Abstract
Objective
Grb2-associated binder 1 (Gab1), a scaffolding adaptor protein, plays an important role in transmitting key signals that control cell growth, differentiation and function from multiple tyrosine kinase receptors. The study was designed to investigate the role of endothelial Gab1 in angiogenesis and underlying molecular mechanisms.
Methods and Results
Using cre-loxp technology, we generated endothelial-specific Gab1 knockout (Gab1-ecKO) mice. Gab1-ecKO mice are viable and showed no obvious developmental defects in the vascular system. To analyze the role of Gab1 in postnatal angiogenesis, we used hindlimb ischemia and Matrigel plug models. We found that loss of endothelial Gab1 in mice dramatically impaired postnatal angiogenesis. Gab1-ecKO mice had impaired ischemia-initiated blood flow recovery, exhibited reduced angiogenesis and were associated with marked limb necrosis. We further observed significant EC death in the ischemic hindlimb of Gab1-ecKO mice. Matrigel plug assay showed that hepatocyte growth factor (HGF)-mediated angiogenesis was inhibited in Gab1-ecKO mice. In vitro studies showed that Gab1 was required for HGF-induced EC migration, tube formation and microvessel sprouting. Mechanistically, HGF stimulated Gab1 tyrosine phosphorylation in ECs, leading to activation of ERK1/2 and Akt, which are angiogenic and survival signaling.
Conclusions
Gab1 is essential for postnatal angiogenesis through mediating angiogenic and survival signaling.
Keywords: Gab1, angiogenesis, hindlimb ischemia, hepatocyte growth factor, endothelial cells
Angiogenesis, the formation of new blood vessels from existing vascular network, begins with the activation, migration, and proliferation of endothelial cells (ECs) in the existing vessels.1 Many growth factors, including vascular endothelial growth factor (VEGF), are shown to regulate EC function and angiogenesis.2 Besides VEGF, hepatocyte growth factor (HGF) is a potent angiogenic factor in vivo and stimulates vascular EC migration, proliferation, and organization into capillary-like tubes in vitro.3–6 HGF binds its receptor c-Met and stimulates c-Met kinase activation, which triggers transphosphorylation of c-Met and downstream signaling events.7, 8 HGF/c-Met pathway has emerged as a promising therapeutic target to promote or inhibit angiogenesis.7–9 However, the mechanisms by which HGF mediates angiogenesis have not been fully understood.
Grb2-associated binder protein 1 (Gab1), a member of the insulin receptor substrates 1 (IRS1)-like multi-substrate docking protein family, is highly expressed in vascular ECs. Gab1 is found to be directly or indirectly recruited to numerous activated receptor tyrosine kinases (RTK), including c-Met and VEGF receptor 2 (VEGFR2, also named as Flk1 or KDR).10–15 Upon phosphorylation by RTK, Gab1 then recruits and activates phosphatidylinositol 3-kinases (PI3K)/Akt and protein tyrosine phosphatase SHP2/ERK1/2 pathways, which are crucial to cell proliferation and differentiation.13, 14, 16–23 However, the role of Gab1 in growth factors/RTKs-mediated angiogenesis remains largely unclear.
Gab1 deficiency in mice is early embryonic lethal due to heart and placental defects.24, 25 In order to directly explore the role of Gab1 in angiogenesis, we generated Gab1 endothelial-specific knockout mice (Gab1-ecKO). Using hindlimb ischemia, Matrigel plug and tumor angiogenesis models, we showed defective postnatal angiogenesis in Gab1-ecKO mice. We further found that Gab1 was required for EC survival in vivo and in vitro under stress conditions. Moreover, HGF-induced Akt and ERK1/2 activation and cell survival were impaired in Gab1 deficient ECs. Thus our data demonstrate that Gab1 is crucial for postnatal angiogenesis through mediating angiogenic and survival signaling.
Methods
Animals
Gab1flox/flox mice (C57BL/6J background) described previously26 andTie2-Cre transgenic mice from Jackson laboratory (C57BL/6J background, stock number 008863) were used. All protocols for animal experiments were approved by the University Committee on Animal Resource of University of Rochester.
Hindlimb Ischemia Model
Hindlimb ischemia was generated by resection of the femoral artery in male WT or Gab1-ecKO mice as previously described.27 Laser Doppler perfusion imaging was used to record perfusion of both right and left limbs at different time points as indicated.
