Abstract
Taste is an early stage in food and drink selection for most animals [1, 2]. Detecting sweetness indicates the presence of sugar and possible caloric content. However, sweet taste can be an unreliable predictor of nutrient value because some sugars cannot be metabolized. In addition, discrete sugars are detected by the same sensory neurons in the mammalian [3] and insect gustatory systems [4, 5], making it difficult for animals to readily distinguish the identity of different sugars using taste alone [6–8]. Here we used an appetitive memory assay in Drosophila [9–11] to investigate the contribution of palatability and relative nutritional value of sugars to memory formation. We show that palatability and nutrient value both contribute to reinforcement of appetitive memory. Non-nutritious sugars formed less robust memory that could be augmented by supplementing with a tasteless but nutritious substance. Nutrient information is conveyed to the brain within minutes of training when it can be used to guide expression of a sugar-preference memory. Therefore flies can rapidly learn to discriminate between sugars using a post-ingestive reward evaluation system and they preferentially remember nutritious sugars.
Appetitive olfactory memory formation in hungry adult Drosophila is very efficient with a single two minute pairing of odorant and sucrose being sufficient to form memory lasting for days [10, 11]. Although there is evidence that other insects can learn to associate visual and olfactory cues with specific nutrients, carbohydrates or proteins [12–15], it is unclear how taste and the respective nutrient value of these components contributes to the processes of learning and memory. Here we investigated whether nutrient value of sugar contributes to the reinforcement of appetitive olfactory memory in fruit flies.
Adult Drosophila feed on soft rotting fruits that are rich in sucrose, fructose and glucose, eg. apples, peaches, grapes and pears. In a classic series of experiments, Wigglesworth [16] determined that these sugars provide energy for adult flight. Flies that were depleted of muscle glycogen by flying them to exhaustion, resumed flight within two minutes when fed glucose, sucrose, fructose, mannose, maltose or trehalose. In contrast, some sugars such as arabinose were completely ineffective. A similar relative value of sugars was established by Hassett [17] who counted the number of flies that survived when provided these sugars as the sole food source. Sucrose, glucose and fructose supported survival whereas arabinose and xylose were very poor.
Lead by these prior studies [16, 17] we chose D-sucrose and D-fructose as nutritious sugars and D-arabinose and D-xylose, which are both abundant in fruits containing large amounts of pectin, as less nutritious sugars. We verified the relative nutritional value of each sugar for our wild-type Canton-S fly strain (Figure 1A) by housing flies in food vials with either 1% agarose (as a source of water) or 1% agarose containing 3M sucrose, fructose, arabinose or xylose as their sole source of food. Consistent with previous studies [16, 17], the majority of flies housed on sucrose and fructose remained alive for 4 days whereas most flies housed with water, arabinose or xylose were dead within 4 days. We also noted, as did Hassett [17], that flies housed on arabinose died even quicker than those on water or xylose, suggesting that prolonged arabinose feeding may be detrimental to flies. We conclude that sucrose and fructose provide nutritional benefit whereas arabinose and xylose do not.
Figure 1. Fruit flies conditioned with palatable and nutritious sugars form robust persistent memory.
