Abstract
The maintenance of normal body weight either through dietary modification or being habitually more physically active is associated with reduced incidence of nonalcoholic fatty liver disease (NAFLD). However, the means by which weight gain is prevented and potential mechanisms activated remain largely unstudied. Here, we sought to determine the effects of obesity prevention by daily exercise vs. caloric restriction on NAFLD in the hyperphagic, Otsuka Long-Evans Tokushima Fatty (OLETF) rat. At 4 wk of age, male OLETF rats (n = 7–8/group) were randomized to groups of ad libitum fed, sedentary (OLETF-SED), voluntary wheel running exercise (OLETF-EX), or caloric restriction (OLETF-CR; 70% of SED) until 40 wk of age. Nonhyperphagic, control strain Long-Evans Tokushima Otsuka (LETO) rats were kept in sedentary cage conditions for the duration of the study (LETO-SED). Both daily exercise and caloric restriction prevented obesity and the development of type 2 diabetes observed in the OLETF-SED rats, with glucose tolerance during a glucose tolerance test improved to a greater extent in the OLETF-EX animals (30–50% lower glucose and insulin areas under the curve, P < 0.05). Both daily exercise and caloric restriction also prevented excess hepatic triglyceride and diacylglycerol accumulation (P < 0.001), hepatocyte ballooning and nuclear displacement, and the increased perivenular fibrosis and collagen deposition that occurred in the obese OLETF-SED animals. However, despite similar hepatic phenotypes, OLETF-EX rats also exhibited increased hepatic mitochondrial fatty acid oxidation, enhanced oxidative enzyme function and protein content, and further suppression of hepatic de novo lipogenesis proteins compared with OLETF-CR. Prevention of obesity by either daily exercise or caloric restriction attenuates NAFLD development in OLETF rats. However, daily exercise may offer additional health benefits on glucose homeostasis and hepatic mitochondrial function compared with restricted diet alone.
Keywords: nonalcoholic fatty liver disease, Otsuka Long-Evans Tokushima Fatty, type 2 diabetes, mitochondrial function, glucose tolerance, physical activity
poor dietary choices and sedentary lifestyles are leading to a weight gain epidemic in Westernized societies. A critical complication of the obesity epidemic is the development of type 2 diabetes and nonalcoholic fatty liver disease (NAFLD). NAFLD affects ∼30% of all U.S. adults and 75–100% of obese and morbidly obese individuals (3, 5) and is now considered the hepatic representation of the metabolic syndrome (10).
Caloric restriction and exercise are commonly recommended for prevention and amelioration of obesity and lifestyle-related diseases such as NAFLD (6). Recent investigations suggest that being habitually more physically active (32), doing higher amounts of physical activity during leisure time (50), and routinely performing or increasing ones physical activity levels to ≥150 min/wk (43) is inversely associated with NAFLD. Having higher cardiorespiratory fitness also is inversely associated with NAFLD and nonalcoholic steatohepatitis (NASH) (8, 21). Furthermore, maintenance of normal body weight is associated with lower incidence of NAFLD (5). In addition, interventions aimed at lowering body weight by caloric restriction alone or in combination with exercise significantly lower intrahepatic fat content in obese adults (41) and improves liver histology in biopsy-proven NASH patients (35). Unfortunately, studies to date have not directly compared the effects and mechanistic differences between weight loss induced by exercise vs. weight loss induced by caloric restriction on development of NAFLD.
We have recently characterized the development of NAFLD in the Otsuka Long-Evans Tokushima Fatty (OLETF) rat. Selectively bred for null expression of the cholecystokinin-1 receptor, OLETF rats exhibit a within-meal feedback defect for satiety, resulting in hyperphagia, obesity, and insulin resistance and type 2 diabetes (18, 26). OLETF rats also develop hepatic steatosis at a young age, with a progression to hepatocyte ballooning and increased fibrosis and collagen deposition by 40 wk of age in a progressive pattern similar to the human disease (36–38). However, unlike most obese animal models (45), the OLETF rat will exercise voluntarily on running wheels, which we have shown to attenuate the development of NAFLD (36, 37). While NAFLD prevention with exercise was associated with increased hepatic fatty acid oxidation and reduced hepatic lipogenesis in these animals, daily exercise also attenuated weight gain (36, 37), suggesting that the observed protective effects may be due in part to an attenuation in adiposity.
Recently, it has been suggested that, because of the absence of an effective drug therapy, lifestyle modifications aimed at arresting NAFLD development and progression should be made in younger individuals (29). Here we directly compared the effectiveness of daily exercise vs. daily caloric restriction on the prevention of obesity and NAFLD in the OLETF rat. We also sought to determine whether daily exercise has additional benefits beyond weight reduction achieved by caloric restriction alone. In addition, the design allowed us to compare and contrast the mechanisms by which each therapy prevents NAFLD. We hypothesized that both preventative therapies would attenuate NAFLD, but with a greater upregulation in hepatic mitochondrial content and function in the exercising OLETF rats.
