Abstract
It has long been known that mammalian enterocytes coexpress two members of the fatty acid-binding protein (FABP) family, the intestinal FABP (IFABP) and the liver FABP (LFABP). Both bind long-chain fatty acids and have similar though not identical distributions in the intestinal tract. While a number of in vitro properties suggest the potential for different functions, the underlying reasons for expression of both proteins in the same cells are not known. Utilizing mice genetically lacking either IFABP or LFABP, we directly demonstrate that each of the enterocyte FABPs participates in specific pathways of intestinal lipid metabolism. In particular, LFABP appears to target fatty acids toward oxidative pathways and dietary monoacylglycerols toward anabolic pathways, while IFABP targets dietary fatty acids toward triacylglycerol synthesis. The two FABP-null models also displayed differences in whole body response to fasting, with LFABP-null animals losing less fat-free mass and IFABP-null animals losing more fat mass relative to wild-type mice. The metabolic changes observed in both null models appear to occur by nontranscriptional mechanisms, supporting the hypothesis that the enterocyte FABPs are specifically trafficking their ligands to their respective metabolic fates.
Keywords: lipid, gut, chylomicron, fatty acid, monoacylglycerol
dietary fat is composed primarily of triacylglycerols (TG). After absorption of the end products of TG digestion, fatty acids (FA) and monoacylglycerols (MG), the intestinal epithelial cells resynthesize TG and package them into chylomicrons, which are subsequently delivered to peripheral tissues. The intestine also takes up FA and MG from the bloodstream; these are not utilized primarily for chylomicron TG, but rather for phospholipid (PL) synthesis and FA oxidation (12, 18, 44).
Fatty acid-binding proteins (FABPs) are a large family of intracellular proteins with high affinity for long-chain FA and, in general, are expressed at high levels in their native tissues. Liver FABP (LFABP; FABP1) and intestinal FABP (IFABP; FABP2) are unique in that substantial levels of both proteins are expressed in a single cell type, the enterocyte (30). LFABP, but not IFABP, is also expressed in hepatocytes. The reasons underlying coexpression of the two FABPs in the intestinal absorptive cell remain unclear (38). Indeed, both LFABP and IFABP expression first appear at parturition (13, 31), and the distributions of both vary directly with dietary fat absorption (16), although LFABP expression is maximal at a more proximal localization than IFABP (3, 8, 13, 34). Finally, both IFABP and LFABP bind long-chain FA with high affinity (37).
Functional specificity for these FABPs has been suggested by important differences in their in vitro properties. First, LFABP binds not only FA but also additional ligands including lysophospholipids and MG (41, 43). Second, we have shown that the kinetic mechanisms of FA transfer from LFABP and IFABP to model membranes are markedly different, with transfer from IFABP occurring by a collisional mechanism and that from LFABP by a diffusional mechanism (14). Additional evidence for functional specificity has been provided by studies of transfected fibroblasts, in which LFABP-expressing cells increased FA targeting to both TG and PL but IFABP expression resulted in FA targeting primarily to TG (24, 35, 36). Finally, both the model membrane and cell transfection studies showed faster rates of FA transfer for IFABP relative to LFABP (14, 24).
One line of IFABP-null mice and two independent lines of LFABP-null mice have been generated (9, 28, 47). Male but not female IFABP−/− mice were heavier than their wild-type (WT) counterparts on a chow diet (47). On a high-fat diet, the increase in body weight in males was somewhat attenuated, and the female IFABP−/− mice gained less weight than WT. Male but not female IFABP−/− mice developed fatty livers on high-fat diets (1), and IFABP−/− mice were found to be hyperinsulinemic and modestly hypertriglyceridemic (47). No changes in intestinal morphology were observed (2). For the LFABP-null mouse, work has focused primarily on the hepatic effects of LFABP deletion, with results showing that LFABP−/− hepatocytes exhibit impaired FA uptake, oxidation, and incorporation into TG (9, 28). No body weight differences in chow-fed LFABP-null mice relative to C57BL/6J control mice were found (9, 22), although a number of reports have indicated increased or decreased weight gain in the LFABP-null mice depending on dietary fat level and composition; a consistent pattern has not emerged (19–21, 29).
Since both LFABP and IFABP are expressed in small intestinal mucosal cells, in the present studies we directly compared IFABP- and LFABP-null mice, focusing on intestine-specific consequences of the gene knockouts. We present evidence that each of the enterocyte FABPs participates in specific pathways of intestinal lipid metabolism. The absence of accompanying changes in lipid metabolic gene expression suggests that the enterocyte FABPs function in the trafficking of lipid ligands to their respective metabolic fates.
EXPERIMENTAL PROCEDURES
Materials.
Oleic acid and sn-2-monoolein were obtained from NuChek Prep (Elysian, MN). [3H]oleic acid ([9,10-3H]oleic acid, 26.3 Ci/mmol) and [14C]oleic acid ([1-14C]oleic acid, 54 mCi/mmol) were obtained from Perkin Elmer-New England Nuclear (Stelton, CT). [3H]monoolein (sn-2-[9,10-3H]monoolein, 40–60 Ci/mmol) was from American Radiochemical (St. Louis, MO). Authentic neutral lipid and PL standards were purchased from Doosan Serdary Research Laboratories (Toronto, ON, Canada) and Avanti Polar Lipids (Alabaster, AL), respectively. Sodium taurocholate was purchased from Calbiochem (La Jolla, CA), and FA-free BSA was obtained from Sigma Aldrich (St. Louis, MO). Thin-layer chromatography (TLC) plates (Silica Gel G, 250 μm, 150 Å) were obtained from Whatman (South Plainfield, NJ). Rabbit antibodies to purified rat LFABP and IFABP (45) were generated in rabbits by Affinity Bioreagents (Golden, CO). All other materials were reagent grade or better.
Animals.
LFABP−/− mice on a C57BL/6J background were generated by Martin and coworkers (22). The mice were backcrossed with C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) for six generations to create LFABP−/− mice and WT littermates to serve as controls. Some experiments included mice that had been backcrossed a seventh time; results from these experiments were consistent with the previous ones. IFABP−/− mice on a C57BL/6J background were created by Vassileva and coworkers (47) and were backcrossed with C57BL/6J mice six times. WT mice from Jackson Laboratories were used as controls. Mice were used at 3–4 mo of age and 25–30 g body weight. Experiments were performed in the fasted state, typically between 8 AM and 11 AM when food had been removed 48 h earlier. Experiments were performed in 48-h-fasted mice to mimic the protocol and therefore directly compare the effects of intestinal LFABP deletion with the effects of hepatic LFABP deletion reported by Newberry et al. (28), and to directly compare the effects of IFABP deletion. Unless otherwise indicated, animals were housed three or four per cage, maintained on a 12:12-h light-dark cycle, and allowed ad libitum access to standard rodent chow (Purina Laboratory Rodent Diet 5001). All animal work was approved by the Rutgers animal care review committee.
Mouse genotyping.
Genotyping was performed by PCR as described by Martin et al. (22).
Preparation of lipids for bloodstream and intraduodenal administration.
Lipids were prepared as described previously (44). In brief, for bloodstream administration, stock solutions were prepared by drying (per mouse) 7.5 μCi of either [14C]oleate (140 nmol) or [3H]monoolein (125 nmol) under a nitrogen stream and then adding 0.5% (final volume) ethanol and 150 μl of a solution containing 0.1 M NaCl and mouse serum (1:1). For intraduodenal administration, stock solutions were prepared by drying (per mouse) 1.5 μCi of either [14C]oleate (28 nmol) or [3H]monoolein (25 nmol) under a nitrogen stream and then adding 150 μl of 10 mM sodium taurocholate in 0.1 M NaCl. Radiochemicals were checked for purity before use.
Surgical procedures.
