Abstract
Syngeneic graft vs. host disease (SGVHD) was first described as a graft vs. host disease-like syndrome that developed in rats following syngeneic bone marrow transplantation (BMT) and cyclosporin A (CsA) treatment. SGVHD can be induced by reconstitution of lethally irradiated mice with syngeneic bone marrow cells followed by 21 days of treatment with the immunosuppressive agent CsA. Clinical symptoms of the disease appear 2–3 wk following cessation of CsA therapy, and disease-associated inflammation occurs primarily in the colon and liver. CD4+ T cells have been shown to play an important role in the inflammatory response observed in the gut of SGVHD mice. Time-course studies revealed a significant increase in migration of CD4+ T cells into the colon during CsA therapy, as well as significantly elevated mRNA levels of TNF-α, proinflammatory chemokines, and cell adhesion molecules in colonic tissue of CsA-treated animals compared with BMT controls, as early as day 14 post-BMT. Homing studies revealed a greater migration of labeled CD4+ T cells into the gut of CsA-treated mice at day 21 post-BMT than control animals via CsA-induced upregulation of mucosal addressin cell adhesion molecule. This study demonstrates that, during the 21 days of immunosuppressive therapy, functional mechanisms are in place that result in increased homing of CD4+ T effector cells to colons of CsA-treated mice.
Keywords: cell adhesion molecules, β7-integrin, chemokines, T cell homing
syngeneic graft vs. host disease (SGVHD) was first described by Glazier et al. (28) as a graft vs. host disease (GVHD)-like syndrome that developed in rats following syngeneic bone marrow (BM) transplantation (BMT) and cyclosporin A (CsA) treatment. In the mouse, clinical symptoms of the disease appear 2–3 wk after cessation of CsA therapy, and disease-associated inflammation occurs primarily in the colon and liver (6, 7). The development of murine SGVHD is a multistep process resulting from the cooperative interaction of various effector cell populations, including NK cells, macrophages (25, 26), and T cells (4, 8, 9). Bryson et al. (9) demonstrated a role for CD4+, but not CD8+, T cells in the induction of colon inflammation in murine SGVHD. In vivo depletion of CD4+ T cells during, but not after, CsA therapy inhibited significantly the induction of SGVHD (9). Finally, SGVHD could be adoptively transferred with CD4+ T cells from diseased animals into secondary recipients (4).
The recruitment of leukocytes from the vascular compartment into the extravascular space is crucial for the development of inflammation. This movement involves cell adhesion interactions that are regulated by several types of cell adhesion molecules (CAMs), which are expressed on the surface of vascular endothelial cells and leukocytes (11, 20, 36). Homing of lymphocytes is governed by their lymphocyte trafficking properties, which include tissue-specific adhesion and chemokine interactions (10, 27, 41, 51). Migration into the inflamed gut requires lymphocyte trafficking through the mesenteric lymph nodes (MLN) (29). Naïve T cells enter the MLN via high endothelial venules and are able to encounter foreign antigens by their interaction with dendritic cells (DC) that arrive from the gut. As a consequence, these lymphocytes become activated, express surface markers, and acquire a gut-homing phenotype that enables them to reach the site of inflammation, where they can exert their effector activities (10). Proinflammatory chemokines, released by the tissue along with their corresponding receptors, found on activated lymphocytes, form an important axis that directs lymphocytes to the gut (31, 41).
Vascular addressins are expressed on the surface of vascular endothelial cells and serve as receptors, allowing leukocyte homing to particular tissues (22, 45). Mucosal addressin CAM-1 (MAdCAM) is a vascular addressin that is expressed on mucosal endothelium lining blood vessels and serves to guide the entry of lymphocytes into mucosal lymphoid tissues such as the gut. It binds to α4β7-integrin, expressed on the surface of activated T lymphocytes, allowing their homing into the gut, where they can initiate an inflammatory response (22). Additional adhesion molecules that can participate in migration of leukocytes to the gut include ICAM and VCAM. Consistent with this, immunoblockade of the CAM or their counterligand has been shown to be successful in reducing gut inflammation in murine models of inflammatory bowel disease (IBD) (2, 27, 39).
Previously published studies report an increase in colonic CD4+ T cells in CsA-treated mice by day 21 post-BMT, suggesting enhanced T cell migration into the colon during the CsA treatment period (9). Studies were undertaken to investigate CD4+ T cell migration into the colon during the induction of SGVHD. Tissues from C3H/HeN mice were taken at various times during CsA treatment and analyzed for the presence of CD4+ T cells, CAMs, chemokines, and proinflammatory cytokines. Results indicate increased levels of inflammatory markers and increased numbers of CD4+ T cells in colonic tissue of mice treated with CsA in the immediate post-BMT period. Immunoblockade studies using a carboxyfluorescein succinimidyl ester (CFSE)-labeled SGVHD CD4+ T cell line showed that increased lymphocyte homing into the gut of CsA-treated mice at day 21 post-BMT compared with control animals occurred via enhanced expression of MAdCAM. Collectively, these data suggest a functional role for the increased levels of CAMs in the enhanced migration of CD4+ T cells into the colons of CsA-treated animals.
MATERIALS AND METHODS
Mice.
C3H/HeN mice (Harlan, Indianapolis, IN) were purchased at 20–21 days of age and used within 1 wk of arrival. The mice were given acidified water, fed autoclaved laboratory food ad libitum, and housed in sterile microisolator cages (Lab Products, Maywood, NJ). The animal use protocol was approved by the University of Kentucky Institutional Animal Use and Care Committee.
Induction of SGVHD.
BM was isolated from the femurs and tibias of syngeneic age-matched mice. The donor BM suspensions were prepared in RPMI 1640 (Cellgro, Herndon, VA) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO). The resulting cell suspensions were resuspended in cytotoxic medium (RPMI 1640 containing penicillin and 0.3% BSA fraction V) to a final concentration of 5 × 107 cells/ml. BM cells were treated with MAb to Thy1.2 (HO-13-4), to deplete Thy1+ cells, for 60 min on ice. Thy1.2-depleted BM cells were washed three times with cytotoxic medium and treated with Low-Tox-M rabbit complement (Cedarlane Laboratories, Westbury, NY) at a concentration of 5 × 107 cells/ml for 1 h in a 37°C water bath. Recipient mice were lethally irradiated with 900 cGy in a 137Cs irradiator (Mark I, J. L. Shepard, Glendale, CA) 4–6 h before transplantation. Irradiated mice were reconstituted with 5 × 106 T cell-depleted BM cells intravenously in 0.1 ml of PBS via tail vein injection. Beginning on the day of transplantation, groups of mice were injected intraperitoneally daily for 21 days with 0.1 ml of 15 mg/kg CsA in olive oil diluent (Sigma-Aldrich, St. Louis, MO) or diluent alone. CsA was purchased through the Division of Laboratory Animal Resources, University of Kentucky. After cessation of CsA, the animals were weighed three times per week and observed for clinical signs of the development of SGVHD (weight loss, diarrhea). Clinical symptoms were typically observed by 2–3 wk after cessation of CsA therapy.
Isolation of immune cells from the colon.
Intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) were isolated according to a modification of the method of Lefrancois and Lycke (33). Briefly, colons were cleaned by removal of debris and all fecal matter with CMF solution [HBSS, Ca2+- and Mg2+-free 100 mM HEPES (Sigma-Aldrich), 250 mM sodium bicarbonate (Fisher Scientific, Pittsburgh, PA), and 2% FBS (Atlanta Biologicals, Norcross, GA)]. Tissue from two to four mice was pooled within a treatment group, cut longitudinally and then laterally into ∼0.5-cm sections, washed with CMF solution, and placed into CMF solution containing 1 mM DTT (Research Products, Mt. Prospect, IL) and 1 mM EDTA (Sigma-Aldrich). The tissue was then placed in a flask and shaken for 30 min at 37°C. After the flask was shaken, cell-containing supernatant was removed and transferred into 50-ml centrifuge tubes and put on ice. CMF-DTT-EDTA was added to the tissue, and the sample was incubated for an additional 30 min. The supernatant represents the epithelial fraction and contains the IEL. Cells were pooled within a group and concentrated by centrifugation and resuspended in 10% complete medium (RPMI 1640, Cellgro) containing 10% FBS (Atlanta Biologicals), penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively; Life Technologies-Invitrogen, Carlsbad, CA), and 5 mM 2-mercaptoethanol (Sigma). LPL suspensions were prepared by treatment of the deepithelialized intestinal tissue with 20 ml of 10% complete medium containing 240 U/ml collagenase (Sigma) and 2 U/ml DNase I (Roche, Indianapolis, IN) on a shaker for 1 h at 37°C. Supernatant was collected and concentrated by centrifugation. The pellet containing the LPL was resuspended in 10 ml of cold 10% complete medium and kept on ice. The resuspended IEL and LPL were then passed over a 0.2-g nylon wool (Robbins Scientific, Sunnyvale, CA) column and eluted using 5% complete medium. After concentration by centrifugation, the cells were resuspended in 44% Percoll (Sigma-Aldrich), layered onto a 67% Percoll cushion in a 14-ml polystyrene centrifuge tube, and then centrifuged (2,000 rpm) for 30 min at room temperature. IEL and LPL were removed from the 44%–67% Percoll interface and washed with 5% complete medium. The resulting cell population was counted using Trypan blue exclusion and analyzed by flow cytometry, as described below.
Flow cytometry analysis.
Isolated lymphoid cells (1 × 106) from the spleens and colons were washed once using staining buffer (PBS containing 1% FCS and 0.1% NaN3) and incubated for 15 min on ice with 1 μg of Fc Block, an antibody against CD16/CD32 (catalog no. 2.4G2, BD PharMingen, San Diego, CA), to reduce nonspecific staining. The cells were then stained with 1 μg of fluorochrome-conjugated MAb against various lymphoid surface markers and incubated on ice for 30 min. Conjugated MAb against CD4 (CT-CD4) was purchased from Caltag (Burlingame, CA), while conjugated MAb against CD44 (IM7), αβ-T cell receptor complex (TCR) (H57-597), and CD62L (MEL-14) were purchased from BD PharMingen. After incubation, the antibody-cell mixture was washed once with PBS containing 0.1% NaN3 (Sigma) and fixed with 1% paraformaldehyde (Sigma). These MAb were used for two- and three-color analyses using a BD Biosciences FACS Calibur flow cytometer (San Jose, CA).
Tissue immunofluorescence.
For immunofluorescence labeling, tissue was embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) and snap-frozen in liquid nitrogen. Tissues were cut into 10-μm sections, mounted on glass slides, and stored at −20°C. At the time of staining, frozen sections were fixed in 4% paraformaldehyde and then treated with blocking solution containing 2 mg/ml normal donkey serum (Jackson ImmunoResearch, West Grove, PA) and 0.3% Triton X-100 in PBS for 30 min at 4°C. All incubations were carried out in a humidified chamber. After the excess blocking solution was drained, the slides were randomly selected for incubation with appropriate primary antibody. The tissues were stained overnight with primary MAb against FITC-conjugated CD4 (1:1,000 dilution; L3T4, eBioscience, San Diego CA), FITC-conjugated rat IgG1 (1:1,000 dilution; eBioscience), or MAdCAM (1:1,000 dilution; MECA-367, BD PharMingen). For MAdCAM staining, slides were washed twice for 30 min, and a biotin-labeled anti-rat IgG secondary antibody (1:1,000 dilution; anti-rat IgG, BD PharMingen) was applied for 1 h. Sections were washed and covered with color-conjugated streptavidin-peroxidase (1:1,000 dilution; streptavidin-phycoerythrin, Sigma). Primary antibodies were replaced by PBS in negative controls. For CD4 and IgG staining, after incubation with primary antibody, slides were washed twice with PBS for 30 min. Samples were visualized with a ×100 objective on a microscope (Carl Zeiss) and digitized with an AxioCam HR Vision camera (Carl Zeiss MicroImaging, Thornwood, NY). For each experiment, three to four animals were evaluated per experimental group; for each tissue, four to five fields were analyzed.
Real-time PCR.
Total colon RNA was isolated using TRIzol reagent (Invitrogen, Grand Island, NY). From each group, 1 μg of RNA was reverse-transcribed into cDNA using the Promega RT system (Madison, WI). PCR was then performed by combination of 2.5 μl of cDNA with 10 μl of Master Mix [0.5 U of platinum Taq (Invitrogen), dNTPs at 0.2 nM each, 0.2 mM PCR buffer (Idaho Technology, Salt Lake City, UT), and 1× SYBR Green (Molecular Probes, Eugene, OR)] and 1 μM primer. PCR primers were as follows: 5′-TGCACCACCAACTGCTTA-3′ (sense) and 5′-GGATGCAGGGATGATGTTC-3′ (antisense) for GAPDH, 5′-GACACCAGCTTGGGCAGTGT-3′ (sense) and 5′-CAGCATGCCCCGTACAGAG-3′ (antisense) for MAdCAM, 5′-CTGAATACAAAACGATCGCTCAA-3′ (sense) and 5′-GCGTTTAGTGGGCTGTCTATCTG-3′ (antisense) for VCAM-1, 5′-GGGACCACGGAGCCAATT-3′ (sense) and 5′-CTCGGAGACATTAGAGAACAATGC-3′ (antisense) for ICAM, and 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ (sense) and 5′-TGGGAGTAGACAAGGTACAACCC-3′ (antisense) for TNF-α. All primers were purchased from Integrated DNA Technologies (Coralville, IA). Real-time RT-PCR was performed on a LightCycler (Roche Diagnostics, Indianapolis, IN). PCR conditions were as follows: 10 min at 95°C for an initial denaturation step, followed by 50 cycles of 30 s at 95°C (denaturation), 30 s at 60°C (annealing), and 30 s at 72°C (elongation). The amount of TNF-α, VCAM, MAdCAM, and ICAM was normalized to GAPDH calculated by the comparative cycle threshold (CT) method.
Total cell lysis and Western blotting.