METHODS
Animal protocol.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. Male OLETF rats at 4 wk of age were kindly supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan) and upon arrival were randomly assigned to either sedentary cage conditions with ad libitum feeding (OLETF-SED), sedentary cage conditions with food intake adjusted to 70% of food intake by the OLETF-SED animals studied in parallel (OLETF-CR; 70% pair feeding), or immediately housed in cages equipped with voluntary running wheels (OLETF-EX) outfitted with a Sigma Sport BC 606 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) for measuring daily running activity. Nonhyperphagic, control strain Long-Evans Tokushima Otsuka (LETO) rats were kept in the sedentary cage conditions for the duration of the study (LETO-SED). All animals were individually caged throughout the entire experiment. Cages were in temperature-controlled animal quarters (21°C) with a 0600–1800 light and 1800–0600 dark cycle. The OLETF-SED, OLETF-EX, and LETO-SED animals were provided standard rodent chow (Formulab 5008; Purina Mills, St. Louis, MO) for ad libitum feeding in new cages at the beginning of each week. Standard rodent chow also was provided to the OLETF-CR animals daily based on ∼70% of the satiated OLETF-SED rat food consumption, an amount equal to the volume of food consumed by the LETO control animals, as previously reported (23). Body mass was measured weekly throughout the investigation. OLETF-EX animals were given free access to voluntary running wheels 24 h/day, and the daily running activity was obtained between 0800 and 1000 each morning. OLETF-EX and LETO-SED rats also had ad libitum access to food and water until the designated time of death (40 wk of age). At 40 wk of age, rats were anesthetized with pentobarbital sodium (100 mg/kg) and then exsanguinated by removal of the heart 5 h after locking of wheels; the sedentary rats (OLETF-SED, OLETF-CR, and LETO-SED) were killed at the same time. All animals were fasted for 5 h before death.
Dual-energy x-ray absorptiometry.
Whole body composition was measured using a Hologic QDR-1000/w dual-energy X-ray absorptiometry machine calibrated for rats as previously described (23).
Tissue homogenization procedure and fatty acid oxidation.
Livers were quickly excised from anesthetized rats and either flash-frozen in liquid nitrogen, placed in 10% formalin, or placed in ice-cold isolation buffer (in mM: 100 KCl, 40 Tris·HCl, 10 Tris-Base, 5 MgCl2·6H2O, 1 EDTA, and 1 ATP; pH 7.4). Palmitate oxidation was measured with radiolabeled [1-14C]palmitate (American Radiochemicals) in fresh liver homogenate preparations as previously reported (37). Lignocerate oxidation (a very-long-chain fatty acid, C-22) was measured with radiolabeled [1-14C]lignocerate (American Radiochemicals) in fresh liver homogenate preparations as previously described (30) as an index to assess peroxisomal fatty acid oxidation. Both 14CO2, representing complete fatty acid oxidation, and 14C-labeled acid soluble metabolites, representing incomplete fatty acid oxidation, were collected and counted as previously described (37). Palmitate oxidation experiments were performed in the presence (100 μM) or absence of etomoxir [a specific inhibitor of mitochondrial carnitine palmitoyl-CoA transferease (CPT)-1 and entry into the mitochondria] to examine the relative contribution of mitochondrial (−etomoxir) and extramitochondrial organelles (+etomoxir) in total fatty acid oxidation.
Intrahepatic lipid content, Oil-Red O staining, and liver histology.
Intrahepatic triglyceride (TG) content was determined as previously described (37). Hepatic diacylglycerol (DAG) content was determined by using a modified Folch (11) method. Briefly, liver tissue was homogenized in ice-cold Trizma-EDTA buffer, and lipids were extracted in chloroform-methanol-acetic acid (2:1:0.15). Extracted lipids were then run on a thin-layer chromatography silica plate in a tank containing hexane, diethyl ether, and acetic acid (70:30:1). DAG fractions were scraped and methylated by incubating with toluene, methanol, and acetyl chloride (0.5:1.2:0.1) at 100°C for 60 min and separated in hexane. Fatty acid methyl esters were analyzed by GC (Agilent Technologies). Oil-Red O staining was performed in frozen liver sections as previously described (37). To examine liver morphology, formalin-fixed paraffin-embedded sections of liver were stained with hematoxylin and eosin (H&E). For assessment of hepatic steatosis, histopathological criteria proposed by Kleiner et al. (19) were adopted in five to six animals per group and three views per animal. Hepatic steatosis was graded as follows: <5% (score 0), 5%-33% (score 1), >33%-66% (score 2), or >66% (score 3). Sirius red staining for collagen deposition was performed as previously referenced (38, 49).
Western blotting.
Western blot analyses were performed for the determination of AMP-activated protein kinase-α (AMPKα), AMPKα Thr172 phosphorylation-specific, acetyl-coenzyme A carboxylase (ACC), ACC Ser79 phosphorylation-specific, fatty acid synthase (FAS) (Cell Signaling, Beverly, MA), stearoyl-CoA desaturase-1 (SCD-1; Alpha Diagnostics International, San Antonio, TX), peroxisome proliferator-activated receptor-α (PPARα; Santa Cruz Biotechnology, Santa Cruz, CA), peroxisome proliferator-activated receptor-γ (PPARγ; Santa Cruz Biotechnology), acyl-CoA oxidase 1 (ACOX1; Santa Cruz Biotechnology), cytochrome P-450 A2 (CYP4A; Abcam, Cambridge, MA), sterol regulatory element binding protein 1c (SREBP-1c; Santa Cruz Biotechnology), mammalian target of rapamycin (mTOR; Cell Signaling), phospho-mTOR Ser2448 (Cell Signaling), and oxidative phosphorylation (OXPHOS) complexes I to V of the electron transport chain (MitoProfile Total OXPHOS Rodent WB Antibody Cocktail; MitoSciences, Eugene, OR). Content of phosphoproteins (using phosphospecific antibodies) was calculated from the density of the band of the phosphoprotein divided by the density of the protein (total) using the appropriate antibody (36, 37). To control for equal protein loading and transfer, the membranes were stained with 0.1% amido black (Sigma) as previously described (37). The total protein staining for each lane was quantified, and these values were used to correct for any differences in protein loading or transfer of all band densities.
Fat pad collection and serum assays.