An expanded methods section is available in supplemental data including hindlimb ischemia model, Laser Doppler perfusion imaging, capillary density analysis, TUNEL staining, in vivo Matrigel plug angiogenesis assay, tumor angiogenesis, aortic ring assay for ex vivo angiogenesis, isolation of mouse lung ECs, cell culture and siRNA transfection, wound closure cell migration, Boyden chamber migration, capillary-like tube formation, Western blot analysis, immuno-fluorescent staining and microscopy, MTT cell viability assay, and data statistic analysis.
Results
Generation and characterization of endothelium-specific Gab1 deficient (Gab1-ecKO) mice
In order to explore the role of endothelial Gab1 in vivo, we generated Gab1 endothelium-specific knock-out mice. Gab1flox/+ mice were bred into Tie2-Cre/+ transgenic mice that express Cre in ECs. Tie2-Cre;Gab1flox/flox (Gab1-ecKO) mice were generated by male Tie2-Cre/Gab1flox/+ and female Gab1flox/flox intercross. Gab1-ecKO mice were viable and were born at the expected mendelian ratio (Supplemental Table I). Supplemental Figure I in the Supplemental Data shows the genotyping results for Gab1-ecKO mice and Gab1flox/flox wild-type (WT, control) littermates. Gab1-ecKO mice had no obvious defects on body weight and vascular development. Lung ECs were isolated. EC morphology was normal (Supplemental Figure II) and confirmed with endothelial specific markers PECAM1 (CD31) and VE-cadherin immunostaining (Supplemental Figure III). Immunobloting analysis showed a significant decrease of Gab1 expression in ECs isolated from Gab1-ecKO mice relative to that from WT mice (Supplemental Figure IV). Co-immunostaining Gab1 with EC specific marker vWF in mouse hindlimb muscles further confirmed EC specific Gab1 deficiency in Gab1-ecKO mice (Supplemental Figure V), while relative low expression of Gab1 in muscle fibers in both Gab1-ecKO mice and WT mice were observed. Moreover, we found that hindlimb vasculature and retina vascular network in adult Gab1-ecKO mice are similar to those in WT mice (Supplemental Figure VI and VII). Collectively, our results indicate that Gab1-ecKO mice have no obvious developmental defects in the vascular system.
Gab1 is essential for ischemia-initiated blood flow recovery and angiogenesis
To examine the role of endothelial Gab1 in postnatal angiogenesis, we used a mouse hindlimb ischemia model. The gastrocnemius blood flow was measured using a deep penetrating Laser Doppler probe. As shown in Figure 1A and 1B, the ratio of blood flow of the right limb relative to the left limb was 100% before surgery. After surgery to the right limb, the ratios dropped by 80% in all groups of WT and Gab1-ecKO mice. Blood flow in the gastrocnemius muscle in WT mice recovered in a time dependent manner. However, Gab1-ecKO mice failed to recover the blood flow, suggesting that Gab1 may play a role in postnatal ischemic angiogenesis. Moreover, Gab1-ecKO mice showed marked necrotic limb at 14 days after the femoral artery resection (Figure 1C), which is consistent with the defective recovery of blood flow. We used a clinical scoring system to assess lower-limb function and tissue salvage after surgery.27 As shown in Figure 1D, the deficiency of endothelial Gab1 was associated with severe tissue ischemia after surgery.
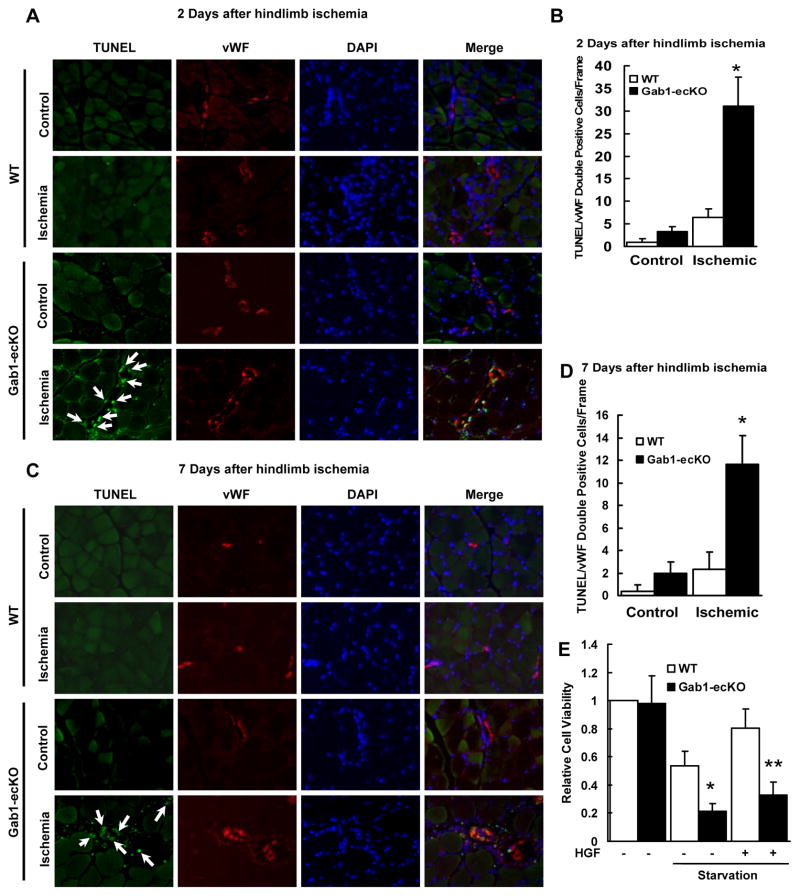
Figure 1. Gab1-ecKO mice have impaired ischemia-initiated blood flow recovery and angiogenesis.
