Abstract
Gut injury and loss of normal intestinal barrier function are key elements in the paradigm of gut-origin systemic inflammatory response syndrome, acute lung injury, and multiple organ dysfunction syndrome (MODS). As hypoxia-inducible factor (HIF-1) is a critical determinant of the physiological and pathophysiological response to hypoxia and ischemia, we asked whether HIF-1 plays a proximal role in the induction of gut injury and subsequent lung injury. Using partially HIF-1α-deficient mice in an isolated superior mesenteric artery occlusion (SMAO) intestinal ischemia reperfusion (I/R) injury model (45 min SMAO followed by 3 h of reperfusion), we showed a direct relationship between HIF-1 activation and intestinal I/R injury. Specifically, partial HIF-1α deficiency attenuated SMAO-induced increases in intestinal permeability, lipid peroxidation, mucosal caspase-3 activity, and IL-1β mRNA levels. Furthermore, partial HIF-1α deficiency prevented the induction of ileal mucosal inducible nitric oxide synthase (iNOS) protein levels after SMAO and iNOS deficiency ameliorated SMAO-induced villus injury. Resistance to SMAO-induced gut injury was also associated with resistance to lung injury, as reflected by decreased levels of myeloperoxidase, IL-6 and IL-10 in the lungs of HIF-1α+/− mice. In contrast, a short duration of SMAO (15 min) followed by 3 h of reperfusion neither induced mucosal HIF-1α protein levels nor caused significant gut and lung injury in wild-type or HIF-1α+/− mice. This study indicates that intestinal HIF-1 activation is a proximal regulator of I/R-induced gut mucosal injury and gut-induced lung injury. However, the duration and severity of the gut I/R insult dictate whether HIF-1 plays a gut-protective or deleterious role.
Keywords: inflammation, inducible nitric oxide synthase, mucosal injury
multiple organ dysfunction syndrome (MODS) is the major cause of morbidity and mortality in critically ill patients. A key paradigm in the development of the systemic inflammatory response syndrome (SIRS) and acute respiratory distress syndrome (ARDS) that culminates in multiple organ dysfunction syndrome (MODS) is loss of intestinal barrier function (10, 12). Intestinal ischemia reperfusion (I/R) injury occurs in different clinical settings, such as trauma, shock, burn, mesenteric artery occlusion, abdominal, cardiac bypass, and thoracic vascular surgery, as well as small intestinal transplantation. Because the gut acts as the “motor” of SIRS, ARDS, and MODS (6) and therapy for the critically ill patient remains largely supportive, studies identifying the underlying pathophysiological factors in the gut that initiate and propagate SIRS, ARDS, and MODS are of critical importance. To date, studies investigating the gut inflammatory/injurious response have primarily focused on the reperfusion phase of the gut I/R insult. Examples include proinflammatory mediators, such as cytokines [(IL-1β) (35)], transcription factors [NF-κB, (3) AP-1 (55)], inducible nitric oxide synthase (iNOS)-derived nitric oxide (50), reactive oxygen species (45), cyclo-oxygenase-2 (22), and poly (ADP-ribose) polymerase (31). However, the induction of many of these factors is secondary to or accentuated by hypoxia, which accompanies ischemia and precedes reperfusion. We hypothesized that the molecular response triggered by hypoxia-ischemia is critical for initiating the sequence of events that leads to the development of gut injury and MODS.
Hypoxia-inducible factor-1 (HIF-1) has emerged as a critical determinant in the pathophysiological response to hypoxia-ischemia in conditions, such as cerebral and myocardial ischemia, and its induction is an early reperfusion-independent component of the inflammatory response (22). Given our observations that HIF-1α levels persisted in the ileal mucosa of male rats subjected to two models of intestinal I/R injury (trauma hemorrhagic shock and superior mesenteric artery occlusion, SMAO), despite restoration of intestinal blood flow and that Pseudomonas aeruginosa was able to induce HIF-1α expression in several enterocytic cell lines under normoxic conditions (30), we hypothesized that prolongation of the intestinal HIF-1 response could be potentially maladaptive/injurious and contribute to loss of gut barrier function and the development of distant organ injury. In this study, we tested whether partial HIF-1α deficiency would attenuate intestinal I/R-induced gut and lung injury.
MATERIALS AND METHODS
Mice.
The generation and genotyping of HIF-1α+/− and HIF-1α+/+ mice on a C57B6/129 genetic background was described previously (25). iNOS knockout mice and their wild-type littermates (C57B6/129 genetic background; Jackson Laboratory) were also used in the experiments. All animal procedures were approved by the Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey and maintained in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
SMAO model.
Male wild-type (WT), HIF-1α+/− and iNOS−/− mice (10–12 wk old) were anesthetized with pentobarbital sodium (50–70 mg/kg ip). In the SMAO groups, the superior mesenteric artery was reversibly occluded at its origin with the aorta for 15 or 45 min using 4–0 suture. Immediate blanching of the small intestine and cecum verified that the blood supply to these intestinal segments had been completely shut off. The intestinal contents were then placed back in the abdomen, and the gut was temporarily closed with a hemostat. After 15 or 45 min of SMAO, the suture was removed, and reperfusion of the intestinal segments was confirmed, after which the incision was closed, and the mice were allowed to awaken 3 h later. Anesthetized mice subjected to sham SMAO, had a midline laparotomy performed, and their loops of bowel eviscerated for 15 min followed by replacement of contents and closure of the abdomen. Three hours after reperfusion, segments of ileum, and samples of lung were harvested for histological examination. Ileal mucosa of the distal ileum was also obtained by gentle scraping with a cover slip.
