Abstract
Fibrosis arises as part of a would-healing response that maintains organ structure and integrity following tissue damage but also contributes to a variety of human pathologies such as liver fibrosis. Liver fibrosis is an abnormal response of the liver to persistent injury with the excessive accumulation of collagenous extracellular matrices. Currently there is no effective treatment, and many patients end up with a progressive form of the disease, eventually requiring a liver transplant. The clarification of mechanisms underlying pathogenesis of liver fibrosis and the development of effective therapy are of clinical importance. Experimental animal models, in particular targeted gene knockouts (loss of function) in mice, have become a powerful resource to address the molecular mechanisms or significance of the targeted gene in hepatic functions and diseases. This review will focus on the recent advances in knowledge obtained from genetically engineered mice that provide novel insights into the pathophysiology of liver fibrosis.
Keywords: liver fibrogenesis, knockout mice, extracellular matrix
Pathogenesis of Liver Fibrosis
Liver fibrosis is defined as an abnormal response of the liver to persistent injury, characterized by the excessive accumulation of collagenous extracellular matrices (ECMs), and therefore involves both wound healing and fibrotic processes. After an injury, parenchymal cells regenerate and replace the necrotic or apoptotic cells, which is associated with an inflammatory response and deposition of ECM. This process requires both a well-orchestrated proliferation of cells and the reconstruction of ECM. If the injury persists, the damaged tissues/organs undergo substitution by overabundant ECM and suffer from extensive, pathological fibrosis. Thus, liver fibrosis is of great clinical importance, since normal liver architecture is disrupted and liver function is ultimately impaired. There are several major causes of liver fibrosis, as described below.
Viral hepatitis fibrosis.
This is caused by chronic hepatitis B, C, or D. Chronic hepatitis C virus infection affects more than 170 million individuals and causes 300,000 deaths annually in the world from cirrhosis and hepatocellular carcinoma (80). Histologically, the liver shows lymphocyte infiltration, piecemeal necrosis of hepatocytes, and fibrosis in the periportal areas.
Alcoholic liver fibrosis.
This type is caused by acetaldehyde, an oxidized metabolite of alcohol. The incidence is positively related to the amount of alcohol consumption in Western countries. The total cases of alcoholic fibrosis in the United States are about three times higher than the number of those arising from hepatitis C. The histological features of alcohol-induced hepatic injury vary, depending on the extent and stage: steatosis (fatty change), lobular inflammation, periportal fibrosis, Mallory bodies, nuclear vacuolation, bile ductal proliferation, and fibrosis or cirrhosis (60).
Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease among children and adults in the United States (95, 100). NAFLD is a clinicopathological entity defined as a presence of hepatic steatosis in individuals who drink little or no alcohol. NAFLD represents a spectrum of liver disease ranging from bland steatosis to nonalcoholic steatohepatitis (NASH). NASH is a progressive form of liver disease that leads to advanced fibrosis. Studies have found that 26–37% of patients with NASH demonstrate progression of fibrosis over time, for periods up to 5.6 years, with up to 9% progressing to cirrhosis (1, 28, 34, 62). Histologically, NASH is characterized by macrovesicular steatosis (the fat globules vary in size from very small to nearly filling the hepatocyte) and ballooning degeneration of hepatocytes with or without Mallory bodies, with or without fibrosis (55).
Biliary fibrosis.
Medicine defines two types: primary and secondary biliary fibrosis. Primary biliary hepatic fibrosis is an autoimmune disorder in which fibrosis is caused by chronic intrahepatic bile retention. It often affects females in the age range of 40 to 60 years old. Histologically, the fibrosis appears around the microbile duct and periportal areas. Secondary biliary fibrosis occurs following the obstruction of bile ducts, which results in the progressive fibrosis in periportal areas.
Parasitic infection-mediated fibrosis.
This type is frequent in developing countries and is often caused by schistosomiasis. The principal immune response is directed against the parasite's eggs and leads to a fibrotic response, whereas the adult worm is weakly immunogenic.
Genetic Studies in Rodents
Models of experimental liver fibrosis.
Several models of experimental liver fibrosis have been established (Table 1).
Table 1.
Hepatotoxic agents for in vivo models of liver fibrosis
| Agents | Effects |
|---|---|
| Chemically induced fibrosis | |
| Carbon tetrachloride (1 ml/kg body wt injected ip 2 times weekly from 4 to 12 wk) (21) | Centrilobular hepatocyte death |
| Thioacetamide (300 mg/l of drinking water or 200 mg/kg of body wt injected ip 2 or 3 times/wk for up to 12 wk) (61) | Centrilobular hepatocyte death |
| Dimethylnitrosamine | Hemorrhagic centrilobular necrosis, destruction of sinusoidal endothelial cells leads to coagulation |
| Cholestatic fibrosis | |
| Bile duct ligation | Periportal hepatocyte death |
| Immunologically mediated fibrosis | |
| Concanavalin A (up to 20 mg/kg of body wt injected iv 1 time/wk for up to 20 wk) (106) | Centrilobular and perisinusoidal fibrosis |
| Schistosoma mansoni (exposure percutaneously to cercariae of S. mansoni) (97) | Granuloma formation and periportal fibrosis |
i.p., intraperitoneally; i.v., intravenously.
CHEMICALLY INDUCED FIBROSIS USING HEPATOTOXIC AGENTS.
Carbon tetrachloride (CCl4) is the most commonly used liver-damaging agent to induce liver fibrosis. The trichloromethyl radical, a metabolite produced by cytochrome P-450 in hepatocytes, leads to lipid peroxidation and membrane damage, which results in a reversible acute centrilobular liver necrosis.
CHOLESTATIC FIBROSIS BY BILE DUCT LIGATION.
Bile duct ligation is a convenient and well-studied experimental disease model that induces biliary fibrosis and cirrhosis such as extrahepatic biliary atresia and primary sclerosing cholangitis.
IMMUNOLOGICALLY MEDIATED FIBROSIS.
Concanavalin A is commonly used to induce immune-mediated liver fibrosis. Histologically, the induced liver fibrosis resembles human chronic hepatitis (54, 63). Schistosoma mansoni infection is another immunologically mediated fibrosis model (19).
EXPERIMENTAL LIVER FIBROSIS USING TRANSGENIC MICE.
