Abstract
The respiratory central pattern generator distributes rhythmic excitatory input to phrenic, intercostal, and hypoglossal premotor neurons. The degree to which this input shapes motor neuron activity can vary across respiratory muscles and motor neuron pools. We evaluated the extent to which respiratory drive synchronizes the activation of motor unit pairs in tongue (genioglossus, hyoglossus) and chest-wall (diaphragm, external intercostals) muscles using coherence analysis. This is a frequency domain technique, which characterizes the frequency and relative strength of neural inputs that are common to each of the recorded motor units. We also examined coherence across the two tongue muscles, as our previous work shows that, despite being antagonists, they are strongly coactivated during the inspiratory phase, suggesting that excitatory input from the premotor neurons is distributed broadly throughout the hypoglossal motoneuron pool. All motor unit pairs showed highly correlated activity in the low-frequency range (1–8 Hz), reflecting the fundamental respiratory frequency and its harmonics. Coherence of motor unit pairs recorded either within or across the tongue muscles was similar, consistent with broadly distributed premotor input to the hypoglossal motoneuron pool. Interestingly, motor units from diaphragm and external intercostal muscles showed significantly higher coherence across the 10–20-Hz bandwidth than tongue-muscle units. We propose that the lower coherence in tongue-muscle motor units over this range reflects a larger constellation of presynaptic inputs, which collectively lead to a reduction in the coherence between hypoglossal motoneurons in this frequency band. This, in turn, may reflect the relative simplicity of the respiratory drive to the diaphragm and intercostal muscles, compared with the greater diversity of functions fulfilled by muscles of the tongue.
Keywords: breathing, coherence, genioglossus, motor units
inspiratory muscles of the thorax (e.g., diaphragm, external intercostal muscles) and upper airway (e.g., tongue, pharyngeal, and laryngeal muscles) are driven by motoneurons that generate bursts of action potentials during the inspiratory phase of the respiratory cycle, with no or little activity during the expiratory phase. These bursts of activity are driven by long-time-scale (e.g., 1–2 Hz in rodents), synchronous activation of motoneurons by the respiratory central pattern generator, presumed to lie in the pre-Botzinger complex (Peever and Duffin 2001; Peever et al. 2002; Sebe and Berger 2008; Sebe et al. 2006). In addition, synchronized oscillations in medium-(15–50 Hz) and high-frequency (50–120 Hz) ranges have been widely reported in pairs of inspiratory muscle motoneurons and in brainstem interneurons with both inspiratory and expiratory discharge patterns (Funk and Parkis 2002; Huang et al. 1993; Huang et al. 1996; Parkis et al. 2003; Peever et al. 2002; Sebe and Berger 2008; van Brederode and Berger 2008).
Most studies of synchronous oscillatory activity in respiratory muscle motoneurons have utilized recordings of left- and right-muscle nerves from hypoglossal, intercostal, or diaphragm motoneuron pools. These recordings provide useful information on the presynaptic modulation of an entire motoneuron pool but do not provide insight into the presynaptic control of identified muscles or features of the presynaptic input that is shared by pairs of motoneurons. For example, each hypoglossal nerve contains the axons of motoneurons innervating seven different tongue muscles, each with different mechanical actions and therefore different force and motor unit recruitment requirements. Similarly, intercostal nerves contain axons that innervate muscles with both inspiratory (external intercostals) and expiratory actions on the thorax (internal intercostals and triangularis sterni). Coherence analysis is a method that examines the correlation between two spike trains but in the frequency domain rather than the time domain. It provides an index of the strength of in-phase (i.e., synchronous) oscillations at each frequency examined. Because the method used to compute coherence removes the autospectra of the individual spike trains, in-phase oscillations that are common to each of the neurons are widely believed to reflect presynaptic inputs (Farmer et al. 1993; Farmer et al. 1997; Fellous et al. 2003; Fellous and Sejnowski 2000; Fellous et al. 2004; Kriener et al. 2008; Sejnowski and Paulsen 2006; Tetzlaff et al. 2008) (and see discussion). Whether the nature of the presynaptic input to hypoglossal or intercostal motoneurons varies across the specific muscles controlled by these motoneuron pools is unknown.
Accordingly, the goal of this study is to examine the nature of the respiration-related presynaptic input that is shared by motor unit pairs in external intercostal muscles, the diaphragm, and two identified tongue muscles (the genioglossus and hyoglossus) with opposing mechanical actions. Comparisons were made within muscles (i.e., recording of two motor units from the same muscle) and also across the genioglossus and hyoglossus muscles (i.e., one motor unit from the genioglossus muscle, one motor unit from the hyoglossus muscle). This approach provided novel information on the composition and distribution of respiration-related presynaptic inputs driving identified muscle motoneuron pools.
METHODS
Experimental preparation.
Studies were done in 61 male Sprague-Dawley rats weighing between 300 and 400 g, and all procedures were approved by the IACUC of The University of Arizona. Anesthesia was initiated by placing animals in a Plexiglas chamber gassed with 3% halothane in oxygen. After induction, the animal was removed from the chamber but received the same anesthetic mixture via a nose cone. A polyethylene catheter was inserted into a femoral vein for the administration of drugs and fluids. As previously described (Bailey et al. 2001; Fuller et al. 1998; Fuller and Fregosi 2000; Fuller et al. 1999), the isoflurane dose was progressively reduced in exchange for deep urethane anesthesia administered intravenously to a final concentration of 1.3 g/kg, with anesthetic depth monitored by applying deep pressure to the paws. Supplemental doses (0.25 g/kg) of urethane were given as needed to maintain analgesia. Colonic temperature was monitored and maintained between 37 and 38°C with a thermoprobe and temperature sensor connected to a servo-controlled heating pad. The genioglossus, hyoglossus, external intercostal, and diaphragm muscles were surgically exposed but left intact, as described previously (Bailey et al. 2001; Fuller et al. 1998; Fuller and Fregosi 2000; Fuller et al. 1999; Janssen and Fregosi 2000).
Electrophysiology.
Motor unit potentials were recorded with high-impedance (10 MΩ) tungsten electrodes (Frederick Haer, Bowdoin, ME) and were differentially amplified (model 7WU16K; Grass Instruments, West Warwick, RI), filtered between 300 and 10,000 Hz, and monitored on a storage oscilloscope and computer screen (John et al. 2005). Amplifier output of the filtered motor unit action potentials were sent to an analog-to-digital converter, which sampled each channel at 20,000 Hz, with all data written to the hard drive of the computer and subsequently backed up on CD ROM discs.
