Abstract
Bypass of the foregut following Roux-en-Y gastric bypass (RYGB) surgery results in altered nutrient absorption, which is proposed to underlie the improvement in glucose tolerance and insulin sensitivity. We conducted a prospective crossover study in which a mixed meal was delivered orally before RYGB (gastric) and both orally (jejunal) and by gastrostomy tube (gastric) postoperatively (1 and 6 wk) in nine subjects. Glucose, insulin, and incretin responses were measured, and whole-body insulin sensitivity was estimated with the insulin sensitivity index composite. RYGB resulted in an improved glucose, insulin, and glucagon-like peptide-1 (GLP-1) area under the curve (AUC) in the first 6 wk postoperatively (all P ≤ 0.018); there was no effect of delivery route (all P ≥ 0.632) or route × time interaction (all P ≥ 0.084). The glucose-dependent insulinotropic polypeptide (GIP) AUC was unchanged after RYGB (P = 0.819); however, GIP levels peaked earlier after RYGB with jejunal delivery. The ratio of insulin AUC to GLP-1 and GIP AUC decreased after surgery (P =.001 and 0.061, respectively) without an effect of delivery route over time (both P ≥ 0.646). Insulin sensitivity improved post-RYGB (P = 0.001) with no difference between the gastric and jejunal delivery of the mixed meal over time (P = 0.819). These data suggest that exclusion of nutrients from the foregut with RYGB does not improve glucose tolerance or insulin sensitivity. However, changes in the foregut response post-RYGB due to lack of nutrient exposure cannot be excluded. Our findings suggest that foregut bypass may alter the incretin response by enhanced nutrient delivery to the hindgut.
Keywords: incretin, gastrostomy tube, mixed meal
the improvement in Type 2 diabetes that occurs in the first 2 yr after bariatric surgery is largely sustained in the longer-term but varies among procedures (3, 29). Roux-en-Y gastric bypass (RYGB) surgery involves formation of a small gastric pouch that is anastomosed to the small intestine at a site that bypasses the duodenum and proximal jejunum. It is an established means to achieve marked and persistent weight loss, leading to reversal of insulin resistance (5, 21) and an ∼80% remission rate of Type 2 diabetes (3). Adjustable gastric banding, a gastric restrictive procedure, improves Type 2 diabetes in ∼55% of patients (3). Rates of remission for both procedures are further dependent on the severity of the disease (28). Although this differential effectiveness of bariatric procedures on Type 2 diabetes mirrors the greater weight loss achieved with RYGB (3), a body of evidence suggests that weight loss is not the sole determining factor. For example, Schauer et al. (28) reported that ∼30% of patients with Type 2 diabetes (duration <10 yr) discontinued all antidiabetic medications after RYGB upon hospital discharge. Biochemical markers of Type 2 diabetes, such as elevated fasting glucose and insulin levels, are also strikingly improved in the days following surgery before substantial weight loss has occurred (16, 26). An improved postprandial glucose profile has also been reported 1 mo post-RYGB (7, 10, 11).
Several lines of evidence attribute the early metabolic effects of RYGB to alterations resulting from bypass of the foregut (i.e., duodenum and proximal jejunum). RYGB is associated with a greater improvement in glucose tolerance and insulin responses than patients that undergo gastric banding (9, 24). In animals, bypass of the duodenum and jejunum without gastric restriction has been shown to improve glucose tolerance and insulin resistance (25, 27). Recent evidence is emerging, however, that counters the notion that bypass of the foregut is responsible for the improved glucose tolerance and insulin sensitivity after RYGB. Sleeve gastrectomy produces a similar rapid improvement in insulin resistance by partial stomach resection in the absence of foregut bypass (23). Furthermore, Kindel et al. (8) found that duodenal-jejunal bypass without gastric restriction in high-fat-fed rats did not result in weight loss nor improve glucose tolerance or insulin sensitivity as assessed by a hyperinsulinemic-euglycemic clamp. Our recent data suggest that the improvement in insulin sensitivity following RYGB can be replicated with an equivalent caloric restriction (6).
