Abstract
We have studied apoptosis of gastrointestinal epithelial cells by examining the receptor-mediated and DNA damage-induced pathways using TNF-α and camptothecin (CPT), respectively. TNF-α requires inhibition of antiapoptotic protein synthesis by cycloheximide (CHX). CHX also results in high levels of active JNK, which are necessary for TNF-induced apoptosis. While CPT induces apoptosis, the increase in JNK activity was not proportional to the degree of apoptosis. Thus the mechanism of activation of JNK and its role in apoptosis are unclear. We examined the course of JNK activation in response to a combination of TNF-α and CPT (TNF + CPT), which resulted in a three- to fourfold increase in apoptosis compared with CPT alone, indicating an amplification of apoptotic signaling pathways. TNF + CPT caused apoptosis by activating JNK, p38, and caspases-8, -9, and -3. TNF-α stimulated a transient phosphorylation of JNK1/2 and ERK1/2 at 15 min, which returned to basal by 60 min and remained low for 4 h. CPT increased JNK1/2 activity between 3 and 4 h. TNF + CPT caused a sustained and robust JNK1/2 and ERK1/2 phosphorylation by 2 h, which remained high at 4 h, suggesting involvement of MEKK4/7 and MEK1, respectively. When administered with TNF + CPT, SP-600125, a specific inhibitor of MEKK4/7, completely inhibited JNK1/2 and decreased apoptosis. However, administration of SP-600125 at 1 h after TNF + CPT failed to prevent JNK1/2 phosphorylation, and the protective effect of SP-600125 on apoptosis was abolished. These results indicate that the persistent activation of JNK might be due to inhibition of JNK-specific MAPK phosphatase 1 (MKP1). Small interfering RNA-mediated knockdown of MKP1 enhanced TNF + CPT-induced activity of JNK1/2 and caspases-9 and -3. Taken together, these results suggest that MKP1 activity determines the duration of JNK1/2 and p38 activation and, thereby, apoptosis in response to TNF + CPT.
Keywords: camptothecin, caspase-3, caspase-9, MEKK4/7
mitogen-activated protein (MAP) kinases (MAPKs) are key components of cellular signal transduction pathways and are activated in response to a wide variety of external stimuli. They can be subdivided into the growth factor-activated MAPKs (ERK1 and ERK2) and the stress-activated MAPKs [JNK, SAPK1, and p38 (SAPK2)] (1, 8, 26, 39, 51). MAPKs relay, amplify, and integrate signals to elicit appropriate cellular responses, such as cell proliferation, differentiation, inflammation, and apoptosis. MAPK activity is dependent on phosphorylation of the threonine and tyrosine residues of the TXY motif within the activation loop of the specific upstream kinase. Phosphorylation of both residues is required for activity, and dephosphorylation of either residue is sufficient to inactivate them. ERK1/2 and JNK1/2 are activated by the upstream kinases MEKK1 and MEKK4/7, respectively. Their activation leads to the phosphorylation of ERK1/2 and JNK1/2 and the subsequent activation of transcription factors and other cellular targets (8, 13, 31, 34). Protein tyrosine phosphatases, serine/threonine-specific protein phosphatases, or dual-specificity (threonine/tyrosine) protein phosphatases dephosphorylate and inactivate MAPKs (10, 16).
The prototypic dual-specificity MAPK phosphatase MKP1 is able to dephosphorylate and inactivate mitogen- and stress-activated isoforms of MAPK in vitro and in vivo (2, 20, 29, 36, 43, 50). MKP1, an inducible nuclear phosphatase, dephosphorylates MAPKs with varying affinity, such as p38MAPK >>≥ JNK >> ERK1/2 (10, 16, 17), with a higher affinity for ERK2 than ERK1 (41). MKP1 forms physical complexes with MAPK isoforms, resulting in increased catalytic activity of MKP1. Franklin and Kraft (17) showed that the induction of MKP1 expression in U937 cells preferentially inhibited JNK. Moreover, expression of MKP1 protected cells against JNK-induced apoptosis (18, 40). Thus the duration and magnitude of the activation state of MAPKs depend on the relative levels of activated MEKKs and MKPs.
TNF-α, a pleiotropic cytokine, induces cell death in some types of cells but can also elicit a wide range of physiological responses, such as inflammation, proliferation, and differentiation (11, 31, 32, 46). Although TNF-α induces apoptosis in many cell types, TNF-α alone does not induce apoptosis in intestinal epithelial (IEC-6) cells. Inhibition of de novo protein synthesis by cycloheximide (CHX) induced apoptosis in response to TNF-α, which was accompanied by activation of ERK1/2 and JNK1/2 (4–6, 37). However, ERK activity decreased, while JNK activity increased, with time during apoptosis (5). Inhibition of MEK1 and MEKK4/7, activators of ERK1/2 and JNK1/2, by U-0126 and SP-600125, respectively increased and inhibition of MEK1 by SP-600125, an inhibitor of MEKK4/7, prevented apoptosis in response to TNF-α + CHX. These results confirmed the pro- and antiapoptotic nature of JNK1/2 and ERK1/2, respectively (4, 5). Various studies, including our own, showed that inhibition of transcription or translation unleashed the proapoptotic effects of TNF-α by activating JNK (4, 5, 12, 23, 46). Interestingly, TNF-α or CHX alone could not activate sufficient JNK1/2 and failed to induce apoptosis. Thus the effects of transcription and translation inhibitors were attributed to prevention of the synthesis of antiapoptotic proteins, which allowed TNF-α to form a death complex and fulfill its apoptotic potential. Although the involvement of JNK in TNF-α + CHX-induced apoptosis is established, the mechanism by which sustained activation of JNK occurs in response to TNF-α is unknown. We previously showed that camptothecin (CPT) induced apoptosis predominantly by activating p53-dependent Bax expression and that JNK inhibition by SP-600125 sensitized cells to CPT-induced apoptosis (7). These results suggest that basal JNK activation is required for survival of cells.
We attempted to find an agent, other than CHX, that, in combination with TNF-α, would activate JNK and induce apoptosis to understand the mechanism and to eliminate the complexity of the de novo protein inhibitor. Since mitochondrial permeability transition occupies a central position in receptor- and DNA damaging agent-induced apoptosis, we speculated that CPT might sensitize cells to TNF-α by augmenting JNK activity. Therefore, we examined the duration and strength of JNK activity in response to a death receptor ligand (TNF-α) and a DNA-damaging agent (CPT). CPT alone induced apoptosis without appreciable activation of JNK1/2. Apoptosis was significantly enhanced when TNF-α was administered along with CPT, which produced a concomitant activation of ERK1/2 and JNK1/2. We used this model to understand the regulatory mechanisms controlling the activity of JNK1/2. Our results show that MEKK4/7 and MKP1 augment the activities of JNK1/2 and p38, leading to increased apoptosis in response to TNF + CPT in IEC-6 cells.