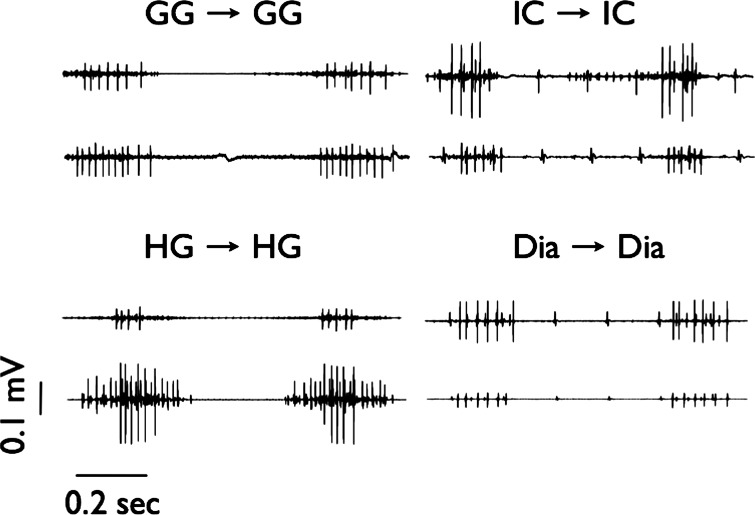
In all experiments, two microelectrodes were inserted into a single muscle using micromanipulators (Narishige, Tokyo, Japan) to study within-muscle events or into each of two different muscles to examine across-muscle events. On the basis of several years of experience with these muscles in the rat model, we have learned to limit the penetration of the microelectrodes to distances that are less than the thickness of the muscle (John et al. 2005). Once we identified consistent discharge from two motor units on both electrodes (see Fig. 1), a 10–15-min recording period commenced. We then moved one or both electrodes to record from a presumptively different motor unit pair, and the protocol was repeated. We conducted postmortem analysis at the conclusion of six experiments to confirm electrode placement within the targeted muscle.
Fig. 1.
Representative dual motor unit recordings from within 4 muscles: the genioglossus (GG-GG), hyoglossus (HG-HG), inspiratory intercostal (IC-IC), and diaphragm (Dia-Dia). The phasic discharge is confined largely to the inspiratory phase of the respiratory cycle.
Motor unit discrimination.
Motor unit potentials (Fig. 1) were discriminated on the basis of waveform shape and amplitude (Spike II software; Cambridge Electronic Design, Cambridge, UK), as described previously (John et al. 2005). In the urethane-anesthetized rat, the bursts of activity recorded from the tongue, diaphragm, and intercostal muscles are purely inspiratory (Bailey and Fregosi 2004; 2006; Bailey et al. 2005; Bailey et al. 2001; Fuller et al. 1998; Janssen and Fregosi 2000; Janssen et al. 2000). To compute discharge rate we calculated the inverse of the mean interspike interval of all action potentials generated during each burst, obtained the average frequency of each burst, and then averaged across 20 such bursts for each motor unit. To obtain an estimate of discharge rate variability, we computed the coefficient of variation of the interspike intervals for each motor unit. This was done by dividing the standard deviation of the interspike interval by the average interspike interval for that unit and expressing the data as a percentage. Cycles containing nonrespiratory behaviors such as swallows or sighs, which are easy to identify because they cause obvious changes in discharge as well as prolongation of the expiratory period (Janssen et al. 2000), were excluded from analysis.
Coherence analysis.
Coherence analysis is a frequency-domain technique that is commonly applied to dual motor unit recordings to reveal the frequency of oscillations that are common to each of the motor units (Farmer et al. 1993; Farmer et al. 1997; Myers et al. 2004). Computation of the coherence function produces a dimensionless number between 0 and 1, which reflects the strength of the correlation between the activities of the two actively discharging motor units at each frequency. Spike times of each unit were transformed into a continuous signal (sampling rate 1,000 Hz) with each spike represented as a 1-ms pulse. The coherence between two spike trains was calculated with Matlab software using unweighted, nonoverlapping data segments 2,048 ms in length, resulting in a frequency resolution of 0.49 Hz. These data were used to compute the magnitude-squared coherence at each 0.49-Hz interval over a frequency range of 0–500 Hz. However, because we found no evidence of coherent discharge at frequencies above 50 Hz, we focus on the 0–50-Hz frequency range.
Statistical evaluation of coherence was done in two ways. First, we analyzed the proportion of motor unit pairs showing significant coherence at each frequency between 0 and 50 Hz. To do this, we first obtained the 95% confidence level for each motor unit pair according to the equation 1 − 0.05∧[1/(N − 1)] where N is the number of disjoint time segments used in the coherence estimation (Amjad et al. 1989; Rosenberg et al. 1989). We then derived the proportion of motor unit pairs showing significant coherence at each frequency and subsequently compared this result both within and across muscles using the χ2-test, followed by post hoc comparisons with Fisher's exact test.
Second, the magnitude-squared coherence for all motor unit pairs was derived by converting raw coherence values into Z-scores using Fisher's transform [z = atanh(√C)], where C represents the magnitude-squared coherence; for simplicity, in the remainder of the article, magnitude-squared coherence is referred to as coherence magnitude. The Z-scores for each motor unit pair were then averaged across 2-Hz bins, and a one-way ANOVA was used to compare the values in each frequency bin. Two-tailed, unequal variance t-tests were used for pair-wise post hoc comparisons. Finally, for each motor unit pair, the maximum unbinned Z-value was calculated for the 10–20-Hz frequency band to test for correlation between coherence and the geometric mean firing rate of each motor unit pair.
RESULTS
Number of motor unit pairs and average motor unit discharge rates.
We recorded the activity of 167 motor unit pairs from 61 animals. Within-muscle comparisons included recordings from 33 genioglossus motor unit pairs, 54 hyoglossus pairs, 14 external intercostal muscle pairs, and 13 diaphragm motor unit pairs. We also studied 22 hyoglossus-genioglossus pairs. We used an average of 7,344 ± 5,690 (mean ± SD) spikes from each motor unit to construct the coherence spectra. The average breathing frequency across all animals ranged from ∼80 to 100 cycles per minute (93.81 ± 20.31, mean ± SD), or 1.33 to 1.66 Hz, consistent with findings reported previously in spontaneously-breathing, urethane-anesthetized rats (Bailey and Fregosi 2004; Bailey et al. 2006; Bailey et al. 2005; Bailey et al. 2001; Fregosi and Fuller 1997; Fuller et al. 1998; Fuller and Fregosi 2000; Fuller et al. 1999; Janssen et al. 2000).