Retroperitoneal and omental fat pads were removed from exsanguinated animals and weighed. Serum glucose (Sigma, St. Louis, MO), TG (Sigma), free fatty acids (FFA; Wako Chemicals, Richmond, VA), and insulin (Linco Research, St. Charles, MO) were measured using commercially available kits according to the manufacturer's instructions after a 12-h overnight fast and 12-h wheel lock in the OLETF-EX rats. This 12-h overnight fast occurred at 38–39 wk of age and coincided with the glucose tolerance test (described below). Serum alanine aminotransferase (ALT) and hemoglobin A1c (HbA1c) concentrations were measured as previously described (23, 37).
Intraperitoneal glucose tolerance test.
Intraperitoneal glucose tolerance tests (IPGTTs) were performed at 38–39 wk of age (n = 6/group). Food was removed from the cages 12 h before each received an intraperitoneal injection of dextrose (50% solution, 2 g/kg body wt). The running wheels in the OLETF-EX rats were locked 12 h before the IPGTT procedure began. Venipuncture blood samples were collected from the lateral tail vein immediately before (0 min) dextrose administration and 15, 30, 45, 60, and 120 min after injection. After centrifugation at 4°C at 3,000 g, serum samples were stored at −80°C until glucose and insulin measurement by a glucose oxidase kit (Thermo Electron, Louisville, CO) and ELISA (Millipore, Billerica, MA), respectively. Insulin sensitivity was estimated as the product of the areas under the curve (AUCs) for glucose and insulin calculated using the trapezoidal method (46).
Reduced and oxidized glutathione.
Reduced (GSH) and oxidized (GSSG) glutathione concentrations were determined by a fluorometric method as previously described by our group (38).
Superoxide dismutase, catalase, citrate synthase, β-hydroxyacyl-CoA dehydrogenase, and glutathione reductase activity.
Superoxide dismutase (SOD) activity in liver homogenate was determined by commercially available methods (Cayman Chemicals, Ann Arbor, MI). Catalase activity was determined by commercially available methods (Sigma) as previously described (47). Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined in whole liver homogenate using the methods of Srere et al. (42) and Bass et al. (2), respectively, as previously described (36, 37). Glutathione reductase activity was assessed by commercially available kits (Cayman Chemicals).
Statistics.
Each outcome measure was examined in six to eight animals. For each outcome measure, a one-way ANOVA was performed (SPSS/15.0; SPSS, Chicago, IL). Significant main effects (P < 0.05) were followed up with Fisher least-significant difference post hoc comparisons. Values are reported as means ± SE, and a P value <0.05 denotes a statistically significant difference.
RESULTS
Animal characteristics.
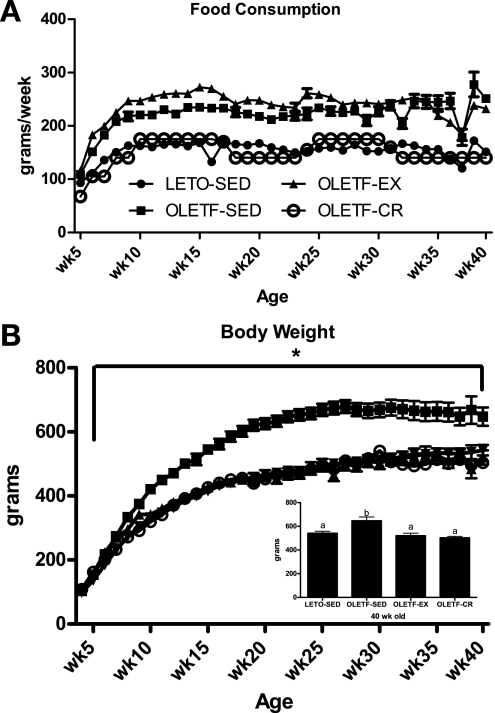
Similar to our previous reports (23, 27), OLETF-EX animals displayed initial running distances of ∼4 km/day (∼150 min/day) at 4 wk of age and ∼12 km/day (∼275 min/day) at 10 wk of age, then declining to ∼7 km/day (∼200 min/day) at 20 wk of age and ∼4 km/day (∼150 min/day) by 40 wk of age (data not shown). By design, absolute food consumption of the OLETF-CR animals was ∼70% of OLETF-SED and equal to the amount consumed by LETO-SED animals, and ad libitum food consumption was similar between OLETF-SED and OLETF-EX (Fig. 1A). Caloric restriction and daily wheel running resulted in significantly lower body weights in the OLETF-CR and OLETF-EX rats compared with OLETF-SED (70% of OLETF-SED values; P < 0.001; final body weights are shown in Fig. 1B, inset), which were equal to LETO-SED values. Voluntary running and caloric restriction suppressed fat pad mass (omental and retroperitoneal) and percent body fat gain compared with OLETF-SED (P < 0.01) to the level of the LETO-SED (Table 1). OLETF-EX animals also displayed a higher heart weight-to-body weight ratio, indicating that adequate stimulus to produce training adaptations was achieved (Table 1).
Fig. 1.
Weekly food consumption (A) and body weight gain (B). Values are means ± SE (n = 6–8 animals in each group). *Otsuka Long-Evans Tokushima Fatty sedentary (OLETF-SED) significantly different from other animal groups at respective ages (P < 0.001). Inset, values with different superscripts are significantly different (P < 0.001). OLETF-EX, exercised OLETF rats; LETO-SED, sedentary Long-Evans Tokushima Otsuka rats; OLETF-CR, calorie-restricted OLETF rats.
Table 1.
