Abstract
Muscarinic receptors (CHRM) are overexpressed in colon cancer. To explore a role for muscarinic receptor signaling in colon cancer metastasis, we used human H508 and HT29 colon cancer cells that coexpress epidermal growth factor (ERBB) and CHRM3 receptors. In a wound closure model, following 8-h incubation of H508 cells with 100 μM ACh we observed a threefold increase in cell migration indistinguishable from the actions of epidermal growth factor (EGF). Atropine blocked the actions of ACh but not of EGF. In SNU-C4 colon cancer cells that express ERBB but not CHRM, EGF caused a threefold increase in migration; ACh had no effect. ACh-induced cell migration was attenuated by chemical inhibitors of ERBB1 activation, by anti-ERBB1 antibody, and by inhibitors of ERK and phosphatidylinositol 3-kinase (PI3K) signaling. Consistent with matrix metalloproteinase-7 (MMP7)-mediated release of an ERBB1 ligand, heparin binding epidermal growth factor-like growth factor (HBEGF), ACh-induced migration was inhibited by an MMP inhibitor and by anti-MMP7 and -HBEGF antibodies. ACh-induced cell migration was blocked by inhibiting RhoA and ROCK, key proteins that interact with the actin cytoskeleton. ACh-induced RhoA activation was attenuated by agents that inhibit ERBB1, ERK, and PI3K activation. Collectively, these findings indicate that ACh-induced cell migration is mediated by MMP7-mediated release of HBEGF, an ERBB ligand that activates ERBB1 and downstream ERK and PI3K signaling. In a cell invasion model, ACh-induced HT29 cell invasion was blocked by atropine. In concert with previous observations, these findings indicate that muscarinic receptor signaling plays a key role in colon cancer cell proliferation, survival, migration, and invasion.
Keywords: acetylcholine, muscarinic receptors, mitogen-activated protein kinase, actin cytoskeleton, tumor metastasis
for most cancers, the critical event resulting in host death is migration of tumor cells to vital organs (e.g., liver, lungs, and brain), a complex process commonly referred to as tumor metastasis (13). For tumor metastasis to occur, cells must be able to move from the tissue of origin, navigate the vasculature or lymphatics, attach to extracellular matrix, and thrive in the tissue of destination (42). A small minority of primary tumor cells survive this transition. Hence, identifying attributes of tumor cells that confer metastatic potential is an area of intense investigation (9). Genetic and epigenetic alterations that give tumor cells the ability to move, travel, and survive outside their native environment are important. Nonetheless, factors in the extracellular milieu that comprise the cellular microenvironment also play key roles in this process. For example, growth factor-activated intracellular signaling regulates cytoskeletal changes required for cell migration (13).
Emerging evidence supports a pivotal role in promoting colon cancer for growth factors that stimulate muscarinic receptor activation. Muscarinic receptor expression, particularly of the M3 subtype (CHRM3), is increased in colon cancer (43) and in mice treated with azoxymethane, an intestine-selective carcinogen, genetic ablation of Chrm3 attenuates both the number and size of colon tumors (25). In the course of studying the role of muscarinic ligands and receptors in promoting colon cancer, we observed that acetylcholine (ACh) stimulates anchorage-independent growth of H508 human colon cancer cells, a cell line that robustly expresses muscarinic receptors (41). Moreover, previous studies in nonintestinal tissues and Chinese hamster ovary (CHO) cells transfected with human muscarinic receptors indicated that ACh stimulates myosin-containing stress fiber formation (14, 38, 40, 45). In addition to their role as growth factors that promote cell proliferation (34), these observations suggested to us that muscarinic receptor ligands may also stimulate colon cancer cell migration and invasion.
In the gut, it is likely that ACh, the prototypical muscarinic receptor ligand, derives primarily from enteric neurons. Nonetheless, some colon cancer cell lines, including H508 cells derived from a moderately differentiated cecal adenocarcinoma, express choline acetyltransferase and other enzymes that confer the ability to produce and release ACh (6). Thus ACh can act as a neurocrine, paracrine, and autocrine growth factor (6). Cholinergic agonists stimulate colon cancer cell proliferation by a mechanism involving activation of matrix metalloproteinases (MMP) with subsequent release of ligands that activate plasma-membrane bound receptor tyrosine kinases: the epidermal growth factor (EGF) receptor (ERBB) family (7, 8, 41). Ligand binding to ERBB tyrosine kinases, primarily ERBB1 (EGFR), activates postreceptor signaling cascades that regulate cell proliferation and survival (7, 8, 41). In H508 colon cancer cells, both ACh-induced cell proliferation and anchorage-independent growth require activation of matrix metalloproteinase-7 (MMP7), release of an ERBB ligand, heparin binding epidermal growth factor-like growth factor (HBEGF), and stimulation of ERBB1 signaling (7, 41).
On the basis of these collective observations, we hypothesized that muscarinic receptor ligands, important growth factors that promote intestinal neoplasia, also stimulate colon cancer cell migration and invasion. To test this hypothesis and investigate cellular mechanisms that mediate muscarinic receptor ligand-induced colon cancer cell migration, we studied the effects of ACh in a cell culture “wound closure” model and in a Matrigel invasion chamber. In these cell models, robust ACh-induced actions on human colon cancer cells were blocked by atropine. Moreover, the studies described herein provide evidence that muscarinic receptor ligands stimulate migration of human colon cancer cells by mechanisms downstream of ERBB1 activation that are both extracellular signal-related kinase (ERK) and phosphatidylinositol 3-kinase (PI3K) dependent. Post-ERBB1 ERK and PI3K signaling induces RhoA activation, thereby stimulating myosin reorganization and cell migration. Some data presented here were published previously in abstract form (4).
MATERIALS AND METHODS
Materials.
Materials used were purchased as follows: PD168393, AG1478, PD98059, wortmannin, GM6001, NC-GM6001, GSK-3 inhibitor IX, Y27632, and exoenzyme C3 (ExoC3) from Calbiochem (San Diego, CA); U0126 and LY294002 from Cell Signaling; recombinant human MMP7, HBEGF, anti-ERBB1 neutralizing antibody clone LA-1 from Millipore; and neutralizing anti-MMP7 antibody from R&D Systems (Minneapolis, MN). Atropine, ACh, carbamylcholine (carbachol), and mitomycin C were from Sigma. Rhodamine-phalloidin, Antifade Mounting Medium, and RhoA G-Lisa activation kit were from Cytoskeleton (Denver, CO). Antibodies to total and phospho-AKT (p-AKT), total and phospho-p44/42 mitogen-activated protein kinase (ERK1/2), and RhoA were from Cell Signaling (Boston, MA). Anti-HBEGF antibody was from Fisher (Waltham, MA). Rabbit IgG, horseradish peroxidase (HRP)-linked antibody (from donkey), and mouse IgG, HRP-linked antibody (from sheep) were obtained from GE Health Care Biosciences (Buckinghamshire, UK).
Cell culture.
Human colon cancer cell lines (H508, SNU-C4) were grown in RPMI 1640 supplemented with 10% fetal bovine serum. HT29 cells were grown in McCoy's 5A medium supplement with 10% fetal bovine serum. Adherent cultures were passaged weekly at subconfluence after trypsinization. Cultures were maintained in incubators at 37°C in an atmosphere of 5% CO2 and 95% air.
Cell migration assay.
