Abstract
The subepithelial intestinal myofibroblast is an important cell orchestrating many diverse functions in the intestine and is involved in growth and repair, tumorigenesis, inflammation, and fibrosis. The myofibroblast is but one of several α-smooth muscle actin-positive (α-SMA+) mesenchymal cells present within the intestinal lamina propria, including vascular pericytes, bone marrow-derived stem cells (mesenchymal stem cells or hematopoietic stem cells), muscularis mucosae, and the lymphatic pericytes (colon) and organized smooth muscle (small intestine) associated with the lymphatic lacteals. These other mesenchymal cells perform many of the functions previously attributed to subepithelial myofibroblasts. This review discusses the definition of a myofibroblast and reconsiders whether the α-SMA+ subepithelial cells in the intestine are myofibroblasts or other types of mesenchymal cells, i.e., pericytes. Current information about specific, or not so specific, molecular markers of lamina propria mesenchymal cells is reviewed, as well as the origins of intestinal myofibroblasts and pericytes in the intestinal lamina propria and their replenishment after injury. Current concepts and research on stem cell therapy for intestinal inflammation are summarized. Information about the stem cell origin of intestinal stromal cells may inform future stem cell therapies to treat human inflammatory bowel disease (IBD).
Keywords: pericytes, bone marrow stem cells, hematopoietic stem cells, mesenchymal stem cells, mesenchymal cells, stromal cells, muscularis mucosae, lymphatic lacteal, α-smooth muscle actin, thymic antigens, injury and repair, inflammatory bowel disease, Crohn's disease, bone marrow transplantation, Thy-1, NG2, fibroblast-specific protein 1, patched, periostin, fibroblast activation protein, epimorphin, bone marrow transplantation
in the past decade, the subepithelial intestinal myofibroblast has come to be regarded as an important cell orchestrating many diverse functions in the intestine in health and disease, including growth and repair, tumorigenesis and cancer progression, inflammation or peripheral immune tolerance, and fibrosis. These concepts have been summarized in several thoughtful and informative reviews (5, 21, 33, 46, 97–99). Our own research indicates that the intestinal myofibroblast is but one of several mesenchymal cells in the intestinal lamina propria, and many of the functions attributed to subepithelial myofibroblasts might be due to other α-smooth muscle actin-positive (α-SMA+) mesenchymal cells such as pericytes, bone marrow-derived stem cells [mesenchymal stem cells (MSCs) or hematopoietic stem cells (HSCs)], muscularis mucosae, and the lymphatic pericytes (colon) and organized smooth muscle (small intestine) associated with the lymphatic lacteals (93, 99).
There are three goals for this review. The first is to review the definition of a myofibroblast and reconsider whether the α-SMA+ subepithelial cells in the intestine are myofibroblasts or other types of mesenchymal cells, i.e., pericytes.
A second goal is to compile current information about specific, or not so specific, molecular markers of lamina propria mesenchymal cells. Because advances in our understanding of the role of these cells in health and disease depend on our ability to identify them in human and animal tissues and to ablate them in disease models, we review here the marker molecules that might be useful in this regard.
The third goal is to review our current understanding of the origins of intestinal myofibroblasts and pericytes in the intestinal lamina propria and their replenishment after injury. Tissue stromal cells have been postulated to play a fundamental role in tissue regeneration and wound repair (112), and stem cell transplantation has been shown to repair the inflamed intestine (35). Information about the stem cell origin of intestinal stromal cells may inform attempts to develop stem cell therapies to treat human inflammatory bowel disease (IBD).
Myofibroblasts
Eyden (30) has challenged the idea that the subepithelial cells in the intestine are true myofibroblasts, and, following a review of our own histological material and the literature, we believe he is correct in questioning this concept. Eyden defines myofibroblasts in detail and with strict criteria: spindle-shaped or stellate cells that are α-SMA positive, vimentin positive, smooth muscle myosin negative but non-smooth muscle myosin positive, fibronectin positive, and very weakly positive or negative for desmin. On electron microscopy (EM), the cells possess abundant cytoplasm and pericellular matrix and contain a prominent rough endoplasmic reticulum and a Golgi apparatus that produces collagen granules. The cells are attached to adjacent myofibroblasts by gap junctions and specialized adherens junctions containing OB-cadherin (47) and, most importantly, are attached to the surrounding matrix through a fibronexus and not basal lamina. The fibronexus is an adhesion structure connecting the myofibroblast to the matrix through extracellular fibronectin filaments that run parallel to and then cross into the cytoplasm of the myofibroblast, forming a plaquelike structure where fibrils enter the cell membrane. These adhesions to matrix and to each other make them a powerful contractile network, which might have a teleological purpose of reducing the size of a wound. According to this strict definition, “true” myofibroblasts would be relegated to wound-healing granulation tissue and the stroma of neoplasms (30).
Pericytes
Pericytes, also called mural cells and vascular smooth muscle cells, were described over 100 years ago. EM and immunohistochemical studies have shown them to be cells with a prominent nucleus and long processes aligned longitudinally along the axis of the vessel with smaller, thin circumferential processes that partially engirdle the vascular wall. In histological sections these processes might not be seen, giving the cells a fusiform morphology like myofibroblasts (10, 67). The mural cells are embedded within and contribute to the formation of the basement membrane of microvessels. Gap junctions directly connect pericytes with each other and with the underlying endothelial cells, and adhesion plaques to matrix augment this attachment. The cells stain positive for α-SMA, desmin, melanoma chondroitin sulfate proteoglycan (MCSP, also called NG2, see Melanoma chondroitin sulfate proteoglycan below), PDGF receptor (PDGFR)-β, and regulator of G protein signaling-5 (RGS5). Pericytes also express monocyte-macrophage markers such as CD11b, and, upon stimulation with interferon (IFN)-γ, they express major histocompatibility complex (MHC) class II molecules and B7 family costimulatory molecules CD80 and CD86, suggesting that they are replenished by circulating fibrocytes (see Hematopoietic stem cells or fibrocytes below). The ability of cultured fibrocytes to support angiogenesis/vasculogenesis by cultured endothelial cells enhances this idea (42), since angiogenesis is known to require both endothelial cells and pericytes. Pericytes also express receptors for and respond to both cholinergic and adrenergic agents, angiotensin II and endothelin-1 (ET-1) (as do cultured subepithelial myofibroblasts).
Hepatic stellate cells (Ito cells) and renal mesangial cells are examples of pericytes, and pericytes are prominent in the stroma of neoplasms, where they probably play a role in tumor angiogenesis (10).
Organization of Intestinal Mesenchymal (Stromal) Cells
α-SMA+ subepithelial cells.
The subepithelial fibroblast-like mesenchymal cells were first defined as fibroblasts or reticulohistiocytic cells (22, 27, 68). Between 1968 and 1971, a series of papers described this organelle in the colon and named it the pericryptal fibroblastic sheath: a group of fusiform-shaped fibroblasts embedded in collagen and mucopolysaccharide ground substance immediately subjacent to the epithelial basement membrane, encircling the colonic crypts and ending in the collagen table just under the colonic surface epithelium. In the basal one-third of the crypts, the fibroblast sheath was observed to be two or three cells thick, often overlapping like shingles. In this location, the cells did not appear to contain a complex cytoplasm with active Golgi. At the mouth of the crypts, the layer is one cell thick and more sparse and fenestrated (stellatelike), and the cells demonstrate long cytoplasmic processes with a rich endoplasmic reticulum and Golgi apparatus (55, 56, 90).
Marsh and Trier defined the anatomy of the subepithelial sheath in the mouse small intestine, noting that the fibroblastic sheath surrounding the small intestinal crypts has an appearance similar if not identical to the surrounding colonic crypts. They reported (76, 77) the lack of a well-formed sheath in the villi, where little pericellular collagen was found and the fibroblasts seemed associated with endothelial and immune cells. Using 3H-methyl-thymidine as a tracer of mitotic activity, studies in the rabbit colon (90) and mouse small intestine (77) suggested that the fibroblasts of the sheath divided first in the crypt region and migrated up the villus like the epithelium. These observations suggested that lamina propria fibroblasts, like the epithelium, were replaced by dividing stem cells. Subsequent similar studies in mouse intestine and colon failed to confirm this idea, as thymidine-labeled cells were seen randomly in the subepithelial sheath (83). These labeling studies do indicate a capability of the subepithelial fibroblast-like cells to divide and contribute to their stability as an organelle in the intestine.