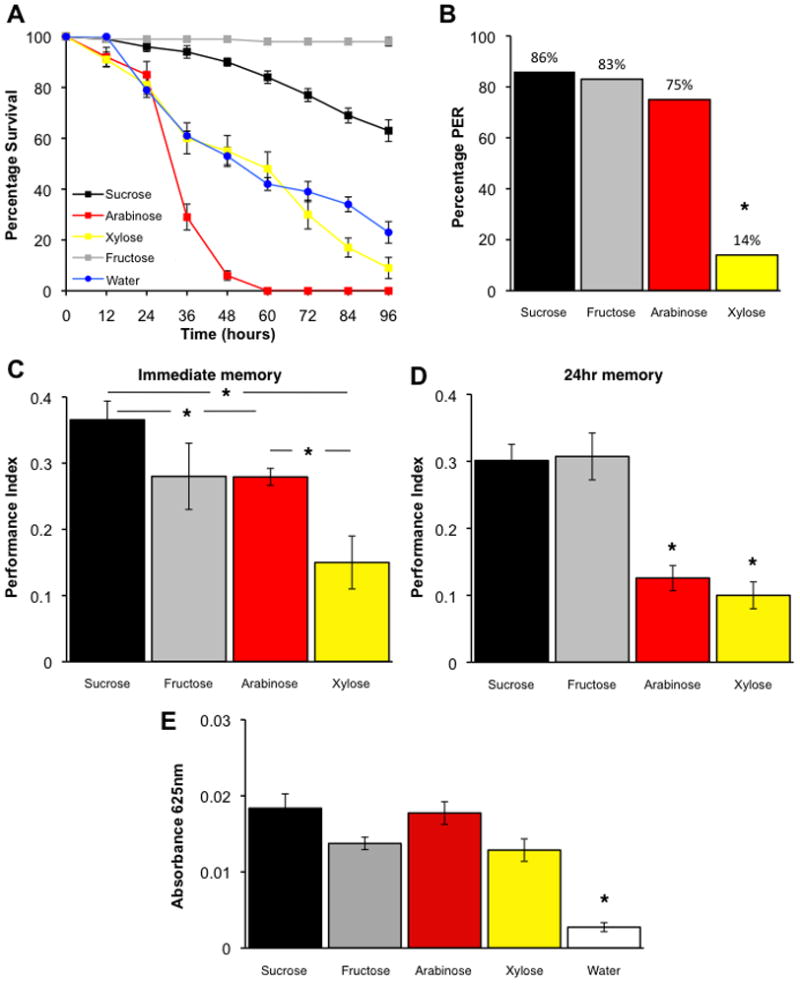
(A) Sucrose and fructose support fruit fly survival for several days but xylose and arabinose do not. Survival on fructose or sucrose was statistically different from water (1% agar) at all time points after 24hr. Xylose was not statistically different to water (all p>0.13) except at 84hr (p=0.04). Arabinose was statistically different to water at all time points after 24hr (all p<0.01, T-test). All sugars were 3M in 1% agar. Data are mean ± standard error of the mean (SEM). n=10 for each data point.
(B) Sucrose, fructose, arabinose and to a lesser extent xylose, elicit proboscis extension behavior. Flies were presented with all sugars as 3M solutions to the front leg. Performance of xylose exposed flies is statistically different from all other groups (all p<0.01, chi-squared test, marked by asterisk). n≥20 flies for each sugar.
(C) Short-term appetitive memory following conditioning with sucrose, fructose, arabinose and xylose. Performance of sucrose conditioned flies is statistically different from arabinose and xylose conditioned flies (both p<0.01 and p<0.005). Arabinose performance is also statistically different to xylose (p<0.04, ANOVA). Data are mean ± SEM. n≥14 except xylose n=6.
(D) Sucrose and fructose form robust 24hr memory but arabinose and xylose do not. Asterisks denote significant difference between marked groups and all others (all p<0.01). There is no statistical difference between arabinose and xylose performance (p=0.6, ANOVA). Data are mean ± SEM. n≥16.
(E) A similar amount of each dyed sugar is consumed during a 5min mock training session. Each sugar was mixed with dye and presented dried on filter paper in the conditioning apparatus. No statistical differences were observed between sugars (all p>0.05, ANOVA) although all were statistically different to dye alone (water, p<0.01). Data are mean ± SEM. n≥8.