A, Representative images of Laser Doppler blood flow before, immediately after, and on days 1, 3, 7, and 14 after femoral artery resection. B, Blood flow in ischemic hind limb was measured. Results were expressed as a ratio of the right (ischemic) to left (control, nonischemic) limb perfusion. WT (n=13), and Gab1-ecKO (n=10). *P<0.05 vs WT. C, Gab1-ecKO mice developed necrotic toes at 7 days to 14 days after femoral artery resection while WT littermate mice did not. Representative images for necrotic toes (arrow) at 14 days after surgery were shown. D, Clinical score at 14 days after femoral arteriectomy as an index of severity of limb ischemia: 0, normal; 1, pale foot or gait abnormalities; 2, less than half of foot necrotic; 3, more than half of foot necrotic without lower limb necrosis; 4, more than half of foot necrotic with some lower limb necrosis; 5, necrosis or autoamputation of entire lower limb. WT (n=13), and Gab1-ecKO (n=10). * P<0.05 vs WT. E. Quantification of capillary density, calculated as the number of capillaries per muscle fiber. For each animal, 6–7 randomly selected fields from 3–5 sections were counted. WT (n=13), and Gab1-ecKO (n=10). *P < 0.05 vs WT+control. **P < 0.05 vs WT + ischemic.
To characterize the ischemia-initiated angiogenesis, we quantified the capillary density of gastrocnemius muscle fibers from both WT and Gab1-ecKO mice before and after the hindlimb ischemia. As shown in Figure 1E, ischemia-initiated angiogenesis resulted in increased capillary density relative to the baseline in WT mice, while Gab1-ecKO mice had dramatically lower capillary density, less than that in the nonischemic control side (Supplemental Figure VIII shows color images of capillary CD31 staining). Taken together, these results established a critical role of endothelial Gab1 in ischemia-initiated angiogenesis.
Gab1 is critical for EC survival under stress conditions
Notably, Figure 1E showing a dramatically decreased capillary density in Gab1-ecKO mice suggests the potential involvement of EC survival in vivo under hypoxia which results in defective angiogenesis and vascular regression. To directly examine the role of Gab1 in cell survival, we performed TUNEL staining on tissues from Gab1-ecKO mice and WT mice after hindlimb ischemic surgery. Endothelial marker gene von Willebrand Factor (vWF) and nuclear DNA DAPI staining were also performed to identify ECs and cell nuclei. As shown in Figure 2, there were significantly more TUNEL-positive ECs in gastrocnemius muscle in Gab1-ecKO mice than in those of WT mice after ischemia. EC apoptosis in the arterioles were further comfirmed by triple immunostaining with TUNEL, vWF and smooth muscle alpha-actin (Supplemental Figure IX). It is worth noting that EC death started as early as 2 days (Figure 2A and 2B) and persisted at 7 days (Figure 2C and 2D) after ischemia in Gab1-ecKO mice, revealing a pivotal role of Gab1 in EC survival under ischemic stress.
Figure 2. Gab1-ecKO mice have enhanced vascular EC death in ischemic limb muscle.
A and B, Representative TUNEL and vWF double staining for EC death from sections of the gastrocnemius/soleus muscles at 2 days (A) and 7 days (B) after femoral resection in WT and Gab1-ecKO mice. Green: TUNEL positive; Red: vWF positive; Blue: DAPI nuclear staining; arrow: apoptotic ECs. Magnification: ×200. C and D, Numbers of TUNEL/vWF double staining positive cells. *P < 0.05 vs WT + ischemic, n=4. E, Gab1 deficient ECs isolated from Gab1-ecKO mice decreased HGF (10 ng/mL)-mediated cell survival under starvation conditions analyzed by MTT cell viability assay, compared that in WT ECs isolated from WT mice. *P < 0.05 vs WT + starvation; **P < 0.05 vs WT + starvation + HGF, n=4.