Over the past decade, targeted gene knockouts (loss of function) in mice have become a powerful strategy to address the basis of mono- and polygenic disorders. A great advance is the targeting of stable or inducible gene disruption exclusively to the liver using liver-specific or cell type-specific gene promoters such as albumin to target hepatocytes. The use of these liver-specific promoters in the Cre-loxP system not only permits the conditional expression and silencing of genes in the liver but also makes it possible to control the temporal expression/silencing of genes by fusing Cre with a mutant estrogen receptor (Cre-ERT2) in which Cre recombinase is induced by the injection of the estrogen analog tamoxifen (44). Considerable data on candidate genes for hepatic fibrogenesis have been accumulated through targeted gene disruption in mice (Table 2).
Table 2.
Genetic factors associated with liver fibrogenesis in knockout mouse models
| Disrupted Gene and Effects | Agents to Induce Fibrosis | References |
|---|---|---|
| Increasing liver fibrogenesis | ||
| IL-6 | Carbon tetrachloride | Streetz et al. (101) |
| IL-6 | Bile duct ligation | Ezure et al. (26) |
| gp130 | Carbon tetrachloride | Streetz et al. (101) |
| gp130/STAT3 | DDC | Plum et al. (79) |
| SOCS3 | Concanavalin A | Ogata et al. (76) |
| SOCS1 (±) | Dimethylnitrosamine | Yoshida et al. (120) |
| Stat5 | Carbon tetrachloride | Hosui et al. (40) |
| Bcl-xL | Carbon tetrachloride | Takehara et al. (103) |
| IL-10 | Carbon tetrachloride | Louis et al. (64) |
| Fibroblast growth factor receptor 4 | Carbon tetrachloride | Yu et al. (123) |
| Tissue-type plasminogen activator | Carbon tetrachloride | Hsiao et al. (41) |
| Adiponectin | Carbon tetrachloride | Kamada et al. (51) |
| Telomerase RNA | Carbon tetrachloride | Rudolph et al. (85) |
| p53 | Carbon tetrachloride | Krizhanovsky et al. (56) |
| Cannabinoid receptor type 2 | Carbon tetrachloride | Julien et al. (50) |
| Angiotensin II type 2 receptor | Carbon tetrachloride | Nabeshima et al. (74) |
| Early growth response-1 | Carbon tetrachloride | Pritchard et al. (81) |
| Decreasing liver fibrogenesis | ||
| TNF-α | Bile duct ligation | Gabele et al. (29) |
| TNF receptor type 1 | Carbon tetrachloride | Sudo et al. (102) |
| TNF receptor 1 and 2 | Carbon tetrachloride | Simeonova et al. (96) |
| FasL | Bile duct ligation | Canbay et al. (17) |
| Cathepsin B | Bile duct ligation | Canbay et al. (16) |
| CHOP | Bile duct ligation | Tamaki et al. (104) |
| p21 | Bile duct ligation | Lunz III et al. (65) |
| IL-1 receptor | Thioacetamide | Gieling et al. (30) |
| IL-13 | Schistosoma mansoni infection | Kaviratne et al. (53) |
| Toll-like receptor 4 | Carbon tetrachloride/bile duct ligation/thioacetamide | Seki et al. (93) |
| CD14 | Bile duct ligation | Isayama et al. (47) |
| LPS-binding protein | Bile duct ligation | Isayama et al. (47) |
| Integrin αβ6 | Bile duct ligation | Wang et al. (112) |
| Smad3 | Dimethylnitrosamine | Latella et al. (57) |
| NADPH oxidase (p47phox) | Bile duct ligation | Bataller et al. (10) |
| Angiotensin II type 1A receptor | Carbon tetrachloride | Kanno et al. (52) |
| Bile duct ligation | Yang et al. (119) | |
| Galectin 3 | Carbon tetrachloride | Henderson et al. (36) |
| Cannabinoid receptor type 1 | Carbon tetrachloride/bile duct ligation/thioacetamide | Teixeira-Clerc et al. (105) |
| Fibroblast growth factor 1 and 2 | Carbon tetrachloride | Yu et al. (122) |
| Leptin | Carbon tetrachloride | Leclercq et al. (59) |
| Thioacetamide | Honda et al. (39) | |
| Complement 5 | Carbon tetrachloride | Hillebrandt et al. (37) |
| Tenascin C | Concanavalin A | EI-Karef et al. (25) |
| CC chemokine receptor 1, 5 | Carbon tetrachloride/bile duct ligation | Seki et al. (91) |
| CC chemokine receptor 2 | Carbon tetrachloride/bile duct ligation | Seki et al. (92) |
| CC chemokine receptor 21 | Thioacetamide | Bonacchi et al. (13) |
| Matrix metalloproteinase 12 | Schistosoma mansoni infection | Madala et al. (66) |
| Matrix metalloproteinase 13 | Bile duct ligation | Uchinami et al. (107) |
| Plasminogen activator inhibitor | Bile duct ligation | Wang et al. (113) |
| No effects | ||
| TNF receptor type 2 | Carbon tetrachloride | Sudo et al. (102) |
| TIMP1, TIMP2 | Schistosoma mansoni infection | Vaillant et al. (109) |
IL, interleukin; gp, glycoprotein; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor; CHOP, CCAAT/enhancer-binding protein (C/EBP) homologous protein; LPS, lipopolysaccharide; NADPH, nicotinamide adenine dinucleotidephosphate; TIMP, tissue inhibitor of matrix metalloproteinase.
Target signaling cascades/molecules for liver fibrosis.
HEPATOCYTE APOPTOSIS/NECROSIS.