For all muscles, single-motor unit activities exhibited consistent, inspiratory-related activity, and all recordings were characterized by high signal-to-noise ratios (see Fig. 1). Average inspiratory-related discharge rates (means ± SD) were 42.3 ± 7.9 Hz (N = 78), 52.3 ± 11.6 Hz (N = 128), 36.8 ± 7.8 Hz (N = 30), and 31.5 ± 6.1 Hz (N = 32) for genioglossus, hyoglossus, intercostal, and diaphragm motor units, respectively. One-way ANOVA revealed significant differences in inspiratory-related discharge rate (F = 53.65, P < 0.001) with hyoglossus > genioglossus > intercostal and diaphragm (all P < 0.001 by Bonferroni post hoc tests).
Discharge-rate variability.
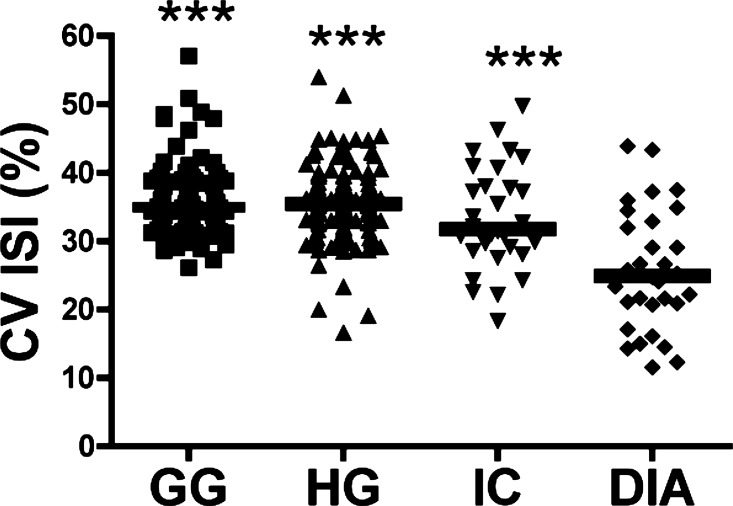
We determined within-muscle discharge-rate variability by computing the coefficient of variation of the interspike intervals for all motor units (Fig. 2). ANOVA revealed significant differences (F = 24.21, P = 0.0018) between the diaphragm and genioglossus (P < 0.001), diaphragm and hyoglossus (P < 0.001), and diaphragm and external intercostal muscles (P < 0.001); however, there were no differences between intercostal and either genioglossus or hyoglossus muscles, or between genioglossus and hyoglossus muscles.
Fig. 2.
The coefficient of variation (CV) of the interspike interval (ISI) for all recorded motor units from the GG, HG, IC, and diaphragm muscles. The horizontal bars indicate the mean value. The CV of ISI in diaphragm motor units was significantly lower that in all other muscles. ***P < 0.001 vs. diaphragm.
Coherence of motor units within a muscle.
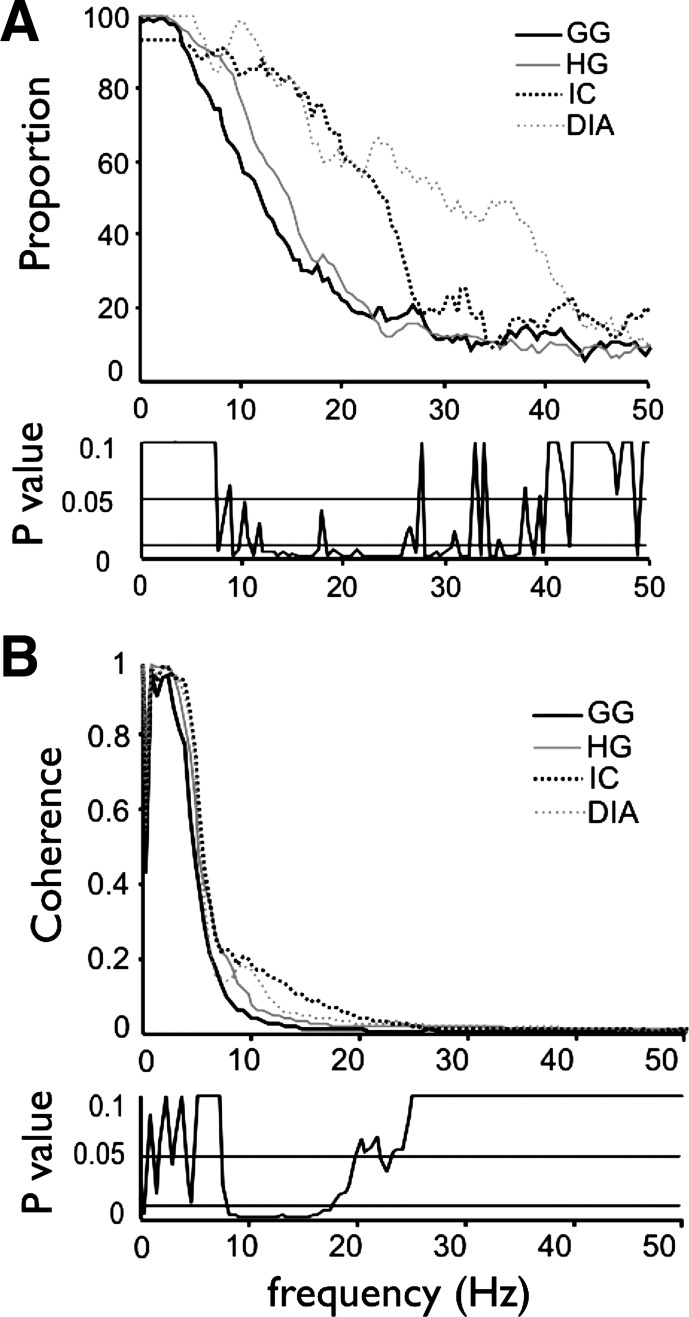
Simultaneous recordings of two motor units within a muscle allowed us to determine the proportion of the motor unit pairs of the muscle that show coherent discharge at each frequency from 0–50 Hz (Fig. 3A). For all four muscles, the proportion of coherent motor unit pairs was very high at low frequencies and fell monotonically from ∼8–50 Hz. The curves for intercostal and diaphragm muscle motor units were right shifted compared with the tongue-muscle curves, such that the frequency at which 50% of the motor unit pairs showed significant coherence was 12 and 15 Hz for genioglossus and hyoglossus vs. 23 and 30 Hz for the intercostal and diaphragm-muscle motor units.
Fig. 3.
Proportion of coherent motor unit pairs and average coherence magnitude for all within-muscle comparisons. A: proportion of within-muscle motor unit pairs showing significant coherence at each frequency, for all 4 muscles. The proportion of coherent motor unit pairs in each of the 4 muscles and at each frequency was compared statistically (see methods), and the results of this analysis are depicted as P values in the lower section of A. B: average coherence magnitude between motor unit pairs within each of the muscles, with the P values derived from one-way ANOVA located beneath the coherence data. There were significant differences in coherence magnitude across the 9–20-Hz bandwidth.
