Abstract
Errors in sound localization, associated with age-related changes in peripheral and central auditory function, can pose threats to self and others in a commonly encountered environment such as a busy traffic intersection. This study aimed to quantify the accuracy and precision (repeatability) of free-field human sound localization as a function of advancing age. Head-fixed young, middle-aged, and elderly listeners localized band-passed targets using visually guided manual laser pointing in a darkened room. Targets were presented in the frontal field by a robotically controlled loudspeaker assembly hidden behind a screen. Broadband targets (0.1–20 kHz) activated all auditory spatial channels, whereas low-pass and high-pass targets selectively isolated interaural time and intensity difference cues (ITDs and IIDs) for azimuth and high-frequency spectral cues for elevation. In addition, to assess the upper frequency limit of ITD utilization across age groups more thoroughly, narrowband targets were presented at 250-Hz intervals from 250 Hz up to ∼2 kHz. Young subjects generally showed horizontal overestimation (overshoot) and vertical underestimation (undershoot) of auditory target location, and this effect varied with frequency band. Accuracy and/or precision worsened in older individuals for broadband, high-pass, and low-pass targets, reflective of peripheral but also central auditory aging. In addition, compared with young adults, middle-aged, and elderly listeners showed pronounced horizontal localization deficiencies (imprecision) for narrowband targets within 1,250–1,575 Hz, congruent with age-related central decline in auditory temporal processing. Findings underscore the distinct neural processing of the auditory spatial cues in sound localization and their selective deterioration with advancing age.
Keywords: presbycusis, spatial localization, binaural hearing, spatial channels, temporal processing
errors in sound localization can impede communication and may pose threats to self and others in a commonly encountered environment such as a busy intersection. One of many factors that can hinder effective utilization of auditory spatial input is advancing age. Both peripheral and central age-related auditory deficits, such as high-frequency hearing loss or decline in temporal processing, can impact the utilization of the auditory spatial cues involved in sound localization. For instance, high-frequency peripheral hearing loss in older listeners has been associated with degradation in vertical and front-back discrimination of auditory space (Abel et al. 2000; Noble et al. 1994; Rakerd et al. 1998). However, well-documented deficits in central binaural and monaural temporal processing in the elderly have not yet been linked to a corresponding impairment in horizontal sound localization (Babkoff et al. 2002; Cranford et al. 1993; Frisina and Frisina 1997; Herman et al. 1977; Lister and Roberts 2005; Martin and Jerger 2005; Mazelova et al. 2003; Roberts and Lister 2004; Ross et al. 2007; Schneider and Hamstra 1999; Strouse et al. 1998; von Wedel et al. 1991). Indeed, despite the rich history of sound localization research, comprehensive quantification of age-related changes in human sound localization, selectively utilizing each spatial cue, remains to be explored.
The auditory spatial cues involved in sound localization include interaural time (and/or phase) and intensity differences (ITDs and IIDs, respectively) in azimuth and pinna/head-generated spectral cues in elevation that also assist front-back discrimination (Algazi et al. 2001; Asano et al. 1990; Middlebrooks 1992; Musicant and Butler 1984; Oldfield and Parker 1984b; Rayleigh 1907; Wightman and Kistler 1989b). For pure tones, ITDs are accompanied by interaural phase differences (IPDs) that are reliable only up to ∼1.5 kHz in humans due to phase ambiguity coupled with declining neuronal temporal coding (phase-locking) at higher frequencies (Dreyer and Delgutte 2006; Johnson 1980; Joris and Yin 1992; Klumpp and Eady 1956; Zwislocki and Feldman 1956). However, ITDs can also be extracted from the envelope of complex high-frequency signals, amplitude-modulated sounds, and filtered transients (Bernstein and Trahiotis 2002; Hafter et al. 1975; Henning 1974; Klumpp and Eady 1956; McFadden and Pasanen 1976; Middlebrooks and Green 1990; Yost et al. 1971). While ITDs dominate in horizontal localization when all spatial cues are available, IIDs and spectral cues are effective primarily at higher frequencies (Macpherson and Middlebrooks 2002; Middlebrooks and Green 1990, 1991; Shaw 1997; Shaw 1974; Wightman and Kistler 1992).
The frequency-dependent perceptual channels for ITDs, IIDs, and spectral cues have been investigated using band-limited auditory targets in young adults. As sound bandwidth is reduced, auditory spatial performance declines primarily in elevation and front-back discrimination (Butler 1986; Carlile et al. 1999; Middlebrooks 1992). Many studies largely agree that the distinct deterioration in vertical localization and front-back discrimination at narrower bandwidths results from the degradation or loss of useful high-frequency spectral cues, particularly spectral notches in the 6- to 12-kHz range (Burlingame and Butler 1998; Butler and Humanski 1992; Hebrank and Wright 1974; Hofman and Van Opstal 2003; King and Oldfield 1997; Langendijk and Bronkhorst 2002; Middlebrooks 1992; Oldfield and Parker 1984b; Roffler and Butler 1967; Shaw 1997), and this similarly occurs with advancing age (Abel et al. 2000; Noble et al. 1994; Rakerd et al. 1998).
Clearly, the dependence of spectral cues and IIDs upon high-frequency spectral information renders them vulnerable to age-related high-frequency hearing loss. Yet, surprisingly, IID thresholds obtained under headphones do not appear to worsen with age (Babkoff et al. 2002; Herman et al. 1977). Therefore, one might expect that free-field horizontal sound localization utilizing IIDs would not decline appreciably in older individuals, but this has not been verified to our knowledge.
In addition to impaired auditory performance due to peripheral hearing loss in the elderly, the findings also suggest central age-related deficits in temporal processing, including elevated gap detection and ITD/IPD thresholds (Babkoff et al. 2002; Cranford et al. 1993; Frisina and Frisina 1997; Grose and Mamo 2010; Herman et al. 1977; Lister and Roberts 2005; Martin and Jerger 2005; Mazelova et al. 2003; Pichora-Fuller and Schneider 1991; Roberts and Lister 2004; Ross et al. 2007; Schneider and Hamstra 1999; Strouse et al. 1998; von Wedel et al. 1991; Warren et al. 1978). In particular, the study by Ross et al. (2007), using both electrophysiological and behavioral measures, reported that older individuals exhibit processing changes in IPDs of amplitude-modulated tones at lower frequencies than younger listeners. Similar results have more recently been reported by Grose and Mamo (2010). Despite these findings, related deficits in free-field horizontal sound localization dependent on ITDs have not yet been documented in older listeners.
The main goal of the present study was to quantify the influence of aging on free-field sound localization reliant on different spatial cues in isolation and in combination. We predicted that sound localization would generally deteriorate in older individuals from a combination of age-related high-frequency hearing loss and central spatial processing deficits. Furthermore, based on the intriguing findings of Ross et al. (2007) and Grose and Mamo (2010), we predicted a deterioration in horizontal sound localization starting at lower frequencies within the ITD-relevant range in aging listeners even though hearing thresholds for our subjects in this range were very similar to young controls.
METHODS
Subjects
Volunteers were recruited from the Rochester community to participate in the current experiments. Nineteen young (9 male, 10 female; ages 19–41), 11 middle-aged (5 male, 6 female, ages 45–66), and 12 elderly (5 male, 7 female; ages 70–81) subjects took part in experiment 1, which addressed the influence of age on sound localization of wideband stimuli. Four young subjects from this group as well as three additional subjects (3 male, 4 female; ages 20–36) participated in a follow-up study (experiment 1A) to clarify the influence of stimulus level on sound localization for the same wideband stimuli. Eight young (4 male, 4 female; ages 19–37), seven middle-aged (4 male, 3 female; ages 51–66), and six elderly (3 male; 3 female; ages 71–81) volunteers participated in experiment 2, which focused on how aging influences the utilization of low-frequency ITD cues in horizontal sound localization of narrowband targets. Six young, seven middle-aged, and five elderly listeners took part in both studies.
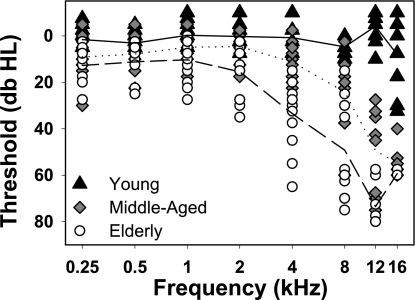
All subjects were free of neurologic or sensory abnormalities. Audiometric thresholds were obtained for all subjects (Fig. 1). Young listeners showed normal performance (ISO 2000). Middle-aged and elderly subjects had normal thresholds for their age, with mild hearing loss at low frequencies, and moderate to significant hearing loss with increasing age at higher frequencies. All underwent an initial practice visit (∼1 h) of acclimation to the laboratory and the experimental tasks.
Fig. 1.
Pure-tone audiograms for subjects in each age group. Each symbol represents a listener's hearing level threshold (in dB HL, right and left ear averaged) at a particular pure-tone frequency. Solid, dotted, and dashed lines indicate group average thresholds as a function of frequency within the young, middle-aged, and elderly group, respectively.
The study was performed in accordance with the 1964 Declaration of Helsinki. The protocols were approved by the University of Rochester Research Subjects Review Board. All subjects gave informed consent and were reimbursed for their participation.
Experimental Chamber, Target Apparatus, and Positioning
The experimental environment has been described previously (Cui et al. 2010a,b; Razavi et al. 2007; Zwiers et al. 2003). Subjects were seated in a fully enclosed and darkened room (3.0 x 3.7 × 2.7 m) lined with 3-in. acoustic foam (SONEXtextile, Illbruck Acoustic, Minneapolis, Minnesota). This material has a sound absorption index of 0.3 at 125 Hz and >0.99 at and >250 Hz (ASTM C 423–77). Beneath the acoustic foam, the room walls were seamlessly lined with a 1/8-in. (0.64 cm) layer of high-density vinyl (PROSPEC Barrier, 1 lb/ft2, 4.8 kg/m2; Illbruck Acoustic, Minneapolis, MN) to reduce intrusion of exogenous sounds (>125 Hz). The reverberation time (RT60) for a Gaussian broadband (0.1–20 kHz) sound source averaged ∼10 ms, irrespective of location. RT60 for a 250-Hz pure tone varied somewhat with location but did not exceed ∼35 ms.