The major proposed mechanism whereby bypass of the foregut improves glucose tolerance is via an enhanced incretin response (1). The two major incretins, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), enhance glucose-dependent insulin release and exhibit beneficial glucoregulatory actions (22). GLP-1 is primarily secreted from L cells concentrated in the ileum, and GIP is primarily secreted from K cells concentrated in the duodenum, although both incretins are detected throughout the intestine (18, 31). A large increase in postprandial GLP-1 immediately after RYGB has been definitively described by us (6) and others (7, 10, 12, 17), and is sustained in the long-term (2, 16, 32). The effect of RYGB on GIP is less consistent (1). The increase in GLP-1 levels 1 mo after RYGB coincides with an enhanced insulin response to enteral glucose and incretin effect (7, 10). Such data have positioned the incretins as the primary mediator of immediate post-RYGB improvements in insulin sensitivity. The comparative absence of such changes with caloric restriction in the absence of altered intestinal anatomy (i.e., gastric banding and diet) (9, 11, 24) has placed further attention on the bypass of the foregut as the critical component of enhanced post-RYGB glucose metabolism. However, we have demonstrated that during the first postoperative week insulin secretion is diminished despite elevated GLP-1 levels (6).
Two prevailing schools of thought exist regarding how altering nutrient exposure to the gut contributes to the enhanced glucose tolerance and insulin sensitivity subsequent to RYGB: 1) exclusion of the foregut from nutrient exposure vs. 2) faster delivery of nutrients to the hindgut. These two theories have been studied in and supported by several animal studies (19, 20, 25, 27, 33). The goal of the present study was to explore the role of foregut bypass on glucose tolerance, insulin sensitivity, and the incretin response in humans. To this end, we measured meal-stimulated glucose, insulin, incretin, and ghrelin levels in subjects before and after RYGB; the meal was administered orally preoperatively and both orally and via a gastrostomy tube postoperatively. The gastrostomy tube allowed nutrient delivery into the gastric remnant with nutrient transit via the foregut, simulating preoperative oral meal ingestion. On the other hand, the oral ingestion of nutrients following RYGB allowed delivery into the distal gastrointestinal tract bypassing most of the foregut.
MATERIALS AND METHODS
Subjects.
The study protocol was approved by the Vanderbilt University Institutional Review Board and conformed to the standards of the Declaration of Helsinki. This study is registered with ClinicalTrials.gov, NCT00765596. All subjects provided written, informed consent to participate in the study and for the placement of the gastrostomy tube. Subjects were recruited from the Center for Surgical Weight Loss at Vanderbilt University Medical Center after approval for RYGB. Placement of the gastrostomy tube was determined by the surgeon for ease of delivery of nutrients in the postoperative period and for possible delivery of medications as needed in the immediate postoperative period. These criteria for gastrostomy tube placement were determined by each patient's surgeon prior to a patient being offered enrollment in the study. The average age of the subjects was 41 ± 11 yr and included three men and six women. A preoperative clinical diagnosis of Type 2 diabetes by the referring primary care physician was present in five of the nine study participants, with an average duration between diagnosis and surgery of 3.7 ± 4.7 yr. Preoperative antidiabetes medications for these subjects consisted of metformin alone; glimepiride alone; combination of metformin and pioglitazone; and combination of metformin, Humalog insulin, glipizide, and pioglitazone. Subjects were asked to discontinue antidiabetes medications for 3 days prior to the preoperative visit and the 6-wk postoperative study visit. After surgery, medications were discontinued for the duration of the patient's stay in the hospital per the surgeon's orders and were kept on hold until after the first week postoperative study visit. The average preoperative hemoglobin A1c for all nine subjects was 6.5 ± 1.3%. Partial data on this subject population describing the effect of caloric restriction on the metabolic improvements in the first week after RYGB were previously published (6).
Study protocol.
Subjects were studied preoperatively and again in the early postoperative period during the first week (4 ± 1.4 days, range 2–6 days) and at 6 wk (41 ± 3.5 days, range 34–44 days). For each study visit, subjects were admitted to the Vanderbilt Clinical Research Center after a 12-h overnight fast for measurement of fasting and meal-induced metabolic and hormonal responses. Blood samples were collected from a heated forearm vein at 0700 (baseline), immediately after completion of a meal, and every subsequent hour for 4 h. The meal was a standardized 250 kcal liquid mixed meal containing 40 g carbohydrates, 6 g fat, and 9 g protein (8 oz. Ensure; Abbott Nutrition, Columbus, OH) and was administered over a 20-min period. Preoperatively the meal was given orally (gastric). For the postoperative studies, subjects were randomized to receive the meals initially orally (jejunal) or via the gastrostomy tube (gastric) (Fig. 1). The mixed meal was then delivered through the alternate route the following day.
