Abstract
Acupuncture is shown to be effective in producing analgesia in ankle sprain pain in humans and animals. To examine the underlying mechanisms of the acupuncture-induced analgesia, the effects of electroacupuncture (EA) on weight-bearing forces (WBR) of the affected foot and dorsal horn neuron activities were examined in a rat model of ankle sprain. Ankle sprain was induced manually by overextending ligaments of the left ankle in the rat. Dorsal horn neuron responses to ankle movements or compression were recorded from the lumbar spinal cord using an in vivo extracellular single unit recording setup 1 day after ankle sprain. EA was applied to the SI-6 acupoint on the right forelimb (contralateral to the sprained ankle) by trains of electrical pulses (10 Hz, 1-ms pulse width, 2-mA intensity) for 30 min. After EA, WBR of the sprained foot significantly recovered and dorsal horn neuron activities were significantly suppressed in ankle-sprained rats. However, EA produced no effect in normal rats. The inhibitory effect of EA on hyperactivities of dorsal horn neurons of ankle-sprained rats was blocked by the α-adrenoceptor antagonist phentolamine (5 mg/kg ip) but not by the opioid receptor antagonist naltrexone (10 mg/kg ip). These data suggest that EA-induced analgesia in ankle sprain pain is mediated mainly by suppressing dorsal horn neuron activities through α-adrenergic descending inhibitory systems at the spinal level.
Keywords: acute ankle sprain, α-adrenergic descending inhibition
clinical and experimental studies have suggested that acupuncture at certain acupoints has therapeutic effects in various painful conditions (Kim et al. 2010; Manheimer et al. 2010). It has been reported that the number of individuals who received alternative medical care was estimated to be 2.1 million in America in 2002 (Tindle et al. 2005). The use of acupuncture treatment for joint sprains, in particular, increased ∼30% between 1990 and 1997 (Eisenberg et al. 1998). Thus, there is an increasing trend of using acupuncture for pain caused by musculoskeletal problems. According to the survey of American physicians who use alternative medical treatments, 76% of them have used acupuncture treatment for patients with ankle sprain and 88% of them indicated that acupuncture was effective (Diehl et al. 1997). While acupuncture is gaining popularity as a therapeutic intervention in the United States, the efficacy or therapeutic benefits are still debatable (Linde et al. 2005; Manheimer et al. 2009; Vickers et al. 2004a and 2004b), and the underlying mechanisms are not well understood.
Ankle sprain is one of the most common sports-related injuries (∼25% of all sports-related injuries) and involves trauma to the lateral ankle ligament complex (Hume and Gerrard 1998; Puffer 2001). Approximately one-third of the subjects that once had ankle sprain later experience recurring symptoms, such as swelling and persistent pain (Konradsen et al. 2002). Two separate components can contribute to persistent pain after ankle sprain: sensitization of nociceptors due to joint inflammation (peripheral sensitization) and sensitization of dorsal horn neurons (central sensitization) after intense nociceptive inputs. The contribution of peripheral sensitization in joint inflammation has been studied extensively, and inflammatory agents from damaged tissues and/or activated fine sensory fibers (neurogenic inflammation) (Ferrell et al. 1997; McDougall et al. 1997, 2006; Schaible et al. 2009) have been well documented. On the other hand, the central mechanism of ankle sprain pain has received much less attention.
In a previous study (Koo et al. 2002), our laboratory developed a rat model of ankle sprain with the severity equivalent to grade I or II of human ankle sprain. In this model, electroacupuncture (EA) applied to the SI-6 acupoint on the contralateral forelimb produced a significant analgesic effect (Koo et al. 2002). Behavioral studies with pharmacological manipulations indicated that the analgesic effects of EA on ankle sprain pain were through the noradrenergic system (Kim et al. 2010; Koo et al. 2002 and 2008). The aim of this study was to determine the effect of EA on dorsal horn neuron responses to ankle movements using the characteristics of these neurons in normal and acute ankle-sprained rats described in our companion paper (Kim et al. 2011). In addition, the parameters of effective EA and specific acupoints that produce changes in dorsal horn neurons excitability were examined.
MATERIALS AND METHODS
Experimental animals.
Adult male Sprague-Dawley rats (Harlan Sprague Dawley, Houston, TX) were used in this study (200∼350 g body wt). Rats were divided into two groups: normal and acute ankle sprained. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch. Animals were housed in plastic cages with soft bedding and were provided with free access to food and water under a 12:12-h reversed light-dark cycle. All animals were adapted for 7 days before the experiment.
Induction of ankle sprain.
Ankle sprain was induced manually as previously described (Koo et al. 2002; Kim et al. 2010) under halothane anesthesia (3% in air for induction and 1.5∼2% for maintenance). Briefly, the left hindfoot was overextended repeatedly in the direction of simultaneous inversion and plantarflexion for 60 times during a 1-min period with a gradual increase of bending force. The same procedures were repeated one more time. At the end, the ankle could be rotated to a position of 180° inversion. Anesthesia was discontinued, and rats recovered within 5–10 min. The resulting ankle sprain was similar to a grade I–II in humans with stretched and/or partially torn ligaments without complete rupture (Cotler 1984; Koo et al. 2002).
Behavioral testing.
To estimate the level of pain in ankle-sprained rats, the amount of weight-bearing force on the affected foot was measured on 1 day after the ankle sprain. On the day of behavioral testing, each rat was weighed and then allowed to walk through a long rectangular plastic tube (10-cm width, 10-cm height, and 60-cm length) with a scale (Acculab Pocket Pro 250-B, Newton, PA) located midway down the tube that only underlay the righthand half of the tube. Thus, when the rat walked in one direction, the weight on the left foot could be determined, and when the rat came down the other way, the weight placed on the right foot could be measured. The weight signals from the scale were fed into an oscilloscope, and the weight-bearing forces were calculated using a data-acquisition system (CED 1401 with the Spike 2 program, CED). To obtain a valid weight-bearing force of the foot, weight-bearing forces were measured six times, and the values were averaged for each measurement.
Drug administration.