MATERIALS AND METHODS
Reagents.
TNF-α was obtained from Pharmingen (San Diego, CA). CPT and CHX were obtained from Sigma (St. Louis, MO). The fluorometric caspase substrates IETD-aminotrifluoromethylcoumarin (AFC; caspase-8), LEHD-AFC (caspase-9), and DEVD-AFC (caspase-3) were purchased from Biomol Research Laboratories (Plymouth Meeting, PA). The cell-permeable caspase inhibitors IETD-fluoromethylketone (FMK), LEHD-FMK, and DQMD-FMK, SP-600125 (a JNK1/2 inhibitor), and SB-203580 (a p38MAPK inhibitor) were purchased from Calbiochem (La Jolla, CA). Mouse anti-phospho-JNK1/2, rabbit anti-JNK1/2, rabbit anti-procaspase-3, rabbit anti-phospho-ERK1/2, rabbit anti-ERK1/2, rabbit anti-cleaved caspase-3, and mouse anti-caspase-9 were purchased from Cell Signaling (Beverly, MA). Mouse anti-actin antibody was purchased from Millipore (Billerica, MA). The MKP1 small interfering RNA (siRNA) and antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Thr/Tyr-phosphorylated MAP-(177-189) peptide and malachite green reagents were purchased from Enzo Life Sciences (Plymouth Meeting, PA). The Cell Death Detection ELISA Plus kit was purchased from Roche Diagnostics (Indianapolis, IN). Mammalian protein extraction reagent (M-PER) and the bicinchoninic acid (BCA) protein assay reagent kit were purchased from Pierce (Rockford, IL). The enhanced chemiluminescence (ECL) Western blot detection system was purchased from DuPont-New England Nuclear (Boston, MA). The IEC-6 cell line (CRL 1592) was obtained from the American Type Culture Collection (Manassas, VA) at passage 13. This cell line is derived from normal rat intestine and was developed and characterized by Quaroni et al. (35). These cells are nontumorigenic, originate from the intestinal crypt as judged by morphological and immunologic criteria, and retain the undifferentiated character of epithelial stem cells. Tests for Mycoplasma were always negative. All chemicals were of the highest purity commercially available.
Cell culture.
The stock cell culture was grown in DMEM containing 5% heat-inactivated FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate in T-150 flasks and incubated at 37°C in a humidified atmosphere of 90% air-10% CO2. Stock cells were passaged once weekly and fed three times per week, and passages 15–22 were used. During the experimental setup, cells were trypsinized with 0.05% trypsin and 0.53 mM EDTA and counted using a Coulter counter (model Z1, Beckman). For the 4-day experimental setup, cells were grown in DMEM-5% dialyzed FBS to confluence for 3 days; on day 4, they were washed once with HBSS and placed in serum-free medium for 24 h prior to experiments.
Apoptosis.
Cells were plated (day 0) at a density of 6.25 × 104 cells/cm2 in DMEM-dialyzed FBS, with triplicate samples for each group. Cells were fed on day 2. On day 3, the cell culture medium was removed and replaced with serum-free medium. On day 4, TNF-α (10 ng/ml), CPT (20 μM), or TNF-α + CPT (TNF + CPT) was added to the serum-free medium for the indicated time period. In some experiments, cells were preincubated with the inhibitors IETD-FMK (25 μM) and LEHD-FMK (25 μM).
Quantitative DNA fragmentation ELISA.
The protocol for quantitative DNA fragmentation ELISA was similar to that described previously (23). Briefly, cells were grown in 24-well plates for DNA fragmentation ELISA assay and protein determination. After the treatments, cells were washed with dialyzed PBS, lysed, and centrifuged to remove the nuclei. An aliquot of the nuclei-free supernatant was placed in streptavidin-coated wells and incubated with anti-histone-biotin antibody and anti-DNA peroxidase-conjugated antibody. After incubation, the sample was removed, the wells were washed three times with incubation buffer, 100 μl of substrate buffer containing 2,2′-azino-di(3-ethylbenzthiazolin sulfonate) were added to each well, and samples were incubated for an additional 5–10 min. Absorbance was read at 405 nm in a microplate reader. After protein concentrations of each samples were determined by the BCA method, triplicates of the samples were combined and kept at −80°C for further immunoblot analysis. Results were expressed as absorbance at 405 nm per milligram protein per minute.
Caspase activity assay.
After the treatments, monolayers were washed with dialyzed PBS, scrapped, and collected into Eppendorf tubes. The cells were collected by centrifugation, incubated with ice-cold cell lysis buffer, and centrifuged at 10,000 g for 10 min at 4°C. The supernatant fraction was used to measure the activities of caspases. Briefly, 10 μl of cell lysate and 90 μl of assay buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 100 mM NaCl, 10 mM DTT, and 1 mM EDTA) containing caspase-3, -8, or -9 fluorometric substrate at a final concentration of 18 μM were placed in each well in a 96-well plate, which was incubated at 37°C for 2 h. Release of AFC from peptide substrate was monitored at an excitation wavelength of 400 nm and an emission wavelength of 520 nm. Protein concentration was determined by the BCA method. The index of casapse activation was calculated as relative fluorescence units (RFU) per milligram protein per minute.
Cell lysate preparation.
The cell monolayers were washed twice with ice-cold dialyzed PBS, pH 7.4, and 350 μl of M-PER buffer containing protease inhibitor and phosphatase inhibitors were added to the plate. The cells were incubated on ice for 15 min, harvested using a rubber scraper, transferred to 1.5-ml microfuge tubes, and centrifuged at 14,000 g for 10 min at 4°C, and supernatants were collected. BCA protein assay reagents, with BSA used as a standard, were used to determine protein concentration.
MKP1 activity.
IEC-6 cells left untreated or treated with TNF-α, CPT, or TNF + CPT were washed with Tris-buffered saline, and lysates were prepared. Twenty microliters of cell lysate were incubated with or without diphosphopeptide substrate (200 μM) in a 96-well plate. A diphosphopeptide, with the sequence DHTGFLpTEpYVATR, corresponding to MAPK residues 177–189 inclusive of putative activation sites, was used as a substrate. The amount of inorganic phosphate released upon hydrolysis of substrate peptide by phosphatase was measured using the malachite green reagent according to the manufacturer's instructions. Appropriate controls were included to validate the assay.
Small interfering RNA transfection.