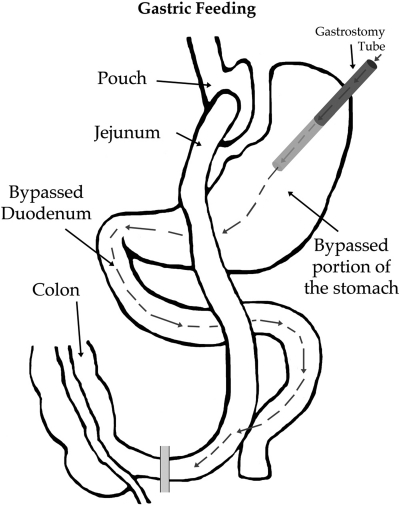
Fig. 1.
Gastric feeding by gastrostomy tube following Roux-en-Y gastric bypass (RYGB) surgery.
Surgical procedure.
Subjects underwent either open or laparoscopic RYGB. A small (∼25 ml) gastric pouch was created and gastrointestinal continuity was established via a Roux-en-gastrojejunostomy. The vagus nerve trunks were preserved. A gastrostomy tube was placed in the excluded stomach fundus with external access through the abdominal wall. All gastrostomy tubes were removed following the last study (∼6 wk) with no adverse effects.
Sample collection and analysis.
Blood was collected in chilled EDTA tubes and immediately centrifuged to separate plasma, which was stored at −80°C until analysis. Glucose was measured bedside via the glucose oxidase method using a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA). The plasma designated for GLP-1 measurement was supplemented with aprotinin (1,000 KIU/ml) and dipeptidyl peptidase-4 inhibitor (20 μl/ml plasma; Millipore, St. Charles, MO). Plasma designated for acylated ghrelin measurement was acidified with 1 N hydrochloric acid (50 μl/ml plasma) and treated with phenylmethanesulfonyl fluoride (0.1 mg/ml plasma). Plasma insulin, leptin, active GLP-1 (7-37 and 7-36 amide), and adiponectin were measured by multiplex immunoassays (Luminex xMAP, Millipore). Total and acylated ghrelin were determined by radioimmunoassay (Millipore). Plasma concentrations of total GIP were measured by enzyme-linked immunosorbent assay (Millipore).
Calculations.
Whole-body insulin resistance was estimated using the insulin sensitivity index (ISI) composite as well as the homeostasis model assessment of insulin resistance (HOMA-IR). The ISI composite, or Matsuda index, takes into account liver insulin sensitivity (fasting glucose and insulin levels) as well as peripheral insulin sensitivity (glucose and insulin concentrations following the meal) (14). HOMA-IR is derived from fasting glucose and insulin levels and was calculated by using the HOMA2 calculator based on Levy's nonlinear computer model (13) (available for download at www.dtu.ox.ac.uk/homa). Total area under the curve (AUC) and peak response level for each individual were calculated according to the trapezoidal rule via the program GraphPad Prism v5.02.
Statistical analyses.
Data are presented as means ± sd, except for data in graphs, which are means ± SE. Linear mixed-effects models were used to test the effect of time post-RYGB and feeding route (oral vs. gastrostomy tube) on outcome measures. Analyses were performed by use of SPSS 18 statistical software.
RESULTS
Body weight and fasting metabolic parameters after RYGB.
Body weight and body mass index both decreased by ∼12.5% over the 6-wk study period (both P < 0.0001) (Table 1). Average weight lost was 1.4 ± 5.3 kg during the first postoperative week and 18.8 ± 7.2 kg at 6 wk. Fasting plasma glucose (P < 0.0001) and insulin (P = 0.058) also improved over time. This led to improvements in insulin resistance (∼34%), as determined by HOMA-IR, which was evident by the first week post-RYGB and sustained throughout the 6-wk study (P = 0.046). Fasting levels of GLP-1 remained consistent at all study time points (P = 0.726), whereas GIP declined slightly within the first week, with no further variation (P = 0.130). Fasting concentrations of acyl ghrelin decreased over time after RYGB (P = 0.017), whereas the decrease in total ghrelin levels did not achieve significance (P = 0.348). Two measured adipokines displayed disparate trends early after RYGB; fasting leptin decreased within the first week with subsequent stabilization (P = 0.017), whereas fasting adiponectin remained at preoperative levels at all post-RYGB time points (P = 0.348).
Table 1.