For experiments that required fasting, each mouse was individually housed at the time of food removal. The surgical procedures were performed as previously described (12, 44). In brief, on the day of the experiment the mice were weighed and anesthetized with ketamine-xylazine-acepromazine (80, 100, 150 mg/kg ip, respectively). For intravenous administration of lipids, the jugular vein was exposed and cannulated and a 28-gauge needle with the injection solution was secured in place by surgical string. For intraduodenal administration, a small section of the intestine was exposed and a small incision was made with microsurgical scissors within 1 cm of the pylorus. A blunt-tip 18-gauge needle was passed into the intestine via the incision and secured in place by surgical string. Next, for both methods of delivery, 2 min after the injection the intestine was removed, measured lengthwise, rinsed with 60 ml of ice-cold 0.1 M NaCl, and opened longitudinally, and mucosa was scraped with a glass microscope slide into tubes in dry ice and immediately stored at −80°C. Typically, total recovery of the administered radiochemical in the mucosa was 50% and 0.1% for intraduodenal and intravenous administration, respectively, consistent with previous findings (44). There were no differences in recovery among the groups.
Immunoblotting.
Mucosa was harvested as described above and homogenized in 20 vols. of PBS pH 7.4 with 0.5% (vol/vol) protease inhibitors (Sigma 8340) on ice with a Potter-Elvejhem homogenizer. Where indicated, a total membrane fraction was obtained by ultracentrifugation (100,000 g, 1 h at 4°C). Protein concentration was determined by the Bradford assay (6). Fifty micrograms of total cell protein or ten micrograms of membrane protein was loaded onto 12% polyacrylamide gels and separated by SDS-PAGE. The proteins were transferred onto polyvinylidene difluoride membranes with a semidry transfer system (Bio-Rad) for 1 h at 20 V. The membranes were incubated in a 5% nonfat dry milk or 2% gelatin blocking solution overnight at 4°C and then probed with primary antibody (1:30,000) for 1 h. After thorough washing, blots were then incubated with anti-rabbit IgG-horseradish peroxidase conjugate (1:30,000), as necessary, for 1 h and then developed by chemiluminescence (ECL reagent, GE Healthcare, Piscataway, NJ). Protein expression was quantified by densitometric analysis with ImageJ software (National Institutes of Health).
Mucosal lipid extraction and acute lipid metabolism.
The frozen mucosal samples were thawed, diluted with 20 ml of PBS pH 7.4 per gram (wet weight), and homogenized by 20 strokes with a Potter-Elvejhem homogenizer on ice, had protein concentration determined, and were stored at −80°C for lipid extraction (11) within 2 days. The homogenate was diluted to a protein concentration of 1 mg/ml, and 1 ml was uniformly used for lipid extraction in order to ensure equivalent extraction yields (44). Lipids were extracted twice with 10 ml of chloroform-methanol (2:1), and the aqueous-phase nonlipid fractions were discarded. The organic lipid layer was dried under a nitrogen stream, resuspended in chloroform-methanol (2:1), and spotted onto Silica Gel G TLC plates along with authentic standards. The TLC plate was developed in a nonpolar solvent system consisting of hexanes, diethyl ether, and acetic acid (70:30:1). For experiments in which 14C-labeled substrate was used, radioactivity was visualized by exposure to phosphorimager plates and analyzed by the Storm 840 Phosphorimager. When 3H-labeled substrate was used, the lipid spots were visualized by exposure to iodine vapors and scraped into scintillation vials containing 5 ml of scintillation fluid. The scintillation vials were vortexed and allowed to settle overnight before analysis in a scintillation counter.
Mucosal lipid composition.
To determine mucosal lipid composition, the intestinal mucosa was harvested as described above; however, the intestine was divided equally into two segments (proximal and distal) before the mucosa was scraped. Lipid extraction and TLC were performed as described above, and the iodine-stained TLC plates were scanned by a Hewlett-Packard scanner. Absolute values for the masses of individual lipid subclasses were obtained by densitometric analysis with ImageJ software using standard curves for each lipid metabolite.
Fatty acid oxidation.
FA oxidation was measured as described by Storch et al. (44). In brief, the sample homogenate containing metabolized [14C]oleate from the acute lipid metabolism experiments was incubated with 7% perchloric acid (PCA), and a tissue paper soaked in 1 M benzethonium hydroxide was used to capture released 14CO2. The radioactivity of the tissue paper and an aliquot from the 3,000 g supernatant of the acidified homogenate was analyzed by scintillation counting. The sum of the radioactivity contained in the supernatant and in the tissue paper was divided by the total amount of radioactivity contained in the initial sample homogenate to determine the percentage of FA oxidized.
Quantitative RT-PCR for mRNA expression analysis.
The protocol for mRNA acquisition and analysis was adapted from Chon et al. (7). Briefly, tissues were homogenized in 4 M guanidinium thiocyanate, 25 mM sodium citrate, and 0.1 M β-mercaptoethanol with several strokes of a Polytron. Total RNA was further purified by phenol extraction and the RNeasy cleanup kit (Qiagen, Valencia, CA) along with DNase treatment to minimize genomic DNA contamination. Reverse transcription was performed with 1 μg of RNA, random primers, an RNase inhibitor, and reverse transcriptase (Promega, Madison, WI) in a total volume of 25 μl. Primer sequences were retrieved from Primer Bank (Harvard Medical School QPCR primer database) and are shown in Supplemental Table S1.1 The efficiency of PCR amplification was analyzed for all primers to confirm similar amplification efficiency. Real-time PCR reactions were performed in triplicate with an Applied Biosystems 7300 instrument. Each reaction contained 80 ng cDNA, each primer at 250 nM, and 12.5 μl of SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in a total volume of 25 μl. Relative quantification of mRNA expression was calculated with the comparative threshold cycle (Ct) method normalized to β-actin.
Body weight.
Mice were housed three or four per cage, maintained on a 12:12-h light-dark cycle, and fed ad libitum standard rodent chow. Body weight was measured weekly on a continuous basis over the course of 4–6 mo after birth.
Food intake.
For the measurement of food intake, mice were individually housed in wire mesh-bottomed metabolic cages and a known amount of food was given to each mouse. The remaining food plus collected “crumbs” were weighed weekly for 1–2 wk per mouse, and weekly results were averaged. Week-to-week measurements were consistent.
Fecal composition.
Two days worth of feces were collected at various time points while the mice were individually housed, for analysis of fecal weight and lipid composition. The feces were dried and weighed, and then 1 mg (dry weight) was dissolved in water overnight and lipid was extracted and analyzed as described above.
Body composition.
Mice were anesthetized with ketamine-xylazine-acepromazine (80, 100, 150 mg/kg ip, respectively). Body composition was analyzed by dual-energy X-ray absorptiometry (DEXA; PIXImus, GE-Lunar, Madison, WI) in fed mice and after 48-h food deprivation. Total fat, fat-free, and bone mineral mass were evaluated excluding the head and tail. The instrument was regularly calibrated.
Energy expenditure/respiratory quotient.
Energy expenditure was assessed by the University of Cincinnati's Mouse Metabolic Phenotyping Center. Mice were placed in an indirect calorimetry chamber 3 h before the dark phase (3 PM), and oxygen consumption and carbon dioxide production were measured for 24 h. The data obtained from 6 AM to 3 PM the following day were averaged and represent “fed” values. At 3 PM on day 2, food was removed and measurements continued for 18 h. The data obtained from 6 AM to 9 AM the following day (15–18 h after food was removed) were averaged and represent “fasted” values. Metabolic rate data are expressed as kilocalories per hour and kilocalories per hour per kilogram of body weight, and gas exchange data are expressed as milliliters per kilogram per minute.
Oral fat tolerance test.