Cell migration was measured by using a validated monolayer wound closure assay with minor modifications (2, 20). Briefly, human H508 colon cancer cells that robustly express both ERBB1 and CHRM3 (8) were grown as confluent monolayers in six-well plates in RPMI 1640 supplemented with 10% fetal bovine serum. Cell monolayers were wounded by scraping with a disposable 200-μl pipette tip, washed twice with fresh serum-free medium, and incubated in serum-free medium in the absence or presence of test agents. The rate of cell migration (movement of cells at the anterior edges of the wounded monolayer) was determined from serial photographs taken at indicated times after wounding with an Axiovert 200 microscope (Carl Zeiss) (total magnification, ×100) by an investigator masked to treatment conditions. Identical regions were examined at each time point by premarking the base of the plates to facilitate alignment. Twelve measurements were performed per field by placing a transparent grid over the photograph and measuring the distance that cells in the leading edge moved from the original wound line. Results were expressed as means ± SE of distance migrated (arbitrary units). When chemical inhibitors or antibodies were used, these were incubated with cells for 30 min before wounding. To inhibit cell proliferation, a potential confounding variable, in all wounding assays cells were preincubated for 1 h with mitomycin C (10 μg/ml), a concentration that inhibited cell proliferation. Incubation with EGF and serum-free media were used as positive and negative controls, respectively.
Cell invasion assay.
Cell invasion assays were performed using BD Biocoat Matrigel Invasion Chambers (BD Biosciences, Bedford, MA) following the manufacturer's recommendations. Briefly, HT29 human colon cancer cells were trypsinized and resuspended in serum-free McCoy's 5A medium and placed in the upper chamber of Transwell inserts (5 × 104 cells/well) and treated with indicated agents or vehicle (control). McCoy's 5A medium containing 10% FBS was placed in the lower chamber. Cells were incubated for 24 h in a humidified atmosphere with 95% air and 5% CO2 at 37°C. Invasive cells were fixed and stained with Hema 3 stain (Fisher). Noninvasive cells in the upper chamber were removed by wiping with a cotton swab. Cells on the lower surface of the insert that had penetrated Matrigel were counted in five randomly selected high-power fields via a light microscope. Each experiment was performed in triplicate. Cell migration assays using BD Biocoat Matrigel Invasion Chambers were also performed with HT29 cells, but without the Matrigel inserts.
Rhodamine phalloidin staining of the actin cytoskeleton.
Serum-starved H508 colon cancer cells, 60% confluent, were incubated for 8 h with serum-free media or ACh (100 μM), washed once with PBS, and incubated in 4% formaldehyde in PBS for 10 min. Cells were washed with PBS for 30 s, permeabilized with 0.5% Triton X-100 in PBS for 5 min, and washed with PBS for 30 s (all at room temperature). Cells were incubated in the dark with rhodamine-phalloidin (14 mM in methanol) for 30 min at room temperature. Cells were washed three times in PBS and allowed to set in Antifade Mounting Medium in the dark for 1 h before photography with fluorescence microscopy at 535 nm excitation, 585 nm emission (Axiovert 200 microscope, Carl Zeiss) at ×400 total magnification with water immersion.
q-PCR.
Cells were subcultured in six-well plates at 106 cells per well. Total cellular RNA was isolated from cells using TRIzol reagent (Invitrogen). First-strand cDNAs were synthesized by use of the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen). ERBB primers for quantitative real-time PCR (q-PCR) were designed by using the National Center of Biotechnology Information nucleotide database, SIM-4 gene alignment program, and online software (www.Genscript.com/ssl-bin/app/primer); CHRM3 primers were designed by Drs. Erica Rosemond and Jürgen Wess (National Institute of Diabetes and Digestive and Kidney Diseases) (33) (Table 1). q-PCR was performed by using the 7900 HT Fast System (ABI) with Power SYBR Green Master Mix (ABI) and 20 ng primer, and cDNA was synthesized from 50 ng total RNA. PCR conditions included 5 min at 95°C followed by 35 cycles of 95°C for 15 s, 60°C for 20 s, and 72°C for 40 s and a final cycle at 95°C for 15 s, 60°C for 15 s, and 95°C for 15 s. q-PCR data were analyzed by using ABI instrument software SDS 2.1.
Table 1.
Primers for qPCR of ERBB and CHRM3 mRNA and predicted amplicon sizes
| Primer Sequences(5′-3′) |
|||
|---|---|---|---|
| Genes | Forward | Reverse | Size, bp |
| ERBB1 (EGFR) | TTCTTGCAGCGATACAGCTC | GGGAACGGACTGGTTTATGT | 102 |
| ERBB2 | TGGTCAAATGTTGGATGATTG | CATGCGGGAGAATTCAGAC | 74 |
| ERBB3 | CCAGTTGGAACACTTAATCGG | TTCACTGCTCCCAGAAACTG | 129 |
| ERBB4 | CTATGCGAGACAAACCCAAA | GGATGCATTGTGATATTCGG | 119 |
| CHRM3 | CGAGACGAGAGCCATCTACTCC | GACCAGGGACATCCTTTTCCGC | 70 |
| GAPDH | CCCCATGGTGTCTGAGCG | CGACAGTCAGCCGCATCTT | 67 |
Immunoblotting.
After the treatments described, cells were rinsed with PBS and lysed in 50 mM Tris·HCl, 150 mM NaCl, 1% Nonidet-P40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate; pH 8.0 (RIPA; Millipore, Temecula, CA) with dithiothreitol (Promega, Madison, WI) and Protease Inhibitor Cocktail (Roche). Cell lysates were cleared by centrifugation and protein estimated by the BCA method (Pierce, Rockford, IL). Cell lysates (40 μg) were separated by 4–12% SDS-PAGE, transferred to nitrocellulose membranes and probed with antibodies to ERBB subtypes, phospho-ERK (p44/42 MAPK), phospho-AKT and RhoA as indicated, and incubated overnight at 4°C. After washing six times for 5 min each, membranes were incubated for 1 h at room temperature with the corresponding HRP-conjugated secondary antibody, and signal intensities of target proteins were detected by use of the enhanced chemiluminescence system (Supersignal kit, Pierce). To verify equal protein loading, blots were stripped and reprobed with antibody to β-actin. Signal strength was evaluated by densitometry, and ERK and AKT activation (phosphorylation) were calculated relative to the strength of the signal for total protein under the same conditions (e.g., pERK/total ERK).
Detection of RhoA activation.
Following the treatments described, RhoA activation was determined by using an ELISA-based assay according to the manufacturer's instructions (G-LISA kit, Cytoskeleton, Denver, CO) (19). Briefly, growth medium was removed when cells reached 50% confluence, and cells were serum starved for 24 h before addition of ACh, alone or 30 min following preincubation with inhibitors. After incubation for the indicated times, cells were washed with PBS and lysates were added to 96-well plates containing Rho-GTP-binding protein. After washing, RhoA activation was measured in triplicate by absorbance at 490 nm (Spectramax Plus384, Molecular Devices, Sunnyvale, CA). For detection of RhoA activation by this assay, DMSO could not be used to dissolve water-insoluble test agents because this solvent interfered with the assay (data not shown). Instead, water-insoluble agents were dissolved in 0.5% methanol, which did not interfere with the assay.
Statistical analysis.
All immunoblots shown are representative of at least three independent experiments. All graphs show mean ± SE of at least three independent experiments. Statistical analysis of cell migration data between the control and treated groups was performed by ANOVA (GraphPad, Instat 3.0, La Jolla, CA) with posttests (Tukey's test). For the RhoA activation assay, the unpaired t-test was used. Statistical significance is given by the number of asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). P < 0.05 was considered statistically significant.
RESULTS
ACh stimulates human colon cancer cell migration in a time- and concentration-dependent fashion.
Previous studies in H508 colon cancer cells showed that interaction of muscarinic ligands with CHRM3 results in activation of ERBB1 with consequent stimulation of cell proliferation (8). To explore the role of ACh in stimulating H508 colon cancer cell migration, we used a cell culture wound closure model. To a confluent monolayer of H508 cells in serum-free medium containing either ACh (100 μM), carbachol (100 μM), EGF (10 μg/ml), or vehicle (phosphate-buffered saline), a linear wound was made by use of a micropipette tip. To exclude confounding effects of ligand-induced cell proliferation, we blocked proliferation by adding mitomycin C (10 μg/ml) to the incubation solution 1 h before wounding and test agent treatment. No differences in ligand-induced cell migration were observed when comparing experiments performed with and without adding mitomycin C (data not shown). After wounding, progressive movement of cells at the edge of the wound was documented by photography at 0, 4, 8, and 24 h. Cell movement was measured from these photographs as described in materials and methods.
