Abstract
The brush border domain at the apex of intestinal epithelial cells is the primary site of nutrient absorption in the intestinal tract and the primary surface of interaction with microbes that reside in the lumen. Because the brush border is positioned at such a critical physiological interface, we set out to create a comprehensive list of the proteins that reside in this domain using shotgun mass spectrometry. The resulting proteome contains 646 proteins with diverse functions. In addition to the expected collection of nutrient processing and transport components, we also identified molecules expected to function in the regulation of actin dynamics, membrane bending, and extracellular adhesion. These results provide a foundation for future studies aimed at defining the molecular mechanisms underpinning brush border assembly and function.
Keywords: cytoskeleton, intestine, myosin, actin, microvilli
the intestinal tract is lined by a continuous sheet of transporting epithelial cells, also known as “enterocytes,” which are responsible for processing and moving nutrients from the lumenal space into the vasculature for distribution to peripheral tissues. In the fully differentiated state, each enterocyte is characterized by a striking apical structure that consists of thousands of microvilli (61). These actin-based protrusions extend from the surface of the cell into the lumen, forming a cytoskeletal scaffold that is capable of supporting an extraordinarily large apical membrane surface area. In addition to the clear benefit with regard to housing membrane-bound enzymes, transporters, and channels required for enterocyte function, recent studies have shown that microvilli also function as vesicle-generating organelles, releasing membranes laden with host defense machinery into the intestinal lumen (55).
All membrane-associated hydrolases, proteases, and lipases required for nutrient processing traffic from the trans-Golgi to the base of the brush border using the microtubule cytoskeleton (74). There they are incorporated into the brush border membrane with a topology that positions their catalytic domains on the outside of the cell (40). The numerous channels and transporter proteins involved in moving nutrients from the lumen into the enterocyte are also highly enriched in the brush border membrane. The catalytic activities and functions of nutrient processing/transporting enzymes have been the focus of study for decades, in part because of the abundance and accessibility of these proteins for purification from native tissues (39). Moreover, investigators have been exploring the biosynthetic mechanisms that enable specific membrane proteins to enrich in the brush border membrane for many years (19, 32). While these aspects of the brush border have received a great deal of experimental attention, a number of fundamental questions about brush border assembly and function remain unanswered and, in many cases, entirely unexplored.
For example, it is now generally assumed that after their delivery to the base of the brush border, membrane proteins are distributed along the microvillar axis through interactions with myosin motor proteins (100). Indeed, recent studies have shown that myosin-1a, one of the most abundant motors expressed in the enterocyte, is capable of exerting plus end-directed force on the microvillar membrane (56). However, “intramicrovillar transport” has yet to be observed directly in living cells, and the regulatory machinery that controls the distribution of membrane-associated proteins is still poorly understood. Another example of a critical unanswered question relates to the assembly of the brush border during enterocyte differentiation. The brush border represents one of the most highly ordered F-actin arrays known to biologists; microvilli are so tightly packed in terminally differentiated enterocytes that there is no free space between adjacent structures. When viewed in cross section, one can observe the hexagonal arrays that reveal the tight packing of these protrusions. Remarkably, there is little or no information on how microvillar actin bundles are nucleated, how microvillar length is controlled, or how the microvilli achieve perfect hexagonal packing during enterocyte differentiation.
One promising approach for developing our understanding of brush border assembly and function is to elucidate the brush border proteome. Indeed, previous studies (5, 23) have already taken steps in this direction with the proteomic analysis of microvillar membrane vesicles or lipid raft preparations. Additional studies (17, 69, 94) have analyzed brush border membranes isolated from other organs including placenta and kidney. These studies have revealed that a remarkably diverse complement of membrane-associated signaling, scaffolding, and motor proteins resides in this domain. One major limitation associated with all previous studies is that these data sets were collected using material that was purposefully depleted in cytoskeletal components. To date, the proteome of the entire brush border, i.e., the intact actin cytoskeleton complete with the overlaying apical membrane, has not been described.
Here, we report the complete brush border proteome obtained using two-dimensional (2-D) liquid chromatography tandem mass spectrometry (LC-MS/MS). Intact brush borders were isolated from adult mice using a well-established combination of differential and density gradient centrifugation (56). We applied stringent criteria to the resulting data set so that listed proteins were expected to have a high likelihood of validation through other methods. The utility of this strategy was supported by immunofluorescence validation of specific novel proteins in a human intestinal epithelial cell culture model. Similar to previous analyses of microvillar membrane fractions (5, 23, 69), numerous proteins involved in nutrient processing and transport were identified. Our analysis also revealed several proteins implicated in the regulation of actin dynamics, membrane/actin interactions, membrane bending, and extracellular adhesion. In addition to accelerating our understanding of brush border assembly, maintenance, and function, these data are expected to provide insights into the mechanisms underlying intestinal pathologies characterized by brush border damage or effacement, including celiac disease and enteric infections (e.g., enteropathogenic Escherichia coli) (78, 79).
MATERIALS AND METHODS
Brush Border Isolation From the Mouse Small Intestine
All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted. Brush borders were isolated from mouse small intestinal tissues using protocols originally developed by Miller and Crane (59) over 50 yr ago (Fig. 1). During a single brush border preparation, 20–25 adult 129 sv/j mice were euthanized with CO2 followed by cervical dislocation in accordance with Vanderbilt Institutional Animal Care and Use Committee-approved protocols. The full length of the small intestine was rapidly dissected, flushed in cold saline (150 mM NaCl, 2 mM imidazole-Cl, and 0.02% Na-azide), and then soaked in cold dissociation solution (200 mM sucrose, 0.02% Na-azide, 12 mM EDTA-K, 18.9 mM KH2PO4, and 78 mM Na2HPO4; pH 7.2) for at least 30 min. A coarse screen was used to separate released enterocytes from remnant tissue; cells were then washed using multiple rounds of low-speed sedimentation (200 g for 10 min, X-15R centrifuge, Beckman Coulter) and resuspended in fresh dissociation solution. Washed cell pellets were resuspended in cold homogenization buffer (10 mM imidazole, 4 mM EDTA-K, 1 mM EGTA-K, 0.02% Na-azide, 1 mM DTT, and 1 mM Pefabloc-SC; pH 7.2) and homogenized in a blender (Waring) with 4 × 15-s pulses. At this point in the preparation, we typically used light microscopy to confirm that brush borders were physically separated from each other, nuclei, and other cellular fragments. Brush borders were collected from the enterocyte homogenate by centrifugation at 1,000 g for 10 min. The resulting pellets were enriched primarily with brush borders and nuclei; this material was washed several times with solution A (75 mM KCl, 10 mM Imidazole, 1 mM EGTA, 5 mM MgCl2, and 0.02% Na-azide; pH 7.2). To separate nuclei from intact brush borders, sucrose was added to this fraction to a final concentration of 50%. Samples were overlayed with 40% sucrose in solution A and centrifuged at 130,000 g for 1 h at 4°C (L8–70M, Beckman Coulter). The resulting 40%/50% interface (Fig. 2A) contained isolated brush borders that were mostly free of nuclei. Brush borders were washed several times with fresh solution A to remove residual sucrose and then stored on ice if the material was to be used immediately or at −80°C for longer-term storage. A total of five replicate brush border preparations were carried out to create material for shotgun proteomic analysis.
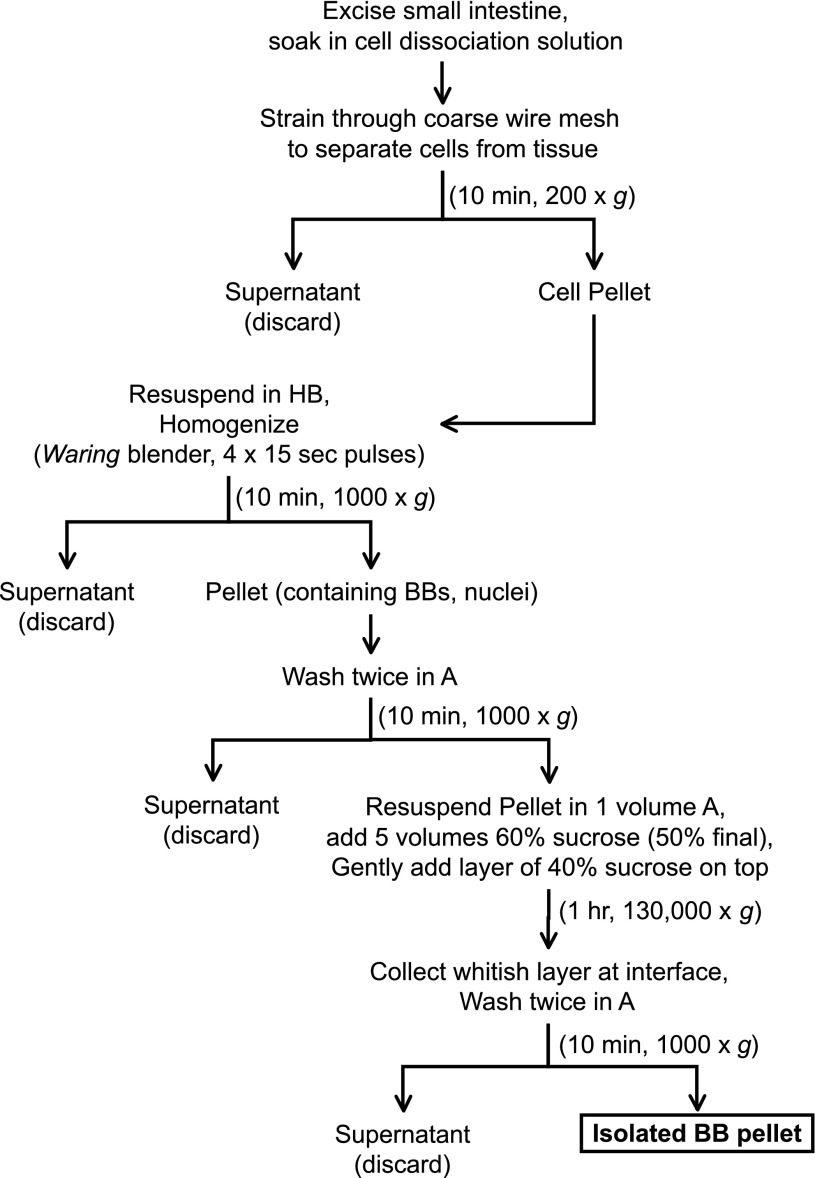
Fig. 1.