Mouse colons were cut into small pieces and then homogenized using a tissue pestle. The tissue was placed in lysis buffer (150 mM NaCl, 1% NP-40, 0.5% sodium dexoycholate, 0.1% SDS, 50 mM Tris, pH 8.0, 10 μg/ml leupeptin, and 1 mM PMSF) and incubated on ice for 2 h with intermittent shaking. Total cell lysates where obtained from the supernatant following centrifugation at 12,000 rpm for 10 min at 4°C in a microcentrifuge (accuSpin, Fisher Scientific). After separation of 30 μg of protein from the total cell lysates on a NuPAGE 10% Bis-Tris polyacrylamide gel, the protein was transferred to a nitrocellulose membrane (IBlot Gel Transfer System, Invitrogen). The membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (PBST). Membranes were then incubated with anti-MAdCAM (1:200 dilution; BD PharMingen) in 5% nonfat milk in PBST overnight at 4°C, washed with PBST, and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The antibody-protein complex was visualized using the Amersham ECL Western Blotting Analysis System (GE Healthcare, Buckinghamshire, UK) and developed onto X-ray film. Membrane-bound protein molecular weight was estimated by comparison against a Western protein standard (MagicMark XP, Invitrogen). To confirm loading, the membranes were stripped (62.5 mM Tris, pH 6.8, 100 mM 2-mercaptoethanol, and 2% SDS at 50°C for 10 min) and reprobed with anti-β-actin (1:10,000 dilution; Sigma-Aldrich). Densitometry of Western blot images was achieved through ImageJ version 1.43 [National Institutes of Health (http://rsb.info.nih.gov/ij/index.html)] analysis. The relative density (fold change) of MAdCAM was calculated as the intensity of the MAdCAM band divided by the intensity of β-actin, giving a protein expression ratio.
SG6 cell line.
The SG6 cell line was established from peripheral lymphoid cells isolated from SGVHD mice exhibiting clinical symptoms of disease. Animals were euthanized, and the spleen and mesenteric lymph nodes were removed and placed into 5% complete medium [RPMI 1640 (Cellgro) containing 5% FBS (Atlanta Biologicals) and penicillin (100 U/ml)-streptomycin (100 μg/ml) (Life Technologies)]. Single-cell suspensions were prepared from pooled spleen, and MLN and the red blood cells were lysed. CD4+ T cells were positively selected using MACS magnetic beads, with the cells being selected on an AutoMACS system (Miltenyi Biotech, Auburn, CA). The purity of the MACS-isolated donor cells was monitored by flow cytometry and found to be >92% CD4+ T cells. Purified CD4+ T cells (1–2 × 106), resuspended in 10% complete medium, were cultured in 24-well, flat-bottomed microtiter plates with 2 × 106 (2,000 cGy) irradiated cecal antigen-pulsed splenic antigen-presenting cells. The cecal antigen was prepared according to the procedure described by Cong et al. (17). Briefly, the ceca were removed from animals and placed in a petri dish containing PBS. The cecal contents were collected by low-speed centrifugation and resuspended in PBS containing DNase I (10 μg/ml; Sigma-Aldrich). Glass beads equal to one-fifth of the volume of the material in the tube were added, and the preparation was sonicated for 5 min (15 s on-15 s off) in a Fisher 550 Dismembrator (Thermo Fisher, Waltham, MA). After sonication, the antigen preparation was centrifuged for 15 min at 10,000 rpm in a Fisher accuSpin microcentrifuge (Thermo Fisher) to remove debris. The supernatant was removed, and the filter was sterilized and analyzed for protein content by Bio-Rad assay (Hercules, CA). Spleen cells isolated from normal C3H/HeN mice were cultured overnight with 200 μg of cecal antigens, washed, irradiated (2,000 cGy), and utilized as described. After 8–10 days of culture, the cells were harvested over Lympholyte M (Atlanta Biologicals). The viable cells were counted and restimulated as described.
Labeling of SG6 cells with CFSE.
Cells were labeled with CFSE according to the manufacturer's instructions (CellTrace CFSE Cell Proliferation Kit, Invitrogen, Eugene, OR). Briefly, a 5 mM CellTrace CFSE stock solution was prepared by dissolving the contents in 18 μl of DMSO (Sigma). Then 5 × 106 cells were incubated with 2.5 μM CFSE in PBS for 6 min at 37°C. After incubation, cells were washed once with 10% complete medium [RPMI 1640 containing penicillin-streptomycin-glutamine and 10% FBS (Atlanta Biologicals)]. The pellet was diluted with five volumes of complete medium, and cells were further incubated for 15 min at 37°C to remove excess dye. The cells were washed with complete medium and resuspended in PBS for use in homing studies.
In vivo homing of labeled SG6 cells.
The in vivo migration of CFSE-labeled SG6 cells was analyzed in tissues of control and CsA-treated mice at day 22 post-BMT. A total of 5 × 106 cells suspended in 0.1 ml of PBS were injected intravenously into the tail of olive oil- or CsA-treated mice at day 21 post-BMT. Mice were killed after 24 h, and the distribution of CFSE-labeled SG6 cells in different organs was measured in snap-frozen tissues microscopically. To determine the role of MAdCAM in the migration of T cells into the colons of CsA-treated mice, animals were irradiated and reconstituted with syngeneic BM and treated with diluent or CsA. Beginning on day 2 after BMT, control and CsA-treated mice were treated intraperitoneally with 100 μg of control rat IgG or anti-MAdCAM (MECA-367, BioXcell, West Lebanon, NH) every other day through day 21 post-BMT. At 2 h before SG6 cell transfer, mice were treated with control Ig or anti-MAdCAM. Tissues were removed from euthanized animals and immediately embedded in OCT compound and frozen in liquid nitrogen. Samples were cut into 10-μm tissue sections, mounted on glass slides, and observed under a fluorescence microscope. Each labeled SG6 cell was counted in 4–10 microscopic fields at ×100 magnification.
Statistical analysis.
Statistical differences between control and CsA-treated samples were determined using unpaired Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Enhanced CD4+αβTCR+ T cells in colons of CsA-treated mice compared with control BMT animals.