Drosophila primarily sense sugars using gustatory receptor neurons (GRNs) on their tarsae and mouthparts [18]. Tarsal contact with desirable sugars drives proboscis extension whereas stimulation of sweet-sensing gustatory neurons on the labellum of the proboscis promotes food acceptance and ingestion. The physiological response of labellar gustatory receptor neurons to some of these sugars has been reported. Electrophysiological recordings from the L1 sensilla on the proboscis showed that sucrose more strongly activated sweet-sensing neurons than fructose and arabinose whereas xylose did not evoke a response [19]. Using Ca2+ imaging, sucrose, fructose and arabinose were shown to evoke similarly strong responses in sweet-sensing Gr5a-expressing neurons [4, 5] whereas xylose was not tested [20]. We tested whether each sugar was detected as favorable using a proboscis extension reflex (PER) assay [4, 8, 19, 21] (Figure 1B). We applied sugar solutions to the front leg of restrained flies and determined the frequency of the PER. Whereas sucrose, fructose and arabinose elicited high levels of PER that were statistically indistinguishable, xylose-evoked PER was significantly lower. Sugars applied directly to the labellum evoked a very similar PER profile (Figure S1). Therefore, published data [16, 17, 19, 20] and those presented here suggest that sucrose and fructose are nutritionally beneficial sugars that strongly activate sweet-sensing GRNs whereas arabinose, and to a lesser extent xylose are detected as sweet but provide no obvious nutritional benefit.
We next used each of these sugars as reinforcement in an olfactory conditioning assay [10] and measured appetitive olfactory memory formation and persistence. Memory tested immediately after training revealed clear differences in performance between flies reinforced with the different sugars (Figure 1C). Performance followed a similar rank order to the robustness of PER evoked by each sugar. Immediate memory performance of flies conditioned with sucrose was indistinguishable from those trained with fructose but was statistically greater than those trained with arabinose or xylose. The performance of arabinose-conditioned flies was statistically indistinguishable from flies conditioned with fructose but was statistically different from those trained with xylose. Therefore short-term memory can be formed with non-nutritious sugars suggesting that sensation of sweetness is sufficient for memory formation. We also tested whether memory persisted 24hr after training. Strikingly, the nutritious sugars, sucrose and fructose, formed robust 24hr memory whereas the non-nutritious sugars, arabinose and xylose, did not (Figure 1D). Memory formed with sucrose and fructose was statistically different from that formed with arabinose and xylose whereas memory formed with arabinose was statistically indistinguishable from xylose conditioned memory.
Published data from functional Ca2+ imaging registered a significant response for arabinose in bitter-sensing Gr66a-expressing neurons as well as in sweet-sensing Gr5a-expressing neurons [20]. We therefore tested whether the poor 24hr memory observed following conditioning with arabinose resulted from an integration of bitter and sweet signals. We arabinose-conditioned flies in which output from the Gr66a-expressing neurons [4, 5] was blocked prior to and during training with the dominant temperature-sensitive uas-shibirets1 (shits1) transgene [22]. shits1 blocks membrane recycling and thus synaptic vesicle release at the restrictive temperature of 31°C. The 24hr memory performance of these flies was statistically indistinguishable from wild-type and all control genotype flies (Figure S2A). Therefore, activation of bitter-sensing neurons is unlikely to be responsible for poor 24hr memory performance observed following conditioning with arabinose reinforcement. In addition, although prolonged exposure of flies to arabinose appears to be detrimental (Figure 1A and [17]), the exposure during the 2min training session does not impair short-term memory performance when compared to fructose (Figure 1C), or alter longevity (Figure S2B).
A reduced amount of sugar ingested during conditioning could also determine the strength of memory formation. To address this possibility, we added tasteless dye to each sugar and measured dye uptake using spectrophotometry (Figure 1E). Flies were deprived of food as if to prepare them for conditioning, and given a mock 5min training session with each of the dyed sugars (the amounts ingested during the usual 2min training session were beyond the limits of detection). Importantly, ingested material takes more than 5min to pass through the fly [23]. Despite differences in PER evoked by each sugar, the amount of dye ingested with each sugar in 5min was statistically indistinguishable. These data suggest that arabinose is not aversive and that the amount of each sugar ingested during training is unlikely to account for differences in memory performance. We concluded that differences could result from an additional reinforcing effect of nutritional value with sucrose and fructose.