Outline of the brush border (BB) isolation procedure. All steps were carried out on ice using prechilled buffers. The entire preparation can be completed in a single afternoon. HB, homogenization buffer.
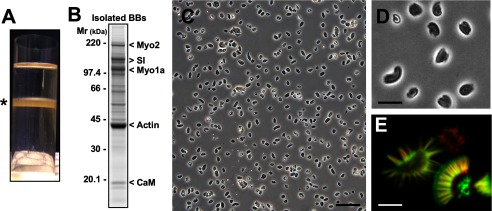
Fig. 2.
Validation of brush border isolation. A: picture of the 40%/50% sucrose gradient centrifuge tube. The whitish layer that forms at the interface (*) is enriched in brush borders and depleted in nuclei. B: SDS-PAGE analysis of isolated brush borders, which was separated using a 4–12% polyacrylamide gel and stained with Coomassie brilliant blue. Prominent protein bands are labeled to the right. Myo, myosin; SI, sucrase-isomaltase; CaM, calmodulin. C and D: phase-images of the isolated brush border fraction (gradient interface highlighted in A); individual brush borders are highly refractive. E: laser scanning confocal micrograph of isolated brush borders labeled with Alexa 488-concanavalin A (green) to mark membranes and Alexa 568-phalloidin (red) to label F-actin. Close inspection showed that the microvillar actin bundles are tightly enclosed by the apical membrane. Core actin bundle rootlets extend below the apical membrane into the terminal web; this produced the red band at the base of the brush border in the lateral view presented here. Membrane-containing structures adjacent to the terminal web likely represent endosomes, vesicles, or mitochondria that remained associated with the brush border throughout the isolation procedure. Scale bars = 60 μm in C, 12 μm in D, and 2 μm in E.
Multidimensional LC-MS/MS
We used multidimensional (2-D) LC-MS/MS for shotgun proteomic analysis of brush border samples (50, 95, 98). To prepare samples for shotgun analysis, isolated brush borders were resuspended in Laemmlli SDS sample buffer and partially separated on a 10% NuPage gel (Invitrogen). After samples had been run into the gel by ∼2 cm, the gel was stained with Coomassie blue G-250 (Bio-Rad) and then destained with sterile Milli-Q water. The protein-containing region was excised from the gel, minced, and then submitted to the Vanderbilt University Mass Spectrometry Core for trypsinization. This partial separation via SDS-PAGE is an efficient sample clean-up step with high recovery that also provides an effective tryptic digest of hydrophobic proteins (45). For shotgun proteomic analysis, tryptic peptide extracts were fractionated by strong cation exchange offline, which provides for replicate reverse-phase analyses of selected ion exchange fractions of interest without repeating the entire multidimensional separation. Protein digests were separated on a 75-mm × 70-cm, 5-mm Partisphere SCX strong cation exchange column (Whatman). Peptides were eluted with a 10–200 mM (pH 3.0–8.0) ammonium formate gradient (1 ml/min) in 25% acetonitrile as previously described (1) with the addition of a final step of 0.5 M ammonium formate during the last 10 min of the 65-min gradient to ensure that all peptides were eluted. Fractions were collected into autosampler vials using a Probot fraction collector (Dionex). Each SCX fraction was analyzed by reverse-phase HPLC-coupled MS using a Thermo Scientific LTQ linear ion-trap MS instrument equipped with a Thermo Surveyor LC system and operated with Xcalibur 1.4 and Bioworks 3.1 software. Electrospray ionization of peptides was done with a Thermo nanospray source modified for automated vented column injection as previously described (49).
Bioinformatics
LC-MS/MS data were transcoded to mzML format by the msconvert tool of the ProteoWizard library (41) using settings for 32-bit precision and zip compression. Tandem mass spectra were identified using the mouse RefSeq database (release 44, 30,041 sequences, downloaded on December 6, 2010). Seventy-one contaminant protein sequences were added to this database, and the database was doubled so that each protein appeared in both forward and reverse orientations. The MyriMatch database search algorithm (version 1.6.79) matched peptides derived from these proteins to the tandem mass spectra, allowing for a precursor mass tolerance of 1.25 m/z and a fragment tolerance of 0.5 m/z (84). Dynamic modifications included oxidation of Met, loss of ammonia from NH2-terminal Gln, carbamidomethyl Cys, and deamidation of Asn-Gly motifs. Peptides were allowed to have either one or two trypsin-conformant termini but could contain no more than two missed proteolytic sites. XCorr and mzFidelity scores were computed for the best five matches by the MVH score. IDPicker 2.6 build 206 used all three of these scores to sort confident identifications away from erroneous ones, filtering to a 5% false discovery rate (FDR), where this value was estimated as (2 × reverse)/(reverse + forward) (52). For final reporting, each protein group was required to contribute a minimum of two distinct peptide sequences and five spectra across the data sets, corresponding to the five MudPIT experiments combined in a single report. Parsimony routines were applied to remove subset and subsumable proteins, resulting in an overall protein FDR of 1.2%. Manual annotation of the resulting list was carried out to eliminate multiple entries of the same protein under different RefSeq identifiers. In addition, there were instances where IDPicker was unable to discriminate between different isoforms of the same protein based on the identified peptides. While all identifications were included in the final IDPicker report (Supplemental Material, Supplemental Report I), these entries were reduced to a single listing in the final annotated data table of 646 brush border proteins (Supplemental Table S1).1
Validation of Identified Proteins
We carried out validation of newly identified proteins at two levels. For a larger subset of proteins that had not been reported as brush border residents before, we used the Human Protein Atlas (http://www.proteinatlas.org/) to determine if these components were expressed in the small intestine and targeted to the brush border domain. In addition, a smaller subset of proteins was validated using an immunofluorescence approach with CACO-2BBE cells, a human intestine-derived epithelial cell culture model (70). To prepare CACO-2BBE cells for immunofluorescence staining, cells were grown on glass coverslips for at least 10 days to ensure a sufficient level of differentiation. Cells were washed with warm PBS followed by fixation in 4% paraformaldehyde (Electron Microcopy Sciences) in PBS for 15 min at room temperature. The fixative was washed away with fresh PBS, and cells were then permeabilized in 0.1% Triton X-100 and PBS for 5 min at room temperature. Cells were washed again followed by blocking in 5% BSA and PBS at 37°C for 1 h. The blocking solution was washed from samples with fresh PBS, which was followed by the application of primary antibody at 37°C for 1 h [anti-mucin-like protocadherin (MLPCDH), 1:250, Sigma, HPA009081; anti-protocadherin (PCDH)24, 1:75, Sigma, HPA012569; and anti-Usher syndrome 1C (Ush1c), Sigma, HPA027398]. This was followed by an additional wash and the application of goat anti-rabbit secondary antibodies (Invitrogen, 1:200) and Alexa 568-phalloidin (Invitrogen, 1:200) at 37°C for 1 h. Fully stained coverslips were mounted onto slides using ProLong anti-fade (Invitrogen). All samples were viewed on an Olympus FluoView1000 with a ×100 objective. All images were contrast enhanced, pseudocolored, and cropped with ImageJ (version 1.42h, National Institutes of Health).
RESULTS
Brush Border Isolation
The combination of hypotonic lysis, differential centrifugation, and sucrose density gradient centrifugation described here represents a well-established approach for isolating intact brush borders (Fig. 1) (59). Indeed, when we used phase-contrast microscopy to examine the material recovered from the sucrose density gradient 40%/50% interface (Fig. 2A), >95% of the objects in the visual field were recognizable as brush borders (Fig. 2, C and D). To assess the quality of this preparation at a higher level of resolution, isolated brush borders were stained with Alexa 488-phalloidin and Alexa 568-concanavalin A to label F-actin and the apical membrane, respectively. These samples were then examined using laser scanning confocal microscopy. The resulting images clearly showed that the isolated structures contained an intact actin cytoskeleton with tightly packed microvillar actin bundles of uniform length (Fig. 2E). Microvillar actin bundles were also enveloped in the apical membrane. Some brush borders demonstrated subapical concanavalin A staining, suggesting the cofractionation of the subapical endosome or vesicles involved in the biosynthetic delivery of cargo to the brush border. SDS-PAGE analysis of isolated brush borders revealed the dominant bands typical for such a preparation: ∼42 kDa (actin), ∼100 kDa (myosin-1a), ∼120–140 kDa [sucrase-isomaltase (SI) and other transmembrane proteins], and ∼ 220 kDa (myosin-2) (Fig. 2B). A lower-molecular-weight (<20 kDa) band representing calmodulin, a light chain that binds to numerous myosin motor proteins, was also observed (Fig. 2B).