It has been reported that Gab1 is involved in VEGF-induced EC survival.28 HGF is also a potent survival growth factor.29 We observed that expression of both VEGF and HGF were increased in ischemic hindlimb muscles (Supplemental Figure X). Interestingly, we found that HGF strongly induced Gab1 tyrosine phosphorylation in ECs (Supplemental Figure XI). To examine whether Gab1 mediates HGF-induced EC survival under stress conditions, we used the MTT cell viability assay and mouse lung ECs isolated from Gab1-ecKO mice and WT mice. Under serum starvation for 24 hours, cell viability of Gab1 deficient ECs dropped by almost 55% relative to the control WT ECs (Figure 2E). While HGF significantly protected WT ECs from death, it failed to maintain EC survival in Gab1 deficient ECs (Figure 2E). Less extent effects were observed when VEGF was used to protect ECs from death (Supplemental Figure XII). We also examined the levels of cleaved caspase 3 for the induction of apoptosis, and found that increased active caspase 3 in response to serum starvation was much higher in Gab1 deficient ECs than that in WT ECs (Supplemental Figure XIII). These data show that Gab1 is required for EC survival under serum starvation.
Gab1 is necessary for HGF-induced angiogenesis revealed by Matrigel plug assay
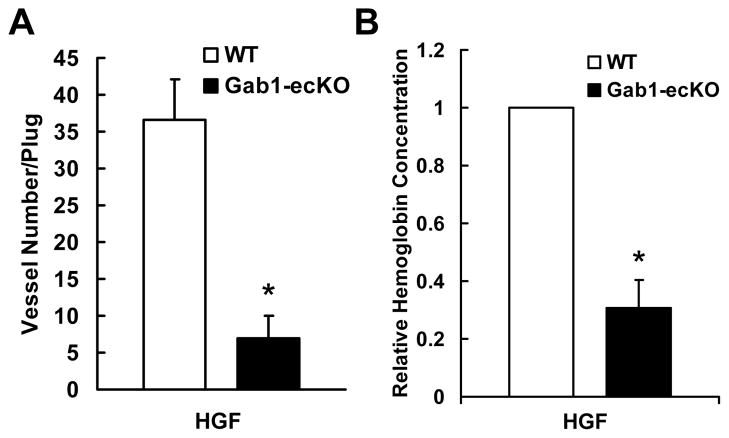
In order to dissect the role of Gab1 in HGF-induced angiogenesis in vivo, we used the Matrigel plug model, in which Matrigel mixed with HGF was subcutaneouslyinjected to Gab1-ecKO mice and WT mice. Matrigel plugs were harvested one week later, and stained for CD31. As shown in Figure 3A, deficiency of endothelial Gab1 in mice significantly reduced invading ECs and the density of microvessels in the Matrigel plugs (Supplemental Figure XIV shows color images of capillary CD31 staining). Hemoglobin concentration in the Matrigels was measured to assess the functional vessels in the Matrigel plugs. As shown in Figure 3B, hemoglobin concentration was much lower in Matrigel plugs implanted in Gab1-ecKO mice than that in Gab1-WT mice, consistent with the results of CD31 staining for microvessels in Matrigel plugs. These data demonstrate that Gab1 is essential for HGF-induced angiogenesis in vivo. Relatively less effects of endothelial Gab1 deficiency on VEGF-induced angiogenesis were observed by the Matrigel plug assay using Gab1-ecKO mice and WT mice (Supplemental Figure XV). In addition, we examined the role of Gab1 in tumor angiogenesis and found that endothelial Gab1 deficiency inhibited the tumor angiogenesis and tumor growth (see Supplemental Figure XVI and XVII).
Figure 3. Gab1 is required for HGF-mediated angiogenesis.
A, Number of sprouting vessels in the Matrigel plugs in the presence or absence of 100 ng/mL HGF after 7 days. *P < 0.05 vs WT + HGF, n=4. B, Hemoglobin measurement in Matrigel plugs by Drabkins’ assay. *P < 0.05 vs WT + HGF, n=5.