Intraperitoneal injections of two pharmacological receptor blockers were tested on EA-induced effects on weight-bearing forces and dorsal horn activities. These were an opioid receptor blocker, naltrexone hydrochloride (10 mg/kg, Sigma, St. Louis, MO), and an α-adrenoceptor antagonist, phentolamine hydrochloride (5 mg/kg, Sigma).
EA.
The results of our previous studies (Koo et al. 2002 and 2008) showed that the contralateral SI-6 is the acupoint where the most powerful EA-induced analgesic effect was produced in ankle sprain pain. Thus, we chose this acupoint to study the EA effect on pain behaviors as well as the responses of dorsal horn neurons to ankle manipulations. In addition, the ST-36 acupoint, which did not produce an analgesic effect on ankle sprain pain, was used as a control EA point.
Under general anesthesia (1.0–1.5% halothane), a pair of stainless steel needles (0.3 mm in diameter) separated by 1 mm were inserted transcutaneously to a depth of 5 mm into either the SI-6 acupoint (Yanglao; the dorsolateral aspect of the head of ulna) of the contralateral forelimb or the ST-36 acupoint (Zusanli; on the proximal part of the tibialis anterior muscle ∼10 mm below the knee joint) of the ipsilateral hindlimb. For EA, various trains of pulses [2, 10, or 100 Hz, 1-ms pulse width, 2-mA intensity (10 times of the muscle twitch threshold)] were applied to the inserted needles for 30 min with an electrical stimulator (A300 and A385, WPI). The delivered current was monitored at all times, and the polarity was reversed every 60 s to prevent polarization of the electrodes. Immediately after the termination of EA, anesthesia was discontinued, and rats usually resumed full activity within 5–10 min. For control rats, needles were inserted at the same acupoint, but electrical stimulation was not applied. Behavior tests were done 30 min before and several times after the termination of EA.
Surgical preparations for electrophysiological recording.
Recordings were performed in normal rats (n = 17) and ankle-sprained rats 1 day after ankle sprain (n = 30). Rats were anesthetized with urethane (1.5 g/kg ip), and supplemental urethane (200 mg/kg ip) was given when a sign of low anesthesia level was detected. The depth of anesthesia was monitored by observing the heart rate, end-tidal CO2 level, and pupil size. The trachea was cannulated to ensure adequate respiration. The rat was paralyzed with pancuronium bromide (1 mg/kg iv for initiation and 0.4–0.6 mg·kg−1·h−1 iv for maintenance) and artificially ventilated at a rate of 80–100 breaths/min to maintain end-tidal Pco2 levels at 3.5–4.5%. A laminectomy was done to expose L4-L6 spinal cord segments, and the rat was then secured in a stereotaxic frame. The vertebral column was held tight by clamps caudal and rostral to the laminectomy region, and the dura was opened. The spinal cord was continually bathed in a pool of warm mineral oil. The core body temperature was monitored and maintained at 36.5–37°C by a heating blanket connected to a rectal thermal probe via an automatic feedback control unit. At the end of the experiment, rats were euthanized with an overdose of urethane (3 g/kg), and death was confirmed by opening the chest.
Recording of spinal dorsal horn neurons.
In ankle-sprained rats, extracellular single cell recordings were made from dorsal horn neurons in the spinal cord ipsilateral to the sprained ankle. Control recordings were made from the same region in normal rats. Recordings were made with a carbon fiber microelectrode (0.4–0.8 MΩ, Kation Scientific). The electrode was lowered into the cord using an electronic micromanipulator (Burigh), which allowed us to measure the depth of the recording site from the dorsal surface. Electrophysiological activities of dorsal horn neurons were fed into a preamplifier and an amplifier (CYBERAMP 320, Axon Instruments, Foster City, CA) and were displayed on an oscilloscope. The amplified signals were also fed into a window discriminator (WPI) with the Spike 2 program (version 4, CED) to isolate single unit recordings. Responses to ankle movement stimuli were stored in a computerized data-acquisition system (CED 140l, CED) for further analysis. Poststimulus histograms were then generated. To examine EA-induced neuronal changes, EA was applied to the contralateral SI-6 acupoint at 10 Hz, 1-ms pulse width, and 2 mA for 30 min.
Mechanical stimulations to the ankle.
Three different types of stimuli were used to activate deep sensory fibers in the ankle joint: plantarflexion, inversion, and compression. A special device equipped with a linear potentiometer was patched to the plantar surface of the rat hindpaw and used to measure movement angles. For plantarflexion stimulation, the foot was first brought to a tibiotarsal-tarsometatarsal angle of 90° as the starting point and then moved the foot to the 190° angle, the maximum plantarflexed position. For inversion stimulus movement, the foot was rotated medially from the resting position (0° on the horizontal plane) up to 120°. For each movement stimulus, the foot was slowly moved from the starting position to the maximally moved position during the first 2 s, held at the maximally moved position for 12 s, and then moved back to the starting position during the last 1 s. The range of each movement was set to where the joint showed appreciable resistance felt at the maximum point but did not induce excessive tissue injury. For the compression stimulus, the pressure was applied mediolaterally to the ankle using a pair of large blunt forceps (20-cm long, contact area: 4 × 4 mm) equipped with strain gauges (Yu et al. 2002). Each compression stimulus started from 0 g intensity but quickly reached to 1,500 g within 2 s and was then held at the maximum intensity for 13 s and quickly released.
Experimental design for evoked neuronal response measurement.
The deep dorsal horn neurons responding exclusively to plantarflexion, inversion, or compression stimuli to the ankle were examined. The neurons responding to cutaneous brush and/or pinch stimuli of the foot skin were thus excluded from the study. Once the neuron that responded to a specific stimulus was identified, spontaneous activities were recorded for 60 s before foot stimulation. These “background activities” were then subtracted from the responses to each stimulus. To establish reliable responses of each neuron, neuronal responses were recorded initially two to three times by repeating the same mechanical stimulus at 10-min intervals. When the responses to each stimulus produced no more than 10% variations from the original, they were considered as stable responses. Once the stable responses were recognized, the recordings were made three times with the same stimulus repeated three times, and the average of the three recordings was used as the average response to that stimulus.
Data analysis and statistics.