Protein Identification
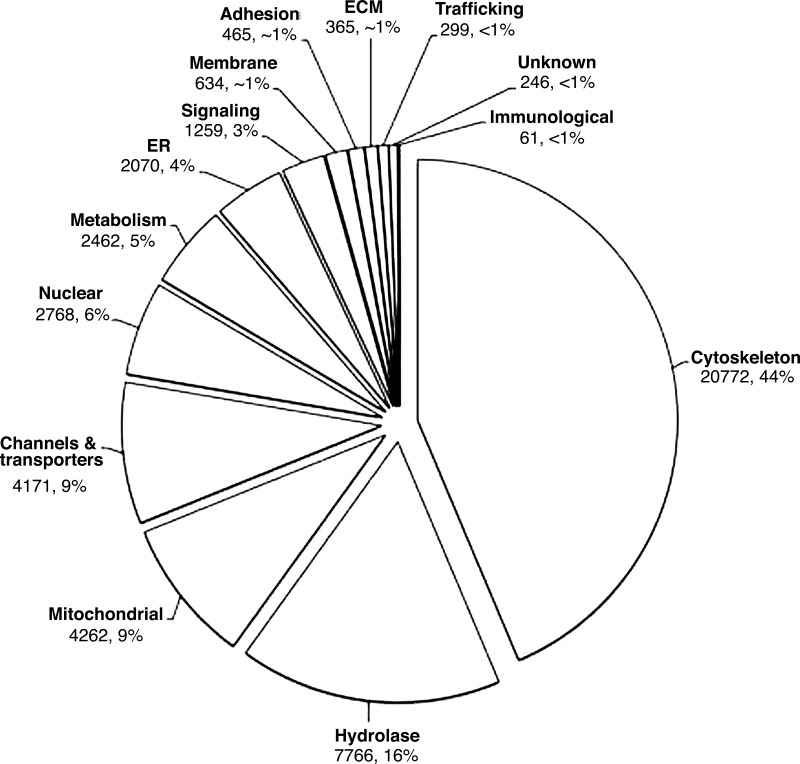
A total of 646 proteins were identified in isolated brush borders. To be included in the final tally, we required that proteins contribute a minimum of two distinct peptide sequences and five spectra to the cumulative data set obtained from five distinct brush border preparations. Of the protein classes identified, the most numerous were cytoskeletal proteins (n = 101), which made up 44% of total spectral counts (20,772/47,600; Fig. 3). Other abundant protein classes include hydrolases (16%) and membrane channels and transporters (9%) that are established players in nutrient processing and transport through the mucosa (Fig. 3). All other proteins were binned into one of the following functional categories: mitochondrial, nuclear, metabolic, endoplasmic reticulum, signaling, membrane, adhesion, extracellular matrix, trafficking, and immunological (Fig. 3 and Supplemental Table S1). Nine proteins were of unknown function (Supplemental Table S1). As the brush border isolation procedure described here uses the full length of the small intestine, isolated organelles will exhibit a protein composition that reflects their tissue of origin (duodenum vs. ileum) (60). Therefore, the proteome that we report was “averaged” along the length of the small intestine.
Fig. 3.
Classification of the brush border proteome into 14 distinct functional classes. The pie chart shows the percentage of total spectral counts contributed by each functional group for all proteins identified in this analysis. Spectral counts and the corresponding percentages of the total are shown with the label for each group. ER, endoplasmic reticulum; ECM, extracellular matrix.
Nutrient Processing Machinery
Hydrolases identified in this analysis included numerous disaccharidases, peptidases, and lipases associated with the brush border membrane (Table 1). Of particular note, the disaccharidases maltase-glucoamylase, SI, and lactase made up a significant portion of our data set, together accounting for ∼7% of total spectral counts. Prominent peptidases, such as aminopeptidase N, dipeptidyl peptidase 4, and two isoforms of angiotensin-converting enzyme (64), were also well represented in our data set, comprising ∼4% of the total spectra (Table 1). Enzymes involved in the metabolism of lipids were prominent in the data set, including the lipases alkaline sphingomyelinase (ectonucleotide pyrophosphatase-phosphodiesterase-7) and neutral ceramidase. In addition to roles in metabolism, many of the identified lipases are critical intermediates in lipid signaling, including phospholipases A2, B1, and D1 (Table 1).
Table 1.
Hydrolases
| Description | Coverage, % | Total Spectra | Number of Preparations | Refseq ID |
|---|---|---|---|---|
| Sucrase-isomaltase, intestinal | 54 | 1,398 | 5 | NP_001074606.1 |
| Aminopeptidase N | 57 | 1,346 | 5 | NP_032512.2 |
| Maltase-glucoamylase | 50 | 1,220 | 5 | NP_001164474.1 |
| Lactase-phlorizin hydrolase preproprotein | 29 | 570 | 5 | NP_001074547.1 |
| Glutamyl aminopeptidase | 46 | 510 | 5 | NP_031960.1 |
| Neprilysin | 53 | 379 | 5 | NP_032630.2 |
| Intestinal alkaline phosphatase | 56 | 321 | 5 | NP_001074551.1 |
| N-acetylated-α-linked acidic dipeptidase-like protein | 50 | 313 | 5 | NP_001009546.1 |
| Angiotensin-converting enzyme 2 precursor | 58 | 288 | 5 | NP_001123985.1 |
| Dipeptidyl peptidase 4 | 45 | 236 | 5 | NP_034204.1 |
| Angiotensin-converting enzyme isoform 1 | 40 | 210 | 5 | NP_997507.1 |
| Meprin A β-subunit precursor | 33 | 189 | 5 | NP_032612.2 |
| Neutral ceramidase | 37 | 149 | 5 | NP_061300.1 |
| Trehalase precursor | 42 | 123 | 5 | NP_067456.1 |
| Intestinal alkaline phosphatase precursor | 40 | 108 | 5 | NP_031458.2 |
| Dipeptidase 1 precursor | 56 | 94 | 5 | NP_031902.2 |
| Phospholipase B1, membrane-associated isoform 1 | 25 | 71 | 5 | NP_001074876.1 |
| Aminopeptidase P | 29 | 52 | 5 | NP_573476.2 |
| γ-Glutamyltranspeptidase 1 | 20 | 36 | 5 | NP_032142.1 |
| Ectonucleotide pyrophosphatase/phosphodiesterase 7 | 27 | 32 | 5 | NP_001025462.1 |
| Neutral α-glucosidase AB | 20 | 31 | 5 | NP_032086.1 |
| Sphingosine-1-phosphate lyase 1 | 23 | 22 | 5 | NP_033189.2 |
| Meprin A α-subunit | 10 | 20 | 5 | NP_032611.2 |
| Phosphatidylinositide phosphatase SAC1 | 25 | 18 | 5 | NP_109617.1 |
| Phospholipase D1 | 16 | 18 | 5 | NP_001157528.1 |
| Membrane primary amine oxidase | 11 | 6 | 3 | NP_033805.1 |
| Phospholipase A2, group IVC | 9 | 6 | 3 | NP_001161976.1 |
Brush Border Channels and Transporters
We identified a total of 53 channels and transporters (Supplemental Table S1), a subset of which are highlighted in Table 2. Many of the identified channels and transporters are driven by electrochemical gradients, such as Na+-glucose transporter (SGLT)1 and SGLT3b (3), proton-coupled peptide transporter 1, and the potential cell volume regulators voltage-dependent anion-selective channel protein (VDAC)1 (67) and VDAC2 (87), whereas others are regulated by transient ionic potentials, such as Ca2+-activated Cl− channel anactomin-6 (77). Concentrations of these key ions are controlled by ion transport machinery in the brush border, such as Na+/H+ exchanger (NHE)3, Na+-K+-ATPase, and their regulatory factors NHE member 3 regulator (NHERF)1 and NHERF3 (42, 103) (Table 2). NHERF1 has also been shown to regulate another transporter present in our data set, multidrug resistance protein 2 (Table 2) (48), a pharmacologically important enzyme responsible for the secretion of a wide range of drugs, toxins, and endogenous compounds (38). Enzymes involved in the uptake of lipids were also particularly abundant in our data set, including those responsible for chylomicron assembly [microsomal triglyceride transfer protein (MTTP), protein disulfide isomerase family A member 3, apoliprotein A, and apolipoprotein B] and cholesterol transport [Niemann-Pick C1-like protein 1, ATP-binding cassette subfamily G (Abcg)5, and Abcg8] (Table II). Mutations in three of the identified lipid transporters have been shown to cause the malabsorptive diseases sitosterolemia (Abcg5 or Abcg8) (43) and abetalipoproteinemia (MTTP) (97).
Table 2.