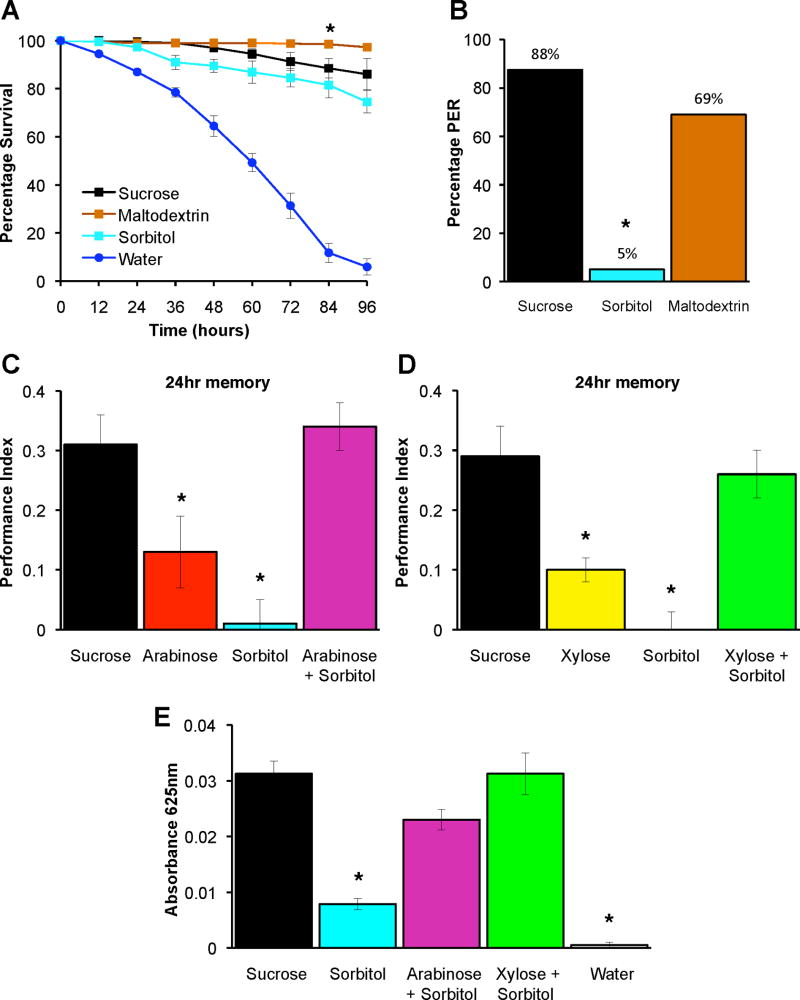
To further test a role for nutritional value in memory reinforcement we trained flies with supplemented arabinose or xylose. Hassett [17] reported that the polyhydric alcohol sorbitol and polysaccharides such as starch/maltodextrin were nutritionally valuable to Drosophila. He also demonstrated phagostimulation of these compounds by mixing them with low doses of simple sugars [17]. We therefore verified the nutritional value of these supplements and assayed their palatability using the PER. Survival on medium containing sorbitol or maltodextrin was statistically indistinguishable to survival on sucrose across four days (with the exception of a single time point, Figure 2A). PER experiments revealed that flies respond significantly to maltodextrin but they respond very rarely to sorbitol (Figure 2B). We suspect strong maltodextrin driven PER results from the 10% contaminating simple sugars in maltodextrin that come from partial hydrolyzation of this polymer of glucose. We therefore favored the use of ‘tasteless’ sorbitol as a supplement in our memory experiments.
Figure 2. Olfactory conditioning with arabinose or xylose supplemented with nutritious sorbitol forms robust 24hr memory.
(A) Sorbitol, maltodextrin and sucrose support fruit fly survival for several days. The number of flies alive on sorbitol or maltodextrin was statistically indistinguishable to those on sucrose at all time points (p>0.07) except at 84hr where maltodextrin was different to sucrose (p=0.03, asterisk). Survival on sorbitol, maltodextrin and sucrose was statistically different to on water at all time points (all p<0.01). Data are mean ± SEM. n=10 for each data point.