The head was positioned and fixed by a personalized bite-bar 2 m from a cylindrical screen of acoustically transparent, black speaker cloth that concealed the target apparatus. The subject's “cyclopean eye” (midpoint between the eyes) was positioned at the geometric origin of the screen. Subjects on the bite-bar were instructed to align their head (nose) toward the center of the screen marked by a red light-emitting diode (LED; 0° horizontal; 0° vertical), thereby defining a subject's perceptual reference of ∼0° yaw and roll. Head pitch was aligned by setting Reid's baseline (an imaginary line extending from the inferior margin of the orbit to the superior aspect of the auditory meatus) parallel with the horizontal plane of the target presentation apparatus. The same head orientation was replicated across experimental sessions using each subject's custom bite-bar as a positioning reference.
The target assembly consisted of an 8-cm diameter (2.2° subtended angle) two-way coaxial loudspeaker (Blaupunkt PCx 352; Hildesheim, Germany) for presenting acoustic targets and a concentrically mounted red LED for back-projecting visual targets onto the screen (2-mm diameter, 0.1° subtended angle). The speaker/LED assembly was mounted on a two-axis servo-controlled robotic arm that rapidly and accurately positioned the target in cylindrical coordinates, covering a substantial portion of frontal audiovisual space (±60° horizontal; ±25° vertical) most relevant to multisensory and motor interaction. Prediction of target location was obscured by a random two-step target positioning algorithm to decouple target travel time from the actual change in target location across trials. Further, potential localization cues emanating from the motors and mechanical linkages during target movements between trials were masked by nondirectional Gaussian white noise [65 dB sound pressure level (SPL), A-weighted] broadcast from two loudspeakers that were positioned in the far periphery (±75° horizontal; 20° vertical; ∼40 cm behind the target screen), thereby minimizing concerns about auditory after effects (Carlile et al. 2001; Getzmann 2003; Thurlow and Jack 1973). The ambient background noise level was 35 dBA with the robotics stationary (i.e., during target presentation) and 55 dBA during target positioning (i.e., between trials).
Stimulus Characteristics
The auditory stimulus consisted of a train of band-limited, flat-spectrum Gaussian noise bursts (150-ms duration, 10-ms cos2 rise-fall time, 5-Hz repetition rate), whose waveforms were frozen within a trial, but freshly regenerated across trials. Stimuli were synthesized digitally (100-kHz sampling rate) using SigGenRP software and presented using TDT System III hardware (Tucker-Davis Technologies, Alachua, FL). Continuous presentation of the train of noise bursts throughout a trial was chosen to provide baseline estimates of sound localization accuracy and precision under optimal (“closed-loop”) conditions, i.e., without involving spatial memory, as occurs with transient (“open-loop”) stimulus presentation. Band-limited stimuli were originally created in the frequency domain with a steep roll-off (>80 dB/octave). The loudspeaker's output was calibrated between 0.1–20 kHz using a one-half inch MK224 Type I condenser microphone (ACO Pacific, Belmont, CA) mounted at the location of the subject bite-bar. Stimuli were equalized using the inverse Fourier transformation of the speaker's spectral response to within ±3 dB across the bandwidth of each target type and were presented at 75 dB SPL (root mean square sound pressure level re 20 μPa) unless otherwise stated.
The visual target was used only for system calibration and task control in the current experiments. It consisted of a flashing red spot (150-ms duration, 5-Hz repetition rate) back-projected onto the target screen from the LED mounted at the center of the loudspeaker.
Response Measures
Subjects guided a two-axis joystick to project a laser-LED beam onto the target screen at the perceived location of each target. The laser pointer (<0.03° subtended angle) was readily visible throughout the field. The joystick was a featureless cylinder (9-cm diameter × 11-cm height) devoid of angular position cues, located out of sight beneath the bite-bar. The laser-LED pointer was centered at the joystick's axes of rotation ∼41 cm directly below the cyclopean eye. Horizontal and vertical joystick position (i.e., subject response) was digitally encoded (at a resolution of <0.1°). It is important to note that subjects were free to move their eyes to aid pointing with foveal vision (“target fixation” paradigm; Razavi et al. 2007) in all experiments in this study. Target fixation yields results distinctly different from those obtained with central ocular fixation in which peripheral vision guides pointing (Razavi et al. 2007).
Data Acquisition
The experiment was controlled by custom software written using LabVIEW RT (v 7.0; National Instruments). This program transmitted commands to the robotic arm to position the target, to the TDT system for producing sound stimuli, and to data acquisition modules that handled experiment timing, data acquisition, and robotic control. An Excel spreadsheet (Microsoft) served as the operator interface for the software, and provided a user-friendly environment for implementing experimental paradigms and storing all data.
Experimental Paradigms
A trial began with the onset of the masking noise and the positioning of the speaker. Once the speaker reached the target location, the masker was extinguished and the stimulus was presented. Listeners were encouraged to localize targets quickly yet accurately using their preferred hand to manipulate the joystick pointer and the other hand to press the button indicating the response endpoint. Most responses occurred within 3–6 s of stimulus onset. Once response endpoint was registered, target and pointer positions were recorded and the stimulus was extinguished, thereby ending the trial. No feedback was provided to indicate performance.
Experiment 1.
This experiment investigated sound localization of wideband targets in young, middle-aged, and elderly listeners. Six auditory targets were tested: broadband (BB: 0.1–20 kHz), limited broadband (limBB: 0.1–10 kHz), low-pass (LP: 0.1–1 kHz), high-pass (HP: 3–20 kHz), limited HP (limHP: 3–10 kHz), and ultra HP (uHP: 10–20 kHz). BB and limBB stimuli activated all known spatial channels (ITD, IID, and elevation-dependent spectral cues). LP targets provided ITDs but no usable IIDs as the target array was located sufficiently far (>1 m away) from listeners (Brungart and Rabinowitz 1999; Shinn-Cunningham et al. 2000). HP, limHP, and uHP targets selectively emphasized IID and spectral cues and were broad enough in bandwidth to preclude utilization of envelope ITD cues in free-field localization (Macpherson and Middlebrooks 2002; McFadden and Pasanen 1976). The spectra of limBB and limHP targets were specifically chosen to investigate how loss of frequencies >10 kHz, a common characteristic of advancing age, would affect sound localization in healthy young listeners. Furthermore, uHP targets elucidated the contribution of spatial cues at high frequencies available mostly to young, healthy listeners. Potential elevation-dependent loudness cues as well as any idiosyncratic amplitude cues related to target location were made unreliable by randomly varying stimulus amplitude from trial to trial between 70–75 dB SPL in 1-dB intervals. An exception was made for uHP targets, which were presented at 80–85 dB SPL to all elderly subjects in an attempt to roughly compensate for their high-frequency hearing loss in the 10- to 20-kHz target range.
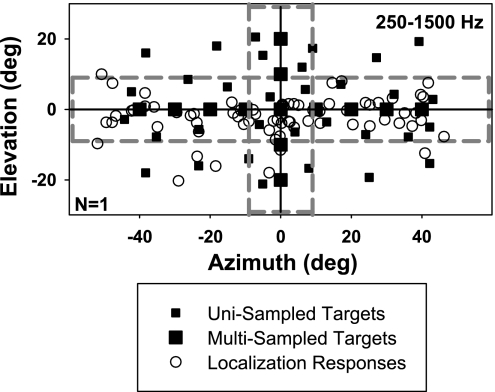
For each stimulus, 13 locations (Fig. 2, large filled squares) at 10° intervals along the horizon (±40°; 0°) and the median vertical plane (0°; ±20°) were presented 3 to 6 times to permit estimates of response precision. Thirty-two additional targets (Fig. 2, small filled squares) were presented once in each session at random locations within the ± 60° × ± 25° field to conceal the multisampled array. Each session lasted for ∼1.5 h and consisted of three blocks of ∼70 trials in which three of the six target bands and all target locations were interleaved. Subjects were given 5- to 10-min breaks between blocks. A total of four sessions per subject were run on separate days to collect the full data set for all target bands and location repetitions.
Fig. 2.
Target array and response distribution. Filled squares represent target positions; larger symbols mark the multisampled locations; open circles indicate a young individual's responses in localizing 250- to 1,500-Hz targets. Only localization responses to targets within the dashed reference lines are included in figures and statistics hereafter.
Experiment 1A.
As a follow-up to experiment 1, this experiment aimed to clarify findings in young adults from the initial wideband study. Free-field audibility thresholds for each stimulus were first measured using single 150-ms noise bursts presented straight ahead (0° horizontal; 0° vertical). A brief LED flash alerted the subject of impending trial onset. A noise burst was presented 0.5–1.5 s after LED offset. Head-fixed listeners were instructed to move the pointer up if they heard the stimulus (positive response), or down if they did not or were unsure (negative response), and record their response with a key press. A rough estimate of threshold was initially obtained by decrementing the stimulus level in 5-dB steps with each presentation from a starting point well above threshold (e.g.. 45 dB SPL) until it was no longer audible (first ∼10 trials). Actual threshold was then estimated using a modified version of the transformed up-down method (Levitt 1971), starting 5 dB above the lowest sound level heard and then decrementing trial by trial in 2-dB steps. When two negative responses occurred in a sequence, the sound level began incrementing until two consecutive positive responses occurred, and so on (using a 2-down, 2-up method). Testing ended after eight such reversals, and the sound levels of the last six reversals were averaged to estimate the detection threshold. Catch trials (no stimulus played) were intermittently presented to verify that listeners performed the task correctly. If two or more false-positive answers occurred, instructions were reiterated and the task was rerun. A total of six blocks (< 10 min each) were run during a single visit to establish detection thresholds for all the wideband targets from experiment 1.
The influence of stimulus level on sound localization in young adults was then systematically studied across frontal space for each stimulus save one (limBB). A reduced target array consisted of the same multisampled locations as experiment 1 but across a narrower (±30°) range in the horizontal plane. Additional randomly distributed targets were presented across the field (including the vertical plane) as before. This smaller target set proved sufficiently rich to prevent perceptual quantization of the multisampled target locations, while revealing loudness effects within an acceptable burden of trials. The lowest sound level tested was 10 dB SL (sensation level in decibels above threshold), while higher levels were fixed across subjects at 45, 60, and 75 dB SPL. These sound levels (3 reiterations each) were interleaved on a trial-by-trial basis in a single session for each target band. A total of five sessions, each consisting of three blocks of ∼50 trials, was run on separate days to collect the data for all targets.
Experiment 2.