Seventy percent confluent IEC-6 cells were transfected with control and MKP1-specific siRNA. Briefly, siRNA complexes prepared using FuGENE 6 HD transfection reagent following the instructions provided by the manufacturer were added drop-wise onto cells in serum-free medium and incubated overnight. Cells left untreated or treated with TNF-α or TNF + CPT were lysed in M-PER containing inhibitors of proteases and phosphatases. Cell lysates were subjected to Western blot analysis for detection of MKP1 to confirm knockdown of MKP1 by siRNA, JNK1/2 and p38 phosphorylation, and activation of caspases.
Western blot analysis.
Proteins (25 μg) precipitated by TCA were dissolved in 1× SDS sample buffer. The protein samples were subjected to 10–15% SDS-PAGE and transferred to Immobilon-P membranes (Millipore). The membranes were blocked with blocking buffer (3–5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20) for 2 h and incubated with the indicated antibodies prepared in blocking buffer overnight at 4°C. All antibodies, except anti-actin, were diluted 1:1,000; anti-actin was diluted at 1:20,000. Membranes were subsequently incubated with appropriate secondary antibody conjugated to horseradish peroxidase at room temperature for 1 h, and immunocomplexes were visualized by the ECL detection system.
Statistics.
All experiments were repeated three times (n = 3). Data are expressed as means ± SE. Experiments involving Western blots were performed three times with similar results, and a representative blot is shown in each case. ANOVA with appropriate post hoc testing was used to determine the significance of the differences between the means of multiple treatments, and Student's t-test was performed to determine the significance of the differences between the means of two treatments. P < 0.05 was regarded as statistically significant.
RESULTS
TNF + CPT causes apoptosis and activates JNK1/2.
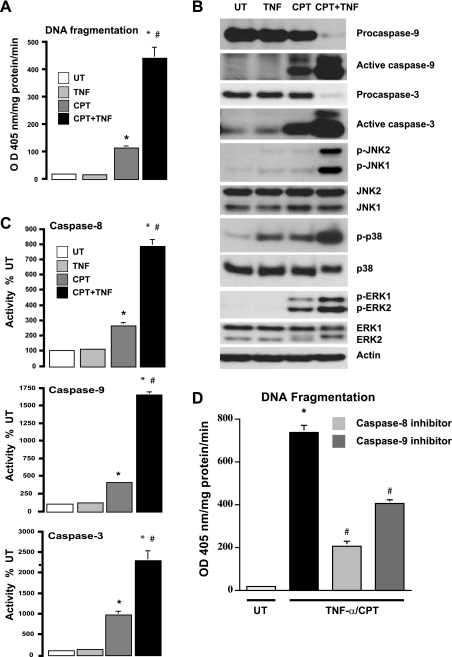
Since CPT induced apoptosis in intestinal epithelial cells with a modest activation of JNK1/2 and TNF-α alone failed to induce apoptosis (4, 37), we examined responses to TNF + CPT. Consistent with our previous studies, CPT induced significant (∼10 fold) apoptosis compared with untreated cells, while TNF-α failed to do so. TNF + CPT significantly increased (∼4 fold) apoptosis over that observed in cells treated with CPT alone (Fig. 1A). Furthermore, apoptosis correlated with increases in the processing of procaspases (Fig. 1B), as judged by decreases in the levels of procaspases-9 and -3, subsequent increases in the levels of active fragments of both caspases (Fig. 1B), and increases in their enzymatic activities (Fig. 1C). In addition, TNF + CPT also increased caspase-8 activity (Fig. 1C). The specific activity of caspase-3 (U·mg protein−1·min−1) was approximately equal to the sum of the activities of caspases-8 and -9, suggesting that caspases-8 and -9 activated caspase-3. Inhibition of caspases-8 and -9 significantly decreased TNF + CPT-induced apoptosis, as judged by the DNA fragmentation assay (Fig. 1D). However, the inhibition of apoptosis was significantly greater in caspase-8-inhibited cells than cells treated with caspase-9 inhibitor (Fig. 1D). Although TNF + CPT increased JNK1/2 and ERK1/2 phosphorylation at 4 h (Fig. 1B), CPT alone increased ERK activity, which was undetectable in TNF-α-treated cells. Interestingly, p38 MAPK (also called SAPK2) activity was significantly augmented by TNF + CPT compared with TNF-α or CPT alone (Fig. 1B). Since we have shown that ERK1/2 protected cells against apoptosis, these results suggest that the increased apoptotic response to TNF + CPT was due to increased activity of JNK1/2 and p38.
Fig. 1.
Effect of camptothecin (CPT), TNF-α, and CPT + TNF on induction of apoptosis. A: intestinal epithelial (IEC-6) cells were left untreated (UT) or exposed to TNF-α (10 ng/ml) (TNF), CPT (20 μM), or TNF-α + CPT (TNF + CPT) for 4 h. DNA fragmentation was measured using a colorimetric ELISA kit. OD, optical density. Values are means ± SE of triplicates. *Significantly different from UT. #Significantly different from CPT. B: cell extracts (25 μg) from experiments described in A were subjected to SDS-PAGE followed by Western blot analysis. Levels of procaspase-9, active caspase-9, procaspase-3, active caspase-3, and total MAPKs (ERK1/2, JNK1/2, and p38) and phosphorylated MAPKs (p-ERK1/2, p-JNK1/2, and p-p38) were determined using specific antibodies. Actin immunoblotting was performed as an internal control for equal loading. Blots are representative of 3 observations. C: confluent serum-starved cells treated as described in A were used to determine enzymatic activities of caspases-8, -9, and -3. Values are means ± SE of triplicates. *Significantly different from UT and TNF-α. #Significantly different from CPT. D: confluent serum-starved cells were treated with TNF + CPT in the presence or absence of inhibitors of caspase-8 or -9 for 4 h. Apoptosis was measured as described in A. Values are means ± SE. *Significantly different from UT. #Significantly different from CPT + TNF.
MAPK activation during apoptosis.