An oral fat tolerance test was performed as described by Newberry et al. (29). Briefly, 10 μCi of [14C]oleate and [3H]monoolein was dried under a nitrogen stream. Five hundred microliters of olive oil was added, and the solution was vortexed vigorously. The olive oil bolus was administered via orogastric gavage to conscious, overnight-fasted mice. Thirty minutes before the gavage, tyloxapol was injected (500 mg/kg ip) to block peripheral lipoprotein clearance. Fifty microliters of blood was taken from the saphenous veins immediately before and at 1, 2, and 4 h after gavage.
Statistical methods.
Statistical comparisons were performed with independent two-sided t-tests or ANOVA. Differences were considered significant if the P value was <0.05.
RESULTS
Energy balance.
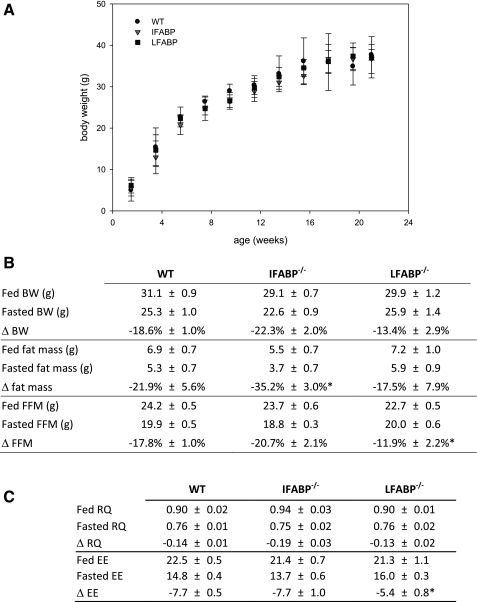
At no point between birth and at least 5 mo of age did the body weight of IFABP−/− or LFABP−/− mice differ from WT (Fig. 1A). In the fed state WT mice weighing 31.1 ± 1 g were composed of 24.2 ± 0.5 g of fat-free mass (78.1 ± 1.8% of total body wt) and 6.9 ± 0.7 g of fat mass (21.8 ± 1.8% of total body wt), and this was essentially unaffected by ablation of either FABP. After fasting body weight decreased by 18.6 ± 1.0% to 25.3 ± 1.0 g (P < 0.01) in WT mice (Fig. 1B), and this was also unchanged in both FABP−/− mice. In WT mice, fat-free mass was reduced by 4.3 ± 0.2 g (17.8 ± 1.0%) to 19.9 ± 0.5 g (P < 0.01) and fat mass was reduced by 1.5 ± 0.4 g (21.9 ± 5.6%) to 5.3 ± 0.7 g (P < 0.01) after food deprivation. Deletion of LFABP, but not IFABP, reduced the amount of fat-free mass lost by >30% from 17.8 ± 1.0% in WT mice to 11.9 ± 2.2% in LFABP−/− mice (P < 0.05). IFABP−/− mice, on the other hand, lost significantly more fat mass than WT mice during food deprivation (35.2 ± 3.0% vs. 21.9 ± 5.6% in WT and IFABP−/−, respectively; P < 0.05). Thus ablation of IFABP enhances fat loss, while ablation of LFABP preserves fat-free mass.
Fig. 1.
Phenotype of wild-type (WT) and fatty acid-binding protein (FABP) knockout mice. A: body weight of male WT, intestinal FABP-null (IFABP−/−), and liver FABP-null (LFABP−/−) mice. Data are means ± SE; n = 10–15/group. B: body weight (BW), fat mass, and fat-free mass (FFM) in fed and 48-h-fasted mice as assessed by dual X-ray energy absorptiometry. Data are means ± SE; n = 5–8/group. C: respiratory quotient (RQ) and total metabolic rate [energy expenditure (EE), kcal·h−1·kg−1] in fed and 18-h-fasted mice as assessed by indirect calorimetry. Data are means ± SE; n = 5 or 6/group. *P < 0.05 vs. WT.
As expected, the respiratory quotient (RQ) was reduced from 0.90 ± 0.02 to 0.76 ± 0.01 upon food deprivation in WT mice, reflecting a greater reliance on fat oxidation to meet energy requirements (Fig. 1C). This was also found in both FABP-null mice, although, in agreement with the enhanced fat loss in fasting IFABP−/− mice, the magnitude of the reduced RQ was modestly, albeit nonstatistically significantly, greater in IFABP−/− mice (−0.14 ± 0.01 vs. −0.19 ± 0.03 in WT and IFABP−/−, respectively, P = 0.08). Total energy expenditure in WT mice was 22.5 ± 0.5 kcal·h−1·kg−1 (0.66 ± 0.02 kcal/h) in the fed state and 14.8 ± 0.4 (0.42 ± 0.01 kcal/h) when fasting. In agreement with the preservation of fat-free mass observed in fasting LFABP−/− mice, LFABP−/− mice maintained energy expenditure better than WT mice (−7.7 ± 0.5 vs. −5.4 ± 0.8 in WT and LFABP−/−, respectively; P < 0.05). Thus the changes in body composition in both FABP−/− mouse models are likely secondary, at least in part, to changes in fuel partitioning and energy expenditure.
WT mice weighing 30.6 ± 0.7 g fed a standard chow diet consumed 2.3 ± 0.1 g/day (6.9 ± 0.2 kcal/day), and ablation of either enterocyte FABP did not alter intake (data not shown). Fecal lipid content and composition were measured as an estimate of fat absorption. Total daily excrement amounted to 0.77 ± 0.06 g in WT mice, of which 8.7 ± 1.6 μg/mg was lipids. The lipid composition was mainly PL (2.9 ± 0.8 μg/mg feces, or 31.2 ± 2.7% of the lipids), cholesterol (1.6 ± 0.3 μg/mg feces, 18.3 ± 1.0%), FA (1.7 ± 0.2 μg/mg feces, 20.0 ± 2.1%), and cholesteryl esters (1.7 ± 0.4 μg/mg feces, 18.3 ± 2.5%). No changes were found in LFABP−/− or IFABP−/− mice.
FABP protein expression.
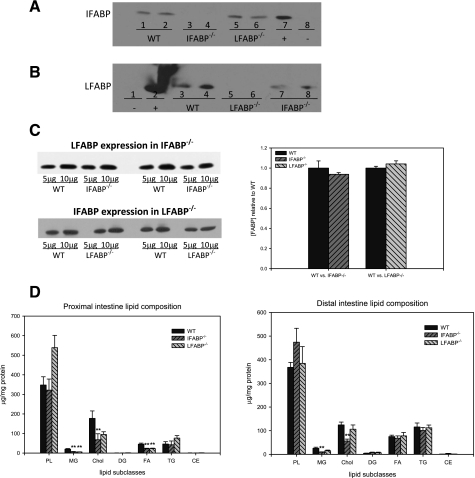
Because of their partially overlapping ligand specificity and similar localization along the gastrointestinal tract, it was possible that ablation of one enterocyte FABP would result in compensatory upregulation of the other. Therefore, we used Western blotting to estimate the amounts of LFABP in the IFABP−/− mice relative to WT, and vice versa. The polyclonal antibodies used were monospecific; as shown in Fig. 2, A and B, the anti-LFABP antibodies did not cross-react with IFABP, and the anti-IFABP antibodies did not cross-react with LFABP. The results showed that IFABP expression in LFABP−/− mice and LFABP expression in IFABP−/− mice were unchanged in 3- to 4-mo-old mouse small intestinal mucosa relative to WT (Fig. 2, C and D).
Fig. 2.
Intestinal phenotype of WT and FABP knockout mice. A: immunoblot for IFABP: 10 μg intestinal mucosa protein from WT (lanes 1 and 2), IFABP−/− (lanes 3 and 4), and LFABP−/− (lanes 5 and 6). Lanes 7 and 8 contain 1 μg purified IFABP (+) and LFABP (−), respectively. B: immunoblot for LFABP: 10 μg intestinal mucosa protein from WT (lanes 3 and 4), LFABP−/− (lanes 5 and 6), and IFABP−/− (lanes 7 and 8). Lanes 1 and 2 contain 1 μg purified IFABP (−) and LFABP (+), respectively. C: representative immunoblots of LFABP expression in the intestinal mucosa of WT and IFABP−/− and IFABP expression in WT and LFABP−/−. Relative quantification of FABP expression in WT and FABP−/− small intestinal mucosa is shown on right. Data are means ± SE; n = 4/group. D: lipid composition of small intestinal mucosa from 3- to 4-mo-old male WT, IFABP−/−, and LFABP−/− mice. Data are means ± SE; n = 4–6/group. **P < 0.01 vs. WT.