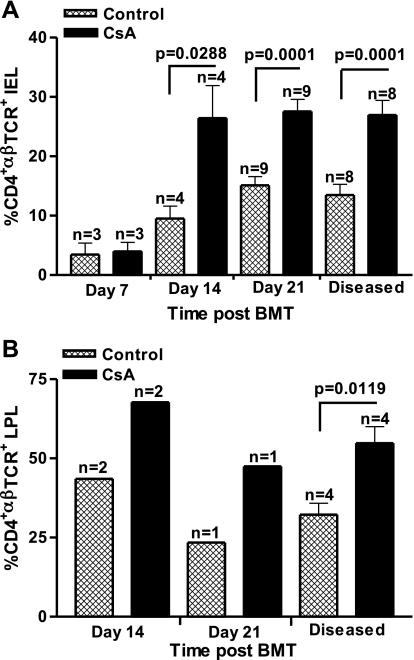
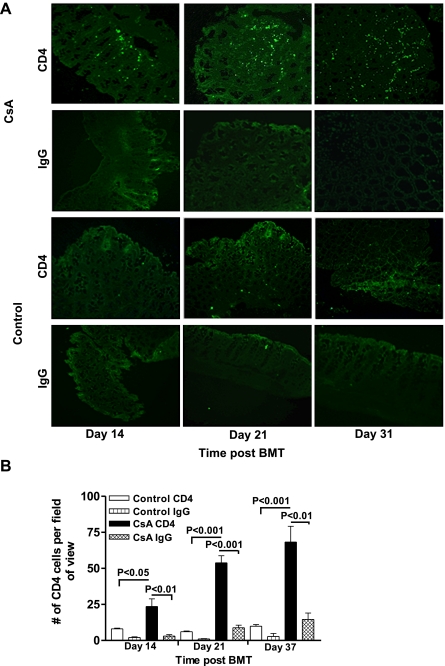
Previous studies showed significant increases in colonic CD4+αβTCR+ T cells isolated from SGVHD mice at 2–4 wk after CsA treatment (5–7 wk post-BMT) relative to control BMT animals, with no changes in the composition of CD8+αβ+ or γδ+ T cells (9). A twofold increase in CD4+ T cells was also observed in the IEL isolated from CsA vs. control BMT mice at 21 days post-BMT at the end of CsA therapy. These studies suggest that increased numbers of CD4+ T cells migrated into the colon of CsA-treated mice during CsA therapy. The functional importance of the migration of CD4+ effector cells into the colon at this time was supported by the findings that in vivo depletion of CD4+, but not CD8+, T cells, during the 21 days of post-BMT CsA therapy inhibited the development of SGVHD (9). Studies were therefore initiated to follow the migration of CD4+ T cells into the colon during the CsA treatment period (days 0–21 post-BMT). For these experiments, colons were isolated from control and CsA-treated mice over the 21 days of CsA therapy, and the IEL and LPLs were isolated and analyzed by flow cytometry for various T cell markers. As shown in Fig. 1A, an increased percentage of CD4+αβTCR+ T cells was found in the colons of CsA-treated mice as early as day 14 post-BMT (P = 0.0288), and this increase was maintained through day 21 post-BMT (P = 0.0001). LPL isolated from CsA-treated vs. control mice also showed an increased percentage of CD4+αβTCR+ T cells (Fig. 1B). IEL and LPL were also analyzed during active disease, confirming previous results of increased infiltration of CD4+ T cells into the large intestine of SGVHD mice compared with control BMT animals (Fig. 1). Immunohistochemical analysis confirmed results from flow cytometric analysis of isolated cells, demonstrating increased CD4+ T cell staining in the colonic tissue of CsA-treated mice compared with control BMT animals at days 14 and 21 post-BMT (Fig. 2A). Similar to the flow cytometry data in Fig. 1, when the CD4+ T cells were counted in immunofluorescence-stained slides, significantly increased numbers of CD4+ cells were found in the CsA-treated vs. control BMT animals at days 14 (P < 0.05), 21 (P < 0.001), and 37 (P < 0.001) post-BMT (Fig. 2B).
Fig. 1.
Enhanced numbers of CD4+ intraepithelial lymphocytes (IEL; A) and lamina propria lymphocytes (LPL; B) isolated from cyclosporin (CsA)-treated animals. C3H/HeN mice were lethally irradiated, reconstituted with syngeneic bone marrow (BM), and treated for 21 days with the diluents, olive oil, or CsA (15 mg/kg ip). At days 7, 14, and 21 after BM transplantation (BMT), colons from 3 animals were isolated from control and CsA-treated mice and pooled within each experimental group. After isolation, IEL and LPL were stained with MAb for CD4 and αβ-T cell receptor complex (TCR) and analyzed by flow cytometry. Data (means ± SE) represent results of pooled experiments; n represents number of experiments.
Fig. 2.
Elevated CD4 expression in colonic tissue of CsA-treated mice compared with BMT controls. Immunofluorescence staining revealed increased CD4+ T cells in colonic tissue of CsA-treated mice. A: colonic tissue from BMT control and CsA-treated animals was collected at days 14, 21, and 37, and frozen sections were stained for the presence of CD4+ T cells. Levels of CD4 staining were increased in colonic tissue from CsA-treated compared with control BMT animals. Data are representative of 6 animals from 2 individual experiments. B: number of CD4+ T cells within each group. Data are from 2–3 fields of view from 3–4 animals within each group. Statistical difference was determined by 1-way ANOVA.
Increased proinflammatory mediators and CAMs during SGVHD.
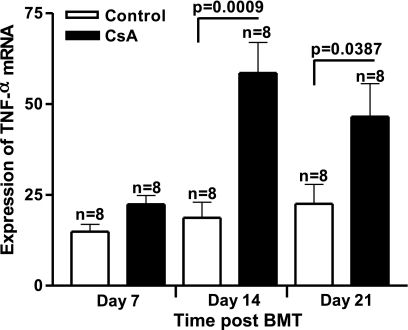
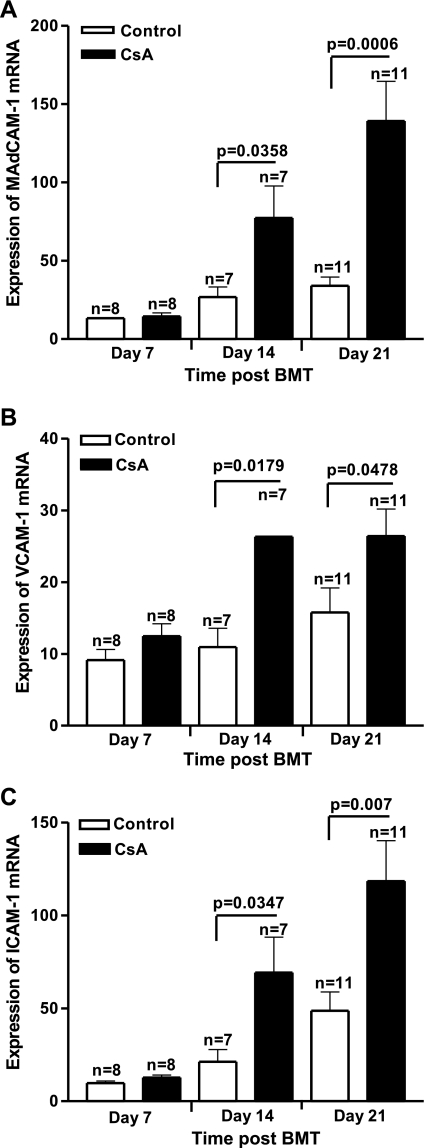
Total body irradiation can affect the severity of many diseases by amplifying the dysregulation of inflammatory cytokines. Total body irradiation, along with allogeneic immune cells, can damage the gastrointestinal (GI) tract and, thereby, allow for increased translocation of LPS into the systemic circulation, leading to increased levels of TNF-α (30). Irradiation has also been shown to stimulate macrophage production of proinflammatory cytokines in a dose-dependent manner (43). Several in vivo and in vitro studies have demonstrated that endothelial cells respond to stimuli such as irradiation, LPS, and TNF-α by becoming activated or undergoing apoptosis (21, 40). In the SGVHD model, increased bacterial leakage into the liver was observed during CsA therapy, suggesting increased permeability of the colon in these animals (25). TNF-α is an inflammatory cytokine capable of inducing cell adhesion molecules such as VCAM, ICAM (14), and MAdCAM (44). Studies were therefore designed to examine the levels of adhesion molecules, TNF-α, and proinflammatory chemokines in colonic tissue of CsA-treated and control BMT mice. Real-time RT-PCR studies were performed to determine if increases in mRNA for TNF-α and CAMs could be detected in the colon of CsA-treated animals. Results from a time-course study revealed significantly increased mRNA levels for TNF-α at days 