Morphological analysis of gut and lung injury.
At death, terminal ileum or lung was fixed in 10% buffered formalin, processed, stained with hematoxylin and eosin, and examined by light microscopy. For each animal, the criteria used to score the degree of villus injury were based on an established grading system (5). The numerical scores were the following: 0 = normal mucosa; 1 = development of subepithelial Gruenhagen's space and vacuolization at the villus tip; 2 = decreased villus height and extension of the subepithelial space with moderate lifting of epithelial layer from the lamina propria; 3 = massive subepithelial lifting/sloughing and increased vacuolization from the tip to midportion of villus; 4 = epithelial lifting and vacuolization from the tip to lower portion of villus; and 5 = mucosal ulceration and disintegration of the lamina propria. All slides were evaluated by two examiners.
Measurement of intestinal permeability.
The everted gut sac method using fluorescein isothiocyanate-labeled 4-kDa dextran (FD4) was performed as previously described (44). Permeability was expressed as the mucosal-to serosal clearance of FD4.
Myeloperoxidase.
Frozen ileal mucosa and lung tissue were homogenized and processed for myeloperoxidase (MPO) activity measurement, as previously described (56). MPO activity in these supernatants was determined by measuring the H2O2-mediated oxidation of o-dianisidine hydrochloride at 460 nm. MPO activity was determined by using a standard curve from MPO derived from human leukocytes (Sigma Aldrich) and normalized to milligram of protein determined by bicinchoninic acid protein assay (Pierce).
Real-time PCR.
Total RNA was prepared from ileal mucosa or lung tissue using RNeasy (Qiagen), and cDNA was synthesized using the high-capacity cDNA reverse transcription kit (Applied Biosystems), according to the manufacturer's protocols. cDNA was then amplified by PCR with TaqMan gene expression Master Mix and predesigned TaqMan probes for murine HIF-1α, TNF-α, IL-1β, IL-6, and IL-10, as recommended by Applied Biosystems. Within each experimental group, mRNA expression was normalized to 18S rRNA amplification (ΔCt) and then fold changes in expression relative to WT sham (ΔΔCt) was determined using 2−(ΔΔCt) (42).
Western blot assays.
Whole cell extracts (WCE) prepared from ileal mucosal scrapings and lung tissue as described previously (30) were resolved by SDS-PAGE and detected by Western blot assays. The following antibodies were used: iNOS (BD Biosciences), HIF-1α (Novus Biologicals), and p42/p44 (Cell Signaling). The Western blot signals were developed with ECL Plus (Amersham) and analyzed by densitometry using an AlphaImager 3400 imaging system and AlphaEase FC software (Alpha Innotech).
HIF-1α ELISA.
Total HIF-1α protein concentrations (pg/ml) were measured in WCE using Human/Mouse Total HIF-1α DuoSet plate (R&D Systems), according to the manufacturer's instructions.
Caspase-3 activity assay.
Caspase-3 activity in the ileal mucosa was determined according to the instructions provided by the caspase-3/CPP32 colorimetric assay kit with DEVD-pNA substrate (Biovision).
Malondialdehyde assay.
Malondialdehyde (MDA) concentration, a marker of lipid peroxidation, was measured in whole ileum tissue that was homogenized in 1.15% KCl buffer, as previously described (1). Ileal homogenate (100 μg) in a 500-μl reaction volume (0.4% SDS, 0.3% thiobarbituric acid, and 0.75% acetic acid, pH 3.5) was incubated at 95°C for 45 min and then centrifuged at 10,000 g for 10 min. The supernatant was collected, and the MDA concentration (μM) was determined spectrophotometrically at 532 nM using a standard curve using malonaldehyde bis (Sigma Aldrich).
Statistical analysis.
Statistical significance among groups for villus injury score, in vivo intestinal permeability, polymorphonuclear leukocytes (PMN) infiltration, and MPO activity was determined by one-way ANOVA analysis followed by the Tukey-Kramer or Neuman-Keuls test. For real-time PCR analysis, data were analyzed by the Mann-Whitney U-test. P values less than 0.05 were considered statistically significant.
RESULTS
Partial HIF-1α deficiency attenuates gut I/R-induced villus injury.