This experiment resembled Experiment 1 but focused on localization of low-frequency narrowband targets presented at 40 dB SL across age groups. Altogether, 10 target bands were synthesized for presentation. Two stimuli had bandwidths spanning nearly all frequencies generating useful ITDs (250–2,205 and 250–1,500 Hz). Five stimuli parsed this ITD range into 250-Hz narrow bands and were used to investigate in detail the frequency-dependent changes in sound localization that we hypothesized might occur with age in the ITD channel (250–500, 500–750, 750–1,000, 1,000–1,250, and 1,250–1,500 Hz). Lastly, three additional targets near the putative upper boundary of the useful ITD range were presented spaced at one-third octave intervals from their starting frequency to assure that equivalent lengths of the basilar membrane (i.e., adjacent critical bands) were stimulated (1,250–1,575, 1,500–1,890, and 1,750–2,205 Hz). Sound level was set to 40 dB SL (see measurement of free-field audibility thresholds above) for each stimulus and subject. In certain cases (see Fig. 9), where 40 dB SL for a given subject and target band slightly exceeded the upper limit of speaker output in the low-frequency range, stimulus amplitude was set at the highest level possible without distortion (75 dB SPL).
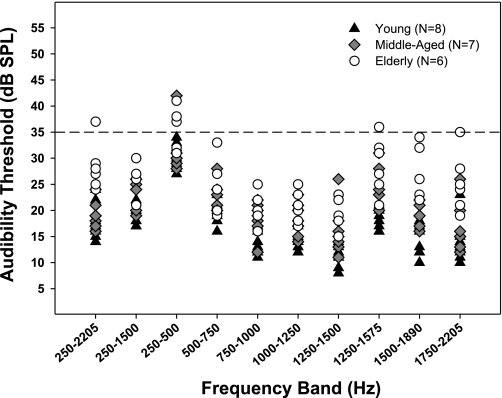
Fig. 9.
Free-field audibility thresholds for low-frequency narrowband targets in young (N = 8; black triangles), middle-aged (N = 7; grey diamonds), and elderly (N = 6; white circles) subjects. Each symbol represents a listener's free-field audibility threshold (in dB SPL) for a particular target band, measured in the experimental booth (see Experimental Paradigms). Dashed line at 35 dB SPL indicates the maximum threshold a subject could have in order for the target band to be presented at 40 dB SL in experiment 2. In rare instances, where a threshold for a given band and subject exceeded 35 dB SPL (symbols above dashed line), stimulus level was set at 75 dB SPL to avoid generating speaker distortions at louder levels for the studied low frequencies.
Data Analysis
Sound sources were presented in a cylindrical coordinate scheme centered at the subject's cyclopean eye, in which azimuth and elevation uniquely defined the coordinates of any target or response. Azimuth is the horizontal angle from the median plane, while elevation is the vertical angle from the horizontal plane (including the interaural line). The terms “azimuth” and “horizontal” are used interchangeably hereafter, as are “elevation” and “vertical.” Positive angles indicate rightward locations in azimuth or upward locations in elevation.
Elevation readouts from the joystick were adjusted trigonometrically by transposing the pointer origin upward to coincide with the cyclopean eye, ensuring that both speaker and pointer shared a common origin, while eliminating tangential error. The results of the visual calibration task showed an error <0.1°, and this small error was used to adjust all of the localization responses.
In experiments 1 and 1A, targets located within 10° of the horizontal and median planes were analyzed (i.e., filled squares within grey dashed reference lines in Fig. 2). In experiment 2, target elevations only within ±10° of the horizontal plane were included in the analysis, since vertical sound localization was profoundly impaired for all low-frequency target bands across age groups due to dearth of useful spectral cues. Localization responses were sorted by spatial plane (horizontal or vertical), stimulus condition (spectral band), and age group (young, middle-aged, elderly). For each target position, localization performance was assessed in terms of accuracy (or error), i.e., the signed difference between response and target location. Each subject's sound localization accuracy across target positions (including both multisampled and randomly dispersed targets within 10° of the studied spatial planes) for a given stimulus condition was quantified using least squares linear regression (Fig. 3) in each spatial plane. Spatial gain (SG) is defined as the slope (first-order coefficient) of the response vs. target location linear regression, while offset denotes the y-intercept (Fig. 3B). SG of 1.0 with offset of 0° represents perfect performance. A positive offset indicates either rightward or upward response bias in azimuth or elevation, respectively. Note that SG of 1 is equivalent to a slope of 0 when response error is plotted as a function of target location. SG below or above 1.0 denotes target location underestimation (“undershoot,” revealed as a negative slope for response error) or overestimation (“overshoot,” positive slope for response error; e.g., Fig. 3B), respectively. Because the slope of the target-response relationship in the horizontal plane in the periphery (beyond ±25°; Fig. 3B) was often clearly different than observed centrally, regression analysis to determine SG was performed for each subject only within central (±25°) horizontal space in all localization experiments. SGs for each target band were averaged across subjects in each age group.
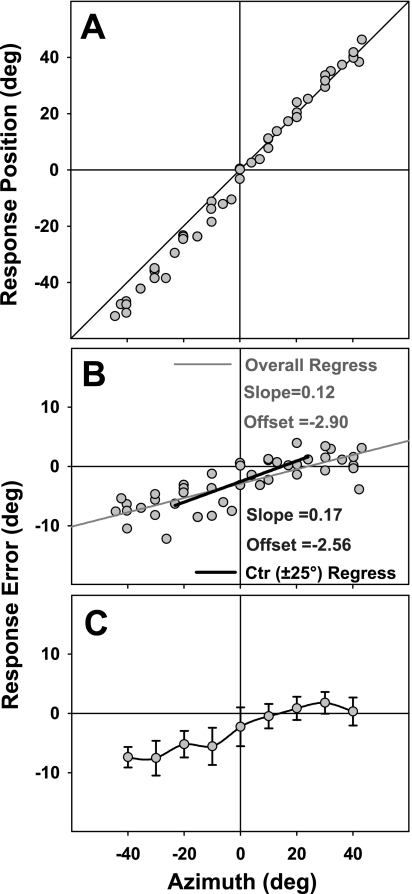
Fig. 3.
Example analysis of sound localization. A: response position as a function of target azimuth for the representative subject from Fig. 2. Diagonal line indicates ideal performance. Each point corresponds to a single trial. B: same data as in A but plotted as signed response error (accuracy). Due to nonlinearity in the target-response relationship beyond ±25°, least-squares regression was performed only within central (±25°) horizontal space (solid black line) hereafter in both experiments 1 and 2. C: response error averaged as a function of binned target locations centered on the nearest multisampled target (large filled squares; see Fig. 2). Error bars denote SD, here equivalent to a measure of precision.
Since the target array was randomly distributed across frontal space, with multisampled locations along the primary horizontal and vertical axes, targets were combined into nonoverlapping rectangular (10° H × 19° V) bins, each centered on the nearest multisampled target (Fig. 2, large filled squares). For each subject and target band, this yielded a measure of localization precision, i.e., SD around mean accuracy as a function of binned target locations (error bars in Fig. 3C). Higher values indicate less repeatable performance (i.e., imprecision). Since precision did not systematically vary as a function of target location, it was pooled across target locations for each target band and subject. Finally, individual subject's precision for each target band was averaged within each age group.
Data processing and statistical analysis (ANOVA) were performed using Matlab (The MathWorks, Natick, MA), Excel (Microsoft, Redmond, WA), and Systat and SigmaPlot (Systat Software, San Jose, CA).
RESULTS
Experiment 1: Wideband Sound Localization
Overview.
In each segment below (horizontal and vertical accuracy; horizontal and vertical precision), baseline sound localization in young adults is addressed first, followed by comparisons with older (middle-aged and elderly) listeners. Overall, subjects of all ages generally demonstrated horizontal overshoot and vertical undershoot as a function of target location across wideband noise targets (Fig. 4). Precision proved poorer in elevation than in azimuth (see Fig. 6). The SG and precision results are summarized (Fig. 5; also see Fig. 7) and analyzed using a 3 × 6 mixed design, repeated-measures ANOVA, where target band serves as the within-subject variable and age group as the between-subject variable.
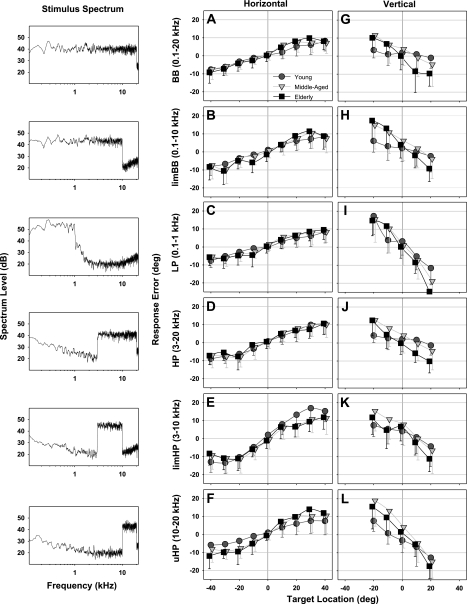
Fig. 4.
Averaged horizontal (center) and vertical (right) sound localization response error (degrees) for the 6 wideband targets as a function of target location in young (N = 19; dark grey circles), middle-aged (N = 11; light grey triangles), and elderly (N = 12; black squares) listeners. Error bars indicate SD around mean error. Symbols are slightly offset on the x-axis for graphic clarity. Stimulus spectra (left) at the subject's head are also shown for the respective wideband targets. Low-frequency energy visible in the spectra in the bottom 3 plots reflects background noise in the test room. BB, broadband; limBB, limited broadband; LP, low-pass; HP, high-pass; limHP, limited HP; uHP, ultra HP.
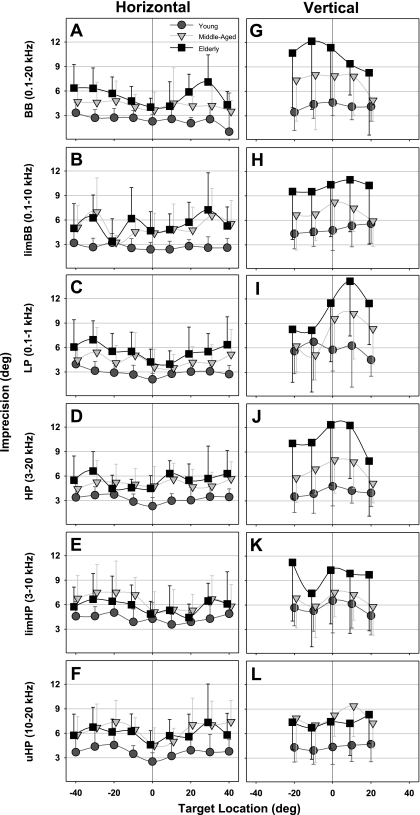
Fig. 6.