Transporters and channels
| Description | Coverage, % | Total Spectra | Number of Preparations | Refseq ID |
|---|---|---|---|---|
| Na+-K+-transporting ATPase α1-subunit | 50 | 743 | 5 | NP_659149.1 |
| Microsomal triglyceride transfer protein large subunit | 56 | 341 | 5 | NP_001156929.1 |
| Na+-K+-transporting ATPase α3-subunit | 21 | 323 | 5 | NP_659170.1 |
| Na+-glucose cotransporter 1 | 29 | 321 | 5 | NP_062784.3 |
| Niemann-Pick C1-like protein 1 | 21 | 190 | 5 | NP_997125.2 |
| Na+-K+-transporting ATPase α4-subunit | 12 | 185 | 5 | NP_038762.1 |
| Proton-coupled peptide transporter (solute carrier family 15 member 1) | 20 | 176 | 5 | NP_444309.2 |
| Na+/H+ exchange regulatory cofactor 3 | 68 | 131 | 5 | NP_067492.2 |
| Na+-glucose transporter 3b (solute carrier family 5 member 4b) | 18 | 112 | 5 | NP_075708.2 |
| Multidrug resistance protein 2 | 21 | 92 | 5 | NP_038834.2 |
| Na+-K+-transporting ATPase β1-subunit | 30 | 81 | 5 | NP_033851.1 |
| Apolipoprotein B precursor | 13 | 80 | 5 | NP_033823.2 |
| Na+/H+ exchange regulatory cofactor 1 | 46 | 75 | 5 | NP_036160.1 |
| Solute carrier family 3, member 1 | 30 | 71 | 5 | NP_033231.2 |
| Cl− intracellular channel protein 5 | 69 | 68 | 5 | NP_766209.1 |
| Na+-dependent neutral amino acid transporter 1 | 14 | 67 | 5 | NP_083154.1 |
| Protein disulfide-isomerase A3 precursor | 50 | 61 | 5 | NP_031978.2 |
| K+-transporting ATPase α-chain 2 | 8 | 60 | 5 | NP_619593.2 |
| Anoctamin-6 | 22 | 57 | 5 | NP_780553.2 |
| Solute carrier family 26, member 6 | 32 | 37 | 5 | NP_599252.2 |
| Voltage-dependent anion-selective channel protein 1 | 41 | 31 | 4 | NP_035824.1 |
| Voltage-dependent anion-selective channel protein 2 | 38 | 31 | 4 | NP_035825.1 |
| Ca2+-activated Cl− channel regulator 4 | 14 | 26 | 5 | NP_997091.3 |
| Na+/H+ exchanger 3 | 17 | 24 | 5 | NP_001074529.1 |
| ATP-binding cassette subfamily G, member 5 | 15 | 23 | 4 | NP_114090.1 |
| ATP-binding cassette subfamily G, member 8 | 15 | 17 | 5 | NP_080456.1 |
| Apolipoprotein A-IV precursor | 33 | 17 | 5 | NP_031494.2 |
Other Membrane-Associated Proteins
In addition to the appearance of numerous nutrient processing enzymes, transporters, and channels in the present dataset, we also identified a number of other membrane-associated proteins that are believed to play important roles in brush border function and maintenance. Although this class only accounted for ∼1% of all identified spectra, a number of functionally significant proteins from this group were observed in all five brush border preparations. Among these were two prominent annexin isoforms, A2 and A13, which were both present at high levels of coverage (104 and 80 total spectra at 57% and 62% coverage, respectively). Annexins are small Ca2+-dependent membrane-binding proteins that have been implicated in a wide range of membrane-related activities, ranging from vesicle fusion with the plasma membrane to budding of vesicles into the multivesicular body (27). We also identified several members of the galectin protein family, including galectin-4, -6, and -9 (56, 38, and 9 total spectra, respectively). Galectins bind to galactosyl groups on transmembrane proteins and have been implicated in a variety of cellular functions ranging from biosynthetic sorting of membrane proteins to organizing apical membrane domains and, potentially, mucosal host defense (18, 20).
The Brush Border Cytoskeleton
A total of 101 cytoskeletal proteins were identified in this analysis (Supplemental Table S1). Due to the inclusion of an intact cytoskeleton in isolated brush borders, this category accounts for almost half of all spectral counts (20,772 or 44%). The three identified actin isoforms represented ∼10% of the total spectra, as might be expected for this extraordinarily actin-rich domain (Table 3, high abundance). We also identified proteins that serve in actin bundling (villin-1 and both isoforms of plastin) and actin-membrane interactions (ezrin, radixin, and harmonin) (12, 14, 31, 76). Actin and some associated cytoskeletal proteins have previously been observed in the proteome of brush border membrane vesicles (23). This prior analysis overlaps with the present data set to a large degree; however, the inclusion of an intact cytoskeleton in our preparation allowed us to identify low-abundance cytoskeletal proteins (<100 total spectra) that were not detected in prior studies (Table 3, low abundance). A number of these newly identified proteins have been shown to interact with actin and/or regulate actin dynamics in other cellular contexts but have never been described in the enterocyte brush border: adenylyl cyclase-associated protein-1 (11), Cordon-bleu (2), insulin receptor tyrosine kinase substrate (IRTKS) (58), cortactin (4), and EGF receptor (Table 3). We also detected components previously implicated in regulating microvillar actin organization, such as Eps8 and its downstream target Rac1 (66) (Table 3, low abundance).
Table 3.
Cytoskeletal components
| Description | Coverage, % | Total Spectra | Number of Preparations | Refseq ID |
|---|---|---|---|---|
| High abundance | ||||
| Actin, cytoplasmic 1 | 81 | 2,512 | 5 | NP_031419.1 |
| Keratin, type II cytoskeletal 8 | 85 | 1,450 | 5 | NP_112447.2 |
| Actin, aortic smooth muscle | 67 | 1,278 | 5 | NP_031418.1 |
| Plectin* | 60 | 986 | 5 | NP_958796.2 |
| Keratin, type I cytoskeletal 19 | 86 | 872 | 5 | NP_032497.1 |
| β-Actin-like protein 2 | 53 | 861 | 5 | NP_780706.1 |
| Villin-1 | 68 | 658 | 5 | NP_033535.2 |
| Plastin-1 | 70 | 529 | 5 | NP_001028382.1 |
| Ezrin | 73 | 453 | 5 | NP_033536.2 |
| Keratin, type I cytoskeletal 20 | 75 | 326 | 5 | NP_075745.1 |
| Desmoplakin | 35 | 244 | 5 | NP_076331.2 |
| Spectrin α-chain, brain isoform 2 | 47 | 226 | 5 | NP_001171138.1 |
| Cortactin | 26 | 202 | 5 | XP_003085904.1 |
| α-Actinin-4 | 62 | 187 | 5 | NP_068695.1 |
| Spectrin β-chain, brain 1 isoform 1 | 38 | 173 | 5 | NP_787030.2 |
| Spectrin β-chain, brain 1 isoform 2 | 42 | 172 | 5 | NP_033286.2 |
| Keratin, type II cytoskeletal 7 | 39 | 169 | 5 | NP_149064.1 |
| Keratin, type I cytoskeletal 18 | 71 | 158 | 5 | NP_034794.2 |
| Clathrin heavy chain 1 | 37 | 146 | 5 | NP_001003908.1 |
| Radixin | 17 | 140 | 5 | NP_033067.2 |
| Keratin, type II cytoskeletal 1b | 7 | 125 | 5 | NP_001003667.1 |
| α1-Catenin | 51 | 111 | 5 | NP_033948.1 |
| Low abundance | ||||
| EGF receptor pathway substrate 8-like 3 | 50 | 87 | 5 | NP_598628.1 |
| EGF receptor pathway substrate | 27 | 26 | 4 | NP_031971.2 |
| Rootletin | 1 | 23 | 5 | NP_742120.2 |
| Actin-related protein 3 | 36 | 22 | 4 | NP_076224.1 |
| Filamin-B* | 9 | 22 | 5 | NP_598841.1 |
| Harmonin* | 25 | 22 | 4 | NP_076138.2 |
| Cordon-bleu* | 8 | 19 | 4 | NP_766084.3 |
| F-actin-capping protein β-subunit | 38 | 15 | 4 | NP_033928.1 |
| Actin-related protein 2 | 17 | 14 | 5 | NP_666355.1 |
| Taperin* | 7 | 12 | 5 | NP_780495.2 |
| Filamin-A | 4 | 11 | 5 | NP_034357.2 |
| F-actin-capping protein α2-subunit | 15 | 10 | 5 | NP_031630.1 |
| Adenylyl cyclase-associated protein 1 | 21 | 8 | 4 | NP_031624.2 |
| F-actin-capping protein α1-subunit | 12 | 7 | 5 | NP_033927.2 |
| Insulin receptor tyrosine kinase substrate* | 13 | 7 | 4 | NP_080109.1 |
| Profilin-1 | 31 | 7 | 3 | NP_035202.1 |
| Tropomyosin α3-chain | 13 | 7 | 3 | NP_071709.2 |
Proteins exhibiting robust staining in the brush border.
Myosin Motor Proteins
A total of 14 different myosin superfamily members were detected in the brush border proteome (Table 4). Many of these proteins have been identified in previous cell biological studies, whereas others are newly identified residents of the brush border. The most abundant myosins, as judged by spectral counts, were class 2 myosins. We obtained strong evidence for the presence of all three nonmuscle myosin-2 isoforms: 2a, 2b, and 2c. Studies have shown that myosin-2 is found in the terminal web, where it connects neighboring actin core rootlets (62), and in the circumferential actin band that wraps around the base of the brush border, at the level of cell-cell contacts (36). In addition, we identified 10 “unconventional” myosins, including the known brush border constituent class 1 myosins (myosins-1a, -1c, -1d, and -1e) (9, 82, 89) as well as myosin-5b, -6, -7b and, at lower levels, myosin-7a (Table 4) (15, 34). Myosins not previously found in the brush border include myosin-15-like protein and myosin-18a (Table 4). We also identified three small polypeptides that are known to function as light chains, including calmodulin. These proteins bind directly to IQ motifs in various myosin heavy chains and regulate the mechanochemical activity of these motors (7, 51, 85).
Table 4.