In 1987, Joyce et al. (51) performed a detailed EM study of the lamina propria of rat small intestine and colon, confirming the anatomy of the crypt area described by previous authors but noting the stellate appearance of the villus subepithelial cells whose processes connected to the matrix with adherence plaques and through adherens junctions to each other, to crypt subepithelial cells, and often to a third dimension of cells in the lamina propria. These cells were weakly SMA, tropomyosin, and both smooth muscle and non-smooth muscle myosin positive (although less so than the smooth muscle of the muscularis mucosae and lymphatic lacteal), indicating their contractile capability. Furuya and Furuya (33) also performed elegant histological studies and demonstrated the contractile nature of this network, confirming with elegant studies its mechanosensitive properties, the connections of the network through dye-permeable junctions, and its contractile response to ET-1 and purinergic transmitters. The report of Joyce et al. and subsequent EM studies confirmed the anatomy of this subepithelial organelle (124). α-SMA immunohistochemistry led to labeling these subepithelial cells in the colon as myofibroblasts (2, 98). In the upper aspect of the crypts and subsurface epithelial region of the colon, the subepithelial cells lose α-SMA staining and become more stellate in shape, i.e., become fibroblasts or stellate cells. Deeper in the core of the colonic lamina propria, some of the α-SMA+ cells are clearly pericytes surrounding microvessels and smaller lymphatic vessels as they make their way to and through the muscularis mucosae (Fig. 1).
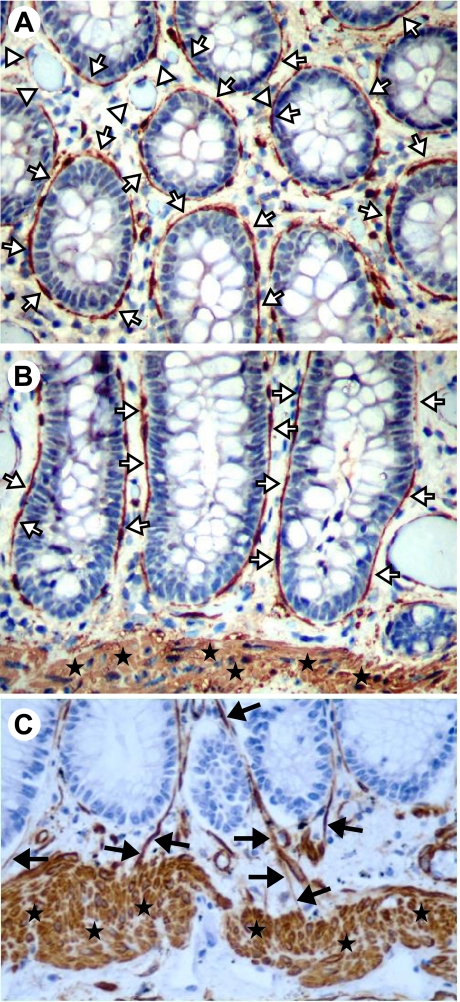
Fig. 1.
α-Smooth muscle actin (α-SMA)-positive cells of the colonic mucosa. Cross section (A) and longitudinal sections (B and C) of the colonic mucosa show positive (brown) α-SMA immunostaining of pericryptal (subepithelial) myofibroblasts (white arrows) and the muscularis mucosae (stars). Vascular and/or lymphatic pericytes (white arrowheads) are also visible. Original magnification ×200. C: pericytes surrounding lymphatics (black arrows) are seen entering the muscularis mucosae in this section. Note that the lamina propria blood vessels between the crypts and muscularis mucosae are also highlighted (original magnification ×400). [A and B taken from Powell et al. (96), with permission. C adapted from Adegboyega et al. (3), with permission].
In the small intestinal villus (Fig. 2), α-SMA staining of subepithelial cells is less than those surrounding the crypts, and may even be lost altogether in the mid- and upper villus. These cells stain weakly for desmin, suggesting that they might be pericytes. Scanning EM of the small intestinal villus after freeze fracture and osmic acid, HCl, NaOH, or enzymatic digestion reveals a subepithelial basement reticular network with embedded fibroblasts that surround fenestrae in the matrix (23, 24, 61, 62, 121). It is likely that these fenestrae are actually the digested remnants of subepithelial microvessels that abut onto the epithelial basement membrane. In scanning EM studies where the villus subepithelial fibroblasts are intact, it is clear that they overlie microvessels (23). Thus we conclude that the predominant α-SMA+ subepithelial cells in the intestinal villus are pericytes, although rare myofibroblasts may also be present.
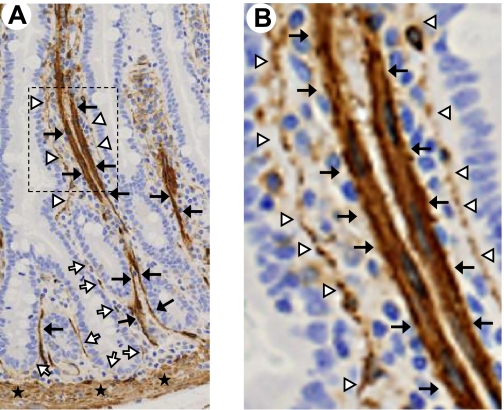
Fig. 2.
α-SMA-positive cells of the small intestinal mucosa. A: longitudinal section of normal human jejunum with positive (brown) α-SMA immunostaining of myofibroblasts (white arrows), pericytes (white arrowheads), lymphatic lacteal-associated smooth muscle (black arrows), and muscularis mucosae (stars). B: magnified image of box shown in A demonstrating intensely α-SMA-positive smooth muscle (black arrows) associated with the lymphatic lacteal and mildly α-SMA-positive cells (white arrowheads) subjacent to the epithelium. These subepithelial cells appear to be predominantly pericytes rather than myofibroblasts. [Courtesy of Patrick Adegboyega, Louisiana State University Health Sciences Center (Shreveport, LA). Adapted from Powell et al. (99) with permission.]
We now believe that in the crypts of the colon and small intestine the subepithelial α-SMA+ cells are activated fibroblasts (myofibroblasts), which are connected by gap or adherens junctions to a second or sometimes third layer of α-SMA− fibroblasts to form a sheath that is located in the lamina propria surrounding the crypt epithelium. These cells appear to be different from α-SMA+ vascular and lacteal pericytes within the lamina propria, but three-dimensional confocal or multiphoton microscopic techniques will be necessary to rule out the possibility that they might also surround vessels in some unseen dimension, and thus might actually be pericytes. Although all of Edyen's criteria have not been satisfied, these α-SMA- and vimentin-positive, desmin-negative cells are not fibroblasts, are probably not pericytes, and are not smooth muscle cells, and so with some license, subepithelial myofibroblast seems to be a good name for this first layer of pericryptal mesenchymal cells. Furthermore, fibronectin immunostaining has not been performed to seek fibronexi associated with these cells, which might better justify naming them myofibroblasts, although adhesion plaques to underlying matrix have been reported (51).
Intestinal fibroblasts.
Intestinal fibroblasts are currently recognized by their morphology and the absence of α-SMA staining. In the colonic mucosa, they may be found in the lamina propria as a second layer of cells adjacent to the myofibroblasts surrounding the colonic crypts. The predominant collection of fibroblasts is in the upper aspects of the colonic crypts, especially in the region under the surface epithelium where the fibroblastic sheath is attenuated and fenestrated. Another area very rich in fibroblasts is the subepithelial region of the middle and upper portions of the small intestinal villus (33). Fibroblasts in many tissues are identified by immunostaining for CD90 (Thy-1) (see Thymus stromal antigens below). However, both Thy-1-positive and Thy-1-negative fibroblasts are reported in the human myometrium and orbit of the eye, and each type shows a fundamentally different response to signaling molecules. For example, only Thy-1-positive orbit and uterine fibroblasts express α-SMA in response to transforming growth factor (TGF)-β (i.e., become myofibroblasts), while only Thy-1-negative cells respond to 15-deoxy-PGJ2 with lipid storage (i.e., become stellate cells) (66). Of interest is the observation that cultured intestinal myofibroblasts assume a stellate morphology in response to elevated intracellular cyclic AMP (124).
Figure 3 is a schematic drawing illustrating our current concepts of the subepithelial cells of the colonic and small intestinal crypts and small intestinal villus. This drawing differs from our previous renditions, pointing out our understanding that only the first layer of subepithelial mesenchymal cells in the colonic crypts are myofibroblasts and that subepithelial cells in the villus of the small intestine are mostly pericytes. These α-SMA+ cells, together with α-SMA− fibroblasts, represent the stromal cells of the intestinal lamina propria.
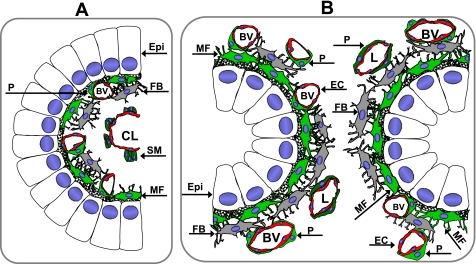
Fig. 3.