Animal and liver characteristics
| Variable | LETO-SED | OLETF-SED | OLETF-EX | OLETF-CR |
|---|---|---|---|---|
| Body fat, % | 20.0 ± 1.0a | 31.6 ± 3.3b | 14.0 ± 1.2a | 19.1 ± 1.7a |
| Fat pad mass, g | 11.9 ± 0.7a | 54.4 ± 6.3b | 10.8 ± 1.6a | 15.7 ± 1.6a |
| Serum glucose, mg/dl | 98.5 ± 5.9a | 169.0 ± 9.4b | 104.7 ± 4.6a | 121.3 ± 5.4c |
| Serum insulin, ng/ml | 1.93 ± 0.28a | 3.13 ± 0.97a,b | 1.48 ± 0.46a | 3.9 ± 0.49b |
| HOMA (ratio) | 83.3 ± 14.0a | 216.8 ± 61.8b | 59.1 ± 24.1a | 211.1 ± 33.0b |
| HbA1c, % | 4.6 ± 0.04a | 7.9 ± 0.8b | 4.4 ± 0.1a | 4.3 ± 0.01a |
| Serum TG, mg/dl | 42.8 ± 4.4a | 237.4 ± 45.8b | 51.5 ± 7.3a | 66.3 ± 4.0a |
| Serum ALTs, U/l | 54.9 ± 2.5 | 69.3 ± 11.2 | 64.9 ± 6.5 | 52.8 ± 5.6 |
| Heart wt-to-body wt ratio, mg/g | 2.54 ± 0.06a | 2.42 ± 0.12a | 3.19 ± 0.11b | 2.62 ± 0.06a |
Values are means ± SE; n = 6–8 rats in each group. Fat pad mass was the combination of omental and retroperitoneal fat pads.
LETO, Long-Evans Tokushima Otsuka; SED, sedentary; OLETF, Otsuka Long-Evans Tokushima fatty; EX, exercised; CR, calorie restricted; TG, triglycerides; ALT, alanine aminotransferase. Homeostasis model assessment (HOMA) = (pmol/l insulin × mmol/l glucose)/22.5 Ref (46). Values with different superscripts are significantly different (P < 0.01).
Fasting glucose was significantly lower in the LETO-SED, OLETF-EX, and OLETF-CR rats compared with OLETF-SED, with LETO-SED and OLETF-EX concentrations also being significantly lower than OLETF-CR (Table 1). OLETF-EX and LETO-SED animals also exhibited significantly lower fasting serum insulin and HOMA calculations (24) compared with OLETF-CR and OLETF-SED animals (Table 1). The lack of differences between OLETF-SED and OLETF-CR and LETO-SED is because of the development of frank type 2 diabetes and a loss of normal pancreatic insulin secretion in the 40-wk-old OLETF-SED rats, as we have previously reported (23). This is supported by the observation of OLETF-SED animals having twofold higher HbA1c levels compared with other groups (P < 0.001; Table 1). In addition, serum TG levels were significantly elevated in the OLETF-SED animals compared with other groups (P < 0.01; Table 1).
Glucose tolerance.
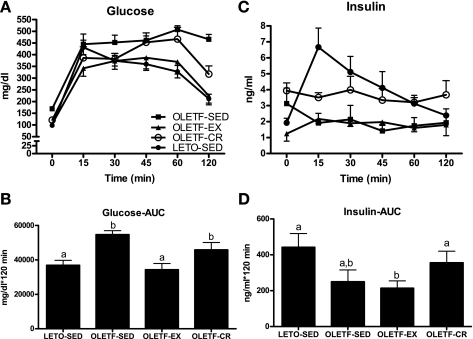
Glucose and insulin responses to an IPGTT are shown in Fig. 2. Glucose AUC was significantly greater in OLETF-SED compared with OLETF-EX and LETO-SED (P < 0.01; Fig. 2, A and B) and tended to be greater compared with OLETF-CR (P = 0.1). In addition, glucose AUC was reduced to a greater extent in the OLETF-EX compared with OLETF-CR animals (P < 0.05; Fig. 2, A and B). Insulin responses to the IPGTT did not differ among LETO-SED, OLETF-SED, and OLETF-CR but were significantly lower in the OLETF-EX compared with LETO-SED and OLETF-CR (P < 0.05; Fig. 2, C and D). Insulin AUCs are significantly elevated in the OLETF-SED compared with LETO-SED, OLETF-CR, and OLETF-EX at 20 wk of age but drop fivefold from 20 to 40 wk in the OLETF-SED animals (39) (data not shown).
Fig. 2.
Systemic glucose homeostasis as assessed by an intraperitoneal glucose tolerance test. Glucose responses across time (A), glucose area under curve (AUC; B), insulin responses across time (C), and insulin AUC (D). Values are means ± SE (n = 5–6). Values with different superscripts are significantly different (P < 0.05).
Liver antioxidative status.
Liver SOD activity was higher in the OLETF-EX and OLETF-CR rats compared with OLETF-SED (P < 0.01; OLETF-SED = 4.11 ± 0.47 U/ml, OLETF-EX = 5.81 ± 0.68, OLETF-CR = 6.78 ± 0.46), whereas catalase activity was lower in OLETF-EX than OLETF-SED and OLETF-CR (P < 0.05; OLETF-SED = 1.65 ± 0.11 μM·μg−1·min−1, OLETF-EX = 1.31 ± 0.06, OLETF-CR = 1.54 ± 0.08). In addition, glutathione reductase activity was significantly reduced in the OLETF-EX compared with OLETF-SED (P < 0.05) and tended to be reduced in OLETF-CR compared with OLETF-SED (P = 0.07; OLETF-SED = 2.58 ± 0.17 nmol/μg/min, OLETF-EX = 2.13 ± 0.10, OLETF-CR = 2.27 ± 0.05). Furthermore, the GSH-to-GSSG ratio was greater in the OLETF-SED compared with OLETF-EX and OLETF-CR (P < 0.01; OLETF-SED = 41.1 ± 6.5, OLETF-EX = 24.9 ± 2.10, OLETF-CR = 26.4 ± 1.7).