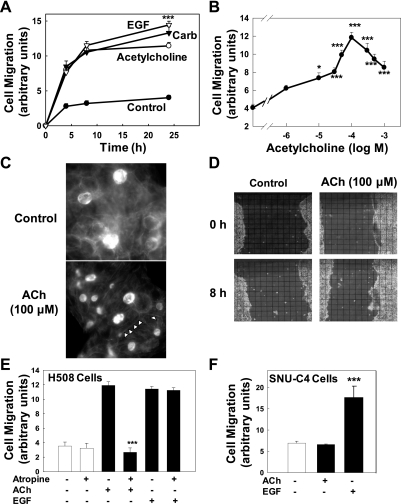
Figure 1A shows the time course of H508 colon cancer cell migration. During the first 8 h, ACh and carbachol stimulated a threefold increase in cell migration compared with vehicle (control); the effects of ACh and carbachol were nearly indistinguishable from those of EGF (Fig. 1A). At 24 h, EGF-induced cell migration was modestly, but significantly (P < 0.001), greater than that observed with ACh, whereas the effects of carbachol and ACh at this time point were not significantly different (Fig. 1A). On the basis of this time course, and particularly the observation that ACh-induced cell migration had reached a plateau at 8 h, we used 8-h incubations for subsequent experiments. Figure 1B shows the dose-response curve for ACh-induced H508 cell migration. Compared with control, a significant increase in cell migration was first detected with 10 μM ACh and was maximal with 100 μM ACh. Cell migration with concentrations of ACh greater than 100 μM was submaximal but remained significantly greater than control (Fig. 1B). This dose-response curve is similar in terms of potency to that observed for ACh-induced H508 cell proliferation (8). Reduced biological effects with supramaximal concentrations of ACh have been observed by us and others when exploring the effects of muscarinic signaling in a variety of cell systems, including H508 colon cancer cell proliferation, pancreatic amylase release, and gastric pepsinogen secretion. It is likely that this is caused by activation of counterstimulatory mechanisms by supramaximal doses of muscarinic receptor ligands; these mechanisms may include activation of protein phosphatases.
Fig. 1.
Acetylcholine (ACh) stimulates colon cancer cell migration in a time- and concentration-dependent manner. Human colon cancer cells were plated at confluence before a linear “wound” was made. As described in materials and methods, photomicrographs were taken immediately after wounding, before test agents were added, and again as described below. A: time course for effects of ACh (100 μM), epidermal growth factor (EGF; 10 μg/ml), and carbamylcholine (Carb; 100 μM) on H508 colon cancer cell migration. Cell migration was measured at the time points indicated. B: dose-response curve for the effects of ACh on H508 cell migration. Plates were incubated with the indicated concentrations of ACh and cell migration was measured at 8 h. The data point on the vertical axis represents cell migration without ACh. C: representative photographs showing that incubation with ACh (100 μM for 8 h) induces stress fiber formation in H508 cells. Arrowheads in bottom image indicate representative stress fibers in ACh-treated cells. D: representative photographs showing cell culture wounding assay immediately after scraping with pipette tip (0 h) and 8 h after incubation with ACh (100 μM) or vehicle (control). The overlying grid used to measure cell migration is shown. E: preincubation with atropine (1 μM) blocked ACh (100 μM)-induced but not EGF (10 μg/ml)-induced H508 cell migration. Cell migration was measured 8 h after addition of test agents. F: in SNU-C4 human colon cancers that do not express muscarinic receptors, cell migration, measured 8 h after addition of test agents, was stimulated by EGF (10 μg/ml) but not ACh (100 μM). Values in A, B, D, and E are means ± SE from at least 3 separate experiments. *, ***P < 0.05 and 0.001, respectively, compared with control (treatment with vehicle alone).
ACh stimulates actin stress fiber formation in H508 cells.
Cell migration involves formation of actin stress fibers that mediate cell contraction (22). Previous work by others using different tissues and cell types indicates that ACh stimulates stress fiber formation (14, 38, 40, 45). We determined whether ACh stimulated actin stress fiber formation in H508 cells. As anticipated, incubation of H508 cells with 100 μM ACh for 8-h stimulated formation of actin stress fibers (Fig. 1C). These findings confirm that ACh induces changes in H508 cell morphology that are necessary for cell contraction and movement (22). Figure 1D shows representative photographs of H508 cell culture plates immediately after linear wounding with a micropipette tip (0 h) and 8 h after incubation with water (control) or 100 μM ACh.
ACh-induced cell migration is dependent on activation and expression of muscarinic receptors.
We used two independent experimental approaches to confirm that the actions of ACh on colon cancer cell migration were mediated by interaction with muscarinic receptors. First, we examined the actions of preincubating cells with a nonselective muscarinic receptor inhibitor, atropine. As shown in Fig. 1E, preincubation with atropine completely blocked the actions of ACh but did not alter cell migration observed with EGF, an ERBB ligand. Second, we examined the actions of ACh and EGF on a human colon cancer cell line, SNU-C4, that expresses ERBB but not muscarinic receptors (8). As shown in Fig. 1F, whereas EGF caused a threefold increase in SNU-C4 colon cancer cell migration, ACh had no effect. These findings provide confirmatory evidence that the actions of ACh on H508 colon cancer cell migration are mediated by functional interaction with muscarinic receptors.
ACh-induced cell migration is dependent on activation of post-ERBB signaling.
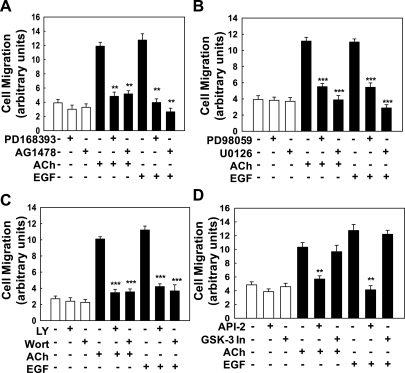
Muscarinic agonist-induced H508 cell proliferation is mediated by cross talk that results in activation of ERBB receptors (8, 35). To determine whether ACh-induced H508 cell migration was mediated by similar mechanisms, we examined the effects of inhibiting ERBB1 activation and that of two key post-ERBB signaling pathways, the ERK and PI3K cascades. Preincubating H508 cells with two ERBB1-selective tyrosine kinase inhibitors, PD168393 and AG1478, abolished both ACh- and EGF-induced cell migration (Fig. 2A). These findings indicate that ACh-induced H508 cell migration is dependent on ERBB1 activation, either as a homo- or heterodimer. To determine the role of post-ERBB signaling via the ERK and PI3K cascades in mediating ACh-induced H508 cell migration, cells were preincubated with two ERK inhibitors (PD98059 and U0126) and two PI3K inhibitors (LY294002 and wortmannin). As shown in Fig. 2, B and C, ACh-induced cell migration was markedly attenuated or abolished by preincubation with either ERK or PI3K inhibitors. Because H508 cell proliferation is ERK, but not PI3K, dependent (5, 8, 41), the actions of PI3K inhibitors on cell migration were unanticipated. To evaluate further the role of PI3K signaling, we examined the downstream roles of AKT and GSK. As shown in Fig. 2D, whereas the AKT inhibitor API-2 also attenuated ACh- and EGF-induced cell migration, a GSK-3 inhibitor (GSK-3 inhibitor IX) did not alter migration induced by either ligand. These findings indicate that both ERK and PI3K signaling mediate the actions of ACh and EGF on H508 colon cancer cell migration. AKT activation, downstream of PI3K, also appears important. However, GSK, a downstream target of AKT, does not play a role in either ACh- or EGF-induced colon cancer cell migration.