Schematic depicting the spatial relationships of the epithelium and mesenchymal elements of the small intestinal villus and colonic crypts. A: cross section through a small intestinal villus showing the epithelium (Epi) and lamina propria. α-SMA+ subepithelial cells are predominantly pericytes (P, green) surrounding small blood vessels (BV). There may be occasional myofibroblasts (MF), although these could all be pericytes in the villus. There are smooth muscle (SM) bundles (dark green) associated with the central lymphatic lacteal (CL). Fibroblasts (FB, gray) are shown. B: cross section through the colonic crypts demonstrating relationships among the various mesenchymal elements in the lamina propria: subepithelial myofibroblasts (MF, green) and pericytes (P, green) surrounding blood vessels (BV) and lymphatics (L). Fibroblasts (F, gray) are found deeper in the lamina propria. EC, endothelial cell.
Identification of Intestinal Stromal Cells
We believe that the subepithelial mesenchymal cells are critical to the initiation and perpetuation of intestinal inflammation and to the therapy of IBD. Either effective therapy may come through medications that inhibit mesenchymal cell-derived inflammatory mediators or processes or, alternatively, novel therapies might be agents that might mimic or promote the salutary mediators and processes (tolerance) for which these stromal cells may be responsible. In light of the effect of anti-TNF antibodies in rheumatoid arthritis, which is directed toward the joint synoviocyte, also an antigen-presenting myofibroblast, it is not unreasonable to propose that the beneficial effects of such therapy in IBD are in part derived from anti-inflammatory effects on intestinal stromal cells, which also possess antigen presenting capability (92, 105). Furthermore, a TNF-overexpressing mouse model develops both chronic inflammatory polyarthritis and Crohn's-like IBD (63) that is dependent on TNF receptor (TNFR)I expression in synovial myofibroblasts and intestinal myofibroblasts (7).
To more definitively study the normal biology of intestinal mesenchymal cells, and to understand their role in human disease, we must be able to identify these cells and ablate them in experimental animal models. Intestinal mesenchymal cells are usually identified by using a panel of molecular markers, hoping to distinguish one cell type from another. However, this approach is rarely definitive given the plasticity of mesenchymal cells and the significant overlap of marker expression among the different cells. For example, desmin expression is often used to distinguish myofibroblasts from smooth muscle, yet pericytes express desmin. Below we discuss some of the more useful markers employed to distinguish intestinal mesenchymal cells. It should become clear that while many of these markers may be useful in the identification of various intestinal mesenchymal cells, none is capable of exclusively defining each population.
α-Smooth muscle actin.
Myofibroblasts have been identified by the expression of α-SMA, vimentin, or desmin, type I collagen maturation enzymes such as prolyl-4-hydroxylase (P4H), and the absence of epithelial cytokeratins (21). α-SMA remains the best single marker of the subepithelial myofibroblast network, but it does not differentiate myofibroblasts from the other mesenchymal elements of the lamina propria; i.e., mural cells (pericytes) of the lamina propria vessels (9, 96), bone marrow-derived (produced) lamina propria stromal stem cells (9, 16), the muscularis mucosae (2), and the smooth muscle of the small intestinal villus core (75). The smooth muscle fibers of the small intestinal villus core are likely the lymphatic lacteals (Fig. 2), a conclusion supported by comparing α-SMA-stained histological sections with published scanning electron micrograph of corrosion casts of intestinal lacteals (86) and immunohistochemical sections stained with antibodies against the lymphatic endothelium (LYVE1, a hyaluronan receptor) (74).
Intermediate filament stains differentiate the myofibroblasts from smooth muscle of the muscularis mucosae or the lymphatic lacteal, the myofibroblasts staining strongly for vimentin and the smooth muscle staining strongly for desmin (2, 3, 75). α-SMA is downregulated by IFN-γ (25, 66), and thus myofibroblasts may be overlooked in inflamed tissues.
Thymus stromal antigens.
Thy-1 (CD90) is a 25- to 37-kDa extracellular glycosylphosphatidylinositol-linked glycoprotein that may also exist in a cleaved, soluble form. Thy-1 is expressed by subsets of fibroblasts, myofibroblasts, vascular pericytes, HSCs and MSCs, activated endothelial cells, and some murine neurons and murine T cells (which limits use of this marker in murine studies) (12, 100, 101). Antibodies against this surface protein identify the human intestinal myofibroblast-fibroblast-pericyte network, where diffuse staining suggests that the extracellular matrix (ECM) may also capture soluble Thy-1 (94).
Intestinal fibroblasts, myofibroblasts, pericytes, and lymphatic stromal cells can also be identified with monoclonal antibodies raised against thymic stromal cells such as ER-TR7 (53, 54, 84, 126) and TE-7 (36, 44). Preliminary studies in our laboratory show reactivity of Thy-1, ER-TR7, and α-SMA against these mesenchymal elements of the lamina propria, and confocal microscopic merged images (Fig. 4) give promise that the combination may be useful in demonstrating the villus smooth muscle (lymphatics) and the subepithelial myofibroblast network. Little is known of the peptides recognized by these antibodies other than their cytoplasmic intracellular location.
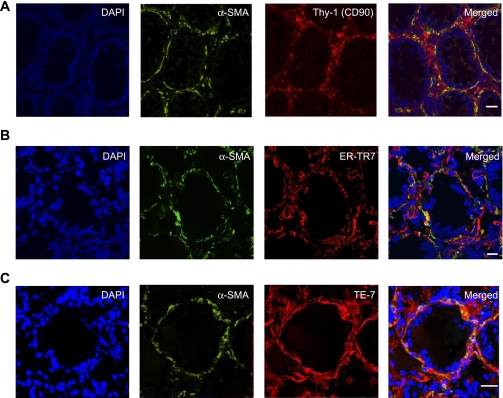
Fig. 4.
Identification of the intestinal mesenchymal cells. A–C: confocal microscopic analysis of multicolor immunofluorescent staining of cross sections of a normal human colonic crypt. CD90, ER-TR7, or TE-7 (red) identify nonhematopoietic mesenchymal cells of the colonic mucosa: fibroblasts, myofibroblasts, and pericytes of the small vessels and lymphatics. The subepithelial myofibroblast network and pericytes are identified on the basis of their morphology, subepithelial or perivascular location, and immunoreactivity to α-SMA (green) and mesenchymal markers CD90, ER-TR7, or TE-7 (red), resulting in orange-yellow color on the merged images. A: cell nuclei (blue) stained with DAPI; α-SMA shown in green; Thy-1 (CD90) shown in red. B: cell nuclei (blue) stained with DAPI; α-SMA shown in green; ER-TR7 shown in red. C: cell nuclei (blue) stained with DAPI; α-SMA shown in green; TE-7 shown in red. Calibration bars are 20 μm.
Melanoma chondroitin sulfate proteoglycan.
MCSP, also known as neuron-glia 2 (NG2) in the rat, is a transmembrane chondroitin sulfate proteoglycan expressed by immature progenitor cells of the oligodendrocyte, chondrocyte, and smooth muscle lineages (111). In the adult intestine, MCSP expression is observed within myofibroblasts, pericytes, and smooth muscle cells of the muscularis mucosae and tunica muscularis in both the large and small intestine. MCSP and α-SMA colocalize within intestinal tissues (119). MCSP is not expressed by fibroblasts and is rarely found in cells expressing fibroblast-specific protein 1 (FSP-1; see Fibroblast-specific protein-1 below) (114). MCSP functions as a coreceptor for PDGF-AA, basic (b)FGF, and integrins, effectively decreasing the threshold dose for these ligands in MCSP-expressing cells (37). The PDGF α-receptor is unresponsive to PDGF-AA in smooth muscle cells derived from the MCSP/NG2-null mouse (38). MCSP expression is often enhanced within tumors, and we have observed increased MCSP expression in stromal myofibroblasts isolated from human colorectal cancers, relative to normal colonic tissue (R. C. Mifflin, unpublished observation). In an elegant series of experiments, Huang et al. (48) have recently demonstrated that MCSP/NG2-negative stromal vascular pericytes severely limit the ability to assemble functional tumor vasculature. The MCSP/NG2-null mouse demonstrates defects in both pericyte recruitment and pericyte/endothelial interactions resulting in reduced establishment of new blood vessels (discussed in Ref. 48).
Fibroblast-specific protein-1.