Daily exercise and caloric restriction attenuate NAFLD progression in OLETF rats.
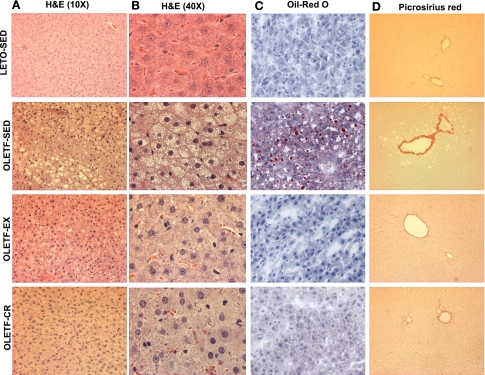
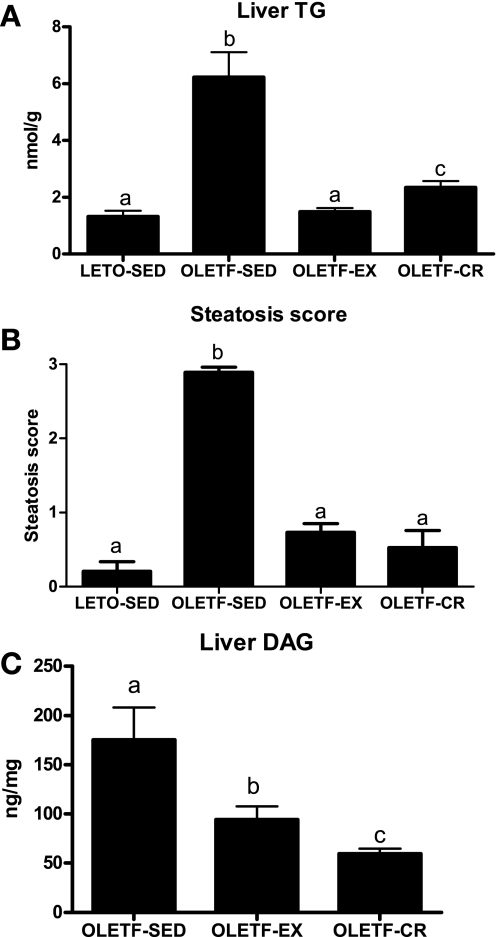
Representative images of randomly selected sections of the liver stained for H&E (both at ×10 and 40 magnification), Oil-Red O, and picrosirius red are shown in Fig. 3. OLETF-EX and OLETF-CR animals did not exhibit the significantly (P < 0.001) increased levels of macro- and microvesicular steatosis (Fig. 3, A–C), hepatocyte ballooning (Fig. 3B), or the increased fibrosis and collagen deposition in perivenular (around terminal hepatic veins) regions (Fig. 3D) that occurred at 40 wk in the OLETF-SED animals. Both preventative therapies normalized liver histological changes to that observed in the lean, LETO-SED controls. Liver injury was modest at this stage of development, since serum ALT levels did not differ among groups (Table 1). Biochemical analysis revealed significantly lower hepatic TG content in the OLETF-EX and OLETF-CR rats compared with OLETF-SED, with further attenuation in the OLETF-EX compared with OLETF-CR (Fig. 4A), to the level of the LETO-SED controls. In addition, steatosis scoring was significantly greater in the OLETF-SED animals compared with LETO-SED, OLETF-EX, or OLETF-CR rats (P < 0.001; Fig. 4B). Furthermore, DAG content was significantly lower in both intervention groups, with levels in OLETF-CR rats significantly lower than OLETF-EX animals (P < 0.05; Fig. 4C).
Fig. 3.
Representative images of hematoxylin and eosin (H&E) (×10 and 40 fields of view; A and B), Oil-Red O (C), and picrosirius red (D) staining. Note the large lipid vacuoles (A and B), macro- and microvesicular steatosis (A-C), hepatocyte ballooning and nuclear displacement (A and B), and perivenular fibrosis (C) in the 40-wk-old OLETF-SED animals compared with the other animal groups.
Fig. 4.
Quantification of hepatic triglyceride (TG) and steatosis scores is shown in A and B and hepatic diacylglycerol (DAG) content is shown in C. Values are means ± SE (n = 6–8). Values with different superscripts are significantly different (P < 0.01).
Daily exercise increased hepatic mitochondrial function in OLETF rats.
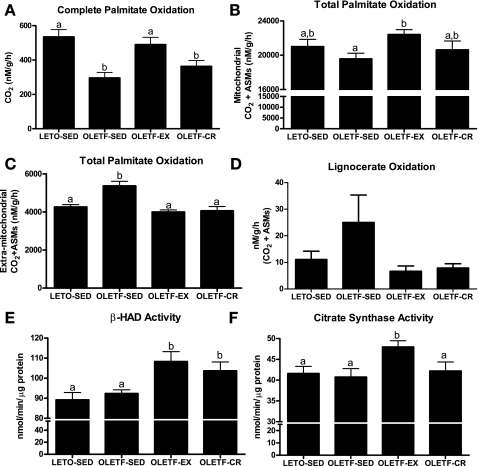
Similar to what we have previously reported (38), complete palmitate oxidation to CO2 was significantly reduced in OLETF-SED compared with LETO-SED animals (Fig. 5A), and only daily exercise normalized complete palmitate oxidation to the LETO-SED levels (Fig. 5A). In addition, the mitochondrial contribution (the etomoxir-inhibitable portion) to whole homogenate total palmitate oxidation (CO2 + acid soluble metabolites; Fig. 5B) was significantly higher in the OLETF-EX animals compared with OLETF-SED, whereas extramitochondrial organelle oxidation (non-etomoxir-inhibitable portion) was 25–30% higher in the OLETF-SED compared with LETO-SED, OLETF-EX, and OLETF-CR (P < 0.01; Fig. 5C). Furthermore, oxidation of the very-long-chain fatty acid lignocerate tended to be increased in the OLETF-SED animals compared with LETO-SED, OLETF-EX, and OLETF-CR (P values ranging from 0.1 to 0.2; Fig. 5D). Daily wheel running also increased hepatic citrate synthase activity compared with OLETF-SED, LETO-SED, and OLETF-CR animals (Fig. 5F) while β-HAD activity was elevated with daily exercise and caloric restriction compared with OLETF-SED and LETO-SED animals (Fig. 5E). The lack of observed differences in β-HAD and citrate synthase activities between OLETF-SED and LETO-SED in whole liver homogenate in the current report differs from our previous findings in isolated hepatic mitochondria (38).