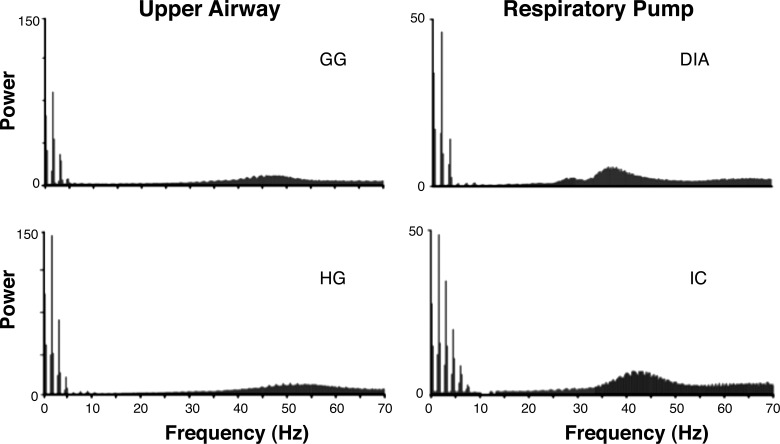
Average coherence magnitudes for motor unit pairs recorded within a muscle are shown over the 0–50-Hz frequency range in Fig. 3B. Note that all muscles showed very high coherence values over the 1–8-Hz bandwidth, reflecting the fundamental respiratory burst frequency and the first three-four harmonics of that frequency, as shown by analysis of the power spectra of individual motor units (see representative examples in Fig. 4).
Fig. 4.
Power-spectral density of representative single motor unit discharges from each of the muscles. Note the large power values at the fundamental respiratory frequency and the first 2–3 harmonics of this fundamental frequency. The power in the 30–60-Hz range reflects the approximate mean firing rate of the motor units.
The average coherence magnitude of motor unit pairs from each of the four muscles was compared statistically by converting raw coherence values to Z-scores followed by one-way ANOVA (see methods), and the results are provided in Fig. 3B, bottom. ANOVA revealed systematic and highly significant differences throughout the 10–20-Hz frequency band, whereas post hoc analyses confirm that the 10–20-Hz differences were dominated by contrasts between tongue and chest-wall muscles.
Coherence of motor units across the genioglossus and hyoglossus muscles.
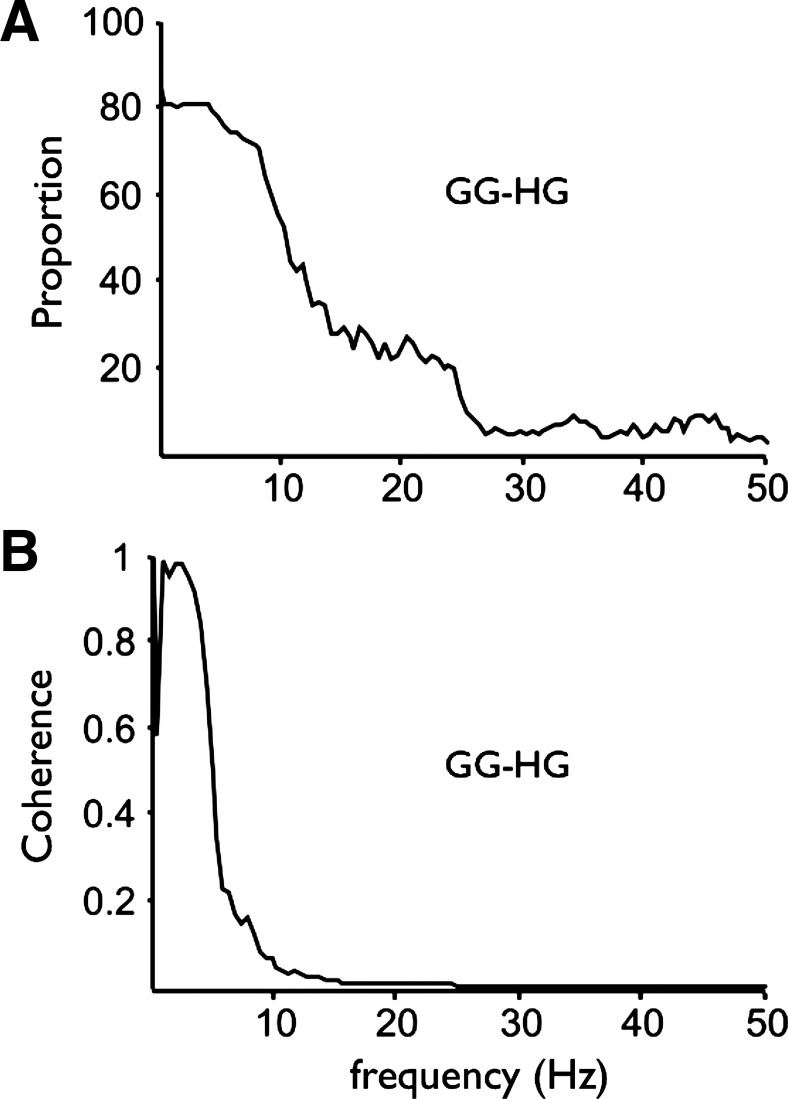
The proportion of genioglossus-hyoglossus motor unit pairs showing significant coherence at each frequency is shown in Fig. 5A. As for the within-muscle comparisons shown in Fig. 3A, the proportion of coherent motor unit pairs across the genioglossus and hyoglossus muscles was very high at low frequencies but in this case fell more sharply. The frequency at which 50% of the motor unit pairs showed significant coherence was in the range of 9–11 Hz. Coherence magnitude between hyoglossus and genioglossus muscle motor units falls off very sharply at frequencies above 8 Hz, suggesting that almost all of the coherent synchronization of motor units in the genioglossus and hyoglossus muscles arises in the respiratory central pattern generator (Fig. 5B).
Fig. 5.
Proportion and average coherence magnitude for motor units recorded simultaneously from the GG and HG. A: proportion of GG-HG motor unit pairs showing significant coherence at each frequency. B: average coherence-magnitude coherence between GG-HG motor unit pairs. Both the proportion of coherent GG-HG pairs and the coherence magnitude for these across-muscle comparisons are very similar to the within-muscle comparisons (GG-GG and HG-HG) shown in Fig. 3A.
Coherence: comparison of tongue muscles with muscles of the chest wall.