(B) Sucrose and maltodextrin elicit robust proboscis extension behavior but sorbitol does not. Flies were presented with 3M solutions of sucrose or sorbitol or 1.25M maltodextrin to the front leg. Performance of sorbitol exposed flies is statistically different from other groups (p<0.01, chi-squared test, marked by asterisk). n≥20 flies for each sugar.
(C) Training with sorbitol supplemented arabinose forms persistent memory. 24hr appetitive memory performance of flies trained with sorbitol supplemented arabinose is not significantly different to flies trained with sucrose (p>0.6). Asterisks denote significant difference between marked groups and others (p< 0.05, arabinose; p<0.01 sorbitol, ANOVA). Data are mean ± SEM. n≥14.
(D) Training with sorbitol supplemented xylose forms persistent memory. 24hr appetitive memory performance of flies trained with sorbitol supplemented xylose is not significantly different to flies trained with sucrose (p>0.6). Asterisks denote significant difference between marked groups and others (p <0.05, xylose; p<0.01 sorbitol, ANOVA). Data are mean ± SEM. n≥14.
(E) More dyed sorbitol is consumed in 5min when mixed with arabinose or xylose. Each substance or combination was mixed with dye and presented dried on filter paper in the conditioning apparatus. The amount ingested with arabinose+sorbitol or xylose+sorbitol was statistically different to sorbitol (p<0.05 and p<0.01 respectively) or water alone (both p<0.01, ANOVA). Consumption of dye with sorbitol was not statistically different from dye with water (p>0.05). Data are mean ± SEM. n≥8.
We conditioned flies using odorants and sucrose, arabinose, xylose or sorbitol alone or with arabinose or xylose mixed with sorbitol, as reinforcement. Training with arabinose, xylose or sorbitol formed little to no 24hr memory (Figure 2C & D). Memory performance was statistically different to that formed with sucrose reinforcement. However, 24hr memory formed with sorbitol supplemented arabinose or xylose was very robust and was statistically indistinguishable from flies trained with sucrose. We also trained flies with maltodextrin supplemented arabinose (Figure S3). As in previous experiments, training with arabinose alone did not form robust 24hr memory whereas arabinose supplemented with maltodextrin, exhibited 24hr memory that was indistinguishable from sucrose conditioned flies. We also tested the amount of dyed sorbitol ingested in 5min when either presented alone, or mixed with arabinose or xylose. Flies ingested significantly more dye mixed with arabinose+sorbitol or xylose+sorbitol than with sorbitol alone (Figure 2E). These data are consistent with previous results [17] and with the notion that flies ingest insufficient amounts of sorbitol to form 24hr memory when sorbitol is presented alone for 2min. However, using non-nutritious sugars to stimulate sorbitol (or maltodextrin) ingestion apparently provides sufficient reinforcement to form robust 24hr memory. Therefore these data support a role for nutrient value in the reinforcement of persistent appetitive memory.