Myosin motor proteins
| Description | Coverage, % | Spectra | Number of Preparations | Refseq ID |
|---|---|---|---|---|
| Heavy chains | ||||
| Nonmuscle myosin-2c | 70 | 2,468 | 5 | NP_082297.1 |
| Myosin-1a | 74 | 1,582 | 5 | NP_001074688.1 |
| Nonmuscle myosin-2a | 63 | 793 | 5 | NP_071855.2 |
| Smooth muscle myosin-2 | 59 | 569 | 5 | NP_038635.2 |
| Myosin-7b | 47 | 362 | 5 | NP_115770.2 |
| Myosin-1d | 49 | 265 | 5 | NP_796364.2 |
| Nonmuscle myosin-2b | 15 | 202 | 5 | NP_780469.1 |
| Myosin-6 | 45 | 179 | 5 | NP_001034635.2 |
| Myosin-5b | 23 | 58 | 5 | NP_963894.1 |
| Myosin-1c | 16 | 34 | 5 | NP_001074243.1 |
| myosin-15-like (predicted) | 18 | 23 | 4 | XP_003085561.1 |
| Myosin-1e | 7 | 12 | 4 | NP_851417.2 |
| Myosin-18a* | 4 | 9 | 3 | NP_035716.1 |
| Myosin-7a | 2 | 6 | 4 | NP_032689.2 |
| Light chains | ||||
| Myosin light polypeptide 6 | 76 | 88 | 5 | NP_034990.1 |
| Myosin regulatory light chain 12B | 62 | 41 | 5 | NP_075891.1 |
| Calmodulin | 48 | 23 | 5 | NP_031615.1 |
Proteins exhibiting robust staining in the brush border.
Adhesion Proteins
As isolated brush borders contain portions of the junctional complex, we expected to identify proteins with established functions in intercellular adhesion. Indeed, our analysis revealed the tight junction components zonula occludens (ZO)-1, -2, and -3 as well as claudin-3 and -7 (53) (Table 5). We also identified cadherin-1 (also known as E-cadherin) and cadherin-17, both of which are localized to the junctional complex and basolateral membrane (10, 35) (Table 5). MLPCDH and PCDH24 were also present at significant levels (Table 5). Although MLPCDH has previously been listed in the proteome of brush border membrane vesicles (23), the presence of PCDH24 is a novel finding. Given their abundant spectral counts and appearance in all five brush border preparations, MLPCDH and PCDH24 were included as targets for validation.
Table 5.
Adhesion molecules
| Description | Coverage, % | Total spectra | Number of Preparations | Refseq ID |
|---|---|---|---|---|
| Mucin-like protocadherin (cadherin-related family member 5) isoform 2* | 30 | 75 | 5 | NP_082345.1 |
| δ1-Catenin isoform 1 | 35 | 73 | 5 | NP_001078919.1 |
| Cadherin-17 precursor | 31 | 72 | 5 | NP_062727.1 |
| Epithelial cell adhesion molecule | 34 | 62 | 5 | NP_032558.2 |
| Mucin-13 precursor | 14 | 44 | 5 | NP_034869.1 |
| Protocadherin-24 (cadherin-related family member 2)* | 8 | 17 | 5 | NP_001028536.2 |
| Carcinoembryonic antigen-related cell adhesion molecule 1 | 17 | 16 | 5 | NP_001034275.1 |
| α2-Catenin isoform 1 | 10 | 14 | 5 | NP_663785.2 |
| Cell surface A33 antigen precursor | 20 | 14 | 4 | NP_067623.1 |
| Cingulin | 9 | 13 | 4 | NP_001032800.2 |
| Tetraspanin 8 | 10 | 12 | 4 | NP_666122.1 |
| Cadherin-1 | 4 | 10 | 5 | NP_033994.1 |
| Claudin-7 | 8 | 9 | 4 | NP_001180548.1 |
| Carcinoembryonic antigen-related cell adhesion molecule 20 | 10 | 8 | 4 | NP_082115.2 |
| Tight junction protein zonula occludens-1 | 5 | 8 | 5 | NP_033412.2 |
| Tight junction protein zonula occludens-3 | 6 | 7 | 2 | NP_038797.2 |
| Tight junction protein zonula occludens-2 | 3 | 6 | 3 | NP_001185914.1 |
| Claudin-3 | 9 | 5 | 3 | NP_034032.1 |
Proteins exhibiting robust staining in the brush border.
Validation of Identified Proteins
As a first step toward validating newly identified proteins, we selected a subset of actin- and membrane-associated proteins from our list for cross-referencing with the Human Proteome Atlas (http://www.proteinatlas.org/). Remarkably, all cross-referenced proteins demonstrated strong expression in human intestinal tissue sections (duodenal and general small intestinal samples), with many exhibiting robust staining in the brush border (see proteins marked with asterisks in Tables 3–5). Proteins validated with the Human Proteome Atlas included IRTKS, Cordon-bleu, harmonin, filamin-B, taperin, PCDH24, MLPCDH, and myosin-18a.
Some proteins were chosen for further validation using immunofluorescence microscopy. For these experiments, we chose to focus on the poorly characterized PCDH isoforms that were identified with significant spectral counts: PCDH24 and MLPCDH. Because harmonin has been shown to localize to the brush border (12) and has been implicated as a binding partner for cadherin superfamily members in hair cell stereocilia (13), we stained for this component as well. Antibodies to PCDH24, MLPCDH, and harmonin were obtained from the Sigma Prestige collection and used to stain fully differentiated CACO-2BBE cells, a human-derived intestinal epithelial cell line (70). All three probes produced striking punctate staining at the apical surface, representative of microvillar labeling (Fig. 4). PCDH24, MLPCDH, and harmonin exhibited mosaic staining; cells exhibited a wide range of expression levels with some cells expressing little or no antigen. Mosaic expression has been observed for other well-characterized apical membrane proteins in this cell line, including SI and intestinal alkaline phosphatase (70, 91). These validation experiments suggest that PCDH24 and MLPCDH may be bona fide components of the enterocyte microvillus.
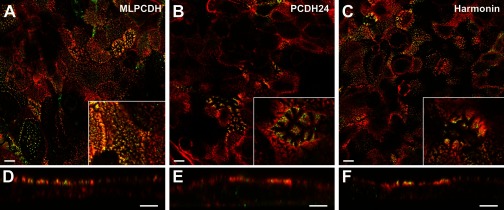
Fig. 4.
Immunofluorescent validation of selected novel brush border components. CACO-2BBE cells were stained with Alexa 568-phalloidin (red) and antibodies directed against mucin-like protocadherin (MLPCDH; A and D), protocadherin-24 (PCDH24; B and E), or harmonin (C and F), which were detected with Alexa 488-conjugated secondary antibodies (green). A–C: en face views taken near the apical surface of the cell monolayer showed that all three proteins demonstrated a punctate staining pattern that was highly mosaic. All three proteins localized strongly to the apical surface of the cells, at the top of the monolayer, as viewed in the vertical sections (D–F). Scale bars = 10 μm in A–C and 5 μm in D–F. Insets show magnified views of individual cells. Box width is 20 µm in all cases.
DISCUSSION
Because the enterocyte brush border has been the focus of biochemical characterization for decades, many of the nutrient processing and transporting proteins identified in our analysis were established as enriched components of this domain long ago. More recent proteomic analyses of apical membrane vesicles or lipid raft preparations (5, 23) has focused on cataloguing membrane channels and transporters. Our analysis identified many of these components, including ATP-binding cassette family proteins, intracellular Cl− channel isoforms, solute carrier family members, multidrug resistant protein isoforms, a diverse array of ion-coupled transporters, and others (Table 2). The approach presented here also enabled us to identify established brush border residents that were not readily detected in previous studies. For example, previous analyses of brush border membrane vesicles were unable to identify SI, one of the principle sugar-processing hydrolases in the brush border (23). Our analysis revealed 1,398 total spectra that matched to SI, with an average of ∼280 per preparation. The lack of SI in previous results may be related to a partitioning of SI-depleted lipid domains into vesicles isolated using the cationic precipitation procedure.
The true utility of analyzing the intact brush border was demonstrated by the abundant spectral counts of cytoskeletal proteins that are well-characterized residents of this domain (Table 3). A total of 3,790 spectra representing the two major actin isoforms (cytoplasmic/β-actin and aortic smooth muscle actin) were detected in this analysis. Other well-established cytoskeletal proteins that were readily detected include the actin bundling proteins villin and plastin/fimbrin (658 and 529 total spectra, respectively) and the two major keratin isoforms expressed in the enterocyte, cytoplasmic keratin-8 and -19 (1,450 and 872 total spectra, respectively). Carrying out shotgun MS on intact brush borders also enabled us to discover cytoskeletal components that are unlikely to be enriched in membrane/vesicle fractions examined in previous studies, including lower abundance myosin motors, proteins involved in controlling actin dynamics, membrane bending machinery, and extracellular adhesion molecules. Each of these is discussed in more detail below.
Mitochondrial and Metabolic Machinery
A large number of mitochondrial components and cytosolic enzymes that function in general metabolism were present in our samples, representing 14% of all spectra identified in this analysis. Although soluble metabolic machinery could conceivably be captured in the microvillus during the brush border isolation, the mitochondrial signals were most likely derived from fragments of this compartment that remain associated with the brush border throughout the isolation process (see Fig. 4a in Ref. 56). The close association of mitochondria with isolated brush borders may reflect the need to maintain high ATP levels, which would be required to sustain the proper function of motor proteins, channels, and transporters that are enriched in this domain. Indeed, previous proteomic analyses of hair cell stereocilia revealed numerous metabolic proteins (e.g., creatine kinase) that were shown to be bona fide components of the hair bundle critical for supporting the metabolic demands of the mechanotransduction machinery (80). While many of the metabolic enzymes listed here likely come from contaminating mitochondrial fragments, others may represent as-yet-unidentified brush border components and have therefore been included here for completeness.
Myosin Motor Proteins
Conventional myosins.