Body weight and fasting metabolic parameters in the early postoperative period
| Baseline | 1st week (2–6 days) | 6 wk (34–44 days) | P Value | |
|---|---|---|---|---|
| Weight, kg | 153 ± 32 | 152 ± 33 | 134 ± 32 | <.0001 |
| BMI, kg/m2 | 51.9 ± 6.0 | 51.4 ± 6.6 | 45.3 ± 5.7 | <.0001 |
| HOMA-IR | 5.0 ± 3.1 | 3.3 ± 2.1 | 3.0 ± 2.1 | 0.046 |
| Glucose, mg/dl | 115 ± 28 | 109 ± 32 | 97 ± 17 | 0.001 |
| Insulin, μU/ml | 39.4 ± 26.5 | 25.8 ± 17.0 | 23.4 ± 17.3 | 0.058 |
| GLP-1, pg/ml | 34.9 ± 32.4 | 36.5 ± 32.6 | 34.6 ± 32.2 | 0.726 |
| GIP, pg/ml | 58.0 ± 31.6 | 42.4 ± 21.1 | 38.1 ± 19.2 | 0.130 |
| Leptin, pg/ml | 72.4 ± 15.3 | 49.5 ± 15.1 | 42.8 ± 23.2 | 0.017 |
| Adiponectin, μg/ml | 7.3 ± 3.0 | 6.4 ± 2.0 | 8.1 ± 2.3 | 0.157 |
| Acyl Ghrelin, pg/ml | 68.2 ± 33.6 | 48.5 ± 26.9 | 40.2 ± 18.0 | 0.017 |
| Total Ghrelin, pg/ml | 585 ± 272 | 414 ± 107 | 447 ± 89 | 0.348 |
Data are means ± SD for 9 subjects, measured after an overnight fast. BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; GLP-1, glucagon-like peptide 1; GIP, gastric inhibitory peptide. P value is for the effect of time after gastric bypass surgery.
Glucose and insulin responses to a jejunal and gastric delivery of a mixed meal after RYGB.
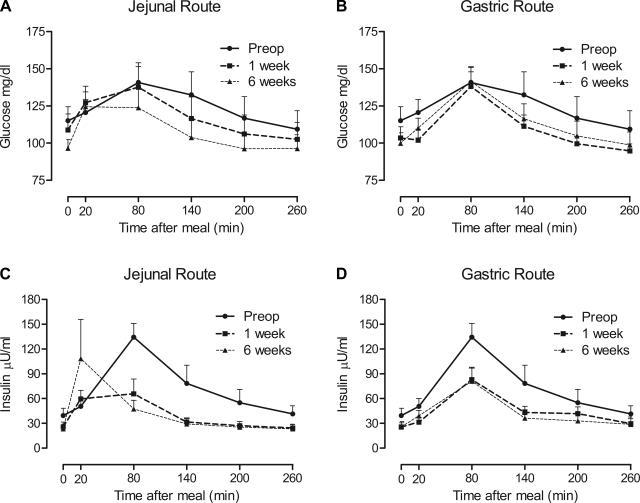
Jejunal delivery of a mixed meal after RYGB was associated with more rapid increases in plasma glucose levels during the first week and persisted through the 6-wk study period compared with gastric delivery (Fig. 2, A and B). The disappearance of plasma glucose was more rapid following RYGB for both delivery routes (Fig. 2, A and B). The glucose AUC improved over time after RYGB (P < 0.0001), with no effect of delivery route over time (P = 0.084) (Table 2). Peak levels of glucose attained after the mixed meal did not change over time (P = 0.070) (Table 2). Compared with baseline, jejunal but not gastric feeding resulted in a more rapid increase in plasma insulin; however, both routes were associated with a more rapid decline to fasting levels (Fig. 2, C and D). As such, the insulin AUC was decreased over the 6 wk following RYGB (P = 0.018), with no route × time interaction detected (P = 0.932) (Table 2). Peak levels of insulin attained after the mixed meal did not change over time (P = 0.981) (Table 2).
Fig. 2.
Time course of plasma glucose (A and B) and insulin (C and D) levels after jejunal (A and C) or gastric (B and D) delivery of a liquid mixed meal preoperatively (Preop) and at 1 and 6 wk after RYGB. Preoperative mixed meal delivery was oral (gastric) and is displayed on all graphs. Gastric delivery after RYGB was via a gastrostomy tube in the stomach fundus, allowing nutrient contact with the bypassed foregut. Data are means of 9 subjects ± se.
Table 2.