Fsp-1, also referred to as S100 calcium binding protein A4 (S100A4), is an intracellular protein identified by Strutz et al. (113) to be expressed in renal fibroblasts but not epithelium. Subsequent studies have demonstrated Fsp-1 expression by intestinal lymphocytes and macrophages and malignant colorectal cancer epithelial cells (116, 118), but a fibroblast-specific transcriptional element has been identified (87) that has been used in a series of elegant studies to target transgene expression within fibroblasts (11, 88). Fsp-1 appears to be a useful marker to aid in the identification of fibroblasts but is expressed at low levels, if at all, in myofibroblasts, pericytes, muscularis mucosae, and smooth muscle of the lamina propria (116, 118). Sugimoto et al. (114) have pointed out that within tumor specimens Fsp-1 antibodies identify a unique population of fibroblasts with minimal overlap in expression of α-SMA, MCSP, and PDGFR-β. Thus while Fsp-1 is useful for characterization and identification of fibroblast populations, it is of little use as a myofibroblast marker.
Patched.
The receptors for hedgehog (Hh) growth and differentiation factors are 12-span transmembrane receptors of the patched (Ptch) family (125). Vertebrates contain two Ptch genes, Ptch 1 and Ptch 2. Ptch normally interacts in an inhibitory fashion with another transmembrane receptor, smoothened (Smo), and Hh binding to Ptch disrupts this interaction. Active Smo initiates a signaling cascade culminating in the activation and nuclear translocation of the transcription factors Gli1, Gli2, and Gli3. Ptch 1 and Gli1 are themselves transcriptional targets of Gli transcription factors. In the intestine of adult mice and humans, Ptch 1 (and Gli1) expression occurs predominantly within the myofibroblasts, pericytes, and smooth muscle of the lamina propria and muscularis mucosae. Hh signaling is crucial for the proper development of myofibroblasts, muscularis mucosae smooth muscle, and lamina propria smooth muscle since mice engineered to contain low levels of intestinal Hh signaling contain significantly lower numbers of these cell types. In these mutant Hh mice, the epithelial compartment is deranged, displaying an increased fraction of proliferating cells (65, 75, 132). These observations underscore the importance of lamina propria and muscularis mucosae α-SMA+ cells to proper epithelial differentiation.
Periostin.
Periostin is an ECM protein enriched in collagen-containing structures such as ligaments, fascia, and cardiac valves. Periostin is important for proper assembly of ECM components in tissues exposed to high amounts of mechanical stress (40). Periostin aids in the incorporation of tenascin-C into the ECM to generate a “meshwork” architecture of the matrix (58). While a great deal has been published on the functions of periostin in other tissues, little is known of its expression and function within the intestine. Kikuchi et al. (59) noted the synthesis and secretion of periostin by pericryptal myofibroblasts and demonstrated a tight relationship between α-SMA and periostin expression in normal colonic mucosa. The concordance between α-SMA and periostin expression diminishes in actively inflamed regions of ulcerative colitis, where periostin is observed throughout the lamina propria and α-SMA expression remains in the pericryptal region and is decreased (59). Much like what occurs in other epithelial cancers (81, 103, 115, 120, 134, 135), periostin expression was observed within the cancer-associated myofibroblasts of advanced colorectal adenocarcinoma (59). Periostin-expressing fibroblasts, when cocultured with HCT116 epithelial cancer cells in a three-dimensional collagen matrix, promote an increase in the number and size of HCT116 colonies (59). Thus while periostin expression represents an additional mechanism whereby cancer-associated myofibroblasts promote tumorigenesis, periostin is a suitable marker for intestinal myofibroblasts only under limited circumstances.
Fibroblast activation protein.
Fibroblast activation protein (FAP) is a type II transmembrane glycoprotein with post-proline dipeptidyl aminopeptidase activity (4). FAP is highly expressed by myofibroblasts and pericytes within the stroma of numerous cancers (106, 129), including colorectal adenocarcinoma (127), but is generally undetectable within normal tissues (104, 106). FAP-deficient mice display retarded growth of lung and colon tumors relative to the parental strain, and pharmacological or immunologic inhibition of FAP activity also leads to decreased tumor growth in animal models (19, 45, 70, 106, 127). FAP depletion retards tumorigenesis by causing elevated collagen accumulation, decreased myofibroblast content, and decreased blood vessel density (106). FAP expression has also been observed in myofibroblasts within tissues undergoing fibrotic episodes such as idiopathic pulmonary fibrosis, hepatic cirrhosis, and rheumatoid arthritis and within strictures resulting from Crohn's disease (104). Bae et al. (8) have determined that MSCs derived from bone marrow, adipose tissue, and umbilical cord blood constitutively express FAP. Immunoselection using a FAP monoclonal antibody yielded a homogeneous population of MSCs (8). Thus while FAP can be used as a marker of activated myofibroblasts and pericytes within tumors and fibrotic tissues, it may also be used for enrichment of their immediate precursors, MSCs.
Epimorphin.
Epimorphin/syntaxin 2 is a membrane-associated protein that is homologous to the syntaxin family of vesicle-docking proteins in neurons and pancreas, where they take part in calcium-mediated exocytosis. Epimorphin levels increase in the fetal gut mesenchyme during lumen formation and villous morphogenesis (32). In the intestine, epimorphin is predominantly expressed by subepithelial myofibroblasts and vascular pericytes (6), although expression by other stromal cell types cannot be ruled out (109). Epimorphin regulates the cellular response to Hh ligands, and myofibroblasts isolated from Epi−/− mice have a reduced response to Hh, and thus decreased Bmp signaling (109). Myofibroblasts engineered to overexpress epimorphin secrete increased levels of Bmp4, which promotes epithelial differentiation.
Origin of Lamina Propria Mesenchymal Cells
Newly proposed therapy for severe and nonresponsive IBD, especially Crohn's disease, is bone marrow transplantation or stem cell therapy (see Targeting Subepithelial Mesenchymal Cells for Stem Cell Therapy below). There are many mechanisms by which stem cell therapy might be effective for autoimmune diseases (also see below): replacement of diseased pericryptal myofibroblasts, which form the epithelial stem cell niche and also regulate epithelial cell differentiation; the replacement of diseased pericytes and endothelial cells, which are necessary for angiogenesis; and even engraftment and transformation of stem cells into neoepithelial cells are theoretically possible. Effective therapy by stem cells requires knowledge of which bone marrow stem cell, mesenchymal (MSC) or hematopoietic (HSC), is critical for the normal stem cell repair process in the intestine as well as how these cells induce repair.
Origin in the embryo.
In the human intestine, lamina propria α-SMA+ mesenchymal cells are present in the embryo as early as 21 wk, and probably earlier (107), and in the mouse they appear at embryonic day 18.5 (80). These cells have the appearance of myofibroblasts. Proper development of subepithelial mesenchymal cells is dependent on epithelium-derived Hh signaling (64, 75, 125, 132). Proper localization of lamina propria mesenchymal cells during development requires PDGF-A and PDGFR-α, while PDGF-BB and PDGFR-β are needed for proper pericyte formation in the kidney and skin (the intestine was not well examined) (52, 73). There is controversy (reviewed in Ref. 99) over whether these subepithelial mesenchymal cells originate from neural crest cells, which has been demonstrated for mesenchyme and pericytes of the thymus (31, 82, 131), or from the serosal mesothelium (128).
Subepithelial mesenchymal cell proliferation is driven by PDGF-BB, EGF, bFGF, and IGF-I and -II and better still in a combinatorial signaling process achieved by pairing PDGF with IL-1β or vasoactive polypeptide (VIP) (49). Combinations of TGF-β and EGF or IGF-II may achieve significant fibroblast proliferation through a connective tissue growth factor (CTGF)-dependent pathway (39).
Origin in the adult.
Gabbiani (46), one of the early pioneers of myofibroblast biology, proposes that other mesenchymal cells in organs such as α-SMA− fibroblasts and α-SMA+ stellate cells, pericytes, and even smooth muscle cells might become myofibroblasts in the postnatal animal through processes of differentiation, activation, and dedifferentiation (reviewed also in Ref. 99). Furthermore, both epithelium and endothelium might transdifferentiate to myofibroblasts through the process of epithelial to mesenchymal transition (EMT) (95, 133). Finally, subepithelial α-SMA+ cells may be replenished in the postnatal animal by stem cells from the bone marrow. Brittan et al. (14) described pericryptal myofibroblast replacement in female recipients by male bone marrow transplantation through identification of the Y chromosome. This group later demonstrated multiple organ engraftment after bone marrow transplantation (26) and neoplastic recruitment of transplanted bone marrow cells (15). Engraftment of transplanted cells into tissue “myofibroblasts” can be increased by wounding the tissue epithelium, and the percentage of donor-derived colonic myofibroblasts and vascular pericytes can reach 39% within a week of transplantation following trinitrobenzenesulfonic acid (TNBS) colitis (13). A similar phenomenon occurs after damage to lung, kidney, stomach and skin, where engrafted mesenchymal cells can occupy 42%, 24%, 64%, and 4% of resident subepithelial mesenchymal cells, respectively, within 10 wk of transplantation (26). Thus the role for bone marrow stem cell replacement of damaged intestinal stromal cells is clear, but these experiments raise two important questions: What is the nature of the stem cell aiding in tissue repair? Do the stem cells only replace lamina propria mesenchymal cells or do bone marrow stem cells also replace damaged epithelial cells?