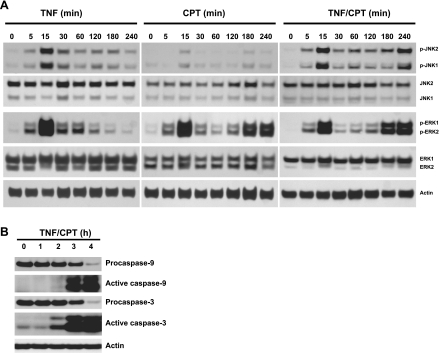
We examined the time courses of JNK1/2 and ERK1/2 activation by TNF-α, CPT, and TNF + CPT to clarify their involvement in apoptosis. JNK1/2 and ERK1/2 activities were increased to a similar extent by TNF-α within 15 min and declined thereafter (Fig. 2A). JNK activity remained slightly elevated until 180 min compared with untreated cells. Unlike TNF-α, ERK1/2 activity was cyclic in nature in response to CPT. CPT had a moderate effect on JNK1/2 activity (Fig. 2A). JNK1/2 activity was induced by TNF + CPT at 15 min (Fig. 2A), was sustained until 120 min, and further increased at 3 and 4 h (Fig. 2A). ERK1/2 activity followed a pattern of activation similar to that of the CPT-treated cells. Increased JNK1/2 activity correlated with activation of caspases-9 and -3, as seen by the decrease and increase in the levels of pro- and active caspases-9 and -3, respectively (Fig. 2B). Interestingly, caspase-3 activation began at 2 h and increased thereafter (Fig. 2B).
Fig. 2.
Activation of JNK1/2, ERK1/2, and caspases in response to CPT, TNF, and TNF + CPT. A: confluent serum-starved IEC-6 cells were treated with TNF-α (10 ng/ml), CPT (20 μM), or TNF + CPT for 0–240 min. Whole cell extracts (25 μg) were subjected to 12% or 15% SDS-PAGE followed by Western blot analysis for detection of total and phosphorylated forms of JNK1/2 and ERK1/2 using specific antibodies. B: levels of pro- and active caspases-9 and -3 were determined using specific antibodies. Actin immunoblotting was performed as an internal control for equal loading. Blots are representative of 3 observations.
Role of MEKKs.
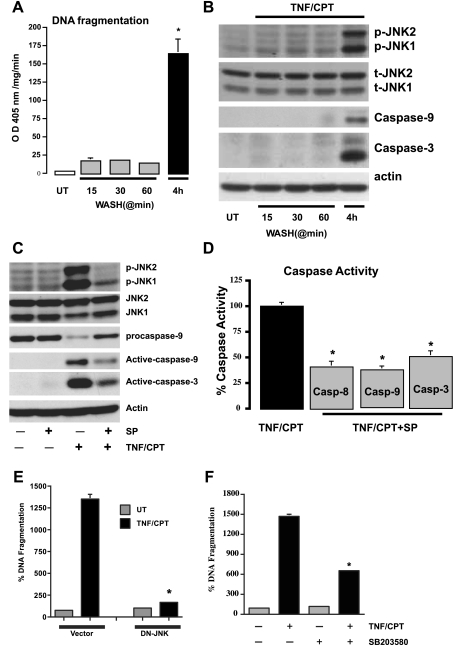
Since TNF-α increased JNK1/2 within 15 min, cells treated with TNF + CPT for 15, 30, and 60 min were washed and further incubated in a medium without treatment (total exposure time 4 h) to evaluate apoptosis. TNF + CPT exposure for up to 60 min failed to induce apoptosis and only slightly increased JNK1/2 activity (Fig. 3, A and B). However, TNF + CPT exposure for 4 h significantly increased apoptosis and JNK1/2 activity and caspase-9 and -3 activities (Fig. 3, A and B). These results indicate that the initial phase of JNK1/2 activation within 15 min is insufficient to induce apoptosis and that sustained activation at 3 h is essential.
Fig. 3.
Persistent signaling by TNF + CPT is essential for the activation of JNK1/2. A: control cells left untreated or exposed to TNF + CPT for indicated time periods followed by washing with control medium were further incubated without TNF + CPT for up to 4 h. DNA fragmentation was measured to assay apoptosis. Values are means ± SE of triplicates. *Significantly different from all groups. B: cells were treated as described in A, and whole cell extracts (25 μg) were subjected to electrophoresis (SDS-PAGE) followed by Western blot analysis for detection of phosphorylated JNK1/2 and respective total JNK1/2 (t-JNK1/2) proteins using specific antibodies. Levels of active caspases-9 and -3 were determined using specific antibodies. Actin immunoblotting was performed as an internal control for equal loading. Blots are representative of 3 observations. C: cells were exposed to TNF + CPT in the presence or absence of SP-600125 (10 μM) for 4 h. Whole cell extracts (25 μg) were subjected to SDS-PAGE followed by Western blot analysis for detection of phosphorylated JNK1/2 and respective total proteins using specific antibodies. Levels of pro- and active caspases-9 and -3 were determined using specific antibodies. D: cells were exposed to TNF + CPT in the presence or absence of SP-600125 (10 μM) for 4 h. Enzymatic activities of caspases-8, -9, and -3 (Casp-8, -9, and -3) were determined from extracts prepared as described in materials and methods. Values are means ± SE of triplicates. *Significantly different from TNF + CPT. E: vector and dominant-negative (DN) JNK-transfected cells were treated with TNF + CPT for 4 h, and DNA fragmentation was measured to assay apoptosis. Values are means ± SE of triplicates. *Significantly different from TNF + CPT-treated vector cells. F: cells were exposed to TNF + CPT in the presence or absence of SB-203580 (10 μM) for 4 h, and DNA fragmentation was measured to assay apoptosis. Values are means ± SE of triplicates. *Significantly different from TNF + CPT-treated cells.
SP-600125, a specific inhibitor of MEKK4/7 (2, 3) and, thereby, JNK1/2, was used to determine the role of JNK1/2 in the TNF + CPT-induced activation of caspases. SP-600125 (10 μM) inhibited JNK1/2 phosphorylation, decreased the processing of procaspase-9, and decreased the levels of active caspases-9 and -3 (Fig. 3C). Enzymatic activities of caspases-8, -9, and -3 were also inhibited in SP-600125-treated cells compared with TNF + CPT-treated cells (Fig. 3D). We confirmed the proapoptotic nature of JNK activation in response to TNF + CPT using a dominant-negative (DN) JNK-transfected stable cell line. TNF + CPT failed to cause DNA fragmentation in DN-JNK cells compared with vector-transfected cells (Fig. 3E). We previously reported that DN-JNK prevented TNF + CHX-induced apoptosis (4), which is similar to the response to TNF + CPT. Since TNF + CPT induced p38, we determined whether p38 inhibition prevented apoptosis. SB-203580, a specific inhibitor of p38, significantly decreased TNF + CPT-induced apoptosis (Fig. 3F). These results suggest an important role for the upstream kinase in the regulation of JNK1/2 and p38 (SAPKs) phosphorylation and its subsequent function in cell survival or apoptosis.