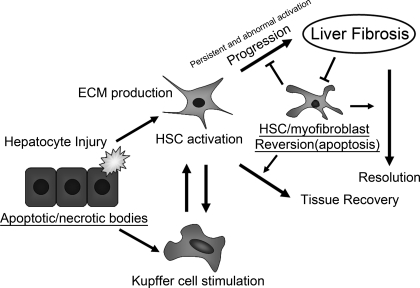
Hepatocytes are damaged by hepatotoxic reagents, including alcohol, bile acids, and viral infection. Upon tissue damage, injured hepatocytes release reactive oxygen species (ROS) and fibrogenic mediators, which induce recruitment of white blood cells. Damaged hepatocytes that undergo apoptosis can be phagocytosed by macrophages and Kupffer cells, which stimulates the fibrogenic actions of liver myofibroblasts (15). An interesting observation is that DNA from apoptotic hepatocytes can act as a mediator of hepatic stellate cell (HSC) differentiation and inhibits platelet-derived growth factor-mediated chemotaxis via Toll-like receptor 9 in vitro, suggesting that such an apoptotic hepatocyte DNA provides a stop signal to retain HSCs at sites of cellular apoptosis (114). Activation of two alternative mechanisms can result in hepatocyte apoptosis: a death receptor-mediated pathway or an intrinsic organelle-dependent pathway (27). The hepatocyte damage mediated by a death receptor is a common mechanism for apoptosis, and it requires activation of Fas/CD95 or tumor necrosis factor (TNF) receptor-1. Fas-deficient mice show decreased fibrogenesis after bile duct ligation (17). In contrast, hepatocyte-specific Bcl-xL (antiapoptotic member of the Bcl-2 family)-deficient mice show spontaneous and continuous apoptosis in hepatocytes and develop liver fibrosis at an advanced age (103). The intrinsic organelle-dependent pathway is caused by lysosomal permeabilization and the release of cathepsin B into the cytoplasm, which causes mitochondrial damage and subsequent hepatocyte death. Indeed, in cathepsin B-null mice, hepatocyte apoptosis, liver damage, and liver fibrosis are attenuated after bile duct ligation (16). CCAAT/enhancer-binding protein homologous protein (CHOP), also known as growth arrest- and DNA damage-inducible gene 153, is a transcriptional regulator induced by endoplasmic reticulum (ER) stress and is a key factor in the ER stress-mediated apoptotic pathway. CHOP-deficient mice have been shown to be resistant to apoptosis in various disease models. In the cholestasis-induced liver fibrosis model, CHOP-null mice show reduced hepatocyte cell death and subsequent liver fibrogenesis (104). Thus, apoptotic signals in the injured liver play an important role in the progression of liver fibrosis (Fig. 1).
Fig. 1.
A proposed model showing how apoptosis in different cell types interacts with the inflammatory process and the progression of liver fibrosis. Proapoptotic stimuli and/or hepatotoxic reagents induce hepatocyte apoptosis/necrosis. Whereas proapoptotic signals (e.g., Fas/CD95, tumor necrosis factor-α, cathepsin B, CCAAT/enhancer-binding protein homologous protein, reactive oxygen species, etc.) enhance hepatocyte damage, antiapoptotic signals (e.g., Bcl-xL) attenuate its damage. These apoptotic/necrotic cells stimulate hepatic stellar cells (HSCs) and Kupffer cells. HSCs undergo activation, transdifferentiate into myofibroblast-like cells, and produce extracellular matrix (ECM) for reconstruction of damaged tissues. Stimulated Kupffer cells and activated HSCs produce profibrogenic cytokines (e.g., transforming growth factor-β) that, in turn, upregulate ECM production in activated HSCs. If injury ceases, the activated HSCs revert to a quiescent state (e.g., apoptosis). If injury persists, the activated HSCs and/or myofibroblasts continuously produce ECM, and a stiffer ECM further promotes ECM production in these cells. The upregulated expression of tissue inhibitor of matrix metalloproteinase-1 inhibits matrix metalloproteinase-mediated ECM degradation and renders activated HSCs and/or myofibroblasts relatively resistant to apoptotic stimuli. Such a cycling results in the progression of liver fibrosis.
PROFIBROGENIC GROWTH FACTORS.
Transforming growth factor-β (TGF-β) regulates a wide variety of cellular processes, including apoptosis of hepatocytes, activation and recruitment of inflammatory cells into injured liver, and transdifferentiation of some liver-resident cells, such as quiescent HSCs, into collagen-producing myofibroblasts (6, 88). Three TGF-β isoforms (β1, β2, and β3) have been identified, but only TGF-β1 is linked to liver fibrogenesis (35). Investigators studying conditional TGF-β1 transgenic mice using the tetracycline regulatory system (5, 31) have achieved elegant observations: although the overexpression of TGF-β1 spontaneously induces HSC activation and liver fibrosis without any treatments, the switching off of TGF-β production in the advanced stages of liver fibrosis results in the reversal of fibrosis (108).
TGF-β is secreted in a biologically inactive (latent) form in a complex with TGF-β latency-associated protein and latent TGF-β-binding proteins. The tissue concentration of these latent complexes is maintained at a constant level (84). In response to injury, local latent TGF-β complexes are converted into active TGF-β. There are several mechanisms for activation, such as via chaotropic agents, proteases, integrins (αvβ6, αvβ8), and thrombospondin-1, all of which are likely to be tissue specific (3, 42). The expression level of integrin αvβ6 is specifically increased on cholangioepithelial cells after bile duct ligation, and, in integrin β6-null mice, periductal fibrogenesis is attenuated with decreased TGF-β signaling (112).
In TGF-β superfamily mediated signaling, all TGF-β ligands bind to cell surface TGF-β receptors. Upon ligand binding, the activated TGF-β receptor phosphorylates the transcription factor Smads, which then translocate to the nucleus to regulate gene expression (69, 72). Smad3-null mice exhibit reduced liver fibrosis with reduced myofibroblast activation and ECM production in response to liver injury. Moreover, the disruption of Smad7, an inhibitory Smad that downregulates TGF-β signaling, results in an enhancement of liver damage and fibrogenesis after chronic injury by CCl4 (33). Furthermore, the hepatocyte-specific overexpression of Smad7 attenuates TGF-β-mediated Smad signaling, which thereby reduces TGF-β-dependent epithelial-mesenchymal transition of hepatocytes and fibrogenesis after chronic injury induced by CCl4 (24).
It has been demonstrated that the persistent activation of epidermal growth factor receptor (EGFR) causes the pathogenesis of tissue fibrosis in several organs, including lung and kidney (58, 67). Activation of the EGFR conveys survival signals to hepatocytes. Amphiregulin, one of the EGFR ligands, is specifically induced upon liver injury and stimulates cell proliferation and inhibits apoptosis of hepatic myofibroblasts and HSCs in vitro. Amphiregulin-null mice exhibit a remarkably reduced fibrosis compared with wild-type controls after chronic liver injury induced by CCl4, which was associated with reduced expression of tissue inhibitor of matrix metalloproteinase (TIMP)-1 and connective tissue growth factor (78). These results indicate a novel role of the EGFR system in hepatic fibrogenesis and suggest that the amphiregulin/EGFR signaling system could be a new therapeutic target for liver fibrosis.
RENIN-ANGIOTENSIN SYSTEM AND NADPH.