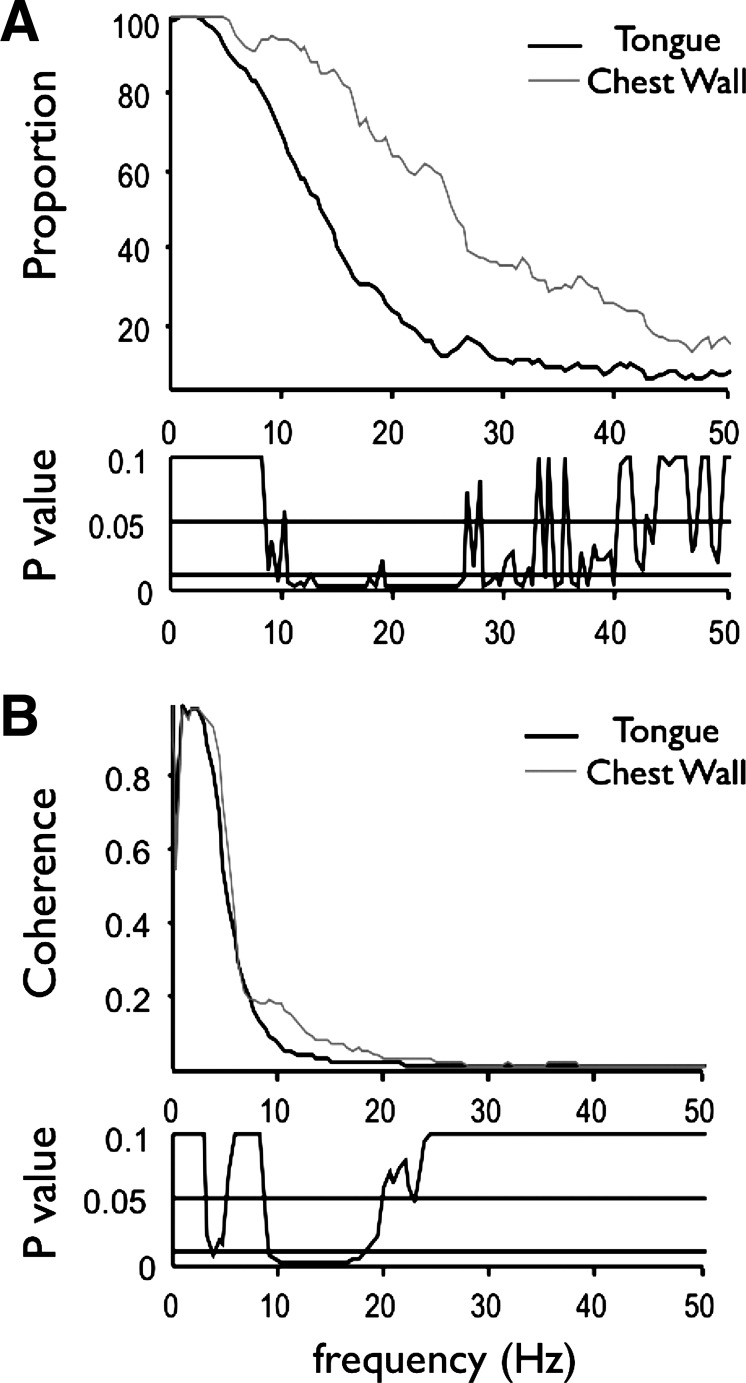
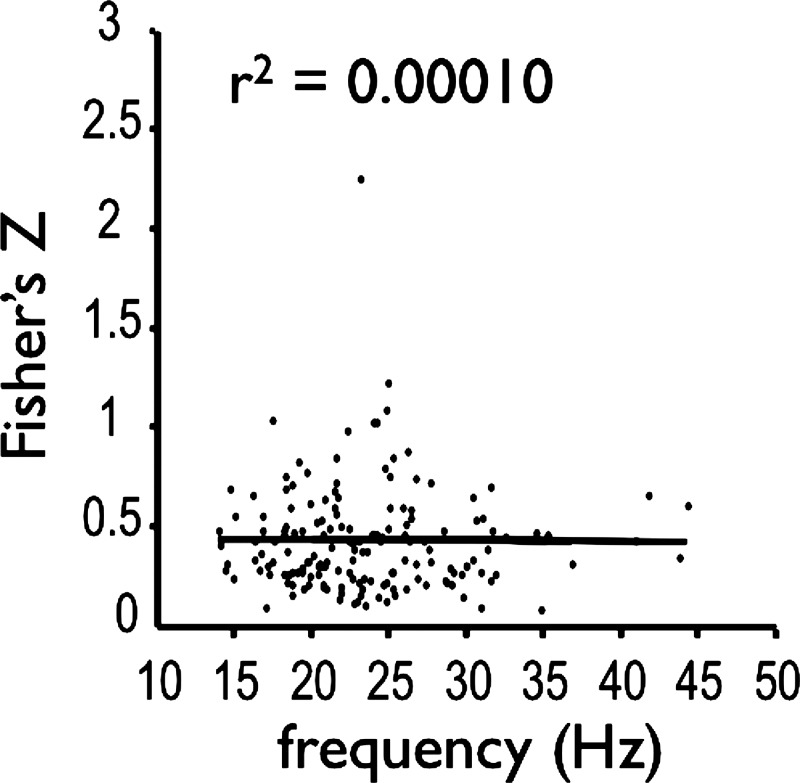
We combined all motor unit pairs recorded within each of the tongue muscles and all motor unit pairs recorded within each of the chest wall muscles to assess differences in motor unit coherence between spinal and cranial motoneurons (Fig. 6). Motor units of chest-wall muscles are more likely to show coherent oscillations than are tongue-muscle motor units over the 10–40-Hz bandwidth (Fig. 6A). The frequency where 50% of the pairs showed significant coherence was about 13 Hz for tongue-muscle motor units and 26 Hz for motor units from the chest-wall muscles. Significant differences in coherence magnitude between tongue and chest-wall motor units were detected in the 3–5-Hz and 10–20-Hz bandwidths, with chest-wall motor unit pairs exhibiting significantly higher coherence values than tongue-muscle motor unit pairs (Fig. 6B). Because previous studies in limb muscles show that the computation of coherence can be influenced by discharge rate (Christou et al. 2007; Lowery and Erim 2005), we examined the relationship between coherence magnitude and firing rate for all motor unit pairs by transforming coherence into Fisher's Z-scores, computing the geometric mean discharge rate for each pair of motor units, and performing a Pearson-correlation analysis. As shown in Fig. 7, the relationship between coherence and discharge rate is flat (r2 = 0.00010).
Fig. 6.