Targeting Subepithelial Mesenchymal Cells for Stem Cell Therapy
Bone marrow transplantation and stem cell therapy have been proposed as a new therapy for severe and nonresponsive IBD, especially Crohn's disease. The rationale behind such therapy is multiconceptual (Table 1). HSC transplantation (HSCT) can accomplish immune reset, i.e., the generation of new self-tolerant lymphocytes after chemotherapy-induced ablation or reduction (conditioning chemotherapy) of self-reactive lymphocytes (33). In the case of allogeneic (donor) transplants, the replacement of genetically abnormal bone marrow precursors with “healthy” HSCs is also possible. The idea that MSC therapy might topically engraft and be used to replace diseased subepithelial mesenchymal cells with healthy transplanted stromal cells, or that stem cells might replace and transdifferentiate into epithelial cells, is a distinct possibility (69). Alternatively, because MSCs demonstrate significant immunosuppressive properties when infused in vivo (see Mesenchymal stem cells below), this property may be used as a mechanism of therapeutic response to MSC therapy in autoimmune diseases. Effective therapy with stem cells requires knowledge of which bone marrow stem cell, mesenchymal (MSC) or hematopoietic (HSC), is most critical for the normal stem cell repair process in intestine and the mechanism of that response.
Table 1.
Therapeutic effects of stem cell therapy in IBD
| Hematopoietic stem cell transplantation |
| “Immune reset”: generation of new self-tolerant lymphocytes after bone marrow ablation or conditioning chemotherapy |
| Replacement of genetically abnormal bone marrow precursors through allogeneic transplantation |
| Stromal cell engraftment in the lamina propria with healthy cells, increasing growth factor production, which creates a healthy stem cell niche and promotes angiogenesis/vasculogenesis |
| Possibly engraftment followed by EMT to replenish epithelial cells |
| Mesenchymal stem cell transplantation |
| Immune suppression: MSCs are immunoprivileged cells |
| Stromal cell engraftment in the lamina propria with healthy cells, increasing growth factor production, which creates a healthy stem cell niche and promotes angiogenesis/vasculogenesis |
| Possibly engraftment of MSCs followed by EMT to replenish epithelial cells |
IBD, inflammatory bowel disease; EMT, epithelial to mesenchymal transition; MSC, mesenchymal stem cell.
Hematopoietic stem cells or fibrocytes.
HSCs express a set of characteristic molecules: the leukocyte common antigen CD45 (pan-hematopoietic marker), progenitor markers CD34 and CD105, monocyte markers such as CD11, CD13, and CD14, and antigen-presenting molecules such as MHC class II and the T lymphocyte B7 family costimulatory molecules CD80, CD86, and also CD40. Like MSCs, they express vimentin, chemokines and chemokine receptors, adhesion molecules, and integrins. They do not normally express MSC markers such as CD90, CD44, CD70, CD73, and CD271.
A recent review details current therapeutic outcomes with myeloablative (chemotherapeutic ablation of both the hematopoietic and lymphopoietic lineages in the bone marrow) HSCT performed for treatment of cancer in patients with concomitant IBD (25 patients in 10 separate reports). Described also are the outcomes of the transplantation of 19 Crohn's disease patients in 5 reports who underwent nonmyeloablative, “conditioning” chemotherapy, which depletes self-reactive lymphocytes. Clinical remissions were noted in all and lasted at least 6 mo and up to 7 yr (35). The benefits of these transplantations were thought to be due to the “immune reset” that occurs with hematopoietic cell replacement (Table 1). Is there experimental evidence of intestinal mesenchymal or epithelial engraftment after HSC transplantation?
Using carefully performed, single-cell cloning of green fluorescent protein (GFP)-positive HSCs, Ogawa et al. (85) have demonstrated that HSCs can repopulate pericytes in the kidney (mesangial cells), brain, and heart. While it is possible that the HSC itself populated these distal sites (78, 110), it is likely that tissue engraftment occurs through an intermediate cell, a hematopoietically derived monocyte derivative called the fibrocyte (reviewed in Refs. 9 and 20).
Fibrocytes are a distinct population of bone marrow-derived cells that become progenitors for mesenchymal cells. They express hematopoietic markers of a monocyte origin and also produce fibroblast/myofibroblast ECM molecules and matrix-modifying enzymes such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (9) (Fig. 5). In the vascular compartment, where they are estimated to represent 0.1–0.5% of circulating cells, they express CD45, CD34, CD105, CD11, MHC class II, CD80 and CD86, and CD13, as do HSCs, but are only weakly positive for the monocyte marker CD14 and negative for CD16. They are also negative for B-cell and T-cell markers and do not express dendritic markers CD1a, CD10, or CD83. They express chemokine receptors CCR1, 3, 4, 5, 7, and 9 as well as CXCR1, 3, and 4. Importantly, they express fibrocyte/mesenchymal markers vimentin, collagen 1, fibronectin, and MMP-9. It is unclear whether they express the fibroblast marker CD90 (Thy-1) (9). In the human it is thought that they are derived from a small subset of CD14+, CD16− monocytes that bear surface chemokine receptor CCR2 (the analogous murine counterpart would be Ly-6Chigh, CRR2+). These low-abundance cells belong to a group of white blood cells termed “inflammatory monocytes” that normally replenish tissue macrophages and dendritic cells. In culture, and presumably in vivo, after direct contact with T cells a subgroup of these cells becomes fibrocytes and there is downregulation of CD14 and upregulation of collagen synthesizing enzymes, MMPs, IL-1, and PDGF-AA (1). In inflamed tissue where they are exposed to TGF-β and ET-1, there is upregulation of α-SMA and downregulation of CD34 and CD45 and the cells become myofibroblasts. These cells have been incriminated in several pathological processes and diseases including hypertrophic scar and keloid formation, airway remodeling in asthma, interstitial pulmonary fibrosis, renal fibrosis, pancreatic fibrosis, and even atherosclerosis (1, 9, 108).
Fig. 5.
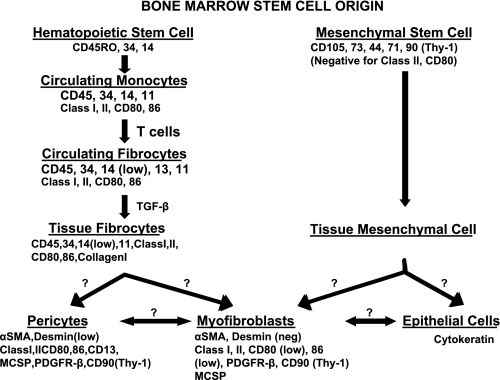
Bone marrow stem cell origin of intestinal mesenchymal stromal cells. Diagram demonstrates possible stem cell origin and routes for derivation of lamina propria mesenchymal cells (and epithelial cells) from bone marrow precursors. See text for discussion. TGF-β, transforming growth factor-β; MCSP, melanoma chondroitin sulfate proteoglycan; PDGFR-β, PDGF receptor-β.
Recently, Uehara et al. (123) have published evidence that fibrocytes may be involved in colonic repair in colitis. Seven days after the induction of dextran sodium sulfate (DSS) colitis, there was an influx of CD45-positive, collagen 1-positive ovoid cells into the inflamed tissue. By day 14, α-SMA-positive spindle-shaped myofibroblasts were present in the tissue. Unfortunately, the study does not definitively demonstrate the proposed sequence of fibrocytes becoming myofibroblasts because the lack of cell labeling of the bone marrow precursor cells does not allow lineage tracing and thus definitive identification of the HSC origin of the fibrocytes in this intriguing experiment.
Mesenchymal stem cells.