The renin-angiotensin system, an important factor in regulating the blood pressure and body fluid homeostasis, is involved in liver fibrogenesis. Among vasoactive cytokines, angiotensin II is likely to play a major role in liver fibrogenesis. Angiotensin II is an effector peptide in the renin-angiotensin system and a major regulator for arterial pressure homeostasis in humans. The key components of this system, angiotensinogen, renin, and angiotensin-converting enzyme, are expressed in response to chronic liver damage, and angiotensin II is produced by activated HSCs (9, 77). Angiotensin II induces hepatic secretion of proinflammatory cytokines and stimulates cellular proliferation, migration, and an array of fibrogenic actions such as collagen synthesis in activated HSCs (7–8, 10). Angiotensin II receptors are pharmacologically differentiated into two distinct types, type 1 and type 2. The disruption of the angiotensin II type 1A receptor reduces the hepatic inflammation and fibrosis after CCl4 treatments or bile duct ligation (52, 119). On the other hand, the disruption of the angiotensin II type 2 receptor exacerbates the liver damage and fibrosis after CCl4 treatments, which is accompanied by increased ROS production (74). These findings indicate that liver fibrogenesis associated with the renin-angiotensin system is determined by the balance between these two types of angiotensin receptor. The profibrogenic effect of angiotensin II is largely mediated by ROS generated by a nonphagocytic form of nicotinamide adenine dinucleotidephosphate (NADPH) oxidase, which plays a pivotal role in liver fibrogenesis (22). A functionally active form of the NADPH oxidase is expressed not only in phagocytic cell types such as Kupffer cells but also in nonphagocytic cell types such as HSCs. Disruption of the NADPH subunit p47phox markedly attenuates both angiotensin II-mediated liver injury and fibrosis after bile duct ligation, which is accompanied by reduced expression of the profibrogenic cytokine TGF-β and activated HSC marker α-smooth muscle cell actin (10). Thus, the nonphagocytic form of NADPH oxidase functions as an important mediator of angiotensin II on HSC activation and following liver fibrogenesis.
IMMUNE RESPONSE AND INFLAMMATORY CYTOKINES.
Inflammation is an important element in the initiation and progression of liver fibrosis. TNF is a pleiotropic inflammatory cytokine and is involved in cell death/apoptosis (4). Increased levels of TNF are observed in the liver after acute injury induced by CCl4 treatment (117). TNF-α can activate HSCs in vitro through stress-activated protein kinase pathways (via p38 mitogen-activated protein kinase) (83). The disruption of TNF-α attenuates liver fibrogenesis after bile duct ligation (29). Furthermore, disruption of the TNF type 1 receptor also attenuates liver fibrogenesis after CCl4 treatments (102). Thus, TNF-α acts as a profibrogenic cytokine during the progression of liver fibrosis.
After partial hepatectomy, IL-6/glycoprotein 130 (gp130) signaling promotes hepatocyte proliferation like TNF-α does. In contrast, IL-6/gp130 and TNF-α show opposite effects on liver fibrogenesis. The disruption of IL-6 induces more advanced biliary fibrosis than control livers after bile duct ligation with less phosphorylation of the signal transducer and activator of transcription 3 (STAT3) and hepatocyte proliferation (26). There is an interesting observation using a conditional knockout model for gp130: while the knockout of gp130 from hepatocytes does not affect liver fibrogenesis during chronic injury induced by CCl4, its knockout in all cell types in the liver results in more advanced fibrosis. These findings indicate that the IL-6/gp130-dependent pathway in nonparenchymal cells of the liver plays a protective role in the pathogenesis of liver fibrosis after liver injury by CCl4 treatments (101). In a sclerosing cholangitis model using 3,5-diethoxycarbonyl-1,4-dihydrocollidine, the livers of hepatic gp130-knockout mice (gp130Δhepa) and hepatic gp130-knockin mice expressing a truncated gp130-knockin allele that lacks the STAT1 and -3 activation regions (gp130ΔhepaSTAT) show a high level of TNF-α expression, more hepatocyte apoptosis, enhanced HSC activation, and advanced liver fibrosis than control livers. Interestingly, mice lacking hepatic gp130 and STAT signaling show increased and earlier mortality (79). Thus, IL-6/gp130/STAT3 signaling is likely to play a protective role in liver fibrogenesis.
Toll-like receptors (TLR), a component of the innate immune system, comprise a highly conserved family of receptors that recognize pathogen-associated molecular patterns and thus allow the host to detect microbial infection. TLR4 acts as a receptor for lipopolysaccharide (LPS), which is a cell wall component of Gram-negative bacteria and is the strongest known inducer of inflammation. Several reports suggest that gut-derived LPS is an important mediator of hepatic fibrogenesis (47, 93). The levels of plasma LPS increase in response to liver injury induced by CCl4, bile duct ligation, or thioacetamide, and pretreatment with a cocktail of nonabsorbable broad-spectrum antibodies inhibits such an increase (82, 93). Kupffer cells are among the first cells in the liver to be hit by gut-derived toxins and are involved in the uptake of LPS (90, 94). CD14 is a LPS receptor and is a 55-kDa glycosyl-phosphatidylinositol-anchored glycoprotein constitutively expressed on the surface of mature monocytes, macrophages, and neutrophils. The disruption of CD14 attenuates liver fibrogenesis after bile duct ligation with decreased macrophage/monocyte infiltration and α-smooth muscle actin expression, as a marker for activated HSCs (47). There is an important observation showing the association between innate immune responses mediated by TLR4 and TGF-β-driven fibrogenesis via activated HSCs. Quiescent HSCs express high levels of TLR4 and are highly sensitive to LPS. Activation of TLR4 signaling by LPS downregulates the TGF-β pseudoreceptor, bone morphogenic protein, and the activin membrane-bound inhibitor (Bambi) via the MyD88-NF-κB pathway on quiescent HSCs, which thereby augments TGF-β-mediated HSC activation and collagen production and enhances hepatic fibrogenesis (93).
Chemokines are potential leukocyte chemoattractants that cooperate with profibrotic cytokines such as IL-13 and TGF-β in the development of fibrosis by recruiting macrophages and other effector cells. Chemokines and their receptors are upregulated in response to liver injury (2, 13, 68). The CC-chemokine family has been shown to play an important role in hepatic fibrogenesis. In vitro evidence suggests that three CC chemokine members [regulated upon activation, normal T-cell expressed and secreted, monocyte chemoattractant protein (MCP)-1, and CC chemokine ligand 21] promote proliferation and migration of HSCs (89). The expression levels of several chemokines such as MCP-1, macrophage inflammatory protein-2, and IL-8/CINC and their receptors, including CXC chemokine receptor 3, CC chemokine receptor (CCR) 5, and CCR7, are upregulated in HSCs during their activating process (13–14, 89). The disruption of CCR1 and/or CCR5 reduces liver fibrogenesis and macrophage infiltration after chronic liver injury induced by CCl4 or bile duct ligation (91). Interestingly, while CCR1 mediates profibrogenic effects in bone marrow-derived cells (primarily Kupffer cells), CCR5 mediates profibrogenic effects in resident liver cells (mainly HSCs). Another chemokine, CCR2, functions to recruit macrophages/Kupffer cells to the site of inflammation and is highly expressed both in HSCs and Kupffer cells in fibrotic liver. The disruption of CCR2 attenuates liver fibrogenesis with the decreased number of activated HSCs after chronic injury induced by CCl4 or bile duct ligation (92). Thus CC-chemokines play an important role in pathogenesis of liver fibrosis.