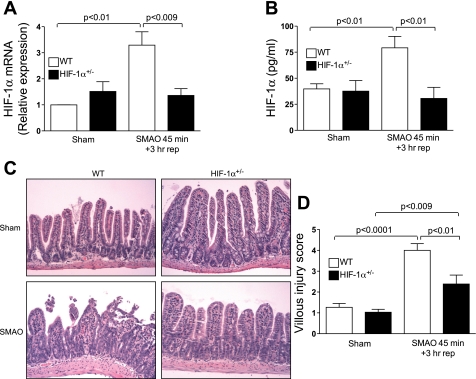
To elucidate the functional significance of the intestinal HIF-1α response to intestinal I/R in vivo, an isolated SMAO intestinal I/R injury model was used in which HIF-1α+/− and WT littermate mice were subjected to SMAO or sham surgery for 45 min and killed after 3 h of reperfusion. As reported previously, mice homozygous for a null (knockout) allele at the locus encoding HIF-1α die on embryonic day 10, whereas HIF-1α+/− mice, which are heterozygous for the knockout allele, develop normally and are indistinguishable from their WT littermates under normoxic conditions (25, 40, 48). We first determined HIF-1α mRNA levels in ileal mucosal scrapings of sham- and SMAO-operated WT and HIF-1α+/− mice by real-time PCR analysis. As shown in Fig. 1A, HIF-1α mRNA levels were markedly upregulated in WT mice subjected to SMAO compared with their sham counterparts (P < 0.001), whereas there was no induction of HIF-1α mRNA expression in HIF-1α+/− mice subjected to SMAO. There was a significant difference in HIF-1α mRNA levels between WT and HIF-1α+/− mice subjected to SMAO (P < 0.009). Using an ELISA that measured the concentration of HIF-1α, we found that compared with Sham, SMAO increased intestinal HIF-1α protein levels in WT mice (Fig. 1B). In contrast, HIF-1α protein levels were not elevated in HIF-1α+/− mice subjected to SMAO.
Fig. 1.
Partially hypoxia-inducible factor-1α (HIF-1α) deficiency attenuates gut ischemia-reperfusion (I/R)-induced villus injury. Wild-type (WT) and HIF-1α+/− mice were subjected to superior mesenteric artery occlusion (SMAO) or sham for 45 min and 3 h of reperfusion. A: real-time PCR analysis measured HIF-1α mRNA expression in the ileal mucosa using the ΔΔCt method. HIF-1α expression was normalized to 18S, and the relative expression was compared with WT sham controls. Values were expressed as means ± SE (n = 5 or 6 mice/group). B: HIF-1α protein levels (pg/ml) in the ileal mucosa were quantified in a HIF-1α ELISA. Values are expressed as means ± SE (n = 6–11 mice/group). C: representative sections of hematoxylin-and-eosin (H&E) staining of the distal ileum (×200). The arrows depict subepithelial lifting, sloughing, and mucosal edema in the villi. D: histological scoring of villus damage. Values are expressed as means ± SE (n = 13–16 mice/group).
We then determined the effects of partial HIF-1α deficiency on SMAO-induced villus injury. Histological evaluation of early ileal mucosal damage revealed extensive blunting of the villi tips, appearance of subepithelial lifting, congestion, and sloughing of the villi tips in WT mice subjected to SMAO compared with their sham counterparts (Fig. 1C). The mean villus injury score for the WT SMAO and WT sham group were 4 ± 0.32 and 1.3 ± 0.18, respectively (Fig. 1D). In contrast, the mucosal damage in the ileal mucosa of SMAO-operated HIF-1α+/− mice was not pronounced, manifesting an injury score of 2.4 ± 0.43 compared with 1.0 ± 0.14 in the HIF-1α+/− sham group. The most prominent observation was the absence of blunting and sloughing of villi tips and congestion in HIF-1α+/− mice subjected to SMAO. Thus, partial HIF-1α deficiency conferred significant protection against SMAO-induced villus injury.
HIF-1 activation is involved in gut barrier dysfunction and the mucosal inflammatory response.
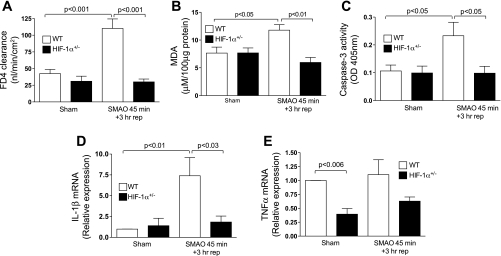
To provide additional evidence that HIF-1 plays a maladaptive role in SMAO-induced gut injury, we examined the effects of HIF-1 on loss of intestinal barrier integrity. Because increased intestinal permeability is causally related to intestinal I/R (16), we tested the hypothesis that HIF-1α activation contributes to I/R-induced increased gut permeability. As shown in Fig. 2A, intestinal permeability to FD4 in the ex vivo everted gut sacs of the WT mice subjected to SMAO was three-fold higher relative to the sham WT group (P < 0.001). In contrast, there was no pronounced change in intestinal permeability in HIF-1α+/− mice subjected to SMAO (P < 0.001 vs. WT SMAO). Our results demonstrate that the SMAO-induced intestinal HIF-1 response modulated intestinal permeability.
Fig. 2.
HIF-1 is involved in gut barrier dysfunction and the mucosal inflammatory response. WT and HIF-1α+/− mice were subjected to SMAO or sham for 45 min and 3 h of reperfusion. A: ex vivo everted gut sac permeability was performed, and permeability was expressed as mucosal to serosal clearance of FD4 (nl·min−1·cm−2). Values are expressed as means ± SE (n = 4–7 mice/group). B: MDA levels (μM/100 μg protein) were measured in whole ileal homogenates. Values are expressed as means ± SE (n = 4 or 5 mice/group). C: caspase-3 activity is expressed as absorbance at 405 nm/50 μg of protein. Values are expressed as the means ± SE (n = 4 or 5/group). D and E: real-time PCR analysis of proinflammatory mediators in the ileal mucosa was performed using the ΔΔCt method. IL-1β (D) and TNF-α (E) were normalized to 18S, and the relative expression was compared with WT sham controls. Values are expressed as means ± SE (n = 4–6 mice/group).