Fig. 5.
Effects of daily exercise and caloric restriction on hepatic complete palmitate oxidation to CO2 (A), total mitochondrial palmitate oxidation [CO2 and acid soluble metabolites (ASMs); B], total extramitochondrial palmitate oxidation (C), total lignocerate oxidation (CO2 and ASMs; D), β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity (E), and citrate synthase activity (F). Values are means ± SE (n = 6–8). Values with different superscripts are significantly different (P < 0.05).
Impact of daily exercise and caloric restriction on markers of hepatic mitochondrial and extramitochondrial protein content.
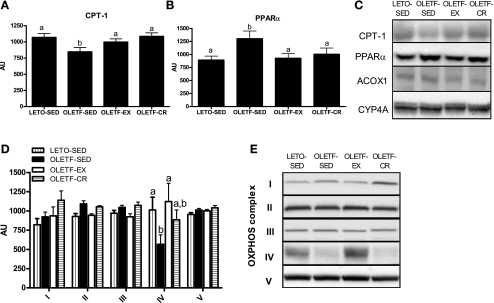
Hepatic CPT-1 protein content, the rate-limiting step in mitochondrial fatty acid entry, was significantly suppressed in the OLETF-SED rats compared with the other animal groups (P < 0.05; Fig. 6A). On the other hand, PPARα protein content was significantly elevated in the OLETF-SED animals (P < 0.05; Fig. 6B). However, PPARα-regulated proteins ACOX1 and CYP4A did not differ among groups (representative Western blot shown in Fig. 6C). Hepatic protein content for OXPHOS I, II, III, and V did not differ among groups; however, mitochondrial-encoded OXPHOS IV (subunit I) was significantly decreased in OLETF-SED animals but normalized to LETO-SED levels with daily EX (P < 0.05; Fig. 6, D and E).
Fig. 6.
Effects of daily exercise and caloric restriction on hepatic carnitine palmitoyl-CoA transferease (CPT)-1 protein content (A), peroxisome proliferator-activated receptor (PPAR)-α protein content (B), and oxidative phosphorylation (OXPHOS) complex I-V protein content (D). Representative Western blots are shown in C and E. Values are means ± SE (n = 6–8). Values with different superscripts are significantly different (P < 0.05). AU, arbitrary units; ACOX1, acyl-CoA oxidase 1; CYP4A, cytochrome P-450 A2.
Impact of daily exercise and caloric restriction on markers of hepatic lipogenesis.
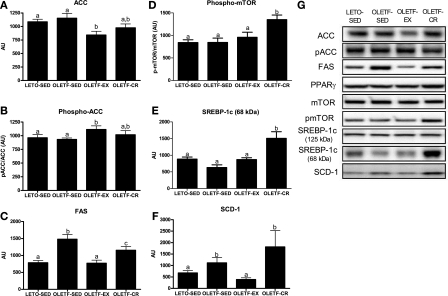
Hepatic protein content of ACC and ACC Ser79 phosphorylation did not differ between LETO-SED and OLETF-SED but was significantly reduced in OLETF-EX animals (P < 0.05; Fig. 7, A and B). However, hepatic FAS was significantly elevated in OLETF-SED animals and reduced with exercise and caloric restriction, with content being reduced to a greater extent in OLETF-EX animals (P < 0.05; Fig. 7C). On the other hand, phospho-mTOR and the active, mature form of SREBP-1c (68-kDa form) were both significantly elevated in the OLETF-CR animals compared with other animal groups (P < 0.01; Fig. 7, D and E). In addition, SCD-1 was significantly elevated in OLETF-SED and OLETF-CR rats compared with LETO-SED and OLETF-EX (P < 0.01; Fig. 7F). Hepatic protein content of AMPKα and AMPKα Thr172 phosphorylation did not differ among groups (data not shown), and hepatic PPARγ, SREBP-1c (125-kDa form), and total mTOR protein content did not differ among groups (representative Western blots shown in Fig. 7G).
Fig. 7.
Effects of daily exercise and caloric restriction on the following hepatic markers of de novo lipogenesis: acetyl-coenzyme A carboxylase (ACC, A), phospho (p)-ACC (B), fatty acid synthase (FAS, C), phospho-mammalian target of rapamycin (mTOR, D), sterol regulatory element binding protein 1c (SREBP-1c, E), and stearoyl-CoA desaturase-1 (SCD-1, F). Representative Western blots are shown in G. Values are means ± SE (n = 6–8). Values with different superscripts are significantly different (P < 0.05).