Fig. 2.
ACh-induced colon cancer cell migration is dependent on ERBB1 activation and post-ERBB1 signaling. Human colon cancer cells were plated at confluence before a linear wound was made. As described in materials and methods, photomicrographs were taken immediately after a linear wound was made and again 8 h after addition of test agents. A: ACh (100 μM)- and EGF (10 μg/ml)-induced migration of H508 colon cancer cells was attenuated by adding ERBB1 activation inhibitors (PD168393, AG1478; both 10 μM). B: ACh (100 μM)- and EGF (10 μg/ml)-induced migration of H508 colon cancer cells was attenuated by adding ERK activation inhibitors (PD98059 and U0126; both 10 μM). C: ACh (100 μM)- and EGF (10 μg/ml)-induced migration of H508 colon cancer cells was attenuated by adding phosphatidylinositol 3-kinase (PI3K) activation inhibitors [10 μM LY294002 (LY) and 50 nM wortmannin (Wort)]. D: ACh (100 μM)- and EGF (10 μg/ml)-induced migration of H508 colon cancer cells was attenuated by adding an inhibitor of AKT activation (5 μM API-2) but not by adding a GSK-3 inhibitor (50 nM GSK-3 inhibitor IX). Values are means ± SE from at least 3 separate experiments. **, ***P < 0.01 and 0.001, respectively, compared with control (treatment with vehicle alone).
MMP7 activation and release of the ERBB ligand HBEGF mediate ACh-induced colon cancer cell migration.
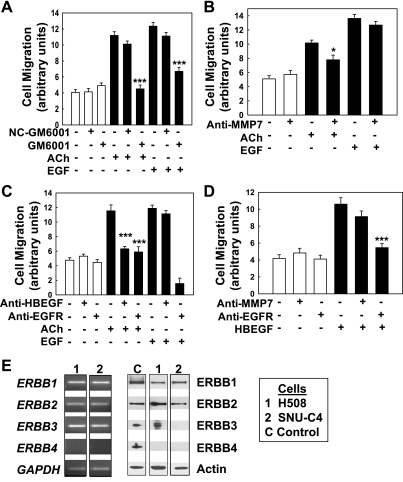
Our previous work indicates that muscarinic ligand-induced H508 cell proliferation is mediated by activation of MMP7. Activated MMP7 cleaves pro-HBEGF, which is expressed on the cell membrane of H508 cells, thereby releasing HBEGF, an ERBB ligand (5, 7, 41). To determine whether H508 cell migration is mediated by a similar mechanism, we first examined the effects on ACh- and EGF-induced cell migration of adding GM6001, a nonselective MMP inhibitor. As shown in Fig. 3A, ACh-induced cell migration was abolished by GM6001, but by not an inert control agent, NC-GM6001. Surprisingly, EGF-induced cell migration was also attenuated by the MMP inhibitor (Fig. 3A). This observation was unanticipated because the actions of EGF do not require MMP-mediated release of another ERBB ligand (15) and GM6001 does not block EGF-induced H508 cell proliferation (7). A likely explanation is that other MMPs blocked by GM6001 play key roles in cell migration, perhaps by degrading extracellular matrix.
Fig. 3.
ACh-induced colon cancer cell migration is mediated by activation of matrix metalloproteinases and release of an ERBB ligand. Human colon cancer cells were plated at confluence before a linear wound was made. As described in materials and methods, photomicrographs were taken immediately after a linear wound was made and again 8 h after adding test agents. A: a broad-spectrum matrix metalloproteinase inhibitor (10 μM GM6001) abolished ACh-induced H508 cell migration. GM6001 also attenuated EGF-induced cell migration. In contrast, a structurally similar control agent (10 μM NC-GM6001) did not alter ACh- or EGF-induced cell migration. B: anti-MMP7 antibody (5 μg/ml) attenuated the actions of ACh but did not alter EGF-induced cell migration. C: anti-HBEGF (1 μg/ml) and anti-ERBB1 antibodies attenuated ACh-induced cell migration. Whereas anti-ERBB1 antibody blocked the actions of EGF, anti-HBEGF antibody did not alter EGF-induced cell migration. D: recombinant HBEGF (20 ng/ml) stimulated H508 cell migration. These actions were blocked by anti-EGFR antibody (0.02 μg/ml) but not by anti-MMP7 antibody (5 μg/ml). E: expression of ERBB subtypes in human colon cancer cells. Left: quantitative real-time PCR (q-PCR) demonstrates expression of mRNA for ERBB subtypes in H508 and SNU-C4 human colon cancer cells. Right: immunoblots demonstrate expression of protein for ERBB subtypes in lysates of H508 and SNU-C4 cells. Values in A–D are means ± SE from at least 3 separate experiments. *, ***P < 0.05 and 0.001, respectively, compared with control (treatment with vehicle alone).
To determine more specifically the role of MMP7 in mediating ACh-induced H508 cell migration, we used neutralizing anti-MMP7 antibody. As anticipated, anti-MMP7 antibody attenuated the actions of ACh but not those of EGF (Fig. 3B). Anti-HBEGF antibody also abolished ACh-induced, but not EGF-induced, cell migration (Fig. 3C), whereas treatment with anti-ERBB1 antibody inhibited both ACh- and EGF-induced cell migration (Fig. 3C). These observations are consistent with MMP7-mediated release of HBEGF and functional interaction of the ERBB ligand with ERBB1. To confirm that HBEGF can stimulate H508 cell migration, we examined the actions of the ERBB ligand alone and in the presence of anti-MMP7 and anti-ERBB1 antibodies. As shown in Fig. 3D, HBEGF stimulated a robust increase in H508 cell migration that was of similar magnitude to that observed with ACh and EGF (Fig. 3C). As anticipated, adding anti-MMP7 antibody did not alter HBEGF-induced cell migration. In contrast, HBEGF-induced cell migration was blocked by adding anti-ERBB1 antibody (Fig. 3D). Collectively, these observations support the hypothesis that ACh-induced cell migration is mediated by activation of MMP7 and release of the ERBB ligand HBEGF. ERBB1 chemical inhibitors and neutralizing anti-ERBB1 antibody block activation of either homo- or heterodimers comprising ERBB1. To compare expression patterns for ERBB subtypes in H508 and other commonly studied human colon cancer cells we used q-PCR and immunoblotting.
Pattern of ERBB gene and protein expression in human colon cancer cells.
The ERBB family consists of four structurally related members: ERBB1 (EGFR, HER1), ERBB2 (HER2, NEU), ERBB3 (HER3), and ERBB4 (HER4). Functional activity of ERBB subtypes requires formation of either homo- or heterodimers before or after ligand binding (44). HBEGF is a ligand for ERBB1 homodimers, ERBB4 homodimers, ERBB1/ERBB2 heterodimers, and ERBB2/ERBB4 heterodimers (44). To determine the pattern of ERBB receptor expression in the human colon cancer cell lines used in these studies, we measured mRNA (Fig. 3E, left) and protein (Fig. 3E, right) expression for ERBB family members. Primers for q-PCR of ERBB genes and predicted amplicon sizes are shown in Table 1.