Intestinal morphology and lipid composition.
In WT mice, the total length of the intestine, from the pyloric sphincter to the cecum, was 39.9 ± 0.7 cm (1.6 ± 0.1 cm/g body wt). This was unaffected by ablation of IFABP (38.8 ± 0.07 cm, 1.8 ± 0.1 cm/g body wt) or LFABP (40.1 ± 1.0 cm, 1.5 ± 0.1 cm/g body wt).
The lipid composition of the proximal and distal intestinal mucosa in the fasted mouse intestine consisted primarily of PL, cholesterol, and TG representing ∼54%, 26%, and 10% of the total lipids, respectively, in WT mice. Neither PL nor TG were altered by ablation of either enterocyte FABP; however, a decrease in cholesterol content was found in IFABP−/− proximal and distal mucosa, and mucosal MG content was also reduced in both intestinal segments (Fig. 2D). MG were also reduced in LFABP−/− proximal mucosa.
Metabolism of diet-derived fatty acids.
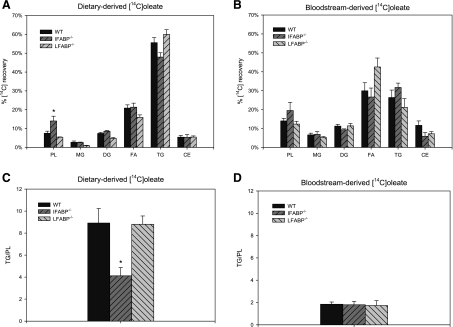
After intraduodenal administration of [14C]oleate, mucosal 14C recovery was predominantly in TG (55.6% ± 2.6%) (Fig. 3A) in WT mice. In IFABP−/− mice, significantly increased 14C recovery in PL and moderately reduced recovery in TG [not significant (NS)] were observed, resulting in a 54% reduction in the TG-to-PL ratio (P < 0.05) (Fig. 3C). To determine whether this alteration in FA partitioning was regulated by feeding status, we directly compared fed and fasted WT and IFABP−/− mice. In agreement with previous results (44), a ∼32% decrease in TG/PL was observed in fasted compared with fed WT mice, driven primarily by increased incorporation of [14C]oleate into PL (data not shown). The effect of feeding was unchanged in the IFABP−/− mice, in which a ∼45% reduction in TG/PL in the fed state relative to WT mice was found (data not shown). In contrast to IFABP−/−, the metabolism of [14C]oleate was essentially unaffected in LFABP−/− mice.
Fig. 3.
Fatty acid metabolism in mouse small intestinal mucosa. A: recovery of diet-derived [14C]oleate in fat-soluble metabolites in small intestinal mucosa. Data are means ± SE; n = 7/group. B: recovery of bloodstream-derived [14C]oleate in fat-soluble metabolites in the small intestinal mucosa. Data are means ± SE; n = 5–10/group. C: incorporation of diet-derived [14C]oleate in triacylglycerols relative to phospholipids in small intestinal mucosa. D: incorporation of bloodstream-derived [14C]oleate in triacylglycerols (TG) relative to phospholipids (PL) in the small intestinal mucosa. Data are means ± SE; n = 7/group. *P < 0.05 vs. WT.
Metabolism of bloodstream-derived fatty acids.
We (44) and others (12) have shown previously that the anabolic fate of bloodstream-derived [14C]oleate in intestinal enterocytes displays a markedly different pattern of assimilation compared with gastrointestinal tract administration. For WT mice, recovery in TG was reduced by 58% (P < 0.01) and incorporation into PL doubled for bloodstream-derived [14C]oleate relative to diet-derived [14C]oleate (Fig. 3, A and B), with the resultant TG/PL reduced ∼80% for bloodstream- compared with diet-derived FA (Fig. 3, C and D) (8.9 ± 1.3 vs. 1.8 ± 0.2; P < 0.01 for all comparisons). Unlike apically delivered FA, incorporation of bloodstream-derived [14C]oleate into complex lipids was largely unaffected by IFABP ablation, and TG/PL was unchanged from WT (Fig. 3C). Thus IFABP ablation alters the metabolic fate of dietary/apical- but not bloodstream/basolateral-derived FA in the small intestinal mucosa, whereas incorporation of [14C]oleate into complex lipids was unaffected by LFABP ablation regardless of its site of administration.
Metabolism of diet-derived monoacylglycerols.
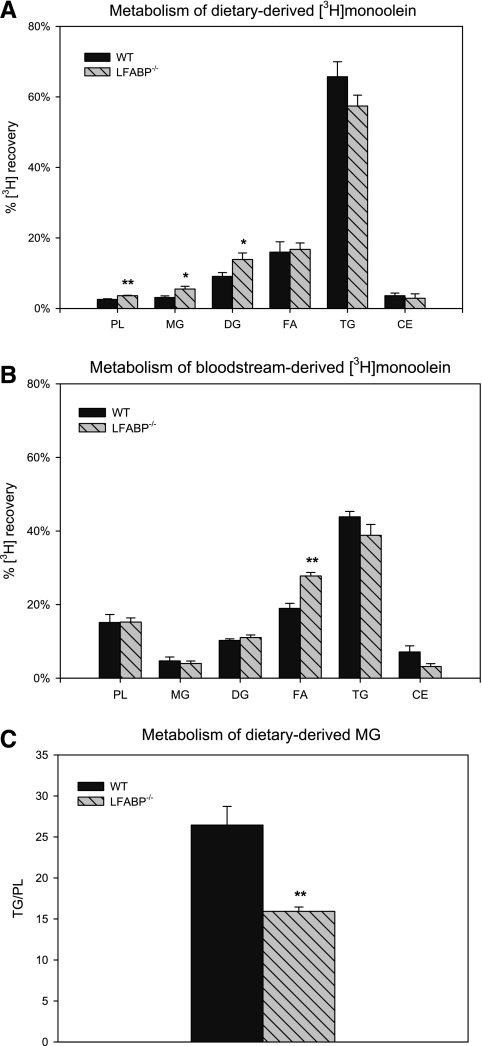
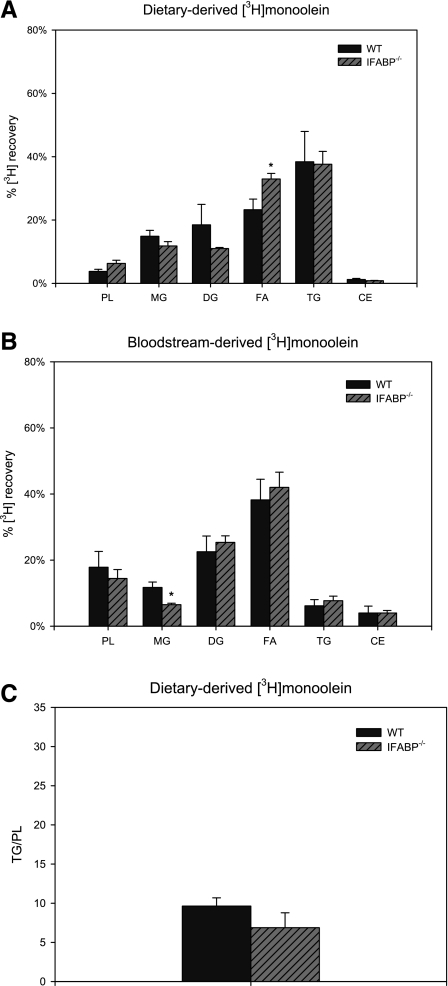
After intraduodenal administration of [3H]monoolein in WT mice, mucosal 3H was recovered mainly in TG (65.7% ± 4.2%) (Fig. 4A), recovery in PL was lower for [3H]monoolein than for [14C]oleate (2.6 ± 0.2% vs. 7.5 ± 1.1%, P < 0.01), and TG/PL was threefold greater for monoolein than for oleate (26.4 ± 2.3 vs. 8.9 ± 1.3, P < 0.01) (Figs. 3C and 4C); these results are in agreement with our previous findings (44). In LFABP−/− small intestinal mucosa, recovery of mucosal 3H from intraduodenally administered [3H]monoolein was increased by ∼41% in PL, by ∼78% in MG, and by ∼53% in diacylglycerols (P < 0.05) relative to WT. The recovery of [3H]monoolein in triacylglycerols was minimally changed (reduced by ∼13%, NS) in LFABP−/− (Fig. 4A). Thus LFABP ablation resulted in a ∼40% reduction in TG/PL (26.4 ± 2.3 vs. 15.9 ± 0.5 for WT and LFABP−/−, respectively; P < 0.01), reflecting greater partitioning of substrate into PL. Ablation of IFABP, on the other hand, resulted in a significantly increased recovery of 3H in free FA (Fig. 5A) but did not affect TG/PL (Fig. 5C).