Chronic hepatitis C virus infection is characterized by relentless activation of the complement system (111). In response to injury, hepatic complement 5 (C5) levels increase and C5 receptor 1 expression occurs in various hepatic cell types, including HSCs (37, 87). Recently, the gene Hc (encoding complement factor C5) has been identified as a quantitative trait gene that modifies liver fibrogenesis by experimental intercrosses between fibrosis-susceptible BALB/cJ and fibrosis-resistant A/L inbred strains (37). Hc−/− strains show less liver fibrosis than Hc+/+ strains after treatment with CCl4. The intravenous administration of the C5 receptor 1 antagonist reduces liver fibrogenesis in Hc+/+ BALB/cJ mice but not Hc-null A/L mice (37).
ENDOGENOUS OPIOIDS.
Endogenous opioids such as cannabinoids have a profibrogenic activity (23, 105). The increased expression of opioid receptors and met-enkephalin, which is an endogenous opioid neurotransmitter/neuromodulator with agonist activity to μ and δ opioid receptors, is observed in the injured liver (23, 73). The activation of opioid receptors results in the increased proliferation and collagen production of activated HSCs in vitro. Indeed, the opioid antagonist naloxone reduces the expression of the activated HSC marker α-smooth muscle cell actin and collagen deposition of HSCs in dimethylnitrosamine-treated rats (23). Cannabinoids are the active component of marijuana and act via two G protein-coupled receptors, cannabinoid receptor type 1 (CB1) and type 2 (CB2). The CB1 receptor is highly induced in myofibroblastic cells of the cirrhotic liver, whereas the receptor is only faintly expressed in quiescent HSCs. The liver lacking CB1 receptor or treated with CB1 receptor antagonist SR141716A inhibits the progression of fibrosis in chronic liver injury with decreased hepatic content of profibrogenic cytokine TGF-β1 (105). The CB2 receptor is also highly upregulated in myofibroblasts of human cirrhotic liver, as well as in cultured HSCs and hepatic myofibroblasts. Activation of the CB2 pathway by Δ9-tetrahydrocannabinoid induces growth inhibition and apoptosis of myofibroblasts and HSCs in vitro, and, indeed, CB2 receptor-null mice show enhanced liver fibrogenesis in chronic liver injury induced by CCl4 (50). Therefore, in contrast to CB1 signaling, the CB2 pathway has an anti-fibrotic role in chronic liver injury. Thus, it would be attractive to pursue combined anti-fibrotic approaches such as the use of CB1 receptor antagonists with CB2 receptor agonists.
CELLULAR SENESCENCE.
Cellular senescence, an important mechanism of tumor suppression, functions by blocking the proliferation of damaged cells at risk of malignant transformation. A recent study has documented that cellular senescence acts to prevent the accumulation of ECM in chronic liver fibrosis (56): the senescent cells in fibrogenic liver are derived primarily from activated HSCs, and they reduce secretion of ECMs and enhance secretion of ECM-degrading enzymes. Furthermore, natural killer cells preferentially kill senescent activated HSCs and facilitate the resolution of fibrosis. However, in mice lacking key senescence regulators (p53 and/or INK4a/ARF), activated HSCs continue to proliferate, leading to excessive liver fibrosis. Thus, cellular senescence plays a part in the pathogenesis of liver fibrosis as a self-defense mechanism. Another study shows that telomere shortening contributes to the pathogenesis of liver cirrhosis (85): telomerase-deficient mice, null for the essential telomerase RNA (mTR) gene, exhibit an accelerating development of liver cirrhosis after chronic injury by CCl4 treatments. Furthermore, adenoviral delivery of the mTR gene into cirrhotic mTR-null mice alleviates cirrhotic pathology and improves liver function. Interestingly, significant telomere shortening is observed in hepatocytes but not HSCs in human cirrhotic liver, and this condition is correlated with cellular senescence in hepatocytes (116). These findings indicate that telomerase therapy could be beneficial for liver fibrosis.
Roles of ECM in liver fibrosis.
FIBRONECTIN AND PROVISIONAL MATRIX.
An essential response to wound healing is the reorganization of ECMs, which provide strength and temporary structure to damaged tissue. A paradigm of adult liver ECM remodeling during wound healing is that, like other tissues, the initial “provisional matrix” formation between plasma type fibronectin and fibrinogen stabilizes wounded areas, which acts as a nidus for subsequent collagen fibrillogenesis (20). Fibronectin is a large dimeric glycoprotein that exists in blood plasma in its soluble form and in its insoluble form as a part of the ECM of almost every tissue in an organism. Plasma fibronectin is produced solely by hepatocytes in the liver (43, 71). A prominent expression of fibronectin is observed during tissue repair. Because experimental evidence has documented that skin wounds heal normally in mice lacking plasma-type fibronectin (86), an absolute requirement for fibronectin and the role of fibronectin in response to adult tissue damage has been speculative. To define the functional identity of fibronectin in the initial stage of adult tissue remodeling, we recently established a null condition for both fibronectin isoforms (plasma and cellular types) from adult mouse liver. We have uncovered that, although it has been postulated that ECM organization and assembly depends on the fibronectin matrix in culture (98–99, 110), the lack of both fibronectin isoforms does not actually interfere with reconstruction and resolution of collagen fibril organization after liver injury induced by CCl4. Furthermore, TGF-β-signaling and type V collagen were identified as essential elements for collagen fibrillogenesis during remodeling of adult liver tissue (70). Thus, our results have wiped out the long-standing concept that collagen fibril organization requires the prior assembly of fibronectin matrix (98–99, 110) and the further interpretation that fibronectin matrix is probably serving as a scaffold for collagen fibril organization. Fibronectin scaffold is not always essential for tissue remodeling, and a certain cell type can assemble collagen fibril networks in the complete absence of fibronectin in vivo.
COLLAGENS AND MATRIX METALLOPROTEINASES.