We then measured the extent of lipid peroxidation in ileal mucosal extracts as a marker of oxidant-induced mucosal damage after SMAO. Specifically, polyunsaturated fatty acids in membranes are sensitive to oxidative damage and are broken down into the peroxidation product, MDA. As shown in Fig. 2B, the intestinal MDA concentration was increased in WT mice subjected to SMAO compared with sham-operated WT mice (P < 0.05). There was no significant change in MDA levels in HIF-1α+/− mice after SMAO, thereby indicating that reduced HIF-1α afforded protection from oxidant-induced intestinal injury.
We also studied caspase-3 activation since it has been implicated in gut injury models of I/R (28, 29), hypoxia (13), and ischemia (2, 18), and the caspase 3 promoter has been reported to contain a functional HIF-1 response element (52). After SMAO, caspase-3 activity was 2.5-fold higher in the ileal mucosa of WT mice relative to HIF-1α+/− mice (P < 0.05, Fig. 2C). The caspase-3 activity in HIF-1α+/− mice subjected to SMAO was identical to sham controls. This observation suggests that HIF-1 manifests a proapoptotic role during intestinal I/R injury.
Lastly, we asked whether the intestinal HIF-1 response modulated the mucosal inflammatory response. As shown in Fig. 2D, IL-1β mRNA levels were augmented in the ileal mucosa of WT mice but not in HIF-1α+/− mice after SMAO (P < 0.03). Although TNF-α expression was not affected by SMAO, endogenous TNF-α mRNA levels were significantly lower in HIF-1α+/− mice compared with WT mice (P < 0.006, Fig. 2E). These observations suggest that HIF-1 modulates intestinal injury and inflammation in response to an acute gut ischemic insult.
HIF-1 mediates gut I/R injury via an iNOS response.
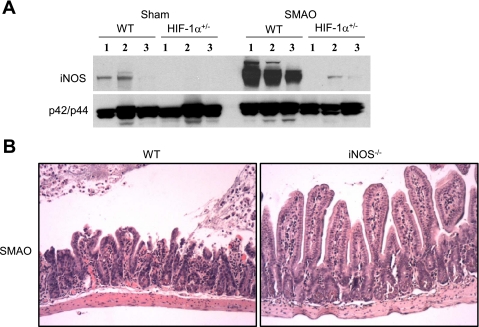
Since iNOS is a downstream target of HIF-1 (33, 34, 38) that has been implicated as a critical inflammatory and injurious mediator of I/R-induced gut barrier failure (17, 50, 54), we asked whether HIF-1 modulated iNOS expression during intestinal I/R injury. A marked difference in the induction of iNOS protein expression was observed in the ileal mucosa between HIF-1α+/− and WT mice after SMAO (Fig. 3A). Endogenous iNOS protein levels were undetectable or low in sham-operated HIF-1α+/− and WT mice, respectively, and increased markedly in response to SMAO in WT mice only. As shown in Fig. 3B, histological examination of the ileum from WT mice subjected to SMAO showed mucosal ulceration, subepithelial lifting, and sloughing, whereas examination of the ileum from iNOS−/−-deficient mice after SMAO revealed no obvious mucosal damage. Taken together, our results suggest that induction of HIF-1 is a proximal event that is necessary for the induction of intestinal iNOS, a key mediator of intestinal injury.
Fig. 3.
Inducible nitric oxide synthase (iNOS) mediates the gut injurious effects of HIF-1α. A: WT and HIF-1α+/− mice were subjected to SMAO or sham for 45 min and 3 h of reperfusion. Whole cell extracts prepared from mucosal scrapings from the distal ileum were examined for iNOS expression by Western blot analysis. Total p42/p44 expression was done to verify equal loading of our samples. The numbers refer to individual mice. B: WT and iNOS−/− mice were subjected to SMAO or sham for 45 min and 3 h of reperfusion. Representative sections of H&E staining of the distal ileum in mice subjected to SMAO (n = 5–7 mice/group).
HIF-1 plays a role in gut I/R-induced lung injury.
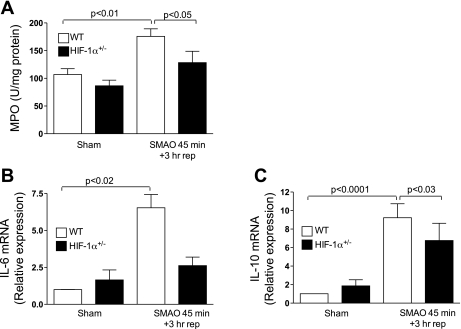
Because numerous studies have shown that intestinal I/R injury leads to the development of a systemic inflammatory response and lung injury (6, 12), we asked whether SMAO-induced lung inflammation and injury were dependent on HIF-1. As shown in Fig. 4A, the sequestration of PMN in the lung was assessed by measuring MPO levels in lung homogenates. Increased MPO activity was evident in lungs of WT mice subjected to SMAO compared with sham controls (P < 0.01). MPO levels in the lungs of SMAO-induced HIF-1α+/− mice were not significantly increased compared with sham controls. We also determined whether HIF-1 modulated the lung inflammatory response that was induced as a consequence of intestinal I/R injury. As shown in Fig. 4, B and C, increased IL-10 and IL-6 mRNA levels were found in lungs of WT mice subjected to SMAO relative to sham controls. Although IL-10 mRNA levels were also increased after SMAO in HIF-1α+/− mice compared with sham controls, partial HIF-1α deficiency significantly reduced IL-10 induction (P < 0.03). Additionally, SMAO did not induce IL-6 expression in HIF-1α+/− mice. Collectively, our results suggest the involvement of HIF-1 in SMAO-induced lung injury and inflammation.