The proportion (A) and magnitude (B) of coherence in motor unit pairs from tongue and chest-wall muscles. For this analysis we combined all motor unit pairs from both tongue muscles and all pairs from the two chest-wall muscles to compare the proportion and magnitude of coherence in cranial and spinal motoneurons. Note that coherent oscillations in chest-wall muscle motor units are significantly higher than tongue-muscle units over the 8–20-Hz bandwidth.
Fig. 7.
Coherence magnitude (transformed into Fisher's Z-scores) as a function of discharge rate for all motor unit pairs studied. The mean discharge rate was computed as the geometric mean of the average discharge rate of each motor unit in a pair. The regression line and r2 values are shown.
Because we did not measure blood gases in our experiments, it is possible that changing anesthetic levels and thus blood gases, both within and across experiments, may have biased our results. Accordingly, for each pair of motor units studied, we measured the animal's respiratory frequency as an index of anesthetic depth and computed the correlation between the magnitude-squared coherence of each motor unit pair and the respiratory frequency. The results of that analysis show no relationship between breathing rate and coherence in the 10–20-Hz range (r2 = 0.0008, P = 0.76). In addition, to insure that there were no systematic differences in anesthetic depth during recordings of chest-wall-muscle and tongue-muscle motor unit pairs, we compared the average breathing frequency associated with all recordings of diaphragm, intercostal, hyoglossus, and genioglossus motor unit pairs. The results of that analysis also revealed no significant differences (F = 0.169, P = 0.174).
DISCUSSION
Summary.
Phasically driven motor units in muscles of the tongue and chest wall show strong correlated activity at frequencies between ∼1.5 and 8 Hz. Coherence at these low frequencies represents synchronized presynaptic oscillations at the fundamental frequency of the respiratory central pattern generator and harmonics of this fundamental frequency. These observations were expected; as in our preparation the motor units are driven spontaneously by an exceptionally strong, stereotyped input function that is distributed concurrently to spinal (intercostal, diaphragm) and cranial (tongue muscles) motoneurons. Although there were small differences in the coherence profiles of tongue vs. chest-wall motor units in the 3–5-Hz range, the largest and most consistent differences were between about 10 and 20 Hz, with chest-wall-muscle motor units showing higher levels of coherence throughout this frequency band. We propose that the lower coherence in tongue-muscle motor units over this range reflects a larger constellation of presynaptic inputs, which collectively lead to a reduction in the coherence between hypoglossal motoneurons in this frequency band. This, in turn, may reflect the relative simplicity of the respiratory drive to the diaphragm and intercostal muscles, compared with the greater diversity of functions fulfilled by muscles of the tongue.
Motor unit discharge rates and variability.
Tongue-muscle motor units had significantly higher discharge rates than the two chest-wall muscles, with the hyoglossus muscle having the highest of all and the diaphragm the lowest. This is consistent with recent data in human subjects, showing rates of 10–18 Hz in diaphragm, 8–11 Hz in external intercostal muscles, and 14–30 Hz in the genioglossus muscle (Saboisky et al. 2007a; Saboisky et al. 2007b). In adult rats, the input resistance of phrenic and hypoglossal motoneurons is about 2 and 12 Mohms, respectively (Hayashi and Fukuda 1995; Viana et al. 1995). Similarly, rheobase current ranges from 5–14 nA in phrenic motoneurons (Jodkowski et al. 1987) and 1–2 nA in hypoglossal motoneurons (Takata et al. 1980). Thus equivalent levels of synaptic input should result in higher discharge rates in hypoglossal motoneurons, as they are intrinsically more excitable.
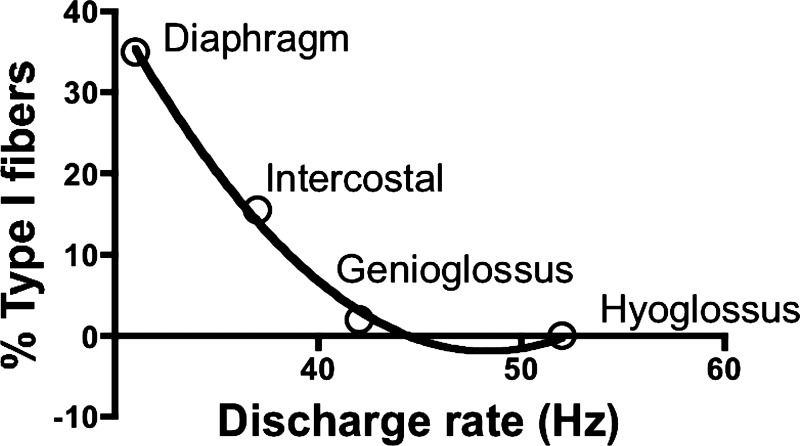
Interestingly, the coefficient of variation of motor unit discharge rates is also significantly lower in the diaphragm compared with the other muscles. Given that the variability of interspike intervals increases as a function of the amplitude and frequency content of synaptic noise and the duration of the afterhyperpolarization current (Powers et al. 2002), this observation is consistent with increased synaptic noise and/or different intrinsic motoneuron properties in tongue compared with diaphragm muscles. Taken together, these observations suggest that motor units with lower intrinsic excitability have slower and more uniform firing profiles. Interestingly, using our average firing rate data together with published data on the proportion of type I muscle fibers for each of the muscles yields an inverse, monotonic relationship (Fig. 8). It is noteworthy that the tongue muscles have either no type I fibers (hyoglossus) or just a few (genioglossus) inasmuch as histochemical fiber type correlates with muscle-shortening velocity, axon diameter, and input resistance (Sawczuk et al. 1995). This relationship is expected but is consistent with the significant differences in firing rates in the tongue and chest-wall muscles that we observed here.
Fig. 8.
Relationship between the average discharge rate that we recorded in rodent hyoglossus, genioglossus external intercostal, and diaphragm muscles and the percentage of type I muscle fibers in each muscle, as reported by others (Cunningham et al. 1991; LaFramboise et al. 1992; Prakash et al. 2000; Smith et al. 2005; Sutlive et al. 2000).
Coherence of motor units within a muscle.