Metabolic parameters after a mixed meal by delivery route before and after gastric bypass surgery
| Gastric Route |
Jejunal Route |
|||||
|---|---|---|---|---|---|---|
| Preop | 1st week | 6 wk | 1st week | 6 wk | P Values | |
| Glucose AUC, mg•l−1•min−1 | 3,317 ± 953 | 2,827 ± 418 | 3,012 ± 657 | 3,087 ± 1022 | 2,827 ± 737 | T < .0001 |
| R 0.909 | ||||||
| R × T 0.084 | ||||||
| Peak glucose, mg/dl | 153 ± 38 | 132 ± 25 | 143 ± 26 | 144 ± 46 | 139 ± 36 | T 0.070 |
| R 0.852 | ||||||
| R × T 0.444 | ||||||
| Insulin AUC, μU•l−1•min−1 | 19.0 ± 11.1 | 13.0 ± 5.5 | 11.7 ± 7.4 | 10.8 ± 5.5 | 11.6 ± 8.7 | T 0.018 |
| R 0.632 | ||||||
| R × T 0.932 | ||||||
| Peak Insulin, μU/ml | 124 ± 58 | 91 ± 37 | 86 ± 47 | 81 ± 51 | 128 ± 143 | T 0.981 |
| R 0.797 | ||||||
| R × T 0.233 | ||||||
| GLP-1 AUC, pg•l−1•min−1 | 9.2 ± 8.4 | 11.2 ± 7.5 | 14.8 ± 13.7 | 12.2 ± 5.5 | 15.5 ± 11.1 | T 0.006 |
| R 0.911 | ||||||
| R × T 0.840 | ||||||
| Peak GLP-1, pg/ml | 45 ± 37 | 69 ± 33 | 94 ± 74 | 85 ± 33 | 132 ± 91 | T < .0001 |
| R 0.785 | ||||||
| R × T 0.249 | ||||||
| GIP AUC, pg•l−1•min−1 | 29.0 ± 11.9 | 35.5 ± 12.1 | 33.4 ± 17.6 | 23.5 ± 8.6 | 24.4 ± 8.8 | T 0.819 |
| R 0.060 | ||||||
| R × T 0.236 | ||||||
| Peak GIP, pg/ml | 227 ± 115 | 246 ± 83 | 258 ± 134 | 193 ± 111 | 242 ± 131 | T 0.231 |
| R 0.416 | ||||||
| R × T 0.895 | ||||||
| AG AUC, pg•l−1•min−1 | 19.5 ± 9.0 | 14.4 ± 7.6 | 9.7 ± 4.6 | 12.0 ± 6.8 | 11.1 ± 6.2 | T 0.001 |
| R 0.645 | ||||||
| R × T 0.571 | ||||||
| Peak AG, pg/ml | 100.1 ± 47.3 | 85.0 ± 52.5 | 51.4 ± 23.7 | 66.0 ± 32.3 | 58.9 ± 33.9 | T 0.002 |
| R 0.511 | ||||||
| R × T 0.537 | ||||||
| TG AUC, pg•l−1•min−1 | 145 ± 53.4 | 102 ± 25.2 | 117 ± 31.7 | 112 ± 36.0 | 123 ± 22.6 | T 0.120 |
| R 0.923 | ||||||
| R × T 0.691 | ||||||
| Peak TG, pg/ml | 702 ± 283 | 511 ± 116 | 567 ± 105 | 532 ± 152 | 573 ± 105 | T 0.110 |
| R 0.802 | ||||||
| R × T 0.866 | ||||||
Data are means ± SD for 9 subjects. AUC, area under the curve; AG, acyl ghrelin; TG, total ghrelin; P values are for the effects of time (T) after gastric bypass surgery, route (R) of meal administration (duodenal vs. jejunal), and the interaction between route and time (R × T).
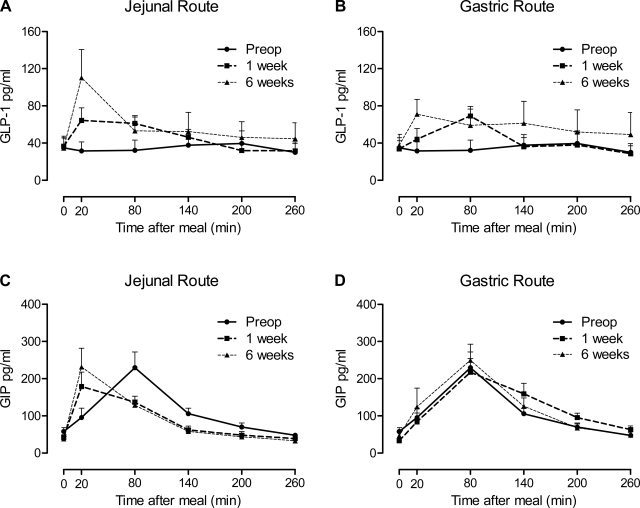
Incretin responses to a jejunal and gastric delivery of a mixed meal after RYGB.