Fig. 4.
Monoacylglycerol (MG) metabolism in fasted WT and LFABP−/− small intestinal mucosa. A: recovery of diet-derived [3H]monoolein in fat-soluble metabolites in small intestinal mucosa of WT and LFABP−/− mice. Data are means ± SE; n = 5–7/group. *P < 0.05, **P < 0.01 vs. WT. B: recovery of bloodstream-derived [3H]monoolein in fat-soluble metabolites in small intestinal mucosa. Data are means ± SE; n = 5/group. **P < 0.01 vs. WT. C: incorporation of diet-derived [14C]monoolein in TG relative to PL in small intestinal mucosa. Data are means ± SE; n = 5–7/group. **P < 0.01 vs. WT. DG, diacylglycerols; FA, fatty acids; CE, cholesteryl esters.
Fig. 5.
MG metabolism in fed WT and IFABP−/− small intestinal mucosa. A: recovery of diet-derived [3H]monoolein in fat-soluble metabolites in small intestinal mucosa of WT and IFABP−/− mice. Data are means ± SE; n = 4/group. B: recovery of bloodstream-derived [3H]monoolein in fat-soluble metabolites in small intestinal mucosa. Data are means ± SE; n = 5 or 6/group. C: incorporation of diet-derived [3H]monoolein in TG relative to PL in small intestinal mucosa. Data are means ± SE; n = 4/group. *P < 0.05.
Metabolism of bloodstream-derived monoacylglycerols.
In contrast to dietary-derived lipids, the processing of bloodstream-derived [3H]monoolein was largely unaffected in LFABP−/− intestinal mucosa. In both WT and null mice, the recovery in TG was reduced for basolateral compared with apical [3H]monoolein, and the incorporation into PL increased ∼fivefold for basolateral relative to apical [3H]monoolein (5.9× for WT and 4.2× for LFABP−/−). In both genotypes, the resultant TG/PL was thus reduced ∼85% for bloodstream compared with dietary monoolein (P < 0.01 for all comparisons). Finally, the recovery of bloodstream-derived [3H]monoolein in the free FA fraction was significantly increased in LFABP-null mice (19.0 ± 1.4% vs. 27.8 ± 0.9% for WT and LFABP−/−, respectively, P < 0.01). Recovery of 3H in MG was reduced in IFABP−/− mice (Fig. 5B), and TG/PL was unaffected compared with WT (Fig. 5C).
No effect of FABP ablation on expression of intestinal mucosa lipid synthetic genes.
To determine whether any of the observed changes in the acute metabolism of FA and MG were due to alterations in the expression of lipid synthetic genes in the FABP-null animals, the RNA levels of endoplasmic reticulum glycerol-3-phosphate acyltransferase (erGPAT), mitochondrial glycerol-3-phosphate acyltransferase (mtGPAT), monoacylglycerol acyltransferase-2 (MGAT2), diacylglycerol acyltransferase-1 (DGAT1), diacylglycerol acyltransferase-2 (DGAT2), and monoacylglycerol lipase (MGL) were analyzed by quantitative PCR (qPCR). No significant differences were found between genotypes in any of the genes examined (Supplemental Fig. S1). These findings indicate that IFABP and LFABP ablation alter the metabolism of dietary FA and MG, respectively, by a nontranscriptional mechanism.
Impaired oxidation of bloodstream-derived fatty acids in LFABP−/− intestinal mucosa.
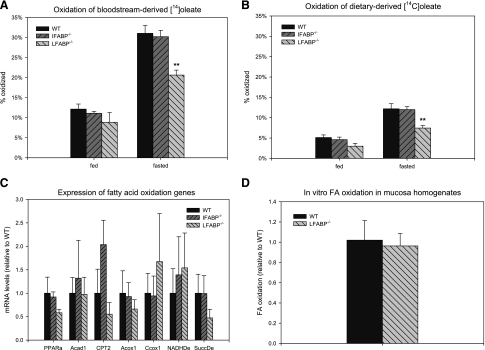
FA oxidation was measured by quantifying the appearance of 14CO2 and 14C-labeled acid-soluble metabolites of [14C]oleate in the same experiments used to examine anabolic metabolism. In the intestinal enterocytes of fed animals, 12.1 ± 1.3% of the [14C]oleate was oxidized within 2 min of administration of the label into the jugular vein of fed mice (Fig. 6A). Food deprivation increased the oxidation of bloodstream-derived [14C]oleate to 31.2 ± 1.6% (P < 0.01). Interestingly, this was significantly lower in LFABP−/− mice, in which only 20.6 ± 1.2% of the [14C]oleate was oxidized (P < 0.01) in food-deprived animals. No changes were found in IFABP−/−. As expected, intraduodenal administration of [14C]oleate resulted in a significantly lower recovery in 14C-labeled acid-soluble products and 14CO2 compared with intravenous administration (12.1 ± 1.3% vs. 5.1 ± 0.6%, for bloodstream- and diet-derived FA, respectively, P < 0.01) (Fig. 6B). Food deprivation more than doubled the oxidation of dietary FA in WT mice, to 12.2 ± 1.3% (P < 0.01). This was also impaired in LFABP−/− mice, where only 7.5 ± 0.6% was oxidized (P < 0.01). Thus while fasting increased intestinal FA oxidation in LFABP−/− mice, the increase was significantly blunted compared with WT mice; the defective oxidation was independent of the site of FA entry into the enterocyte.
Fig. 6.
Fatty acid oxidation in mouse small intestinal mucosa. A: oxidation of bloodstream-derived [14C]oleate in small intestinal mucosa of fed and 48-h-fasted mice. Data are means ± SE; n = 7/group. **P < 0.01 vs. WT. B: oxidation of diet-derived [14C]oleate in small intestinal mucosa in fed and 48-h-fasted mice. Data are means ± SE; n = 5–10/group. **P < 0.01 vs. WT. C: expression of fatty acid oxidation genes in small intestinal mucosa of WT and LFABP−/− mice. Data are means ± SE; n = 3 or 4/group. D: fatty acid oxidation in homogenates from small intestinal mucosa of 48-h-fasted male mice as described in experimental procedures. Data are means ± SE; n = 3 or 4/group.
Fatty acid oxidation defect in LFABP−/− mice is not due to altered oxidative capacity.
