauch U, Obermeier F, Lopez R, Spector L, Fire E, Yarden J, Rogler G, Dotan N, Klebl F. Serum anti-glycan antibodies predict complicated Crohn's disease behavior: a cohort study. Inflamm Bowel Dis 16: 1367–1375, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Rigby RJ, Hunt MR, Scull BP, Simmons JG, Speck KE, Helmrath MA, Lund PK. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut 58: 1104–1112, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rivera RM, Bennett LB. Epigenetics in humans: an overview. Curr Opin Endocrinol Diabetes Obes 17: 493–499, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol 3: 99–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol 28: 321–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med 152: 159–166, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 28: 429–436, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Ruiz-Perez B, Chung DR, Sharpe AH, Yagita H, Kalka-Moll WM, Sayegh MH, Kasper DL, Tzianabos AO. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc Natl Acad Sci USA 102: 16753–16758, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature 461: 218–223, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterology 137: 1912–1933, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Sivakumar P, Das AM. Fibrosis, chronic inflammation and new pathways for drug discovery. Inflamm Res 57: 410–418, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Stevens EA, Bradfield CA. Immunology: T cells hang in the balance. Nature 453: 46–47, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Strohacker K, McFarlin BK. Influence of obesity, physical inactivity, and weight cycling on chronic inflammation. Front Biosci (Elite Ed) 2: 98–104, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R. Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol 21: 2069–2080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Swaminathan S, Shah SV. New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol 18: 2636–2643, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Szabo H, Fiorino G, Spinelli A, Rovida S, Repici A, Malesci AC, Danese S. Review article: anti-fibrotic agents for the treatment of Crohn's disease — lessons learnt from other diseases. Aliment Pharmacol Ther 31: 189–201, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Taskar V, Coultas D. Exposures and idiopathic lung disease. Semin Respir Crit Care Med 29: 670–679, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Van Tol EA, Holt L, Li FL, Kong FM, Rippe R, Yamauchi M, Pucilowska J, Lund PK, Sartor RB. Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am J Physiol Gastrointest Liver Physiol 277: G245–G255, 1999 [DOI] [PubMed] [Google Scholar]
- 60. Weersma RK, Stokkers PC, van Bodegraven AA, van Hogezand RA, Verspaget HW, de Jong DJ, van der Woude CJ, Oldenburg B, Linskens RK, Festen EA, van der Steege G, Hommes DW, Crusius JB, Wijmenga C, Nolte IM, Dijkstra G. Molecular prediction of disease risk and severity in a large Dutch Crohn's disease cohort. Gut 58: 388–395, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol 31: 110–119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wood MJ, Powell LW, Ramm GA. Environmental and genetic modifiers of the progression to fibrosis and cirrhosis in hemochromatosis. Blood 111: 4456–4462, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol 179: 6988–7000, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan X, Huang Y, Wang H, Du M, Hess BW, Ford SP, Nathanielsz PW, Zhu MJ. Maternal obesity induces sustained inflammation in both fetal and offspring large intestine of sheep*. Inflamm Bowel Dis 2010. [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]