As shown in Fig. 3E (left), mRNA for ERBB1, ERBB2, and ERBB3 was expressed in both H508 and SNU-C4 colon cancer cells. In contrast, signal for ERBB4 was not detected in either cell line. In terms of ERBB protein expression, both ERBB1 and ERBB2 were expressed in H508 and SNU-C4 cells (Fig. 3E, right). ERBB3 protein expression was not observed in SNU-C4 cells and neither colon cancer cell line expressed protein for ERBB4. In H508 cells, these findings exclude ERBB4 as an HBEGF target receptor. Moreover, on the basis of these findings, it appears that HBEGF can stimulate migration of H508 and SNU-C4 cells (Fig. 1E) by binding either to ERBB1 homodimers, to ERBB1/ERBB2 heterodimers, or to a mixture of both types of ERBB receptors. In either case, these findings provide additional evidence that ERBB1 plays a key role in mediating the actions of muscarinic receptor ligands.
ACh stimulates ERBB-dependent activation of both ERK and AKT.
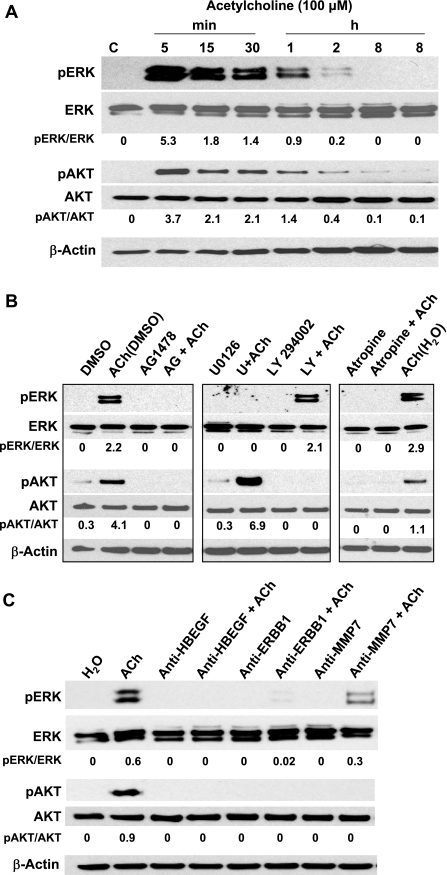
Results shown in Figs. 2 and 3 implicate post-ERBB1 signaling by both ERK and PI3K signaling in ACh-induced colon cancer cell migration. We confirmed that ACh activates these signaling cascades in H508 colon cancer cells. After treating cells with ACh alone or in combination with selective inhibitors, we immunoblotted cell extracts for phosphorylated (activated) ERK and AKT; the latter is a key signaling molecule downstream of PI3K. As shown in Fig. 4A, ACh stimulated time-dependent increases in phosphorylation of both ERK and AKT. ACh-induced phosphorylation of ERK and AKT peaked within 5 min and returned to baseline by 2 h (Fig. 4A). Expression of unphosphorylated ERK and AKT did not change during the course of the 8-h incubation (Fig. 4A). β-Actin was used as a loading control.
Fig. 4.
In H508 colon cancer cells, ACh activates ERBB1-dependent ERK and PI3K signaling. ERK and AKT activation (phosphorylation) was determined by immunoblotting proteins from H508 cell lysates as described in materials and methods. A: representative immunoblot showing that ACh (100 μM) stimulates time-dependent ERK and AKT phosphorylation (pERK and pAKT) but no change in expression of total ERK and AKT. Densitometry was used to compare signals for phosphorylated (activated) compared with total proteins. B: representative immunoblot exploring upstream signaling pathways that mediate ACh-induced AKT and ERK activation. ACh-induced phosphorylation of AKT is attenuated by inhibitors of ERBB1 (10 μM AG1478) and PI3K (10 μM LY294002), but not by an ERK inhibitor (10 μM U0126). ACh-induced phosphorylation of ERK is attenuated by ERBB1 and ERK inhibitors, but not by a PI3K inhibitor. ACh-induced activation of both AKT and ERK was blocked by atropine (5 μM), a muscarinic receptor inhibitor. DMSO (0.5%), a solvent for U0126 and LY294002, increased ACh-induced AKT phosphorylation but did not alter ACh-induced ERK phosphorylation. C: representative immunoblot showing that in H508 cells, ACh-induced ERK and AKT activation (phosphorylation) was abolished or attenuated by anti-HBEGF, anti-ERBB1 and anti-MMP7 antibodies. For each condition, the ratio of signal intensity for activated ERK and AKT, compared with that for total ERK and AKT, respectively, was calculated. β-Actin was used as a loading control. All immunoblots are representative of at least 3 separate experiments. C, control (treatment with vehicle alone).
ACh-induced AKT phosphorylation was attenuated by ERBB1 and PI3K inhibitors (AG1478 and LY294002, respectively), but not by U0126, an inhibitor of MEK, the kinase immediately upstream of ERK (Fig. 4B). ACh-induced ERK activation was attenuated by preincubation of H508 cells with an ERBB1 inhibitor (AG1478) and by an inhibitor of ERK signaling (U0126), but not by a PI3K inhibitor (LY294002) (Fig. 4B). In fact, incubation of ACh plus U0126 resulted in increased phosphorylation of AKT (Fig. 4B). This finding provides evidence for cross talk between ERK and AKT signaling in these colon cancer cells. As anticipated, ACh-induced activation of both AKT and ERK was blocked by atropine, a nonselective muscarinic receptor inhibitor (Fig. 4B). As shown in Fig. 4C, ACh-induced activation of ERK was abolished by preincubation with anti-HBEGF and -ERBB1 antibodies and strongly attenuated (50% decrease in signal intensity) by preincubation with anti-MMP7 antibodies. ACh-induced activation of AKT was abolished by preincubation with anti-HBEGF, -ERBB1, and -MMP7 antibodies. We cannot explain why anti-MMP7 antibodies were more effective at blocking AKT compared with ERK activation. Collectively, these findings are consistent with ACh-induced activation of both ERK and PI3K signaling downstream of ERBB1 activation and confirm the selectivity of the chemical inhibitors used in the cell migration studies.
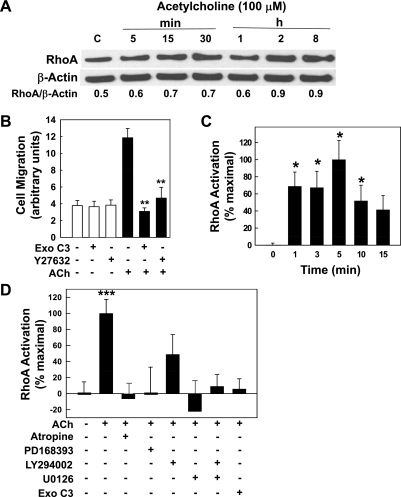
Effects of Rho and ROCK activation on ACh-induced cell migration.
In CHO cells, Strassheim et al. (38) reported that activation of transfected human M3 muscarinic receptors induces myosin light chain (MLC) phosphorylation and the formation of myosin-containing stress fibers. Members of the Rho family of GTPases play a major role in regulating cell morphology and reorganizing the cell cytoskeleton in response to external stimuli such as muscarinic receptor agonists (28, 40). In particular, RhoA plays a key role in muscarinic receptor agonist-induced formation of actin stress fibers and focal adhesions (16, 27, 29, 38). ROCK 1 and 2, downstream targets of RhoA, phosphorylate (activate) MLC and inactivate MLC-phosphatase, thereby increasing actinomyosin assembly.