To distinguish a global reduction in mucosal oxidative capacity from a trafficking defect, FA oxidative capacity was assessed by two methods. First, mRNA expression of key FA oxidative enzymes was assessed by qPCR. The results showed that genes involved in mitochondrial β-oxidation (acyl-CoA dehydrogenase-1, carnitine palmitoyltransferase-2), peroxisomal FA oxidation (acyl-CoA oxidase-1), electron transport (cytochrome-c oxidase, NADH dehydrogenase, succinate dehydrogenase), or peroxisome proliferator-activated receptor-α (PPARα), were not altered by LFABP ablation (Fig. 6C). Next, FA oxidation was analyzed in mucosa homogenates in vitro with [14C]oleic acid bound to albumin as the substrate. The use of a homogenate and albumin circumvents any trafficking defect that might be imposed by LFABP ablation and tests total oxidative capacity directly. In accord with the hypothesized trafficking function of LFABP, oxidative capacity was unaffected in homogenates derived from LFABP−/− intestinal mucosa (Fig. 6D).
Oral fat tolerance test.
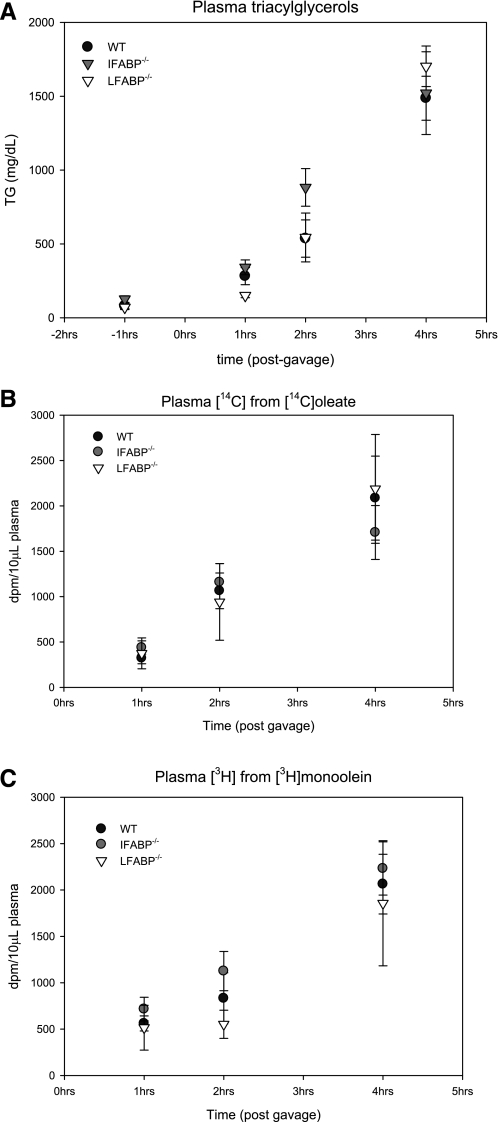
To assess the intestinal processing of a large lipid load, an oral fat tolerance test (OFTT) was performed. An intraperitoneal injection of tyloxapol was employed to block peripheral TG clearance; thus the increase in plasma TG after an oral dose reflects specifically intestinal TG secretion. Fasting plasma TG were 79.6 ± 19.0 mg/dl in WT mice, and this was unaffected by deletion of either enterocyte FABP. After an orogastric gavage of 500 μl of olive oil containing [14C]oleate and [3H]monoolein, plasma TG and radioactivities rose steadily in WT and both FABP−/− mice, although the LFABP−/− mice displayed a trend toward lower TG secretion (Fig. 7).
Fig. 7.
Oral fat tolerance test. A: plasma TG levels before and 1, 2, and 4 h after orogastric gavage of 500 μl olive oil, [14C]oleate, and [3H]monoolein. B: plasma 14C radioactivity 1, 2, and 4 h after orogastric gavage of 500 μl olive oil, [14C]oleate, and [3H]monoolein. C: plasma 3H radioactivity 1, 2, and 4 h after orogastric gavage of 500 μl olive oil, [14C]oleate, and [3H]monoolein. Data are means ± SE; n = 3 or 4/group.
Expression of membrane transporters in intestinal mucosa.
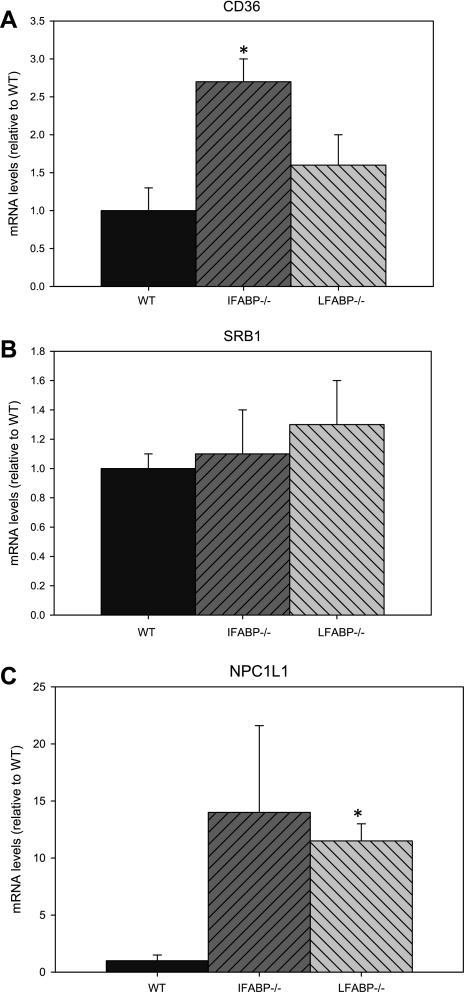
We compared the RNA expression of the putative FA transporter CD36 and sterol transporters NPC1L1 and SRB1 in intestinal mucosa from WT and the two FABP-null mice. The results showed that ablation of IFABP was associated with a significant increase in CD36 expression. Furthermore, while no changes in SRB1 expression were observed, mRNA levels of NPC1L1 were increased in both LFABP−/− and IFABP−/− mucosa; while there was large variability for the IFABP−/− samples in particular, every sample was greater than WT (range 1.6- to 10.2-fold) (Fig. 8).
Fig. 8.
Expression of CD36 (A), SRB1 (B), and NPC1L1 (C) mRNA in mouse small intestinal mucosa. Analyses were performed as described in experimental procedures. Data are means ± SE; n = 5 or 6/group. *P < 0.05 vs. WT.
DISCUSSION
The two enterocyte FABP-null models responded differently to food deprivation. Deletion of IFABP, but not LFABP, significantly enhanced fat loss during fasting. In agreement with this change in body composition, indirect calorimetry showed that the reduction in RQ upon fasting was greater for IFABP−/− mice relative to WT, reflecting a greater shift toward fat oxidation, although the RQ difference did not reach statistical significance. In contrast, food deprivation in LFABP-ablated mice did not alter fat mass relative to WT; rather in LFABP−/− mice, the loss of fat-free mass was significantly blunted. As fat-free mass is the primary driver of metabolic rate, the fasting-induced decline in energy expenditure was also found to be significantly reduced in LFABP−/− mice relative to WT. On the other hand, the absolute level of energy expenditure was comparable to WT. Although indirect calorimetry clearly detected the expected decline in RQ in fasted relative to fed LFABP−/−, it likely lacks the sensitivity to detect the change expected from the differences in fat-free mass in LFABP−/− mice compared with WT. Since it has been found that LFABP−/− livers were smaller than WT after 48 h of food deprivation (28), it is likely that the component of fat-free mass spared in fasting LFABP−/− mice in the present studies is skeletal muscle. Thus combined inhibition of the two FABPs may provide a mechanism for enhancing fat loss while sparing lean body mass during weight reduction protocols.