Two different response parameters were analyzed: mean evoked responses and afterdischarges. All evoked responses were adjusted by subtracting the averaged background discharges that were recorded without mechanical stimuli. The mean evoked response values were the averaged evoked discharges during the entire period of mechanical stimulation. Some neurons maintained increased activities after the stimuli were removed, and those were considered as afterdischarges. When afterdischarges were present, activities were recorded for 20 s after removal of the stimulus, and averaged values were then presented in the data. Mean neuronal response were measured three times at 10-min intervals, and averaged values of those three were then normalized as 100% and used as the baseline values before EA application. All data are presented as means ± SE. Statistical significance was analyzed using two-way ANOVA with one repeated factor followed by Duncan's multiple-comparison post hoc test using SigmaStat (version 3.0, SPSS). P values of <0.05 were considered to be significant.
RESULTS
EA at SI-6 produced analgesic effects through spinal adrenergic systems.
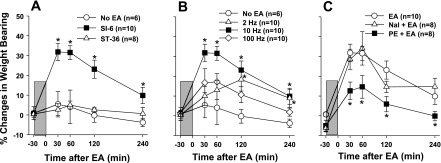
Three separate features of EA-induced analgesia were tested in this series of experiments: point specificity, stimulus parameters, and potential neurotransmitters involved. To test the point specificity, the effect of EA applied to two different locations, SI-6 or ST-36, on weight-bearing forces on the ankle-sprained hindlimb was examined; the data are shown in Fig. 1A. Since our previous studies (Koo et al. 2002 and 2008) showed that the contralateral SI-6 acupoint produced a powerful EA-induced analgesic effect in ankle sprain pain, we chose this acupoint to study the EA effect on pain behaviors as well as the responses of dorsal horn neurons to ankle manipulations. Data are presented as percent changes of weight-bearing forces before and after EA application. The weight-bearing forces 1 day after acute ankle sprain and immediately before EA application were used as a baseline value zero. Thirty minutes after EA application (1-ms pulses, 2 mA, 10 Hz, for 30 min) at the contralateral SI-6 acupoint, the weight-bearing forces of the affected limb increased significantly, and this analgesic effect lasted at least 4 h. On the other hand, EA applied to the ipsilateral ST-36 acupoint did not show significant change. Thus, the data indicate that there is a specificity of acupoint that induces an analgesic effect on ankle sprain pain.
Fig. 1.
Effects of electroacupuncture (EA) application (1-ms pulse, 2 mA, for 30 min) on pain behaviors in rats 1 day after ankle sprain. The EA period is indicated by the shaded boxes. Pain levels were determined by measuring the weight-bearing forces on the affected limb. A: point specificity of the EA-induced analgesic effect. The effect of EA (with 10 Hz) applied to the contralateral SI-6 acupoint was compared with that of the ipsilateral ST-36 acupoint. Only EA at SI-6 was effective in producing the analgesic effect on ankle sprain pain. B: frequency dependency of the EA-induced analgesic effect. EA with variable frequencies was applied to the SI-6 point. EA with 10-Hz stimulation produced a more powerful analgesic effect than either 2- or 100-Hz stimulation. C: effect of phentolamine (PE; 5 mg/kg ip) and naltrexone (Nal; 10 mg/kg ip) on EA-induced analgesia. Drugs were administered 30 min before EA application. The α-adrenoceptor antagonist PE but not the opioid receptor antagonist Nal was effective in blocking the EA-induced analgesic effect on ankle sprain pain. Data are presented as percent changes in weight-bearing forces (means ± SE) using the pre-EA (- 30 m) baseline value as zero. *Values were significantly different from the control group (no EA in A and B and EA in C).
To test whether the EA analgesic effect depends on stimulus parameters, the effects of EA with three different frequencies were tested. EA was applied to the contralateral SI-6 acupoint, and weight-bearing forces were then measured before and several times after EA application. The data of this experiment are shown in Fig. 1B. EA with 10-Hz stimulus frequency was the most effective in increasing the weight-bearing forces on the affected foot compared with those with 2 or 100 Hz. The data indicate that the stimulus parameter of EA is critical for the generation of effective analgesia and 10 Hz seems to be the optimum frequency of stimulation.
The third experiment was to identify the possible endogenous inhibitory system involved in EA analgesia. The possible involvement of two endogenous inhibitory systems (opioid and adrenergic) was examined using pharmacological receptor blockers. Either naltrexone (10 mg/kg), an opioid receptor antagonist, or phentolamine (5 mg/kg), an α-adrenoceptor antagonist, was administered intraperitonially 30 min before EA application. EA (1-ms pulses, 2 mA, 10 Hz) was applied to the contralateral SI-6 point for 30 min, and weight-bearing forces were measured before and several times after the EA application. As shown in Fig. 1C, systemic naltrexone did not block the EA-induced increase in weight-bearing forces of the ankle-sprained foot. On the other hand, systemic phentolamine significantly reduced the EA-induced increase in weight-bearing forces. The data suggest that EA-induced analgesia is mediated by the endogenous adrenergic inhibitory system and not by the opioid inhibitory system.
Dorsal horn neuron responses were reduced after EA.
Extracellular recordings were made from the deep dorsal horn (lamina IV-VII) neurons that responded to a specific ankle stimulus. Recordings were made 30 min before, during, and 0, 15, 30, and 60 min after the termination of EA application (shaded boxes in graphs) at the SI-6 acupoint for 30 min (Figs. 2–4). Responses were recorded in a total of 36 neurons (1 neuron/rat) from 17 normal rats and 19 ankle-sprained rats (1 day after ankle sprain). As in the study of the companion paper (Kim et al. 2011), the majority of neurons in both the normal and ankle-sprained rats responded exclusively to a specific afferent modality: plantarflexion, inversion, or compression. Neurons responding to multiple stimuli were rare and were excluded in this study.