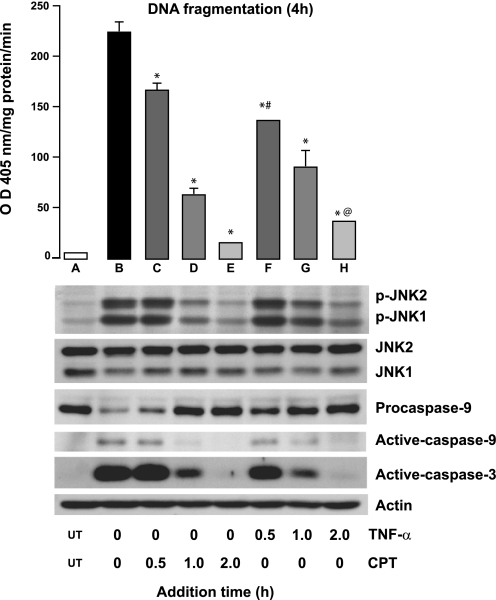
Although the above-described results demonstrate that upstream kinases are essential for the regulation of SAPKs, it is unclear whether TNF-α or CPT activates them. To gain insight into the process, cells were exposed to TNF-α or CPT at 0 h and CPT or TNF-α, respectively, was added at different time points. At the end of 4 h of DNA fragmentation, JNK1/2 phosphorylation and the levels of active caspases-9 and -3 were measured. Addition of TNF + CPT at 0 h caused maximum apoptosis (DNA fragmentation), JNK1/2 phosphorylation, and activation of caspases-9 and -3 (Fig. 4, lane B). When CPT was given at different time points to the cells exposed to TNF-α at 0 h, DNA fragmentation decreased significantly in a time-dependent manner (Fig. 4, bars C–E) compared with cells exposed to TNF + CPT for 4 h. Addition of CPT at 2 h failed to cause apoptosis in TNF-α-exposed cells (Fig. 4, bar E). The extent of apoptosis directly correlated with the activation of JNK1/2 and caspases-9 and -3. Although a similar pattern was observed when cells were exposed to CPT at 0 h followed by TNF-α (Fig. 4, bars F–H), the extent of DNA fragmentation was significantly lower. Procaspase-9 cleavage and caspase-3 activation were more pronounced when TNF-α was administered at 0 h followed by CPT after 30 min. However, procaspase-9 cleavage and caspase-3 activation decreased when CPT was given at 0 h followed by addition of TNF-α at 30 min (Fig. 4, bars C and F). These results suggest that the signaling events initiated by TNF + CPT during the first 30-min exposure lead to maximal activation of JNK1/2 and apoptosis. Thus it appears that TNF-α-induced JNK1/2 activity was sustained by CPT.
Fig. 4.
JNK1/2 activation during TNF + CPT-induced apoptosis. Confluent serum-starved cells were exposed to TNF-α (10 ng/ml) or CPT (20 μM) at 0 h, CPT and TNF-α, respectively, were added at indicated times, and cells were further incubated until 4 h. TNF + CPT at 0 h served as control. At the end of 4 h, apoptosis was evaluated by DNA fragmentation assay (top), and cell extracts were analyzed by Western blot for detection of phosphorylated JNK1/2, total JNK1/2, procaspase-9, and active caspases-9 and -3 (bottom). Values are means ± SE of 3 observations. *Significantly different from A. #Significantly different from C. @Significantly different from D.
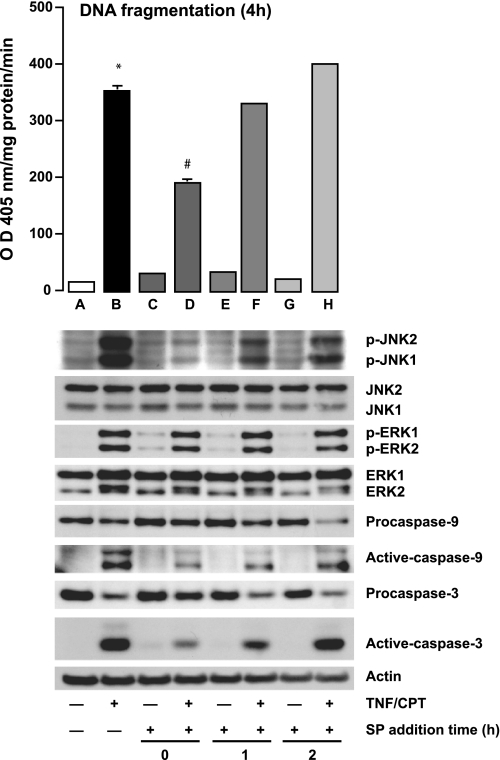
We further examined the role of upstream kinases in an experiment in which cells treated with TNF + CPT were exposed to SP-600125 at different time points. Figure 5 shows that SP-600125 prevented JNK1/2 activation and apoptosis only when added along with TNF + CPT (Fig. 5, bar D). Although SP-600125 addition 1 h after TNF + CPT failed to prevent apoptosis, the levels of phosphorylated JNK1/2 were higher than in untreated cells but lower than in TNF + CPT-treated cells. SP-600125 added 1 h after TNF + CPT failed to prevent the processing of procaspases-3 and -9 and, thereby, their activation as judged by the increased levels of active fragments. Interestingly, SP-600125 had no effect on the activation of ERK1/2. The levels of total JNK1/2 and ERK1/2 remained unchanged throughout the experiment. Thus the levels of activated JNK1/2 in the SP-600125-treated cells (1 h after TNF + CPT) are sufficient to induce apoptosis and may be sustained by processes other than activation by MEKK4/7.
Fig. 5.
Effect of MEKK4/7 inhibition on TNF + CPT-induced JNK1/2 and ERK1/2 activation. Serum-starved cells were treated with TNF + CPT for 4 h, 10 μM SP-600125 (SP) was added at 0, 1, and 2 h, and cells were further incubated. At the end of incubation (4 h), apoptosis was determined by DNA fragmentation assay (top), and cell extracts were analyzed by Western blot for detection of phosphorylated and total JNK1/2 and ERK1/2, procaspases-9 and -3, and active caspases-9 and -3 (bottom). Actin immunoblotting was performed as an internal control for equal loading. Representative blots from 3 experiments are shown. Values are means ± SE of 3 observations. *Significantly different from A. #Significantly different from B.
Role of MKP1 in JNK1/2 activation.