Because of their coexpression in absorptive enterocytes and overlap in ligand binding specificities, upregulation of LFABP or IFABP in the intestinal mucosa of IFABP−/− or LFABP−/− mice, respectively, would not have been unexpected; however, this did not occur in either case, in agreement with previous reports (22, 47). Nevertheless, the presence of another FABP in the intestinal cells of either enterocyte FABP−/− mouse could still, because of the high total FABP concentration, metabolically compensate for the loss of one FABP. It is possible that the relatively modest effects on steady-state mucosal lipid composition reflect such redundancy. There were several subtle changes in mucosal composition, however. For example, the steady-state level of FA was reduced in proximal mucosa from both IFABP- and LFABP-null mice. It is possible that the observed increase in expression of the putative apical transmembrane FA transporter CD36 (25) in IFABP-null mucosa, and the trend toward increased expression in LFABP−/−, are perhaps indicative of a compensatory mechanism for ensuring high levels of mucosal FA uptake. It was also found that MG were reduced in LFABP−/− proximal, but not distal, mucosa. Interestingly, LFABP expression is higher in proximal than distal mucosa (3), and LFABP has been shown to bind MG (Ref. 41; Lagakos et al., unpublished observations). In addition, the cholesterol content of IFABP-null mucosa was significantly reduced, and this was accompanied by increased expression of the intestinal sterol transporter NPC1L1, perhaps representing another compensatory mechanism for maintaining normal lipid absorption. Since IFABP does not itself bind cholesterol (42), it is likely that the increased NPC1L1 expression is secondary to alterations in FA levels, as decreased NPC1L1 expression by polyunsaturated FA has been observed in both cultured cells and hamster small intestine (5, 23). Studies are under way to determine whether a high-fat diet will lead to more marked alterations in mucosal lipid composition and metabolism, perhaps by exceeding the substrate binding capacity of total enterocyte FABP content.
The acute metabolic fate of lipid substrates in the enterocyte is dependent on whether they enter across the apical (AP) or basolateral (BL) plasma membrane of the cell (46). For both FA and MG, greater incorporation into TG occurs for AP- relative to BL-administered lipid and greater incorporation into PL occurs for BL- relative to AP-administered lipid (12, 44). In IFABP−/− mucosa, a significant >50% reduction in the ratio of oleate incorporated into TG relative to PL was observed for AP-administered FA, but not for BL administration. No changes in oleate incorporation into complex lipids were found in LFABP−/− mucosa, regardless of its site of cellular entry. On the basis of our previous in vitro kinetics studies using purified proteins and model membranes, we had proposed that the enterocyte FABPs could both function as intracellular transporters for FA, albeit via different mechanisms (14, 43, 45). The absence of any genotype-dependent differences in the expression of intestinal lipid synthetic genes rules out a transcriptional mechanism as causing the observed changes in lipid metabolism and supports a trafficking role for IFABP, in particular suggesting that IFABP may be involved in the trafficking of dietary-derived FA away from PL and toward TG. This is in accord with the findings of Alpers et al. (4), who showed that IFABP bound to more apically administered FA than to basolaterally administered FA, as well as those of Murphy and coworkers (35, 36), who reported that IFABP transfection in fibroblasts resulted in FA targeting to TG. A trafficking role for IFABP was also suggested by fluorescence photobleaching studies, in which the cytoplasmic diffusion rate of a fluorescent FA in IFABP-expressing cells was higher than in control cells (24).
Although FA oxidation is neither an important source of energy for enterocytes (49), accounting for only 3% of mucosal CO2 production (50), nor a quantitatively important component of total body energy expenditure (<0.1%) (10), it is nevertheless present in the enterocyte and is a regulated process. As in other tissues (48), food deprivation significantly increased oxidation of [14C]oleate to CO2 and water-soluble metabolites in WT small intestinal mucosa. The present experiments used the fasting protocol of Newberry et al. (28) in order to directly compare the effects of LFABP deletion in intestine with the effects reported in liver (27), and to determine whether IFABP has a role in this process. In LFABP−/−, but not IFABP−/− mice, intestinal FA oxidation was markedly attenuated for both dietary and bloodstream-derived FA. As the expression of genes involved in mitochondrial β-oxidation, peroxisomal FA oxidation, or electron transport, as well as PPARα, were unchanged relative to WT, and the long-chain FA oxidative capacity was unchanged in LFABP−/− intestinal mucosa homogenates in vitro, these results suggest a nontranscriptional effect of LFABP ablation, indirectly supporting our previous hypothesis that LFABP plays a role in intracellular lipid transport (14, 43, 45). Erol and colleagues (9) observed a similar phenotype in LFABP−/− livers, in which palmitate oxidation was reduced in intact hepatocytes but not liver homogenates and FA oxidation genes were unchanged. Collectively, these results imply that LFABP is involved in trafficking long-chain FA toward oxidative pathways.
We have shown that LFABP but not IFABP binds MG (Ref. 41; Lagakos et al., unpublished observations). After intraduodenal delivery of [3H]monoolein, recovery in mucosal PL and diacylglycerols was significantly increased in LFABP−/− mice, with a concomitant reduction in TG, and the resulting TG/PL was thus markedly reduced. The higher incorporation of apically administered MG into PL suggests that LFABP is involved in the transport of MG away from PL synthesis and toward TG synthesis. As noted above, the genes involved in complex lipid synthesis showed no significant genotype-induced alterations; thus the effects on MG partitioning support a trafficking function for LFABP. Similarly, the observed increase in [3H]monoolein recovery in the MG fraction was not due to decreased expression of MGL, thus suggesting a slower assimilation of newly arrived MG, likely secondary to decreased transport. In contrast to MG, no effects were found on the incorporation of FA into complex lipids in LFABP−/− mice. As discussed above, this may be due to the high expression of IFABP in the intestine, which binds FA but not MG.
While we noted a tendency toward reduced lipemic response in LFABP-null mice following an oral fat gavage, the difference from WT was not statistically significant. This is at variance with the results of Newberry et al. (29), who found that LFABP-null mice had significantly reduced lipemia in similar experiments. The mice used in that study were female, however, and as LFABP-null mice have been reported to exhibit sex dimorphism in lipid metabolism (9, 19), a sex difference could possibly account for the varying extent of the effects. Furthermore, Mansbach and coworkers (26) recently demonstrated a critical requirement for LFABP in the synthesis of prechylomicron transport vesicles, which also suggests a role for LFABP in the intestinal processing of dietary lipids.
In addition to monitoring total plasma TG levels during the OFTT, we included [14C]oleate and [3H]monoolein in the orogastric gavage. [3H]monoolein was included because it is assimilated primarily by the MGAT pathway (15, 32–34), and LFABP ablation affected intestinal metabolism of diet-derived MG. Theoretically, a defect in the glycerol-3-phosphate (G3P)-TG synthesis pathway would selectively reduce the appearance of 14C relative to 3H, whereas a defective MGAT pathway would reduce both 14C and 3H. FABP ablation had no effect on the appearance of 14C (from [14C]oleate) and 3H (from [3H]monoolein) in plasma after an oral lipid load. This was not unexpected because the reduced incorporation of [14C]oleate and [3H]monoolein into TG relative to PL in FABP-null mice was caused primarily by increased incorporation into newly synthesized PL, which are selected against for incorporation into chylomicrons and intestinal secretion after a fat load (17, 39).
This report presents the first direct comparison of LFABP- and IFABP-null mice, with studies focusing on the small intestinal mucosa where the proteins are coexpressed. The results indicate that there is overlap in the functions of IFABP and LFABP in the enterocyte; however, the present results also provide evidence supporting divergent functions for IFABP and LFABP in intestinal lipid metabolism, as well as divergent effects of IFABP and LFABP on whole body energy homeostasis. IFABP appears to transport FA from the apical pole of the enterocyte, away from PL synthesis and toward TG synthesis. Although this supports the hypothesis that IFABP functions as an intracellular FA-trafficking protein, precisely which lipid substrates are ultimately acylated by IFABP-bound FA (e.g., G3P, lysophosphatidic acid, phosphatidic acid-derived diacylglycerol, MG-derived diacylglycerol, etc.), and their end product (e.g., TG for storage, chylomicron TG, etc.) remain to be determined. IFABP, which is expressed only in intestine, appears to function in preserving fat mass during food deprivation, underscoring the emerging understanding of important connections between gut lipid metabolism and systemic energy balance (40).