Gab1 mediates microvessel sprouting, capillary-like tube formation and EC migration
To further investigate the role of Gab1 in angiogenesis, we performed the aorta ring ex vivo angiogenesis assay, in vitro angiogenesis capillary-like tube formation and EC migration. In the aorta ring assay, we found that the number of sprouting microvessels from the aorta rings isolated from Gab1-ecKO mice in the presence of HGF was dramatically reduced (Figure 4A and 4B). In the capillary-like tube formation assay, we used mouse lung ECs isolated from Gab1-ecKO mice and WT mice. As shown in Figure 4C and 4D, the capillary-like tube formation in the presence of HGF was significantly enhanced in WT ECs, while HGF-induced capillary-like tube formation in Gab1 deficient ECs was impaired. Cell migration was measured by the modified Boyden’s chamber method and wound-healing migration assay. In the modified Boyden’s chamber method, HGF-induced EC migration was impaired in Gab1 deficient ECs compared to that in WT ECs (Figure 4E). Less effects of endothelial Gab1 deficiency on VEGF-induced EC migration were observed (Supplemental Figure XVIII). In the wound-healing migration assay, Gab1 deficient ECs failed to close the wound areas in the presence of HGF (Figure 4F and 4G). In addition, we infected adenoviruses encoding mouse Gab1-WT or LacZ (control) in Gab1 deficient ECs. Introducing mouse Gab1 but not LacZ rescued the ability of wound closure for these ECs in response to HGF (Figure 4H–J).
Figure 4. Gab1 deficiency in ECs impaired microvessel sprouting, tube formation and migration.
A and B, Microvessel sprouting in aortic ring assay. Representative micrographs and statistic results of sprouting microvessels from aortic ring grown in the presence and absence of 100 ng/mL HGF after 7 days were shown. *P < 0.05 vs WT + HGF, n=4. C and D, tube formation. Gab1-KO ECs showed impaired HGF (10 ng/mL)-induced capillary-like tube formation in the Matrigel analyzed by in vitro tube formation assay. *P < 0.05 vs WT + HGF, n=4. E, EC migration measured by the Boyden chamber method with Gab1-KO ECs and WT ECs. Bar graph represents averaged data, expressed as migrated cell number counted per 10 fields (X200). *P < 0.05 vs WT + HGF, n=4. F–J, EC migration measured by wound-healing assay with Gab1-KO ECs and WT ECs (F–G) or Gab1-KO ECs infected with Adenoviral LacZ or Gab1 (H–J). Representative images and percent increase in migrated cell number over that in unstimulated cells (control) were shown. *P < 0.05 vs WT + HGF, n=4. # P < 0.05 vs LacZ + HGF, n=4.
Gab1 is required for angiogenic and survival signaling in ECs
The PI3K/Akt and ERK signaling pathways are involved in EC survival and in vitro angiogenesis. To investigate how Gab1 mediates HGF-induced EC survival and migration, we assessed the activation of Akt and ERK1/2 in response to HGF stimulation using human umbilical endothelial cells (HUVECs) transfected with Gab1 siRNA or scrambled siRNA (control). Gab1 siRNA efficiently reduced the level of Gab1 expression in HUVECs (Figure 5A). Knockdown Gab1 substantially decreased the levels of Akt and ERK1/2 phosphorylation in response to HGF (Figure 5A–C). We also used Gab1 deficient ECs to examine HGF-induced Akt and ERK1/2 signaling and observed similar results (Figure 5D and 5E). Gab1 deficiency did not affect HGF receptor c-Met tyrosine phosphorylation (Supplemental Figure XIX). Moreover, we infected HUVECs with various Gab1 mutants to dissect the Gab1 signaling pathways. The overexpression of adenoviral Flag-Gab1-ΔPI3K (three PI3K binding sites tyrosine were replaced by phenylalanine) in HUVECs greatly inhibited HGF-mediated Akt activation but not ERK1/2 activation, while the overexpression of adenoviral Flag-Gab1-ΔSHP2 (two SHP2 binding sites tyrosine were replaced by phenylalanine) in HUVECs blocked HGF-mediated ERK1/2 activation but not Akt activation (Figure 5F–H).Taken together, these results establish a critical role of endothelial Gab1 in angiogenic and survival signaling.
Figure 5. Gab1 mediated angiogenic and survival signaling in ECs.
A–C, Knockdown of Gab1 by siRNA in HUVECs inhibited HGF (10 ng/mL)-mediated time-dependent activation Akt and ERK1/2. *P < 0.05 vs control siRNA + HGF, n=3. D and E, Gab1 deficient ECs isolated from Gab1-ecKO mice had impaired Akt and ERK1/2 activation in response to HGF (10 ng/mL) for 10 min. *P < 0.05 vs WT + HGF, n=3. F–H, Overexpression of Gab1 mutants in HUVECs affected Akt and ERK1/2 activation in response to HGF (10 ng/mL) for 10 min. *P < 0.05 vs WT + HGF, n=3.