14 and 21 post-BMT in colonic tissue of CsA-treated mice compared with BMT controls (P = 0.0009 and P = 0.0387 at days 14 and 21 post-BMT, respectively; Fig. 3). To confirm the PCR finding of increased TNF-α mRNA levels in CsA-treated animals in the early post-BMT period, colon explant cultures were initiated with tissue obtained from control and CsA-treated animals, and the supernatant was analyzed 24 h later by ELISA for TNF-α (see Supplemental Fig. S1 in Supplemental Material for this article, available online at the Journal website). TNF-α was increased in these cultures as early as day 7 post-BMT and increased through day 21 post-BMT. Concomitant with the increase in this proinflammatory cytokine, mRNA levels of CAMs were increased in colons of CsA-treated mice as early as day 14 post-BMT compared with control mice (Fig. 4). MAdCAM levels analyzed by real-time RT-PCR revealed significantly increased mRNA at days 14 and 21 post-BMT in CsA-treated mice compared with BMT controls (P = 0.0358 and P = 0.0006 at days 14 and 21 post-BMT, respectively; Fig. 4A). Similar results were observed for VCAM (P = 0.0179 and P = 0.0478 at days 14 and 21 post-BMT, respectively; Fig. 4B) and ICAM (P = 0.0347 and P = 0.0070 at days 14 and 21 post-BMT, respectively; Fig. 4C). To determine if increased mRNA for CAM resulted in increased protein expression, changes in MAdCAM expression at days 7, 14, and 21 post-BMT in tissues from normal, normal CsA-treated, control BMT, and CsA-treated BMT animals were determined by Western blot analysis. As shown in Fig. 5A, the level of MAdCAM increased in the CsA-treated BMT animals with time through day 21 post-BMT compared with normal, control BMT, and normal CsA-treated animals. MAdCAM expression was significantly increased in the colons obtained from CsA-treated BMT animals at days 14 and 21 post-BMT (Fig. 5B). Interestingly, while not significant, MAdCAM was consistently elevated in normal CsA-treated animals relative to normal or control BMT mice. These results, as well as those obtained from immunofluorescence staining of MAdCAM-1 (see Supplemental Fig. S2), confirmed elevated levels of CAMs in colonic tissue of CsA-treated mice.
Fig. 3.
Increased production of TNF-α in colonic tissue of CsA-treated mice. C3H/HeN mice were induced for syngeneic graft vs. host disease (SGVHD). Tissue isolated from distal colon at days 7, 14, and 21 during CsA treatment was analyzed by real-time RT-PCR for the presence of mRNA for TNF-α. Data are means ± SE; n represents number of animals analyzed in each group pooled from 2 independent experiments.
Fig. 4.
Increased mRNA expression of cell adhesion molecules (CAMs) in colon of CsA-treated animals. RNA was isolated from colons of control BMT and CsA-treated animals at days 7, 14, and 21 post-BMT. Real-time RT-PCR was performed for mucosal addressin CAM (MAdCAM; A), VCAM (B), and ICAM (C). Data (means ± SE) represent pooled results of 2 experiments; n represents number of animals analyzed in each group.
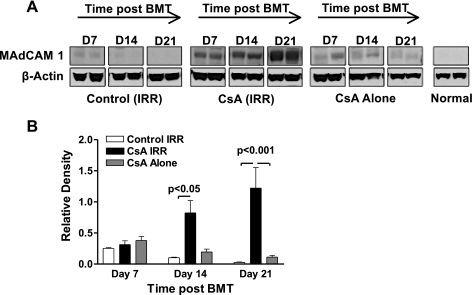
Fig. 5.
Expression of MAdCAM after BMT and CsA treatment. A: total cell lysates were extracted from colons of treated mice at days 7, 14, and 21 post-BMT. Cell proteins were separated electrophoretically on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Expression of MAdCAM protein was identified using specific antibody. Blot shows 2 individual animals that are representative of 4 animals analyzed within each treatment group. Control (IRR) indicates irradiated, bone marrow transplant control mice and CsA (IRR) indicates irradiated, bone marrow transplant CsA-treated mice. B: relative densities of Western blot bands quantified using ImageJ version 1.43 (National Institutes of Health). Relative density was calculated as density of MAdCAM divided by density of β-actin for each individual sample. Data represent results from 4 mice within each treatment group. Statistical difference was determined by 1-way ANOVA.
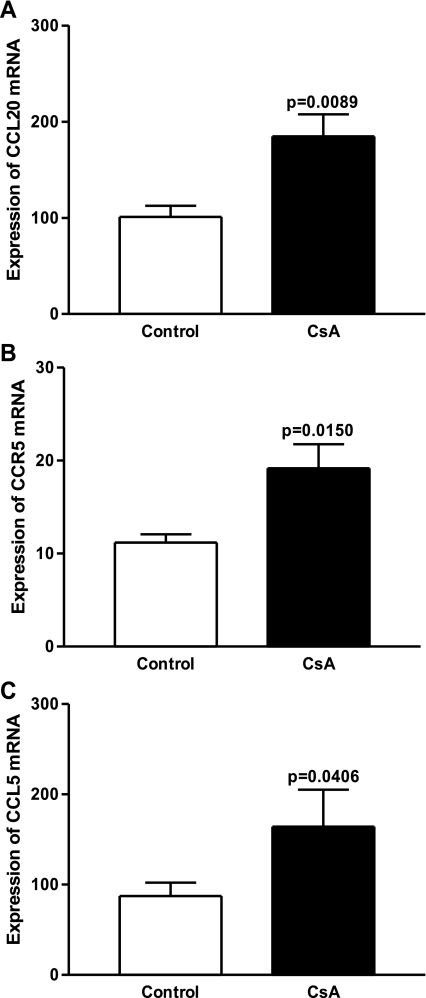
Chemokines play an important role in lymphocyte trafficking to specific sites, regulating immune responses and homeostasis. Radiation therapy is capable of disrupting the epithelial integrity, leading to an inflammatory response and, hence, increased expression of proinflammatory cytokines and chemokines (12, 21, 40). The constitutive presence of these regulators allows for the trafficking of lymphocytes into the GI mucosal compartment, disrupting the physiological balance and contributing to pathogenesis such as IBD (31, 41). Since many studies have confirmed increases in proinflammatory chemokines (i.e., CCL20, CCR6, CCR5, CCL5) during gut inflammation, experiments were performed to investigate if chemokines associated with mucosal homing were upregulated during CsA treatment. Figure 6 shows significant increases in mRNA levels of CCL20 (P = 0.0089) and CCR5 and its ligand CCL5 (P = 0.0150 and P = 0.0406, respectively) in the gut of CsA-treated mice compared with controls at day 21 post-BMT. This significant increase in mediators and ligands in CsA-treated vs. control BMT mice suggests a role for these molecules in the enhanced migration of CD4+ T cells into the colon of CsA-treated animals.
Fig. 6.