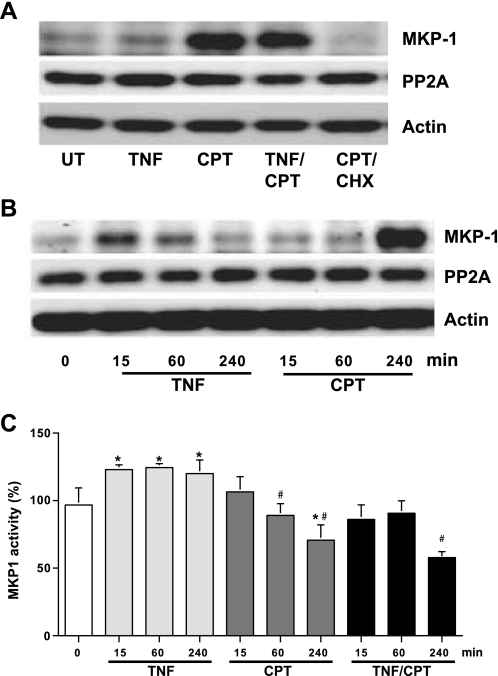
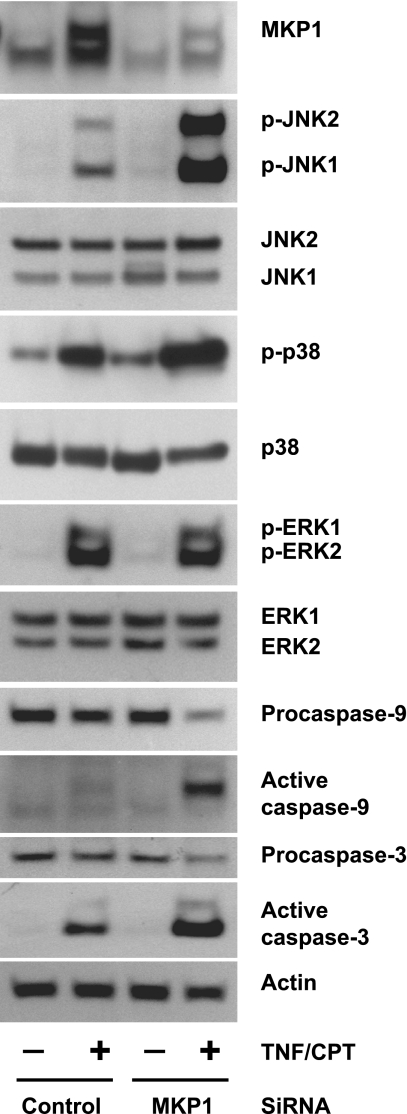
Our results confirm the involvement of MEKKs in the regulation of JNK1/2 and also suggest the requirement of another regulatory protein for the maintenance of phosphorylated JNK1/2. The increased phosphorylation of JNK1/2 in the presence of SP-600125 could be due to the inhibition of JNK1/2 dephosphorylation. Since MKP1 is known to dephosphorylate MAPKs, we determined the levels of MKP1 and its catalytic activity. TNF-α slightly increased the levels of MKP1, while CPT and TNF + CPT greatly induced MKP1 at 4 h (Fig. 6A). However, MKP1 induction was detectable in response to TNF only during the early phase of treatments (15 and 60 min; Fig. 6B). CHX completely inhibited the expression of MKP1 in response to CPT, confirming the inducible nature of MKP. The levels of protein phosphatase 2A were unchanged in all the treatment groups. MKP1 activity was significantly increased by TNF-α alone within 15 min and remained higher throughout the time course (Fig. 6C). Although MKP1 protein levels were increased by CPT, MKP1 activity was significantly lower than in untreated and TNF-α-treated cells (Fig. 6C). MKP1 enzymatic activity was significantly decreased in TNF + CPT-treated cells at 4 h compared with cells treated with TNF-α and CPT alone (Fig. 6C). These results suggest that the enzymatic activity of MKP1 determines the levels of JNK activity and, thereby, apoptosis in response to TNF + CPT. To confirm the involvement of MKP1 in TNF + CPT-induced JNK1/2 activity, cells were transfected with MKP1-specific and control siRNAs. We used a mixture of three MKP1-specific siRNAs for the effective knockdown of MKP1 expression. TNF + CPT induced massive apoptosis within 2 h in MKP1 siRNA-transfected cells compared with similarly treated control cells. Therefore, the experiment was terminated at 2 h. TNF + CPT significantly increased MKP1 protein, and basal, as well as the TNF + CPT-induced, MKP1 protein decreased in cells transfected with MKP1 siRNA compared with control cells (Fig. 7). Furthermore, TNF + CPT-induced JNK1/2 and p38 phosphorylation was manyfold higher when the phosphatase was blocked than in similarly treated control cells. The activities of JNK1/2 and p38 correlated with the activities of caspases-9 and -3. However, MKP1 knockdown had no effect on TNF + CPT-induced ERK1/2 activity (Fig. 7). MKP1 siRNA failed to induce JNK1/2 activity and apoptosis in cells treated with TNF-α alone (data not shown).
Fig. 6.
Effect of TNF-α and CPT on MAPK phosphatase 1 (MKP1) activity. Confluent serum-starved IEC-6 cells were treated with TNF-α (10 ng/ml), CPT (20 μM), or TNF + CPT for 0–240 min. Whole cell extract (20 μg protein) was used to detect levels of MKP1, protein phosphatase 2A (PP2A), and actin (A and B) and to determine phosphatase activity using Thr/Tyr-phosphorylated MAP-(177–186) peptide as a substrate (C). Amount of inorganic phosphate released from substrate was measured as described in materials and methods. Values are means ± SE of 3 observations. *Significantly different from 0 min. #Significantly different from respective TNF treatment.
Fig. 7.
MKP1 small interfering RNA (siRNA) potentiates TNF + CPT-induced JNK1/2 activity and apoptosis. IEC-6 cells grown to 70% confluence were transfected with MKP1-specific and control siRNA and serum-starved for 24 h. Transfected cells were left untreated or exposed to TNF + CPT for 2 h. Cell extracts were analyzed by Western blot to detect levels of MKP1, phosphorylated and total JNK1/2, p38, and ERK1/2, procaspases-9 and -3, and active caspases-9 and -3. Actin immunoblotting was performed as an internal control for equal loading. Representative blots from 3 experiments are shown.
DISCUSSION
The SAPKs play crucial roles in regulating cellular responses to various forms of stress (31) and determine whether cells survive or undergo apoptosis in response to a variety of stimuli. JNK is regulated by upstream kinases, including MEKK4/7, which activate it by phosphorylating Thr183 and Tyr185 (25). JNK-mediated phosphorylation of c-Jun, ATF2, p53, and Bad is implicated in the induction of apoptosis and survival (14, 15, 19, 21). The degree and duration of JNK activity are determined by several MAPK phosphatases, as well as by scaffold proteins, including JIP1-4 and POSH (22, 24, 47, 49).