LFABP appears to traffic diet-derived MG away from PL and toward TG. Additionally, LFABP directs FA, regardless of their site of entry into the intestinal cells, toward catabolic pathways. LFABP may also contribute to the loss of skeletal muscle during food deprivation. It is important to consider the effects of LFABP ablation as arising potentially from alterations in liver metabolism in addition to intestinal mucosal effects. Thus, at present, it is unknown whether the liver LFABP, the intestinal LFABP, or LFABP from both tissues, is mediating this effect. Tissue-specific knockout of LFABP will be used to address solely the contributions of enterocyte LFABP in the presence of normal levels of liver LFABP.
Given the observed effects of enterocyte FABP loss on body composition during food deprivation, with IFABP ablation resulting in increased fat loss and LFABP ablation resulting in decreased lean body mass loss, it is possible that pharmacological inhibition of both could be used to increase loss of fat mass and decrease loss of lean mass during a weight reduction regimen.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-38389 (J. Storch) and funds from the New Jersey Agricultural Experiment Station (J. Storch).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Malcolm Watford (Rutgers University) for valuable discussions.
Present address of W. S. Lagakos: Dept. of Medicine, University of California, San Diego, CA 92037.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Agellon LB, Drozdowski L, Li L, Iordache C, Luong L, Clandinin MT, Uwiera RR, Toth MJ, Thomson AB. Loss of intestinal fatty acid binding protein increases the susceptibility of male mice to high fat diet-induced fatty liver. Biochim Biophys Acta 1771: 1283–1288, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Agellon LB, Li L, Luong L, Uwiera RR. Adaptations to the loss of intestinal fatty acid binding protein in mice. Mol Cell Biochem 284: 159–166, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Agellon LB, Toth MJ, Thomson AB. Intracellular lipid binding proteins of the small intestine. Mol Cell Biochem 239: 79–82, 2002 [PubMed] [Google Scholar]
- 4. Alpers DH, Bass NM, Engle MJ, DeSchryver-Kecskemeti K. Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim Biophys Acta 1483: 352–362, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Alvaro A, Rosales R, Masana L, Vallve JC. Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids. J Nutr Biochem 21: 518–525, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7. Chon SH, Zhou YX, Dixon JL, Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem 282: 33346–33357, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Drozdowski L, Clement L, Keelan M, Niot I, Clandinin MT, Agellon L, Wild G, Besnard P, Thomson AB. Dietary lipids modify intestinal lipid-binding protein RNA abundance in diabetic and control rats. Digestion 70: 192–198, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB J 18: 347–349, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Fan YK, Croom WJ, Jr, Eisen EJ, Daniel LR, Black BL, McBride BW. Selection for growth does not affect apparent energetic efficiency of jejunal glucose uptake in mice. J Nutr 126: 2851–2860, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 12. Gangl A, Ockner RK. Intestinal metabolism of plasma free fatty acids. Intracellular compartmentation and mechanisms of control. J Clin Invest 55: 803–813, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon JI, Elshourbagy N, Lowe JB, Liao WS, Alpers DH, Taylor JM. Tissue specific expression and developmental regulation of two genes coding for rat fatty acid binding proteins. J Biol Chem 260: 1995–1998, 1985 [PubMed] [Google Scholar]
- 14. Hsu KT, Storch J. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J Biol Chem 271: 13317–13323, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Lehner R, Kuksis A. Biosynthesis of triacylglycerols. Prog Lipid Res 35: 169–201, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Luxon BA, Milliano MT. Cytoplasmic transport of fatty acids in rat enterocytes: role of binding to fatty acid-binding protein. Am J Physiol Gastrointest Liver Physiol 277: G361–G366, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Mansbach CM., 2nd The origin of chylomicron phosphatidylcholine in the rat. J Clin Invest 60: 411–420, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mansbach CM, 2nd, Dowell RF. Uptake and metabolism of circulating fatty acids by rat intestine. Am J Physiol Gastrointest Liver Physiol 263: G927–G933, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty-acid-binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J 391: 549–560, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol Gastrointest Liver Physiol 290: G36–G48, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Martin GG, Atshaves BP, McIntosh AL, Payne HR, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem 324: 101–115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem 278: 21429–21438, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mathur SN, Watt KR, Field FJ. Regulation of intestinal NPC1L1 expression by dietary fish oil and docosahexaenoic acid. J Lipid Res 48: 395–404, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L cells. Am J Physiol Gastrointest Liver Physiol 275: G244–G249, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM., 2nd Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem 282: 17974–17984, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-Fabp −/− mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest Liver Physiol 294: G307–G314, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem 278: 51664–51672, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology 44: 1191–1205, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Ockner RK, Manning JA. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J Clin Invest 54: 326–338, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ockner RK, Manning JA. Fatty acid binding protein. Role in esterification of absorbed long chain fatty acid in rat intestine. J Clin Invest 58: 632–641, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oxley A, Jutfelt F, Sundell K, Olsen RE. sn-2-Monoacylglycerol, not glycerol, is preferentially utilised for triacylglycerol and phosphatidylcholine biosynthesis in Atlantic salmon (Salmo salar L.) intestine. Comp Biochem Physiol B Biochem Mol Biol 146: 115–123, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P, Niot I. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 48: 278–287, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Phan CT, Tso P. Intestinal lipid absorption and transport. Front Biosci 6: D299–D319, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Prows DR, Murphy EJ, Moncecchi D, Schroeder F. Intestinal fatty acid-binding protein expression stimulates fibroblast fatty acid esterification. Chem Phys Lipids 84: 47–56, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-cells. Lipids 30: 907–910, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem 269: 23918–23930, 1994 [PubMed] [Google Scholar]
- 38. Richieri GV, Ogata RT, Zimmerman AW, Veerkamp JH, Kleinfeld AM. Fatty acid binding proteins from different tissues show distinct patterns of fatty acid interactions. Biochemistry 39: 7197–7204, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Scow RO, Stein Y, Stein O. Incorporation of dietary lecithin and lysolecithin into lymph chylomicrons in the rat. J Biol Chem 242: 4919–4924, 1967 [PubMed] [Google Scholar]
- 40. Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab 297: E10–E18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Storch J. Diversity of fatty acid-binding protein structure and function: studies with fluorescent ligands. Mol Cell Biochem 123: 45–53, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr 28: 73–95, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Storch J, Veerkamp JH, Hsu KT. Similar mechanisms of fatty acid transfer from human anal rodent fatty acid-binding proteins to membranes: liver, intestine, heart muscle, and adipose tissue FABPs. Mol Cell Biochem 239: 25–33, 2002 [PubMed] [Google Scholar]
- 44. Storch J, Zhou YX, Lagakos WS. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J Lipid Res 49: 1762–1769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thumser AE, Storch J. Liver and intestinal fatty acid-binding proteins obtain fatty acids from phospholipid membranes by different mechanisms. J Lipid Res 41: 647–656, 2000 [PubMed] [Google Scholar]
- 46. Trotter PJ, Storch J. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J Lipid Res 32: 293–304, 1991 [PubMed] [Google Scholar]
- 47. Vassileva G, Huwyler L, Poirier K, Agellon LB, Toth MJ. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J 14: 2040–2046, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Veerkamp JH, van Moerkerk HT. Peroxisomal fatty acid oxidation in rat and human tissues. Effect of nutritional state, clofibrate treatment and postnatal development in the rat. Biochim Biophys Acta 875: 301–310, 1986 [DOI] [PubMed] [Google Scholar]
- 49. Watford M, Lund P, Krebs HA. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J 178: 589–596, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Windmueller HG, Spaeth AE. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem 253: 69–76, 1978 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.