Previous studies have examined the correlated discharge of pairs of motor units during volitional contractions in human subjects and used coherence analysis to estimate the extent to which in-phase oscillations synchronize motoneuron discharge (Baker et al. 1999; Farmer et al. 1997; Laine and Bailey 2011; Lowery et al. 2007). Similarly, in vitro studies have shown that in-phase oscillations in the 20–50-Hz range are involved in spike-timing precision in phrenic motoneurons (Parkis et al. 2003) and that the reliability of action potential discharge in hypoglossal motoneurons is highest when the input frequency of an injected sinusoid is in the 3–25-Hz range (van Brederode and Berger 2008). Our data are consistent with these observations inasmuch as the frequency at which 50% of the motor unit pairs showed significant coherence is about 13 and 26 Hz for tongue and chest-wall muscles, respectively (Fig. 6A). However, we have also demonstrated that the incidence and magnitude of inspiratory-phase coherence in the 10–20-Hz bandwidth is consistently and significantly greater in muscles of the chest wall compared with muscles of the tongue (Fig. 6, A and B). Although the reason for this difference is unknown, it is clear that statistically significant coherence in this frequency band would require a reasonably strong in-phase oscillation that is common to each of the motor units being analyzed. However, if one motoneuron pool receives more sources of input that contain activity in the 10–20-Hz range, and if those inputs arrive out of phase with each other, they will summate destructively, leading to relatively weaker coherence. On the basis of this idea, we suggest that, in addition to the inputs from the respiratory central pattern generator that appear to be shared equally by both motoneuron pools, the hypoglossal motoneurons receive a wider constellation of presynaptic inputs than phrenic/intercostal motoneurons, possibly attributable to the wider range of functions performed by tongue muscles compared with diaphragm or intercostal muscles. Some evidence for this conclusion includes robust, inspiratory-phase GABAergic and glycinergic inputs to hypoglossal motoneurons from the reticular formation (Fenik et al. 2004; Fenik et al. 2005; O'Brien et al. 2004; Remmers et al. 1980; Withington-Wray et al. 1988; Woch and Kubin 1995) and also the nucleus of Roller (Marchetti et al. 2002; O'Brien et al. 2004), which is a relay site for sensory afferents. Importantly, our previous work in the same preparation showed that pulmonary stretch receptors evoke much stronger inhibition of tongue compared with intercostal muscles (Bailey et al. 2001; Fregosi and Fuller 1997; Janssen et al. 2000), consistent with stronger inhibitory synaptic inputs to hypoglossal compared with intercostal motoneurons.
Coherence between genioglossus and hyoglossus muscle motor units.
In the last decade, our laboratory has documented respiratory-related cohactivation of protrudor and retractor tongue muscles in the rat (Bailey and Fregosi 2004; Bailey et al. 2001, 2005; Fuller et al. 1998; Janssen and Fregosi 2000; Janssen et al. 2000), observations that were subsequently confirmed in human subjects (Mateika et al. 1999). The present results show very low coherence at frequencies above those that are due to the fundamental respiratory frequency and the first few harmonics of this fundamental frequency. These results suggest that presynaptic input from the central pattern generator is distributed broadly to the hypoglossal premotor neuron pool, leading to synchronized excitation of hypoglossal motoneurons that drive both protrudor and retractor muscles of the tongue. Previous anatomic studies have shown that motoneurons driving the genioglossus and hyoglossus muscles in the rat are somatotopically organized within the hypoglossal motor nucleus (Dobbins and Feldman 1995; Gilliam and Goldberg 1995; Guo et al. 1996; McClung and Goldberg 1999, 2000, 2002), but we do not know whether the hypoglossal premotor neurons that convey excitatory synaptic input from the respiratory central pattern generator have unique projections to genioglossus and hyoglossus motoneurons, or whether they branch extensively to innervate motoneurons in two or more muscle motoneuron pools. Peever et al. (2001) used cross-correlation analysis of inspiratory bursts in medial and lateral hypoglossal nerve branches (which innervate the genioglossus and hyoglossus muscles, respectively) and found strongly correlated activity. In contrast, they failed to find significant correlations between hypoglossal and phrenic nerve activities. They interpreted the data as evidence for a common drive from the hypoglossal premotor neuron pool, which agrees with our findings of similar coherence profiles among genioglossus and hyoglossus motor units. These observations and the present ones suggest that excitatory synaptic input from the respiratory central pattern generator is distributed broadly to the hypoglossal premotor neurons, leading to respiration-related coactivation of genioglossus and hyoglossus muscles.
Conclusions.
Phasically driven motor units in muscles of the tongue and chest wall show strong correlated activity at frequencies between ∼1.5 and 8 Hz, reflecting presynaptic oscillations from the respiratory central pattern generator and harmonics of this fundamental frequency. Chest-wall muscle motor units consistently showed higher levels of coherence than tongue muscle motor units over the 10–20-Hz frequency band. We propose that the lower coherence in tongue-muscle motor units over this range reflects a larger constellation of presynaptic inputs, which collectively lead to a reduction in the coherence between hypoglossal motoneurons in this frequency band. This, in turn, may reflect the relative simplicity of the respiratory drive to the diaphragm and intercostal muscles, compared with the greater diversity of functions fulfilled by muscles of the tongue.
GRANTS
This work was supported by of NIDCD grant R01DC007692.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. E. Fiona Bailey for use of analysis software and a critique of the manuscript and Seres J. Bennett-Cross for technical assistance.