Averaged horizontal (A–F) and vertical (G–L) sound localization imprecision (intrasubject variability; degrees) for wideband targets as a function of target location for the 3 age groups, organized as in Fig. 4.
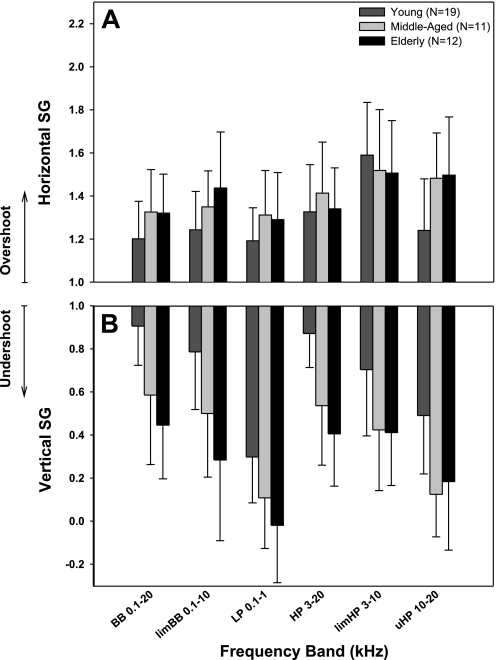
Fig. 5.
Averaged horizontal (A) and vertical (B) spatial gain (SG) for each wideband target in young (N = 19; dark grey bars), middle-aged (N = 11; light grey bars), and elderly (N = 12; black bars) listeners. SG greater or less than 1 reflects overshoot and undershoot, respectively. Error bars denote SD around mean SG.
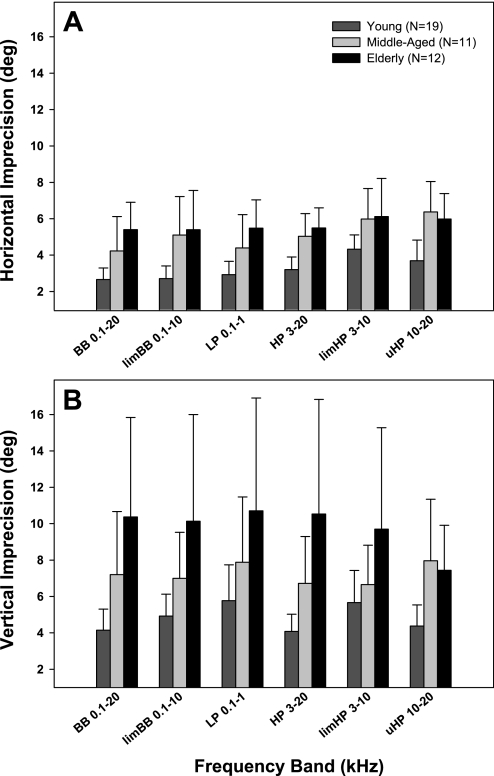
Fig. 7.
Averaged horizontal (A) and vertical (B) imprecision for each wideband target in the 3 age groups, organized as in Fig. 5.
Localization accuracy.
Horizontal sound localization SG showed a significant main effect of target band [F(5/247) = 32.6, P < 0.001] but not age (P > 0.05) and a significant interaction between age and target band [F(10/247) = 5.4, P < 0.001; Fig. 5A]. In particular, horizontal localization in young adults (pair-wise Holm-Sidak t-test) demonstrated enhanced overshoot for HP targets (SGHP = 1.33; Fig. 4D) relative to BB and LP (P < 0.001) and furthermore for limHP targets (SGlimHP = 1.59; Fig. 4E) relative to all other bands (P < 0.001). In addition, horizontal SG in young listeners proved different (pair-wise Holm-Sidak t-test) from both middle-aged (P < 0.01) and elderly (P < 0.02) subjects for uHP targets (Fig. 4F).
Vertical SG revealed significant main effects of both target band [F(5/247) = 52.7, P < 0.001] and age [F(2/247) = 16.7, P < 0.001] but there were no significant interactions (Fig. 5B). Vertical accuracy in young listeners (pair-wise Holm-Sidak t-test) showed dramatic undershoot for LP (SGLP = 0.28; Fig. 4I) and uHP (SGuHP = 0.47; Fig. 4L) targets relative to all other bands (P < 0.001) and less so for limHP (SGlimHP = 0.69; Fig. 4K) re BB (P < 0.001) and HP (P < 0.005) targets. Vertical SG of all target bands became further underestimated in both middle-aged (PBB,HP,uHP < 0.002; PlimBB,limHP < 0.006) and elderly (all P < 0.003) subjects relative to young controls (Fig. 5B).
Localization precision.
Horizontal precision showed significant main effects of both target band [F(5/247) = 23.4, P < 0.001] and age [F(2/247) = 17.3, P < 0.001], as well as an interaction between target band and age [F(10/247) = 2.8, P < 0.005; Fig. 7A]. In young adults, horizontal precision proved poorest (pair-wise Holm-Sidak t-test) for limHP targets (average of 4.3°; Fig. 6E) relative to all others (all P < 0.001) except uHP (Fig. 6F), which in turn was worse than BB, limBB, and LP targets (all P < 0.001). Compared with young controls, horizontal precision consistently worsened for all target bands (Fig. 7A) in the elderly (all P < 0.001) and in middle-aged listeners (all P < 0.006). Middle-aged and elderly subjects had comparable horizontal precision for all targets except BB (P < 0.05; Fig. 6A).
Vertical precision demonstrated significant main effects of age [F(2/247) = 10.4, P < 0.001] and target band [F(5/247) = 2.5, P < 0.05] as well as an interaction between age and target band [F(10/247) = 2.4, P < 0.02; Fig. 7B]. Young adults had poorest vertical precision for LP targets (5.7°; Fig. 6I; pair-wise Holm-Sidak t-test; P < 0.005 re BB, HP, and uHP) and limHP targets (5.6°; Fig. 6K; P < 0.001 re BB and HP). Vertical precision was systematically worse in the elderly (Fig. 7B) compared with young adults for all targets (all P < 0.002), and compared with middle-aged subjects only for BB and HP targets (P < 0.05). Middle-aged subjects differed in vertical precision from young adults in localizing BB, HP, and uHP targets (all P < 0.05; Fig. 6, G, J, and L, respectively).
Localization offset.
In addition to accuracy and precision across target locations, we examined the perception of auditory space straight ahead. This was quantified in two ways: the average sound localization error at the central oversampled point (0° horizontal; 0° vertical) and the y-intercept from regressions that produced SG noted above. Ideally, these would provide the same values, but the regression intercept is inevitably affected by peripheral responses and disproportionally more so with eccentricity. Thus the more refined method is the direct assessment of localization error straight ahead. This central error averaged 0.5° ± 2.13° SD horizontally and 1.91° ± 6.52° SD vertically in young subjects for BB sound, both proving statistically indistinguishable from 0°. Other target bandwidths were also indistinguishable from zero with the exception of limHP, which showed a vertical central error of 4.15° ± 7.36° SD (P < 0.05, t-test). The middle-aged and elderly age groups revealed similar results, with limHP targets eliciting the greatest (and only significant) vertical error straight ahead. The reason for this remains mysterious and presumably reflects aspects of the limited spatial cues available in that particular bandwidth. Finally, intercepts from regressions were typically within 1° horizontally and 2° vertically of the direct measures of straight ahead.
Experiment 1A: Influence of Stimulus Level on Wideband Sound Localization in Young Adults
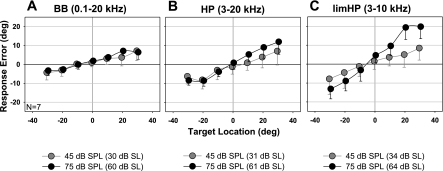
The robust overshoot of horizontal localization for limHP targets in young adults (Fig. 4E) is particularly puzzling given that the spectral range of limHP targets overlapped considerably with some of the other target bands (BB, limBB, and HP). One possible explanation for this enhanced overshoot might be differences in the audibility of the target bands. To address this possibility, we quantified horizontal and vertical sound localization in young listeners for the same wideband targets at several different stimulus levels.
An important first step was to obtain free-field thresholds for each target band. We did so for each subject using the target presentation loudspeaker placed straight ahead of the subject (0° horizontal; 0° vertical). Not surprisingly, thresholds were higher for stimuli at the lower and upper extremes of hearing (LP and uHP) than for stimulus bands that also contained mid-frequencies (not shown).
Figure 8 highlights the main findings of sound localization as a function of stimulus level (not all conditions shown). To our surprise, simply reducing suprathreshold sound level significantly diminished horizontal overshoot for limHP targets (ΔSG = 0.34, P < 0.001; Fig. 8C) and less so for HP targets (ΔSG = 0.13, P < 0.05; Fig. 8B). By contrast, horizontal SG of all other target bands (only BB shown for comparison; Fig. 8A) and vertical SG in general were not affected by stimulus level, except for small idiosyncratic worsening near threshold (10 dB SL; not shown).
Fig. 8.
Averaged response error (degree) as a function of target location in young adults (N = 7) for BB (A), HP (B), and limHP (C) targets at 45 and 75 dB SPL (grey and black symbols, respectively). Error bars indicate SD around mean error. Symbols are slightly offset on the x-axis for clarity.
Experiment 2: Low-Frequency Narrowband Horizontal Sound Localization
Experiment 2 further investigated age-related changes in sound localization specifically focusing on low-frequency ITD cues. Although experiment 1A showed no effect of stimulus level on horizontal localization of LP targets, we decided to take the extra precaution of presenting auditory stimuli at a fixed suprathreshold sensation level (40 dB SL) in experiment 2 to assure equal audibility across subjects and target bands.
Figure 9 shows individual subjects' free-field audibility thresholds for all 10 low-frequency target bands presented. As expected, thresholds were somewhat elevated in older listeners and in rare instances (symbols above dashed line at 35 dB SPL) exceeded the level at which a target could be presented at 40 dB SL with our apparatus. When this occurred, the stimulus amplitude for the particular target band was set at 75 dB SPL (equivalent to ≥33 dB SL), which was the maximum level the speaker could generate without distortions in that band. This small reduction in sensation level, mostly limited to the very low-frequency 250–500 Hz targets, did not correlate with changes in localization performance.