Fibrillar collagens (especially type I and III) and elastin are the most abundant ECM components. The amount of type I collagen in scar tissues is significantly increased compared with normal tissues (32): the quantity of ECMs in fibrotic liver can be up to eightfold higher than that of normal liver. Fibrosis reflects a balance between production and degradation of ECM. Matrix metalloproteinases (MMPs) and their specific inhibitors (TIMPs) play a pivotal role in both fibrogenesis and fibrolysis. MMPs are a family of zinc-dependent enzymes and comprise collagenases, gelatinases, stromelysins, and membrane-type MMPs based on their substrates (11, 75). MMPs are secreted from cells into the extracellular space as proenzymes, which are then activated by a number of specific, usually cell surface-associated, cleavage mechanisms. ECM degradation is mediated by interstitial collagenase, and MMP-1 and -13 (in humans) or MMP-13 (in rodents) are the main proteases that can degrade type I collagen. In chronic injury, the hepatic expression of MMP-13 mRNA is increased during the development of fibrosis but is decreased once the fibrosis becomes prominent. On the other hand, its expression level is increased in the recovery phase of liver fibrosis (115, 118). After bile duct ligation, MMP-13 mRNA is upregulated in isolated HSCs (107). Unexpectedly, MMP-13-null mouse liver attenuates cholestasis-induced liver fibrosis after bile duct ligation, accompanied by significantly decreased expression of the inflammatory mediator TNF-α and the fibrogenic cytokine TGF-β1. Thus, despite the known function of MMPs in degrading the ECM in liver fibrosis, MMP-13 can contribute to the cholestatic liver fibrogenesis by modulating the initial inflammation (107).
MMP activities are inhibited by a family of TIMP (TIMP-1 to -4). The expression of TIMP-1 in the liver is markedly upregulated in both the murine fibrosis model as CCl4 treatments or bile duct ligation and humans fibrotic liver (12, 45). TIMP-1 has an antiapoptotic effect in activated HSCs in culture, and the overexpression of TIMP-1 in the mouse liver shows significantly attenuated spontaneous resolution of chronic liver fibrosis induced by CCl4 (121). MMP-1 and -8 and stromelysin (MMP-3) are activated by the serine protease plasmin, which is generated from circulating plasminogen by urokinase plasminogen activator (uPA) or tissue plasminogen activator (tPA) (46). The conversion from plasminogen to plasmin is inhibited by plasminogen activator inhibitor-1 (PAI-1). The disruption of uPA attenuates liver fibrogenesis after chronic injury induced by CCl4 with increased TIMP1 expression (41). The disruption of PAI-1 also attenuates liver fibrosis after bile duct ligation accompanying increased tPA but not uPA activity (113). Therefore, a balance of ECM production and degradation by MMPs and TIMPs plays a pivotal role in liver fibrogenesis.
ECM stiffness and liver fibrosis.
Accumulating evidence shows that ECM stiffness is an important indicator for determining HSC behaviors (38). Continuous ECM stiffness induces transdifferentiation and maturation of myofibroblastic phenotypes in activated HSCs. If such a stiffer ECM persists, it leads to higher myofibroblast contraction and ECM secretion, which leads to further ECM stiffening. Consequently, these stiff ECMs dramatically impede organ architecture and function. Furthermore, these stiff ECMs have a correlation to the reversion of activated HSCs. In mouse liver expressing mutated type I collagen, which confers resistance to collagenase digestion, apoptosis of activated HSCs decreased during the recovery period following liver injury (49). As predicted from this study, the degradation of ECM promotes apoptosis in activated HSCs (48). Clinically, new diagnostic modalities such as transient elastography suggest a positive correlation between the stiffness of liver and the fibrosis stage and could be useful tools to monitor fibrosis progression and regression as a noninvasive evaluation of liver fibrosis (18).
Future Directions
Gene knockout technology has led to many breakthroughs in liver fibrogenesis research. In contrast, if and how each phenotype in these experimental animal models reflects aspects of human liver fibrogenesis is still poorly understood. The functional links of each target molecule during hepatic fibrogenesis need to be clarified. To date, many studies using gene knockout mice have focused on the progression of liver fibrogenesis. Indeed, most antifibrotic approaches have focused on myofibroblasts, including HSCs, since the key pathogenic event in liver fibrosis is the activation of these cell types. Relatively few studies have focused on the resolution of liver fibrosis. The pertinent questions in this regard are whether liver fibrosis can completely regress to normal liver and whether all types of liver fibrosis achieve this regression to normal by the same molecular mechanisms. Indeed, in contrast to the traditional view that liver cirrhosis is an irreversible disease, accumulating evidence indicates that even advanced fibrosis is reversible. In animal models of liver injury, regression of fibrosis has been well documented after the cessation of liver damage. Although emerging antifibrotic interventions have a therapeutic effect in experimental animal models of liver fibrosis, their efficacy and safety in humans remains to be elucidated in the clinical setting. Nevertheless, the translation of basic antifibrotic research into improved therapeutic approaches is an essential step in the management of patients with chronic liver diseases, having a significant beneficial public health impact.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Health Grant R01 DK-074538 (to T. Sakai).
DISCLOSURES
No conflict of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We acknowledge many outstanding contributions of investigators in the field whose work could not be cited because of space constraints.