DISCUSSION
The maintenance of normal body weight either through dietary modification or being habitually more physically active is associated with reduced incidence of NAFLD. In addition, weight control and exercise are commonly prescribed therapies for NAFLD (6, 14). In fact, being more physically active (32, 43, 50) or targeting weight loss by energy restriction alone or in combination with exercise training (33, 35, 41, 48) are associated with reduced NAFLD. However, a direct comparison between the effectiveness of preventing NAFLD by controlling body weight with caloric restriction alone or with exercise alone is lacking. Here we used the OLETF rat, a hyperphagic rodent model that develops obesity and NAFLD (38), to gain mechanistic insight into dietary and exercise preventative therapies targeting weight reduction. A novel, clinically relevant finding was that preventing weight gain with either daily exercise or daily caloric restriction effectively prevented NAFLD development seen in sedentary OLETF rats. However, despite similar hepatic phenotypes, OLETF rats that engaged in daily exercise also exhibited better systemic glucose tolerance; enhanced mitochondrial fatty acid oxidation, oxidative enzyme function, and protein content; and further suppression of hepatic de novo lipogenesis proteins compared with caloric restriction. This would collectively suggest distinct advantages of obesity prevention through daily exercise over caloric restriction in overall metabolic health.
NAFLD prevention through daily exercise was associated with an upregulation in hepatic mitochondrial fatty acid oxidation and a tighter coupling of β-oxidation and the tricarboxylic acid cycle, resulting in a more complete degradation of fatty acids compared with OLETF-SED and OLETF-CR animals. This occurred despite the fact that hepatic CPT-1, the rate-limiting step in mitochondrial fatty acid entry, was significantly elevated in both the OLETF-EX and OLETF-CR animals. Whether or not upregulated mitochondrial function and fatty acid oxidation has a beneficial impact on overall health has not been examined directly, but it previously has been demonstrated that overexpression of CPT-1 increases fatty acid oxidation and reduces hepatic TG accumulation (44). This documents that deficiencies in mitochondrial fatty acid entry and oxidative capacity play a role in NAFLD development.
In the current report, both daily exercise and caloric restriction independently reduce hepatic PPARα protein content and extramitochondrial fatty acid oxidation but did not alter hepatic ACOX1 and CYP4A protein content. Mitochondrial abnormalities are closely related to the pathogenesis of NAFLD (7, 16, 38, 40), and β-oxidative capacity is thought to be decreased (12, 31, 38) in NAFLD but not always in NASH (40). However, peroxisomal β-oxidation is elevated in the diabetic liver and in livers from fatty rats (1, 15). Our findings suggest that peroxisomal/microsomal activity, but not likely peroxisomal or microsomal content, is activated in the OLETF-SED animals to compensate for reduced mitochondrial content and function, which could lead to the accumulation of lipotoxic lipid moieties, factors known to activate hepatic mitochondrial oxidative stress (28). This would explain the compensatory increases in the GSH-to-GSSG ratio and glutathione reductase activity and the reduced SOD activity observed in the liver of the OLETF-SED rats.
Recent stable isotope tracer work demonstrates that ∼26% of hepatic TG accumulation in NAFLD patients can be accounted for by de novo lipogenesis (9), highlighting the importance of this metabolic pathway in NAFLD. In addition, it has been implicated that, with the apparent uncoupling and reductions in complete mitochondrial fatty acid oxidation, incomplete oxidation products are likely being directed to fatty acid biosynthesis pathways (20, 28). Here, daily exercise globally impacted several measures of de novo fatty acid synthesis, including reduced ACC and FAS and increased phosphorylation of ACC (inactive when phosphorylated). In addition, daily exercise also dramatically reduced hepatic SCD-1 protein content compared with OLETF-SED rats. In combination with our previous report of reduced hepatic malonyl-CoA levels with exercise in OLETF rats (36), daily exercise appears to beneficially alter substrate partitioning and correct mitochondrial insufficiencies in this animal model.
Through the conversion of saturated fatty acids to monounsaturated fatty acids, hepatic SCD-1 contributes to abnormal partitioning of fatty acids by increasing ACC activity and decreasing fatty acid oxidation, shunting substrate toward fatty acid synthesis. There is also increasing evidence for a role of mTOR in inducing lipogenesis (22). The action of insulin on SCD-1-induced lipogenesis is thought to be mediated through regulation of mTOR and SREBP-1c (25), and the activation of lipogenesis and SREBP1c requires complex 1 of the target of rapamycin (34). Here we report that OLETF-CR animals exhibited significant increases in the active, mature form of SREBP-1c, increases in SCD-1, and increased catalytic activation of mTOR because of an increase in its phosphorylation status. This was independent of changes in PPARγ protein content. These findings suggest that, as opposed to the exercising animals that remained hyperphagic, the caloric-restricted animals have upregulated the machinery necessary for lipogenesis. These findings warrant future, more in-depth investigation.
Our findings suggest that daily exercise upregulates processes involved in hepatic mitochondrial flux and turnover to buffer the hypercaloric environment seen in this animal model, thereby oxidizing excess substrate, preventing de novo lipogenesis, and limiting hepatic TG and DAG accumulation that is seen in the hyperphagic, sedentary OLETF rat. Conversely, caloric restriction appears to prevent NAFLD not through enhanced mitochondrial flux but rather through elimination of hyperphagia and limiting available substrate to accumulate in the liver. An apparent balance between hepatic uptake, oxidation, and storage of fatty acids occurs under both preventative conditions but not in the OLETF-SED animals, whose hypercaloric status was not properly matched with an increase in mitochondrial β-oxidation, resulting in an increased need for extramitochondrial oxidative support and an excess accumulation of hepatic fat.
Our results document that both caloric restriction and daily exercise attenuate the loss of glycemic control and both prevent the type 2 diabetes development observed in the OLETF-SED rats, but with exercise being superior to caloric restriction despite similar reductions in body weight and adiposity between groups. Elevated release of FFAs from visceral adipose tissue into the portal circulation is thought to contribute to NAFLD and NASH (4). In the hyperphagic, sedentary, diabetic OLETF rat, insulin-mediated suppression of adipose tissue lipolysis is likely blunted, leading to an increased delivery of FFAs to the liver. Thus it is likely that reducing visceral adiposity and insulin resistance through daily exercise and caloric restriction had a significant impact on NAFLD in this rodent model.