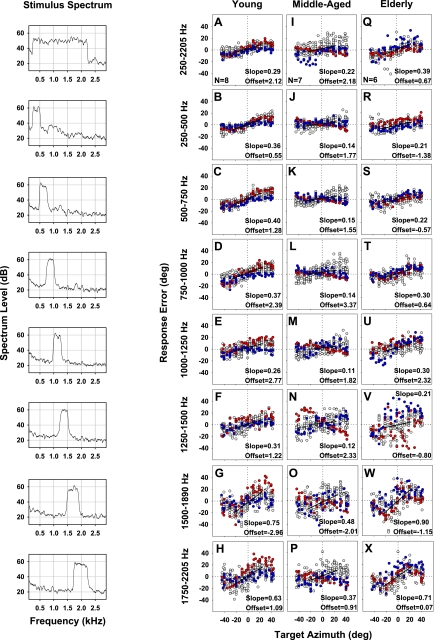
Sound localization of low-frequency narrowband targets generally demonstrated horizontal overshoot that fluctuated somewhat unsystematically for target bands between 250–2,205 Hz (Fig. 10). Localization responses of two individual listeners in each age group are highlighted (red and blue symbols) to illustrate changes in intra- as well as intersubject variability in accuracy across target bands. Results showed a striking increase in localization variance (i.e., imprecision) for 1,250–1,500 Hz (and 1,250–1,575 Hz; not shown) targets in middle-aged (Fig. 10N) and elderly (Fig. 10V) listeners, compared with lower frequency bands in the same age groups or to young listeners within the 1,250- to 1,575-Hz target range. Localization of 1,500- to 1,890-Hz and 1,750- to 2,205-Hz targets was also significantly more variable, but now across all age groups, and this was accompanied by a noticeable increase in overshoot, relative to lower frequencies.
Fig. 10.
Horizontal response error in localizing narrowband targets in the range from 250 to 2,205 Hz by young (N = 8; A–H), middle-aged (N = 7; I-P), and elderly (N = 6; Q–X) listeners. Each symbol represents a single localization response by a subject on a given trial. Localization responses of 2 individual listeners in each age group are highlighted (red and blue circles) to illustrate intrasubject variability. As in Fig. 3B, regressions (solid lines) were fit only through the central ±25° of target azimuth due to localization nonlinearity in peripheral space. Stimulus spectrum is also shown (left) for each target band, similar to Fig. 4.
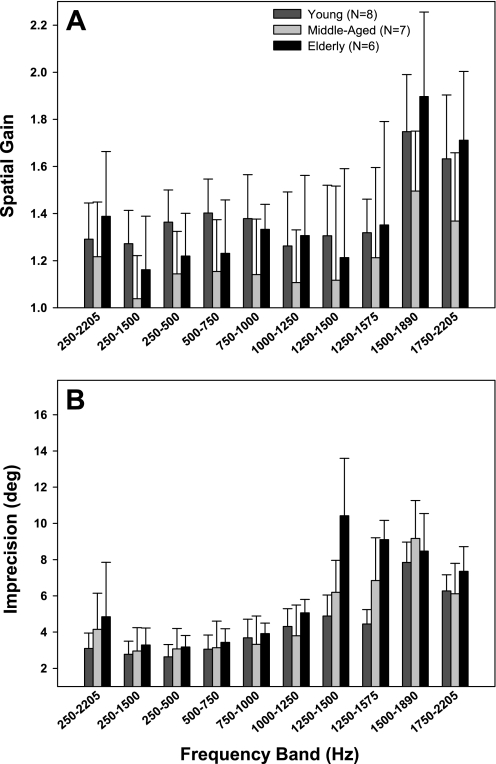
We summarized these results by averaging SG and precision across subjects within each age group for each target band (Fig. 11). Statistical assessment using a 3 × 10 mixed design, repeated-measures ANOVA showed a main effect of target band [F(9/209) = 20.4, P < 0.001] but not age, and no significant interaction, on SG of horizontal sound localization. In young listeners, horizontal SG demonstrated dramatically enhanced overshoot for 1,500- to 1,890-Hz (SG = 1.75; P < 0.001 re all target bands except 1,750–2,205 Hz) and 1,750- to 2,205-Hz targets (SG = 1.63; P < 0.001 re all target bands except 250–500, 500–750, 750–1,000, and 1,500–1,890 Hz; pair-wise Holm-Sidak t-test). Horizontal SG of the elderly group was statistically indistinguishable from young controls. It was notable that middle-aged listeners generally showed lower SG across all target bands than the other two age groups, but with the high level of variability this trend reached significance only for 1,500- to 1,890-Hz (P = 0.005) and 1,750- to 2,205-Hz (P < 0.02) targets relative to the elderly.
Fig. 11.
Summary bar graphs for horizontal accuracy (SG; A) and imprecision (B) for each low-frequency stimulus band in young (N = 8), middle-aged (N = 7), and elderly (N = 6) listeners. Error bars denote intersubject variability (SD around mean).
Repeated-measures ANOVA also revealed significant main effects of target band [F(9/209) = 60.6, P < 0.001] and age [F(9/209) = 5.1, P < 0.02], as well as an interaction between target band and age [F(9/209) = 5.1, P < 0.001], on precision of sound localization. Holm-Sidak t-tests showed that precision for 1,500- to 1,890-Hz targets in young adults was significantly poorer than all other target bands (all P < 0.001) except 1,750–2,205 Hz. Precision for 1,750- to 2,205-Hz targets in young listeners was compromised relative to all other stimuli (P < 0.001) except 1,250- to 1,500 Hz and 1,250- to 1,575-Hz targets, while precision for 1,250–1,500 Hz targets was poorer than for 250- to 1,500-Hz, 250- to 500-Hz, and 500–750-Hz targets (all P < 0.001). Localization precision significantly worsened with advancing age for 1,250- to 1,500-Hz and 1,250- and 1,575-Hz targets but not for the other target bands. In particular, precision for 1,250- to 1,575-Hz targets significantly worsened in middle-aged relative to young listeners (P = 0.002) and in the elderly relative to both middle-aged (P < 0.01) and young (P < 0.001) subjects. Precision for 1,250- to 1,500-Hz targets was significantly worse (P < 0.001) in the elderly compared with both young and middle-aged subjects, which were statistically indistinguishable.
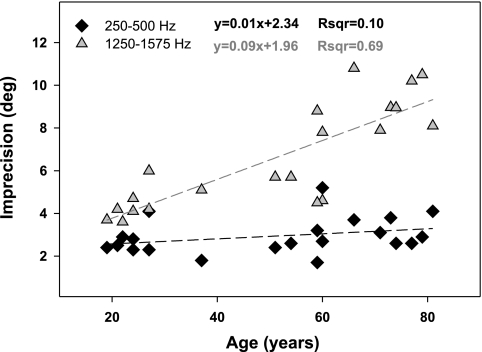
Figure 12 highlights the selective deterioration in precision as a function of age when localizing 1,250- to 1,575-Hz targets (1,250–1,500 Hz not shown but nearly identical) relative to stimuli containing lower frequencies, depicted here only for 250- to 500-Hz targets for clarity. The slopes of the precision vs. age functions proved distinct and highly significant for 1,250- to 1,500-Hz and 1,250- to 1,575-Hz targets (both slopes = 0.09, P < 0.001), unlike all other targets, which showed no age effect (P > 0.2).
Fig. 12.
Horizontal precision deteriorates with age within 1,250–1,575 Hz. Dotted lines denote linear regressions, characterizing the relationship (equations shown) between imprecision and age. Each symbol represents an individual subject's imprecision averaged across horizontal target locations for a given target band. Imprecision in localizing 250- to 500-Hz targets served as control for comparison.
DISCUSSION
Wideband Sound Localization in Young Listeners
The objective of experiment 1 was to quantify the influence of aging on the accuracy and precision of free-field human sound localization reliant on ITDs, IIDs, and spectral cues. We compared localization performance in different spectral bands selectively filtered to activate known spatial channels. Young subjects generally showed horizontal overestimation (spatial gain, SG >1.0) and vertical underestimation (SG <1.0) of auditory target location, the extent of which varied with frequency band and sound level.
Horizontal localization.
The general overshoot we observed in horizontal sound localization is in agreement with many previous reports (Choisel and Zimmer 2003; Lewald and Ehrenstein 1998; Oldfield and Parker 1984a; Seeber 2002; Vliegen and Van Opstal 2004; Wightman and Kistler 1989b). However, other studies have reported horizontal undershoot in free-field sound localization, but the conditions in these studies differed in important ways from ours (Carlile et al. 1997; Makous and Middlebrooks 1990; Perrott et al. 1987; Rakerd et al. 1998). For example, Rakerd et al. (1998) employed tonal stimuli that have been shown to produce undershoot compared with noise stimuli, likely due to a limited spectral content (Recanzone et al. 1998). The remaining studies showing undershoot used head pointing with extensive training to minimize eye-in-head misalignment. Yet, the persistence of undershoot in their results suggests that there was a residual contribution of the eyes in the head despite training, and that performance might have been improved if eye position had been measured and taken into account.
It should also be noted that the deflection of the eyes in the head toward auditory targets during localization in the present study, though brief, likely contributed to an increase in SG. This rapid adaptation of auditory space in the direction of eye position in the head applied equally to all experiments reported here and is unlikely to have biased the comparisons across conditions. Eye-position-dependent adaptation has been addressed at length previously by our group (Cui et al. 2010a; Dobreva 2010; Razavi et al. 2007) and by others (Bohlander 1984; Lewald 1998, 1997; Lewald et al. 2000; Lewald and Ehrenstein 1998, 1996; Lewald and Getzmann 2006; Weerts and Thurlow 1971; Yao and Peck 1997).