REFERENCES
- Amjad AM, Breeze P, Conway BA, Halliday DM, Rosenberg JR. A framework for the analysis of neuronal networks. Prog Brain Res 80: 243–255; discussion 239–242, 1989. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol 101: 609–617, 2006. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Huang Y, Fregosi RF. The anatomic consequences of intrinsic tongue muscle activation. J Appl Physiol 101: 1377–1385, 2006. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Janssen PL, Fregosi RF. PO2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med 171: 1403–1407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res 128: 109–117, 1999. [DOI] [PubMed] [Google Scholar]
- Christou EA, Rudroff T, Enoka JA, Meyer F, Enoka RM. Discharge rate during low-force isometric contractions influences motor unit coherence below 15 Hz but not motor unit synchronization. Exp Brain Res 178: 285–295, 2007. [DOI] [PubMed] [Google Scholar]
- Cunningham JM, Kaiser KK, Sanes JR. Rostrocaudal variation of fiber type composition in rat intercostal muscles. Histochemistry 95: 513–517, 1991. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol 357: 376–394, 1995. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol 470: 127–155, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods 74: 175–187, 1997. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Rudolph M, Destexhe A, Sejnowski TJ. Synaptic background noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. Neuroscience 122: 811–829, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus 10: 187–197, 2000. [DOI] [PubMed] [Google Scholar]
- Fellous JM, Tiesinga PH, Thomas PJ, Sejnowski TJ. Discovering spike patterns in neuronal responses. J Neurosci 24: 2989–3001, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch Ital Biol 142: 237–249, 2004. [PubMed] [Google Scholar]
- Fenik VB, Davies RO, Kubin L. Noradrenergic, serotonergic and GABAergic antagonists injected together into the XII nucleus abolish the REM sleep-like depression of hypoglossal motoneuronal activity. J Sleep Res 14: 419–429, 2005. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 110: 295–306, 1997. [DOI] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. J Appl Physiol 88: 2123–2130, 2000. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519: 601–613, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Parkis MA. High frequency oscillations in respiratory networks: functionally significant or phenomenological? Respir Physiol Neurobiol 131: 101–120, 2002. [DOI] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol 74: 547–555, 1995. [DOI] [PubMed] [Google Scholar]
- Guo Y, Goldberg SJ, McClung JR. Compartmental organization of styloglossus and hyoglossus motoneurons in the hypoglossal nucleus of the rat. Brain Res 728: 277–280, 1996. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Fukuda Y. Electrophysiological properties of phrenic motoneurons in adult rats. Jpn J Physiol 45: 69–83, 1995. [DOI] [PubMed] [Google Scholar]
- Huang WX, Christakos CN, Cohen MI, He Q. Possible network interactions indicated by bilaterally coherent fast rhythms in expiratory recurrent laryngeal nerve discharges. J Neurophysiol 70: 2192–2196, 1993. [DOI] [PubMed] [Google Scholar]
- Huang WX, Cohen MI, Yu Q, See WR, He Q. High-frequency oscillations in membrane potentials of medullary inspiratory and expiratory neurons (including laryngeal motoneurons). J Neurophysiol 76: 1405–1412, 1996. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol 89: 1345–1351, 2000. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Williams JS, Fregosi RF. Consequences of periodic augmented breaths on tongue muscle activities in hypoxic rats. J Appl Physiol 88: 1915–1923, 2000. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol 58: 105–124, 1987. [DOI] [PubMed] [Google Scholar]
- John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med 172: 1331–1337, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriener B, Tetzlaff T, Aertsen A, Diesmann M, Rotter S. Correlations and population dynamics in cortical networks. Neural Comput 20: 2185–2226, 2008. [DOI] [PubMed] [Google Scholar]
- LaFramboise WA, Watchko JF, Brozanski BS, Daood MJ, Guthrie RD. Myosin heavy chain expression in respiratory muscles of the rat. Am J Respir Cell Mol Biol 6: 335–339, 1992. [DOI] [PubMed] [Google Scholar]
- Laine CM, Bailey EF. Common synaptic input to the human hypoglossal motor nucleus. J Neurophysiol 105: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery MM, Erim Z. A simulation study to examine the effect of common motoneuron inputs on correlated patterns of motor unit discharge. J Comput Neurosci 19: 107–124, 2005. [DOI] [PubMed] [Google Scholar]
- Lowery MM, Myers LJ, Erim Z. Coherence between motor unit discharges in response to shared neural inputs. J Neurosci Methods 163: 384–391, 2007. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Pagnotta S, Donato R, Nistri A. Inhibition of spinal or hypoglossal motoneurons of the newborn rat by glycine or GABA. Eur J Neurosci 15: 975–983, 2002. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec 260: 378–386, 2000. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of motoneurons in the dorsal hypoglossal nucleus that innervate the retrusor muscles of the tongue in the rat. Anat Rec 254: 222–230, 1999. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of the hypoglossal motoneurons that innervate the horizontal and oblique components of the genioglossus muscle in the rat. Brain Res 950: 321–324, 2002. [DOI] [PubMed] [Google Scholar]
- Myers LJ, Erim Z, Lowery MM. Time and frequency domain methods for quantifying common modulation of motor unit firing patterns (Abstract). J Neuroeng Rehabil 1: 2, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Sebe JY, Berger AJ. GABA(B) modulation of GABA(A) and glycine receptor-mediated synaptic currents in hypoglossal motoneurons. Respir Physiol Neurobiol 141: 35–45, 2004. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J Neurosci 23: 8152–8158, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Duffin J. Respiratory control of hypoglossal motoneurons. Adv Exp Med Biol 499: 101–106, 2001. [DOI] [PubMed] [Google Scholar]
- Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflügers Arch 442: 78–86, 2001. [DOI] [PubMed] [Google Scholar]
- Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience 110: 711–722, 2002. [DOI] [PubMed] [Google Scholar]
- Powers RK, Turker KS, Binder MD. What can be learned about motoneurone properties from studying firing patterns? Adv Exp Med Biol 508: 199–205, 2002. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol 89: 563–572, 2000. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Anch AM, deGroot WJ, Baker JP, Jr, Sauerland EK. Oropharyngeal muscle tone in obstructive sleep apnea before and after strychnine. Sleep 3: 447–453, 1980. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Walsh LD, Gandevia SC. New display of the timing and firing frequency of single motor units. J Neurosci Methods 162: 287–292, 2007a. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol 102: 772–780, 2007b. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Intrinsic properties of motoneurons. Implications for muscle fatigue. Adv Exp Med Biol 384: 123–134, 1995. [PubMed] [Google Scholar]
- Sebe JY, Berger AJ. Inspiratory-phase short time scale synchrony in the brainstem slice is generated downstream of the pre-Botzinger complex. Neuroscience 153: 1390–1401, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, van Brederode JF, Berger AJ. Inhibitory synaptic transmission governs inspiratory motoneuron synchronization. J Neurophysiol 96: 391–403, 2006. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J Neurosci 26: 1673–1676, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Goldberg SJ, Shall MS. Phenotype and contractile properties of mammalian tongue muscles innervated by the hypoglossal nerve. Respir Physiol Neurobiol 147: 253–262, 2005. [DOI] [PubMed] [Google Scholar]
- Sutlive TG, Shall MS, McClung JR, Goldberg SJ. Contractile properties of the tongue's genioglossus muscle and motor units in the rat. Muscle Nerve 23: 416–425, 2000. [DOI] [PubMed] [Google Scholar]
- Takata M, Shohara E, Fujita S. The excitability of hypoglossal motoneurons undergoing chromatolysis. Neuroscience 5: 413–419, 1980. [DOI] [PubMed] [Google Scholar]
- Tetzlaff T, Rotter S, Stark E, Abeles M, Aertsen A, Diesmann M. Dependence of neuronal correlations on filter characteristics and marginal spike train statistics. Neural Comput 20: 2133–2184, 2008. [DOI] [PubMed] [Google Scholar]
- van Brederode JF, Berger AJ. Spike firing resonance in hypoglossal motoneurons. J Neurophysiol 99: 2916–2928, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Repetitive firing properties of developing rat brainstem motoneurones. J Physiol 486: 745–761, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience 25: 1041–1051, 1988. [DOI] [PubMed] [Google Scholar]
- Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport 6: 2085–2088, 1995. [DOI] [PubMed] [Google Scholar]