In IEC-6 cells, TNF + CPT induced apoptosis and activated JNK1/2 analogous to TNF + CHX, while CPT alone was less effective. We have shown that inhibition of JNK1/2 sensitized cells to CPT, while CHX inhibited CPT-induced apoptosis and p21Waf1/Cip1 expression but had no effect on p53 phosphorylation (7). CPT induced apoptosis by increasing the expression and phosphorylation of p53, which in turn increased p21Waf1/Cip1, Bax, and apoptosis. Recent reports indicating a role for p21Waf1/Cip1 as an inhibitor of JNK1/2 may explain the lack of JNK induction by CPT (19, 37). TNF-α is known to activate caspase-8 through the TNF receptor-mediated death complex. Activated caspase-8 directly activates caspase-3 and initiates Bid-mediated cytochrome c release from mitochondria, leading to the activation of caspase-9 and, subsequently, additional caspase-3, thus amplifying apoptotic signaling (15, 33, 38, 44, 46). In our experiments, the activities of caspases followed the order caspase-3 > caspase-9 > caspase-8, and the activity of caspase-3 was approximately the sum of the activities of caspases-8 and -9 (Fig. 1C). Furthermore, the more pronounced inhibition of apoptosis induced by TNF + CPT by the caspase-8 inhibitor than by the caspase-9 inhibitor suggests a dominant role for caspase-8 (Fig. 1, C and D). JNK1/2 has been shown to augment caspase-8-mediated cleavage of Bid, leading to the formation of a novel product called jBid, which increases mitochondria-mediated caspase-9 activation and apoptosis (15, 42). In this study, we have not explored the mechanism by which JNK1/2 induces apoptosis; however, we showed previously that TNF-α + CHX induced apoptosis in a JNK-dependent manner by activating caspase-8, leading to the release of cytochrome c from mitochondria and subsequent activation of caspase-9 (4, 5). Since JNK1/2 is activated downstream of the TNF receptor, inhibition of caspase-8 activity by SP-600125 suggests a role for JNK1/2 in caspase-8-mediated cleavage of Bid. Although CPT alone induced caspase-8 activity largely independently of JNK1/2 (37), activation was approximately twofold higher in response to TNF + CPT, further confirming the involvement of JNK1/2 in caspase-8-mediated apoptosis (Figs. 1C and 3D).
Inhibition of JNK1/2 sensitized cells to CPT-induced apoptosis, suggesting that basal JNK1/2 activity is essential to survival of cells (Fig. 4), confirming our previous study (7). The transient early activation of JNK1/2 and ERK1/2 in response to TNF-α might trigger survival signals and, therefore, failed to induce apoptosis. Furthermore, the failure of exposure of cells to TNF + CPT for 30 min (Fig. 3, A and B) to induce apoptosis suggests that the activation of initial signaling events was not sufficient. The increase in JNK1/2 and ERK1/2 activities within 15 min by TNF + CPT, but not by CPT, suggests that TNF-α activates their upstream kinases, MEKK4/7 and MEK1, respectively. These data imply that MEKK4/7 might be crucial for the sustained activation of JNK1/2 and, thereby, apoptosis. Although exposure to TNF + CPT for 4 h is essential for the induction of apoptosis and JNK1/2 activation, the predominant role of TNF-α appears to be in the initial activation of JNK1/2. JNK1/2 and caspase-9 and -3 activities were similar in cells exposed to TNF for 4 h when CPT was present at 0 and 0.5 h (Fig. 4, bars B and C). However, delaying the exposure to TNF-α for 30 min in CPT-exposed cells resulted in less apoptosis and activation of caspases than in the cells in which CPT exposure was delayed (Fig. 4, cf. bars C and F).
Inhibition of MEKK4/7 significantly decreased (∼45%) TNF + CPT-induced apoptosis and decreased the activation of caspases (Fig. 5), reaffirming the role of MEKK4/7-mediated JNK1/2 activation in apoptosis. However, inhibition of MEKK4/7 after the onset of JNK1/2 activity (1 h after TNF + CPT) had little effect on DNA fragmentation (Fig. 5, bar F). Furthermore, after 1 h of exposure to TNF + CPT, the JNK inhibitor failed to eliminate JNK1/2 activity, caspase-3 activity, and apoptosis (Fig. 5, bar H). These results imply that the sustained phosphorylation of JNK1/2 was largely independent of MEKK4/7 and suggested that the deactivation of JNK1/2 might be inhibited.
It is well known that dual-specificity MKPs play an important role in regulating the activities of the MAPKs (27). The balance between MAPKs and MKPs determines whether cells survive or undergo apoptosis (43, 45, 50). MKP1 is overexpressed in many types of cancer cells (28, 30). The inducible nuclear phosphatase MKP1 was originally characterized as an ERK2 phosphatase in vitro and in vivo (17, 29, 36). However, it was subsequently shown to have activity against JNK (SAPK1) and p38 (SAPK2) in vitro and in vivo (9, 41). MKP1 is able to form physical complexes with MAPK isoforms, which results in the catalytic activity of MKP1. The correlation of the substrate selectivity, quantitative binding, and catalytic activation of MKP1 indicates that JNK is a preferred substrate for MKP1 in vivo (41). Expression of MKP1 protects cells against JNK-mediated apoptosis (18, 39, 40, 48). ERK1/2 and JNK1/2 have been shown to phosphorylate and, thereby, stabilize MKP1 (9, 10, 27). Thus the increased levels of MKP1 in response to CPT might be due to its stabilization by active ERK1/2 in TNF + CPT-treated cells (Figs. 2D and 6A). As stated above, the catalytic activity of MKP1 depends on the availability of specific substrate. The lack of active JNK1/2 in response to CPT might explain the decreased MKP1 activity in CPT-treated cells (Fig. 6C). Consistent with the above-described findings, we found that decreased expression of MKP1 enhanced JNK1/2 and p38 activity, the activation of caspases-9 and -3, and apoptosis in response to TNF + CPT (Fig. 7). Furthermore, increased MKP1 activity by TNF-α within 15 min might be responsible for preventing the sustained JNK1/2 activity and apoptosis (Fig. 6). However, CPT significantly inhibited MKP1 activity (Fig. 6). Thus TNF-α appears to activate MEKK1/MEKK4/7 and, thereby, increases ERK1/2 and JNK1/2 activities, and CPT prevents the deactivation of TNF-induced JNK1/2 activity. These data indicate that MKP1 is responsible for the sustained activation of JNK1/2 over the 4-h response to TNF + CPT.