The most notable finding in young adults from experiment 1 and 1A is that horizontal localization of limHP targets with energy in the 3- to 10-kHz range resulted in a remarkable central overshoot (SG = 1.59), but this overshoot was sharply reduced (though not eliminated) by extending the spectrum upward and downward (HP: 3–20 kHz, SGHP = 1.33; BB: 0.1–20 kHz, SGBB = 1.20). Moreover, localization of LP (0.1–1 kHz) and uHP (10–20 kHz) targets, both of which have spectra outside the range of limHP targets, proved similar in accuracy to BB targets containing all localization cues. Furthermore, we were surprised to find that localization of limHP and HP targets improved in accuracy (i.e., showed much less overshoot) at lower suprathreshold sound levels, whereas spatial performance proved relatively stable across the same range of sound levels for the other wideband targets.
Although past studies have investigated horizontal sound localization of band-passed stimuli (Butler 1986; Carlile et al. 1999; Lewald and Ehrenstein 1998; Middlebrooks 1992), none have specifically employed a stimulus comparable to our limHP target. Our findings suggest that spatial information >10 kHz is important, but the precise nature of the cues available in this upper region of the audible spectrum remains unclear. Studies have shown that IIDs are a complex function of both source frequency and direction, particularly at higher (>8 kHz) frequencies (Kuhn 1977; Middlebrooks et al. 1989; Shaw 1974; Wightman and Kistler 1989a). Interestingly, for any given horizontal location, IIDs systematically vary in magnitude as a function of frequency, exhibiting a prominent peak ∼7 kHz and a trough ∼11 kHz attributed to pinna diffraction (Duda 1997). The fact that our limHP sound stimulus included the peak but not the trough in IID magnitude may explain the resulting exaggerated horizontal overshoot. This possibility is supported by the results from HP targets (3–20 kHz), which included both the IID peak and trough and yielded less overshoot. By contrast, the uHP target (10–20 kHz), which contained only the trough, yielded smaller IIDs and even less overshoot.
Other studies have recognized the contribution of monaural spectral cues (Musicant and Butler 1984), including covert spectral peaks (Butler et al. 1990), to horizontal sound localization. Regardless of which cues might be available at high frequencies, our findings raise the possibility that clinically “normal” hearing listeners with good sensitivity up to 8–10 kHz, but moderate to severe hearing loss above that (e.g., our elderly subjects), will suffer from impaired (and less consistent) sound localization in the horizontal plane unless low-frequency cues are present in the stimulus to provide ITD cue reinforcement.
The dependence of horizontal accuracy on suprathreshold stimulus level in localizing limHP and HP targets is a novel, though puzzling, finding, since previous reports generally agree that sound level does not appreciably impact the localization of broadband, long-duration (>100 ms) sounds, except near threshold (Altshuler and Comalli 1975; Davis and Stephens 1974; Hartmann and Rakerd 1993; Inoue 2001; Macpherson and Middlebrooks 2000; Sabin et al. 2005; Su and Recanzone 2001; Vliegen and Van Opstal 2004). One possible explanation for this discrepancy is that the majority of past studies employed broadband noise or clicks providing all the potential spatial cues for estimating sound location. It is possible that spatial gain would grow at higher suprathreshold sensation levels (e.g.. > ∼65 dB SL) when localizing any target that provides only IID cues. Unfortunately, we were unable to test this prediction with our uHP stimulus, because such a high sensation level would require a stimulus of ∼90 dB SPL, a level that our loudspeaker cannot generate in this spectral band without distortion and that in any case would be uncomfortably loud for our listeners. Even if this speculation proves true, the physiological mechanism accounting for such a nonmonotonic increase in horizontal localization overshoot of high-pass targets at high levels remains unclear, largely because such levels are rarely presented in studies of binaural processing.
Vertical localization.
Our findings confirm previous reports, showing that a broad frequency spectrum, inclusive of frequencies ≥3 kHz, facilitates vertical sound localization (Carlile et al. 1999; Carlile et al. 1997; King and Oldfield 1997). Limiting the spectrum to <10 kHz resulted in greater undershoot, indicating that vertical spatial performance is less accurate for lower frequency targets and would likely be compromised in listeners with high-frequency hearing loss.
Importantly, localization of wideband stimuli lacking frequencies <10 kHz, such as uHP (10–20 kHz) targets, also demonstrated profound undershoot, in agreement with previous reports (King and Oldfield 1997; Middlebrooks 1992). Clearly, frequencies between 10 and 20 kHz contribute to, but are not alone sufficient for, accurate vertical sound localization. This is likely due to the absence of prominent pinna/head-generated spectral notches in the 5- to 10-kHz range that are critical for sound localization within the studied ±20° vertical range. In combination, these observations suggest that vertical localization is compromised by any limitation in the bandwidth of the audible stimulus spectrum available to the listener and is optimized by the availability of multiple spectral peaks and notches across the entire upper-frequency range (3–20 kHz).
Influence of Aging on Wideband Sound Localization
Experiment 1 showed that older subjects differed from young listeners in wideband sound localization performance, manifested as increased horizontal overshoot (limited to one target band only) and vertical undershoot across target bands, all coupled with a general drop in precision. This effect was most prominent in the elderly group, while the middle-aged group reflected a transition between young and elderly.
Accuracy of horizontal localization reliant primarily on IID information demonstrated a noticeable increase in SG with age for uHP (10–20 kHz) but not for HP or limHP targets. Given the poor high-frequency hearing sensitivity in older listeners, the enhanced overshoot likely reflected near-monaural listening conditions, which would shift the perceived sound source laterally toward the ipsilateral ear as the level at the contralateral ear approaches threshold (Butler 1986; Slattery and Middlebrooks 1994). Indeed, the sensation levels of uHP targets presented to both elderly and middle-aged subjects were low enough to allow for this possibility (∼15 and 20 dB SL, respectively). Unfortunately, we could not verify this by testing whether performance improved with higher stimulus levels due to the aforementioned power handling limitations of our loudspeaker.
Precision of horizontal localization deteriorated by nearly the same degree across target bands with age (particularly so in the elderly group), irrespective of the spatial channels utilized. This uniform decline was somewhat surprising given that IID processing is not thought to degenerate with age, in contrast to ITD processing (Babkoff et al. 2002; Herman et al. 1977). The comparable decline in precision for sound localization utilizing either IIDs or ITDs in older individuals implies that the observed aging effect may be exerted at levels above the initial stage of binaural cue processing in the brainstem.
The profound deterioration in vertical sound localization accuracy and precision with age is in accord with past studies (Noble et al. 1994; Rakerd et al. 1998). The deficit has been mostly attributed to age-related high-frequency hearing loss, which in turn compromises utilization of spectral elevation cues. Interestingly, vertical sound localization of LP targets, which was poor in all subjects due to paucity of elevation cues, was significantly worse in the elderly than in young adults. Although thresholds ≤1 kHz in the elderly listeners were on average only slightly elevated compared with young listeners (Fig. 1), LP targets presented at 75 dB SPL were at a sufficient sensation level in elderly and young subjects (∼50 dB SL and ∼55 dB SL, respectively) to preclude concerns about audibility. Instead, these findings might provide evidence for age-related decline in processing of spectral cues that is not fully explained by hearing loss.
Horizontal Sound Localization of Low-Frequency Narrowband Targets in Young Adults
Young listeners demonstrated a decline in horizontal localization performance for 1,500- to 1,890-Hz and 1,750- to 2,205-Hz targets relative to lower frequency bands, likely reflecting an upper boundary of ITD processing. Indeed, past studies have reported that the human auditory system is insensitive to interaural differences in the ongoing temporal fine structure of pure tone stimuli above ∼1,500 Hz (Dreyer and Delgutte 2006; Johnson 1980; Joris and Yin 1992; Klumpp and Eady 1956; Zwislocki and Feldman 1956). Since our stimuli were narrow bands of noise, not pure tones, it is possible that listeners were able to extract useful, though not particularly salient, envelope cues for processing ITDs beyond 1,500 Hz, as other studies have similarly shown (McFadden and Pasanen 1976; Yost et al. 1971). It is also likely that listeners began utilizing weak IID cues (nominally most salient above 2 kHz) to aid their performance. Nevertheless, the relative weakness of both ITD and IID cues in the 1,500- to 2,200-Hz frequency region is reflected by much poorer localization performance, confirming the classical study of Stevens and Newman (1936).
Influence of Aging on Horizontal Sound Localization of Low-Frequency Narrowband Targets
We found a striking and novel aging effect on sound localization, wherein middle-aged and elderly listeners suffered a noticeable decline in horizontal precision relative to young listeners for targets in the 1,250- to 1,575-Hz range. This increase in variance reflected both intra- and intersubject variability. Indeed, listeners often freely admitted that the “higher-pitched” stimuli were harder to pinpoint. Our findings complement Ross et al. (2007) who examined cortical potentials evoked by changes in IPDs of amplitude-modulated tones as a function of stimulus frequency. Responses to phase disparities in the carrier occurred for frequencies up to 1,225 Hz in young controls but only up to 940 Hz in middle-aged and 760 Hz in elderly listeners. Behavioral thresholds on a related task demonstrated a comparable trend but with more variability. Their results indicate that the upper frequency limit for processing of temporal cues declines with advancing age, as recently confirmed by another group (Grose and Mamo 2010). We extend these physiological and behavioral threshold findings by demonstrating that the age-related decline in the upper frequency limit for effective use of ITD cues impairs free-field sound localization as well.
To our knowledge, there are only a few relevant studies that have addressed the influence of aging on horizontal human spatial localization (Abel et al. 2000; Abel and Hay 1996; Cranford et al. 1993). Abel and Hay (1996) as well as Abel et al. (2000) found age-related increases in front-back confusion when localizing 300-ms broadband noise or one-third-octave noise bands centered at 0.5 and 4 kHz. Results were attributed to a decline in spectral processing with age. These findings do not contradict ours, since their narrowband stimuli were centered at a sufficiently low (0.5 kHz) or high (4 kHz) frequency to provide robust ITD or IID cues for left-right discrimination. Indeed, aside from front-back confusions, localization of the noise band centered at 0.5 kHz did not indicate difficulties in left-right discrimination with age in agreement with our data.
Cranford et al. (1993) reported age-related deficits in fusing clicks in a precedence effect task, suggestive of a breakdown in auditory temporal acuity (i.e., resolution), also noted in many other studies (Babkoff et al. 2002; Herman et al. 1977; Lister and Roberts 2005; McCroskey and Kasten 1982; Roberts and Lister 2004; Robin and Royer 1989; Ross et al. 2007; Schneider and Hamstra 1999; Strouse et al. 1998; von Wedel et al. 1991; Warren et al. 1978). Despite vastly different methodologies and focal questions of interest, these findings agree with ours in providing general evidence for age-related decline in temporal processing.