Fig. 4.
HIF-1 plays a role in gut I/R-induced lung injury. WT and HIF-1α+/− mice were subjected to SMAO or sham for 45 min and 3 h of reperfusion. A: MPO activity (U/mg of protein) was measured in the lung homogenates. Values expressed as means ± SE (n = 5–8/group). B: real-time PCR analysis of cytokines in the lung was performed using the ΔΔCt method. IL-6 and IL-10 expression was normalized to 18S, and the relative expression was compared with WT sham controls. Values are expressed as means ± SE (n = 4–6 mice/group).
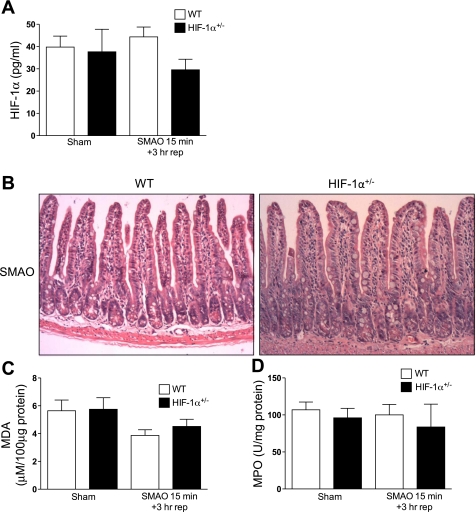
Duration of the intestinal ischemic insult is required to activate HIF-1 and downstream responses.
Since studies have shown that the severity of intestinal I/R injury is directly proportional to the duration of the ischemic insult, we determined the effects of short duration of SMAO (15 min) followed by 3 h of reperfusion. As shown in Fig. 5A, HIF-1α protein levels were not elevated in the ileal mucosa of WT mice after SMAO compared with control shams. Histological analysis did not show pronounced blunting, subepithelial lifting in the villi tips, or mucosal ulceration in WT or HIF-1α+/− mice subjected to 15 min SMAO and 3 h of reperfusion (Fig. 5B) in contrast to WT mice subjected to 45 min SMAO and 3 h of reperfusion (Fig. 1C). In addition, lipid peroxidation of the ileum was not elevated in WT or HIF-1α+/− mice subjected to 15 min SMAO and 3 h of reperfusion compared with their respective sham controls (Fig. 5C). Finally, there was also no evidence of pulmonary PMN sequestration (Fig. 5D) in WT or HIF-1α+/− mice subjected to 15-min SMAO. Taken together, our results suggest that the duration of the SMAO insult determines whether HIF-1 is activated and triggers a pathogenic response to intestinal I/R.
Fig. 5.
Duration of the intestinal ischemic insult dictates the functional sequelae of the intestinal HIF-1 response. WT and HIF-1α+/− mice were subjected to SMAO or sham for 15 min and 3 h of reperfusion. A: HIF-1α protein levels (pg/ml) in the ileal mucosa were quantified in a HIF-1α ELISA. Values are expressed as means ± SE (n = 8–11 mice/group). B: representative sections of H&E staining of the distal ileum (n = 8–16 mice/group). C: MDA levels (μM/100 μg protein) were measured in whole ileal homogenates. Values are expressed as means ± SE (n = 6 mice/group). D: MPO activity (U/mg of protein) was measured in the lung homogenates (n = 4–8 mice/group).
DISCUSSION
Gut injury and loss of normal intestinal barrier function are key elements in the paradigm of gut-origin SIRS and MODS. The gut is a unique organ because it is a major target for injury during stress states (51) and a source of factors that are responsible for the development of acute lung injury after major trauma and shock. Several factors have been proposed linking gut injury and inflammation to the development of systemic inflammation and distant organ dysfunction. These factors include cytokines and chemokines, nitric oxide, and oxidants, as well as translocating bacteria and their products, such as endotoxin (6). Many of these same factors may also be involved in transducing gut I/R to gut inflammation. Our earlier studies have demonstrated that gut ischemia in vivo induces an intestinal mucosal HIF-1 response that does not disappear upon reoxygenation of the intestine and that this persistence of the HIF-1 response appears to be, at least in part, mediated by translocating intestinal bacteria and/or bacterial products, such as lipopolysaccaride (30). The present study provides four lines of evidence that HIF 1 acts as a proximal mediator of intestinal injury and subsequent lung injury and inflammation in response to gut I/R. First, using partially HIF-1α-deficient mice, we have established a direct relationship between HIF-1 activation and intestinal I/R injury. Second, our SMAO results demonstrate that HIF-1 activation is associated with loss of gut barrier function (increased intestinal permeability, lipid peroxidation, and apoptosis), pronounced villus injury, and a marked gut-derived inflammatory response. Third, our results indicate that the deleterious effects of HIF-1 in gut ischemic states may be due, at least in part, to the induction of iNOS. Fourth, these studies also demonstrate that resistance to intestinal I/R injury is associated with resistance to lung injury and inflammation in partially HIF-1α-deficient mice. The direct relationship between HIF-1 activation and SMAO-induced intestinal I/R injury validates our recent study demonstrating that HIF-1 activation-mediated gut and lung injury in a global trauma-hemorrhagic shock model (15).