Numerous nonmuscle myosin-2 isoforms (2a, 2b, 2c, and smooth muscle) were detected in isolated brush borders (Table 4). Although myosin-2 is a well-known component of the circumferential band of actin that lines the periphery of the cell at the base of the brush border, the functional significance of multiple isoforms remains to be established. Based on the diversity of catalytic and mechanical properties of these motors (44, 65), one possibility is that different isoforms carry out distinct functions. This would be consistent with recent studies on non-muscle myosins-2a and -2b (83), which independently regulate adhesion receptor function and F-actin organization at cell-cell contacts, respectively. An alternate idea is that all class 2 myosins contribute to the same function, but by controlling expression levels and targeting of functionally distinct isoforms to the junctional complex, enterocytes are able to fine tune the contractility of this domain. Intriguingly, physiological studies have shown that barrier function of the intestinal mucosa is intimately linked with the activity of myosin-2 in the junctional complex (88, 102).
Unconventional myosins.
This analysis also revealed the presence of a variety of unconventional (nonclass 2) myosin isoforms, including myosin-1a, -1c, -1d, -1e, -5b, -6, -7b, -7a, and -18a and a myosin-15-like protein. While many of these are well characterized and have been linked to the enterocyte brush border through previous biochemical or immunocytological analyses (6, 15, 34), the full brush border proteome did provide some interesting insights. For example, both myosin-7b and -7a have been implicated as enterocyte motors (8), but the present data set clearly shows that myosin-7b is much more abundant in the brush border based on spectral counts (Table 4). This is potentially interesting in light of cell biological studies that have shown that myosin-7b enriches in the distal half of microvilli (15). With tandem MyTH4/FERM motifs in its tail domain, myosin-7b is well equipped to bind a variety of cargoes and serve as a major plus end-directed transporter in the microvillus.
We also identified myosins not previously recognized as brush border constituents, including myosin-18a. Myosin-18a diverges significantly from most myosins but is somewhat related to class 2 myosins, possessing a motor domain, a single IQ motif, a coiled-coil region, and a COOH-terminal globular tail domain (25). A unique feature of myosin-18a is the presence of an NH2-terminal PDZ domain that binds actin in an ATP-insensitive manner (37). The mechanochemical properties of this motor are also likely to be unique, as a critical highly conserved glutamate residue in the motor domain is substituted by a glutamine (37). This glutamate is thought to be involved in regulating phosphate release after ATP hydrolysis (47), the rate-limiting step of the myosin mechanochemical cycle. In fact, a conserved glutamate to aspartate mutation in this position has been shown to uncouple the chemical and mechanical properties of myosin-1a (101). It will be interesting to determine if myosin-18a is a catalytically active, actin-activated ATPase, like all other members of the myosin superfamily characterized to date.
Peptides matched to a myosin-15-like protein were also found in four of the five brush border preparations. The myosin-15-like entry that matched these peptides (XP_003085561.1) is a predicted protein that contains a single MyTH4/FERM domain. Interestingly, none of the peptides identified in our analysis matched to a recognizable myosin-15 motor domain structure. If the myosin-15-like protein expressed in enterocytes is in fact “headless,” it may be playing a regulatory role by modulating cargo binding of other MyTH4/FERM domain-containing motors, such as myosin-7b. Future studies will be needed to further characterize and investigate the functional consequence of this myosin-15-like protein.
Regulators of Actin Assembly
One of the most striking features of the enterocyte brush border is the high density of microvilli that extend off the apical surface into the intestinal lumen. Remarkably, there is little information on how the numerous microvilli that comprise the brush border are nucleated to grow or how their growth is coordinated to create protrusions of the same length. To control the spatial and temporal distribution of actin filaments, cells typically control the targeting and activity of proteins that function to overcome the rate-limiting step in filament formation: nucleation. Our analysis did identify components of the actin-related protein (Arp)2/3 complex, the nucleation machinery that gives rise to the meshwork of actin filaments at the leading edge of motile cells and the cell cortex (71). Because Arp2/3 is a branched nucleator that functions to initiate new filament growth off the side of preexisting “mother” filaments, this complex is unlikely to function in the growth of microvillar actin bundles. Arp2/3 components are more likely to reside in the junctional band of actin filaments that surround the base of the brush border; fragments of this array were included in brush borders isolated using our preparation (56).
Intriguingly, our analysis also identified Cordon-bleu, a newly identified multi-WH2 domain-containing protein that functions in nucleating actin filaments (72). Cordon-bleu contains three WH2 domains, and each one has the ability to bind to a single G-actin monomer (2, 72). As such, current models suggest that a single molecule of Cordon-bleu brings together three G-actin monomers to form the energetically unfavorable trimer, after which F-actin polymerization proceeds rapidly. Importantly, in vitro experiments have suggested that Cordon-bleu can support the polymerization of unbranched actin filaments (2), making it a good candidate for nucleating the production of parallel actin bundles found in microvilli.
Even in the fully differentiated state, microvilli are highly dynamic structures, with actin bundles undergoing continuous treadmilling: actin monomers incorporate into filament plus ends at the microvillus tip and dissociate from minus ends at the base (90). The brush border proteome contains two proteins that may regulate the length of microvillar actin bundles by controlling the rate of actin polymerization: the actin bundling and capping proteins Eps8 and Eps8-like 3 (Table 4) (22). Eps8 has previously been shown to be critical for regulating the length and organization of intestinal microvilli in Caenorhabditis elegans (16), and yet Eps8 knockout mice display only slight perturbations to microvillar morphology (86). An explanation for this difference is that C. elegans possesses only one Eps8 homolog, whereas vertebrates possess four: Eps8 and Eps8-like 1, 2, and 3 (66). Our data indicate that Eps8-like 3 is the predominant member of this family in the enterocyte brush border (Table 4), further suggesting that this isoform may be responsible for regulating microvillar length in vertebrates.
Membrane Bending
This analysis also revealed the presence of IRTKS in the brush border (also known as brain-specific angiogenesis inhibitor 1-associated protein 2-like protein 1). IRTKS belongs to the IRSp53/MIM family of inverse BAR domain proteins (58); inverse BAR domain proteins have been implicated in the bending and support of outward membrane protrusions such as filopodia (58, 99). IRTKS also contains a WH2 domain that may enable direct interactions with actin filaments (58). This finding might hold special relevance in the context of the gut as studies with the adherent pathogenic bacterium, enterohemorrhagic E. coli, have implicated IRTKS in the formation of actin-rich cell surface pedestals, which are critical for the virulence of this organism (93, 96). In the context of normal enterocyte physiology, IRTKS could play a role in deforming the apical membrane during brush border assembly, stabilizing the curvature of microvillar membrane, or linking the microvillar membrane directly to supporting core actin bundles.
Peripheral Membrane Binding
Other membrane-associated proteins identified in this data set include members of the annexin (annexin A2 and A13) and galectin (galectin-4, -6, and -9) families. Annexin A2 has been shown to play important roles at the interface of the actin cytoskeleton and the plasma membrane (57), where it is thought to help polymerize actin in a phosphatidylinositol 4,5-bisphosphate-regulated manner (33). This coupling of the membrane to polymerizing actin is thought to help drive phagocytosis in macrophages and rod outer segments (24, 46). Because annexin A2 is strongly localized to the terminal web region of the brush border where endocytosis and exocytosis occur (54), it is a likely candidate for regulating vesicle trafficking to and from the brush border. Galectins have also been shown to play important roles in the apical trafficking of membrane proteins (20); indeed, knockdown of just one galectin, galectin-4, in HT-29 cells resulted in a dramatic reduction in the delivery of all glycosylated proteins to the apical surface (63). The importance of annexins and galectins in apical transport is underscored by findings suggesting that changes in their expression levels are contributing factors to a number of gastrointestinal diseases ranging from inflammation to cancer (21, 81). Recently, it has been shown that multiple types of annexins and galectins are enriched in vesicles released from the tips of microvilli, suggesting that these protein families may also function in this novel form of secretion from the brush border (55).
Adhesion Molecules
One of the most surprising aspects of this analysis relates to the presence of putative extracellular adhesion molecules in the brush border. Although some of the adhesion molecules identified here are well-established players in junctional adhesion (cadherins-1 and -17 and ZO-1, -2, and -3), others, such as MLPCDH and PCDH24, are poorly characterized. Nevertheless, staining of CACO-2BBE cells showed robust labeling for both MLPCDH and PCDH24 isoforms in microvilli (Fig. 4). Although little is known about these molecules, both are type I transmembrane proteins and contain varying numbers of extracellular “EC” repeats, which are characteristic of the cadherin protein superfamily (eight for PCDH24 and four for MLPCDH) (28, 29, 68). The isoform 2 variant of MLPCDH identified here also contains a juxtamembrane mucin-like domain with tandem repeats of threonine, serine, and proline (28, 29). Transfection of cultured cells with MLPCDH increased aggregation in a Ca2+-dependent manner, suggesting that this molecule is capable of forming bona fide adhesion complexes (28). Although the role of these PCDHs in brush border function remains to be established, an intriguing possibility is that MLPCDH and/or PCDH24 create adhesion between microvilli, contributing to the tight packing of these protrusions observed in fully differentiated enterocytes.
The presence of adhesion molecules in the brush border is reminiscent of hair bundle architecture on the apical surface of mechanosensory hair cells. In the cochlea, hair bundles are clusters of stereocilia that are organized into three rows of graded height (30). Adjacent stereocilia are connected between rows by thread-like links composed of other cadherin superfamily members, PCDH-15 and cadherin-23 (75). These links are also connected to ion channels so that stereocilia deflections give rise to transduction currents. By analogy, brush border microvilli may also build physical links between adjacent protrusions, which could facilitate brush border function or assembly. Interestingly, the brush border proteome also included harmonin, taperin, and clarin, three proteins that are expressed in hair cells and play significant roles in the mechanotransduction process (26, 73, 92). The physiological importance of these proteins in the context of the brush border is further suggested by the finding that patients harboring mutations in harmonin frequently suffer from inflammatory enteropathy (12).