Discussion
The novel finding of the present study is that endothelial Gab1 plays an essential role in postnatal angiogenesis in mice. Mechanistically, we found that Gab1 is critical for mediating HGF angiogenic signaling and survival of ECs under stress conditions in vivo and in vitro. To the best of our knowledge, this is the first study that revealed a key role for Gab1 in angiogenesis and HGF-mediated signaling in ECs.
Several genetic and biochemical studies have shown that Gab1 is involved in mouse development, oncogenic transformation and metabolism.24–26, 30 In particular, Gab1 deficient mice showed developmental defects in the heart, placenta, and skin, and died between E13.5 and E18.5.25 However, the role of Gab1 in vascular endothelial biology and angiogenesis have not been explored. Using cre-loxp system, we first generated Gab1-ecKO mice, which are viable and have no obvious vascular development defects. Using three angiogenic models including hindlimb ischemia, Matrigel plug and tumor angiogenesis in Gab1-ecKO mice, we showed that Gab1 regulates postnatal angiogenesis in vivo. Specifically, we found that ischemia-induced vessel growth and blood flow recovery were severely impaired in Gab1-ecKO mice compared to those in WT mice. HGF-mediated EC infiltration and microvessel growth in the implanted Matrigel plugs were blocked in Gab1-ecKO mice. Moreover, tumor angiogenesis and growth were inhibited in Gab1-ecKO mice. In agreement with our in vivo results, our in vitro studies showed that Gab1 is required for HGF-induced microvessel sprouting, EC migration and tube formation, which are essential components in angiogenic processes in vivo. Taken together, our studies clearly demonstrate an indispensable role of endothelial Gab1 in mediating angiogenesis in vitro and in vivo.
It has been shown that tissue ischemia/hypoxia induces expression of growth factors, which stimulates EC proliferation and migration resulting in neovascularization.31 Meanwhile, ischemia/hypoxia blocks nutrition supply and generates reactive oxygen species that may promote cell death, suggesting that EC survival could play important roles in ischemia-initiated angiogenesis. In this study, we observed that ischemic hindlimb in Gab1-ecKO mice underwent severe toe necrosis and had much lower capillary density in the ischemic limb than that in the nonischemic control, indicating that Gab1-ecKO mice not only have impaired angiogenesis but also show potential regression of preexisting vasculature. Therefore we reasoned that a Gab1-mediated cell survival mechanism may maintain preexisting vasculature and regulate angiogenesis under ischemic conditions. Indeed, our TUNEL and vWF double staining studies demonstrate that there is significant EC death in the ischemic hindlimb of Gab1-ecKO mice. In particular, we detected vascular EC death in the ischemic hindlimb of Gab1-ecKO mice, starting as early as 2 days and persistent at 7 days after femoral artery resection. To our knowledge, this is the first evidence showing the potential role of EC survival in hindlimb ischemia–induced angiogenesis and the role of Gab1 in EC survival in vivo. Consistent with these observations, we found that ECs isolated from Gab1-ecKO mice are more sensitive to starvation-induced cell death than those isolated from WT mice. The levels of cleaved caspase 3, a critical executioner of apoptosis, were enhanced in ECs isolated from Gab1-ecKO mice under starvation conditions, further supporting the concept that Gab1 acts as a survival factor against EC death. Collectively, our results indicate that Gab1-mediated EC survival is involved in ischemia-initiated angiogenesis.
Akt and ERK1/2 activation have been shown to regulate cell migration and survival signaling pathways.32 Several studies have suggested that Akt and ERK1/2 pathways are involved in HGF-mediated angiogenesis in vitro.33, 34 Gab1, an adaptor protein, plays an important role in mediating growth factors-induced activation of Akt and ERK1/2 through recruiting PI3K and SHP2 in a tyrosine phosphorylation-dependent manner.35, 36 However, the role of Gab1 in HGF-mediated signaling in ECs remains unclear. We first found that HGF strongly stimulated Gab1 tyrosine phosphorylation in ECs. Using Gab1 deficient ECs isolated from Gab1-ecKO mice and Gab1 siRNA knocked-down HUVECs as well as Gab1 phosphotyrosine mutants, we further found that Gab1 mediated HGF-induced Akt and ERK1/2 activation in ECs. The data provide signal mechanisms by which Gab1 mediates growth factors-induced angiogenesis.