In summary, past studies that assessed the influence of aging on horizontal sound localization utilized stimuli including nearly all useful ITD and, in some cases, also IID cues. Thus older listeners were able to extract accurate localization information from a broad range of useful frequencies. However, in the present study, when the spectrum of low-frequency targets was restricted to selected portions of the ITD-relevant range and approached the upper limit of ITD processing, a binaural temporal processing deficit was exposed in the sound localization of aged individuals that could not be compensated.
Our findings have implications for the ability of older individuals to orient and navigate in space. One interpretation of the current results is that advancing age expands the mid-frequency region within which horizontal sound localization becomes less certain, since neither ITD nor IID cues are particularly robust (Stevens and Newman 1936). Importantly, these affected frequencies are in the spectral band particularly relevant for speech and may help explain age-related difficulties in concurrent sound source segregation (i.e., the cocktail-party effect). Furthermore, even though average horizontal localization accuracy within the affected spectral range is similar across age groups, greater trial-to-trial variability (imprecision) in older individuals implies a higher probability of spatial errors on any given occasion. These idiosyncratic localization mistakes could pose risks to self and others during demanding navigation tasks such as driving.
Concluding remarks: Central vs. Peripheral Etiology of Age-Related Deficits in Horizontal Localization of Low-Frequency Targets
It is virtually impossible to fully disentangle the influence of peripheral hearing loss from central aging on sound localization. Ideally, to investigate whether central auditory aging influences sound localization, older subjects should have comparable hearing thresholds to normal young adults. In reality, however, even the healthiest aged listeners, such as those selected for the current study, demonstrated noticeable threshold elevation, particularly at high frequencies. More importantly for our study, even though aged subjects had clinically “normal” hearing in the range <2 kHz, their thresholds were still somewhat higher than in young adults. This pattern of hearing loss fits the profile of combined “metabolic” or “flat” presbycusis, which affects all frequencies roughly equally, and “sensory” presbycusis, which is a progressive high-frequency loss (Schmiedt 2009). Irrespective of the precise factors contributing to our aged listeners' hearing loss, we attempted to compensate for this deficit at least partially by setting the stimulus level at 40 dB SL in experiment 2 to assure equal audibility across target bands and listeners. Despite this effort, it is clear that peripheral presbycusis was not fully compensated for the purposes of sound localization by simply raising the sensation level, and that compromised horizontal sound localization of low-frequency narrowband targets in older listeners likely also involves a central etiology.
Although our findings cannot definitively identify the locus of robust age-related decline in localization shown in experiment 2, they offer unequivocal evidence for impairment in older listeners in utilizing portions of the ITD-relevant low-frequency range to represent horizontal space. Since ITDs are processed centrally, the present findings provide supporting evidence for aging of the central auditory system, reported in other studies (Frisina and Frisina 1997; Martin and Jerger 2005; Mazelova et al. 2003; Ross et al. 2007; Schneider and Hamstra 1999; Strouse et al. 1998). Importantly, documentation of age-related deficits in horizontal sound localization may prove valuable in future efforts to assist aging adults in overcoming this limitation.
GRANTS
This work was supported by National Institute on Aging Grant R01-AG-16319 and National Institute on Deafness and Other Communication Disorders Grant P30 DC-05409 (Center for Navigation and Communication Sciences).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Martin Gira and Robert Schor for technical assistance, John Housel, Brendan Guercio, and Emily Clark for help in data collection; Babak Razavi and Scott H. Seidman for assistance with data analysis; and Paul Allen for useful feedback on an earlier version of this manuscript.
REFERENCES
- Abel SM, Giguere C, Consoli A, Papsin BC. The effect of aging on horizontal plane sound localization. J Acoust Soc Am 108: 743–752, 2000 [DOI] [PubMed] [Google Scholar]
- Abel SM, Hay VH. Sound localization. The interaction of aging, hearing loss and hearing protection. Scand Audiol 25: 3–12, 1996 [DOI] [PubMed] [Google Scholar]
- Algazi VR, Avendano C, Duda RO. Elevation localization and head-related transfer function analysis at low frequencies. J Acoust Soc Am 109: 1110–1122, 2001 [DOI] [PubMed] [Google Scholar]
- Altshuler MW, Comalli PE. Effect of stimulus intensity and frequency on median horizontal plane sound localization. J Aud Res 15: 262–265, 1975 [Google Scholar]
- Asano F, Suzuki Y, Sone T. Role of spectral cues in median plane localization. J Acoust Soc Am 88: 159–168, 1990 [DOI] [PubMed] [Google Scholar]
- ASTM C 423–77 Sound Absorption and Sound Absorption Coefficients by the Reverberation Room Method. Philadelphia, PA: American Society for Testing and Materials, 1977 [Google Scholar]
- Babkoff H, Muchnik C, Ben-David N, Furst M, Even-Zohar S, Hildesheimer M. Mapping lateralization of click trains in younger and older populations. Hear Res 165: 117–127, 2002 [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Trahiotis C. Enhancing sensitivity to interaural delays at high frequencies by using “transposed stimuli.” J Acoust Soc Am 112: 1026–1036, 2002 [DOI] [PubMed] [Google Scholar]
- Bohlander RW. Eye position and visual attention influence perceived auditory direction. Percept Mot Skills 59: 483–510, 1984 [DOI] [PubMed] [Google Scholar]
- Brungart DS, Rabinowitz WM. Auditory localization of nearby sources. Head-related transfer functions. J Acoust Soc Am 106: 1465–1479, 1999 [DOI] [PubMed] [Google Scholar]
- Burlingame JA, Butler RA. The effects of attenuation of frequency segments on binaural localization of sound. Percept Psychophys 60: 1374–1383, 1998 [DOI] [PubMed] [Google Scholar]
- Butler RA. The bandwidth effect on monaural and binaural localization. Hear Res 21: 67–73, 1986 [DOI] [PubMed] [Google Scholar]
- Butler RA, Humanski RA. Localization of sound in the vertical plane with and without high-frequency spectral cues. Percept Psychophys 51: 182–186, 1992 [DOI] [PubMed] [Google Scholar]
- Butler RA, Humanski RA, Musicant AD. Binaural and monaural localization of sound in two-dimensional space. Perception 19: 241–256, 1990 [DOI] [PubMed] [Google Scholar]
- Carlile S, Delaney S, Corderoy A. The localisation of spectrally restricted sounds by human listeners. Hear Res 128: 175–189, 1999 [DOI] [PubMed] [Google Scholar]
- Carlile S, Hyams S, Delaney S. Systematic distortions of auditory space perception following prolonged exposure to broadband noise. J Acoust Soc Am 110: 416–424, 2001 [DOI] [PubMed] [Google Scholar]
- Carlile S, Leong P, Hyams S. The nature and distribution of errors in sound localization by human listeners. Hear Res 114: 179–196, 1997 [DOI] [PubMed] [Google Scholar]
- Choisel S, Zimmer K. A pointing technique with visual feedback for sound localization experiments. In: Audio Engineering Society 115th Convention. New York: Journal of the Audio Engineering Society, Oct. 10–13, 2003, p. 1–15 [Google Scholar]
- Cranford JL, Andres MA, Piatz KK, Reissig KL. Influences of age and hearing loss on the precedence effect in sound localization. J Speech Hear Res 36: 437–441, 1993 [DOI] [PubMed] [Google Scholar]
- Cui QN, O'Neill WE, Paige GD. Advancing age alters the influence of eye position on sound localization. Exp Brain Res 206: 371–379, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui QN, Razavi B, O'Neill WE, Paige GD. Perception of auditory, visual, and egocentric spatial alignment adapts differently to changes in eye position. J Neurophysiol 103: 1020–1035, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ, Stephens SD. The effect of intensity on the localization of different acoustical stimuli in the vertical plane. J Sound Vibration 35: 223–229, 1974 [Google Scholar]
- Dobreva MS. The influence of aging, memory, and stimulus characteristics on human auditory and visual spatial localization (PhD thesis). In: Dissertations & Theses. Rochester, NY: University of Rochester, 2010 [Google Scholar]
- Dreyer A, Delgutte B. Phase locking of auditory-nerve fibers to the envelopes of high-frequency sounds: implications for sound localization. J Neurophysiol 96: 2327–2341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda RO. Elevation dependence of the interaural transfer function. In: Binaural and Spatial Hearing in Real and Virtual Environments, edited by Gilkey RH, Anderson TR. Mahwah, NJ: Lawrence Erlbaum, 1997, p. 49–75 [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res 106: 95–104, 1997 [DOI] [PubMed] [Google Scholar]
- Getzmann S. The influence of the acoustic context on vertical sound localization in the median plane. Percept Psychophys 65: 1045–1057, 2003 [DOI] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear 31: 755–760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafter ER, De Maio J, Hellman WS. Difference thresholds for interaural delay. J Acoust Soc Am 57: 181–187, 1975 [DOI] [PubMed] [Google Scholar]
- Hartmann WM, Rakerd B. Auditory spectral discrimination and the localization of clicks in the sagittal plane. J Acoust Soc Am 94: 2083–2092, 1993 [DOI] [PubMed] [Google Scholar]
- Hebrank J, Wright D. Spectral cues used in the localization of sound sources on the median plane. J Acoust Soc Am 56: 1829–1834, 1974 [DOI] [PubMed] [Google Scholar]
- Henning GB. Detectability of interaural delay in high-frequency complex waveforms. J Acoust Soc Am 55: 84–90, 1974 [DOI] [PubMed] [Google Scholar]
- Herman GE, Warren LR, Wagener JW. Auditory lateralization: age differences in sensitivity to dichotic time and amplitude cues. J Gerontol 32: 187–191, 1977 [Google Scholar]