Concluding Remarks
This report is the first to describe the full proteome of the enterocyte brush border. As such, we expect these results to provide a rich source of data that will stimulate the development of new hypotheses and studies on the assembly, maintenance, and function of the enterocyte apical domain. Future studies must focus on validating the novel identifications made here and probing functions of important molecules not previously known to reside in the brush border.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-DK-075555 (to M. J. Tyska) and R01-CA-126218 (to D. L. Tabb), American Heart Association predoctoral fellowships (to R. E. McConnell and A. E. Benesh) and Grant 09GRNT2310188 (to M. J. Tyska), the VUMC Digestive Diseases Research Center (pilot funds from National Institutes of Health Grant P30-DK-058404, to R. M. Peek), and a Vanderbilt University Innovation and Discovery in Engineering And Science award (to M. J. Tyska).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank all members of the Tyska laboratory for advice and support, Dr. Amy Ham and Dr. David Friedman of the Vanderbilt University Medical Center (VUMC) Mass Spectrometry Research Center for outstanding core technical support, and the VUMC Cell Imaging Shared Resource.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Gastrointestinal and Liver Physiology website.
REFERENCES
- 1. Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics 1: 947–955, 2002. [DOI] [PubMed] [Google Scholar]
- 2. Ahuja R, Pinyol R, Reichenbach N, Custer L, Klingensmith J, Kessels MM, Qualmann B. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell 131: 337–350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aljure O, Diez-Sampedro A. Functional characterization of mouse sodium/glucose transporter type 3b. Am J Physiol Cell Physiol 299: C58–C65, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton 65: 687–707, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Babusiak M, Man P, Petrak J, Vyoral D. Native proteomic analysis of protein complexes in murine intestinal brush border membranes. Proteomics 7: 121–129, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Bahler M, Kroschewski R, Stoffler HE, Behrmann T. Rat myr 4 defines a novel subclass of myosin I: identification, distribution, localization, and mapping of calmodulin-binding sites with differential calcium sensitivity. J Cell Biol 126: 375–389, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett 513: 107–113, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Bement WM, Hasson T, Wirth JA, Cheney RE, Mooseker MS. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc Natl Acad Sci USA 91: 11767, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benesh AE, Nambiar R, McConnell RE, Mao S, Tabb DL, Tyska MJ. Differential localization and dynamics of class I myosins in the enterocyte microvillus. Mol Biol Cell 21: 970–978, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berndorff D, Gessner R, Kreft B, Schnoy N, Lajous-Petter AM, Loch N, Reutter W, Hortsch M, Tauber R. Liver-intestine cadherin: molecular cloning and characterization of a novel Ca2+-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol 125: 1353–1369, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bertling E, Hotulainen P, Mattila PK, Matilainen T, Salminen M, Lappalainen P. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol Biol Cell 15: 2324–2334, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bitner-Glindzicz M, Lindley KJ, Rutland P, Blaydon D, Smith VV, Milla PJ, Hussain K, Furth-Lavi J, Cosgrove KE, Shepherd RM, Barnes PD, O'Brien RE, Farndon PA, Sowden J, Liu XZ, Scanlan MJ, Malcolm S, Dunne MJ, Aynsley-Green A, Glaser B. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat Genet 26: 56–60, 2000. [DOI] [PubMed] [Google Scholar]
- 13. Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, Houdusse A, Legrain P, Wolfrum U, Richardson G, Petit C. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J 21: 6689–6699, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bretscher A, Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci USA 76: 2321–2325, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen ZY, Hasson T, Zhang DS, Schwender BJ, Derfler BH, Mooseker MS, Corey DP. Myosin-VIIb, a novel unconventional myosin, is a constituent of microvilli in transporting epithelia. Genomics 72: 285–296, 2001. [DOI] [PubMed] [Google Scholar]
- 16. Croce A, Cassata G, Disanza A, Gagliani MC, Tacchetti C, Malabarba MG, Carlier MF, Scita G, Baumeister R, Di Fiore PP. A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat Cell Biol 6: 1173–1179, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Cutillas PR, Biber J, Marks J, Jacob R, Stieger B, Cramer R, Waterfield M, Burlingame AL, Unwin RJ. Proteomic analysis of plasma membrane vesicles isolated from the rat renal cortex. Proteomics 5: 101–112, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Danielsen EM, Hansen GH. Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem 64: 377–382, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Danielsen EM, Hansen GH. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim Biophys Acta 1617: 1–9, 2003. [DOI] [PubMed] [Google Scholar]
- 20. Delacour D, Koch A, Jacob R. The role of galectins in protein trafficking. Traffic 10: 1405–1413, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Demetter P, Nagy N, Martin B, Mathieu A, Dumont P, Decaestecker C, Salmon I. The galectin family and digestive disease. J Pathol 215: 1–12, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Disanza A, Carlier MF, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol 6: 1180–1188, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Donowitz M, Singh S, Salahuddin FF, Hogema BM, Chen Y, Gucek M, Cole RN, Ham A, Zachos NC, Kovbasnjuk O, Lapierre LA, Broere N, Goldenring J, deJonge H, Li X. Proteome of murine jejunal brush border membrane vesicles. J Proteome Res 6: 4068–4079, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Fan X, Krahling S, Smith D, Williamson P, Schlegel RA. Macrophage surface expression of annexins I and II in the phagocytosis of apoptotic lymphocytes. Mol Biol Cell 15: 2863–2872, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foth BJ, Goedecke MC, Soldati D. New insights into myosin evolution and classification. Proc Natl Acad Sci USA 103: 3681–3686, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geng R, Geller SF, Hayashi T, Ray CA, Reh TA, Bermingham-McDonogh O, Jones SM, Wright CG, Melki S, Imanishi Y, Palczewski K, Alagramam KN, Flannery JG. Usher syndrome IIIA gene clarin-1 is essential for hair cell function and associated neural activation. Hum Mol Genet 18: 2748–2760, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol 6: 449–461, 2005. [DOI] [PubMed] [Google Scholar]
- 28. Goldberg M, Peshkovsky C, Shifteh A, Al-Awqati Q. μ-Protocadherin, a novel developmentally regulated protocadherin with mucin-like domains. J Biol Chem 275: 24622–24629, 2000. [DOI] [PubMed] [Google Scholar]
- 29. Goldberg M, Wei M, Tycko B, Falikovich I, Warburton D. Identification and expression analysis of the human μ-protocadherin gene in fetal and adult kidneys. Am J Physiol Renal Physiol 283: F454–F463, 2002. [DOI] [PubMed] [Google Scholar]
- 30. Grillet N, Kazmierczak P, Xiong W, Schwander M, Reynolds A, Sakaguchi H, Tokita J, Kachar B, Muller U. The mechanotransduction machinery of hair cells. Sci Signal 2: pt5, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Grimm-Gunter EM, Revenu C, Ramos S, Hurbain I, Smyth N, Ferrary E, Louvard D, Robine S, Rivero F. Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol Biol Cell 20: 2549–2562, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hauri HP, Quaroni A, Isselbacher KJ. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci USA 76: 5183–5186, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayes MJ, Shao DM, Grieve A, Levine T, Bailly M, Moss SE. Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5,-bisphosphate. Biochim Biophys Acta 1793: 1086–1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heintzelman M, Hasson T, Mooseker M. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci 107: 3535–3543, 1994. [DOI] [PubMed] [Google Scholar]
- 35. Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 129: 489–506, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirokawa N, Tilney LG, Fujiwara K, Heuser JE. Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J Cell Biol 94: 425–443, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Isogawa Y, Kon T, Inoue T, Ohkura R, Yamakawa H, Ohara O, Sutoh K. The N-terminal domain of MYO18A has an ATP-insensitive actin-binding site. Biochemistry 44: 6190–6196, 2005. [DOI] [PubMed] [Google Scholar]
- 38. Jemnitz K, Heredi-Szabo K, Janossy J, Ioja E, Vereczkey L, Krajcsi P. ABCC2/Abcc2: a multispecific transporter with dominant excretory functions. Drug Metab Rev 42: 402–436, 2010. [DOI] [PubMed] [Google Scholar]
- 39. Kenny AJ, Booth AG, Macnair RD. Peptidases of the kidney microvillus membrane. Acta Biol Med Ger 36: 1575–1585, 1977. [PubMed] [Google Scholar]
- 40. Kenny AJ, Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev 62: 91–128, 1982. [DOI] [PubMed] [Google Scholar]
- 41. Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics 24: 2534–2536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, Barati M, Rane MJ, Lederer E. Parathyroid hormone regulation of Na+,K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J Am Soc Nephrol 16: 2598–2607, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol 4: 21, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kovacs M, Thirumurugan K, Knight PJ, Sellers JR. Load-dependent mechanism of nonmuscle myosin 2. Proc Natl Acad Sci USA 104: 9994–9999, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292: G1249–G1262, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Law AL, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, Wavre-Shapton ST, Moss SE, Hayes MJ. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol Biol Cell 20: 3896–3904, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lawson JD, Pate E, Rayment I, Yount RG. Molecular dynamics analysis of structural factors influencing back door pi release in myosin. Biophys J 86: 3794–3803, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, Boyer JL. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. J Biol Chem 285: 19299–19307, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Licklider LJ, Thoreen CC, Peng J, Gygi SP. Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column. Anal Chem 74: 3076–3083, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR, 3rd Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol 17: 676–682., 1999. [DOI] [PubMed] [Google Scholar]