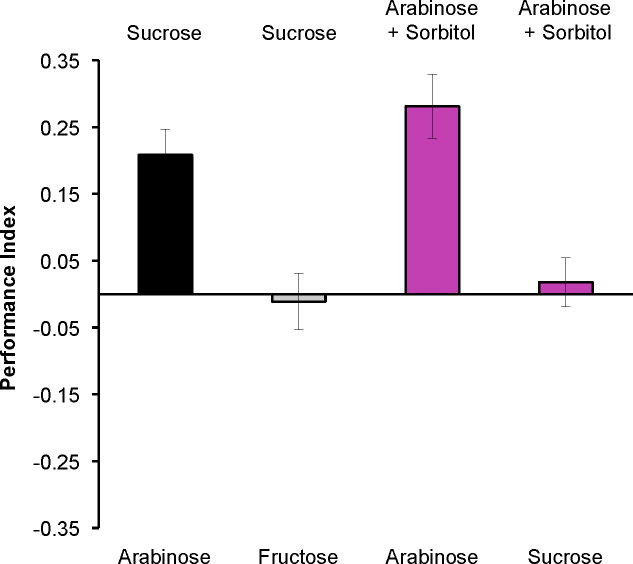
Conditioned taste aversion learning in mammals is noteworthy for the long delay (up to 12hr) between the presentation of the tastant and the induction of nausea [24]. One might imagine a post-ingestive nutritive affect would also develop slowly. However, in the flying to exhaustion studies, Wigglesworth [16] observed that flies resumed flight 30–45sec after feeding with glucose and 60–90sec after feeding with sucrose. This work suggests that energy resources can be internalized and utilized extremely fast. We therefore assessed the speed of nutrient detection in our memory assay by testing flies for discrimination between odors immediately following a differential conditioning protocol pairing one odor with a nutritious sugar and the other odor paired with a non-nutritious sugar (Figure 3). We first trained flies with sucrose versus arabinose as reinforcement or sucrose versus fructose for comparison. Flies showed an immediate preference for the odor previously paired with sucrose rather than arabinose. However flies showed no preference when differentially trained with the two nutritious sugars, sucrose and fructose. We next trained flies with odor paired with arabinose versus arabinose plus sorbitol or with odors paired with arabinose plus sorbitol versus sucrose. Flies showed immediate preference for the odor previously paired with sorbitol supplemented arabinose over arabinose alone. Strikingly, flies showed no preference for either odor when trained with arabinose plus sorbitol versus sucrose. Therefore, nutrient value is very quickly assigned to memory processing so that flies can exhibit preference behavior to the nutrient associated odorant less than two minutes after training. These experiments also suggest that nutrient content contributes to immediate memory performance in this behavioral choice assay.
Figure 3. Nutrient information is rapidly coded and can be used to guide preference behavior immediately after training.
Flies were differentially conditioned by pairing one odor with one sugar and the other odor with a different sugar, or supplemented sugar. They were then immediately tested for olfactory preference. Flies always exhibited preference for the odor that had been previously paired with a substance with nutrient value – sucrose over arabinose; arabinose+sorbitol over arabinose alone. They showed no preference when both odors were paired with nutritious substance – sucrose versus fructose; sucrose versus arabinose+sorbitol. Both of these scores were not statistically different from zero (both p>0.6, Mann Whitney U test). Data are mean ± SEM. n≥10.
In conclusion, we show that efficient appetitive memory formation in Drosophila involves signals representing nutrient value. Whereas sweet taste is sufficient to form short-term memory, persistent memory appears preferentially formed with sugars that also provide nutrient benefit. Strikingly, supplementing inadequate sugars with nutritious but tasteless sorbitol forms stronger 24hr memory suggesting that nutrition is a key element for memory persistence. This model is supported by a previous study in which bees only formed persistent memory if they were allowed to ingest sugar during conditioning [25]. Using a post-ingestive mechanism overcomes the shortcomings of the gustatory system to discriminate between sugars [8] and of potentially being fooled by certain sweet-tasting substances that an animal may not be able to usefully metabolize.
The ability to assign nutrient information to sources of food, and remember them, seems very valuable for an animal to forage effectively. Our data suggest that nutrient information can be very rapidly and accurately assigned to a particular food source. This rapidity contrasts to conditioned taste aversion memory formation in mammals [24] and the honeybee [26], both of which involve a post-ingestive mechanism that is delayed and appears to be less accurately assigned to food sources to be avoided. Although conditioned taste aversion has not yet been demonstrated in fruit flies, it seems logical that an animal might benefit from accurately learning nutritious food sources while being more conservative in learning to avoid sources that are potentially dangerous.