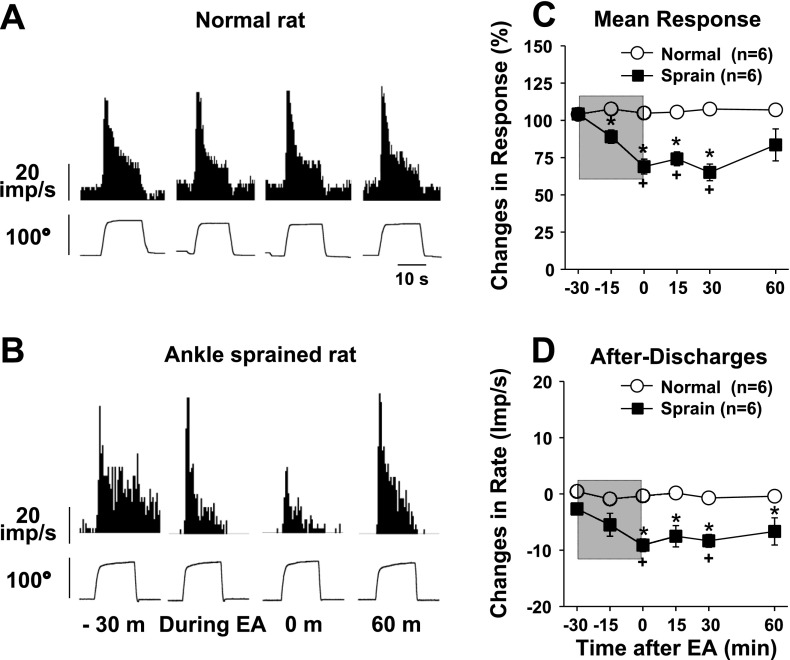
Fig. 2.
Changes in dorsal horn neuron responses to plantarflexion of the foot before and after EA in normal rats (n = 6) and ankle-sprained rats (n = 6). The EA period is indicated by the shaded boxes. A and B: examples of recorded activity evoked by plantarflexion movement (bottom traces show changes of the plantarflexion angle) in a normal rat (A) and an ankle-sprained rat (B). C and D: summary data of six normal rats and six ankle-sprained rats. m, minute. *Values were significantly different from the normal group; +values were significantly different from the pre-EA value (−30 min).
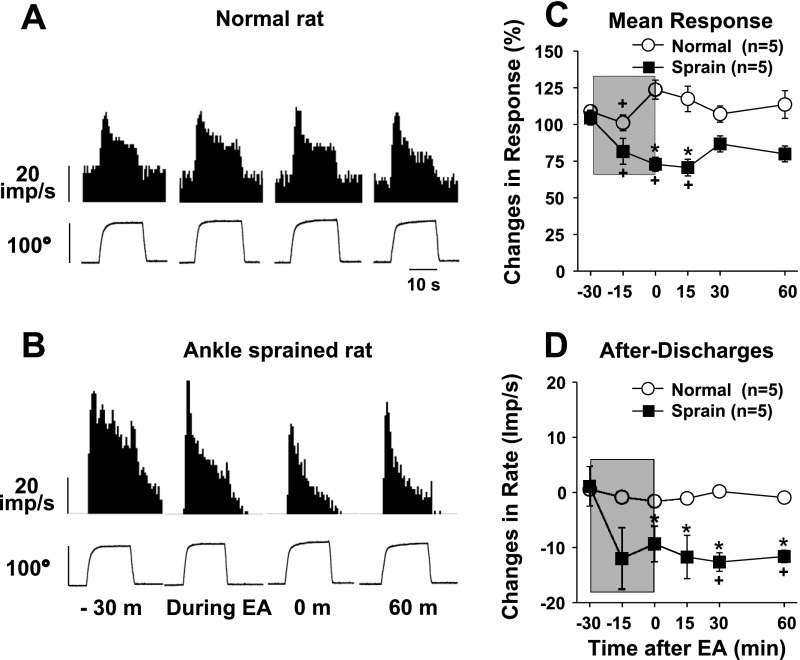
Fig. 3.
Changes in dorsal horn neuron responses to inversion of the foot before and after EA in normal rats (n = 5) and ankle-sprained rats (n = 5). The EA period is indicated by the shaded boxes. A and B: examples of recorded activity evoked by inversion movement (bottom traces show changes of the inversion angle) in a normal rat (A) and an ankle-sprained rat (B). C and D: summary data of five normal rats and five ankle-sprained rats. *Values were significantly different from the normal group; +values were significantly different from the pre-EA value (−30 min).
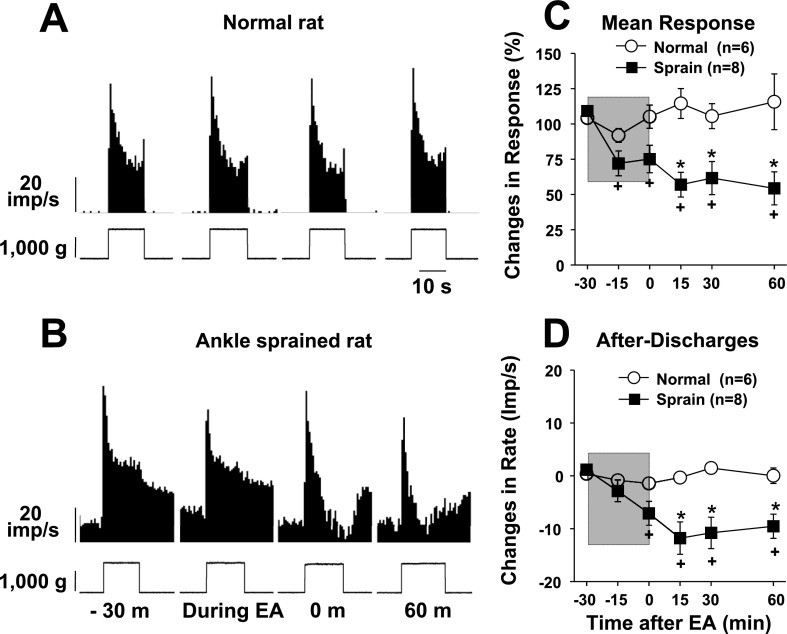
Fig. 4.
Changes in dorsal horn neuron responses to compression applied to the ankle joint before and after EA in normal rats (n = 6) and ankle-sprained rats (n = 8). The EA period is indicated by the shaded boxes. A and B: examples of recorded activity evoked by compression applied to the ankle joint (bottom traces show changes in the compression force) in a normal rat (A) and an ankle-sprained rat (B). C and D: summary data of six normal rats and eight ankle-sprained rats. *Values were significantly different from the normal group; +values were significantly different from the pre-EA value (−30 min).