The HIF-1 signaling pathway has emerged as a major regulator of intestinal homeostasis and appears to manifest a dichotomous role in gut inflammatory disease (7). Our findings in the 45-min SMAO model indicating that HIF-1 plays a maladaptive role in an acute gut inflammatory and injury response stand in contrast to reports that HIF-1 attenuates intestinal inflammation and induces the expression of gut barrier-protective genes in several chronic colonic inflammatory bowel disease models (7). Conditional deletion of HIF-1α in the colon resulted in the loss of gut barrier function and amplification of intestinal inflammation in 2,4,6-trinitrobenzene sulfonic acid-induced colitis (27), oxazolone-induced colitis (27), and Clostridium difficile-induced colitis (23). Additionally, the pharmacological activation of HIF-1 by prolyl hydroxylase inhibitors conferred mucosal protection in several murine models of colitis (8, 43). There are several potential model-related reasons for these differences. We have investigated an acute I/R model that involves the small intestine and not the colon. In contrast, the colitis studies involved chemical-induced injury of the colon that evolves over days. Adding complexity to the potential roles of HIF-1 in gut injury, investigators using a specific epithelial HIF-1α-deficient mouse found that the hyperinflammatory response in dextran sodium-sulfate-induced colitis model was augmented rather than ameliorated (49).
In the context of acute intestinal I/R injury, previous work has demonstrated that HIF-1-dependent activation of ecto-5′-nucleotidase and A2B adenosine receptor ameliorated SMAO-induced gut I/R injury (20, 21). This work used a 15-min SMAO model, which is at odds with our findings, since we did not observe significant histological damage or oxidant-induced injury in the ileum in WT or HIF-1α+/− mice after a short duration of SMAO (15 min) followed by 3 h of reperfusion. We did not find mucosal HIF-1α protein levels to be increased in WT mice after this short period of SMAO. Additionally, there was no evidence of gut I/R-induced lung injury. The reason for the differences between these studies and our results is not clear. However, our observation that a longer period of SMAO (45 min) followed by 3 h of reperfusion was associated with pronounced intestinal and lung injury and inflammation is consistent with studies demonstrating that the magnitude of apoptosis and severity of mucosal injury is directly proportional to the duration of intestinal ischemic insult (4, 37). Furthermore, real-time analysis of acute mucosal events in the villus epithelium demonstrated that although strong intracellular acidification and increased mitochondrial energy production were evident in the jejunum within 5 min of ischemia, the adverse effects of a short period of ischemia (15 min) were reversible upon reperfusion, whereas after a long period of ischemia (40–50 min), mucosal damage was irreversible upon reperfusion (19). Taken together, these results suggest that the duration and severity of the intestinal ischemic insult are critical determinants of whether HIF-1 plays a gut-protective or deleterious role.
Given that HIF-1 regulates iNOS expression (33, 34, 38) and iNOS-derived nitric oxide (NO) impairs gut barrier function in shock states (11, 17, 54), our results indicate that the deleterious effects of HIF-1 in gut I/R injury may be due, at least in part, to the induction of iNOS expression. Ileal mucosal iNOS expression was reduced in partially deficient HIF-1α mice subjected to 45-min SMAO and 3 h of reperfusion compared with WT mice. Additionally, mucosal HIF-1α expression was not reduced in iNOS−/− mice subjected to SMAO, despite the attenuation of villus injury (data not shown). HIF-1 is, therefore, proximal to iNOS, and HIF-1-induced increases in iNOS expression are, at least, partly responsible for the injurious effects of HIF-1. Consistent with our results, studies have shown that HIF-1-induced iNOS mediates the cytotoxic effects of HIF-1 in astrocytes resulting in neuronal cell death (53) and that HIF-1 binds to the iNOS promoter in the ischemic tissue after cerebral ischemia (33). In addition to the brain, crosstalk between iNOS and HIF-1α has also been demonstrated in trauma hemorrhagic shock-induced hepatic injury (26). Furthermore, recent studies have demonstrated caspase-3 activation in models of hemorrhagic shock (28, 29), hypoxia (13), and ischemia (2, 18) and that increased caspase-3 protein and activity are regulated via iNOS (28, 36). Thus, our results showing that HIF-1 activation is associated with both increased iNOS expression and caspase-3 activation in the ileal mucosa are consistent with this published work, as well as studies showing that a functional HIF-1 response element is present within the caspase-3 promoter (52). These findings suggest that prolonged HIF-1 activation may contribute to impaired gut barrier function by promoting intestinal cell death. While our results suggest that iNOS and caspase-3 may be necessary in HIF-1-mediated gut injury, these results do not exclude the possibility that other HIF-1 regulated genes, as well as reactive oxygen species and HIF-1-independent factors, are also involved in I/R-induced impaired gut barrier function.