The properties of MSCs in animal and humans and their role in normal health and disease have been reviewed elsewhere (17, 29, 122). MSCs are cultured from bone marrow stromal cells by adhesion to plastic in 10% calf serum. They can serve as feeder layers for the culture of HSCs and, with proper conditions and the addition of proper growth factors, can self renew and differentiate into mesodermal tissue such as adipocytes, chondrocytes, osteocytes, and other tissue stromal cells. They also show multipotentiality by transdifferentiating, under the influence of different factors, into cells of other germ line lineages such as endoderm (lung and intestinal epithelial cells and muscle cell) and ectoderm (skin epithelial cells and neurons). Whether such transdifferentiation occurs in vivo has been controversial, but this seems likely under conditions of organ damage and repair (112). MSCs express characteristic molecules such as CD90 (Thy-1), CD44 (hyaluronan receptor), CD105 (SH2), CD73 (SH3/4), CD90, CD71, CD271, and STRO1. Cultured MSCs are defined also by their failure to express HSC markers such as CD45 (pan-hematopoietic marker), CD34 (stem cell progenitor marker), CD14 (monocyte marker), CD11 (monocyte/dendritic marker), and CD4 T-cell antigen-presenting molecules such as MHC class II and the costimulatory molecules CD80, CD86, and CD40. MSCs express several adhesion molecules such as VCAM-1, activated leukocyte adhesion molecule (ALCAM), ICAM-1, ICAM-3, endoglin (CD105, TGF-β coreceptor) and various α1–5- and β1,3,4-integrins as well as many of the CC, CXC, CX3C, and XC chemokine receptors, which aid in their ability to home to and adhere to damaged tissues. It is important to note that in some animal species MSCs may not express the same molecules as human MSCs, e.g., mouse MSCs may show variable CD34 expression, and MSC expression patterns in vivo may not be the same as in established culture. Although typically cultured from bone marrow, isolation of MSCs has been accomplished from fetal blood, umbilical cord, amniotic fluid, and adipose tissue (17).
In addition to their characteristic ability to support HSC growth and differentiation, and their ability to differentiate into mesenchymal tissues, another fundamental property of MSCs is their immunomodulatory properties, with effects on both innate and adaptive immunity (reviewed in Refs. 29, 99, and 122). The MSC is known as the “immunoprivileged cell” or “universal donor.” It is important to understand that all mature mesenchymal tissue stromal cells share these immunoregulatory properties (50). Some investigators have proposed that tissue MSCs might gain MHC class II molecules with the ability to present antigens and promote inflammation (91) or suppress inflammation through expression of PD-L1 immune suppressor molecules, and this bivalent immune function might be governed by the level of ambient IFN-γ (18). Most of the salutary effects of infused MSCs in animal models of organ transplant rejection, central nervous system (CNS), kidney, heart, liver, and pancreas, are attributable to these immunomodulatory effects (29, 130). In other disease models of heart, lung, and CNS the beneficial effects of transplanted MSCs have been attributed to the trophic effects of MSC-derived growth factors that induce proliferation and differentiation of local, host progenitor cells and not to topical engraftment of these MSCs with subsequent incorporation into the tissue (17, 35, 69, 122). Recently MSCs have been used to treat graft versus host disease, type 1 diabetes, and Crohn's disease, but it is too early to determine therapeutic efficacy.
There have only been a few studies of the ability of MSCs to engraft and repair colitis. Brown et al. (16) in a model of DSS colitis recognized a COX-2-expressing “stromal cell” that moved from the upper regions of the colonic lamina propria to the basal regions of the lamina propria adjacent to the location of epithelial stem cells. This repositioning was dependent on MYD88 signaling, indicating that toll-like receptors were important to the directional movement of this prostaglandin-secreting cell. Furthermore, this study showed that prostaglandins were critical in initiating epithelial proliferation and repair. Unfortunately, there was no confirmation of the origin of this cell: it could be an MSC or a tissue fibrocyte.
In mice (57) or rats (117) with DSS-induced colitis or in rats with TNBS-induced colitis (43), infusion of MSCs into nonmyeloablated animals improved the severity and shortened the duration of colitis. The MSCs were topically implanted into the lamina propria. The beneficial effects were ascribed to the provision of growth factors and angiogenic/vasculogenic factors or differentiation into endothelial cells and other lamina propria mesenchymal cells (fibroblast, myofibroblasts, and smooth muscle cells).
There is evidence that transplanted bone marrow stem cells may engraft topically into the gut epithelium as well as into the lamina propria. With Y chromosome identification, bone marrow-derived cells can be found in the intestinal epithelium of humans (79, 89). In experimental animal models, damage to the epithelium is thought to create chemokines that enhance homing of stem cells, and when GFP-labeled bone marrow cells were transplanted into a TNBS mouse model of colitis, donor cells were found in 38% of colonic epithelial cells after transplantation (60). While the lineage of these engrafting cells is uncertain, Yabana et al. (130) used male donor MSCs derived from GFP engineered rats and infused them into wild-type, DSS-colitis female mice either with normal marrow or with busulfan myeloablation. The transplanted cells were identified with colocalization of eGFP and Y-FISH analysis. Transplanted MSCs were found in the lamina propria adjacent to the crypts (presumably subepithelial myofibroblasts or pericytes); however, the bulk of the cells were found engrafted into the epithelium: 2% of colonic crypts and 10% of colonic epithelial cells bore the GFP marker in the group with normal marrow and 8% of crypts and 42% of epithelial cells in the myeloablated model (130). These investigators also demonstrated improved barrier function in the MSC transplanted animals. Whether there is direct engraftment of bone marrow stem cells into the epithelium after infusion of MSCs or whether EMT of engrafted subepithelial myofibroblasts occurred is uncertain.
Mesenchymal cells of fibrotic lesions.
A major consequence of the chronic inflammation and tissue injury associated with IBD, particularly Crohn's disease, is tissue fibrosis (102). Fibrosis results from excessive local accumulation of ECM molecules laid down by activated myofibroblasts and fibroblasts or by resident stellate cells. These activated stromal cells may be derived locally via stimulation or induction of proliferation, or by EMT driven by proinflammatory cytokines (102). Additionally, resident myofibroblasts and fibroblasts may migrate to sites of inflammation in response to chemotactic gradients of PDGFs, IGF-I, or TGF-β. As mentioned above, stromal cells derived from MSCs, fibrocytes, or HSCs may also home to sites of inflammation and, in the presence of ongoing inflammation, become activated myofibroblasts/fibroblasts and contribute to tissue fibrosis.
The key to therapy directed at preventing fibrosis in IBD is to eliminate or curb the chronic inflammation. The immunomodulatory capacity of MSCs and their ability to provide angiogenic/vasculogenic factors and trophic factors for replacement of damaged epithelium suggest that MSC transplantation may play a useful role in the treatment of IBD-related intestinal fibrosis. Additionally, it is possible that mesenchymal cells newly arrived as stem cells and then integrated into the lamina propria might be less profibrogenic than those present during chronic inflammation. While the exact mechanism is unclear, preliminary studies using animal models of hepatic, lung, and cardiac fibrosis support the conclusion that stem cell therapy may be useful antifibrotic treatment (28, 41, 71, 72).
Conclusions
Mesenchymal cells of the intestinal lamina propria represent a spectrum of phenotypes that perform distinct functions based upon their location. The first layer of subepithelial cells surrounding the crypts of the colon and small intestine are apparently myofibroblasts, although no doubt pericytes are present in the lamina propria also. In the surface of the colon and villus of the small intestine, the subepithelial cells are predominantly pericytes. Additional pericytes are found surrounding blood vessels and lymphatics in the core of the villus, and these cells seem to be connected to subepithelial cells as part of a three-dimensional network. The second layer of mesenchymal cells surrounding crypts, the mesenchymal cells of the villus, and the subsurface areas of the colon, the interconnecting mesenchymal cells of the villus core cells lose α-SMA staining and become fibroblasts. Lymphatic smooth muscle and pericytes are also present in the lamina propria.
Immunologic markers exist to aid in the identification of these cells, but none is absolutely specific or definitive. Lack of desmin expression appears to help separate α-SMA+ myofibroblasts from pericytes (weakly positive for desmin) and from the smooth muscle of the muscularis mucosae and lymphatic lacteal (strongly positive for desmin). Vimentin staining is less intense or absent in these smooth muscle bundles. Coupling α-SMA staining with antibodies marking thymus stromal cell antigens appears to be useful in separating myofibroblasts and pericytes from fibroblasts.
The primary origin of lamina propria mesenchymal cells in the embryo remains uncertain. In the postnatal animal subepithelial mesenchymal cells (myofibroblasts and pericytes) can be recruited from circulating precursor pools during inflammation and carcinogenesis, but it is unclear whether the important pool is originally from an HSC origin, from a MSC origin, or from both. Subtle functional differences may exist among cells derived from either MSCs or HSCs, but such differences have yet to be identified.
MSCs and HSCs represent future therapeutic hopes for intestinal injury and chronic intestinal inflammatory states. Current stem cell therapies are directed toward the immune aspect of intestinal inflammatory diseases. However, studies in animal models suggest that stem cell therapies might replace lost stromal cells (and perhaps epithelial cells) after injury. Thus an important aspect of stem cell therapeutic potential may represent true regenerative medicine and be derived from their replenishment of the subepithelial mesenchymal cells that normally play important roles in growth, development, and repair of the intestinal mucosa.