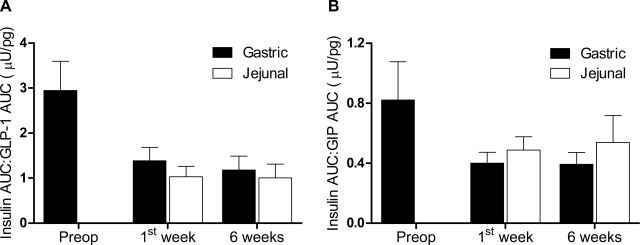
The GLP-1 (active form) response to a mixed meal was absent preoperatively and restored postoperatively by both jejunal and gastric feeding routes (Fig. 3, A and B). The GLP-1 AUC and peak GLP-1 levels both increased over time (P = 0.006 and P < 0.0001, respectively), without any effect of feeding route over time (P = 0.840 and 0.249, respectively) (Table 2). GIP reached peak concentrations earlier and diminished more rapidly after RYGB with jejunal but not gastric feeding (Fig. 3, C and D). There was no change in GIP AUC or peak levels after RYGB (P = 0.819 and 0.231) (Table 2). To determine whether insulin release was altered in relationship to GLP-1 or GIP after RYGB or by feeding route, we calculated the ratio of the AUCs. The insulin AUC-to-GLP-1 AUC ratio (insulin AUC:GLP-1 AUC) decreased by ∼60% after RYGB (P = 0.001), and there was no effect of feeding route (P = 0.696) or route × time interaction (P = 0.937) (Fig. 4A). Similar to GLP-1, the insulin AUC-to-GIP AUC ratio (insulin AUC:GIP AUC) decreased after surgery (∼45%, P = 0.061) and did not vary by feeding route over time (P = 0.646) (Fig. 4B).
Fig. 3.
Time course of active glucagon-like peptide 1 (GLP-1; A and B) and gastric inhibitory peptide (GIP; C and D) levels after jejunal (A and C) or gastric (B and D) delivery of a liquid mixed meal preoperatively and at 1 and 6 wk after RYGB. Preoperative mixed meal delivery was oral (gastric) and is displayed on all graphs. Gastric delivery after RYGB was via a gastrostomy tube in the stomach fundus allowing nutrient contact with the bypassed foregut. Data are means of 9 subjects ± se.
Fig. 4.
Relative insulin and incretin release after a mixed meal delivered to the stomach (■) or jejunum (□). A: ratio of insulin area under the curve (AUC) to GLP-1 AUC. B: ratio of insulin AUC to GIP AUC. Data are means of 9 subjects ± SE. There is an effect of time after gastric bypass on insulin-to-GLP-1 ratio (P < 0.001), and the effect on insulin-to-GIP ratio was marginal (P = 0.061). No effect of route (P = 0.696 and 0.806) or route × time interaction (P = 0.937 and 0.646) was detected.
Ghrelin responses to a jejunal and gastric delivery of a mixed meal after RYGB.
The AUC and peak levels of acyl ghrelin in response to a meal declined after RYGB (P = 0.001 and 0.002, respectively) without a route × time interaction (P = 0.571 and 0.537, respectively) (Table 2); this is reflective of the decrease in fasting acyl ghrelin levels after RYGB since acyl ghrelin did not fluctuate in the 4 h after the meal (Supplementary Fig. S1; the online version of this article contains supplemental data). There was no decline in total ghrelin AUC (P = 0.120) and peak levels (P = 0.110) with no effect of delivery route over time (P = 0.691 and 0.866, respectively) (Table 2) and, similar to acyl ghrelin, a maintenance of fasting levels for 4 h after the mixed meal (Supplementary Fig. S1).
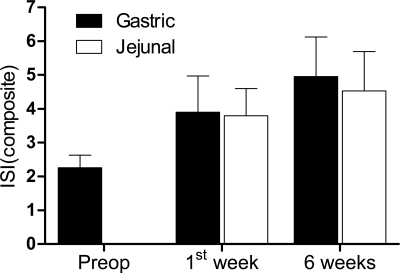
Insulin sensitivity after RYGB.