In H508 cells, we used immunoblotting to examine RhoA expression, RhoA and ROCK inhibitors to examine their role in mediating cell migration, and a RhoA activation assay to identify key signal transduction pathways (Fig. 5). Compared with basal levels, RhoA expression was not altered over the course of an 8-h incubation with 100 μM ACh (Fig. 5A). These findings indicate that muscarinic receptor activation does not alter expression of this key protein. We used two independent methods to determine whether ACh-induced RhoA activation plays a role in mediating colon cancer cell migration: selective chemical inhibitors of RhoA and ROCK activation, and a RhoA activation assay. Preincubating H508 cells with either ExoC3, a RhoA inhibitor (16), or Y27632, a ROCK inhibitor (16, 39), abolished ACh-induced cell migration (Fig. 5B). We used a RhoA activation assay to confirm that incubation with ACh activates RhoA and to investigate the regulatory role of post-ERBB signaling upstream of RhoA. As shown in Fig. 5C, ACh (100 μM) stimulated time-dependent activation of RhoA that was significantly greater than baseline from 1 to 10 min (P < 0.05). Fifteen minutes following addition of ACh, RhoA activation was reduced to near baseline values (Fig. 5C; P = 0.07 for ACh-stimulated RhoA activation at 15 compared with 0 min). To confirm the importance of this rapid pulse of ACh-induced RhoA activation, we compared the effects of adding the RhoA activation inhibitor (ExoC3) before or after ACh. Adding ExoC3 30 min before incubation with ACh blocked colon cancer cell migration (Fig. 5B) whereas adding ExoC3 15 min after ACh did not block ACh-induced cell migration (not shown). These findings are consistent with the time course shown in Fig. 5C. On the basis of this time course, we used 5-min incubations with ACh to examine effects of inhibiting postreceptor signaling (Fig. 5D).
Fig. 5.
Actions of ACh on expression and activation of RhoA and ROCK. A: in H508 colon cancer cells, incubation with 100 μM ACh for up to 8 h did not alter expression of RhoA. RhoA expression was determined by immunoblotting proteins from H508 cell lysates after incubation for the times indicated. For each time point, the ratio of signal intensity for RhoA compared with that for β-actin (loading control) was calculated. Immunoblots shown are representative of 3 others. B: chemical inhibitors of RhoA [exoenzyme C3 (ExoC3), 5 μg/ml] and ROCK (Y27632, 75 μM) abolished ACh-induced H508 cell migration. After preincubation with inhibitors, cells were plated at confluence before a linear wound was made. Cells were incubated with or without ACh (100 μM) for an additional 8 h. As described in materials and methods, photomicrographs were taken immediately after a linear wound was made and again 8 h after addition of ACh. **P < 0.01 compared with ACh alone. Cell migration with ACh plus ExoC3 and Y27632 was not significantly different than control migration with vehicle alone. C: time course for ACh-induced activation of RhoA. In H508 cells, RhoA activation was measured using an ELISA-based assay (materials and methods) before (time = 0 min) and at the indicated times after adding ACh (solid bars). Results are expressed as a percentage of the maximal response obtained with 100 μM ACh (5 min). *P < 0.05 compared with basal RhoA activation (0 min). D: ACh-induced RhoA activation was blocked by inhibitors of muscarinic receptor, ERBB1, ERK, and PI3K signaling. After 5 min incubation with test agents, RhoA activation was measured as described in materials and methods. Bar graph shows effects of adding water-soluble (atropine, ExoC3) and methanol-soluble (PD168393, LY294002, U0126) inhibitors to 100 μM ACh. Vehicle (water and 0.5% methanol), atropine (5 μM), and ExoC3 (5 μg/ml) were used as controls. Basal RhoA activation was not altered by PD168393, LY294002, and U0126 (all 10 μM; data not shown) but these inhibitors all attenuated ACh-induced RhoA activation. ***P < 0.001 for 100 μM ACh compared with vehicle alone (either water or 0.5% methanol). Values shown in bar graphs (B–D) represent means ± SE from at least 3 separate experiments.
In experiments shown in Fig. 5D, we used two solvents for test agents: water for water-soluble inhibitors (atropine, ExoC3) and 0.5% methanol for water-insoluble agents (PD168393, LY294002, U0126). Controls experiments (not shown) confirmed that, compared with water, methanol did not alter basal or ACh (100 μM)-induced RhoA activation (not shown). In contrast, DMSO alone strongly increased basal RhoA activation; this prevented us from testing MMP7 inhibitors that were not soluble in water or methanol. In the absence of ACh, no inhibitor tested in the experiments shown in Fig. 5D altered basal RhoA activation (not shown). As anticipated, muscarinic receptor and RhoA inhibitors (atropine and exoenzyme C3, respectively) abolished ACh-induced RhoA activation (Fig. 5D). Likewise, as anticipated from experiments depicted in Figs. 2 and 3 showing that activation of ERBB1 and downstream ERK and PI3K cascades play key roles in mediating ACh-induced cell migration, inhibitors of ERBB1 (PD168393), ERK (U0126), and PI3K (LY294002) signaling attenuated RhoA activation (Fig. 5D). Because the PI3K inhibitor (LY294002) caused only 50% inhibition of RhoA activation (Fig. 5D), we concluded that this degree of RhoA inhibition was sufficient to block cell migration. Blocking both ERK and PI3K signaling with a combination of U0126 plus LY294002 abolished ACh-induced RhoA activation.
ACh stimulates human colon cancer cell invasion.
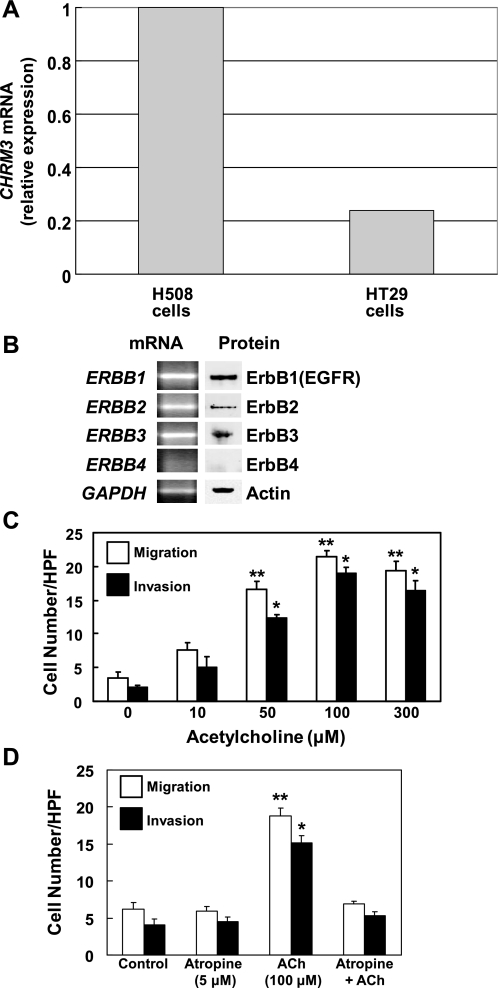
Wound healing (cell migration) and cell invasion may result from different regulatory circuits. Hence, we examined the actions of ACh on human colon cancer cells in a Matrigel invasion assay. Because commercially available invasion chambers consist of a Matrigel-coated insert with an 8-μm pore size, we were unable to conduct these experiments using H508 cells. Because H508 cells have a large diameter and multilobar nuclei they cannot pass through 8-μm pores (6). As an alternative, we used a smaller diameter commonly used human colon cancer cell line, HT29 cells (35). First, we used q-PCR to confirm that HT29 cells express CHRM3 and compared this to CHRM3 mRNA expression in H508 cells. Primers for q-PCR of CHRM3 and predicted amplicon size are shown in Table 1. As shown in Fig. 6A, CHRM3 mRNA is expressed in HT29 cells but relative expression is ∼20% of that in H508 cells. These findings are consistent with previous observations that H508 cells express higher levels of CHRM3 mRNA than other human colon cancers cells, although CHRM3 expression in HT29 cells was not examined in that study (12). We were unable to measure expression of CHRM3 protein because specific antibodies for the M3 muscarinic receptor subtype are not available (18).
Fig. 6.