GRANTS
The research performed in the authors' laboratories mentioned in this article was supported by National Institutes of Health Grants DK-55783 (D. W. Powell) and CA-127229 (D. W. Powell) and an American Gastroenterological Association (AGA) Scholars Award to I. V. Pinchuk.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 126: 829–836, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res 10: 5870–5879, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Aertgeerts K, Levin I, Shi L, Snell GP, Jennings A, Prasad GS, Zhang Y, Kraus ML, Salakian S, Sridhar V, Wijnands R, Tennant MG. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J Biol Chem 280: 19441–19444, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Therapeut 114: 94–106, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Andoh A, Fujino S, Hirai Y, Fujiyama Y. Epimorphin expression in human colonic myofibroblasts. Int J Mol Med 13: 57–61, 2004 [PubMed] [Google Scholar]
- 7. Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 205: 331–337, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bae S, Park CW, Son HK, Ju HK, Paik D, Jeon CJ, Koh GY, Kim J, Kim H. Fibroblast activation protein alpha identifies mesenchymal stromal cells from human bone marrow. Br J Haematol 142: 827–830, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 87: 858–870, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7: 452–464, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303: 848–851, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Bradley JE, Ramirez G, Hagood JS. Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype. Biofactors 35: 258–265, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brittan M, Chance V, Elia G, Poulsom R, Alison MR, MacDonald TT, Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology 128: 1984–1995, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes SJ, Hodivala-Dilke K, Goldman J, Alison MR, Wright NA. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut 50: 752–757, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brittan M, Wright NA. Stem cell in gastrointestinal structure and neoplastic development. Gut 53: 899–910, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of PTGS2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117: 258–269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25: 2739–2749, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood 107: 4817–4824, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng JD, Dunbrack RL, Jr, Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res 62: 4767–4772, 2002 [PubMed] [Google Scholar]
- 20. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA 94: 6307–6312, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 123: 2229–2238, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Deane HW. Some electron microscopic observations on the lamina propria of the gut, with comments on the close association of macrophages, plasma cells, and eosinophils. Anat Rec 149: 453–473, 1964 [DOI] [PubMed] [Google Scholar]
- 23. Desaki J, Fujiwara T, Komuro T. A cellular reticulum of fibroblast-like cells in the rat intestine: scanning and transmission electron microscopy. Arch Histol Jpn 47: 179–186, 1984 [DOI] [PubMed] [Google Scholar]
- 24. Desaki J, Shimizu M. A re-examination of the cellular reticulum of fibroblast-like cells in the rat small intestine by scanning electron microscopy. J Electron Microsc (Tokyo) 49: 203–208, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Desmouliere A, Rubbia-Brandt L, Abdiu A, Walz T, Macieira-Coelho A, Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res 201: 64–73, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 21: 514–520, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Donnellan WL. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterology 49: 496–514, 1965 [PubMed] [Google Scholar]
- 28. Elkhafif N, El Baz H, Hammam O, Hassan S, Salah F, Mansour W, Mansy S, Yehia H, Zaki A, Magdy R. CD133+ human umbilical cord blood stem cells enhance angiogenesis in experimental chronic hepatic fibrosis. APMIS 119: 66–75, 2011 [DOI] [PubMed] [Google Scholar]
- 29. English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell 7: 431–442, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med 12: 22–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol 180: 3183–3189, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Fritsch C, Swietlicki EA, Lefebvre O, Kedinger M, Iordanov H, Levin MS, Rubin DC. Epimorphin expression in intestinal myofibroblasts induces epithelial morphogenesis. J Clin Invest 110: 1629–1641, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furuya S, Furuya K. Subepithelial fibroblasts in intestinal villi: roles in intercellular communication. Int Rev Cytol 264: 165–223, 2007 [DOI] [PubMed] [Google Scholar]
- 35. García-Bosch O, Ricart E, Panes J. Review article: Stem cell therapies for inflammatory bowel disease—efficacy and safety. Aliment Pharmacol Ther 32: 939–952 2010 [DOI] [PubMed] [Google Scholar]
- 36. Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem 56: 347–358, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem 274: 16831–16837, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGFalpha-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci 112: 905–915, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J 18: 469–479, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal 2: 9–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hardjo M, Miyazaki M, Sakaguchi M, Masaka T, Ibrahim S, Kataoka K, Huh NH. Suppression of carbon tetrachloride-induced liver fibrosis by transplantation of a clonal mesenchymal stem cell line derived from rat bone marrow. Cell Transplant 18: 89–99, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15: 2215–2224, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther 326: 523–531, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Haynes BF, Scearce RM, Lobach DF, Hensley LL. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med 159: 1149–1168, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayward SW. Preclinical assessment of fibroblast activation protein as a target for antitumor therapy. Future Oncol 6: 347–349, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15: 4310–4320, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularization in the brain of the NG2 null mouse. Dev Biol 344: 1035–1046, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jobson TM, Billington CK, Hall IP. Regulation of proliferation of human colonic subepithelial myofibroblasts by mediators important in intestinal inflammation. J Clin Invest 101: 2650–2657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol 179: 2824–2831, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Joyce NC, Haire MF, Palade GE. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology 92: 68–81, 1987 [DOI] [PubMed] [Google Scholar]
- 52. Karlsson L, Lindahl P, Heath J, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-alpha deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 127: 3457–3466, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Katakai T, Hara T, Lee JH, Gonda H, Sugai M, Shimizu A. A novel reticular stromal structure in lymph node cortex: an immuno-platform for interactions among dendritic cells, T cells and B cells. Int Immunol 16: 1133–1142, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med 200: 783–795, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kaye GI, Lane N, Pascal RR. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. II. Fine structural aspects of normal rabbit and human colon. Gastroenterology 54: 852–865, 1968 [PubMed] [Google Scholar]
- 56. Kaye GI, Pascal RR, Lane N. The colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. III. Replication and differentiation in human hyperplastic and adenomatous polyps. Gastroenterology 60: 515–536, 1971 [PubMed] [Google Scholar]
- 57. Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology 132: 944–954, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285: 2028–2039, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo A, Fukayama M. Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem 56: 753–764, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Komori M, Tsuji S, Tsujii M, Murata H, Iijima H, Yasumaru M, Nishida T, Irie T, Kawano S, Hori M. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen 13: 109–118, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Komuro T. Re-evaluation of fibroblasts and fibroblast-like cells. Anat Embryol (Berl) 182: 103–112, 1990 [DOI] [PubMed] [Google Scholar]
- 62. Komuro T, Hashimoto Y. Three-dimensional structure of the rat intestinal wall (mucosa and submucosa). Arch Histol Cytol 53: 1–21, 1990 [DOI] [PubMed] [Google Scholar]
- 63. Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10: 387–398, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, Chan TL, Mifflin RC, Powell DW, Yuen ST, Leung SY, Chen X. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci USA 104: 15418–15423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kosinski C, Stange DE, Xu C, Chan AS, Ho C, Yuen ST, Mifflin RC, Powell DW, Clevers H, Leung SY, Chen X. Indian Hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology 139: 893–903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol 163: 1291–1300, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia 58: 1–10, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Lane N, Caro L, Otero Vilardebo LR, Godman GC. On the site of sulfation in colonic goblet cells. J Cell Biol 21: 339–351, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lanzoni G, Roda G, Belluzzi A, Roda E, Bagnara GP. Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol 14: 4616–4626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lebeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther 8: 1378, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee SH, Jang AS, Kim YE, Cha JY, Kim TH, Jung S, Park SK, Lee YK, Won JH, Kim YH, Park CS. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res 11: 16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li L, Zhang Y, Li Y, Yu B, Xu Y, Zhao S, Guan Z. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl Int 21: 1181–1189, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997 [DOI] [PubMed] [Google Scholar]