We estimated whole-body insulin sensitivity by calculating the ISI composite from the mixed meal preoperatively and delivered to the jejunum and duodenum postoperatively. Insulin sensitivity increased approximately twofold in the 6 wk following gastric bypass (P = 0.001), but there was no effect of route (P = 0.902) or route × time interaction (P = 0.819) (Fig. 5).
Fig. 5.
Insulin sensitivity assessed from a mixed-meal tolerance test delivered to the stomach (■) or jejunum (□). Whole-body insulin sensitivity was estimated by calculating the composite (Matsuda) insulin sensitivity index (ISI). Data are means of 9 subjects ± SE. There is an effect of time after gastric bypass on insulin sensitivity (P = 0.001), but no effect of route (P = 0.902) or route × time interaction (P = 0.819).
DISCUSSION
RYGB achieves weight loss through restriction of nutrient intake and nutrient malabsorption. The latter is achieved by surgical bypass of the duodenum and proximal jejunum, allowing direct transport of nutrients from the stomach to the midjejunum. An ancillary result of the surgery is an alteration in pancreatic and gastrointestinal hormone secretions, particularly GLP-1 and GIP (1), which may aid in postprandial glucose control by enhancing insulin release. To explore the role of the foregut in glucose tolerance and insulin resistance in humans, we utilized a unique model in which we introduced a mixed meal post-RYGB via a gastrostomy tube placed intraoperatively in the stomach fundus. This model has several advantages but also has inherent limitations. This procedure allowed us to evaluate the presurgical exposure to food (i.e., duodenum and proximal jejunum) under the conditions of postsurgical caloric restriction (first week) and subsequent weight loss (6 wk). Food delivery through the gastrostomy tube simulates presurgical oral nutrient intake, albeit without the involvement of the cephalic phase and lack of contact with a significant portion (usually 100–150 cm) of proximal jejunum that forms the Roux limb. These aspects of gastrostomy tube delivery could reduce nutrient transit time compared with the oral route. Furthermore, a single food bolus administered via the gastrostomy tube may not fully exclude changes that occur when the foregut is exposed to daily nutrients. However, a recent study in a rodent model of RYGB suggests that intestinal adaptation occurred mostly in regions that were stimulated by nutrients postsurgery (30). Despite these limitations, the findings of the present study provide some insight on the potential role of the foregut and incretins in the improvements in insulin sensitivity observed in the first 6 wk following RYGB.
Based on studies carried out in rats comparing duodenal-jejunal bypass to gastrojejunostomy, Rubino et al. (25) contend that the exclusion of the proximal small intestine from nutrient exposure rather than the rapid delivery of nutrients to the distal small intestine is responsible for improved glucose tolerance after RYGB. A recent study by Kindel et al. (8), however, failed to show an effect of duodenal-jejunal bypass on insulin sensitivity. Unfortunately incretin responses were not measured in these studies (8, 25). Wang et al. (33) reported that in rats transposition of a small segment of ileum to the proximal jejunum was equivalent to duodenal-jejunal bypass in controlling glucose, possibly because ileal transposition resulted in more pronounced increase in GLP-1 secretion. Thus the possibility remains that the foregut and hindgut hypotheses of metabolic improvement after RYGB are not mutually exclusive, and both aspects of the surgery provide benefit. In the present study, both jejunal and gastric postoperative delivery of the mixed meal restored the aberrant preoperative GLP-1 response. These findings are suggestive of more responsive L cells, located primarily in the distal ileum and proximal colon, to the rapid delivery of nutrients to the distal small intestine with both administration routes.
Jejunal delivery of the mixed meal post-RYGB was associated with earlier peak levels of GIP, whereas the GIP response to gastric delivery was almost identical to those observed in the preoperative period. The reasons for the early rises in GIP following RYGB are not known, given that the K cells responsible for secreting GIP are primarily located in the bypassed duodenum and proximal jejunum; however, K cells are dispersed throughout the small intestine (18). It is possible that the earlier exposure of the Roux-jejunal segment to nutrients, which also contains K cells, results in more rapid GIP release.
In response to a mixed meal, both glucose tolerance and postprandial hyperinsulinemia improved in the first 6 wk after RYGB, which was associated with an improvement in insulin sensitivity. Interestingly, delivery of nutrients to the jejunum or stomach in the immediate postoperative period resulted in similar improvements in glucose tolerance and insulin sensitivity. These data argue against bypass of nutrient exposure to the foregut as a primary mechanism in immediate improvements in glucose tolerance following RYGB. Interestingly, despite the greater response in GLP-1 after surgery, the insulin AUC:GLP-1 AUC declined after RYGB, indicating that the increased GLP-1 release was not associated with an increased insulin release. These observations challenge the hypothesis that the improved insulin responses following RYGB are attributed to enhanced GLP-1 and the known insulinotropic role of GLP-1 (22). In fact, the insulin AUC:GLP-1 AUCs were similarly reduced following RYGB by both feeding routes. Similar to GLP-1, the insulin AUC:GIP AUCs also declined after RYGB, minimizing a role for increased GIP release in enhancing insulin release. Our recent data support caloric restriction as an important mediator of early improvements in insulin resistance after RYGB (6).