Induction of SGVHD resulted in increased production of proinflammatory chemokines in the colon. mRNA was isolated from colons of control BMT and CsA-treated animals at day 21 post-BMT. Real-time RT-PCR was performed for CCL20 (A), CCR5 (B), and CCL5 (C). Data are means ± SE from pooled samples from 2 experiments; n = 6.
Increased homing of labeled SGVHD T cells into the colons of CsA-treated mice.
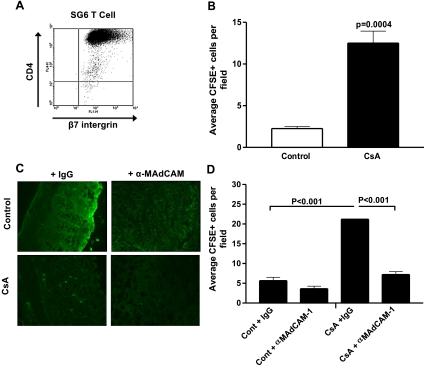
An increase in the migration of CD4+ T cells into the colons of CsA-treated mice compared with control BMT animals was observed as early as day 14 post-BMT, and this increase correlated with an increase in CAMs and chemokines associated with mucosal homing. Studies were undertaken to determine if increases in these molecules were indeed functional. Since increased homing of CD4+ T cells, along with increased β7-integrin expression (unpublished data), was observed in the colonic tissue of CsA-treated mice, we hypothesized that the most likely CAM involved in the trafficking of these lymphocytes to the gut was MAdCAM, which has been shown to be upregulated in gut inflammation (2, 45). For these experiments, a long-term microbial antigen-specific CD4+ T cell line (SG6) was generated from SGVHD animals and used to study the homing of lymphocytes into several organs of control and CsA-treated mice. Phenotypically, the CD4+ T cell line (SG6) was β7-integrin+ (Fig. 7A) and had an activated phenotype (CD62L−, CD44hi; data not shown). To determine if these CD4+β7-integrin+ SGVHD T cells would migrate at a higher frequency to the colon of CsA-treated than control animals, CFSE-labeled SG6 cells were injected intravenously into the tail of control and CsA-treated mice at day 21 post-BMT. Increased numbers of SG6 labeled cells migrated into the colons of CsA-treated mice compared with control BMT animals (Fig. 7B; P = 0.0004). To determine if the enhanced CAM expression that is observed in the CsA-treated mice during CsA treatment mediated increased migration of CD4+ T cells in these mice, animals were treated with anti-MAdCAM or control IgG during the immediate BMT period. At this time (day 21) post-BMT, expression of CAMs and chemokines is increased in CsA-treated mice (Figs. 4–6). Tissues were isolated 24 h after injection of SG6 and analyzed by fluorescence microscopy. Importantly, when immunoblockade of MAdCAM was performed, decreased migration of SG6 cells was observed (Fig. 7C). When labeled SG6 cells were enumerated, a significant increase in the migration of these labeled CD4+ T cells into the colonic tissue of CsA-treated mice (P < 0.001) was observed compared with control BMT animals (Fig. 7D), and immunoblockade of MAdCAM significantly reduced T cell migration (P < 0.001). These results support the observation that increased CAM expression was functional for the migration of CD4+ T cells into the colons of CsA-treated mice during the induction of SGVHD.
Fig. 7.
Immunoblockade of MAdCAM inhibits migration of CD4+β7-integrin+ SGVHD T cell line to colon of CsA-treated animals. A: SGVHD T cell line SG6 was harvested over Ficoll and analyzed by 2-color flow cytometry for CD4 and β7-integrin. Markers were set using unstained cells. Data are representative of 3 independent experiments. B: SG6 cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and injected intravenously into the tail of control or CsA-treated animals at day 21 post-BMT. After 24 h, SG6 cell distribution to the colon was visualized under a fluorescence microscope. Labeled SG6 cells were counted within 4–7 microscope fields. Values are means ± SE from 2 independent experiments; n = 4 per experimental group. C: C3H/HeN mice were induced for SGVHD. Beginning on day 2 post-BMT, recipients were injected intraperitoneally with 100 μg of control rat IgG or anti-MAdCAM (MECA-367, α-MAdCAM) every other day through day 21 post-BMT. SG6 cells were labeled with CFSE injected intravenously into the tail of control or CsA-treated animals at day 21 post-BMT. After 24 h, their distribution to the colon was visualized under a fluorescence microscope (×250 magnification). D: number of labeled SG6 cells within a microscope field (10 fields/tissue). Values are means ± SE from a total of 3 animals per experimental group. Statistical significance was determined by 1-way ANOVA
DISCUSSION
Leukocyte homing from the blood to different compartments of the body is governed by complex signal cascades and interactions of surface molecules on inflamed tissue and their respective ligands on the surface of lymphocytes (10, 20, 36). It has been observed that proportions of CD4+ T cells are increased in colons of SGVHD mice compared with BMT controls and that CD4+ T cells were responsible for the induction of murine SGVHD (3, 4, 9). The role of CD4+ T cells as effector cells in the induction of murine SGVHD was demonstrated in two sets of experimental studies. First, CD4+, but not CD8+, T cell depletion during the 21 days of CsA therapy inhibited SGVHD-induced colitis (9). Additional studies showed that murine SGVHD could be transferred to secondary recipients with CD4+, but not CD8+, T cells isolated from diseased animals (4). Given the role of CD4+ T cells in the induction of SGVHD, studies were designed to follow up on the observation that CD4+ T cells accumulate in the colon during CsA therapy (9) and to study potential mechanisms involved in trafficking of these cells into the colon of CsA-treated mice. Longitudinal studies demonstrated increased numbers of CD4+ T cells in IEL and LPL isolated from CsA-treated mice as early as day 14 post-BMT compared with control transplanted animals. Concurrent with these observations was an increase in the production of proinflammatory cytokines and chemokines and a functional increase in CAM in the colons of CsA-treated animals.
SGVHD is an inducible syndrome that develops after lethal irradiation and reconstitution with syngeneic BM followed by 21 days of treatment with the immunosuppressive agent CsA. Pretransplant radiation and CsA therapy are absolute requirements for induction of SGVHD (4, 6, 15, 23, 28). Radiation conditioning has been shown to induce the upregulation of transcription factors and proinflammatory cytokines (30, 48, 50). The other component of the SGVHD induction protocol, CsA, is a calcineurin inhibitor, which, as a class, mediates toxicities in several tissues via the induction of oxidative stress (13, 46, 47) and transcription factors, including NF-κB (19, 34, 49) and inflammatory cytokines (16, 32, 37). Given the interrelationship between oxidative stress and cytokines and the induction of CAM expression (1, 35, 38, 42, 45), the potential exists that the unique interaction between pretransplant irradiation and CsA therapy could result in the generation of an inflammatory environment, leading to increased CAM expression and increased migration of effector cells into the colon of CsA-treated animals in the early post-BMT period. While it is perhaps surprising that T cells are migrating at increased numbers into the colon during CsA therapy, treatment with anti-T cell MAb after cessation of CsA had no effect on disease induction. However, consistent with the findings presented here, depletion of CD4+ T cells during CsA therapy inhibited disease, demonstrating the functional significance of T cell migration into the intestinal tract during this period (9).