- 51. Lowey S, Trybus KM. Common structural motifs for the regulation of divergent class II myosins. J Biol Chem 285: 16403–16407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ma ZQ, Dasari S, Chambers MC, Litton MD, Sobecki SM, Zimmerman LJ, Halvey PJ, Schilling B, Drake PM, Gibson BW, Tabb DL. IDPicker 2.0: improved protein assembly with high discrimination peptide identification filtering. J Proteome Res 8: 3872–3881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol 5: 119–144, 2010. [DOI] [PubMed] [Google Scholar]
- 54. Massey-Harroche D, Mayran N, Maroux S. Polarized localizations of annexins I, II, VI and XIII in epithelial cells of intestinal, hepatic and pancreatic tissues. J Cell Sci 111: 3007–3015, 1998. [DOI] [PubMed] [Google Scholar]
- 55. McConnell RE, Higginbotham JN, Shifrin DA, Jr, Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol 185: 1285–1298, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol 177: 671–681, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Merrifield CJ, Rescher U, Almers W, Proust J, Gerke V, Sechi AS, Moss SE. Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr Biol 11: 1136–1141, 2001. [DOI] [PubMed] [Google Scholar]
- 58. Millard TH, Dawson J, Machesky LM. Characterisation of IRTKS, a novel IRSp53/MIM family actin regulator with distinct filament bundling properties. J Cell Sci 120: 1663–1672, 2007. [DOI] [PubMed] [Google Scholar]
- 59. Miller D, Crane RK. A procedure for the isolation of the epithelial brush border membrane of hamster small intestine. Anal Biochem 2: 284–286, 1961. [DOI] [PubMed] [Google Scholar]
- 60. Mitschke D, Reichel A, Fricker G, Moenning U. Characterization of cytochrome P450 protein expression along the entire length of the intestine of male and female rats. Drug Metab Dispos 36: 1039–1045, 2008. [DOI] [PubMed] [Google Scholar]
- 61. Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol 1: 209–241, 1985. [DOI] [PubMed] [Google Scholar]
- 62. Mooseker MS, Pollard TD, Fujiwara K. Characterization and localization of myosin in the brush border of intestinal epithelial cells. J Cell Biol 79: 444–453, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morelle W, Stechly L, Andre S, Van Seuningen I, Porchet N, Gabius HJ, Michalski JC, Huet G. Glycosylation pattern of brush border-associated glycoproteins in enterocyte-like cells: involvement of complex-type N-glycans in apical trafficking. Biol Chem 390: 529–544, 2009. [DOI] [PubMed] [Google Scholar]
- 64. Naim HY. Angiotensin-converting enzyme of the human small intestine. Subunit and quaternary structure, biosynthesis and membrane association. Biochem J 286: 451–457, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Norstrom MF, Smithback PA, Rock RS. Unconventional processive mechanics of non-muscle myosin IIB. J Biol Chem 285: 26326–26334, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Offenhauser N, Borgonovo A, Disanza A, Romano P, Ponzanelli I, Iannolo G, Di Fiore PP, Scita G. The eps8 family of proteins links growth factor stimulation to actin reorganization generating functional redundancy in the Ras/Rac pathway. Mol Biol Cell 15: 91–98, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124: 513–526, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Okazaki N, Takahashi N, Kojima S, Masuho Y, Koga H. Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation. Carcinogenesis 23: 1139–1148, 2002. [DOI] [PubMed] [Google Scholar]
- 69. Paradela A, Bravo SB, Henriquez M, Riquelme G, Gavilanes F, Gonzalez-Ros JM, Albar JP. Proteomic analysis of apical microvillous membranes of syncytiotrophoblast cells reveals a high degree of similarity with lipid rafts. J Proteome Res 4: 2435–2441, 2005. [DOI] [PubMed] [Google Scholar]
- 70. Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992. [DOI] [PubMed] [Google Scholar]
- 71. Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465, 2003. [DOI] [PubMed] [Google Scholar]
- 72. Qualmann B, Kessels MM. New players in actin polymerization–WH2-domain-containing actin nucleators. Trends Cell Biol 19: 276–285, 2009. [DOI] [PubMed] [Google Scholar]
- 73. Rehman AU, Morell RJ, Belyantseva IA, Khan SY, Boger ET, Shahzad M, Ahmed ZM, Riazuddin S, Khan SN, Friedman TB. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am J Hum Genet 86: 378–388, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6: 233–247, 2005. [DOI] [PubMed] [Google Scholar]
- 75. Sakaguchi H, Tokita J, Muller U, Kachar B. Tip links in hair cells: molecular composition and role in hearing loss. Curr Opin Otolaryngol Head Neck Surg 17: 388–393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci 103: 131–143, 1992. [DOI] [PubMed] [Google Scholar]
- 77. Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, Kunzelmann K. Expression and function of epithelial anoctamins. J Biol Chem 285: 7838–7845, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shaner NC, Sanger JW, Sanger JM. Actin and α-actinin dynamics in the adhesion and motility of EPEC and EHEC on host cells. Cell Motil Cytoskeleton 60: 104–120, 2005. [DOI] [PubMed] [Google Scholar]
- 79. Shaw RK, Cleary J, Murphy MS, Frankel G, Knutton S. Interaction of enteropathogenic Escherichia coli with human intestinal mucosa: role of effector proteins in brush border remodeling and formation of attaching and effacing lesions. Infect Immun 73: 1243–1251, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron 53: 371–386, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Singh P. Role of annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett 252: 19–35, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Skowron JF, Bement WM, Mooseker MS. Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil Cytoskeleton 41: 308–324, 1998. [DOI] [PubMed] [Google Scholar]
- 83. Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 12: 696–702, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tabb DL, Fernando CG, Chambers MC. MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res 6: 654–661, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Taylor KA. Regulation and recycling of myosin V. Curr Opin Cell Biol 19: 67–74, 2007. [DOI] [PubMed] [Google Scholar]
- 86. Tocchetti A, Soppo CB, Zani F, Bianchi F, Gagliani MC, Pozzi B, Rozman J, Elvert R, Ehrhardt N, Rathkolb B, Moerth C, Horsch M, Fuchs H, Gailus-Durner V, Beckers J, Klingenspor M, Wolf E, Hrabe de Angelis M, Scanziani E, Tacchetti C, Scita G, Di Fiore PP, Offenhauser N. Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS One 5: e9468, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Triphan X, Menzel VA, Petrunkina AM, Cassara MC, Wemheuer W, Hinsch KD, Hinsch E. Localisation and function of voltage-dependent anion channels (VDAC) in bovine spermatozoa. Pflügers Arch 455: 677–686, 2008. [DOI] [PubMed] [Google Scholar]
- 88. Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol Cell Physiol 273: C1378–C1385, 1997. [DOI] [PubMed] [Google Scholar]
- 89. Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell 16: 2443–2457, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J 82: 1869–1883, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vachon PH, Perreault N, Magny P, Beaulieu JF. Uncoordinated, transient mosaic patterns of intestinal hydrolase expression in differentiating human enterocytes. J Cell Physiol 166: 198–207, 1996. [DOI] [PubMed] [Google Scholar]
- 92. Verpy E, Leibovici M, Zwaenepoel I, Liu XZ, Gal A, Salem N, Mansour A, Blanchard S, Kobayashi I, Keats BJ, Slim R, Petit C. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet 26: 51–55, 2000. [DOI] [PubMed] [Google Scholar]
- 93. Vingadassalom D, Kazlauskas A, Skehan B, Cheng HC, Magoun L, Robbins D, Rosen MK, Saksela K, Leong JM. Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation. Proc Natl Acad Sci USA 106: 6754–6759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Walmsley SJ, Broeckling C, Hess A, Prenni J, Curthoys NP. Proteomic analysis of brush-border membrane vesicles isolated from purified proximal convoluted tubules. Am J Physiol Renal Physiol 298: F1323–F1331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Washburn MP, Wolters D, Yates JR, 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol 19: 242–247., 2001. [DOI] [PubMed] [Google Scholar]
- 96. Weiss SM, Ladwein M, Schmidt D, Ehinger J, Lommel S, Stading K, Beutling U, Disanza A, Frank R, Jansch L, Scita G, Gunzer F, Rottner K, Stradal TE. IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe 5: 244–258, 2009. [DOI] [PubMed] [Google Scholar]
- 97. Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258: 999–1001, 1992. [DOI] [PubMed] [Google Scholar]
- 98. Wolters DA, Washburn MP, Yates JR., 3rd An automated multidimensional protein identification technology for shotgun proteomics. Anal Chem 73: 5683–5690, 2001. [DOI] [PubMed] [Google Scholar]
- 99. Yang C, Hoelzle M, Disanza A, Scita G, Svitkina T. Coordination of membrane and actin cytoskeleton dynamics during filopodia protrusion. PLoS One 4: e5678, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yang LE, Maunsbach AB, Leong PK, McDonough AA. Redistribution of myosin VI from top to base of proximal tubule microvilli during acute hypertension. J Am Soc Nephrol 16: 2890–2896, 2005. [DOI] [PubMed] [Google Scholar]
- 101. Yengo CM, Ananthanarayanan SK, Brosey CA, Mao S, Tyska MJ. Human deafness mutation E385D disrupts the mechanochemical coupling and subcellular targeting of myosin-1a. Biophys J 94: L5–L7, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci USA 107: 8237–8241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zachos NC, Li X, Kovbasnjuk O, Hogema B, Sarker R, Lee LJ, Li M, de Jonge H, Donowitz M. NHERF3 (PDZK1) contributes to basal and calcium inhibition of NHE3 activity in Caco-2BBe cells. J Biol Chem 284: 23708–23718, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.