Taken together, our data show that simultaneous activation of MEKK4/7 and MKP1 by TNF-α leads to a transient increase in JNK1/2 activity, which does not cause apoptosis in IEC-6 cells. CPT induced apoptosis by increasing p53-dependent Bax expression and caspase-8, -9, and -3 activities (4, 7). CPT inhibited MKP1 activity and induced apoptosis without activating MEKK4/7 and, thereby, JNK1/2. Thus sustained JNK1/2 activity in response to TNF + CPT results from the activation of MEKK4/7 by TNF-α and the deactivation of MKP1 by CPT. Increased activities of JNK1/2, p38, and caspases-8 and -9 by TNF-α in the presence of CPT indicate that SAPKs (JNK and p38) regulate a step upstream of caspase-8 and -9 activation. Although CPT increased Bax expression, it is sequestered in the cytoplasm by Bcl-2. JNK1/2-mediated phosphorylation of Bcl-2 prevents its association with Bax, allowing the increased translocation of Bax to mitochondria, causing extensive release of cytochrome c, leading to a higher level of caspase-9 activation. Active caspase-9 cleaves procaspase-8, which generates tBid and amplifies the mitochondrial permeability transition and the activation of caspases.
GRANTS
This publication was made possible by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-16505 and was also supported by the Thomas Gerwin endowment.
DISCLAIMER
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Abe MK, Kuo WL, Hershenson MB, Rosner MR. Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localization, and cell growth. Mol Cell Biol 19: 1301–1312, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alessi DR, Smythe C, Keyse SM. The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activity by oncogenic ras in Xenopus oocyte extracts. Oncogene 8: 2015–2020, 1993 [PubMed] [Google Scholar]
- 3. Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharya S, Ray RM, Viar MJ, Johnson LR. Polyamines are required for activation of c-Jun NH2-terminal kinase and apoptosis in response to TNF-α in IEC-6 cells. Am J Physiol Gastrointest Liver Physiol 285: G980–G991, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-α-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol 286: G479–G490, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharya S, Ray RM, Johnson LR. Decreased apoptosis in polyamine depleted IEC-6 cells depends on Akt-mediated NF-κB activation but not GSK3β activity. Apoptosis 10: 759–776, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bhattacharya S, Ray RM, Johnson LR. Role of polyamines in p53-dependent apoptosis of intestinal epithelials. Cell Signal 21: 509–522, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and phosphorylated in response to insulin and NGF. Cell 65: 663–675, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 60: 261–310, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14: 6–16, 2000 [PubMed] [Google Scholar]
- 11. Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science 296: 1634–1635, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNF-α-induced apoptosis. Cell 115: 61–70, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 5198: 682–685, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of Bad couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem 277: 40944–40949, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal 16: 769–779, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Franklin CC, Kraft AS. Conditional expression of mitogen-activated protein kinase (MAPK) phosphatase, MKP-1, preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem 272: 16917–16923, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1, is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci USA 95: 3014–3019, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuchs SY, Alder V, Buschman T, Yin Z, Wu X, Jones SN, Ronai Z. JNK targets p53 ubiquitination and degradation in non-stressed cells. Genes Dev 12: 2658–2663, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J 15: 3621–3632, 1996 [PMC free article] [PubMed] [Google Scholar]
- 21. Gupta S, Campbell D, Derijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signaling pathway. Science 267: 389–393, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Ham YM, Choi JS, Chun KH, Joo SH, Lee SK. The c-Jun N-terminal kinase 1 activity is differentially regulated by specific mechanisms during apoptosis. J Biol Chem 278: 50330–50337, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Jin S, Ray RM, Johnson LR. Rac1 mediates intestinal epithelial cell apoptosis via JNK. Am J Physiol Gastrointest Liver Physiol 291: G1137–G1147, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J 22: 954–965, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kadoya T, Khurana A, Tcherpakov M, Bromberg KD, Didier C, Broday L, Asahara T, Bhoumik A, Ronai Z. JAMP, a jun N-terminal kinase 1 (JNK1)-associated membrane protein, regulates duration of JNK activity. Mol Cell Biol 25: 8619–8630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol 3: 221–227, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signaling. Curr Opin Cell Biol 12: 186–192, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27: 253–261, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-jun N-terminal kinase activity and AP-1 dependent gene activation. J Biol Chem 270: 8377–8380, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Loda M, Capodieci P, Mishra R, Yao H, Corless C, Grigioni W, Wang Y, Magi-Galluzzi C, Stork PJ. Expression of mitogen-activated protein kinase phosphatase-1 in the early phases of human epithelial carcinogenesis. Am J Pathol 149: 1553–1564, 1996 [PMC free article] [PubMed] [Google Scholar]
- 31. Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19: 91–118, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Nakano H. Signaling crosstalk between NF-κB and JNK. Trends Immunol 25: 402–405, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Ozören N, El-Deiry WS. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol 13: 135–147, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De SE, Tang WJ, D'Adamino L, Franzoso G. Gadd45β mediates the NF-κB suppression of JNK signaling by targeting MKK7/JNKK2. Nat Cell Biol 6: 146–153, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase by dual phosphorylation on tyrosine and threonine. J Biol Chem 270: 7420–7426, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Ray RM, Viar MJ, Yuan Q, Johnson LR. Polyamine depletion delays apoptosis of rat intestinal epithelial cells. Am J Physiol Cell Physiol 278: C480–C489, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Salvesen GS, Dixit VM. Caspases: intracellular signaling by proteolysis. Cell 91: 443–461, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Sánchez-Perez I, Murguía JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 16: 533–540, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Perez I, Martinez-Gomariz M, Williams D, Keyse SM, Perona R. CL100/MKP1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene 19: 5142–5152, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38α and JNK MAP kinases mediate catalytic activation and substrate selectivity of MAP kinase phosphatase-1. J Biol Chem 276: 16491–16500, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem 69: 217–245, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487–493, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Thorburn A. Death receptor-induced cell killing. Cell Signal 16: 139–144, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23: 2838–2849, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Whitmarsh AJ, Cavangh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281: 1671–1674, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Wu JJ, Bennett AM. Essential role for mitogen-activated protein (MAP) kinase phosphatase-1 in stress-responsive MAP kinase and cell survival signaling. J Biol Chem 280: 16461–16466, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Xu Z, Kukekov NV, Greene LA. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J 22: 252–261, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng CF, Guan KL. Dephosphorylation and inactivation of the mitogen-activated protein kinase by a mitogen-induced Thr/Tyr phosphatase. J Biol Chem 268: 16116–16119, 1993 [PubMed] [Google Scholar]
- 51. Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem 270: 12665–12669, 1995 [DOI] [PubMed] [Google Scholar]