Numerous studies have shown the important role of CAMs in lymphocyte homing to inflamed tissues (2, 10, 21, 22, 27, 45). Since our studies concentrated on the colon, we focused on the expression of MAdCAM, because it has been demonstrated to be essential in the induction of intestinal GVHD (22). Eyrich et al. (22) showed that irradiation induces the increased expression of VCAM and ICAM, but not MAdCAM, by 3 days after radiation conditioning and syngeneic BMT. Additional signals involved in the induction of allogeneic GVHD were required for the upregulation of MAdCAM in these studies. In the SGVHD model, CsA therapy appeared to provide additional signals that resulted in the increased expression of CAM by day 14 post-BMT in the colons of CsA-treated, BMT animals. Concurrent with these findings, significantly elevated levels of CD4+ T cells were also observed in the colon of CsA-treated mice at this early time point (Figs. 1 and 2). In contrast to the CsA therapy of BMT mice, preliminary studies showed that treatment of normal, nonirradiated animals with CsA did not induce the influx of CD4+ cells into the colon (data not shown), further demonstrating the requirement for pretransplant radiation conditioning and CsA treatment in the development of SGVHD (4, 6, 23, 24). In addition, TNF-α mRNA levels were also significantly elevated by day 14 post-BMT in the colon of CsA-treated mice compared with transplant control animals (Fig. 3). Since the level of this proinflammatory molecule was significantly elevated during CsA treatment, preliminary experiments were performed to analyze the possible role of TNF-α in the induction phase of SGVHD. However, attempts to decrease TNF-α by in vivo neutralization were associated with a high mortality rate in the treated animals. Similar results have been reported in which TNF neutralization in the early post-BMT period resulted in mortality in some of the treated animals (18). These results suggest that TNF-α has a protective effect during the early stages after BMT and is essential for recipient survival.
The mechanism by which effector T cells are recruited into the gut during the inflammatory response of SGVHD likely involves chemokine gradients. Chemokine interactions with their corresponding receptors have been implicated in many inflammatory processes via their ability to attract effector cells to the sites of inflammation (10, 41, 51). Several studies have been able to characterize chemokines highly expressed during gut inflammation, which include CCR5, CCL3, CCL5, and CCL20 (41, 51). mRNA levels of chemokines associated with gut inflammation were significantly increased in the colon of CsA-treated mice by day 21 post-BMT compared with control. While activated lymphocytes expressing CCR5 were present by day 14 post-BMT, its counterligands released by the tissue were not yet upregulated. Thus we cannot discard the possibility that other mediators are involved in the recruitment of these CD4+ T cells to the inflamed tissue of CsA-treated mice, as high levels of redundancy are involved in chemokine receptor function and inflammatory responses (51).
To determine if the increases in CAMs and chemokines were functional during the SGVHD induction period, effector CD4+ T cells isolated from diseased animals were used for lymphocyte trafficking studies. Previous results (4) and unpublished data have demonstrated an increased frequency of reactivity of CD4+ T cells in lymphoid cells isolated from SGVHD animals against microbial antigens isolated from the cecum of normal mice. Phenotypic analysis of these cells showed that they express a β7-integrin, which would allow the interaction between these lymphocytes and the CAM (MAdCAM), which is upregulated in the colonic tissue of CsA-treated mice. Homing studies performed by intravenous inoculation of the CFSE-labeled CD4+ SGVHD T cell line at day 21 post-BMT revealed their preferential migration into the colon of CsA-treated mice compared with control BMT animals. Immunoblockade of MAdCAM during CsA therapy inhibited the enhanced migration of the CD4+ T cell line into the colon of CsA-treated animals, demonstrating a functional role for the increased CAMs and inflammatory mediators present in the intestines of CsA-treated mice.
This study represents a first demonstration that the CD4+ T cells responsible for the pathogenesis observed in murine SGVHD are increased as early as day 14 post-BMT in colons of CsA-treated mice compared with BMT control animals, suggesting that, during the 21 days of immunosuppressive therapy, these CD4+ T cells are migrating into, or expanding in, the intestines of these animals in a CsA-resistant manner. Furthermore, this study demonstrated that increased expression of CAMs and chemokines is involved in the colonic inflammation observed during the induction of SGVHD and that CAMs and chemokines may serve as potential therapeutic targets for novel approaches of GI diseases that are T cell-mediated (i.e., IBD, GVHD). Thus, after conditioning and syngeneic BMT and during CsA therapy, there is an ordered increase in the expression of inflammatory mediators and CAMs that is associated with the increased migration of effector cells into the colon, leading to the development of the colonic inflammation associated with murine SGVHD.
GRANTS
This work was supported by National Cancer Institute Grant PO1 CA-092372 (J. S. Bryson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Ando T, Jordan P, Wang Y, Itoh M, Joh T, Sasaki M, Elrod JW, Carpenter A, Jennings MH, Minagar A, Alexander JS. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis 11: 258–264, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. α4β7-Integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74: 185–195, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Brandon JA, Jennings CD, Perez J, Caywood B, Alapat D, Kaplan AM, Bryson JS. Induction of murine syngeneic graft-versus-host disease by cells of recipient origin. Transplantation 83: 1620–1627, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bryson JS, Jennings CD, Brandon JA, Perez J, Caywood BE, Kaplan AM. Adoptive transfer of murine syngeneic graft-vs.-host disease by CD4+ T cells. J Leukocyte Biol 82: 1393–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bryson JS, Jennings CD, Caywood BE, Kaplan AM. Induction of a syngeneic graft-versus-host disease-like syndrome in DBA/2 mice. Transplantation 48: 1042–1047, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Bryson JS, Jennings CD, Caywood BE, Kaplan AM. Strain specificity in the induction of syngeneic graft-versus-host disease in mice. Transplantation 51: 911–913, 1991 [PubMed] [Google Scholar]
- 8. Bryson JS, Jennings CD, Caywood BE, Kaplan AM. Thy1+ bone marrow cells regulate the induction of murine syngeneic graft-versus-host disease. Transplantation 56: 941–945, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Bryson JS, Zhang L, Goes SW, Jennings CD, Caywood BE, Carlson SL, Kaplan AM. CD4+ T cells mediate murine syngeneic graft-versus-host disease-associated colitis. J Immunol 172: 679–687, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 272: 60–66, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol 72: 209–253, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Cantisani V, Mortele KJ, Viscomi SG, Glickman J, Silverman SG, Ros PR. Rectal inflammation as first manifestation of graft-vs.-host disease: radiologic-pathologic findings. Eur Radiol 13 Suppl 4: L75–L78, 2003 [PubMed] [Google Scholar]