Actions of ACh on HT29 human colon cancer cell migration and invasion. A: relative expression of CHRM3 mRNA in HT29 and H508 colon cancer cells. B: expression of ERBB subtypes in HT29 cells. Left: q-PCR demonstrates expression of mRNA for ERBB subtypes. Right: immunoblots demonstrate expression of protein for ERBB subtypes in HT29 cell lysates. C: ACh stimulates dose-dependent increases in HT29 cell migration and invasion. Cell migration and invasion assays were performed by using BD Biocoat Invasion Chambers without or with the Matrigel insert, respectively. HPF, high-powered field. D: HT29 cell migration and invasion stimulated by 100 μM ACh is blocked by preincubation with 5 μM atropine. Values shown in bar graphs (C and D) represent means ± SE from at least 3 separate experiments. *, **P < 0.05 and 0.01, respectively, compared with control (treatment with vehicle alone). q-PCR primer pairs for experiments in A and B are shown in Table 1.
We also used q-PCR to confirm that HT29 cells express members of the ERBB family. The data shown in Fig. 6B indicate that the same members of the ERBB family are expressed in HT29 cells as observed in H508 cells (Fig. 3E). As shown in Fig. 6B (left), mRNA for ERBB1, ERBB2, and ERBB3 was expressed in HT29 colon cancer cells; ERBB4 signal was not detected. In terms of ERBB protein expression, the results were similar: ERBB1, ERBB2, and ERBB3, but not ERBB4, was expressed in HT29 cells (Fig. 6B, right). Hence, like H508 cells, HT29 cells express CHRM3, ERBB1, and ERBB2.
In Fig. 6C, we show the dose response for ACh-induced HT29 cell migration and invasion. As observed with H508 cells, maximal HT29 cell migration was observed with 100 μM ACh. Moreover, the stoichiometry for ACh-induced HT29 cell invasion was the same as that observed for cell migration; cell invasion was also maximal, approximately fourfold increase compared with basal, with 100 μM ACh (Fig. 6C). The data shown in Fig. 6D confirm that ACh-induced HT29 cell migration and invasion are mediated by activation of muscarinic receptors; both actions were blocked by preincubation with 5 μM atropine.
DISCUSSION
The ability of intestinal epithelial cells to migrate is important for restitution of the integrity of the epithelial lining following mucosal injury. For example, this is necessary to heal intestinal ulcers, thereby preventing progressive injury and translocation of bacteria, chemicals, and other potential toxins from the gut lumen (20, 37). However, an adverse consequence of these attributes is that cell migration and invasion are also critical for colon cancer metastasis; neoplastic epithelial cells must leave the intestine, enter the blood or lymphatic streams, travel to other organs such as the liver, and survive in these foreign extraintestinal milieus (9). Metastatic dissemination of tumor cells is the primary cause of death from colon cancer; disease limited to the colon is readily treated by surgery.
Novel findings described in the present communication reveal that muscarinic receptor ligands robustly stimulate dose- and time-dependent migration of human colon cancer cells that express muscarinic receptors (Fig. 1). Muscarinic ligand-induced cell migration is abolished when muscarinic receptor activation is inhibited by atropine and does not occur in cancer cell lines that do not express muscarinic receptors (i.e., SNU-C4 cells) (Fig. 1). Likewise, in a Matrigel cell invasion assay, ACh stimulates robust, dose-dependent human colon cancer cell invasion that is inhibited by atropine (Fig. 6). Collectively, these findings indicate that muscarinic receptor agonists, particularly those that activate CHRM3, stimulate colon cancer cell migration and invasion, thereby promoting dissemination of tumor cells.
As demonstrated by the use of selective chemical inhibitors and neutralizing antibodies, in H508 human colon cancer cells muscarinic ligand-induced cell migration requires activation of matrix metalloproteinases, specifically MMP7, release of HBEGF, an ERBB ligand, and activation of post-ERBB signaling. Recombinant HBEGF induces cell migration that is of similar magnitude to that observed with ACh and EGF (Fig. 3). HBEGF is capable of activating ERBB1 homodimers and ERBB1/ERBB2 heterodimers (44). In H508 cells, we identified expression of mRNA and protein for both ERBB1 (EGFR) and ERBB2 (Fig. 3). ERBB1 inhibitors used in our experiments (AG1478 and PD168393) and anti-ERBB1 antibody block activation of both ERBB1 homodimer and ERBB1/ERBB2 heterodimer. Hence these experiments cannot distinguish whether HBEGF activates ERBB1 homodimer, ERBB1/ERBB2 heterodimer, or a mixture of both receptor subtypes.
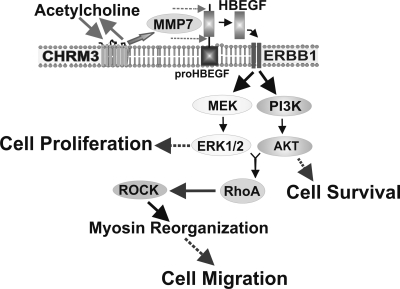
Cell type and species differences may determine the pattern of post-ERBB signaling (24). In H508 human colon cancer cells, post-ERBB signaling involving both ERK and PI3K is required for cell migration; chemical inhibitors or antibodies that block activation of ERBB1, ERK, or PI3K independently attenuated ACh-induced RhoA activation and cell migration (Figs. 2, 5). Our previous work has shown that post-ERBB ERK signaling is a critical regulator of cell proliferation (5, 7, 8). In contrast, PI3K signaling plays only a minor role in stimulating cell proliferation (26). However, PI3K signaling plays a major role in augmenting cell survival; activation of NF-κB, downstream of PI3K, activation is a key downregulator of H508 cell apoptosis (35). These observations indicate that in H508 human colon cancer cells, regulatory pathways for cell migration are different than those for either cell proliferation or survival. Overall, the findings shown in this article are consistent with the model proposed in Fig. 7; ACh-induced colon cancer cell migration is mediated by post-ERBB1, ERK-, and PI3K-dependent RhoA activation.
Fig. 7.
Cartoon illustrating proposed intracellular mechanisms whereby muscarinic receptor activation stimulates human colon cancer cell migration. Muscarinic receptor ligands (e.g., ACh) bind to type 3 muscarinic receptors (CHRM3) on H508 cells, thereby activating matrix metalloproteinase-7 (MMP7) with consequent release of an ERBB receptor ligand (HBEGF). Published work indicates that, downstream of ERBB1 activation, ERK activation stimulates colon cancer cell proliferation and PI3K/AKT activation augments cell survival (5, 7, 8, 26, 35, 41). Novel findings reported in this communication include the observation that post-ERBB1 signaling by both the ERK and PI3K cascades stimulates RhoA activation and colon cancer cell migration.
The importance of post-ERBB1 PI3K signaling for H508 cell migration is consistent with previous observations that EGF-stimulated migration of a murine small intestinal cell line was abolished by a combination of wortmannin and LY294002 (23). In our work, preincubation with either of these two PI3K activation blockers alone was sufficient to abolish both EGF- and ACh-induced H508 cell migration (Fig. 2). Confirmatory evidence for the importance of both ERK and PI3K signaling is provided by the observations that blocking these pathways in H508 colon cancer cells attenuated ACh-induced RhoA activation (Fig. 5). From a clinical translational viewpoint, it may be beneficial therapeutically that blocking either post-ERBB1 signaling pathway, ERK or PI3K, is sufficient to block muscarinic receptor ligand-induced colon cancer cell migration. This is of particular importance for unresectable primary or metastatic colon cancer, where impeding further dissemination of tumor cells may be the only feasible way of prolonging life.
Some cancers possess the capacity for rapid dissemination. For example, at very early stages of development, breast cancer cells can metastasize to the lungs (17) and lung cancer cells can metastasize to the brain (11). In contrast, colon cancer is generally advanced locally before metastasis occurs (9). This observation suggests that colon cancer cells that initially lack competence for metastasis must acquire attributes that facilitate cell migration and colonization. On the basis of previous work showing increased expression of muscarinic receptors in colon cancer (12, 43), ACh-induced anchorage-independent growth of H508 human colon cancer cells (41), and the present results showing that muscarinic receptor ligands stimulate colon cancer cell migration and invasion, we speculate that muscarinic receptor expression and ligand-induced activation of muscarinic receptor signaling are important attributes for metastatic spread of colon cancer.