- 74. Ma B, von Wasielewski R, Lindenmaier W, Dittmar KE. Immmunohistochemical study of the blood and lymphatic vasculature and the innervation of mouse gut and gut-associated lymphoid tissue. Anat Histol Embryol 36: 62–74, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132: 279–289, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Marsh MN, Trier JS. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. I. Structural features. Gastroenterology 67: 622–635, 1974 [PubMed] [Google Scholar]
- 77. Marsh MN, Trier JS. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. II. Radioautographic studies. Gastroenterology 67: 636–645, 1974 [PubMed] [Google Scholar]
- 78. Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131: 994–1008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Matsumoto T, Okamoto R, Yajima T, Mori T, Okamoto S, Ikeda Y, Mukai M, Yamazaki M, Oshima S, Tsuchiya K, Nakamura T, Kanai T, Okano H, Inazawa J, Hibi T, Watanabe M. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology 128: 1851–1867, 2005 [DOI] [PubMed] [Google Scholar]
- 80. McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology 136: 2074–2091, 2009 [DOI] [PubMed] [Google Scholar]
- 81. Michaylira CZ, Wong GS, Miller CG, Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim SB, Herlyn M, Diehl JA, Gimotty P, Rustgi AK. Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res 70: 5281–5292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol 180: 5344–5351, 2008 [DOI] [PubMed] [Google Scholar]
- 83. Neal JV, Potten CS. Description and basic cell kinetics of the murine pericryptal fibroblast sheath. Gut 22: 19–24, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Odaka C. Localization of mesenchymal cells in adult mouse thymus: their abnormal distribution in mice with disorganization of thymic medullary epithelium. J Histochem Cytochem 57: 373–382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: its pathophysiologic implications. Blood 108: 2893–2896, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Ohtani O, Ohtani Y. Organization and developmental aspects of lymphatic vessels. Arch Histol Cytol 71: 1–22, 2008 [DOI] [PubMed] [Google Scholar]
- 87. Okada H, Danoff TM, Fischer A, Lopez-Guisa JM, Strutz F, Neilson EG. Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. Am J Physiol Renal Physiol 275: F306–F314, 1998 [DOI] [PubMed] [Google Scholar]
- 88. Okada H, Inoue T, Kanno Y, Kobayashi T, Watanabe Y, Ban S, Neilson EG, Suzuki H. Selective depletion of fibroblasts preserves morphology and the functional integrity of peritoneum in transgenic mice with peritoneal fibrosing syndrome. Kidney Int 64: 1722–1732, 2003 [DOI] [PubMed] [Google Scholar]
- 89. Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med 8: 1011–1017, 2002 [DOI] [PubMed] [Google Scholar]
- 90. Pascal RR, Kaye GI, Lane N. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. I. Autoradiographic studies of normal rabbit colon. Gastroenterology 54: 835–851, 1968 [PubMed] [Google Scholar]
- 91. Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz) 56: 1–8, 2008 [DOI] [PubMed] [Google Scholar]
- 92. Pinchuk IV, Beswick EJ, Saida JI, Reyes VE, Powell DW. Human colonic myofibroblasts promote the expansion of Cd4+Cd25high Foxp3+ regulatory T cells. Gastroenterology 132: A397–A397, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pinchuk IV, Mifflin RC, Saada JI, Powell DW. Intestinal mesenchymal cells. Curr Gastroenterol Rep 12: 310–318, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, Raju GS, Reyes VE, Powell DW. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 135: 1228–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9: 265–273, 2009 [DOI] [PubMed] [Google Scholar]
- 96. Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol 289: G2–G7, 2005 [DOI] [PubMed] [Google Scholar]
- 97. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol Cell Physiol 277: C1–C9, 1999 [DOI] [PubMed] [Google Scholar]
- 98. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999 [DOI] [PubMed] [Google Scholar]
- 99. Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73: 213–237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J 20: 1045–1054, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta 1763: 991–999, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 6: 228–235, 2009 [DOI] [PubMed] [Google Scholar]
- 103. Riener MO, Fritzsche FR, Soll C, Pestalozzi BC, Probst-Hensch N, Clavien PA, Jochum W, Soltermann A, Moch H, Kristiansen G. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology 56: 600–606, 2010 [DOI] [PubMed] [Google Scholar]
- 104. Rovedatti L, Di Sabatino A, Knowles CH, Sengupta N, Biancheri P, Corazza GR, Macdonald TT. Fibroblast activation protein expression in Crohn's disease strictures. Inflamm Bowel Dis (August 30, 2010). doi: 10.1002/ibd.21446 [DOI] [PubMed] [Google Scholar]
- 105. Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Di Mari JF, Reyes VE, Powell DW. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol 177: 5968–5979, 2006 [DOI] [PubMed] [Google Scholar]
- 106. Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119: 3613–3625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sappino AP, Dietrich PY, Skalli O, Widgren S, Gabbiani G. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Arch A Pathol Anat Histopathol 415: 551–557, 1989 [DOI] [PubMed] [Google Scholar]
- 108. Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 171: 380–389, 2003 [DOI] [PubMed] [Google Scholar]
- 109. Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion. J Clin Invest 120: 2081–2093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 120: 1192–1203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol 31: 423–435, 2002 [DOI] [PubMed] [Google Scholar]
- 112. Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science 324: 1666–1669, 2009 [DOI] [PubMed] [Google Scholar]
- 113. Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130: 393–405, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5: 1640–1646, 2006 [DOI] [PubMed] [Google Scholar]
- 115. Sutton CW, Rustogi N, Gurkan C, Scally A, Loizidou MA, Hadjisavvas A, Kyriacou K. Quantitative proteomic profiling of matched normal and tumor breast tissues. J Proteome Res 9: 3891–3902, 2010 [DOI] [PubMed] [Google Scholar]
- 116. Takenaga K, Nakanishi H, Wada K, Suzuki M, Matsuzaki O, Matsuura A, Endo H. Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clin Cancer Res 3: 2309–2316, 1997 [PubMed] [Google Scholar]
- 117. Tanaka F, Tominaga K, Ochi M, Tanigawa T, Watanabe T, Fujiwara Y, Ohta K, Oshitani N, Higuchi K, Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci 83: 771–779, 2008 [DOI] [PubMed] [Google Scholar]
- 118. Taylor S, Herrington S, Prime W, Rudland PS, Barraclough R. S100A4 (p9Ka) protein in colon carcinoma and liver metastases: association with carcinoma cells and T-lymphocytes. Br J Cancer 86: 409–416, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Terada N, Ohno N, Murata S, Katoh R, Stallcup W, Ohno S. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem Cell Biol 126: 483–490, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Tischler V, Fritzsche FR, Wild PJ, Stephan C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J, Schraml P, Jung K, Moch H, Soltermann A, Kristiansen G. Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer 10: 273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Toyoda H, Ina K, Kitamura H, Tsuda T, Shimada T. Organization of the lamina propria mucosae of rat intestinal mucosa, with special reference to the subepithelial connective tissue. Acta Anat (Basel) 158: 172–184, 1997 [DOI] [PubMed] [Google Scholar]
- 122. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8: 726–736, 2008 [DOI] [PubMed] [Google Scholar]
- 123. Uehara H, Nakagawa T, Katsuno T, Sato T, Isono A, Noguchi Y, Saito Y. Emergence of fibrocytes showing morphological changes in the inflamed colonic mucosa. Dig Dis Sci 55: 253–260, 2010 [DOI] [PubMed] [Google Scholar]
- 124. Valentich JD, Popov V, Saada JI, Powell DW. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. Am J Physiol Cell Physiol 272: C1513–C1524, 1997 [DOI] [PubMed] [Google Scholar]
- 125. van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev 87: 1343–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 126. Van Vliet E, Melis M, Foidart JM, Van Ewijk W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem 34: 883–890, 1986 [DOI] [PubMed] [Google Scholar]
- 127. Wen Y, Wang CT, Ma TT, Li ZY, Zhou LN, Mu B, Leng F, Shi HS, Li YO, Wei YQ. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci 101: 2325–2332, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132: 5317–5328, 2005 [DOI] [PubMed] [Google Scholar]
- 129. Wolf BB, Quan C, Tran T, Wiesmann C, Sutherlin D. On the edge of validation—cancer protease fibroblast activation protein. Mini Rev Med Chem 8: 719–727, 2008 [DOI] [PubMed] [Google Scholar]
- 130. Yabana T, Arimura Y, Tanaka H, Goto A, Hosokawa M, Nagaishi K, Yamashita K, Yamamoto H, Adachi Y, Sasaki Y, Isobe M, Fujimiya M, Imai K, Shinomura Y. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol 218: 350–359, 2009 [DOI] [PubMed] [Google Scholar]
- 131. Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 328: 1129–1135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zacharias WJ, Li X, Madison BB, Kretovich K, Kao JY, Merchant JL, Gumucio DL. Hedgehog is an anti-inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology 138: 2368–2377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119: 1429–1437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang Y, Zhang G, Li J, Tao Q, Tang W. The expression analysis of periostin in human breast cancer. J Surg Res 160: 102–106, 2010 [DOI] [PubMed] [Google Scholar]
- 135. Zhu M, Fejzo MS, Anderson L, Dering J, Ginther C, Ramos L, Gasson JC, Karlan BY, Slamon DJ. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol 119: 337–344, 2010 [DOI] [PubMed] [Google Scholar]