Many growth factors are implicated in the processes of angiogenesis in vivo. Our results showing that VEGF and HGF are induced in response to ischemia, suggest that both VEGF and HGF are involved in ischemia-induced angiogenesis. In vitro studies have shown that Gab1 was tyrosine phosphorylated and mediated angiogenesis in vitro in response to VEGF. Consistent with this concept, we found that VEGF-induced angiogenesis was affected in Gab1-ecKO mice. However, we observed much stronger Gab1 tyrosine phosphorylation in ECs in response to HGF than that in response to VEGF. We further found that HGF-induced angiogenesis in vitro and in vivo was severely impaired in Gab1-ecKO mice. Our results suggest that Gab1 signaling is more important in HGF-mediated angiogenesis than VEGF-mediated angiogenesis.
It has been shown that enhanced secretion of cardiac HGF from myocardial infarct regions is associated with an attenuation of ventricular enlargement and an improvement in cardiac function.37 In fact, administration of recombinant HGF promotes angiogenesis in ischemic hindlimb and heart in various animal models.6, 38, 39 In this study, we uncover an essential role for Gab1 in ischemia-, tumor- and HGF-mediated angiogenesis. Our findings may have clinical implications and suggest that blocking Gab1 function results in the inhibition of tumor angiogenesis and tumor growth, and enhancing Gab1 signaling could be a potential therapeutic strategy to improve ischemic diseases.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported in part by the American Heart Association Postdoctoral Fellowship (to W.W.), Postdoctoral Fellowship (to C.H.H), NIH grant HL076754 and HL097593 (to M.K.J.) and Grant-In-Aid Award 0755916T (to Z.G.J.), the American Diabetes Association Thomas R. Lee Award 1-06-CD-13 (to Z.G.J.), and NIH grant HL80611 (to Z.G.J.).
Footnotes
Disclosures
None
References
- 1.D’Amore PA, Thompson RW. Mechanisms of angiogenesis. Annu Rev Physiol. 1987;49:453–464. doi: 10.1146/annurev.ph.49.030187.002321. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 3.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomita N, Morishita R, Taniyama Y, Koike H, Aoki M, Shimizu H, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenic property of hepatocyte growth factor is dependent on upregulation of essential transcription factor for angiogenesis, ets-1. Circulation. 2003;107:1411–1417. doi: 10.1161/01.cir.0000055331.41937.aa. [DOI] [PubMed] [Google Scholar]
- 5.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 7.Maulik G, Shrikhande A, Kijima T, Ma PC, Morrison PT, Salgia R. Role of the hepatocyte growth factor receptor, c-Met, in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002;13:41–59. doi: 10.1016/s1359-6101(01)00029-6. [DOI] [PubMed] [Google Scholar]
- 8.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–516. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 9.Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–6743. [PubMed] [Google Scholar]
- 10.Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 12.Lock LS, Royal I, Naujokas MA, Park M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J Biol Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroun CR, Holgado-Madruga M, Royal I, Naujokas MA, Fournier TM, Wong AJ, Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 1999;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isakoff SJ, Cardozo T, Andreev J, Li Z, Ferguson KM, Abagyan R, Lemmon MA, Aronheim A, Skolnik EY. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. Embo J. 1998;17:5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunnick JM, Mei L, Doupnik CA, Wu J. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J Biol Chem. 2001;276:24380–24387. doi: 10.1074/jbc.M010275200. [DOI] [PubMed] [Google Scholar]
- 17.Cunnick JM, Dorsey JF, Munoz-Antonia T, Mei L, Wu J. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J Biol Chem. 2000;275:13842–13848. doi: 10.1074/jbc.275.18.13842. [DOI] [PubMed] [Google Scholar]
- 18.Lehr S, Kotzka J, Herkner A, Klein E, Siethoff C, Knebel B, Noelle V, Bruning JC, Klein HW, Meyer HE, Krone W, Muller-Wieland D. Identification of tyrosine phosphorylation sites in human Gab-1 protein by EGF receptor kinase in vitro. Biochemistry. 1999;38:151–159. doi: 10.1021/bi9818265. [DOI] [PubMed] [Google Scholar]