EA effects on the activities of plantarflexion neurons from six normal rats and six ankle-sprained rats are shown in Fig. 2. Examples of actual recordings are shown in Fig. 2, A and B, and the summary of response changes is shown in Fig. 2, C and D. In normal rats, plantarflexion neurons showed strong evoked activity in response to plantarflexion, and the activity stopped when the foot was returned to the resting position (Fig. 2A). Furthermore, the response activities to plantarflexion movement did not change either during or after EA application in normal rats (Fig. 2, A, C, and D). On the other hand, in ankle-sprained rats, EA application significantly reduced the response activities of plantarflexion neurons along with afterdischarges (Fig. 2, B–D). The EA-induced inhibition lasted for at least 30 min after the termination of EA.
The activities of the dorsal horn neurons responding to inversion of the foot were recorded in five neurons in normal rats and five neurons from rats 1 day after ankle sprain. Typical response patterns are shown in Fig. 3A for normal rats and in Fig. 3B for ankle-sprained rats. Averaged data are shown in Fig. 3, C and D. Similar to plantarflexion neurons, EA produced inhibition of the evoked response of inversion neurons recorded from ankle-sprained rats but not those recorded from normal rats. The afterdischarges (which developed in ankle-sprained rats) were also significantly reduced after EA for a long period of time.
The activities of the dorsal horn neurons responding to ankle compression were recorded in six neurons in normal rats and eight neurons in ankle-sprained rats. Typical response patterns are shown in Fig. 4A for normal rats and in Fig. 4B for ankle-sprained rats. Averaged data are shown in Fig. 4, C and D. Again, the response pattern to ankle compression was similar to the results of the study done in the companion paper (Kim et al. 2011). As with plantarflexion and inversion neurons, EA produced inhibition of the evoked responses of compression neurons as well as afterdischarges.
Phentolamine blocked the EA-induced reduction of dorsal horn neuron responses.
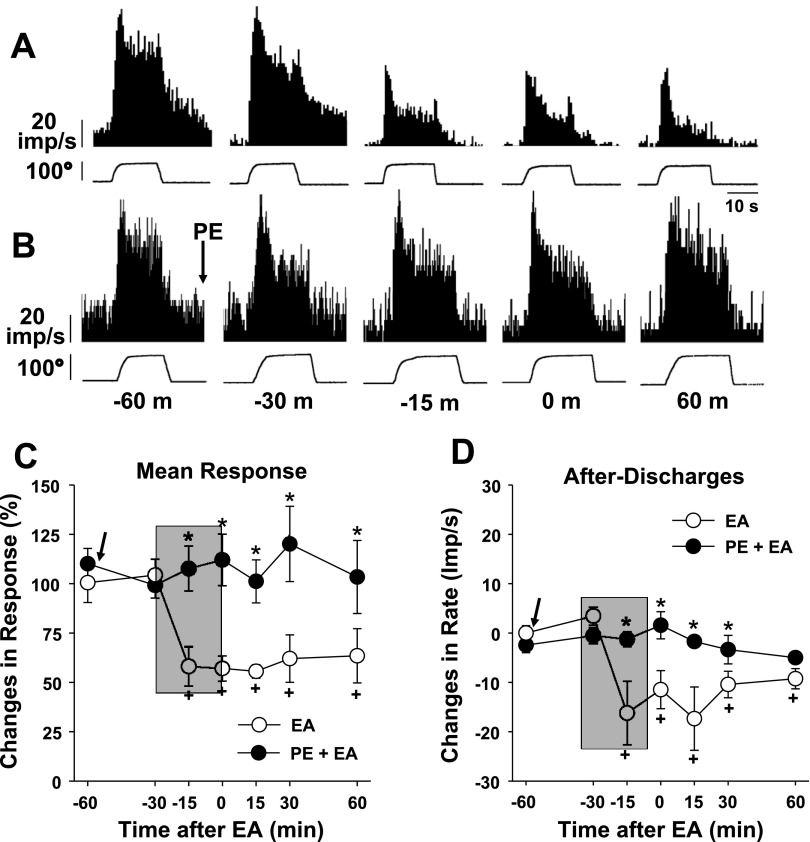
The behavioral experiments showed that the EA-induced analgesia was partially blocked by phentolamine (Fig. 1C), thus suggesting that the EA-induced analgesia may be mediated by α-adrenoceptors. To confirm this in neuronal activity, the effect of phentolamine on dorsal horn neuron responses was examined. In this part of the study, activities of the dorsal horn neurons that responded to plantarflexion (n = 4), inversion (n = 3), or compression (n = 4) were recorded in ankle-sprained rats before, during, and after EA application with or without phentolamine administration (5 mg/kg ip). Two examples of recordings are shown in Fig. 5A (without phentolamine) and Fig. 5B (with phentolamine injection 60 min before EA application). Because EA-induced inhibitions were similar among the neurons responding to plantarflexion, inversion, or compression, the results were pooled together from all 11 neurons recorded regardless of stimulus modality, and the summary data are shown in Fig. 5, C and D. As shown in Fig. 5A, EA produced a significant inhibition of dorsal horn neuron responses to inversion without phentolamine treatment. On the other hand, when the rat was pretreated with phentolamine, EA no longer produced the inhibition (Fig. 5B). The summary data also show that the mean responses and afterdischarges were significantly reduced by EA (4 neurons, open circles), but this EA inhibition was completely blocked by phentolamine pretreatment (7 neurons, filled circles). In contrast, dorsal horn neuron responses to ankle stimuli recorded in the normal rat were not affected either by EA application or with the same dose of phentolamine. Thus, the data suggest that EA-induced inhibition of dorsal horn neuron responses to ankle stimuli is mediated by α-adrenoceptors, confirming the result of the behavioral experiments.
Fig. 5.