More support for the concept that HIF-1 plays an inflammatory role during gut I/R injury stems from our observations that a longer duration of 45 min SMAO followed by 3 h of reperfusion augmented IL-1β and iNOS expression in the ileal mucosa of WT mice, and their upregulation was not evident in HIF-1α+/− mice. On the basis of recent studies showing that cytokines (46), bacteria (30, 39), LPS (30, 47), and nitric oxide (9) are capable of activating HIF-1 in numerous cell types, including enterocytes, it is possible that gut I/R-induced inflammatory mediators act in a positive feedback loop to sustain elevated HIF-1 levels, thereby, shifting the balance from HIF-1 being adaptive to maladaptive. It would also be of interest to deteremine whether HIF-2α manifests a proinflammatory role in our SMAO model using mice devoid of HIF-2α in the intestinal epithelium since HIF-2α has been recently implicated in myeloid- and colonic-mediated inflammatory responses (24, 32, 49).
Our observations showing that SMAO-induced gut injury was associated with an augmented pulmonary inflammatory response, as manifested by increased PMN sequestration and elevated cytokine levels, are consistent with I/R-induced gut injury leading to distant organ injury and dysfunction (10). Thus, the fact that protection from SMAO-induced gut injury was associated with protection from lung injury in the HIF-1α+/− mice is not surprising and fits the gut hypothesis of MODS. However, the lung-protective effects observed in the HIF-1α+/− mice might be more complex than purely their resistance to SMAO-induced gut injury. Specifically, we found that HIF-1 modulated both pulmonary IL-6 and IL-10 expression after SMAO, and both IL-6 and IL-10 have been shown to be regulated by HIF-1 (41, 49). Thus, it is possible that the relative deficiency in pulmonary HIF-1 in the HIF-1α+/− mice could have also exerted a protective effect. Future work will be required to resolve this issue.
In summary, loss of gut barrier function and the induction of the inflammatory response as a consequence of splanchnic ischemia and reperfusion contribute to a HIF-1 response that is maladaptive. Because the mortality rate for patients who manifest ARDS and MODS is 30–50% and current therapy is largely supportive (14), delineating the role of the HIF-1 as both a trigger and mediator of gut I/R injury has the potential to provide novel insights into the pathogenesis of acute organ dysfunction and the development of novel target-based therapies.
GRANTS
This study was supported by National Institutes of Health Grants 1P50GM069790 (to R. Feinman and E. A. Deitch), T32-GM069330 (to F. J. Caputo), and R01-HL55338 (to G. L. Semenza).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Banafsche R, Gunther L, Nefflen JU, Moutsiou S, Knolle PA, Herfarth C, Klar E. NF-κB antisense oligonucleotides reduce leukocyte-endothelial interaction in hepatic ischemia-reperfusion. Transplant Proc 33: 3726–3727, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Chaitanya GV, Babu PP. Activation of calpain, cathepsin-B and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res 33: 2178–2186, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9: 575–581, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Lui VCH, Rooijen NV, Tam PKH. Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut. Gut 53: 1772–1780, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu C, McArdle A, Brown R, Scott H, Gurd F. Intestinal mucosal lesions in low-flow states. Arch Surg 101: 478–483, 1970 [DOI] [PubMed] [Google Scholar]
- 6. Clark J, Coopersmith C. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 28: 384–393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7: 281–287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cummins EP, Seeballuck F, Keely SJ, Mangan NE, Callanan JJ, Fallon PG, Taylor CT. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134: 156–165, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res 315: 1791–1797, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Deitch EA, Sambol JT. The Gut-Origin Hypothesis of MODS. Baltimore: W. B. Saunders, 2002, p. 105–116 [Google Scholar]
- 11. Deitch EA, Shorshtein A, Houghton J, Lu Q, Xu DZ. Inducible nitric oxide synthase knockout mice are resistant to diet-induced loss of gut barrier function and intestinal injury. J Gastrointest Surg 6: 599–605, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Deitch EA, Xu D, Lu Q. Gut lymph hypothesis of early shock and trauma-induced multiple organ dysfunction: A new look at gut origin of sepsis. J Org Dys 2: 70–70, 2006 [Google Scholar]
- 13. Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol 294: H724–H735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Erickson S, Martin G, Davis J, Matthay M, Eisner M. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 37: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feinman R, Deitch EA, Watkins AC, Abungu B, Colorado I, Kannan KB, Sheth S, Caputo FJ, Lu Q, Ramanathan M, Attan S, Badami CJ, Doucet D, Barlos D, Bosch-Marce M, Semenza GL, Xu DZ. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol 299: G833–G843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fink MP, Delude RL. Epithelial barrier dysfunction: A unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. [DOI] [PubMed] [Google Scholar]