REFERENCES
- 1. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 42: 132–138, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Afford SC, Fisher NC, Neil DA, Fear J, Brun P, Hubscher SG, Adams DH. Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. J Pathol 186: 82–89, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 116: 217–224, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene 12: 1–9, 1996 [PubMed] [Google Scholar]
- 5. Baron U, Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol 327: 401–421, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 115: 209–218, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bataller R, Gabele E, Schoonhoven R, Morris T, Lehnert M, Yang L, Brenner DA, Rippe RA. Prolonged infusion of angiotensin II into normal rats induces stellate cell activation and proinflammatory events in liver. Am J Physiol Gastrointest Liver Physiol 285: G642–G651, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bataller R, Gines P, Nicolas JM, Gorbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodes J. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology 118: 1149–1156, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Bataller R, Sancho-Bru P, Gines P, Lora JM, Al-Garawi A, Sole M, Colmenero J, Nicolas JM, Jimenez W, Weich N, Gutierrez-Ramos JC, Arroyo V, Rodes J. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 125: 117–125, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Schoonhoven R, Hagedorn CH, Lemasters JJ, Brenner DA. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 112: 1383–1394, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis 21: 373–384, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 110: 821–831, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, Cosmi L, Romagnani P, Milani S, Failli P, Batignani G, Liotta F, Laffi G, Pinzani M, Gentilini P, Marra F. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology 125: 1060–1076, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M, Gentilini P, Marra F. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem 276: 9945–9954, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology 39: 273–278, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, Gores GJ. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest 112: 152–159, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology 123: 1323–1330, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 48: 835–847, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Cheever AW, Williams ME, Wynn TA, Finkelman FD, Seder RA, Cox TM, Hieny S, Caspar P, Sher A. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol 153: 753–759, 1994 [PubMed] [Google Scholar]
- 20. Clark RAF. The Molecular and Cellular Biology of Wound Repair. New York, NY: Plenum, 1996 [Google Scholar]
- 21. Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med 117: 237–250, 2005 [DOI] [PubMed] [Google Scholar]
- 22. De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys 462: 266–272, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Minicis S, Candelaresi C, Marzioni M, Saccomano S, Roskams T, Casini A, Risaliti A, Salzano R, Cautero N, di Francesco F, Benedetti A, Svegliati-Baroni G. Role of endogenous opioids in modulating HSC activity in vitro and liver fibrosis in vivo. Gut 57: 352–364, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology 135: 642–659, 2008 [DOI] [PubMed] [Google Scholar]
- 25. El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol 211: 86–94, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ezure T, Sakamoto T, Tsuji H, Lunz JG, 3rd, Murase N, Fung JJ, Demetris AJ. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am J Pathol 156: 1627–1639, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Faouzi S, Burckhardt BE, Hanson JC, Campe CB, Schrum LW, Rippe RA, Maher JJ. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem 276: 49077–49082, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 40: 820–826, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Gabele E, Froh M, Arteel GE, Uesugi T, Hellerbrand C, Scholmerich J, Brenner DA, Thurman RG, Rippe RA. TNFalpha is required for cholestasis-induced liver fibrosis in the mouse. Biochem Biophys Res Commun 378: 348–353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol 296: G1324–G1331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89: 5547–5551, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med 10: 76–99, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamzavi J, Ehnert S, Godoy P, Ciuclan L, Weng H, Mertens PR, Heuchel R, Dooley S. Disruption of the Smad7 gene enhances CCI4-dependent liver damage and fibrogenesis in mice. J Cell Mol Med 12: 2130–2144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol 98: 2042–2047, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol 30: 77–87, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 103: 5060–5065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, Schirin-Sokhan R, Wilkens G, Geier A, Lorenzen J, Kohl J, Gressner AM, Matern S, Lammert F. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet 37: 835–843, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Honda H, Ikejima K, Hirose M, Yoshikawa M, Lang T, Enomoto N, Kitamura T, Takei Y, Sato N. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology 36: 12–21, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-β and STAT3 activation. J Exp Med 206: 819–831, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsiao Y, Zou T, Ling CC, Hu H, Tao XM, Song HY. Disruption of tissue-type plasminogen activator gene in mice aggravated liver fibrosis. J Gastroenterol Hepatol 23: e258–e264, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hynes RO. Fibronectins. New York: Springer-Verlag, 1990 [Google Scholar]
- 44. Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res 27: 4324–4327, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 24: 176–184, 1996 [DOI] [PubMed] [Google Scholar]
- 46. Irigoyen JP, Munoz-Canoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci 56: 104–132, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, McKim SE, Parsons C, Rippe RA, Wheeler MD. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol 290: G1318–G1328, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126: 1795–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Issa R, Zhou X, Trim N, Millward-Sadler H, Krane S, Benyon C, Iredale J. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J 17: 47–49, 2003 [DOI] [PubMed] [Google Scholar]
- 50. Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128: 742–755, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology 125: 1796–1807, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Kanno K, Tazuma S, Chayama K. AT1A-deficient mice show less severe progression of liver fibrosis induced by CCl(4). Biochem Biophys Res Commun 308: 177–183, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol 173: 4020–4029, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, Muto Y, Moriwaki H. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol 11: 1491–1500, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Latella G, Vetuschi A, Sferra R, Catitti V, D'Angelo A, Zanninelli G, Flanders KC, Gaudio E. Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int 29: 997–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol 37: 206–213, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Lefkowitch JH. Morphology of alcoholic liver disease. Clin Liver Dis 9: 37–53, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol 36: 488–493, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 39: 770–778, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Louis H, Le Moine A, Quertinmont E, Peny MO, Geerts A, Goldman M, Le Moine O, Deviere J. Repeated concanavalin A challenge in mice induces an interleukin 10-producing phenotype and liver fibrosis. Hepatology 31: 381–390, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A, Deviere J. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology 28: 1607–1615, 1998 [DOI] [PubMed] [Google Scholar]
- 65. Lunz JG, 3rd, Tsuji H, Nozaki I, Murase N, Demetris AJ. An inhibitor of cyclin-dependent kinase, stress-induced p21Waf-1/Cip-1, mediates hepatocyte mito-inhibition during the evolution of cirrhosis. Hepatology 41: 1262–1271, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Madala SK, Pesce JT, Ramalingam TR, Wilson MS, Minnicozzi S, Cheever AW, Thompson RW, Mentink-Kane MM, Wynn TA. Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J Immunol 184: 3955–3963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Madtes DK, Elston AL, Hackman RC, Dunn AR, Clark JG. Transforming growth factor-alpha deficiency reduces pulmonary fibrosis in transgenic mice. Am J Respir Cell Mol Biol 20: 924–934, 1999 [DOI] [PubMed] [Google Scholar]