Effects of PE (5 mg/kg ip) pretreatment on the EA-induced inhibition of dorsal horn neuron activity in ankle-sprained rats. The EA period is indicated by the shaded boxes. A and B: examples of dorsal horn neuron responses to inversion of the foot before (−60 and −30 min), during (−15 min), and after (0 and 60 min) EA stimulation without (A) and with (B) PE pretreatment. C: changes of the averaged mean responses from 11 neurons (4 plantarflexion-, 3 inversion-, and 4 compression-responsive neurons combined). D: changes of averaged afterdischarges. Arrows indicate the time of PE injection on seven neurons (●) but not four neurons (○). *Values were significantly different from the control (EA) group; +values were significantly different from the pre-EA value (at −60 m).
DISCUSSION
The present study examined the EA effect on the activities of dorsal horn neurons in ankle-sprained rats and compared this with the EA effect on pain behaviors. Activities of the deep dorsal horn neurons responding to plantarflexion, inversion, or compression were significantly reduced by EA application to the contralateral SI-6 acupoint in ankle-sprained rats. The EA stimulus parameter of 10 Hz was more effective in inducing analgesia than either a lower (2 Hz) or higher (100 Hz) frequency. In addition, the α-adrenoceptor antagonist phentolamine significantly reduced both EA-induced analgesia and dorsal horn neuron responses. Thus, the data suggest that the reduction of dorsal horn neuron activities is the neural substrate for EA-induced analgesia.
In general, ankle sprain involves a stretch or tear of the lateral ligament complex of the ankle joint, the anterior and posterior talofibular and calcaneofibular ligaments, during an unanticipated sudden hyperplantarflexion and inversion (Safran et al. 1999). Although traditional conservative therapies on ankle sprain are quite effective (Karlsson 1998), about a third of patients suffer from persistent pain, swelling, or recurrent sprain (Anandacoomarasamy and Barnsley 2005; Kern-Steiner et al. 1999; Konradsen et al. 2002; Koo et al. 2002). Recent surveys have indicated that acupuncture has been used for treating musculoskeletal disorders (Cai 2010; Freedman 2002; Song 1993; Yang et al. 2009; Yao-chi et al. 2007; Zhou and Liu 2003), including ankle sprain (Diehl et al. 1997; He and Xu 2006; Park et al. 2004; Zhang and Miao 1990). Although the popularity of acupuncture has increased in recent years, its underlying mechanism is not clear. The main purpose of this study was to establish the basis of neural mechanisms of the effect of acupuncture in ankle sprain pain. After the study in the companion paper (Kim et al. 2011), which characterized the response properties of the dorsal horn neurons to ankle stimulation, the present study examined their responses to EA application. The present study shows that EA produced a significant reversal of enhanced evoked responses as well as afterdischarges developed in ankle-sprained rats. Thus, the data indicate that ankle sprains sensitize dorsal horn neurons and that this sensitization is reversed by EA. On the other hand, EA does not produce any effect on normal dorsal horn neuron responses.
Although it is clear that EA transiently reverses sensitization of dorsal horn neurons, the mechanism underlying how EA produces such a reversal is not clear. The fact that EA applied to the forelimb produces its action on the lumbar dorsal horn neurons suggests that one or more endogenous descending inhibitory systems are involved. One such well-known system is the opioid system; thus, EA may have produced its action through the endogenous opioid inhibitory system. In fact, several previous studies have indicated that EA-induced analgesia might be mediated by the opioid system (Han 2003; Kim et al. 2004; Mayer et al. 1977; Wang et al. 2008; Zhang et al. 2004a) since EA analgesia was blocked by opioid receptor antagonists. In contrast, several other studies have suggested that EA-induced analgesia is mediated by the adrenergic inhibitory system or nonopioid system (Kim et al. 2005; Kim et al. 2010; Koo et al. 2002 and 2008; Laitinen 1982; Zhang et al. 2004b). If the opioid system is the main mechanism underlying EA-induced analgesia in ankle sprain pain, we would have expected that the opioid receptor antagonist naltrexone would have a significant reduction in EA-induced analgesia. However, the behavioral data showed that naltrexone had a minimum effect. On the other hand, an α-adrenoceptor antagonist, phentolamine, produced a significant reduction in EA-induced analgesia, suggesting that adrenoceptors are importantly involved in EA-induced analgesia. Similarly, enhanced responses of the dorsal horn neurons after ankle sprain were significantly reduced by EA. This EA-induced reduction in dorsal horn neuron responses was also blocked by phentolamine. Thus, both the behavioral and electrophysiological experiments suggest that the endogenous descending adrenergic system could be involved in EA-induced analgesia in ankle sprain pain and that the system is mediated by α-adrenoceptors. Another possibility is that the phentolamine effect is through either peripheral or presynaptic α-adrenoceptors (Moon et al. 1999). Considering that EA-induced analgesia in ankle sprain pain is almost completely inhibited by intrathecal phentolamine (Koo et al. 2008), it is likely that this phentolamine effect is primarily through spinal actions. Furthermore, the presence of presynaptic α-adrenoceptors in the spinal cord has not been shown. Thus, we speculate that EA-induced analgesia in ankle sprain pain is mediated by the descending adrenergic system and postsynaptic α-adrenoceptors.
The reasons why some EA-induced analgesia is blocked by α-adrenergic antagonists while others are more affected by opioid inhibitors are not clear. The possible factors include 1) EA applied to different acupoints may activate different inhibitory systems, 2) EA may activate multiple inhibitory systems and different pain conditions (and animal models) may require different inhibitory systems to produce analgesia, and/or 3) different EA stimulus parameters may activate different inhibitory systems. Further studies are warranted to sort out these and other possibilities of the endogenous inhibitory systems that are involved in EA-induced analgesia.
Our data showed that the stimulus frequency of 10 Hz was most effective in producing analgesia in ankle sprain pain compared with 2 or 100 Hz. Thus, the present results suggest that the efficacy of the EA-induced analgesic effect is dependent on stimulus parameters. In previous studies, different analgesic effects were induced depending on the stimulus parameters with EA (Hahm 2007; Lao et al. 2004; Park et al. 2006; Yang et al. 2010) or transcutaneous electrical nerve stimulation (Sluka et al. 2000) in animals. The reasons for the differential efficacy of different stimulus parameters are not entirely clear. One possibility is that different stimulation frequencies may activate different sets of primary afferents and, thus, transmit inputs to different parts of the central nervous system. Another possibility is that a certain frequency of stimulus is more effective in activating a specific population of afferent fibers, which initiate an analgesic effect. Further study is warranted to identify the most effective stimulus parameters of EA and the underlying mechanisms of the differential efficacy.