Two recent independent case reports comparing oral and gastroduodenal feeding routes in patients at 5 wk (4) and 3 yr (15) post-RYGB found a more exaggerated GLP-1 and insulin response with oral feeding. The patient in the later study, however, suffered from severe postprandial hypoglycemia and therefore may represent an extreme post-RYGB incretin response. Additionally, these studies measured total GLP-1, whereas we measured active GLP-1. Further studies including preoperative assessments are necessary to compare these case reports with the present report. In summary, our study comparing oral and gastrostomy tube feeding after RYGB suggests that exclusion of the foregut from nutrient exposure is not responsible for improved glucose tolerance and insulin sensitivity after RYGB. However, changes in the foregut response post-RYGB due to lack of nutrient exposure cannot be excluded, so additional studies using more than a single food bolus are necessary to consider this possibility. Our findings suggest that more rapid delivery of nutrients to the hindgut may be an important beneficial aspect of RYGB. In our study both meal delivery routes occurred with equivalent caloric intake and weight loss; thus further studies will be needed to isolate the contribution of caloric restriction and weight loss from rapid nutrient delivery to the hindgut on the improved glucose metabolism following RYGB.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK070860 to N. N. Abumrad, the Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, DK20593 to the Vanderbilt Diabetes Research and Training Center, DK058404 to the Vanderbilt Digestive Disease Research Center, and T32-DK007061-31A1 to J. M. Isbell.
DISCLOSURES
The authors declare no conflicts of interest.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Marcy Buckley for nursing support and Kareem Jabbour and Nadine Saliba for laboratory assistance. We also thank the patients who volunteered for this study.
Clinical Trial Registration: this study is registered with ClinicalTrials.gov, number NCT00765596.
REFERENCES
- 1. Bose M, Olivan B, Teixeira J, Pi-Sunyer FX, Laferrere B. Do incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: what are the evidence? Obes Surg 19: 217–229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bose M, Teixeira J, Olivan B, Bawa B, Arias S, Machineni S, Pi-Sunyer FX, Scherer PE, Laferrere B. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes 2: 47–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248–256.e5, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Dirksen C, Hansen DL, Madsbad S, Hvolris LE, Naver LS, Holst JJ, Worm D. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion. Diabetes Care 33: 375–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139: 448–455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 33: 1438–1442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 34: 462–471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindel TL, Martins PJ, Yoder SM, Jandacek RJ, Seeley RJ, D'Alessio DA, Obici S, Tso P. Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents. Obesity (Silver Spring) 19: 380–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3: 597–601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with Type 2 diabetes. Diabetes Care 30: 1709–1716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with Type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21: 2191–2192, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 15. McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 95: 1851–1855, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Morinigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg 16: 1594–1601, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 91: 1735–1740, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Mortensen K, Petersen LL, Orskov C. Colocalization of GLP-1 and GIP in human and porcine intestine. Ann NY Acad Sci 921: 469–472, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, Gulla N, Donini A. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery 142: 74–85, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C, Donini A. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 15: 1258–1264, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Pereira JA, Lazarin MA, Pareja JC, de Souza A, Muscelli E. Insulin resistance in nondiabetic morbidly obese patients: effect of bariatric surgery. Obes Res 11: 1495–1501, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A, Hansotia T, Drucker DJ, Wollheim C, Burcelin R, Thorens B. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 113: 635–645, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, Basso N. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg 20: 50–55, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16: 298–305, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 244: 741–749, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240: 236–242, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 239: 1–11, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238: 467–484; discussion 484–465, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Taqi E, Wallace LE, de Heuvel E, Chelikani PK, Zheng H, Berthoud HR, Holst JJ, Sigalet DL. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg 45: 987–995, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290: E550–E559, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, Morinigo R, Lacy AM. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab 94: 884–891, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Wang TT, Hu SY, Gao HD, Zhang GY, Liu CZ, Feng JB, Frezza EE. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg 247: 968–975, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.