- 17. Forsythe RM, Xu DZ, Lu Q, Deitch EA. LPS-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock 17: 180–184, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Giakoustidis AE, Giakoustidis DE, Koliakou K, Kaldrymidou E, Iliadis S, Antoniadis N, Kontos N, Papanikolaou V, Papageorgiou G, Atmatzidis K, Takoudas D. Inhibition of intestinal ischemia/repurfusion induced apoptosis and necrosis via down-regulation of the NF-κB, c-Jun and caspase-3 expression by epigallocatechin-3-gallate administration. Free Radic Res 42: 180–188, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Guan Y, Worrell RT, Pritts TA, Montrose MH. Intestinal ischemia-reperfusion injury: reversible and irreversible damage imaged in vivo. Am J Physiol Gastrointest Liver Physiol 297: G187–G196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J 22: 2784–2797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182: 3965–3968, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ. Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events. Arch Orthop Trauma Surg 121: 219–222, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Hirota SA, Fines K, Ng J, Traboulsi D, Lee J, Ihara E, Li Y, Willmore WG, Chung D, Scully MM, Louie T, Medlicott S, Lejeune M, Chadee K, Armstrong G, Colgan SP, Muruve DA, MacDonald JA, Beck PL. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology 139: 259–269.e3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120: 2699–2714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12: 149–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Raju R, Bland KI, Chaudry IH. Selective inhibition of iNOS attenuates trauma-hemorrhage/resuscitation-induced hepatic injury. J Appl Physiol 105: 1076–1082, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest 114: 1098–1106, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiang JG, Bowman PD, Lu X, Li Y, Wu BW, Loh HH, Tsen KT, Tsokos GC. Geldanamycin inhibits hemorrhage-induced increases in caspase-3 activity: role of inducible nitric oxide synthase. J Appl Physiol 103: 1045–1055, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Kiang JG, Peckham RM, Duke LE, Shimizu T, Chaudry IH, Tsokos GC. Androstenediol inhibits the trauma-hemorrhage-induced increase in caspase-3 by downregulating the inducible nitric oxide synthase pathway. J Appl Physiol 102: 933–941, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Koury J, Deitch EA, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, Feinman R. Persistent HIF-1α activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock 22: 270–277, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Liaudet L, Szabo A, Soriano F, Zingarelli B, Szabo C, Salzman A. Poly (ADP-ribose) synthetase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock 14: 142–143, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular Cell 40: 294–309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matrone C, Pignataro G, Molinaro P, Irace C, Scorziello A, Di Renzo GF, Annunziato L. HIF-1 reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem 90: 368–378, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, Varesio L. Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem 272: 12236–12243, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Michalsky MP, Deitch EA, Ding J, Lu Q, Huang Q. Interleukin-6 and tumor necrosis factor production in an enterocyte cell model (Caco-2) during exposure to Escherichia coli. Shock 7: 139–146, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Murao Y, Loomis W, Wolf P, Hoyt DB, Junger WG. Effect of dose of hypertonic saline on its potential to prevent lung tissue damage in a mouse model of hemorrhagic shock. Shock 20: 29–34, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw T. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 274: G270–G276, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol Lung Cell Mol Physiol 274: L212–L219, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Patel NJ, Zaborina O, Wu L, Wang Y, Wolfgeher DJ, Valuckaite V, Ciancio MJ, Kohler JE, Shevchenko O, Colgan SP, Chang EB, Turner JR, Alverdy JC. Recognition of intestinal epithelial HIF-1α activation by Pseudomonas aeruginosa. Am J Physiol Gastrointest Liver Physiol 292: G134–G142, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J Immunol 178: 7516–7519, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor (HIF) prolyl hydroxylase inhibition. Gastroenterology 134: 144–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rupani B, Caputo F, Watkins A, Vega D, Magnotti L, Lu Q, Xu D, Deitch E. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery 141: 481–489, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Sasaki M, Joh T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J Clin Biochem Nutr 40: 1–12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scharte M, Han X, Bertges DJ, Fink MP, Delude RL. Cytokines induce HIF-1 DNA binding and the expression of HIF-1-dependent genes in cultured rat enterocytes. Am J Physiol Gastrointest Liver Physiol 284: G373–G384, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Scharte M, Han X, Uchiyama T, Tawadrous Z, Delude RL, Fink MP. LPS Increases hepatic HIF-1α protein and expression of the HIF-1-dependent gene aldolase A in rats. J Surg Res 135: 262–267, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 91: 803–806, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, Gonzalez FJ. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology 134: 2036–2048, e2033, 2048.e1–3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suzuki Y, Deitch EA, Mishima S, Lu Q, Xu DZ. Inducible nitric oxide synthase gene knockout mice have increased resistance to gut injury and bacterial translocation after an intestinal ischemia-reperfusion injury. Crit Care Med 28: 3692–3696, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Taylor C, Colgan S. Hypoxia and gastrointestinal disease. J Mol Med 85: 1295–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Van Hoecke M, Pringent-Tessier A, Garnier P, Bertrand N, Filomenko R, Bettaieb A, Marie C, Beley A. Evidence of HIF-1 functional binding activity to caspase-3 promoter after photothrombotic cerebral ischemia. Mol Cell Neurosci 34: 40–47, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Vangeison G, Carr D, Federoff HJ, Rempe DA. The good, the bad, and the cell type-specific roles of hypoxia inducible factor-1α in neurons and astrocytes. J Neurosci 28: 1988–1993, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu DZ, Lu Q, Deitch EA. Nitric oxide directly impairs intestinal barrier function. Shock 17: 139–145, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Yeh KY, Yeh M, Glass J, Granger DN. Rapid activation of NF-κB and AP-1 and target gene expression in postischemic rat intestine. Gastroenterology 118: 525–534, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol 82: 774–780, 2007 [DOI] [PubMed] [Google Scholar]