Traditionally, acupuncture treatment involves the insertion of needles to a certain depth at specific acupoints designated to specific diseases, suggesting the existence of point specificity of acupoints (Zaslawski et al. 2003). The present study shows that EA at contralateral SI-6, but not ipsilateral ST-36, is effective in producing analgesia in ankle-sprained rats. The exact nature of the acupoints is not known. A handful of studies, however, have suggested that the distribution of acupoints is related to peripheral nerve routes and, thus, performing acupuncture is a form of stimulation of a specific peripheral nerve. For example, low-frequency EA applied to the ST-36 acupoint produced μ-opioid-dependent analgesia (Han 2003), and ST-36 is located on the tibial nerve route. Furthermore, low-frequency stimulation of the tibial nerve has been shown to inhibit the activities of spinothalamic tract neurons in an opioid-dependent manner in primates (Chung et al. 1984a and 1984b). Thus, it is possible that the specificity of EA is related to the stimulation of a specific peripheral nerve located at that acupoint. While we still use specific acupoints to treat certain diseases based on previous records or experience, the identity of acupoints and their mechanism of producing specific outcomes need to be explored in the future.
It is interesting to note the difference between opioid- and α-adrenoceptor-mediated analgesic systems. Both morphine (the study in the companion paper) and EA (the present study) reduce responses of dorsal horn neurons to ankle stimulation. However, the two are mediated by different mechanisms since naltrexone reverses morphine but not EA-induced reduction of dorsal horn neuronal responses. Therefore, analgesic effects can be achieved by multiple mechanisms, such as opioid receptor and α-adrenoceptors. However, the EA-induced analgesic effect in ankle sprain pain seems to be mediated by one of such mechanisms, the α-adrenoceptor-mediated analgesic system.
In conclusion, the present study demonstrated that EA at the SI-6 acupoint in the contralateral forelimb produces a long-lasting inhibition of responses of dorsal horn neurons to ankle stimulation in ankle-sprained rats. The suppression was likely accomplished through activation of a descending adrenergic inhibitory system and α-adrenergic receptors in the spinal cord. Future studies are warranted for the delineation of much more detailed mechanisms including an exploration of the supraspinal origin of this EA-induced descending adrenergic analgesic system.
GRANTS
This work was supported by National Institutes of Health Grants R01-AT-01474, P01-NS-11255, and R01-NS-31680. J. H. Kim was supported, in part, by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (no. R13-2008-028-01000-0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Griselda Gonzales for editing the manuscript.
REFERENCES
- Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med 39: e14, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai DF. Warm-needling plus Tuina relaxing for the treatment of carpal tunnel syndrome. J Tradit Chin Med 30: 23–24, 2010. [DOI] [PubMed] [Google Scholar]
- Chung JM, Fang ZR, Hori Y, Lee KH, Willis WD. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain 19: 259–275, 1984a. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Hori Y, Endo K, Willis WD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain 19: 277–293, 1984b. [DOI] [PubMed] [Google Scholar]
- Cotler JM. Lateral ligamentous injuries of the ankle. In: Traumatic Disorders of the Ankle, edited by Hamilton WC. New York, NY: Springer-Verlag, 1984, p. 113– 277–123. [Google Scholar]
- Diehl DL, Kaplan G, Coulter I, Glik D, Hurwitz EL. Use of acupuncture by American physicians. J Altern Complement Med 3: 119–126, 1997. [DOI] [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA 280: 1569–1575, 1998. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, McDougall JJ, Bray RC. Spatial heterogeneity of the effects of calcitonin gene-related peptide (CGRP) on the microvasculature of ligaments in the rabbit knee joint. Br J Pharmacol 121: 1397–1405, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J. Acupuncture for carpal tunnel syndrome. Acupunct Med 20: 39–40, 2002. [DOI] [PubMed] [Google Scholar]
- Hahm TS. The effect of 2 Hz and 100 Hz electrical stimulation of acupoint on ankle sprain in rats. J Korean Med Sci 22: 347–351, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci 26: 17–22, 2003. [DOI] [PubMed] [Google Scholar]
- He XF, Xu HB. Observation on therapeutic effect of acupuncture at Yanglingquan (GB 34) on sprain of external ankle joint. Zhongguo Zhen Jiu 26: 569–570, 2006. [PubMed] [Google Scholar]
- Hume PA, Gerrard DF. Effectiveness of external ankle support. Bracing and taping in rugby union. Sports Med 25: 285–312, 1998. [DOI] [PubMed] [Google Scholar]
- Karlsson J. Ligament injuries of the ankle–what happens later? Non-surgical treatment is effective in 80–90 per cent of cases. Lakartidningen 95: 4376–4378, 1998. [PubMed] [Google Scholar]
- Kern-Steiner R, Washecheck HS, Kelsey DD. Strategy of exercise prescription using an unloading technique for functional rehabilitation of an athlete with an inversion ankle sprain. J Orthop Sports Phys Ther 29: 282–287, 1999. [DOI] [PubMed] [Google Scholar]
- Kim HW, Kwon YB, Han HJ, Yang IS, Beitz AJ, Lee JH. Antinociceptive mechanisms associated with diluted bee venom acupuncture (apipuncture) in the rat formalin test: involvement of descending adrenergic and serotonergic pathways. Pharmacol Res 51: 183–188, 2005. [DOI] [PubMed] [Google Scholar]
- Kim HY, Koo ST, Kim JH, An K, Chung K, Chung JM. Electroacupuncture analgesia in rat ankle sprain pain model: neural mechanisms. Neurol Res 32, Suppl 1: 10–17, 2010. [DOI] [PubMed] [Google Scholar]