- Hofman M, Van Opstal J. Binaural weighting of pinna cues in human sound localization. Exp Brain Res 148: 458–470, 2003 [DOI] [PubMed] [Google Scholar]
- Inoue J. Effects of stimulus intensity on sound localization in the horizontal and upper-hemispheric median plane. J UOEH 23: 127–138, 2001 [DOI] [PubMed] [Google Scholar]
- ISO Acoustics: statistical distribution of hearing thresholds as a function of age. In: ISO 7029. Geneva, Switzerland: International Organization for Standardization, 2000 [Google Scholar]
- Johnson DH. The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am 68: 1115–1122, 1980 [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am 91: 215–232, 1992 [DOI] [PubMed] [Google Scholar]
- King RB, Oldfield SR. The impact of signal bandwidth on auditory localization: implications for the design of three-dimensional audio displays. Hum Factors 39: 287–295, 1997 [Google Scholar]
- Klumpp RG, Eady HR. Some measurements of interaural time difference thresholds. J Acoust Soc Am 28: 859–860, 1956 [Google Scholar]
- Kuhn GF. Model for the interaural time differences in the azimuthal plane. J Acoust Soc Am 62: 157–167, 1977 [Google Scholar]
- Langendijk EH, Bronkhorst AW. Contribution of spectral cues to human sound localization. J Acoust Soc Am 112: 1583–1596, 2002 [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49, Suppl 2: 467+, 1971 [PubMed] [Google Scholar]
- Lewald J. The effect of gaze eccentricity on perceived sound direction and its relation to visual localization. Hear Res 115: 206–216, 1998 [DOI] [PubMed] [Google Scholar]
- Lewald J. Eye-position effects in directional hearing. Behav Brain Res 87: 35–48, 1997 [DOI] [PubMed] [Google Scholar]
- Lewald J, Dorrscheidt GJ, Ehrenstein WH. Sound localization with eccentric head position. Behav Brain Res 108: 105–125, 2000 [DOI] [PubMed] [Google Scholar]
- Lewald J, Ehrenstein WH. Auditory-visual spatial integration: a new psychophysical approach using laser pointing to acoustic targets. J Acoust Soc Am 104: 1586–1597, 1998 [DOI] [PubMed] [Google Scholar]
- Lewald J, Ehrenstein WH. The effect of eye position on auditory lateralization. Exp Brain Res 108: 473–485, 1996 [DOI] [PubMed] [Google Scholar]
- Lewald J, Getzmann S. Horizontal and vertical effects of eye-position on sound localization. Hear Res 213: 99–106, 2006 [DOI] [PubMed] [Google Scholar]
- Lister JJ, Roberts RA. Effects of age and hearing loss on gap detection and the precedence effect: narrow-band stimuli. J Speech Lang Hear Res 48: 482–493, 2005 [DOI] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Listener weighting of cues for lateral angle: the duplex theory of sound localization revisited. J Acoust Soc Am 111: 2219–2236, 2002 [DOI] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Localization of brief sounds: effects of level and background noise. J Acoust Soc Am 108: 1834–1849, 2000 [DOI] [PubMed] [Google Scholar]
- Makous JC, Middlebrooks JC. Two-dimensional sound localization by human listeners. J Acoust Soc Am 87: 2188–2200, 1990 [DOI] [PubMed] [Google Scholar]
- Martin JS, Jerger JF. Some effects of aging on central auditory processing. J Rehabil Res Dev 42: 25–44, 2005 [DOI] [PubMed] [Google Scholar]
- Mazelova J, Popelar J, Syka J. Auditory function in presbycusis: peripheral vs. central changes. Exp Gerontol 38: 87–94, 2003 [DOI] [PubMed] [Google Scholar]
- McCroskey RL, Kasten RN. Temporal factors and the aging auditory system. Ear Hear 3: 124–127, 1982 [DOI] [PubMed] [Google Scholar]
- McFadden D, Pasanen EG. Lateralization of high frequencies based on interaural time differences. J Acoust Soc Am 59: 634–639, 1976 [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Narrow-band sound localization related to external ear acoustics. J Acoust Soc Am 92: 2607–2624, 1992 [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Directional dependence of interaural envelope delays. J Acoust Soc Am 87: 2149–2162, 1990 [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annu Rev Psychol 42: 135–159, 1991 [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Makous JC, Green DM. Directional sensitivity of sound-pressure levels in the human ear canal. J Acoust Soc Am 86: 89–108, 1989 [DOI] [PubMed] [Google Scholar]
- Musicant AD, Butler RA. The influence of pinnae-based spectral cues on sound localization. J Acoust Soc Am 75: 1195–1200, 1984 [DOI] [PubMed] [Google Scholar]
- Noble W, Byrne D, Lepage B. Effects on sound localization of configuration and type of hearing impairment. J Acoust Soc Am 95: 992–1005, 1994 [DOI] [PubMed] [Google Scholar]
- Oldfield SR, Parker SPA. Acuity of sound localization–a topography of auditory space. 1. Normal hearing conditions. Perception 13: 581–600, 1984a [DOI] [PubMed] [Google Scholar]
- Oldfield SR, Parker SPA. Acuity of sound localization–a topography of auditory space. 2. Pinna cues absent. Perception 13: 601–617, 1984b [DOI] [PubMed] [Google Scholar]
- Perrott DR, Ambarsoom H, Tucker J. Changes in head position as a measure of auditory localization performance: auditory psychomotor coordination under monaural and binaural listening conditions. J Acoust Soc Am 82: 1637–1645, 1987 [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA. Masking-level differences in the elderly: a comparison of antiphasic and time-delay dichotic conditions. J Speech Hear Res 34: 1410–1422, 1991 [DOI] [PubMed] [Google Scholar]
- Rakerd B, Vander Velde TJ, Hartmann WM. Sound localization in the median sagittal plane by listeners with presbyacusis. J Am Acad Audiol 9: 466–479, 1998 [PubMed] [Google Scholar]
- Rayleigh L. On our perception of sound direction. Philos Mag 13: 214–232, 1907 [Google Scholar]
- Razavi B, O'Neill WE, Paige GD. Auditory spatial perception dynamically realigns with changing eye position. J Neurosci 27: 10249–10258, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Makhamra SD, Guard DC. Comparison of relative and absolute sound localization ability in humans. J Acoust Soc Am 103: 1085–1097, 1998 [DOI] [PubMed] [Google Scholar]
- Roberts RA, Lister JJ. Effects of age and hearing loss on gap detection and the precedence effect: broadband stimuli. J Speech Lang Hear Res 47: 965–978, 2004 [DOI] [PubMed] [Google Scholar]
- Robin DA, Royer FL. Age-related changes in auditory temporal processing. Psychology and Aging 4: 144–149, 1989 [DOI] [PubMed] [Google Scholar]
- Roffler SK, Butler RA. Factors that influence the localization of sound in the vertical plane. J Acoust Soc Am 43: 1255–1259, 1967 [DOI] [PubMed] [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, Picton TW. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci 27: 11172–11178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AT, Macpherson EA, Middlebrooks JC. Human sound localization at near-threshold levels. Hear Res 199: 124–134, 2005 [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. The physiology of cochlear presbycusis. In: The Aging Auditory System, edited by Gordon-Salant S, Frisina RD, Popper AN, Fay RR. Springer Handbook of Auditory Research vol. 34, New York: Springer, 2009, p. 9– 124–38 [Google Scholar]
- Schneider BA, Hamstra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. J Acoust Soc Am 106: 371–380, 1999 [DOI] [PubMed] [Google Scholar]
- Seeber B. A new method for localization studies. Acta Acustica United Acustica 88: 446–450, 2002 [Google Scholar]
- Shaw E. Acoustical features of the human external ear. In: Binaural and Spatial Hearing in Real and Virtual Environments, edited by Gilkey RH, Anderson TR. Mahwah, NJ: Lawrence Erlbaum, 1997, p. 25–47 [Google Scholar]
- Shaw EA. Transformation of sound pressure level from the free field to the eardrum in the horizontal plane. J Acoust Soc Am 56: 1848–1861, 1974 [DOI] [PubMed] [Google Scholar]
- Shinn-Cunningham BG, Santarelli S, Kopco N. Tori of confusion: binaural localization cues for sources within reach of a listener. J Acoust Soc Am 107: 1627–1636, 2000 [DOI] [PubMed] [Google Scholar]
- Slattery WH, III, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hear Res 75: 38–46, 1994 [DOI] [PubMed] [Google Scholar]
- Stevens SS, Newman EB. The localization of actual sources of sound. Am J Psychol 48: 297–306, 1936 [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am 104: 2385–2399, 1998 [DOI] [PubMed] [Google Scholar]
- Su TI, Recanzone GH. Differential effect of near-threshold stimulus intensities on sound localization performance in azimuth and elevation in normal human subjects. J Assoc Res Otolaryngol 2: 246–256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlow WR, Jack CE. Some determinants of localization-adaptation effects for successive auditory stimuli. J Acoust Soc Am 53: 1573–1577, 1973 [DOI] [PubMed] [Google Scholar]
- Vliegen J, Van Opstal AJ. The influence of duration and level on human sound localization. J Acoust Soc Am 115: 1705–1713, 2004 [DOI] [PubMed] [Google Scholar]
- von Wedel H, von Wedel UC, Streppel M. Monaural and binaural time resolution ability in the aged. Acta Otolaryngol (Stockh) Suppl 476: 161–166, 1991 [PubMed] [Google Scholar]
- Warren LR, Wagener JW, Herman GE. Binaural analysis in the aging auditory system. J Gerontol 33: 731–736, 1978 [DOI] [PubMed] [Google Scholar]
- Weerts TC, Thurlow WR. The effects of eye position and expectation on sound localization. Percept Psychophys 9: 35–39, 1971 [Google Scholar]
- Wightman FL, Kistler DJ. The dominant role of low-frequency interaural time differences in sound localization. J Acoust Soc Am 91: 1648–1661, 1992 [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. Headphone simulation of free-field listening. I: Stimulus synthesis. J Acoust Soc Am 85: 858–867, 1989a [DOI] [PubMed] [Google Scholar]
- Wightman FL, Kistler DJ. Headphone simulation of free-field listening. II: Psychophysical validation. J Acoust Soc Am 85: 868–878, 1989b [DOI] [PubMed] [Google Scholar]
- Yao L, Peck CK. Saccadic eye movements to visual and auditory targets. Exp Brain Res 115: 25–34, 1997 [DOI] [PubMed] [Google Scholar]
- Yost WA, Wightman FL, Green DM. Lateralization of filtered clicks. J Acoust Soc Am 50: 1526–1531, 1971 [DOI] [PubMed] [Google Scholar]
- Zwiers MP, Van Opstal AJ, Paige GD. Plasticity in human sound localization induced by compressed spatial vision. Nat Neurosci 6: 175–181, 2003 [DOI] [PubMed] [Google Scholar]
- Zwislocki J, Feldman RS. Just noticeable differences in dichotic phase. J Acoust Soc Am 28: 860–864, 1956 [Google Scholar]