Taste defective mice can develop preference for nutritious liquids and calorie content activates the dopaminergic reward system [27]. Furthermore, fMRI studies in human subjects administered sucrose, saccharin or maltodextrin concluded a central brain response for the caloric content of carbohydrate that was independent of sweet taste [28]. Therefore, post-ingestive sensing of the nutrient content of carbohydrate is likely to be conserved. Finding that taste and nutrient value of sugar contribute to appetitive memory formation suggests there may be parallel reinforcement pathways for appetitive memory in the fly. Strikingly, bitter taste and post-ingestive effects of toxin provide parallel reinforcement signals for aversive learning in honeybees [26]. It will be important to identify the molecular nature of post-ingestive nutrient detection and the mechanisms through which it is broadcast to the brain. Our work here suggests that the neural circuits of reinforcement and perhaps memory consolidation in the fruit fly brain contain neurons that are receptive to the nutrient signals.
Experimental Procedures
Fly strains
Fly stocks were raised on standard cornmeal food at 25°C and 60% relative humidity. Mixed sex populations of wild-type Canton-S flies that were housed together were used throughout. To disrupt neurotransmission from bitter-sensing neurons (Figure S2A) we crossed Gr66aGAL4 [5] male flies to females harboring a double insertion of uas-shibirets1 [22]. Heterozygous transgenic control flies were from crosses of each transgenic line to wild-type Canton-S flies.
Appetitive Olfactory Conditioning
All flies were food deprived before training by storing them for 16–20hr in glass milk bottles containing water dampened 3MM filter paper to prevent desiccation. The olfactory appetitive paradigm was performed essentially as described [10] with the following modifications. Sugars were prepared as 3M solutions and 2.5ml was pipetted onto a 2.25 inch X 3 inch piece of 3MM paper and allowed to dry. Following training, flies were either tested immediately or stored in vials with standard fly food for 3hr, then transferred into vials containing 1% agar until testing 21 hours later. The performance index (PI) was calculated according to [10]. The odors used were 3-octanol and 4-methylcyclohexanol. Statistical analyses were performed using KaleidaGraph (Synergy Software). Overall analyses of variance (ANOVA) were followed by planned pairwise comparisons between the relevant groups with a Tukey HSD post-hoc test. Unless stated otherwise, all data points in each conditioning experiments represent n≥14.
Survival Assays
Groups of 20 (10 male, 10 female) 24hr old wild-type flies were housed in vials containing either a 3M solution of each substance (sucrose, fructose, arabinose, xylose, sorbitol) or 1.25M maltodextrin in 1% agar or 1% agar alone as a water control. The number of flies still alive in each vial was counted every 12hr. Each data point represents the mean of 10 separate vials per condition.
Proboscis Extension Reflex (PER) Assay
PER was performed similarly to as described in [4, 19, 21] with the following modifications. Groups of 3–7 day old wild-type flies were food deprived as described above for 24hr. The flies were then anesthetized for 1min by placing them in a cold test tube immersed in a 4°C ice bath. Flies were stuck back-down onto non-toxic adhesive fly paper and left to recover for 1.5hr at 25°C/ 60% relative humidity. To assay PER each fly was either presented to the fore-leg or labellum with the following regimen on a rolled Kim-wipe wick: water (negative control), test compound, 3M sucrose (positive control). Test compounds were presented 3 times per fly and each fly was exposed to only one of the test substances flanked by the water and sucrose controls on either side. Data are presented as the percentage of presentations that elicited PER from the total number of presentations. Flies that extended their proboscis to water alone or that failed to extend to 3M sucrose at the end, were discounted from the analysis. Data were analyzed using the Yates’ chi-square test.
Ingestion Assay
This assay was inspired by previous work [29]. All flies were food deprived before testing by storing them for 16–20hr in glass milk bottles containing water dampened 3MM filter paper to prevent desiccation. Sugars were prepared as 3M solutions with 0.4% FD & C Blue No. 1 food dye (Spectrum Chemical) and 2.5ml was pipetted onto a 2.25 inch X 3 inch piece of 3MM paper and allowed to dry. The papers were inserted in the training chamber of the olfactory appetitive conditioning paradigm [10]. 100 flies were loaded and given 5min to feed in the presence of airflow. Flies were then removed from the training chamber, immediately chilled to prevent excretion and homogenized in 1ml of Phosphate Buffered Saline (PBS; 1.86mM NaH2PO4, 8.41mM Na2HPO4 and 175mM NaCl). Following clearance of debris by centrifugation the dye in the supernatant was quantified by measuring the absorbance at 625nm. All n≥8.