- Kim JH, Min BI, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res 998: 230–236, 2004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim HY, Chung K, Chung JM. Responses of spinal dorsal horn neurons to foot movements in rats with a sprained ankle. J Neurophysiol. First published March 9, 2011; doi.10.1152/jn.00852.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T. Seven years follow-up after ankle inversion trauma. Scand J Med Sci Sports 12: 129–135, 2002. [DOI] [PubMed] [Google Scholar]
- Koo ST, Lim KS, Chung K, Ju H, Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain 135: 11–19, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo ST, Park YI, Lim KS, Chung K, Chung JM. Acupuncture analgesia in a new rat model of ankle sprain pain. Pain 99: 423–431, 2002. [DOI] [PubMed] [Google Scholar]
- Laitinen LV. Inhibition of cutaneous nociception by deep musculoskeletal pain. A clinical observation. Pain 13: 373–377, 1982. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang RX, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res 1020: 18–29, 2004. [DOI] [PubMed] [Google Scholar]
- Linde K, Streng A, Jurgens S, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes MG, Weidenhammer W, Willich SN, Melchart D. Acupuncture for patients with migraine: a randomized controlled trial. JAMA 293: 2118–2125, 2005. [DOI] [PubMed] [Google Scholar]
- Manheimer E, Cheng K, Linde K, Lao L, Yoo J, Wieland S, van der Windt DA, Berman BM, Bouter LM. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev: CD001977, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manheimer E, Wieland S, Kimbrough E, Cheng K, Berman BM. Evidence from the Cochrane Collaboration for Traditional Chinese Medicine therapies. J Altern Complement Med 15: 1001–1014, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res 121: 368–372, 1977. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Bray RC, Sharkey KA. Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints. Anat Rec 248: 29–39, 1997. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Larson SE. Nociceptin/orphanin FQ evokes knee joint pain in rats via a mast cell independent mechanism. Neurosci Lett 398: 135–138, 2006. [DOI] [PubMed] [Google Scholar]
- Moon DE, Lee DH, Han HC, Xie J, Coggeshall RE, Chung JM. Adrenergic sensitivity of the sensory receptors modulating mechanical allodynia in a rat neuropathic pain model. Pain 80: 589–595, 1999. [DOI] [PubMed] [Google Scholar]
- Park IB, Ahn CB, Choi BT. Effects of electroacupuncture with different frequencies on the glycoconjugate alterations in articular cartilage in the ankle joints of complete Freund's adjuvant-injected rats. Am J Chin Med 34: 417–426, 2006. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim WY, Baek ST, Lee SD, Kim KS. Comparison of superficial and deep acupuncture in the treatment of ankle sprain: a randomized controlled trial-pilot study. J Kor Acupunc Moxib Soc 21: 137–147, 2004. [Google Scholar]
- Puffer JC. The sprained ankle. Clin Cornerstone 3: 38–49, 2001. [DOI] [PubMed] [Google Scholar]
- Safran MR, Benedetti RS, Bartolozzi AR, 3rd, Mandelbaum BR. Lateral ankle sprains: a comprehensive review: part 1: etiology, pathoanatomy, histopathogenesis, and diagnosis. Med Sci Sports Exerc 31: S429–437, 1999. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, Vazquez E, Segond von Banchet G. Joint pain. Exp Brain Res 196: 153–162, 2009. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain 4: 185–193, 2000. [DOI] [PubMed] [Google Scholar]
- Song Z. Treatment of 1000 cases of lumbar soft tissue injury with acupuncture plus exercise. J Tradit Chin Med 13: 19–21, 1993. [PubMed] [Google Scholar]
- Tindle HA, Davis RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine by US adults: 1997–2002. Altern Ther Health Med 11: 42–49, 2005. [PubMed] [Google Scholar]
- Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, Fisher P, Van Haselen R. Acupuncture for chronic headache in primary care: large, pragmatic, randomised trial. Br Med J 328: 744, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, Fisher P, Van Haselen R, Wonderling D, Grieve R. Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis. Health Technol Assess 8: iii and 1–35, 2004b. [DOI] [PubMed] [Google Scholar]
- Wang SM, Kain ZN, White P. Acupuncture analgesia: I. The scientific basis. Anesth Analg 106: 602–610, 2008. [DOI] [PubMed] [Google Scholar]
- Yang CP, Hsieh CL, Wang NH, Li TC, Hwang KL, Yu SC, Chang MH. Acupuncture in patients with carpal tunnel syndrome: a randomized controlled trial. Clin J Pain 25: 327–333, 2009. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Koo ST, Kim YS, Lee JE, Hwang HS, Lee MS, Choi SM. Contralateral electroacupuncture pretreatment suppresses carrageenan-induced inflammatory pain via the opioid-mu receptor. Rheumatol Int; http://www.springerlink.com/content/1673321570688482/ [DOI] [PubMed]
- Yao-chi W, Bi-meng Z, Chong-miao W, Jun-feng Z, Ping S, Liu GZ. [Observation on short-term and long-term therapeutic effects of electroacupuncture at Houxi (SI 3) on acute lumbar sprain]. Zhongguo Zhen Jiu 27: 3–5, 2007. [PubMed] [Google Scholar]
- Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Methods 115: 107–113, 2002. [DOI] [PubMed] [Google Scholar]
- Zaslawski CJ, Cobbin D, Lidums E, Petocz P. The impact of site specificity and needle manipulation on changes to pain pressure threshold following manual acupuncture: a controlled study. Complement Ther Med 11: 11–21, 2003. [DOI] [PubMed] [Google Scholar]
- Zhang F, Miao Y. Acupuncture treatment for sprains of the ankle joint in 354 cases. J Tradit Chin Med 10: 207–208, 1990. [PubMed] [Google Scholar]
- Zhang RX, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res 1020: 12–17, 2004a. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Zhang JS, Yung KK, Zhang HQ. Non-opioid-dependent anti-inflammatory effects of low frequency electroacupuncture. Brain Res Bull 62: 327–334, 2004b. [DOI] [PubMed] [Google Scholar]
- Zhou S, Liu M. Thirty cases of acute lumbar sprain treated by acupuncture combined with point-injection at tianzhu. J Tradit Chin Med 23: 203–204, 2003. [PubMed] [Google Scholar]