An important consideration that still deserves attention is whether exercise training in the absence of weight loss can be used as an effective therapy in the prevention or treatment of NAFLD. A promising recent report suggests that short-term exercise training reduces hepatic lipid content in previously sedentary obese men and women in the absence of reductions in body weight or insulin resistance (17). These findings need to be confirmed in long-term exercise interventions.
In conclusion, our findings have important human health application, particularly for the 60–80% of Americans who overeat, who are overweight, and who are physically inactive. Our data support preventative strategies that either eliminate the overeating environment (OLETF-CR) or, if overeating is present, an increase in daily physical activity (OLETF-EX) to prevent obesity and NAFLD. In addition, daily exercise may offer health benefits beyond caloric restriction through better systemic glucose homeostasis and upregulation in hepatic mitochondrial function.
GRANTS
This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO. This work was partially supported by National Institutes of Health Grants DK-56345 (J. A. Ibdah), HL-36088 (M. H. Laughlin), and F32 DK-83182 (R. S. Rector), and by institutional funds from the School of Medicine and College of Veterinary Medicine.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The OLETF and LETO rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). We thank Suzie Ridenhour, Craig Meers, and Meghan Ruebel for excellent technical assistance to this work and Whitney Collins and Aaron Bunker for help with animal husbandry. We also thank the Veterinary Medicine Diagnostics Laboratory at the University of Missouri for help with the histological sections and alanine aminotransferase measurements and the Diabetes Diagnostics Lab at the University of Missouri for help with the hemoglobin A1c measurements.
REFERENCES
- 1. Asayama K, Sandhir R, Sheikh FG, Hayashibe H, Nakane T, Singh I. Increased peroxisomal fatty acid beta-oxidation and enhanced expression of peroxisome proliferator-activated receptor-alpha in diabetic rat liver. Mol Cell Biochem 194: 227–234, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969 [DOI] [PubMed] [Google Scholar]
- 3. Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 132: 112–117, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114: 147–152, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Caldwell S, Lazo M. Is exercise an effective treatment for NASH? Knowns and unknowns. Ann Hepatol 8, Suppl 1: S60–S66, 2009 [PubMed] [Google Scholar]
- 7. Caldwell SH, Swerdlow RH, Khan EM, Iezzoni JC, Hespenheide EE, Parks JK, Parker WD., Jr Mitochondrial abnormalities in non-alcoholic steatohepatitis. J Hepatol 31: 430–434, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 130: 2023–2030, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Folch J, Lees M, SloaneStanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 12. Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Munoz-Yague T, Solis-Herruzo JA. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 44: 581–591, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Glynn EL, Lujan HL, Kramer VJ, Drummond MJ, DiCarlo SE, Rasmussen BB. A chronic increase in physical activity inhibits fed-state mTOR/S6K1 signaling and reduces IRS-1 serine phosphorylation in rat skeletal muscle. Appl Physiol Nutr Metab 33: 93–101, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut 56: 1760–1769, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horie S, Ishii H, Suga T. Changes in peroxisomal fatty acid oxidation in the diabetic rat liver. J Biochem (Tokyo) 90: 1691–1696, 1981 [DOI] [PubMed] [Google Scholar]
- 16. Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology 128: 1381–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50: 1105–1112, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41: 1422–1428, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 47: 1158–1166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol 19: R1046–R1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 25. Mauvoisin D, Rocque G, Arfa O, Radenne A, Boissier P, Mounier C. Role of the PI3-kinase/mTor pathway in the regulation of the stearoyl CoA desaturase (SCD1) gene expression by insulin in liver. J Cell Commun Signal 1: 113–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol 48: 360–367, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 104: 708–715, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Muoio DM, Koves TR. Skeletal muscle adaptation of fatty acid depends on coordinated actions of the PPARs and PGC1a: implications for metabolic disease. Appl Physiol Nutr Metab 32: 874–883, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Nobili V, Day C. Childhood NAFLD: a ticking time-bomb? (Abstract) Gut 58: 1442, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Noland RC, Woodlief TL, Whitfield BR, Manning SM, Evans JR, Dudek RW, Lust RM, Cortright RN. Peroxisomal-mitochondrial oxidation in a rodent model of obesity-associated insulin resistance. Am J Physiol Endocrinol Metab 293: E986–E1001, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38: 999–1007, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, Belloni E, Canu T, Terruzzi I, Scifo P, Del Maschio A, Luzi L. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 30: 683–688, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54: 603–608, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8: 224–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 51: 121–129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 17: 2162–2168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969 [Google Scholar]
- 43. St. George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology 50: 68–76, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Stefanovic-Racic M, Perdomo G, Mantell BS, Sipula IJ, Brown NF, O'Doherty RM. A moderate increase in carnitine palmitoyltransferase 1a activity is sufficient to substantially reduce hepatic triglyceride levels. Am J Physiol Endocrinol Metab 294: E969–E977, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Stern JS, Johnson PR. Spontaneous activity and adipose cellularity in the genetically obese Zucker rat (fafa). Metabolism 26: 371–380, 1977 [DOI] [PubMed] [Google Scholar]
- 46. Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 17: 152–154, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol 27: 103–107, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Wei Y, Clark SE, Morris EM, Thyfault JP, Uptergrove GM, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol 49: 417–428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I, Blendis L, Halpern Z, Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology 48: 1791–1798, 2008 [DOI] [PubMed] [Google Scholar]