In summary, our findings indicate that, in addition to attenuating colon cancer cell proliferation (8, 25), blocking muscarinic receptor activation and/or signal transduction reduces the ability of colon cancer cells to migrate and invade, thereby either potentially preventing initiation or extension of metastases. An overall scheme for key steps in the regulation of muscarinic receptor ligand-induced colon cell migration is shown in Fig. 7. Since ERBB receptors are overexpressed in colon cancer and play a key role in many regulatory processes in cancer cells, much clinical investigation has focused on blocking the actions of ERBB ligands. Nonetheless, although the use of monoclonal antibodies and chemical inhibitors that reduce ERBB1 activation was promising in vitro, these agents proved to be only modestly effective in vivo (21, 32). Moreover, even when this approach is efficacious, it is of short duration and limited primarily to tumors that express wild-type KRAS (1).
Our work on muscarinic receptors and ligands in colon cancer identifies several potential molecular targets upstream of ERBB activation (Fig. 7) (5, 7, 8). As demonstrated by our previous work and novel findings in the present communication, these targets include choline acetyltransferase and other molecules involved in ACh production and release (6), CHRM3 (5, 7, 8), matrix metalloproteinases, primarily MMP7 (41), and ERBB proligands and ligands (7). Limitations of targeting these molecules, such as the ubiquity of muscarinic receptor signaling, can be addressed by using agents that do not cross the blood-brain barrier and are otherwise designed to act regionally and selectively on muscarinic receptors expressed in colon cancer cells. Benefits of this approach will likely be limited to colon tumors that overexpress muscarinic receptors, an additional motivation to personalize cancer therapy by indentifying colon tumors that overexpress CHRM3.
The findings described in the present work showing that muscarinic receptor ligands stimulate human colon cancer cell migration by activating post-ERBB1 receptor signaling strengthens the rationale for targeting muscarinic receptors and postreceptor signaling in colon cancer, likely in concert with other antineoplastic therapy. In clinical trials, within 1 year of initial response to anti-ERBB1 therapy, new ERBB1 mutations (3, 30, 31) and other mechanisms (10) increase cancer cell resistance to the actions of these agents (36). Consequently, investigators now focus on multidrug cancer therapies that simultaneously target different portions of key molecules (e.g., ERBB1) or several components of molecular signaling pathways (28). To determine the safety and efficacy of pharmacological inhibitors of muscarinic receptor activation alone or in combination with other treatments, clinical trials in colon cancer appear warranted.
GRANTS
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (J.-P. Raufman) and by National Institutes of Health grants CA-107345 and CA-120407 (J.-P. Raufman). A. Belo was supported by T32 DK-067872 (J.-P. Raufman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26: 1626–1634, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Antonyak MA, Li B, Regan AD, Feng Q, Dusaban SS, Cerione RA. Tissue transglutaminase is an essential participant in the epidermal growth factor-stimulated signaling pathway leading to cancer cell migration and invasion. J Biol Chem 284: 17914–17925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 12: 6494–6501, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Belo A, Cheng K, Shant J, Raufman JP. Acetylcholine-induced colon cancer cell migration is mediated by epidermal growth factor receptor (EGFR)-dependent activation of both ERK and PI3K signaling (Abstract). Gastroenterology 136: S2040, 2009 [Google Scholar]
- 5. Cheng K, Raufman JP. Bile acid-induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol 70: 1035–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cheng K, Samimi R, Xie G, Shant J, Drachenberg C, Wade M, Davis RJ, Nomikos G, Raufman JP. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am J Physiol Gastrointest Liver Physiol 295: G591–G597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol 73: 1001–1012, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng K, Zimniak P, Raufman JP. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res 63: 6744–6750, 2003 [PubMed] [Google Scholar]
- 9. Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med 359: 2814–2823, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Feld R, Rubinstein LV, Weisenberger TH. Sites of recurrence in resected stage I non-small-cell lung cancer: a guide for future studies. J Clin Oncol 2: 1352–1358, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res 5: 2532–2539, 1999 [PubMed] [Google Scholar]
- 13. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 127: 679–695, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gutjahr MC, Rossy J, Niggli V. Role of Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Exp Cell Res 308: 422–438, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 284: 2–13, 2003 [DOI] [PubMed] [Google Scholar]
- 16. He H, Pannequin J, Tantiongco JP, Shulkes A, Baldwin GS. Glycine-extended gastrin stimulates cell proliferation and migration through a Rho- and ROCK-dependent pathway, not a Rac/Cdc42-dependent pathway. Am J Physiol Gastrointest Liver Physiol 289: G478–G488, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell 13: 58–68, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Jositsch G, Papadakis T, Haberberger RV, Wolff M, Wess J, Kummer W. Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 379: 389–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meriane M, Duhamel S, Lejeune L, Galipeau J, Annabi B. Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells 24: 2557–2565, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501–1511, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peeters M, Siena S, Van Cutsem E, Sobrero A, Hendlisz A, Cascinu S, Kalofonos H, Devercelli G, Wolf M, Amado RG. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 115: 1544–1554, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci 120: 3491–3499, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology 114: 493–502, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Polk DB, Tong W. Epidermal and hepatocyte growth factors stimulate chemotaxis in an intestinal epithelial cell line. Am J Physiol Cell Physiol 277: C1149–C1159, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res 68: 3573–3578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raufman JP, Shant J, Guo CY, Roy S, Cheng K. Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell Physiol 215: 538–549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ray RM, Patel A, Viar MJ, McCormack SA, Zheng Y, Tigyi G, Johnson LR. RhoA inactivation inhibits cell migration but does not mediate the effects of polyamine depletion. Gastroenterology 123: 196–205, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, Koutcher JA, Spassova M, Ouerfelli O, Mellinghoff IK, Zakowski MF, Politi KA, Pao W. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest 119: 3000–3010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16: 522–529, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M, Miller VA. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res 12: 839–844, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res 12: 7232–7241, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci USA 99: 1521–1526, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosemond E, Rossi M, McMillin SM, Scarselli M, Donaldson JG, Wess J. Regulation of M3 muscarinic receptor expression and function by transmembrane protein 147. Mol Pharmacol 79: 251–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol 296: C221–C232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shant J, Cheng K, Marasa BS, Wang JY, Raufman JP. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res 315: 432–450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shepard HM, Brdlik CM, Schreiber H. Signal integration: a framework for understanding the efficacy of therapeutics targeting the human EGFR family. J Clin Invest 118: 3574–3581, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silen W, Ito S. Mechanisms for rapid re-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217–229, 1985 [DOI] [PubMed] [Google Scholar]
- 38. Strassheim D, May LG, Varker KA, Puhl HL, Phelps SH, Porter RA, Aronstam RS, Noti JD, Williams CL. M3 muscarinic acetylcholine receptors regulate cytoplasmic myosin by a process involving RhoA and requiring conventional protein kinase C isoforms. J Biol Chem 274: 18675–18685, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology 121: 566–579, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Whitney G, Throckmorton D, Isales C, Takuwa Y, Yeh J, Rasmussen H, Brophy C. Kinase activation and smooth muscle contraction in the presence and absence of calcium. J Vasc Surg 22: 37–44, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Xie G, Cheng K, Shant J, Raufman JP. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol 296: G755–G763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol 17: 559–564, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Yang WL, Frucht H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E(2) production in colon cancer cells. Carcinogenesis 21: 1789–1793, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Yoshimura Y, Yamaguchi O. Calcium independent contraction of bladder smooth muscle. Int J Urol 4: 62–67, 1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.