- 68. Marra F, DeFranco R, Grappone C, Milani S, Pastacaldi S, Pinzani M, Romanelli RG, Laffi G, Gentilini P. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol 152: 423–430, 1998 [PMC free article] [PubMed] [Google Scholar]
- 69. Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev 19: 2783–2810, 2005 [DOI] [PubMed] [Google Scholar]
- 70. Moriya K, Bae E, Honda K, Sakai K, Sakaguchi T, Tsujimoto I, Kamisoyama H, Keene DR, Sasaki T, Sakai T. A fibronectin-independent mechanism of collagen fibrillogenesis in adult liver remodeling.. Gastroenterology In press. DOI: 10.1053/j.gastro.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mosher DF. Fibronectin. San Diego, CA: Academic, 1989 [Google Scholar]
- 72. Moustakas A, Heldin CH. The regulation of TGFβ signal transduction. Development 136: 3699–3714, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Munoz-Luque J, Ros J, Fernandez-Varo G, Tugues S, Morales-Ruiz M, Alvarez CE, Friedman SL, Arroyo V, Jimenez W. Regression of fibrosis after chronic stimulation of cannabinoid CB2 receptor in cirrhotic rats. J Pharmacol Exp Ther 324: 475–483, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nabeshima Y, Tazuma S, Kanno K, Hyogo H, Iwai M, Horiuchi M, Chayama K. Anti-fibrogenic function of angiotensin II type 2 receptor in CCl4-induced liver fibrosis. Biochem Biophys Res Commun 346: 658–664, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999 [DOI] [PubMed] [Google Scholar]
- 76. Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A. Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene 25: 2520–2530, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology 123: 1667–1676, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Castillo J, Goni S, Vespasiani-Gentilucci U, Zagami MG, Lotersztajn S, Prieto J, Berasain C, Avila MA. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology 48: 1251–1261, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Plum W, Tschaharganeh DF, Kroy DC, Corsten E, Erschfeld S, Dierssen U, Wasmuth H, Trautwein C, Streetz KL. Lack of glycoprotein 130/signal transducer and activator of transcription 3-mediated signaling in hepatocytes enhances chronic liver injury and fibrosis progression in a model of sclerosing cholangitis. Am J Pathol 176: 2236–2246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet 362: 2095–2100, 2003 [DOI] [PubMed] [Google Scholar]
- 81. Pritchard MT, Nagy LE. Hepatic fibrosis is enhanced and accompanied by robust oval cell activation after chronic carbon tetrachloride administration to Egr-1-deficient mice. Am J Pathol 176: 2743–2752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004 [DOI] [PubMed] [Google Scholar]
- 83. Reeves HL, Dack CL, Peak M, Burt AD, Day CP. Stress-activated protein kinases in the activation of rat hepatic stellate cells in culture. J Hepatol 32: 465–472, 2000 [DOI] [PubMed] [Google Scholar]
- 84. Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem 280: 7409–7412, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287: 1253–1258, 2000 [DOI] [PubMed] [Google Scholar]
- 86. Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fassler R. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med 7: 324–330, 2001 [DOI] [PubMed] [Google Scholar]
- 87. Schlaf G, Schmitz M, Rothermel E, Jungermann K, Schieferdecker HL, Gotze O. Expression and induction of anaphylatoxin C5a receptors in the rat liver. Histol Histopathol 18: 299–308, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res 307: 1–14, 2002 [DOI] [PubMed] [Google Scholar]
- 89. Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol 285: G949–G958, 2003 [DOI] [PubMed] [Google Scholar]
- 90. Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology 130: 1886–1900, 2006 [DOI] [PubMed] [Google Scholar]
- 91. Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest 119: 1858–1870, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology 50: 185–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 13: 1324–1332, 2007 [DOI] [PubMed] [Google Scholar]
- 94. Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, Adachi K, Futatsugi S, Kuida K, Takeuchi O, Okamura H, Fujimoto J, Akira S, Nakanishi K. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol 166: 2651–2657, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: Hepatitis B, C and NAFLD: summary of a single topic conference. Hepatology 44: 1344–1354, 2006 [DOI] [PubMed] [Google Scholar]
- 96. Simeonova PP, Gallucci RM, Hulderman T, Wilson R, Kommineni C, Rao M, Luster MI. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol 177: 112–120, 2001 [DOI] [PubMed] [Google Scholar]
- 97. Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55: 695–700, 1965 [DOI] [PubMed] [Google Scholar]
- 98. Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 13: 3546–3559, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sottile J, Shi F, Rublyevska I, Chiang HY, Lust J, Chandler J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am J Physiol Cell Physiol 293: C1934–C1946, 2007 [DOI] [PubMed] [Google Scholar]
- 100. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51: 1820–1832, 2010 [DOI] [PubMed] [Google Scholar]
- 101. Streetz KL, Tacke F, Leifeld L, Wustefeld T, Graw A, Klein C, Kamino K, Spengler U, Kreipe H, Kubicka S, Muller W, Manns MP, Trautwein C. Interleukin 6/gp130-dependent pathways are protective during chronic liver diseases. Hepatology 38: 218–229, 2003 [DOI] [PubMed] [Google Scholar]
- 102. Sudo K, Yamada Y, Moriwaki H, Saito K, Seishima M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 29: 236–244, 2005 [DOI] [PubMed] [Google Scholar]
- 103. Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology 127: 1189–1197, 2004 [DOI] [PubMed] [Google Scholar]
- 104. Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol 294: G498–G505, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 12: 671–676, 2006 [DOI] [PubMed] [Google Scholar]
- 106. Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 90: 196–203, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology 44: 420–429, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Ueberham E, Low R, Ueberham U, Schonig K, Bujard H, Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology 37: 1067–1078, 2003 [DOI] [PubMed] [Google Scholar]
- 109. Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol 167: 7017–7026, 2001 [DOI] [PubMed] [Google Scholar]
- 110. Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem 277: 37377–37381, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Walport MJ. Complement. Second of two parts. N Engl J Med 344: 1140–1144, 2001 [DOI] [PubMed] [Google Scholar]
- 112. Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 46: 1404–1412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang H, Zhang Y, Heuckeroth RO. PAI-1 deficiency reduces liver fibrosis after bile duct ligation in mice through activation of tPA. FEBS Lett 581: 3098–3104, 2007 [DOI] [PubMed] [Google Scholar]
- 114. Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology 46: 1509–1518, 2007 [DOI] [PubMed] [Google Scholar]
- 115. Watanabe T, Niioka M, Hozawa S, Kameyama K, Hayashi T, Arai M, Ishikawa A, Maruyama K, Okazaki I. Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J Hepatol 33: 224–235, 2000 [DOI] [PubMed] [Google Scholar]
- 116. Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, Rudolph KL. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J 16: 935–942, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Yamada Y, Fausto N. Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am J Pathol 152: 1577–1589, 1998 [PMC free article] [PubMed] [Google Scholar]
- 118. Yan S, Chen GM, Yu CH, Zhu GF, Li YM, Zheng SS. Expression pattern of matrix metalloproteinases-13 in a rat model of alcoholic liver fibrosis. Hepatobil Pancreat Dis Int 4: 569–572, 2005 [PubMed] [Google Scholar]
- 119. Yang L, Bataller R, Dulyx J, Coffman TM, Gines P, Rippe RA, Brenner DA. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol 43: 317–323, 2005 [DOI] [PubMed] [Google Scholar]
- 120. Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y, Sata M, Nagai H, Yoshimura A. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med 199: 1701–1707, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology 36: 850–860, 2002 [DOI] [PubMed] [Google Scholar]
- 122. Yu C, Wang F, Jin C, Huang X, Miller DL, Basilico C, McKeehan WL. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol 163: 1653–1662, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yu C, Wang F, Jin C, Wu X, Chan WK, McKeehan WL. Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am J Pathol 161: 2003–2010, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]