Supplementary Material
Acknowledgments
We thank Hubert Amrein for flies, Teiichi Tanimura, Geraldine Wright, Robert Gegear and members of the Waddell lab for discussion. This work was supported by NIH MH09883 and MH081982 to S.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dethier VG. The Hungry Fly - a Physiological Study of the Behaviour Associated with Feeding. Harvard University Press; 1976. [Google Scholar]
- 3.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Breslin PA, Kemp S, Beauchamp GK. Single sweetness signal. Nature. 1994;369(6480):447–448. doi: 10.1038/369447a0. [DOI] [PubMed] [Google Scholar]
- 7.Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: implications for the neural coding of T1R ligands. J Neurosci. 2007;27:11242–11253. doi: 10.1523/JNEUROSCI.1227-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masek P, Scott K. Limited taste discrimination in Drosophila. Proc Natl Acad Sci U S A. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colomb J, Kaiser L, Chabaud MA, Preat T. Parametric and genetic analysis of Drosophila appetitive long-term memory and sugar motivation. Genes Brain Behav. 2009;8:407–415. doi: 10.1111/j.1601-183X.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 12.Simpson SJ, White PR. Associative learning and locust feeding: evidence for a ‘learned hunger’ for protein. Animal Behaviour. 1990;40:506–513. [Google Scholar]
- 13.Raubenheimer D, Tucker D. Associative learning by locusts: pairing of visual cues with consumption of protein and carbohydrate. Animal Behaviour. 1997;54:1449–1459. doi: 10.1006/anbe.1997.0542. [DOI] [PubMed] [Google Scholar]
- 14.Bitterman ME, Menzel R, Fietz A, Schafer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J Comp Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- 15.Zhang S, Bock F, Si A, Tautz J, Srinivasan MV. Visual working memory in decision making by honey bees. Proc Natl Acad Sci U S A. 2005;102:5250–5255. doi: 10.1073/pnas.0501440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wigglesworth VB. The utilization of reserve substances in Drosophila during flight. J Exp Biol. 1949;26:150–163. doi: 10.1242/jeb.26.2.150. [DOI] [PubMed] [Google Scholar]
- 17.Hassett CC. The utilization of sugars and other substances by Drosophila. Biological Bulletin. 1948:114–123. [PubMed] [Google Scholar]
- 18.Amrein H, Thorne N. Gustatory perception and behavior in Drosophila melanogaster. Curr Biol. 2005;15:R673–84. doi: 10.1016/j.cub.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. 2007:193. doi: 10.3791/193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 23.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 24.Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- 25.Wright GA, Mustard JA, Kottcamp SM, Smith BH. Olfactory memory formation and the influence of reward pathway during appetitive learning by honey bees. J Exp Biol. 2007;210:4024–4033. doi: 10.1242/jeb.006585. [DOI] [PubMed] [Google Scholar]
- 26.Wright GA, Mustard JA, Simcock NK, Ross-Taylor AAR, McNicholas LD, Popescu A, Marion-Poll F. Parallel reinforcement pathways for conditioned food aversions in the honeybee. Curr Biol. 2010;20:2234–2240. doi: 10.1016/j.cub.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Chambers ES, Bridge MW, Jones DA. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J Physiol. 2009;587:1779–1794. doi: 10.1113/jphysiol.2008.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanimura T, Isono K, Takamura T, Shimada I. Genetic dimorphism in the taste sensitivity to trehalose in Drosophila melanogaster. J Comp Physiol [A] 1982;147:433–437. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.