- 19.Kamikura DM, Khoury H, Maroun C, Naujokas MA, Park M. Enhanced transformation by a plasma membrane-associated met oncoprotein: activation of a phosphoinositide 3′-kinase-dependent autocrine loop involving hyaluronic acid and CD44. Mol Cell Biol. 2000;20:3482–3496. doi: 10.1128/mcb.20.10.3482-3496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laffargue M, Raynal P, Yart A, Peres C, Wetzker R, Roche S, Payrastre B, Chap H. An epidermal growth factor receptor/Gab1 signaling pathway is required for activation of phosphoinositide 3-kinase by lysophosphatidic acid. J Biol Chem. 1999;274:32835–32841. doi: 10.1074/jbc.274.46.32835. [DOI] [PubMed] [Google Scholar]
- 21.Holgado-Madruga M, Moscatello DK, Emlet DR, Dieterich R, Wong AJ. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc Natl Acad Sci U S A. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yart A, Laffargue M, Mayeux P, Chretien S, Peres C, Tonks N, Roche S, Payrastre B, Chap H, Raynal P. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J Biol Chem. 2001;276:8856–8864. doi: 10.1074/jbc.M006966200. [DOI] [PubMed] [Google Scholar]
- 23.Ong SH, Hadari YR, Gotoh N, Guy GR, Schlessinger J, Lax I. Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc Natl Acad Sci U S A. 2001;98:6074–6079. doi: 10.1073/pnas.111114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs M, Brohmann H, Zechner D, Muller T, Hulsken J, Walther I, Schaeper U, Birchmeier C, Birchmeier W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh M, Yoshida Y, Nishida K, Narimatsu M, Hibi M, Hirano T. Role of Gab1 in heart, placenta, and skin development and growth factor- and cytokine-induced extracellular signal-regulated kinase mitogen-activated protein kinase activation. Mol Cell Biol. 2000;20:3695–3704. doi: 10.1128/mcb.20.10.3695-3704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bard-Chapeau EA, Hevener AL, Long S, Zhang EE, Olefsky JM, Feng GS. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat Med. 2005;11:567–571. doi: 10.1038/nm1227. [DOI] [PubMed] [Google Scholar]
- 27.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caron C, Spring K, Laramee M, Chabot C, Cloutier M, Gu H, Royal I. Non-redundant roles of the Gab1 and Gab2 scaffolding adapters in VEGF-mediated signalling, migration, and survival of endothelial cells. Cell Signal. 2009;21:943–953. doi: 10.1016/j.cellsig.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio PM. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. Embo J. 1996;15:6205–6212. [PMC free article] [PubMed] [Google Scholar]
- 30.Nakaoka Y, Nishida K, Narimatsu M, Kamiya A, Minami T, Sawa H, Okawa K, Fujio Y, Koyama T, Maeda M, Sone M, Yamasaki S, Arai Y, Koh GY, Kodama T, Hirota H, Otsu K, Hirano T, Mochizuki N. Gab family proteins are essential for postnatal maintenance of cardiac function via neuregulin-1/ErbB signaling. J Clin Invest. 2007;117:1771–1781. doi: 10.1172/JCI30651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 32.Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31:20–24. doi: 10.1042/bst0310020. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta S, Sellers LA, Li RC, Gherardi E, Zhao G, Watson N, Sasisekharan R, Fan TP. Targeting of mitogen-activated protein kinases and phosphatidylinositol 3 kinase inhibits hepatocyte growth factor/scatter factor-induced angiogenesis. Circulation. 2003;107:2955–2961. doi: 10.1161/01.CIR.0000077501.19266.E5. [DOI] [PubMed] [Google Scholar]
- 34.Makondo K, Kimura K, Kitamura N, Kitamura T, Yamaji D, Jung BD, Saito M. Hepatocyte growth factor activates endothelial nitric oxide synthase by Ca(2+)- and phosphoinositide 3-kinase/Akt-dependent phosphorylation in aortic endothelial cells. Biochem J. 2003;374:63–69. doi: 10.1042/BJ20030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida K, Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 2003;94:1029–1033. doi: 10.1111/j.1349-7006.2003.tb01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu H, Neel BG. The “Gab” in signal transduction. Trends Cell Biol. 2003;13:122–130. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda S, Goto Y, Baba T, Satoh T, Sumida H, Miyazaki S, Nonogi H. Enhanced secretion of cardiac hepatocyte growth factor from an infarct region is associated with less severe ventricular enlargement and improved cardiac function. J Am Coll Cardiol. 2000;36:115–121. doi: 10.1016/s0735-1097(00)00675-6. [DOI] [PubMed] [Google Scholar]
- 38.Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40:47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 39.Futamatsu H, Suzuki J, Mizuno S, Koga N, Adachi S, Kosuge H, Maejima Y, Hirao K, Nakamura T, Isobe M. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis: a potential role for induction of T helper 2 cytokines. Circ Res. 2005;96:823–830. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.