- 13. Chen C, Reddy KS, Johnston TD, Khan TT, Ranjan D. Vitamin E inhibits cyclosporin A and H2O2 promoted Epstein-Barr virus (EBV) transformation of human B cells as assayed by EBV oncogene LMP1 expression. J Surg Res 113: 228–233, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Chen XL, Zhang Q, Zhao R, Ding X, Tummala PE, Medford RM. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-α in endothelial cells. J Pharmacol Exp Ther 305: 573–580, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Cheney RT, Sprent J. Capacity of cyclosporine to induce auto-graft-versus-host disease and impair intrathymic T cell differentiation. Transplant Proc 17: 528–530, 1985 [Google Scholar]
- 16. Chung BH, Li C, Sun BK, Lim SW, Ahn KO, Yang JH, Choi YH, Yoon KH, Sugawara A, Ito S, Kim J, Yang CW. Rosiglitazone protects against cyclosporine-induced pancreatic and renal injury in rats. Am J Transplant 5: 1856–1867, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med 187: 855–864, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, Brewer JP, Ferrara JL. Hyporesponsiveness of donor cells to lipopolysaccharide stimulation reduces the severity of experimental idiopathic pneumonia syndrome: potential role for a gut-lung axis of inflammation. J Immunol 165: 6612–6619, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Corsini E, Viviani B, Marinovich M, Galli CL. Cyclosporin A exacerbates skin irritation induced by tributyltin by increasing nuclear factor-κB activation. J Invest Dermatol 117: 1627–1634, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol 29: 87–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eissner G, Lindner H, Behrends U, Kolch W, Hieke A, Klauke I, Bornkamm GW, Holler E. Influence of bacterial endotoxin on radiation-induced activation of human endothelial cells in vitro and in vivo: protective role of IL-10. Transplantation 62: 819–827, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Eyrich M, Burger G, Marquardt K, Budach W, Schilbach K, Niethammer D, Schlegel PG. Sequential expression of adhesion and costimulatory molecules in graft-versus-host disease target organs after murine bone marrow transplantation across minor histocompatibility antigen barriers. Biol Blood Marrow Transplant 11: 371–382, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Fischer AC, Beschorner WE, Hess AD. Requirements for the induction and adoptive transfer of cyclosporine-induced syngeneic graft-versus-host disease. J Exp Med 169: 1031–1041, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fischer AC, Laulis MK, Horwitz L, Beschorner WE, Hess A. Host resistance to cyclosporine induced syngeneic graft-versus-host disease. Requirement for two distinct lymphocyte subsets. J Immunol 143: 827–832, 1989 [PubMed] [Google Scholar]
- 25. Flanagan DL, Gross R, Jennings CD, Caywood BE, Goes S, Kaplan AM, Bryson JS. Induction of syngeneic graft-versus-host disease in LPS hyporesponsive C3H/HeJ mice. J Leukoc Biol 70: 873–880, 2001 [PubMed] [Google Scholar]
- 26. Flanagan DL, Jennings CD, Bryson JS. Th1 cytokines and NK cells participate in the development of murine syngeneic graft-versus-host disease. J Immunol 163: 1170–1177, 1999 [PubMed] [Google Scholar]
- 27. Gironella M, Molla M, Salas A, Soriano A, Sans M, Closa D, Engel P, Pique JM, Panes J. The role of P-selectin in experimental colitis as determined by antibody immunoblockade and genetically deficient mice. J Leukoc Biol 72: 56–64, 2002 [PubMed] [Google Scholar]
- 28. Glazier A, Tutschka PJ, Farmer ER, Santos GW. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med 158: 1–8, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med 205: 2483–2490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 90: 3204–3213, 1997 [PubMed] [Google Scholar]
- 31. Kang SG, Piniecki RJ, Hogenesch H, Lim HW, Wiebke E, Braun SE, Matsumoto S, Kim CH. Identification of a chemokine network that recruits FoxP3+ regulatory T cells into chronically inflamed intestine. Gastroenterology 132: 966–981, 2007 [DOI] [PubMed] [Google Scholar]
- 32. LaSpina M, Tripathi S, Gatto LA, Bruch D, Maier KG, Kittur DS. An interleukin-6-neutralizing antibody prevents cyclosporine-induced nephrotoxicity in mice. J Surg Res 148: 121–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. In: Current Protocols in Immunology, edited by Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. New York: Wiley, 1997, p. 3.19.11–13.19.16 [DOI] [PubMed] [Google Scholar]
- 34. Lim SW, Li C, Ahn KO, Kim J, Moon IS, Ahn C, Lee JR, Yang CW. Cyclosporine-induced renal injury induces Toll-like receptor and maturation of dendritic cells. Transplantation 80: 691–699, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Lin SJ, Shyue SK, Hung YY, Chen YH, Ku HH, Chen JW, Tam KB, Chen YL. Superoxide dismutase inhibits the expression of vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 induced by tumor necrosis factor-α in human endothelial cells through the JNK/p38 pathways. Arterioscler Thromb Vasc Biol 25: 334–340, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4: 360–370, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Muraoka K, Fujimoto K, Sun X, Yoshioka K, Shimizu K, Yagi M, Bose H, Jr, Miyazaki I, Yamamoto K. Immunosuppressant FK506 induces interleukin-6 production through the activation of transcription factor nuclear factor (NF)-κB. Implications for FK506 nephropathy. J Clin Invest 97: 2433–2439, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oshima T, Pavlick KP, Laroux FS, Verma SK, Jordan P, Grisham MB, Williams L, Alexander JS. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol 281: C1096–C1105, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for β7-integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol 158: 2099–2106, 1997 [PubMed] [Google Scholar]
- 40. Robaye B, Mosselmans R, Fiers W, Dumont JE, Galand P. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am J Pathol 138: 447–453, 1991 [PMC free article] [PubMed] [Google Scholar]
- 41. Scheerens H, Hessel E, de Waal-Malefyt R, Leach MW, Rennick D. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease: IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+ CD45RBhigh T cells. Eur J Immunol 31: 1465–1474, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Sellak H, Franzini E, Hakim J, Pasquier C. Reactive oxygen species rapidly increase endothelial ICAM-1 ability to bind neutrophils without detectable upregulation. Blood 83: 2669–2677, 1994 [PubMed] [Google Scholar]
- 43. Shan YX, Jin SZ, Liu XD, Liu Y, Liu SZ. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: dose-response relationship, mechanisms and implications. Radiat Environ Biophys 46: 21–29, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-α and IL-1. J Immunol 151: 5239–5250, 1993 [PubMed] [Google Scholar]
- 45. Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol 148: 1819–1838, 1996 [PMC free article] [PubMed] [Google Scholar]
- 46. Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A. N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant 14: 923–929, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Wolf A, Trendelenburg CF, Diez-Fernandez C, Prieto P, Houy S, Trommer WE, Cordier A. Cyclosporine A-induced oxidative stress in rat hepatocytes. J Pharmacol Exp Ther 280: 1328–1334, 1997 [PubMed] [Google Scholar]
- 48. Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood 83: 2360–2367, 1994 [PubMed] [Google Scholar]
- 49. Zhang Y, Sun X, Muraoka K, Ikeda A, Miyamoto S, Shimizu H, Yoshioka K, Yamamoto K. Immunosuppressant FK506 activates NF-κB through the proteasome-mediated degradation of IκBα. Requirement for IκBα N-terminal phosphorylation but not ubiquitination sites. J Biol Chem 274: 34657–34662, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Zhou D, Brown SA, Yu T, Chen G, Barve S, Kang BC, Thompson JS. A high dose of ionizing radiation induces tissue-specific activation of nuclear factor-κB in vivo. Radiat Res 151: 703–709, 1999 [PubMed] [Google Scholar]
- 51. Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis 14: 1000–1011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.